Fibromyalgia may predict pain-related worsening of opioid use disorder. Pain-related Opioid Use Disorder Exacerbation Scale is a new scale for pain and addiction research.
Keywords: Central nervous system sensitization, Opioid-related disorders, Fibromyalgia, Chronic pain
Abstract
Fibromyalgia and opioid use disorder (OUD) are highly impactful chronic illnesses with substantially overlapping psychosocial, biological, and clinical features. Little previous research has examined interactions between fibromyalgia and OUD. Limiting such research has been the previous requirement of a clinical examination to diagnose fibromyalgia. The 2011 American College of Rheumatology Fibromyalgia Survey (ACR-FMS) is a validated self-report instrument with high sensitivity and specificity for fibromyalgia intended to enable fibromyalgia research in settings where a clinical examination is impractical. The present observational study uses the ACR-FMS to determine whether fibromyalgia affects odds of acknowledging pain-related OUD exacerbations among a sample of participants with pain and OUD. Participants with pain and OUD (n = 125) were recruited from an academic substance use treatment facility. The ACR-FMS, along with an original scale measuring pain-related OUD exacerbation—the Pain-related OUD Exacerbation Scale—was administered through an electronic survey. The factor structure, internal consistency, and construct validity of Pain-related OUD Exacerbation Scale were tested. In addition, descriptive analyses, multiple hierarchical linear regression, ordinal logistic regression, and multinomial logistic regression analyses were performed. Although all participants had pain, those with fibromyalgia demonstrated significantly greater odds of acknowledging pain-related OUD exacerbations. Pain-related OUD Exacerbation Scale was found to have a single-factor solution, strong internal consistency, and construct validity. This study provides first evidence of fibromyalgia as a risk factor for pain-related exacerbation of OUD and introduces a new scale with promising psychometric properties to measure pain-related OUD exacerbation.
1. Introduction
Fibromyalgia and opioid use disorder (OUD) are highly burdensome chronic diseases with substantially overlapping psychosocial, neurobiological, and clinical features.9,26,45 Adverse childhood events and trauma during adulthood increase risk of fibromyalgia and OUD, and both conditions commonly co-occur with anxiety and mood disorders.31,32,39,58 Although the pathogenesis of fibromyalgia is incompletely understood, sensitization of the central nervous system to normal or subthreshold nociceptive stimuli has been implicated.11,26 The central nervous system pain amplification observed in fibromyalgia has an apparent corollary in opioid-induced hyperalgesia. Unrelated studies of fibromyalgia and hyperalgesia among individuals with OUD have produced remarkably similar patterns of nociceptive responding by quantitative sensory testing.16–18 In addition, several neural substrates may be common to fibromyalgia and OUD.22,26 Of particular interest are structural and functional abnormalities of the anterior cingulate cortex and insula that have been implicated in processing pain affect and drug craving.3,22,26,43
Connections between fibromyalgia and OUD extend beyond observational and experimental research. Patients with both conditions often report widespread pain, fatigue, sleep disturbances, and cognitive and emotional problems.11,47,56 Given such extensive overlap in symptomatology, it is plausible that individuals with comorbid OUD and fibromyalgia might bear greater symptom burden than others with just OUD. If true, this would have implications for OUD disease trajectory, given the well-established role of negative reinforcement in the natural history of OUD.24,33–35 By extension, it is plausible that comorbid fibromyalgia might increase the odds of certain exacerbations of OUD, including pain-related maintenance of OUD (ongoing compulsive opioid use despite harm due to pain-mediated negative reinforcement), escalation of OUD (using ever-greater quantities of opioids for maladaptive pain coping), treatment delay (deferring treatment for fear of pain exacerbation during opioid abstinence), and pain-precipitated relapse. Previous research has documented relationships between pain and the onset, maintenance, and relapse of OUD.4,5,36,53 However, no previous study has sought to determine whether individuals with comorbid fibromyalgia and OUD have greater odds of pain-related OUD exacerbations.
Recently, our group discovered differences in self-report of pain-motivated maintenance, escalation, treatment delay, and fear of relapse based on an established measure of fibromyalgia in a clinical sample of patients with OUD.25 However, our previous study was limited to detection of group differences only. We did not attempt to demonstrate increased odds of acknowledging pain-related addiction exacerbations among individuals with fibromyalgia and OUD. In addition, our previous analysis included a sizable contingent of participants who reported no pain at all, possibly inflating observed differences between those with and without fibromyalgia.
Therefore, the current study aims to extend our previous work by assessing how the presence of fibromyalgia might affect the odds of acknowledging pain-related OUD exacerbations (maintenance of OUD, escalation of OUD, OUD treatment delay, and OUD relapse) among individuals with pain and OUD. We also present first evidence of the validity and reliability of a new scale designed to measure pain-related exacerbation of OUD.
2. Methods
2.1. Participants
A total of 144 participants were recruited between July 7, 2021, and December 10, 2021, from the Ohio State University Wexner Medical Center Talbot Hall. Sample size was calculated a priori for the analyses presented in our original article.19 Talbot Hall is a specialty addiction treatment facility with services including partial hospitalization, intensive outpatient, group and individual counseling, medical withdrawal management, and outpatient clinics offering medications (buprenorphine and naltrexone) for OUD. Recruitment was conducted by trained providers during routine care.
Participants were adults with OUD defined by the presence of at least 2 of 11 OUD criteria during the past 12 months as described in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5).2 The only exclusion criteria were inability to provide informed consent, read, or comprehend survey items. There were no other inclusion or exclusion criteria.
Eight individuals declined participation. One case was excluded for not meeting OUD criteria. Participants who denied bodily pain (n = 16) were excluded from this secondary analysis. In addition, 2 cases were excluded for noncompletion of the model predictor American College of Rheumatology Fibromyalgia Survey (ACR-FMS). The final sample from this secondary analysis included 125 (n = 125) individuals with pain and OUD.
Survey responses were collected using REDCap, a web platform capable of securely collecting personal health information and managing online databases. Participants privately accessed the survey on tablet computers and were not able to interact with one another or view others' responses.
2.2. Measures
The survey included DSM-5 OUD criteria (recorded by an addiction provider at the time of participation), demographics (gender, race, ethnicity, employment status, income, education, housing status, and substance use treatment needs), and the following instruments and original items. Age was unintentionally omitted from the survey.
2.2.1. 2011 Fibromyalgia Survey criteria
Bodily pain and related symptoms were assessed by the ACR-FMS.54–56 The ACR-FMS localizes pain by body region (0-19) and measures the severity of related symptoms including problems thinking, fatigue, and difficulty sleeping (0-12). Epidemiological, community, and clinical studies have shown ACR-FMS to be predictive of pain, disability, and treatment outcomes in diverse clinical populations.7,8,19,30,41,42,57 As a self-report measure, it is robustly indicative of fibromyalgia even in the absence of a clinical examination (ACR-FMS ≥ 13, sensitivity 96.6% and specificity 91.8%) and is suitable for fibromyalgia diagnosis in studies when clinician assessment of fibromyalgia is impractical.55
2.2.2. Pain-related Opioid Use Disorder Exacerbation Scale
A four-item scale was created to measure pain-related exacerbations of OUD. These exacerbations included pain-related maintenance of OUD, “Pain is a major reason why I have kept using opioids;” escalation of OUD, “I find myself needing more and more opioids to control my pain;” treatment delay, “I have put off getting treatment for opioid use disorder because I am afraid my pain will be worse when I stop using opioids;” and fear of pain-precipitated relapse, “I am worried pain will cause me to relapse in the future.” Items were prefaced “Below is a list of statements dealing with pain and OUD. Read each item carefully and indicate whether you strongly agree, agree, feel neutral, disagree, or strongly disagree with each statement.” Responses were scaled as strongly agree (4), agree (3), neutral (2), disagree (1), or strongly disagree (0). The factor structure, internal consistency, and construct validity of this original scale were assessed and are presented in the Results section.
2.2.3. Research and Development-36
Mental health and bodily pain were assessed by the Research and Development (RAND) Corporation RAND 36-Item Health Survey 1.0 (RAND-36), also known as the Short Form 36 (SF-36).27 Research and Development-36 measures health-related quality of life on 8 domains: Mental Health, Bodily Pain, General Health, Physical Functioning, Social Functioning, Vitality, Role Limitations due to Emotional Problems, and Role Limitations due to Physical Health. Research and Development-36 has strong psychometric properties that have been repeatedly replicated in diverse samples.6,13,40,50,51
The present analysis required 2 RAND-36 domains: Mental Health and Bodily Pain. The Mental Health domain includes questions about symptoms of depression and anxiety. These questions are “During the past four weeks: 24) Have you been a very nervous person? 25) Have you felt so down in the dumps that nothing could cheer you up? 26) Have you felt calm and peaceful? 28) Have you felt downhearted and blue? 30) Have you been a happy person?” Responses include all of the time, most of the time, a good bit of the time, some of the time, a little of the time, and none of the time. Items 26 and 30 are reverse coded.
The Bodily Pain domain includes 2 items: (21) “How much pain have you had in the past 4 weeks?” and (22) “During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and housework)?” Question 21 is on a 6-point Likert scale: none, very mild, mild, moderate, severe, and very severe. Question 22 uses a 5-point scale: not at all, a little bit, moderately, quite a bit, and extremely. To score RAND-36, each item is linearly transformed to a range of 0 to 100 and these standardized scores are averaged by domain.14,27 Higher domain scores represent greater health-related quality of life in that domain (ie, better mental health).
2.3. Data analysis
Sample characteristics were assessed by central tendency, frequency, and percent. To aid interpretation of the primary regression analyses, the psychometric properties of our 4-item original scale—hereafter referred to as the Pain-related OUD Exacerbation Scale (PrOUD ES)—were assessed using Cronbach alpha (internal consistency), exploratory factor analysis (factor structure), and Spearman correlations (construct validity). Next, a hierarchical linear multiple regression was conducted to determine the effect of fibromyalgia on PrOUD ES after controlling for potential confounding variables (gender, race, OUD severity, type of opioids used, mental health, and pain severity). Finally, 4 separate cumulative odds ordinal logistic regression analyses with proportional odds were then conducted to determine the effect of fibromyalgia on acknowledgement of pain-related OUD exacerbations. The Mental Health subscale of RAND-36, gender, and race were included as covariates. The predictor variable of primary interest in each model was fibromyalgia (ACR-FMS ≥ 13). Ordinal dependent variables included (1) maintenance of OUD, “Pain is a major reason why I have kept using opioids;” (2) escalation of OUD, “I find myself needing more and more opioids to control my pain;” (3) treatment delay, “I have put off getting treatment for opioid use disorder because I am afraid my pain will be worse when I stop using opioids;” and (4) relapse, “I am worried pain will cause me to relapse in the future.” The assumption of proportional odds was assessed by full likelihood ratio tests comparing each fitted model to a model with varying location parameters. One ordinal model violated test assumptions and was followed up by a separate multinomial logistic regression.
2.4. Ethics
Approval for this study was obtained before initiation from the Ohio State University Wexner Medical Center Institutional Review Board. Participants provided verbal consent and were monetarily compensated for completing the survey.
3. Results
3.1. Sample characteristics
Demographic information was obtained from 117 participants (93.6%). Of those who provided demographics, 49 (41.9%) were female and 68 (58.1%) were male. Twenty-nine reported their race as Black (24.8%), 85 as White (72.6%), and 3 as any other race (2.6%). Most (113, 96.6%) indicated non-Hispanic ethnicity. Age was unintentionally omitted from the questionnaire, but participants were adults between 18 and 88 years. All 125 participants answered a multiple response question regarding the types of substances for which they were seeking treatment. Able to choose more than 1 substance, most reported seeking treatment for addiction to illicitly manufactured fentanyl (96, 76.8%), followed by heroin (43, 34.4%) and prescription opioids (27, 21.6%). Most (121, 96.8%) met criteria for severe OUD (6 or more DSM-5 OUD criteria). Three (2.4%) participants had moderate OUD (4-5 DSM-5 OUD criteria) and 1 (0.8%) had mild OUD (2-3 criteria).
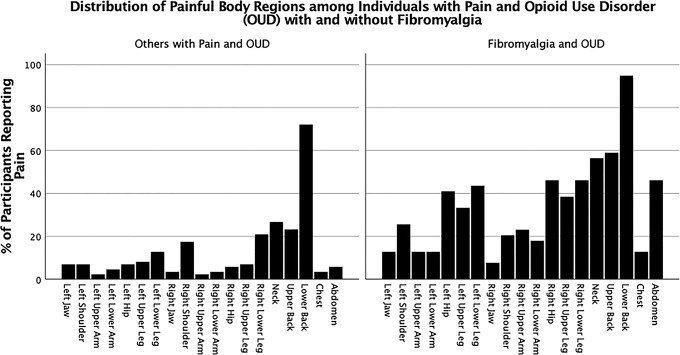
All 125 participants (100%) reported pain in at least 1 body region. Low back pain was most frequently reported (n = 99, 79.2%), followed by neck pain (n = 45, 36%) and upper back pain (n = 43, 34.4%). The mean number of painful body regions was 3.69 ± 2.74. Participants endorsing moderate to severe cognitive symptoms, fatigue, or waking unrefreshed numbered 58 (46.4%), 82 (65.6%), and 87 (69.6%), respectively. The mean total ACR-FMS score was 10.57 ± 4.622. The ACR-FMS scores of 39 (31.2%) participants were equal to or greater than 13 indicating that they met survey criteria for fibromyalgia. Considerable pain burden was noted even among participants without fibromyalgia as evidenced by ACR-FMS distribution skewed toward higher pain and symptom severity scores (refer to “Others with Pain and OUD” in Fig. 1) and low mean scores on the RAND-36 Bodily Pain subscale (lower scores equate to worse pain) among both groups (Table 1) (Fig. 2).
Figure 1.
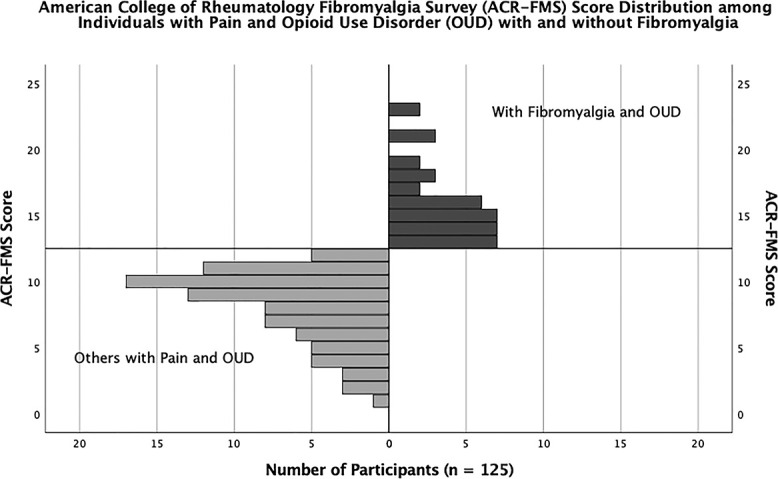
Distribution of American College of Rheumatology Fibromyalgia Survey scores among participants with and without fibromyalgia.
Table 1.
Sample characteristics.
| Characteristic | Participants with OUD and fibromyalgia | Others with pain and OUD | All participants |
|---|---|---|---|
| Gender, n (%) | |||
| Female | 20 (52.6) | 29 (36.7) | 49 (41.9) |
| Male | 18 (47.4) | 50 (63.3) | 68 (58.1) |
| Race, n (%) | |||
| Black | 8 (21.1) | 21 (26.6) | 29 (24.8) |
| White | 28 (73.7) | 57 (72.2) | 85 (72.6) |
| Any other race | 2 (5.3) | 1 (1.3) | 3 (2.6) |
| Ethnicity, n (%) | |||
| Hispanic | 2 (5.3) | 2 (2.5) | 5 (3.8) |
| Non-Hispanic | 28 (73.7) | 77 (97.5) | 113 (96.6) |
| Opioid type, n (%) | |||
| Fentanyl | 27 (69.2) | 69 (80.2) | 96 (76.8) |
| Heroin | 12 (30.8) | 31 (36.0) | 43 (34.4) |
| Prescription opioid | 10 (25.6) | 17 (19.8) | 27 (21.6) |
| OUD severity, mean (SD) | |||
| DSM-5 OUD criteria | 10.5 (1.6) | 10.6 (1.3) | 10.6 (1.3) |
| Mental health, mean (SD) | |||
| RAND-36 Mental Health domain score | 37.6 (14.5) | 49.3 (17.2) | 45 (17.2) |
| Bodily pain, mean (SD) | |||
| RAND-36 Bodily Pain domain score | 31.7 (16.3) | 54.5 (23.1) | 47.3 (23.6) |
| Painful body regions, mean (SD) | |||
| Body regions | 6 (2.9) | 2.4 (1.3) | 3.7 (2.7) |
| Pain-related OUD Exacerbation Scale, mean (SD) | |||
| PROUD ES score | 12.1 (3.6) | 9.5 (4.1) | 10.3 (4.1) |
Ten participants were missing demographics. Age was unintentionally omitted. Greater than 6 Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria indicates severe opioid use disorder (OUD). Research and Development-36 (RAND-36) domains are scaled 0 to 100 with lower scores indicating worse health in that domain (ie, worse pain or mental health). Greater Pain-related OUD Exacerbation Scale scores indicate greater endorsement of pain-related OUD exacerbations.
Figure 2.
Percent of participants with and without fibromyalgia reporting pain in various body regions.
3.2. Psychometric analysis of Pain-related Opioid Use Disorder Exacerbation Scale
Responses to the PrOUD ES were nonnormally distributed with the Shapiro–Wilk test (P < 0.001) and skewed toward greater acknowledgement of pain-related OUD exacerbations −0.666 (standard error = 0.217). The median, minimum, and maximum total PrOUD ES scores were 12.0, 0.0, and 16.0, respectively. Total PrOUD ES scores reflected prevalent acknowledgement of pain-related OUD exacerbations in this sample of individuals with pain and OUD. Eighty-four percent of the sample agreed or strongly agreed with at least 1 PrOUD ES item.
An exploratory factor analysis was run on PrOUD ES (n = 125). Exploratory factor analysis appropriateness was assessed before extraction. A correlation matrix showed all 4 variables had correlation coefficients greater than 0.3. The Kaiser–Meyer–Olkin measure was 0.77 for PrOUD ES as a whole, and individual questionnaire item Kaiser–Meyer–Olkin measures were all greater than 0.7. The Bartlett test of sphericity was statistically significant (P < 0.001). Because of multivariate nonnormality, principal axis factoring was chosen as the extraction method. Exploratory factor analysis revealed 1 factor with an eigenvalue greater than 1. This factor explained 70.39% of the variance. Visual inspection of the scree plot indicated that a single factor should be retained.10 A single-factor solution met the interpretability criterion. Because only 1 factor was retained, the solution was not rotatable. The final structure was consistent with PrOUD ES measuring our construct of interest: pain-related exacerbation of OUD.
Pain-related Opioid Use Disorder Exacerbation Scale exhibited a high level of internal consistency with a Cronbach alpha of 0.859. Construct validity was confirmed by Spearman rank-order correlations between PrOUD ES and (1) the number of painful body regions reported on ACR-FMS and (2) the Bodily Pain subscale of RAND-36. Pain-related Opioid Use Disorder Exacerbation Scale was positively correlated with the number of painful body regions rs (121) = 0.206, P = 0.021, indicating that more sites of bodily pain were associated with greater endorsement of pain-related OUD exacerbations. The pain-related quality of life subscale of RAND-36 was negatively correlated with PrOUD ES rs (121) = −0.391, P < 0.001, suggesting that deleterious impacts of Bodily Pain on life-quality were associated with acknowledgement of pain-related OUD exacerbation. Pain-related Opioid Use Disorder Exacerbation Scale item means, factor loadings, and communalities are shown in Table 2.
Table 2.
Pain-related Opioid Use Disorder Exacerbation Scale.
| Questionnaire item | % Affirmed | Mean (SD) | Factor loading | Communalities |
|---|---|---|---|---|
| Pain is a major reason why I have kept using opioids. | 60.8 | 2.5 (1.3) | 0.769 | 0.591 |
| I find myself needing more and more opioids to control my pain. | 66.4 | 2.7 (1.2) | 0.844 | 0.713 |
| I have put off getting treatment for opioid use disorder because I am afraid my pain will be worse when I stop using opioids. | 60.0 | 2.5 (1.2) | 0.825 | 0.680 |
| I am worried pain will cause me to relapse in the future. | 60.0 | 2.6 (1.2) | 0.673 | 0.454 |
Responses were scaled as strongly disagree (0), disagree (1), neutral (2), agree (3), or strongly agree (4).
% Affirmed = the percentage of the total sample that agreed or strongly agreed with the item. Eighty-four percent of participants affirmed at least 1 pain-related OUD exacerbation.
3.3. Effect of fibromyalgia on Pain-related Opioid Use Disorder Exacerbation Scale
Hierarchical multiple regression was used to determine whether fibromyalgia predicted pain-related exacerbation of OUD (PrOUD ES) after controlling for gender, race, OUD severity (number of DSM-5 OUD criteria present), type of opioid used (illicitly manufactured fentanyl, heroin, or prescription opioid analgesics), mental health (subscale of RAND-36), and pain severity (item 21 of RAND-36). The dependent variable was mean centered before analysis. Partial regression plots and a plot of studentized residuals against predicted values confirmed linearity. There was homoscedasticity on visual inspection of a plot of studentized residuals vs unstandardized predicted values. All tolerance values were greater than 0.1 indicating no multicollinearity. There were no studentized deleted residuals greater than 3 standard deviations or values for the Cook distance exceeding 1. The assumption of normality was met, as assessed by a P–P plot. The full model was statistically significant, R2 = 0.259, F (13, 103) = 2.77, P = 0.002, adjusted R2 = 0.166. Gender, race, OUD severity, and opioid type had no significant effect on the explanatory power of the model. The addition of mental health (model 4) and pain severity (model 5) led to statistically significant increases in R2 of 0.052, F (1, 109) = 6.35, P = 0.013, and 0.109, F (5, 104) = 2.91, P = 0.017, respectively. The introduction of fibromyalgia (model 6) also led to a statistically significant increase in R2 of 0.036, F (1, 103) = 5.03, P = 0.027, above and beyond all previously loaded variables. Table 3 summarizes the multiple hierarchical regression analyses.
Table 3.
Hierarchical multiple regression: Pain-related OUD Exacerbation Scale.
| R | R 2 | Adj. R2 | ΔR2 | ΔF | P | |
|---|---|---|---|---|---|---|
| Model 1 | 0.123 | 0.015 | 0.006 | 0.015 | 1.76 | 0.188 |
| Model 2 | 0.194 | 0.038 | 0.021 | 0.023 | 2.68 | 0.104 |
| Model 3 | 0.250 | 0.063 | 0.012 | 0.025 | 0.733 | 0.571 |
| Model 4 | 0.338 | 0.114 | 0.057 | 0.052 | 6.35 | 0.013 |
| Model 5 | 0.472 | 0.223 | 0.133 | 0.109 | 2.91 | 0.017 |
| Model 6 | 0.509 | 0.259 | 0.166 | 0.036 | 5.03 | 0.027 |
Model 1: gender.
Model 2: gender and race.
Model 3: gender, race, opioid use disorder (OUD) severity, and types of opioids used (heroin, illicitly manufactured fentanyl, or prescription opioids).
Model 4: gender, race, OUD severity, types of opioids used, and Mental Health (Research and Development-36 [RAND-36]).
Model 5: gender, race, OUD severity, types of opioids used, Mental Health (RAND-36), and Pain Severity (RAND-36).
Model 6: gender, race, OUD severity, types of opioids used, Mental Health (RAND-36), Pain Severity (RAND-36), and fibromyalgia.
3.4. Effect of fibromyalgia on specific pain-related opioid use disorder exacerbations
3.4.1. Maintenance of opioid use disorder
A cumulative odds ordinal logistic regression analysis with proportional odds was run to determine the effect of fibromyalgia on acknowledgement of pain-related OUD maintenance. The primary predictor variable was fibromyalgia. Gender, race, and Mental Health (RAND-36) were initially included as covariates. Gender 0.873 (95% CI, 0.433-1.76), race 0.777 (95% CI, 0.364-1.66), and Mental Health 0.985 (95% CI, 0.964-1.01) were found to be insignificant predictors of pain-related OUD maintenance and their inclusion reduced the overall explanatory power of the model to the point of insignificance χ2(4) = 9.14, P = 0.058. However, fibromyalgia was associated with significantly increased odds of pain-related OUD maintenance 2.31 (95% CI, 1.08-4.92), P = 0.031. Therefore, the model was rerun with fibromyalgia as the sole predictor variable.
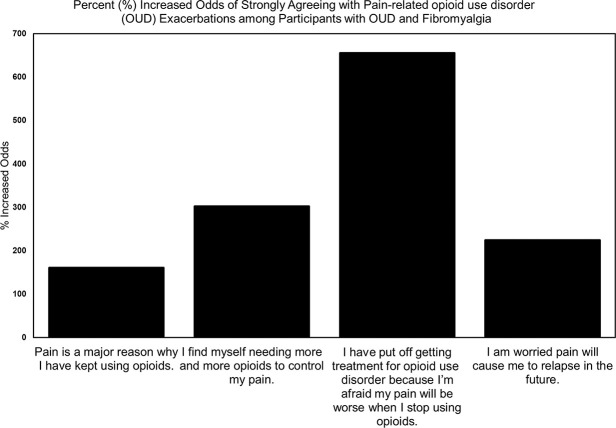
In this second model, the assumption of proportional odds was met, as assessed by a full likelihood ratio test comparing the fit of the proportional odds model to a model with varying location parameters, χ2(3) = 0.149, P = 0.985. A Pearson goodness-of-fit test indicated that the model was a good fit to the observed data, χ2(3) = 0.148, P = 0.986. The model statistically significantly predicted the dependent variable over and above the intercept-only model, χ2(1) = 7.204, P = 0.007. The odds of strongly agreeing with the statement “Pain is a major reason why I have kept using opioids” was 2.612 (95% CI, 1.286-5.306) times higher among participants with fibromyalgia, a statistically significant effect χ2(1) = 7.058, P = 0.008.
3.4.2. Escalation of opioid use disorder
Ordinal logistic regression was conducted to predict odds of pain-related escalation of OUD with fibromyalgia, gender, race, and Mental Health as independent variables. The assumption of proportional odds was met per full likelihood ratio test, χ2(12) = 14.15, P = 0.291. The model statistically significantly predicted the dependent variable better than an intercept-only model, χ2(4) = 21.44, P < 0.001. Odds of strongly agreeing with the statement “I find myself needing more and more opioids to control my pain” was 4.43 (95% CI, 1.98-9.92) times higher among participants with fibromyalgia. This effect was significant χ2(1) = 13.12, P < 0.001. White race also increased odds of pain-related OUD escalation 2.789 (95% CI, 1.28-6.09), P = 0.01. Neither gender 0.989 (95% CI, 0.428-2.03) nor Mental Health 0.993 (95% CI, 0.971-1.02) had a significant effect on endorsement of pain-related OUD escalation.
3.4.3. Opioid use disorder treatment delay
The assumption of proportional odds was violated per full likelihood test, χ2(3) = 8.884, P = 0.031. Therefore, a multinomial logistic regression was conducted to assess the effect of fibromyalgia, gender, race, and mental health on odds of agreement with the statement “I have put off getting treatment for opioid use disorder because I am afraid my pain will be worse when I stop using opioids.” A neutral response was used as the reference category. The model statistically significantly predicted the dependent variable over the intercept-only model, χ2(16) = 44.54, P < 0.001. Gender 2.19 (95% CI, 0.571-8.41), race 1.27 (95% CI, 0.243-6.64), and Mental Health 9.58 (95% CI, 0.915-1.00) had no significant effect on endorsement of pain-related OUD treatment delay. However, fibromyalgia was associated with significantly increased odds of strongly agreeing with pain-related OUD treatment delay 5.70 (95% CI 1.26-25.9). This effect was statistically significant χ2(4) = 12.63, P = 0.013.
3.4.4. Opioid use disorder relapse
Ordinal logistic regression was used to predict odds of fearing pain-related OUD relapse. Fibromyalgia, gender, race, and mental health were independent variables. A full likelihood ratio test indicated the assumption of proportional odds was met, χ2(12) = 16.16, P = 0.184. The full logit model statistically significantly predicted the dependent variable, χ2(4) = 18.36, P = 0.001. The odds of strongly agreeing with the statement “I am worried pain will cause me to relapse in the future” was 2.51 (95% CI, 1.17-5.40) times higher among participants with fibromyalgia, χ2(1) = 10.356, P = 0.001. Race 0.63 (95% CI, 0.292-1.34), gender 1.51 (95% CI, 0.741-3.06), and Mental Health 0.980 (95% CI, 0.959-1.00) were insignificant (Fig. 3).
Figure 3.
Percent increase in odds of pain-related opioid use disorder exacerbations associated with fibromyalgia.
4. Discussion
This study aimed to predict odds of self-reported OUD exacerbations based on fibromyalgia among patients with comorbid pain and OUD. We found fibromyalgia (ACR-FMS ≥ 13) increased the odds of strongly agreeing with 4 questions probing pain-related OUD exacerbation. Despite the fact that all participants had pain in at least 1 body region, those with fibromyalgia were at substantially greater odds of strongly agreeing with the statements “Pain is a major reason why I have kept using opioids,” “I find myself needing more and more opioids to control my pain,” “I have put off getting treatment for opioid use disorder because I am afraid my pain will be worse when I stop using opioids,” and “I am worried pain will cause me to relapse in the future.” Fibromyalgia predicted the odds of acknowledging pain-related OUD exacerbations independently of potential confounders across all 6 regression analyses.
The present work provides evidence of the factor structure, validity, and reliability of a new scale to measure pain-related OUD exacerbations: PrOUD ES. Given great scientific interest in the intersection of pain and OUD embodied by the National Institutes of Health Helping to End Addiction Long-term initiative, PrOUD ES warrants further study as a patient-reported outcome measure of potential importance.
Previous pain and addiction literature gives context to our findings. Complex, sometimes bidirectional, relationships have been observed between pain and OUD.29,48,53 Pain may precede OUD and many individuals with OUD report onset of OUD after exposure to prescribed opioid analgesics.28 Alternatively, chronic opioid use—as in OUD—may produce opioid-induced hyperalgesia or contribute to injury.12,37 Regardless of which precedes the other, pain and OUD are frequently comorbid, complicating their effective management.49
Poorly operationalized definitions of pain, measurement, and sampling issues have stymied the study of pain in the OUD population.46 Nevertheless, a growing body of literature implicates pain in the onset, maintenance, and relapse of OUD and as an important consideration in the treatment of patients with OUD.5,36,46,53 The prevalence of pain in the OUD treatment population is unknown but is estimated to be as high as 36% to 62%.46,52 Pain has been associated with poorer treatment outcomes as well as physical, psychiatric, and social functioning among patients receiving medications for opioid use disorder.15 Despite this, two-thirds of patients with pain and OUD report their pain is unmanaged by their addiction medicine provider and uncontrolled pain is a primary factor contributing to relapse.21 The present study contributes to the literature because it strongly suggests not all pain is equal in increasing odds of OUD exacerbation. Relative to other forms of pain, fibromyalgia may be a uniquely pernicious comorbidity, with implications for OUD treatment.
Scant literature exists regarding fibromyalgia as a comorbidity of OUD. One nationally representative survey from the United States found fibromyalgia and posttraumatic stress had additive effects on OUD risk.4 A retrospective chart review of patients from a tertiary fibromyalgia clinic observed negative psychosocial associations among opioid-treated patients with fibromyalgia including a small number with problematic substance use.23 Another retrospective study found a 12-fold increase in OUD-related hospitalizations among patients with fibromyalgia between 1998 and 2014.44 Given the dearth of extant literature, more research is needed to establish the role of fibromyalgia in OUD. The present work provides a framework for future research by demonstrating the use of a surrogate measure of fibromyalgia (ACR-FMS) that is easily deployable in addiction treatment settings. As a self-report measure, ACR-FMS may facilitate studies of fibromyalgia in OUD even if addiction researchers have insufficient expertise to diagnose musculoskeletal pain conditions.
Study strengths included a relatively large clinical sample, low levels of missingness, and use of a validated self-report measure of fibromyalgia. Several limitations also require discussion. A cross-sectional design prevented testing of relationships between fibromyalgia and observable OUD exacerbations. In addition, our findings have uncertain generalizability. Participants of the larger study were not recruited based on the presence of pain. Pain chronicity is incompletely characterized by ACR-FMS, but endorsement of our original items (particularly pain-related OUD maintenance, escalation, and treatment delay) implies participants' pain was persistent or recurrent. Another potential criticism might be that OUD itself could account for participants' pain, rather than fibromyalgia. However, a diagnosis of fibromyalgia no longer requires the absence of other clinically important illnesses, such as OUD.38,54 In fact, fibromyalgia is often present in individuals with other conditions that produce widespread pain (ie, psoriatic arthritis, systemic lupus erythematosus, or rheumatoid arthritis).20,59 In addition, the International Association for the Study of Pain (IASP) asserts that “A person's report of an experience as pain should be respected” and that “Pain is always a personal experience that is influenced to varying degrees by biological, psychological, and social factors.”1 Therefore, we reject the notion that pain associated with the disease of opioid addiction should be regarded differently from pain associated with other health conditions. Our analysis did not control for pain catastrophizing, which is known to affect fibromyalgia prognosis.11 Finally, the mode (ie, buprenorphine, naltrexone, or level of behavioral care) or length of OUD treatment was not controlled for in this pilot study of fibromyalgia in OUD.
One notable feature of our study that may be considered a limitation or a strength is the use of ACR-FMS in lieu of a provider examination to assess fibromyalgia. The American College of Rheumatology Fibromyalgia Survey is a validated self-report instrument with excellent sensitivity and specificity for fibromyalgia well-suited to our study design.55 Although clinician assessment is not necessary to diagnose fibromyalgia with ACR-FMS, such assessment might have provided additional useful data about other pain diagnoses present in the sample. Our findings do not preclude the possibility that other pain conditions might have similar important implications for OUD disease trajectory.
This study has research and clinical implications. We have demonstrated a promising new scale for measuring pain-related OUD exacerbations (PrOUD ES), as well as a novel methodology for studying fibromyalgia in OUD treatment settings where practitioners may lack specialized knowledge of musculoskeletal pain conditions. American College of Rheumatology Fibromyalgia Survey and PrOUD ES may be considered for use as patient-reported outcome measures in OUD research. Additional studies are needed to further assess the psychometric properties of PrOUD ES and its relationship to pain catastrophizing. The American College of Rheumatology Fibromyalgia Survey might be useful in epidemiological and community-based studies of OUD, including among non–treatment-seeking populations, to establish the role of fibromyalgia in OUD. Although additional research is needed, these instruments might be useful for identifying patients at risk of pain-related OUD exacerbations.
Acknowledgements
The authors thank Mamie Martin, RN, MSN, APRN-CNP, DNP; Leah Brown, MS, MPH, APRN-CNP; Jessica Belser, MSW, MS, PMHNP-BC, CARN-NP; and Megan Ackley APRN-CNP for their support in conducting this study.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Contributor Information
Julie Teater, Email: Julie.Teater@osumc.edu.
Parker Entrup, Email: parker.Entrup@osumc.edu.
Megan Deaner, Email: Megan.Deaner@osumc.edu.
Craig Bryan, Email: craig.bryan@osumc.edu.
Steven E. Harte, Email: seharte@med.umich.edu.
Chelsea M. Kaplan, Email: chelsmar@med.umich.edu.
Kihn Luan Phan, Email: Luan.Phan@osumc.edu.
Daniel J. Clauw, Email: dclauw@med.umich.edu.
Conflict of interest statement
O.T. Hall provided expert opinion regarding the opioid crisis to Lumanity and Emergent BioSolutions. D. J. Clauw has testified in state lawsuits against opioid manufacturers for their role in the opioid overdose crisis. The remaining authors have no conflicts of interest to declare. Funding was provided by the Care Innovation and Community Improvement Plan (CICIP), a program of the Ohio Department of Medicaid. The views expressed in this publication do not necessarily reflect the official policies of the Ohio Department of Medicaid nor does mention of trade names, commercial practices, or organizations imply endorsement by the government of Ohio.
References
- [1].International Association for the Study of Pain. Vol. 2022. Washington: IASP Terminology, 1979. [Google Scholar]
- [2].American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington: American Psychiatric Association, 2013. [Google Scholar]
- [3].Arnold LM, Bennett RM, Crofford LJ, Dean LE, Clauw DJ, Goldenberg DL, Fitzcharles M-A, Paiva ES, Staud R, Sarzi-Puttini P, Buskila D, Macfarlane GJ. AAPT diagnostic criteria for fibromyalgia. J Pain 2019;20:611–628. [DOI] [PubMed] [Google Scholar]
- [4].Bilevicius E, Sommer JL, Asmundson GJG, El-Gabalawy R. Posttraumatic stress disorder and chronic pain are associated with opioid use disorder: results from a 2012-2013 American nationally representative survey. Drug and Alcohol Dependence 2018;188:119–125. [DOI] [PubMed] [Google Scholar]
- [5].Blanco C, Wall MM, Okuda M, Wang S, Iza M, Olfson M. Pain as a predictor of opioid use disorder in a nationally representative sample. Am J Psychiatry 2016;173:1189–1195. [DOI] [PubMed] [Google Scholar]
- [6].Brazier JE, Harper R, Jones NM, O'cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J 1992;305:160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brummett CM, Janda AM, Schueller CM, Tsodikov A, Morris M, Williams DA, Clauw DJ. Survey criteria for fibromyalgia independently predict increased postoperative opioid consumption after lower-extremity joint arthroplasty: a prospective, observational cohort study. Anesthesiology 2013;119:1434–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brummett CM, Urquhart AG, Hassett AL, Tsodikov A, Hallstrom BR, Wood NI, Williams DA, Clauw DJ. Characteristics of fibromyalgia independently predict poorer long‐term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol 2015;67:1386–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 2014;44:68–75. [DOI] [PubMed] [Google Scholar]
- [10].Cattell RB. The scree test for the number of factors. Multivariate Behav Res 1966;1:245–276. [DOI] [PubMed] [Google Scholar]
- [11].Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547–1555. [DOI] [PubMed] [Google Scholar]
- [12].Compton P, Canamar CP, Hillhouse M, Ling W. Hyperalgesia in heroin dependent patients and the effects of opioid substitution therapy. J Pain 2012;13:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Coons SJ, Alabdulmohsin SA, Draugalis JR, Hays RD. Reliability of an Arabic version of the RAND-36 Health Survey and its equivalence to the US-English version. Med Care 36 1998:428–432. [DOI] [PubMed] [Google Scholar]
- [14].Crist RC, Reiner BC, Berrettini WH. A review of opioid addiction genetics. Curr Opin Psychol 2019;27:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Dennis BB, Bawor M, Paul J, Varenbut M, Daiter J, Plater C, Pare G, Marsh DC, Worster A, Desai D, Thabane L, Samaan Z. The impact of chronic pain on opioid addiction treatment: a systematic review protocol. Syst Rev 2015;4:49–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, Dayer P, Vischer TL. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum 2003;48:1420–1429. [DOI] [PubMed] [Google Scholar]
- [17].Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. PAIN 2001;93:155–163. [DOI] [PubMed] [Google Scholar]
- [18].Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. PAIN 2001;90:91–96. [DOI] [PubMed] [Google Scholar]
- [19].Dudeney J, Law EF, Meyyappan A, Palermo TM, Rabbitts JA. Evaluating the psychometric properties of the widespread pain index and the symptom severity scale in youth with painful conditions. Can J Pain 2019;3:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Duffield SJ, Miller N, Zhao S, Goodson NJ. Concomitant fibromyalgia complicating chronic inflammatory arthritis: a systematic review and meta-analysis. Rheumatology 2018;57:1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ellis MS, Kasper Z, Cicero T. Assessment of chronic pain management in the treatment of opioid use disorder: gaps in care and implications for treatment outcomes. J Pain 2021;22:432–439. [DOI] [PubMed] [Google Scholar]
- [22].Elman I, Borsook D. Common brain mechanisms of chronic pain and addiction. Neuron 2016;89:11–36. [DOI] [PubMed] [Google Scholar]
- [23].Fitzcharles M-A, Ste-Marie PA, Gamsa A, Ware MA, Shir Y. Opioid use, misuse, and abuse in patients labeled as fibromyalgia. Am J Med 2011;124:955–960. [DOI] [PubMed] [Google Scholar]
- [24].George O, Koob GF, Vendruscolo LF. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology 2014;231:3911–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hall OT, Teater J, Rood KM, Phan KL, Clauw DJ. Central sensitization in opioid use disorder: a novel application of the American College of Rheumatology Fibromyalgia Survey Criteria. Pain Rep 2022;7:e1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harte SE, Harris RE, Clauw DJ. The neurobiology of central sensitization. J Appl Biobehavioral Res 2018;23:e12137. [Google Scholar]
- [27].Hays RD, Morales LS. The RAND-36 measure of health-related quality of life. Ann Med 2001;33:350–357. [DOI] [PubMed] [Google Scholar]
- [28].Higgins C, Smith BH, Matthews K. Incidence of iatrogenic opioid dependence or abuse in patients with pain who were exposed to opioid analgesic therapy: a systematic review and meta-analysis. Br J Anaesth 2018;120:1335–1344. [DOI] [PubMed] [Google Scholar]
- [29].Hsu WY, Lin CL, Kao CH. Association between opioid use disorder and fractures: a population based study. Addiction 2019;114:2008–2015. [DOI] [PubMed] [Google Scholar]
- [30].Janda AM, As-Sanie S, Rajala B, Tsodikov A, Moser SE, Clauw DJ, Brummett CM. Fibromyalgia survey criteria are associated with increased postoperative opioid consumption in women undergoing hysterectomy. Anesthesiology 2015;122:1103–1111. [DOI] [PubMed] [Google Scholar]
- [31].Kaleycheva N, Cullen AE, Evans R, Harris T, Nicholson T, Chalder T. The role of lifetime stressors in adult fibromyalgia: systematic review and meta-analysis of case-control studies. Psychol Med 2021;51:177–193. [DOI] [PubMed] [Google Scholar]
- [32].Kleykamp BA, Ferguson MC, McNicol E, Bixho I, Arnold LM, Edwards RR, Fillingim R, Grol-Prokopczyk H, Turk DC, Dworkin RH. The prevalence of psychiatric and chronic pain comorbidities in fibromyalgia: an ACTTION systematic review, Proc Semin Arthritis Rheum 2021;51:166–174. [DOI] [PubMed] [Google Scholar]
- [33].Koob GF. Neurobiological substrates for the dark side of compulsivity in addiction. Neuropharmacology 2009;56:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Koob GF. Negative reinforcement in drug addiction: the darkness within. Curr Opin Neurobiol 2013;23:559–563. [DOI] [PubMed] [Google Scholar]
- [35].Koob GF. Drug addiction: hyperkatifeia/negative reinforcement as a framework for medications development. Pharmacol Rev 2021;73:163–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Larson MJ, Paasche-Orlow M, Cheng DM, Lloyd-Travaglini C, Saitz R, Samet JH. Persistent pain is associated with substance use after detoxification: a prospective cohort analysis. Addiction 2007;102:752–760. [DOI] [PubMed] [Google Scholar]
- [37].Lee MO, Sanford Silverman M, Hans Hansen M, Vikram Patel M, Laxmaiah Manchikanti M. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011;3;14:145–161. [PubMed] [Google Scholar]
- [38].Middleton GD, Mcfarlin JE, Lipsky PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum 1994;37:1181–1188. [DOI] [PubMed] [Google Scholar]
- [39].Miró E, Martínez MP, Sánchez AI, Cáliz R. Clinical manifestations of trauma exposure in fibromyalgia: the role of anxiety in the association between posttraumatic stress symptoms and fibromyalgia status. J Traumatic Stress 2020;33:1082–1092. [DOI] [PubMed] [Google Scholar]
- [40].Moorer P, Suurmeijer TP, Foets M, Molenaar IW. Psychometric properties of the RAND-36 among three chronic diseases (multiple sclerosis, rheumatic diseases and COPD) in The Netherlands. Qual Life Res Int J Qual Life aspects Treat Care Rehabil 2001;10:637–645. [DOI] [PubMed] [Google Scholar]
- [41].Neville SJ, Clauw AD, Moser SE, Urquhart AG, Clauw DJ, Brummett CM, Harte SE. Association between the 2011 fibromyalgia survey criteria and multisite pain sensitivity in knee osteoarthritis. Clin J Pain 2018;34:909–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Nicol A, Arnold P, Clauw D. (321) (321) Fibromyalgia-ness in persistent low back pain after lumbar spine surgery: a preliminary investigation. J Pain 2017;18:S55. [Google Scholar]
- [43].Sawaddiruk P, Paiboonworachat S, Chattipakorn N, Chattipakorn SC. Alterations of brain activity in fibromyalgia patients. J Clin Neurosci 2017;38:13–22. [DOI] [PubMed] [Google Scholar]
- [44].Singh JA, Cleveland JD. Time trends in opioid use disorder hospitalizations in gout, rheumatoid arthritis, fibromyalgia, osteoarthritis, and low back pain. J Rheumatol 2021;48:775–784. [DOI] [PubMed] [Google Scholar]
- [45].Smallwood RF, Laird AR, Ramage AE, Parkinson AL, Lewis J, Clauw DJ, Williams DA, Schmidt-Wilcke T, Farrell MJ, Eickhoff SB, Robin DA. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain 2013;14:663–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Speed TJ, Parekh V, Coe W, Antoine D. Comorbid chronic pain and opioid use disorder: literature review and potential treatment innovations. Int Rev Psychiatry 2018;30:136–146. [DOI] [PubMed] [Google Scholar]
- [47].Strang J, Volkow ND, Degenhardt L, Hickman M, Johnson K, Koob GF, Marshall BDL, Tyndall M, Walsh SL. Opioid use disorder. Nat Rev Dis primers 2020;6:3–28. [DOI] [PubMed] [Google Scholar]
- [48].Stumbo SP, Yarborough BJH, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Substance Abuse Treat 2017;73:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug Alcohol Dependence 2004;73:23–31. [DOI] [PubMed] [Google Scholar]
- [50].Zee KI, Sanderman R, Heyink JW, Haes H. Psychometric qualities of the RAND 36-Item Health Survey 1.0: a multidimensional measure of general health status. Int J Behav Med 1996;3:104–122. [DOI] [PubMed] [Google Scholar]
- [51].VanderZee KI, Sanderman R, Heyink J. A comparison of two multidimensional measures of health status: the nottingham health profile and the RAND 36-item health survey 1.0. Qual Life Res 1996;5:165–174. [DOI] [PubMed] [Google Scholar]
- [52].Voon P, Hayashi K, Milloy MJ, Nguyen P, Wood E, Montaner J, Kerr T. Pain among high-risk patients on methadone maintenance treatment. J Pain 2015;16:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Weiss RD, Potter JS, Griffin ML, McHugh RK, Haller D, Jacobs P, Gardin J, II, Fischer D, Rosen KD. Reasons for opioid use among patients with dependence on prescription opioids: the role of chronic pain. J substance abuse Treat 2014;47:140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 revisions to the 2010/2011 fibromyalgia diagnostic criteria, Proc Semin Arthritis Rheum 2016;46:319–329. [DOI] [PubMed] [Google Scholar]
- [55].Wolfe F, Clauw DJ, Fitzcharles M-A, Goldenberg DL, Häuser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol 2011;38:1113–1122. [DOI] [PubMed] [Google Scholar]
- [56].Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res 2010;62:600–610. [DOI] [PubMed] [Google Scholar]
- [57].Wolfe F, Häuser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia–I: examination of rates and predictors in patients with rheumatoid arthritis (RA). PAIN 2011;152:291–299. [DOI] [PubMed] [Google Scholar]
- [58].Yavne Y, Amital D, Watad A, Tiosano S, Amital H. A systematic review of precipitating physical and psychological traumatic events in the development of fibromyalgia, Proc Semin Arthritis Rheum 2018;48:121–133. [DOI] [PubMed] [Google Scholar]
- [59].Zhao SS, Duffield SJ, Goodson NJ. The prevalence and impact of comorbid fibromyalgia in inflammatory arthritis. Best Pract Res Clin Rheumatol 2019;33:101423. [DOI] [PubMed] [Google Scholar]