ABSTRACT
Purpose
We investigated short-term (9 d) exposure to low energy availability (LEA) in elite endurance athletes during a block of intensified training on self-reported well-being, body composition, and performance.
Methods
Twenty-three highly trained race walkers undertook an ~3-wk research-embedded training camp during which they undertook baseline testing and 6 d of high energy/carbohydrate (HCHO) availability (40 kcal·kg FFM−1·d−1) before being allocated to 9 d continuation of this diet (n = 10 M, 2 F) or a significant decrease in energy availability to 15 kcal·kg FFM−1·d−1 (LEA: n = 10 M, 1 F). A real-world 10,000-m race walking event was undertaken before (baseline) and after (adaptation) these phases, with races being preceded by standardized carbohydrate fueling (8 g·kg body mass [BM]−1 for 24 h and 2 g·kg BM−1 prerace meal).
Results
Dual-energy x-ray absorptiometry–assessed body composition showed BM loss (2.0 kg, P < 0.001), primarily due to a 1.6-kg fat mass reduction (P < 0.001) in LEA, with smaller losses (BM = 0.9 kg, P = 0.008; fat mass = 0.9 kg, P < 0.001) in HCHO. The 76-item Recovery–Stress Questionnaire for Athletes, undertaken at the end of each dietary phase, showed significant diet–trial effects for overall stress (P = 0.021), overall recovery (P = 0.024), sport-specific stress (P = 0.003), and sport-specific recovery (P = 0.012). However, improvements in race performance were similar: 4.5% ± 4.1% and 3.5% ± 1.8% for HCHO and LEA, respectively (P < 0.001). The relationship between changes in performance and prerace BM was not significant (r = −0.08 [−0.49 to 0.35], P = 0.717).
Conclusions
A series of strategically timed but brief phases of substantially restricted energy availability might achieve ideal race weight as part of a long-term periodization of physique by high-performance athletes, but the relationship between BM, training quality, and performance in weight-dependent endurance sports is complicated.
Key Words: RED-S, PHYSIQUE MANAGEMENT, ENDURANCE ATHLETE, LOW ENERGY AVAILABILITY, LEA
Low energy availability (LEA) is the exposure variable that underpins the impairment of the health and performance in athletes via the syndromes variously described as relative energy deficiency in sport (RED-S) (1), and female and male athlete triad (Triad) (2–4) energy availability (EA) is defined as the amount of dietary energy remaining to support resting physiological function after subtracting the energy cost of exercise (5). A mismatch between energy intake and energy committed to training and competition, resulting in insufficient energy to maintain normal hormonal and metabolic function, can arise from a number of psychological, biological, and behavioral factors (6). The expert opinion that exposure to LEA can be associated with a variety of health and performance concerns is supported by narrative reviews (7,8), prospective data from interventions undertaken in metabolic ward (9,10), or more free-living (11–13) scenarios, longitudinal observational studies (14), and cross-sectional data (for a review, see [15,16]). Nevertheless, there is an apparent inconsistency between individuals with regard to the type, prevalence, and severity of the impairments of different body systems associated with periods of LEA (15,16). In addition, some of the scenarios in which LEA occurs or is deliberately implemented, such as manipulation of body mass (BM)/composition or blocks of intensified training, are an integral part of endurance sport in terms of expert models of periodization (17) and observed athlete practices (for a review, see [18]). Indeed, although harmful practices related to physique management or weight making are seen in some sports (19), there is also observational evidence that athletes can successfully periodize their body composition within a narrow range, across and between the annual training program, according to their exercise characteristics and competition goals (20–22).
One scenario in which LEA often occurs is during periods of intensified training in the base phase of the annual training plan of the endurance athletes. At the commencement of the training season, the athlete may have reduced aerobic capacity and suboptimal body composition, with goals to address these issues and improve their “power-to-weight/BM” ratio during the first training block. Nevertheless, they may also be scheduled to compete in early season racing with expectations of reasonable performance. Chronic exposure to LEA may impair endurance performance via a reduction in training capacity and adaptation (14) as well as an acute depletion of muscle fuel reserves (23). Perturbations to markers of body function such as the reproductive system (9), iron and immune function (13), bone turnover (24), and muscle fuel stores (23) appear within ~6 d of LEA exposure and merit the investigation of the potential to be restored in a similar timeframe. Nevertheless, it is possible that the periodization of short-duration high-magnitude energy restriction followed by restoration of fuel stores might offer a practical strategy to manipulate small but potentially important shifts in body mass/body fat with short-term but reversible effects on physiological and psychological parameters.
Accordingly, the aim of this study was to investigate the effect of a short-term (9 d) exposure to a substantial reduction in EA, from an EA of ~40 kcal (189 kJ)·kg fat-free mass (FFM)−1·d to 15 kcal (63 kJ)·kg·FFM−1·d−1 in elite endurance athletes during a block of intensified training on race performance after acute restoration of energy and carbohydrate (CHO) intake. We hypothesized that the LEA would be associated with small but transient changes in metabolism (i.e., substrate utilization) training capacity and well-being (e.g., mental stress, perceptions of recovery, and fatigue), while achieving a detectable change in body fat and body mass. However, acute restoration of CHO availability and EA for 24 h, as undertaken according to sports nutrition guidelines and included in the baseline and control group practices (25), would restore exercise capacity while maintaining body composition changes, allowing athletes to achieve similar (or even superior) benefits to race performance as athletes undertaking the same training program supported by HCHO.
METHODS
Participants and overall design
Twenty-three highly trained race walkers (20 males, 3 females) were recruited for one of two ~3-wk research-embedded training camps from which this data set was collected (2019: Canberra, Australia [~580 m altitude] and 2021: Melbourne, Australia [sea level]). Previous work from our group has noted significant changes in the key variable of interest (race performance) from such training camps with sample sizes of ~8–10 athletes (26), including mixed-sex groups (27). The group consisted of athletes in tiers 3–5 of a standardized classification of athletic caliber (tier 3, n = 5; tier 4, n = 15; tier 5, n = 3) (28). One athlete (male, tier 4) was not able to complete the second V̇O2peak assessment or race 2 because of an unrelated injury, leaving a cohort of 22 athletes (19 males, 3 females) for this aspect of the study. The training camps were conducted at the beginning of the annual season, with athletes training for either the 20- or the 50-km race walking events for national and international competition. Athletes were informed of the risks and requirements of the study before providing written informed consent. Ethics approval was obtained from the Ethics Committee of the Australian Institute of Sport (2019; no. 20181203) and the Australian Catholic University (2021; no. 2020-238HC).
This study involved two dietary phases, with testing blocks involving laboratory testing and a field-based race, completed before and after these phases (Fig. 1). During the baseline phase, athletes undertook the initial test protocols over 4 d, while consuming a fixed high-CHO (~8 g·kg−1·d−1)/high-energy (~52 kcal·kg−1·d−1) intake. This was implemented while the characteristics required for EA calculations were assessed, after which all participants switched to a high-CHO/energy diet based on EA (HCHO). On completion, they were then assigned to parallel groups either continuing the HCHO diet (HCHO group; n = 10 males, 2 females) or switching to an LEA (15 kcal·kg−1·d−1) diet (LEA group; n = 10 males, 1 female), accounting for each athlete’s nominated treatment preference (26) while attempting to match each group for individual characteristics (e.g., age, 20-km personal best time, training status, and load). Baseline characteristics of each group (HCHO vs LEA) were as follows: age, 25.5 ± 6.9 versus 29.3 ± 4.5 yr (NS); height, 175.6 ± 7.4 versus 177.1 ± 8.7 cm (NS); body mass (BM), 64.3 ± 7.1 versus 66.4 ± 6.8 kg (NS); V̇O2peak, 61.9 ± 4.7 versus 62.1 ± 5.8 mL·kg−1·min−1 (NS); personal best in a 10-km race walk, 42:32 ± 2:23 versus 40:41 ± 1:35 mm:ss (P < 0.05); and personal best in a 20-km race walk, 1:26:12 ± 5:10 versus 1:21:55 ± 3:28 h:ms:ss (P < 0.05).
FIGURE 1.
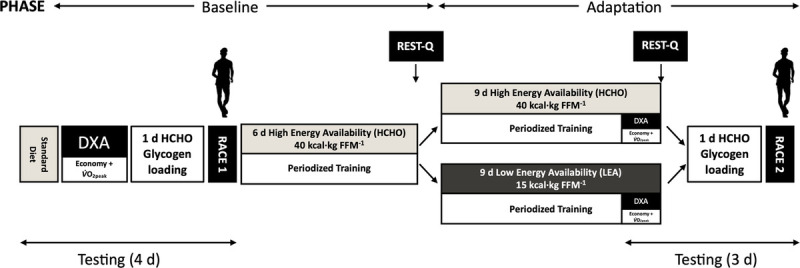
Schematic representation of the study methodology involving highly trained race walkers (n = 20 M, 3 F). The protocol consisted of a baseline phase involving 6 d of exposure to diet with high energy/CHO availability (HCHO), followed by a 9-d adaptation phase in which athletes were allocated to either HCHO or a diet of LEA. Before and after these dietary phases, the two groups of athletes undertook laboratory testing and a real-life 10,000-m race walking competition (Race) preceded by the same 1 d high-CHO glycogen loading diet.
The study was part of a larger investigation developed to investigate the effect of LEA on a number of themes of metabolism, health, and performance (details and results are presented elsewhere [13,29]). This study focuses on outcomes related to physiology (body composition, race walking economy, V̇O2peak) and well-being (perceived stress and recovery), in support of the primary end point of race performance. Because the classic studies of LEA involved 5 d exposure, we commenced a series of test protocols after 6 d, with the performance testing battery (adaptation testing block; Fig. 1) being scheduled at the end, after 9 d exposure. During both dietary phases, athletes followed a semistructured training plan, with key sessions completed as a group and all other training recorded in an electronic training diary. Weekly training involved ~115 km of “on-legs” training (race walking or running cross-training) and included two long walks, a track interval session, a hill session, and 1–2 gym sessions.
Dietary intervention
All menus were developed by accredited sports dietitians/nutritionists and consumed by athletes in a communal living environment in which dietary compliance was monitored. The HCHO diet was targeted at an EA of 40 kcal·kg FFM−1·d−1, with macronutrient ratios of ~65% CHO, 15% protein, and 20% fat to allow CHO intake to scale to the fuel demands of training. The LEA intervention limited EA to 15 kcal·kg FFM−1·d−1, with macronutrient composition of ~60% CHO, 25% protein, and 15% fat. Daily EA was determined as [Energy intake (EI) – exercise energy expenditure (EEE)]/(kg FFM) (5).
To determine target EI, EEE was calculated from each individual athlete’s physiological testing data and training plan. Specifically, oxygen consumption determined during a four-stage submaximal economy test (see below) was averaged and subsequently converted to kilocalories expended per kilometer of race walking. This value was then used to predict the daily energy cost of each athlete’s individual training plan. Here, the daily EEE, which represents the additional energy cost attributed to exercise during a training/test session period rather than the total energy expenditure during the period (30), was calculated by subtracting resting metabolic rate from session energy expenditure. To promote the compliance and the accuracy of implemented EA, actual training logs were reviewed twice daily (at lunch and at dinner), and the remaining food intake for the day was adjusted if the athlete’s actual training resulted in a change in EEE that exceeded an EA of ~2.4 kcal·kg FFM−1 (~2 km of race walking). Data representing both the planned and the actual EEE calculations were collated. Dietary analysis was undertaken using FoodWorks computer software (FoodWorks 9; Xyris Software, Australia).
The standardized meal consumed 2 h before the laboratory testing protocol (economy testing and V̇O2peak) provided a CHO content of 2 g·kg BM−1 for the HCHO treatment, whereas the CHO content of the same meal on the LEA diet provided 1 g·kg BM−1 CHO. Because the aim of the prerace diet was to achieve similar muscle and liver glycogen repletion regardless of the preceding dietary protocol, athletes consumed the same standardized diet, providing 8 g·kg BM−1 CHO for the 24 h before each of the 10,000-m races. In addition, a standardized breakfast providing an additional 2 g·kg BM CHO−1 was consumed 2 h before the race start.
Testing: monitoring of body mass and composition
During the testing protocols, the race walkers undertook dual-energy x-ray absorptiometry (DXA) assessment of body composition. These measurements were undertaken in the early morning in an overnight fasted and rested state as previously described (31). The same DXA technician positioned participants on the iDXA (GE Healthcare, Milwaukee, WI) and analyzed all images (enCore v16, GE Healthcare). The test–retest technical error of measurement for the iDXA at our center is 0.1% for total mass, 0.4% for lean mass, 1.6% for fat mass, and 0.4% for bone mass. Body mass was measured before the commencement of all key training sessions and test protocols. Although some BM fluctuations between sessions were expected due to acute changes in nutrition and hydration status over the day, general trends in BM over the dietary interventions were tracked by focusing on sessions completed under similar conditions, including those with robust standardization of the time of day and feeding (e.g., race day, V̇O2peak test).
Testing: economy and V̇O2peak
The laboratory testing protocols included an incremental exercise test to exhaustion, which was performed 2 h after the intake of the standardized test meal associated with their diet. In camp 1, this was undertaken in the Australian Institute of Sport Laboratory using a customized treadmill as previously reported (26). In camp 2, testing was conducted in the Australian Catholic University Melbourne Campus Performance Laboratory using a motorized treadmill (Pulsar 3p; h/p/cosmos, Nussdorf-Traunstein, Germany). After a 10-min self-selected and individually standardized warm-up, walking economy was assessed during four submaximal stages, with each lasting 4 min and increasing in speed by 1 km·h−1 per stage. The selected starting speeds were 10, 11, or 12 km·h−1 based on each individual’s personal best times for the 20-km road race and increased to 13–15 km·h−1 for the final stage. The speeds of the second and fourth stages corresponded approximately to each individual athlete’s average walking pace for the 50- and 20-km race walk events, respectively. Each stage was followed by 1 min rest for the collection of capillary (fingertip) blood samples to assess blood lactate (Lactate Pro 2; Akray Inc., Kyoto, Japan), ketone bodies (β-hydroxybutyrate, FreeStyle Optium Neo; Abbott Diabetes Care, Victoria, Australia), and glucose (FreeStyle Optium Neo, Abbott Diabetes Care) concentrations, as well as RPE (6–20 Borg scale). Heart rate (HR) was measured continuously throughout the test (Polar Heart Rate Monitor; Polar Electro, Kempele, Finland). In camp 1, expired gas was collected and analyzed using a custom-built indirect calorimetry system described previously (32), whereas in camp 2, expired gas was collected and analyzed via open-circuit spirometry (TrueOne 2400; Parvo Medics, Sandy, UT). For both camps, the final 60 s of gas was collected and accepted as steady state, and rates of O2 consumption (V̇O2) and CO2 production (V̇CO2) were used to calculate the RER.
On completion of the final submaximal walking stage, athletes rested for 5 min before completing a ramp (speed and then gradient) test to volitional fatigue. Treadmill speed was increased by 0.5 km·h−1 every 30 s until the speed corresponding to the individual’s final submaximal stage was reached (13–15 km·h−1), with treadmill gradient then increasing by 0.5% every 30 s thereafter, until exhaustion. Expired gas was collected and analyzed throughout, maximal HR recorded, and capillary blood samples collected 1 min after completion. Rates of CHO and fat oxidation (g·min−1) were calculated from V̇CO2 and V̇O2 values using nonprotein RER values (33). These equations are based on the premise that V̇O2 and V̇CO2 accurately reflect tissue O2 consumption and CO2 production, and that indirect calorimetry is a valid method for quantifying rates of substrate oxidation in well-trained athletes during strenuous exercise of up to ~85% of V̇O2peak (34).
Testing: 10,000-m race
The final component of the testing protocols involved a real-life athletic competition. Athletes competed in a 10,000-m race held on a synthetic 400 m outdoor athletics track (camp 1: AIS Canberra, ACT, Australia; camp 2: Clifton Hill, Victoria, Australia). Each race commenced at 0900 h and was conducted under World Athletics rules, which involved officiating by technical judges, invitation for participation by competitors external to the study, prize money, and the pit-lane rule for technique infringements. Official race times were recorded by photo finish (2019) or hand timing by race officials (2021).
The goals of the race nutrition plan were to standardize the individualized competition practices of each participant. Therefore, each athlete repeated a similar training load during the 48 h before each race (economy/aerobic capacity test schedule and personal training) and, as described previously, consumed the same HCHO diet over the 24-h prerace period and prerace meal. An individualized warm-up was undertaken and repeated for each race. The use of performance supplements was permitted when it did not interfere with the treatment diet and usage during the first race was documented and repeated for the second race. This allowed some athletes to use an identical preevent caffeine protocol for each race. The World Athletics policy to allow a water station in an outside lane in hot weather was implemented due to the environmental conditions in the 2019 study and repeated for consistency in the 2021 camp.
Testing: Recovery–Stress Questionnaire for Athletes
The 76-item Recovery–Stress Questionnaire for Athletes (RESTQ-Sport-76) (35) was administered to athletes at the end of each dietary intervention with instructions to consider the effects of the previous 7 d. This questionnaire gauges the frequency of stress symptoms and recovery-associated activities/states during the specified period and addresses both nonspecific and sport-specific areas of stress and recovery. The questionnaire includes 76 statements that are divided into seven general stress scales, five general recovery scales (e.g., physical recovery), three sport-specific stress scales (e.g., emotional exhaustion), and four sport-specific recovery scales (e.g., self-regulation). The questionnaire has an internal consistency (Cronbach’s α = 0.67–0.89), and previous work has demonstrated a high test–retest reliability (r > 0.79) (35)
Statistical analysis
Results are expressed as mean ± SD. Using R Studio (v1.4.1), all variables were analyzed using general linear mixed models estimated with restricted maximal likelihood. Normality was assessed using residual and qq-plots, with only lactate concentrations showing evidence of nonnormal distribution. These data were therefore log-transformed before analysis. Homoscedasticity was tested with the Fligner–Killeen test, and where applicable, a Welsh’s adjustment implemented. For each model, fixed effects of diet (LEA or HCHO) and test (baseline or adaptation) were used, with random intercepts included for sex, subject identification, and camp. For the graded economy and V̇O2peak test data, an additional fixed effect for stage was incorporated. Models were then optimized by removing nonsignificant interactions and comparing models using Akaike information criteria. Statistical significance of fixed effects was determined using type II Wald tests with Kenward–Roger approximation. Where significant fixed effects were established, pairwise comparisons were identified using the Tukey post hoc adjustments. Significance was accepted at P < 0.05. The required sample size was calculated a priori using 10,000-m race performance as the primary outcome based on our previous work evaluating dietary interventions in similar populations (ref SN2). Specific sample size was calculated using G*Power software version 3.1 (Bonn University, Bonn, Germany). Based on such data, a sample size of seven athletes per group was considered appropriate (n = 8, critical t = 2.179, expected power 0.939, P < 0.05). To account for possible dropouts and/or nonadherence to diets, we attempted to recruit 12 athletes per group (24 total) and were successful in recruiting 23. As noted above, one athlete (male, tier 4) was unable to complete adaptation testing and withdrew from the study leaving a final n = 23.
RESULTS
Dietary intake
The diet and training protocols were implemented according to the study plan, and all athletes complied with the monitoring practices. Actual dietary intakes of the two groups during the baseline and adaptation dietary intervention protocols (summarized in Table 1) showed no differences between groups during the baseline period for EI, EA, or macronutrient intakes (all P > 0.05). These parameters did not differ between the baseline and the adaptation dietary interventions for the HCHO group (all P > 0.05). However, in comparison with both their baseline interventions and to the HCHO group, the dietary intake of the LEA group showed a significant decrease in EA during the adaptation intervention because of a decrease in EI (P < 0.001) and maintenance of EEE. Changes in EI during the LEA diet were achieved by reductions in CHO intake in absolute amounts (P < 0.001) and amounts relative to BM (P < 0.001), with similar changes in characteristics of fat intake (all P < 0.001). Protein intake was maintained in absolute amounts and relative to BM across each phase for both groups, requiring an increase in the contribution of protein to EI during the LEA diet (P < 0.001). Fiber intake was slightly lower during the LEA dietary intervention (P < 0.05) compared with the baseline phase for this group and the corresponding HCHO intervention. The recorded intake of both groups achieved the predetermined targets for EA and macronutrient contribution to EI for both phases (Table 1).
TABLE 1.
Dietary intake during 6-d baseline of high energy/CHO (HCHO) availability then 9-d adaptation to an LEA diet in highly trained race walkers undertaking endurance training.
| Target | HCHO (n = 10 M, 2 F) | LEA (n = 10 M, 1 F) | |
|---|---|---|---|
| Baseline: both groups = high energy/CHO availability diet | |||
| EI (MJ·d−1) | 15.1 ± 3.2 | 15.3 ± 1.7 | |
| EI (kJ·kg·d−1) | 235 ± 27 | 232 ± 7 | |
| EI (kcal·d−1) | 3612 ± 77 | 3660 ± 41 | |
| CHO (g·d−1) | 577 ± 125 | 588 ± 63 | |
| CHO (E%) | 65 | 64 ± 0.4 | 64 ± 0.4 |
| CHO (g·kg·d−1) | 9.0 ± 1.1 | 8.9 ± 0.3 | |
| Protein (g·d−1) | 135 ± 28 | 138 ± 16 | |
| Protein (E%) | 15 | 15 ± 0.3 | 15 ± 0.2 |
| Protein (g·kg·d−1) | 2.1 ± 0.2 | 2.1 ± 0.1 | |
| Fat (g·d−1) | 78 ± 18 | 78 ± 10 | |
| Fat (E%) | 20 | 19 ± 0.5 | 19 ± 0.6 |
| Fat (g·kg·d−1) | 1.2 ± 0.2 | 1.2 ± 0.1 | |
| Fiber (g·d−1) | 44 ± 7 | 41 ± 4 | |
| EEE (kcal·d−1) | 1358 ± 611 | 1292 ± 155 | |
| EA (kcal·kg FFM−1·d−1) | 40 | 42 ± 3 | 42 ± 2 |
| Adaptation: LEA group = LEA diet; HCHO group = high energy/CHO availability diet | |||
| EI (MJ·d−1) | 15.3 ± 3.0 | 9.1 ± 1.3*,** | |
| EI (kJ·kg·d−1) | 237 ± 23 | 138 ± 13*,** | |
| EI (kcal·d−1) | 3660 ± 71 | 2170 ± 31*,** | |
| CHO (g·d−1) | 586 ± 114 | 313 ± 43*,** | |
| CHO (E%) | HCHO: 65; LEA: 60 | 64 ± 0.5 | 57 ± 0.5*,** |
| CHO (g·kg·d−1) | 9.1 ± 0.9 | 4.7 ± 0.4*,** | |
| Protein (g·d−1) | 136 ± 26 | 131 ± 18 | |
| Protein (E%) | HCHO: 15; LEA: 25 | 15 ± 0.4 | 24 ± 0.5*,** |
| Protein (g·kg·d−1) | 2.1 ± 0.2 | 2.0 ± 0.2 | |
| Fat (g·d−1) | 77 ± 18 | 37 ± 8*,** | |
| Fat (E%) | HCHO: 20; LEA: 15 | 19 ± 1.0 | 15 ± 1.3*,** |
| Fat (g·kg·d−1) | 1.2 ± 0.2 | 0.6 ± 0.1*,** | |
| Fiber (g·d−1) | 43 ± 8 | 35 ± 2*,** | |
| EEE (kcal·d−1) | 1321 ± 463 | 1279 ± 301 | |
| EA (kcal·kg FFM−1·d−1) | HCHO: 40; LEA: 15 | 43 ± 4 | 15 ± 2*,** |
Data are presented as mean ± SD.
*P < 0.001, within-group difference between baseline and adaptation.
**P < 0.001, difference between HCHO and LEA.
HCHO, high energy/CHO availability; F, female; M, male.
General changes in body mass and composition
Body mass assessments for all subjects across the days of the baseline and adaptation periods are summarized in Figures 2A (HCHO group) and 2B (LEA group), with Figure 2C displaying the change in BM relative to initial BM recorded across each of the matched activities. In general, BM remained stable in both groups across the baseline phase then demonstrated a substantial decrease (~2 kg) over the adaptation diet in the LEA group (P < 0.001) as well as a small (0.5 kg) decrease in the HCHO group (P < 0.001). Both groups showed a small but nonsignificant BM increase (~0.3 kg; HCHO, P = 0.993; LEA, P = 0.858) over the 24 h of HCHO diet in preparation for race 2.
FIGURE 2.
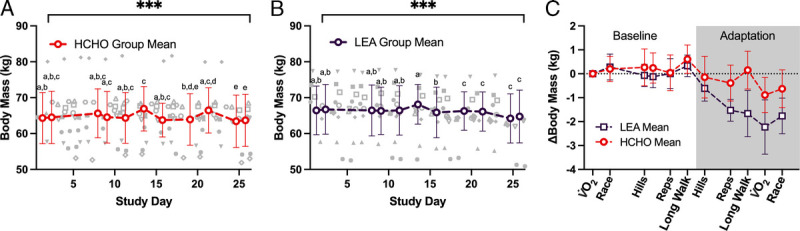
Changes in body mass over the duration of an endurance training camp in highly trained race walkers (n = 23) who were divided in two groups to consume a diet providing high energy/CHO availability (HCHO) for a 6-d baseline phase before being assigned to 9 d adaptation phase of either HCHO (n = 12) (A) or LEA diet (n = 11) (B). Data for the change relative to initial body mass (∆BM) for the two groups matched across study activities (2C). Data are presented as mean ± SD. Points sharing a letter are not different from one another (P > 0.05). ***Main effect of time (P < 0.001).
DXA-derived changes in body composition from the commencement of the baseline phase to the completion of adaptation phase are summarized in Table 2. Substantial changes in BM (2.1 kg, P < 0.001), which was comprised primarily of a 1.6-kg reduction in fat mass (P < 0.001), were seen in the LEA group. Decreases in BM (0.9 kg, P = 0.008), inclusive of a fat mass reduction (0.9 kg, P < 0.001), were also evident in the HCHO group. However, the reduction in both BM (P = 0.005) and fat mass (P = 0.009) was significantly larger in LEA compared with HCHO. The LEA group also had a reduction in FFM (0.4 ± 0.8 kg), whereas the HCHO group remained stable (0.03 ± 0.4 kg); however, these differences were not deemed significant (P = 0.131).
TABLE 2.
Assessment of body composition via dual x-ray absorptiometry during 6-d baseline of high energy/CHO availability then 9-d adaptation to an LEA diet in highly trained race walkers undertaking endurance training.
| HCHO (n = 10 M, 2 F) | LEA (n = 10 M, 1 F) | |
|---|---|---|
| Prebaseline Testing | ||
| Scale mass (kg) | 64.1 ± 7.5 | 66.1 ± 6.7 |
| DXA total mass (kg) | 64.6 ± 7.5 | 66.6 ± 6.6 |
| Lean mass (kg) | 53.0 ± 8.3 | 54.3 ± 5.8 |
| Bone mineral content (kg) | 2.8 ± 0.4 | 2.8 ± 0.3 |
| Fat mass (kg) | 8.9 ± 2.7 | 9.4 ± 2.5 |
| FFM (kg) | 55.7 ± 8.7 | 57.1 ± 6.2 |
| Postadaptation Testing | ||
| Scale mass (kg) | 63.1 ± 7.2* | 64.1 ± 6.8*^ |
| DXA total mass (kg) | 63.7 ± 7.4 | 64.5 ± 6.8*^ |
| Lean mass (kg) | 52.9 ± 8.3 | 53.8 ± 6.6 |
| Bone mineral content (kg) | 2.8 ± 0.4 | 2.8 ± 0.3 |
| Fat mass (kg) | 8.0 ± 2.4* | 7.9 ± 2.3*^ |
| FFM (kg) | 55.7 ± 8.7 | 56.7 ± 6.6 |
Data are presented as mean ± SD.
*P < 0.05, difference between baseline and adaptation.
^P < 0.05, difference in magnitude of change from baseline to adaptation between HCHO and LEA.
M, male; F, female.
Training load
Planned and actual EEE for each day of the baseline and adaptation phases are summarized in Figure 3. Overall, athletes expended ~1300 kcal each day equating to ~19 km of race walking (HCHO vs LEA; baseline phase: 1358 ± 611 vs 1284 ± 161 kcal·d−1; adaptation phase: 1321 ± 463 vs 1301 ± 307 kcal·d−1). The mean difference between planned versus actual training was ~16 kcal·d−1, which equates to ~230 m and was not significant (P = 0.434). Furthermore, there were no differences in planned or actual EEE between diets (P = 0.354) or testing phases (P = 0.939). Values (HCHO vs LEA) for the energy difference between planned and actual EEE were as follows: baseline phase: −14 ± 110 vs −48 ± 139 kcal·d−1; adaptation phase: −11 ± 91 vs −53 ± 89 kcal·d−1. Meanwhile, these differences expressed as training distances were as follows (HCHO vs LEA): baseline phase: −200 vs −690 m·d−1; adaptation phase: −160 vs −770 m·d−1.
FIGURE 3.
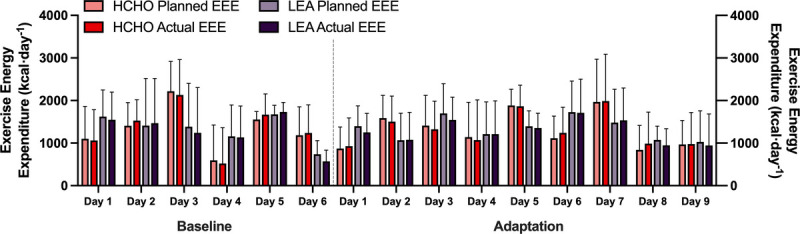
Daily planned vs actual EEE during an endurance training camp of highly trained race walkers (n = 22) who were divided into two groups to consume a diet providing high energy/CHO availability (HCHO) for a 6-d baseline phase before being assigned to a 9-d adaptation phase of either HCHO (n = 12) or LEA diet (n = 10). No differences were seen in either group between planned and actual for either phase. Data are presented as mean ± SD.
Economy and V̇O2peak
Twenty-two race walkers completed the graded economy and V̇O2peak test protocol. The results of these tests are summarized in Supplemental Table 1 (see Supplemental Digital Content, http://links.lww.com/MSS/C829) and Figure 4. There were no differences in absolute or relative V̇O2peak between groups (P = 0.718 and P = 0.793, respectively) or across trials (P = 0.646 and P = 0.078, respectively). Both groups displayed an increase in RER, absolute V̇O2 (L·min−1), HR, and RPE associated with the increase in exercise intensity across all four stages of both economy tests (all P < 0.0001). During the adaptation trial, there was a reduction in absolute V̇O2 (L·min−1) during stages 1–4 (P < 0.001) and lower lactate concentrations after all stages (P = 0.007); however, no diet-associated effects were evident (V̇O2, P = 0.718; lactate P = 0.290). A reduced RER and HR was seen during the adaptation trial for both groups (P < 0.001); however, this decrease was larger in the LEA group compared with the HCHO group (~0.03 vs 0.07 decrease in RER and 5 vs 9 bpm decrease in HR; both P < 0.001). At adaptation, there was also an increase in calculated fat oxidation rates (P < 0.001) and a reduction in CHO oxidation (P < 0.001) across all stages when compared with baseline in both groups. However, this shift was larger for both fat and CHO utilization (both P < 0.001) in the LEA group compared with HCHO.
FIGURE 4.
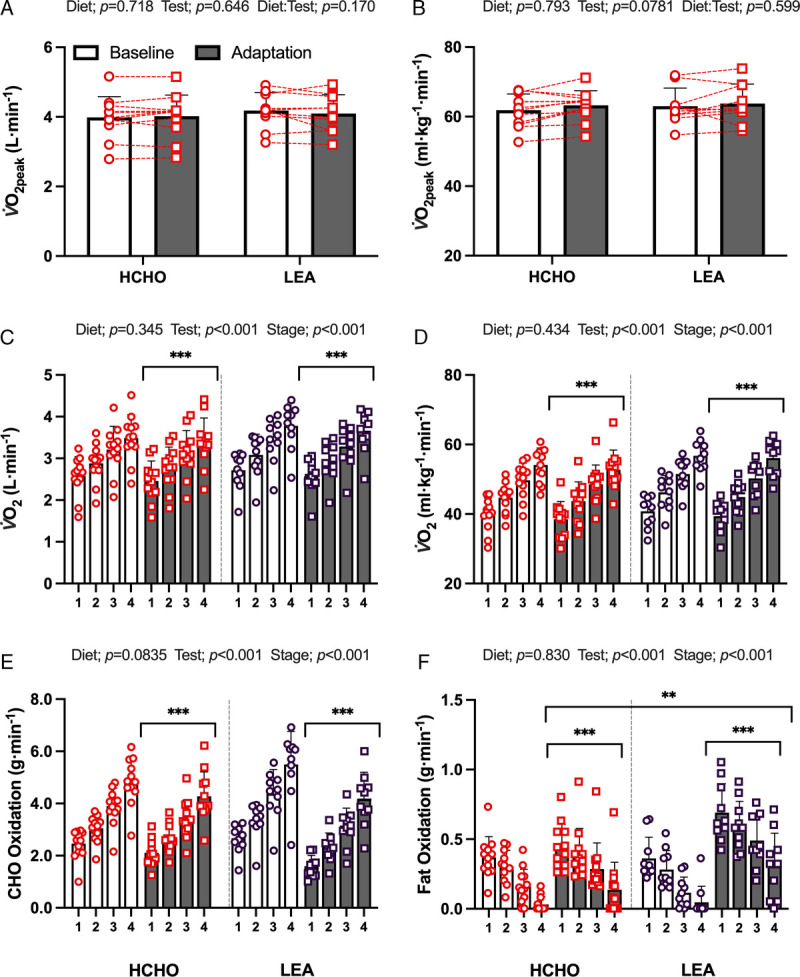
Mean data and individual responses for absolute (A) and relative (B) V̇O2peak obtained during the incremental max test performed before baseline phase or after adaptation to an HCHO (n = 12) or an LEA (n = 10) diet in highly trained race walkers. Absolute (C) and relative (D) oxygen uptake and calculated substrate oxidation rates (E, CHO; F, fat) during the four-stage economy test. Data are presented as mean ± SD. Significant differences: **P < 0.01; ***P < 0.001.
RESTQ-Sport-76
Figure 5 summarizes the results of the RESTQ-Sport-76, undertaken at the end of each dietary phase. There were significant diet–trial effects for the constructs of overall stress (P = 0.021), overall recovery (P = 0.024), sport-specific stress (P = 0.003), and sport-specific recovery (P = 0.012). Specifically, during the adaptation phase, the LEA group reported an increase in both overall stress (P = 0.006, with significant subscales including emotional stress, fatigue, lack of energy, and physical complaints) and sport-specific stress (P = 0.002, with significant subscales including emotional exhaustion and injury, P < 0.005). Furthermore, decreases in both overall recovery (P = 0.014, with significant subscales including physical recovery and general well-being) and sport-specific recovery (P = 0.032, with significant scales including being in shape and self-efficacy) were observed in the LEA group. By contrast, other than a main effect of test noted for social recovery (P = 0.004), no other differences were detected for any scale item between phases in athletes adhering to the HCHO diet (all P > 0.05).
FIGURE 5.
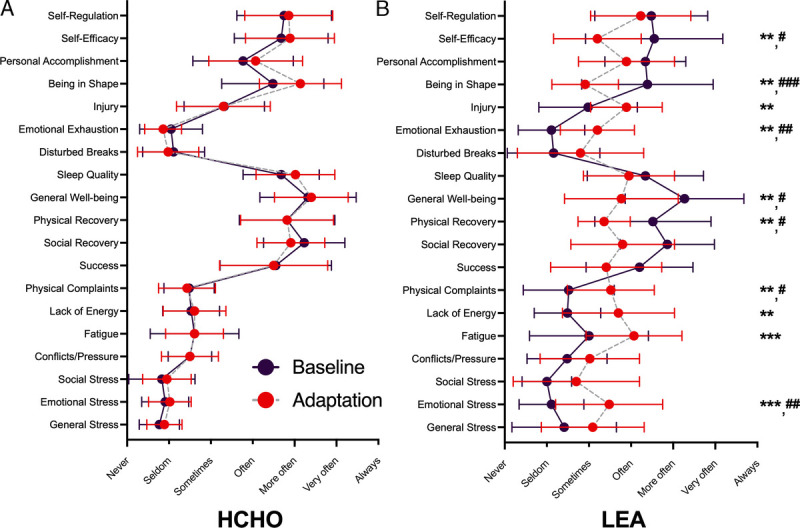
RESTQ-Sport-76 scores during an endurance training camp of highly trained race walkers (n = 23) who consumed a diet providing high energy/CHO availability (HCHO) for a 6-d baseline phase before being assigned to a 9-d adaptation phase of either HCHO (n = 12) (A) or LEA diet (n = 11) (B). Data are presented as mean ± SD. Significant difference between baseline and adaptation for the *P < 0.05; **P < 0.01. Significant difference to HCHO: #P < 0.05; ##P < 0.01; ###P < 0.001.
Race performance
Race day data are summarized in Figure 6. Both HCHO and LEA groups achieved a similar improvement in race performance (Fig. 6A), with the mean improvement (Fig. 6B) being 4.5% ± 4.1% and 3.5% ± 1.8% for HCHO and LEA, respectively (P < 0.001), equivalent to ~127 and ~92 s. BM measured immediately before the start of each race is summarized in Figure 6C and showed that the decrease in BM was greater in LEA (−2.0 ± 0.9 kg) compared with HCHO (−0.9 ± 0.6 kg, P = 0.001). The relationship between changes in performance and changes in prerace BM for all participants (Fig. 6D) was not significant (r = −0.08 [−0.49 to 0.35], P = 0.717). There was a significant interaction (P = 0.049) for postrace RPE, with HCHO showing a small decrease (0.4 a.u.) from baseline to adaptation races whereas the LEA group reported an increase (1.2 a.u.).
FIGURE 6.
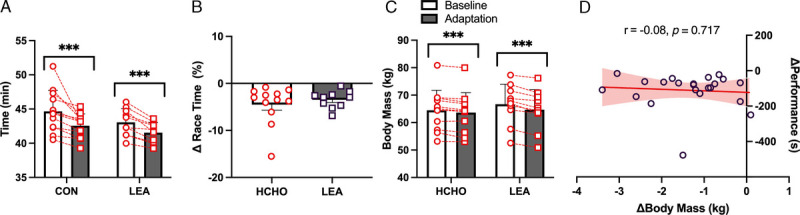
Mean and individual data for performance of 10,000-m race walking event (A) and change in race performance (B) in highly trained race walkers at baseline and after adaptation to an HCHO (n = 12) or an LEA (n = 9) diet. Changes in BM on race day (C) and correlation between change in prerace body mass and 10,000-m race performance (D). Significant differences ***P < 0.001.
DISCUSSION
This is the first investigation of short-term exposure to LEA in highly trained (mostly male) endurance athletes, which involves the manipulation of EA against the background of a real-world scenario of periodized daily training. The novel protocol included personalized meal plans and a continuously updating approach for calculating EEE in the field to adjust EI based on EA targets on a daily and within-day basis. The main findings were as follows: 1) 9 d of substantial restriction of EA was associated with a significant loss of body mass (~3% BM), mostly from body fat, that was maintained when the athletes briefly returned to a high-energy, high-CHO diet; 2) although the period of LEA was associated with increased perception of fatigue and a loss of training quality, the rapid restoration of energy and CHO intake over 24 h to meet guidelines for glycogen restoration allowed athletes to complete a real-world 10,000-m (~40-min) race with a performance improvement that was equal to that of a group who had trained with sustained high energy/CHO availability; and 3) the relationship between changes in BM and improvements in race performance was not significant. These findings suggest that the long-term periodization of physique to achieve ideal body mass might be achieved in a series of strategically timed but brief phases of substantially restricted EA, and that the relationship between BM, training quality, and performance in weight-dependent endurance sports is complex.
The current study investigated a possible model of targeted BM manipulation with particular interest in impairments of the quality of life and training capacity to determine whether these could be easily reversed to preserve competition performance. We monitored the latter by examining the completion of planned training program as well as changes in the validated athlete self-report measuring tool, the RESTQ-Sport-76 (35). Overall, all athletes completed their intended training volume with no differences between groups or training periods in terms of planned versus actual training energy expenditure (mean daily expenditure: ~1300 kcal, equivalent to ~19-km race walking). However, we suspect that during the adaptation period, LEA athletes often completed training sessions despite feeling fatigued, in recognition that failing to finish (and the resultant reduction in EEE) would have led to a meal adjustment to further reduce dietary EI.
Indeed, self-reports from the RESTQ-Sport-76 tool noted marked increases in stress and reductions in recovery scales at the end of the adaptation phase in the LEA group, with negligible changes in the HCHO group from baseline scores over the same period. The RESTQ-Sport-76 is underpinned by theories on physiological and psychological responses to stress and recovery and collects information on general and sports-specific aspects of these scales (35). Athletes exposed to LEA reported a significant increase in overall stress (Fig. 5), with focus on the subscales of emotional stress (irritation, aggression, anxiety, or inhibition), fatigue (time pressures, training, disturbances in work, overfatigue, and loss of sleep), lack of energy (inability to concentrate, make decisions, or lacking energy), and physical complaints (whole body physical indispositions or complaints). Sports-specific stress was also increased, particularly in relation to the subscales of emotional exhaustion (feelings of burnout or wanting to discontinue sport) and injury (perceived acute injury risk or vulnerability). There was a decrease in overall recovery, including the subscales of physical recovery (physical recovery, physical well-being, and fitness) and general well-being (good moods, high well-being, relaxation, and contentment), whereas the subscales of being in shape (feeling fit, efficient, and vital) and self-efficacy (feeling optimally prepared and convinced of proper training) were particularly decreased within the sports-specific recovery scales. These changes expand on the generally negative alterations to psychological status, noted by tools such as the Profile of Mood States during periods of weight making in weight category sports (19).
Significant differences in changes in body composition over the ~3-wk training period were noted between treatment groups. We deliberately set the EA target for the HCHO group at 40 kcal·kg FFM−1·d−1 to allow small changes in body composition (loss of ~0.5 kg fat mass/BM) commensurate with our previous experiences of base phase training in similar groups of elite race walkers (26,27). Indeed, according to the DXA assessment of body composition, the HCHO group showed a small (0.9 kg) loss of BM, principally from body fat over the 3-wk period. However, losses of total BM (2.1 kg) and fat mass (1.6 kg) were doubled in the LEA group, with a small loss of FFM (0.4 kg) [Table 2], which predominantly occurred during the 9-d LEA (15 kcal·kg FFM−1·d−1) intervention. We note that several individuals within the HCHO group showed larger BM loss, including some loss of FFM, with further analysis of our dietary intervention protocol identifying more difficulty in achieving EA targets for athletes with higher training volumes, including significant cross-training (Heikura et al., unpublished data). BM monitoring over the course of the training program showed daily fluctuations commensurate with changes in hydration and recent food intake, with both groups making a small (0.3 kg) gain over the course of the 24-h period of race preparation as would be expected from gains in muscle glycogen and restoration of any fluid deficits. Despite the prerace restoration of these labile components of BM, the LEA group reported to the start of race 2 being ~2 kg (~3% BM) lighter than at the first race, a twofold difference to that of the HCHO group.
Performances of real-world races were improved by ~4% or ~100 s across the 3-wk training camp, with similar improvements in both groups. These results support the complexity of the factors underpinning competition success in high-level endurance athletes. Indeed, although the physics of movement in running events show theoretical and empirical support for the benefits of a lighter BM (20,36), including potential benefits of training at a high BM before reducing weight for competition (37), we did not observe a greater improvement in performance in the LEA group compared with the control high-energy, high-CHO group. Furthermore, we failed to detect any correlation between the change in BM and the change in performance between the two races. This suggests that a combination of factors such as the quality of training, psychological readiness, muscle fuel reserves, and BM changes contributed to the final performance. Although we may have been able to reduce negative effects of the rapid weight loss period in the LEA group on the former characteristics by including a 24-h period of CHO restoration, it is possible that if an equal loss of BM had been achieved by a smaller daily energy deficit spread across an entire training period, different effects on performance might have been seen. We also note, however, that our strategy was incorporated into base phase training, which provided only a 24-h light taper and refueling strategy; different effects on performance might be seen if a rapid BM manipulation was followed by a more focused competition taper. A small loss of lean mass contributed to the change in BM, an outcome that might be reduced by a concurrent increase in event-specific resistance training and/or an increase in the protein content of the diet (38).
The concept of EA was introduced into sport nutrition by Professor Anne Loucks, with EA being defined as the energy remaining when the energy expended in exercise is subtracted from an athlete’s EI, expressed relative to FFM to account for the most metabolically active tissues (5). Elegant studies undertaken on sedentary females under metabolic ward conditions identified that perturbations to biomarkers of a number of body systems, including reproductive hormones, and markers of bone turnover and metabolic rate, and typically associated with health issues in female endurance athletes, were attributable to LEA rather than endurance training per se (9,10,39,40). Although recent evolution of the model of EA in athletes now recognizes that inadequate EI and/or increased exercise loads arise from diverse etiology, purposeful energy restriction for manipulation of body mass/composition is a common cause (6). Although impairments of health and performance are undoubtedly associated with some scenarios of LEA in individual athletes, as explained by the RED-S (1) and Triad (2–4) syndromes, cross-sectional studies of both male and female athletes show variability in the type, prevalence, and severity of the disturbances to different body systems (15,16). Therefore, there is a need for further evolution in these models to accept that some exposure to LEA is normal in human existence and can be at least accommodated, if not associated with beneficial athletic practices (41).
Although LEA interventions undertaken in metabolic wards have contributed to our knowledge, characteristics such as the involvement of previously sedentary participants and the use of liquid meal diets (9,10,39,40) limit the extrapolation to real-life athletes. The current literature provides very few examples of prospective intervention studies of exposure to LEA in highly trained athletes, particularly in free-living situations. Studies have examined 3–5 d of the implementation of LEA involving whole food diets in trained- to well-trained endurance athletes, with EA targets being achieved by providing/prescribing a fixed EI while undertaking a standardized laboratory or field exercise session each day (11,12,23,24,42). Although these protocols provide a practical way to ensure that EA conditions are achieved, they do not mimic real-life protocols in which athletes alter their daily training and dietary practices according to the goals of the micro-, meso-, and macrocycles of a periodized preparation (17). The current intervention study overcame these limitations and manipulated EA in an authentic manner, allowing the highly trained participants to continue their periodized training program (varying the mode, frequency, intensity, and duration of daily workouts) while continually adjusting their EI to achieve the target for high and low EA. The actual methods used to achieve this included 1) provision and supervision of a rigorously controlled dietary plan, 2) individualization of EEE calculations based on the athlete’s prospective training plans, and 3) real-time manipulation of EI over the day according to the actual execution of training plans.
This study was part of a larger project to investigate the effect of LEA on various body systems with the implementation of various study protocols after 6–7 d of adaptation to HCHO or LEA diets; these results are provided elsewhere (13,29). The current protocol involving 9 d of LEA exposure was focused on training outcomes, quality of life, changes in body composition, and race performance, with opportunities to investigate whether a short-term but substantial energy restriction could be undertaken while maintaining the expected enhancement of performance associated with a 3-wk training block at the commencement of the athletic season (26,27). Despite the well-documented pursuits of endurance athletes, particularly those in weight-dependent and gravity-affected sports, to achieve a light and lean physique (43), the systematic investigation of optimal techniques for physique manipulation with evidence of performance benefits in elite athletes is curiously lacking. Indeed, prospective interventions on BM loss in high-performance athletes are focused on the rapid weight loss strategies of weight category sports (19). These examples are anomalous to the present scenario in terms of the type of sporting event, the period of BM loss, and, in the case or rapid weight making, the concomitant use of dehydration (19). Yet systematic reviews (18), prospective observational studies (22), and case studies (20) of changes in energy expenditure, EI, and body composition in highly trained endurance athletes document self-imposed fluctuations across the training season and sporting career to achieve an optimal physique for race performance. Furthermore, the lay literature provides powerful testimonials from individual athletes about the pervasive beliefs within distance athletics (44) and road cycling (45) that a lighter BM and high power-to-weight (BM) ratio are associated with performance improvements.
There is a lack of literature with which to make comparisons of our findings. A longer-term BM intervention in high-performance athletes from different types of sports, achieved via supervised dietary education under free-living conditions, observed the same total BM loss (5% BM) in two groups who targeted a weekly loss of 0.7% vs 1.4% BM, via a reduction 19% or 30% reduction in EI over ~9 and ~5 wk, respectively (46). The group who undertook the slower rate of BM loss achieved an increase in lean mass and greater improvement in strength (maximum repetition bench press). However, no other differences in metrics of performance (countermovement jump, other strength measures, 40-m sprint) were reported between groups based on a slow or faster BM loss (46). Meanwhile, 3 d of exposure to LEA (~19 kcal·kg FFM−1·d−1) in well-trained male distance runners achieved a BM loss of 1.2 kg compared with a similar period of normal EA (~53 kcal·kg FFM−1·d−1) but did not alter time to exhaustion on a treadmill at 90% V̇O2max (23). Here, any advantages of the lower BM may have been offset by the reduction in muscle glycogen content, a factor that we addressed in the current study.
We acknowledge the limitations of this study, including the nonrandomized allocation of the athletes to the treatment groups. However, our view and practice during studies involving elite and world-class athletes (26,27) is that participants should only be assigned to treatments they believe will enhance their health and/or performance. In addition to ethical considerations regarding the support of athletic preparation, we believe that true performance outcomes can only be achieved with standardization of placebo effects and removal of nocebo effects (47) such that each athlete feels truly able to compete at their best. We note that each treatment group includes one to two female athletes and may have been affected by proposed sex differences in at least some of the responses to LEA (11,24). However, our clear-cut results were apparently unaffected by the inclusion of these participants, and we remain committed to including female participants within cohorts or in female-specific studies where applicable to address the underrepresentation of females in sports science research (48). In return, we also note the strength of the study design: the involvement of international-level athletes; the rigorous control and reporting of diet and exercise, which manipulated EA within the real-world periodization of training programs; and the authentic measurement of sports performance.
CONCLUSIONS
In conclusion, highly trained endurance athletes were able to achieve a small weight loss (3% BM) during a training camp in the base preparation phase of the training calendar by undertaking a brief (9-d) period of LEA (15 kcal·kg FFM−1·d−1, equal to a reduction of 40% of EI). Although this was associated with subjective measures of fatigue, stress, and a decrease in recovery, when combined with 24 h of prerace fueling, athletes achieved an improvement in race performance equivalent to a similar cohort who had undertaken the same 3-wk training block with high energy and CHO availability. These results suggest that a reduction in body mass may not be required to improve race performance after an intensified training camp. However, if body composition alterations are required, this protocol might be suitably integrated within the athlete’s annual training plan to assist with body composition manipulation goals while preserving competition performance, either as a periodic activity or as a strategically placed activity to allow recovery before a competition. The importance of adequate energy and CHO availability to acutely support race performance is demonstrated by our study. Furthermore, we note the puzzling lack of investigation of the complicated nature of body composition manipulation and performance in gravity-affected endurance sports despite the overwhelming evidence of such practices by high-performance athletes. Such research is needed to identify any real benefits of reduced BM and increased power-to-BM ratios and to acknowledge the health and performance issues associated with prolonged periods of energy and CHO restriction. Meanwhile, this study showcased a new protocol to achieve various levels of EA, which track the real-life periodization of training energy expenditure.
Supplementary Material
Acknowledgments
This study was funded by a Program Grant from the Australian Catholic University Research Fund awarded to L. M. B. The authors declare no conflicts of interest. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results of the present study do not constitute endorsement by the American College of Sports Medicine. The authors acknowledge the contributions of many people who enabled the laboratory and fieldwork to be completed on this study (Brent Vallance, Steve Langley, Lachlan Marshall, Amelia Carr, Rebecca Hall, Bryce Anderson, Ana Bele Recalde, Sara Forbes, Jess Rothwell, Ola Luszak, Yuka Sanui, and Nikita Fensham). They are in awe and gratitude to the athletes who participated in this study.
Footnotes
L. M. B. and J. W. contributed equally to this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.acsm-msse.org).
Contributor Information
JAMIE WHITFIELD, Email: Jamie.Whitfield@acu.edu.au.
MEGAN L. R. ROSS, Email: meganlrross11@gmail.com.
NICOLIN TEE, Email: nicolin.tee@acu.edu.au.
AVISH P. SHARMA, Email: avish.sharma@vis.org.au.
ANDY J. KING, Email: Andy.King@acu.edu.au.
IDA A. HEIKURA, Email: IHeikura@csipacific.ca.
AIMEE MORABITO, Email: aimee.morabito@ausport.gov.au.
ALANNAH K. A. MCKAY, Email: alannah.mckay@acu.edu.au.
REFERENCES
- 1.Mountjoy M Sundgot-Borgen J Burke L, et al. International Olympic Committee (IOC) consensus statement on Relative Energy Deficiency in Sport (RED-S): 2018 update. Int J sport Nutr Exerc Metab. 2018;28(4):316–31. [DOI] [PubMed] [Google Scholar]
- 2.De Souza MJ Nattiv A Joy E, et al. 2014 Female Athlete Triad Coalition Consensus Statement on treatment and return to play of the female athlete triad: 1st international conference held in San Francisco, California, May 2012 and 2nd international conference held in Indianapolis, Indiana, May 2013. Br J Sports Med. 2014;48(4):289. [DOI] [PubMed] [Google Scholar]
- 3.Fredericson M Kussman A Misra M, et al. The male athlete triad—a consensus statement from the Female and Male Athlete Triad Coalition. Part II. Clin J Sport Med. 2021;31(4):349–66. [DOI] [PubMed] [Google Scholar]
- 4.Nattiv A De Souza MJ Koltun KJ, et al. The male athlete triad-a consensus statement from the Female and Male Athlete Triad Coalition. Part 1: definition and scientific basis. Clin J Sport Med. 2021;31(4):345–53. [DOI] [PubMed] [Google Scholar]
- 5.Loucks AB, Kiens B, Wright HH. Energy availability in athletes. J Sports Sci. 2011;29(Suppl 1):S7–15. [DOI] [PubMed] [Google Scholar]
- 6.Burke L, Fahrenholtz I, Garthe I, Lundy L, Melin A. Low energy availability: challenges and approaches to measurement and treatment. In: Burke L, Deakin V, Minehan M, editors. Clinical Sports Nutrition. 6th ed. Sydney: McGraw-Hill; 2021. pp. 110–43. [Google Scholar]
- 7.De Souza MJ Strock NCA Ricker EA, et al. The path towards progress: a critical review to advance the science of the female and male athlete triad and relative energy deficiency in sport. Sports Med. 2022;52(1):13–23. [DOI] [PubMed] [Google Scholar]
- 8.Areta JL, Taylor HL, Koehler K. Low energy availability: history, definition and evidence of its endocrine, metabolic and physiological effects in prospective studies in females and males. Eur J Appl Physiol. 2021;121(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loucks AB, Verdun M, Heath EM. Low energy availability, not stress of exercise, alters LH pulsatility in exercising women. J Appl Physiol (1985). 1998;84(1):37–46. [DOI] [PubMed] [Google Scholar]
- 10.Ihle R, Loucks AB. Dose–response relationships between energy availability and bone turnover in young exercising women. J Bone Miner Res. 2004;19(8):1231–40. [DOI] [PubMed] [Google Scholar]
- 11.Koehler K Hoerner NR Gibbs JC, et al. Low energy availability in exercising men is associated with reduced leptin and insulin but not with changes in other metabolic hormones. J Sports Sci. 2016;34(20):1921–9. [DOI] [PubMed] [Google Scholar]
- 12.Papageorgiou M Martin D Colgan H, et al. Bone metabolic responses to low energy availability achieved by diet or exercise in active eumenorrheic women. Bone. 2018;114:181–8. [DOI] [PubMed] [Google Scholar]
- 13.McKay AKA Peeling P Pyne DB, et al. Six days of low carbohydrate, not energy availability, alters the iron and immune response to exercise in elite athletes. Med Sci Sports Exerc. 2022;54(3):377–87. [DOI] [PubMed] [Google Scholar]
- 14.Vanheest JL, Rodgers CD, Mahoney CE, De Souza MJ. Ovarian suppression impairs sport performance in junior elite female swimmers. Med Sci Sports Exerc. 2014;46(1):156–66. [DOI] [PubMed] [Google Scholar]
- 15.Logue D, Madigan SM, Delahunt E, Heinen M, Mc Donnell SJ, Corish CA. Low energy availability in athletes: a review of prevalence, dietary patterns, physiological health, and sports performance. Sports Med. 2018;48(1):73–96. [DOI] [PubMed] [Google Scholar]
- 16.Logue DM Madigan SM Melin A, et al. Low energy availability in athletes 2020: an updated narrative review of prevalence, risk, within-day energy balance, knowledge, and impact on sports performance. Nutrients. 2020;12(3):835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mujika I, Halson S, Burke LM, Balague G, Farrow D. An integrated, multifactorial approach to periodization for optimal performance in individual and team sports. Int J Sports Physiol Perform. 2018;13(5):538–61. [DOI] [PubMed] [Google Scholar]
- 18.Heydenreich J, Kayser B, Schutz Y, Melzer K. Total energy expenditure, energy intake, and body composition in endurance athletes across the training season: a systematic review. Sports Med Open. 2017;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke LM, Slater GJ, Matthews JJ, Langan-Evans C, Horswill CA. ACSM expert consensus statement on weight loss in weight-category sports. Curr Sports Med Rep. 2021;20(4):199–217. [DOI] [PubMed] [Google Scholar]
- 20.Stellingwerff T. Case study: body composition periodization in an Olympic-level female middle-distance runner over a 9-year career. Int J Sport Nutr Exerc Metab. 2018;28(4):428–33. [DOI] [PubMed] [Google Scholar]
- 21.Haakonssen EC, Barras M, Burke LM, Jenkins DG, Martin DT. Body composition of female road and track endurance cyclists: normative values and typical changes. Eur J Sport Sci. 2016;16(6):645–53. [DOI] [PubMed] [Google Scholar]
- 22.Fudge BW Westerterp KR Kiplamai FK, et al. Evidence of negative energy balance using doubly labelled water in elite Kenyan endurance runners prior to competition. Br J Nutr. 2007;95(1):59–66. [DOI] [PubMed] [Google Scholar]
- 23.Kojima C Ishibashi A Tanabe Y, et al. Muscle glycogen content during endurance training under low energy availability. Med Sci Sports Exerc. 2020;52(1):187–95. [DOI] [PubMed] [Google Scholar]
- 24.Papageorgiou M Elliott-Sale KJ Parsons A, et al. Effects of reduced energy availability on bone metabolism in women and men. Bone. 2017;105:191–9. [DOI] [PubMed] [Google Scholar]
- 25.Burke LM, Hawley JA, Jeukendrup A, Morton JP, Stellingwerff T, Maughan RJ. Toward a common understanding of diet-exercise strategies to manipulate fuel availability for training and competition preparation in endurance sport. Int J Sport Nutr Exerc Metab. 2018;28(5):451–63. [DOI] [PubMed] [Google Scholar]
- 26.Burke LM Ross ML Garvican-Lewis LA, et al. Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2017;595(9):2785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke LM Sharma AP Heikura IA, et al. Crisis of confidence averted: impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS One. 2020;15(6):e0234027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKay AKA Stellingwerff T Smith ES, et al. Defining training and performance caliber: a participant classification framework. Int J Sports Physiol Perform. 2022;17(2):317–31. [DOI] [PubMed] [Google Scholar]
- 29.Fensham NC, Heikura IA, McKay AKA, Tee N, Ackerman KE, Burke LM. Short-term carbohydrate restriction impairs bone formation at rest and during prolonged exercise to a greater degree than low energy availability. J Bone Miner Res. 2022;37(10):1915–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burke LM, Lundy B, Fahrenholtz IL, Melin AK. Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):350–63. [DOI] [PubMed] [Google Scholar]
- 31.Nana A Slater GJ Hopkins WG, et al. Importance of standardized DXA protocol for assessing physique changes in athletes. Int J Sport Nutr Exerc Metab. 2016;26(3):259–67. [DOI] [PubMed] [Google Scholar]
- 32.Saunders PU Telford RD Pyne DB, et al. Improved running economy in elite runners after 20 days of simulated moderate-altitude exposure. J Appl Physiol (1985). 2004;96(3):931–7. [DOI] [PubMed] [Google Scholar]
- 33.Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16(1):23–9. [PubMed] [Google Scholar]
- 34.Romijn JA, Wolfe RR. Effects of prolonged exercise on endogenous substrate supply and utilization. In: Lamb DR, Gisolfi CV, editors. Perspectives in Exercise Science and Sports Medicine. 5: Energy Metabolism in Exercise and Sport. Dubuque (IA): Brown & Benchmark; 1992. pp. 207–34. [Google Scholar]
- 35.Kellmann M. Preventing overtraining in athletes in high-intensity sports and stress/recovery monitoring. Scand J Med Sci Sports. 2010;20(Suppl 2):95–102. [DOI] [PubMed] [Google Scholar]
- 36.Weyand PG, Davis JA. Running performance has a structural basis. J Exp Biol. 2005;208(Pt 14):2625–31. [DOI] [PubMed] [Google Scholar]
- 37.Bosco C. Adaptive response of human skeletal muscle to simulated hypergravity condition. Acta Physiol Scand. 1985;124(4):507–13. [DOI] [PubMed] [Google Scholar]
- 38.Mettler S, Mitchell N, Tipton KD. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc. 2010;42(2):326–37. [DOI] [PubMed] [Google Scholar]
- 39.Loucks AB, Heath EM. Induction of low-T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol. 1994;266(3 Pt 2):R817–23. [DOI] [PubMed] [Google Scholar]
- 40.Hilton LK, Loucks AB. Low energy availability, not exercise stress, suppresses the diurnal rhythm of leptin in healthy young women. Am J Physiol Endocrinol Metab. 2000;278(1):E43–9. [DOI] [PubMed] [Google Scholar]
- 41.Shirley MK, Longman DP, Elliott-Sale KJ, Hackney AC, Sale C, Dolan E. A life history perspective on athletes with low energy availability. Sports Med. 2022;52(6):1223–34. [DOI] [PubMed] [Google Scholar]
- 42.Ishibashi A Kojima C Tanabe Y, et al. Effect of low energy availability during three consecutive days of endurance training on iron metabolism in male long distance runners. Physiol Rep. 2020;8(12):e14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Burke LM, Close GL, Lundy B, Mooses M, Morton JP, Tenforde AS. Relative energy deficiency in sport in male athletes: a commentary on its presentation among selected groups of male athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):364–74. [DOI] [PubMed] [Google Scholar]
- 44.Cain M. I was the fastest girl in America, until I joined Nike. In: The New York Times. Sect. Opinion; 2019. [Google Scholar]
- 45.Coyle D, Hamilton T. The Secret Race: Inside the Hidden World of the Tour de France: Doping, Cover-ups, and Winning at All Costs. UK: Random House; 2013. [Google Scholar]
- 46.Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot-Borgen J. Effect of two different weight-loss rates on body composition and strength and power-related performance in elite athletes. Int J Sport Nutr Exerc Metab. 2011;21(2):97–104. [DOI] [PubMed] [Google Scholar]
- 47.Raglin J, Szabo A, Lindheimer JB, Beedie C. Understanding placebo and nocebo effects in the context of sport: a psychological perspective. Eur J Sport Sci. 2020;20(3):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith ES McKay AKA Ackerman KE, et al. Methodology review: a protocol to audit the representation of female athletes in sports science and sports medicine research. Int J Sport Nutr Exerc Metab. 2022;32(2):114–27. [DOI] [PubMed] [Google Scholar]


