An ecological study of the United States in 2020 found county-level differences in the rates of COVID-19 cases and deaths and rates of chlamydia, gonorrhea, and syphilis.
Background
Shifts in public health infrastructure to respond to one emerging health threat may have unanticipated consequences for preexisting diseases. Previous research evaluating the impact of COVID-19 on sexually transmitted infections (STIs) has been conducted nationally, with little exploration of the impact on a granular geospatial level. This ecological study seeks to quantify the association between COVID-19 cases or deaths and chlamydia, gonorrhea, and syphilis cases for all US counties in 2020.
Methods
Separate, adjusted multivariable quasi-Poisson models with robust standard errors modeled the county-level association between 2020 COVID-19 cases and deaths per 100,000 and 2020 chlamydia, gonorrhea, or syphilis cases per 100,000. Models were adjusted for sociodemographic characteristics.
Results
Every 1000 additional COVID-19 cases per 100,000 was associated with a 1.80% increase in the average number of chlamydia cases (P < 0.001) and a 5.00% increase in the average number of gonorrhea cases (P < 0.001). Every 1000 additional COVID-19 deaths per 100,000 was associated with a 57.9% increase in the average number gonorrhea cases (P < 0.001) and a 74.2% decrease in the average number of syphilis cases (P = 0.004).
Conclusions
Higher rates of COVID-19 cases and deaths were associated with increased rates of some STIs at the US county level. The underlying reasons for these associations could not be established by this study. The emergency response to an emerging threat may have unanticipated influence on preexisting diseases that varies by level of governance.
Pandemic preparedness is a fundamental tenant of national health and security. The US response to such a threat was tested with the sudden emergence of the novel coronavirus disease 2019 (COVID-19) pandemic, which necessitated a rapid shift in public health resources and funding. Although these actions may have reduced the spread of COVID-19, it is possible that this all-encompassing pivot had unanticipated impacts on other parts of the public health infrastructure. For example, studies suggest that the reported prevalence of other highly contagious diseases decreased between 2019 and 2020, including some sexually transmitted infections (STIs).1,2 Declines in STIs may correspond to stay-at-home orders or other physical distancing policies for COVID-19, which, in turn, had a profound impact on the prevalence and transmission of other diseases.2,3 Alternatively, these declines may have been the result of interruptions in testing, screening, and reporting that occurred during the pandemic.4 This is especially true of STIs, as many clinical services (including sexual health programs) temporarily shifted their resources and focus to COVID-19, decreasing preventive services such as screenings.2 This was further compounded by supply chain concerns that limited access to sample collection kits. National declines in STIs were concomitant with increased COVID-19 cases and deaths, supporting the need to further explore the relationship between the 2 infections.1
It is imperative that STIs be separated and studied individually, as the overall decrease observed among all STIs was not consistent when stratified by individual infection. For example, reported chlamydia cases decreased by 13% between 2019 and 2020 nationally within the United States; in contrast, gonorrhea cases increased by 10%, and primary and secondary syphilis cases increased by 7%.2 However, a study of New York State that excluded New York City identified fewer syphilis cases but greater gonorrhea cases in 2020 compared with the same period in 2019, whereas a study in King County, Washington, found that both syphilis and gonorrhea were lower between January and June 2020 compared with January and July 2019.5,6 Thus, the patterns associated with each STI should be assessed separately. This granular approach can help clarify how changes in legislation at varying levels of government and healthcare infrastructure enacted during the pandemic (such as decreased access to in-person care and increased access to telemedicine consultations) differentially impacted different types of STIs.7
Furthermore, the current literature almost exclusively explores these relationships at the national and state levels, and those that do look at the county level often only focus on a small subset. This has introduced a need for a broader comparative analysis across all counties within the United States. Differences in COVID-19 outcomes by sociodemographic status have already been demonstrated at a county level, and it is possible that these differences may also extend to STIs.8 Given the heterogeneity in counties even within the same state, understanding how STI rates changed at a county level may provide a more granular geospatial understanding of the interplay between STIs and COVID-19, including the impact of varied state-and-local policies and responses to the pandemic. Thus, the aim of this study was to explore the association between COVID-19 cases or deaths and the rates of chlamydia, gonorrhea, and syphilis for county-level US data in 2020.
MATERIALS AND METHODS
Data Collection
County-level COVID-19 cases per 100,000 in 2020 served as the primary exposure. County-level COVID-19 deaths per 100,000 in 2020 were explored as a secondary exposure. Data for both were collected from USA Facts, which combines data from the US Centers for Disease Control and Prevention (CDC) and state-and-local public health agencies.9 The outcomes of interest were the county-level, aggregated rates of chlamydia, gonorrhea, and syphilis (primary and secondary only) per 100,000 in 2020, obtained from the CDC Sexually Transmitted Disease Surveillance, 2020 report.2 Data were available for 3141 county-and-county-level equivalents. These data were not available at the person level, so case breakdowns by gender identity, sex assigned at birth, and sexual orientation were not available.
Covariates used in multivariable models were chosen based on their epidemiological relationship with STI rates. All covariate data were collected at the county level and included age (i.e., percent 19 and less than 19 years, percent between 20 and 64 years, percent 65 and greater years), race (i.e., percent White, Black or African American, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, or other), ethnicity (i.e., percent Hispanic or Latino, not Hispanic or Latino, or other), education (i.e., percent less than high school diploma, high school diploma, or bachelor's degree and higher), rurality (i.e., urban, suburban, rural), percent unemployed, and percent below the poverty line. Age, race, and ethnicity data were obtained using the most recent 5-year estimates from the US Census Bureau American Community Survey. Education, socioeconomic variables, and rurality were obtained from the US Department of Agriculture Economic Research Service.10–12 To put the STI rates reported during the pandemic in 2020 into historical context, national changes in STI rates between 2019 and 2020 were also evaluated.
Statistical Analysis
First, descriptive statistics (i.e., median and interquartile range [IQR]) were computed to compare the change in STI per 100,000 in 2019 and 2020, with changes in rates tested for significance using Wilcoxon signed rank tests. Second, associations between each exposure (county-level rates of COVID-19 cases and deaths) and outcome (county-level rates of chlamydia, gonorrhea, and syphilis) in 2020 were assessed with Poisson regression models, which accounted for the strictly positive, count nature of STI cases. After evaluating the dispersion of the outcome data, the variance and mean were found to be significantly different (all P < 0.001); thus, quasi-Poisson models with robust standard errors were selected to account for overdispersion. Bivariate models that assessed the relationship between COVID-19 cases or deaths and each STI were first evaluated to obtain unadjusted incident rate ratios (IRRs). Then, multivariable models adjusting for age, race, ethnicity, education, socioeconomic status, and rurality were evaluated. Quasi-Poisson models are an alternative to negative binomial models. Both model overdispersed count data, and they typically produce similar point estimates.13 In a sensitivity analysis, each multivariable model was rerun as a negative binomial model (Supplemental Digital Content 1, http://links.lww.com/OLQ/A933). Results suggest that both models produced similar estimates, with all quasi-Poisson estimates biased toward the null. Thus, the results of the quasi-Poisson are presented as a conservative interpretation of findings.
Incident rate ratios for COVID-19 cases or deaths are expressed in terms of 1000 new cases or deaths per 100,000 persons, whereas IRRs for all covariates except rurality are expressed for every 5-percentage-point increase in the variable of interest and 5-percentage-point decrease in the reference for that variable. In all instances, a P value of less than 0.05 (i.e., P ≤ 0.05) was considered statistically significant. All analyses were conducted in R (version 4.2.1) using the RStudio Integrated Development Environment (version 2022.07.1).
RESULTS
National Changes in STI Rates Between 2019 and 2020
Across the United States in 2019, there was a county-level median of 332 (IQR, 219–509) cases of chlamydia per 100,000, 84 (IQR, 40–170) cases of gonorrhea per 100,000, and 2.1 (IQR, 0–7.5) cases of syphilis per 100,000 (Fig. 1). In 2020, the county-level median chlamydia cases fell to 300 (IQR, 206–467) cases per 100,000 for an average county-level decrease of 38 cases (SD, 124). In contrast, the county-level median gonorrhea cases rose to 98 (IQR, 48–191) cases per 100,000 for an average county-level increase of 16 cases (SD, 74.9). County-level syphilis cases rose to 2.8 (IQR, 0–8.5) per 100,000 for an average county-level increase of 0.77 cases (SD, 9.74). The difference between 2019 and 2020 was significant for all diseases (P < 0.001, Fig. 2).
Figure 1.
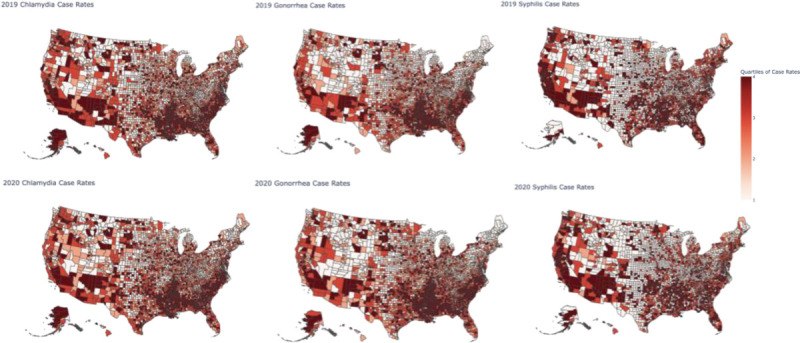
Unadjusted chlamydia, gonorrhea, and syphilis cases per 100,000 in 2019 (top row) and 2020 (bottom row). Counties are categorized by the quartile of severity, with “1” representing the lowest quartile of cases and “4” representing the highest quartile of cases.
Figure 2.
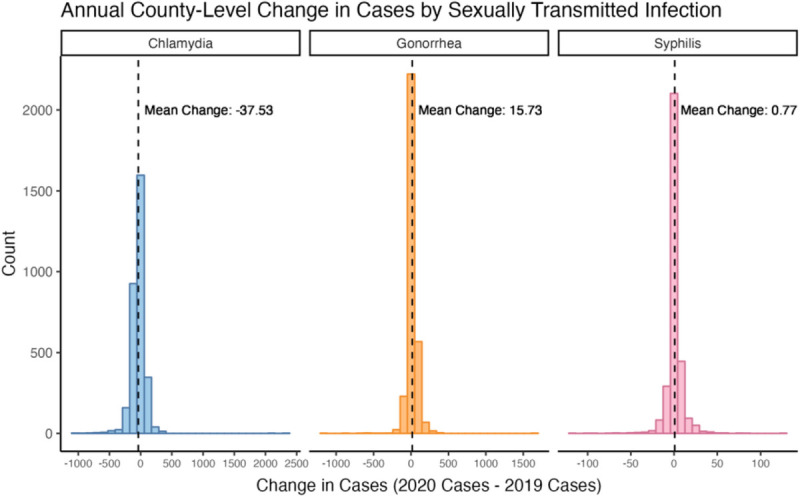
Distribution of the change in chlamydia, gonorrhea, and syphilis cases across US counties. Note that each STI has its own x axis, but the y axis is shared. All changes are expressed as 2020 cases minus 2019 cases. Chlamydia cases decreased by an average of 37.5 cases (SD, 124), gonorrhea increased by an average of 15.7 cases (SD, 74.9), and syphilis increased by an average of 0.77 cases (SD, 9.74).
Bivariate Associations Between STI and COVID-19 Cases and Deaths
In bivariate analyses of county-level rates of COVID-19 cases or deaths in 2020 and county-level STI rates in 2020, every 1000 additional COVID-19 case per 100,000 was associated with a 3.88% increase (IRR, 1.04; 95% confidence interval [CI], 1.03–1.05; P < 0.001) in the average number of chlamydia cases and a 7.15% increase (IRR, 1.07; 95% CI, 1.06–1.09; P < 0.001) in the average number of gonorrhea cases per 100,000 within a county in 2020. However, the bivariate association between COVID-19 cases and syphilis was not significant (IRR, 1.01; 95% CI, 0.99–1.03; P = 0.404). In addition, every 1000 additional COVID-19 deaths per 100,000 was associated with a 196% increase (IRR, 2.96; 95% CI, 2.18–4.01; P < 0.001) in the average number of chlamydia cases and a 526% increase (IRR, 6.26; 95% CI, 4.12–9.52; P < 0.001) in the average number of gonorrhea cases per 100,000 within a county. For every 1000 additional COVID-19 deaths per 100,000, the average number of syphilis cases per 100,000 increased by 35.9%, but this was not statistically significant (IRR, 1.36; 95% CI, 0.64–2.86; P = 0.419).
Multivariable Association Between Chlamydia and COVID-19 Cases or Deaths
After adjusting for covariates, every 1000 additional COVID-19 cases per 100,000 was associated with a 1.80% increase (IRR, 1.02; 95% CI, 1.01–1.03; P < 0.001) in the average number of chlamydia cases per 100,000 within a county in 2020 (Table 1). After adjusting for covariates, the relationship between COVID-19 deaths per 100,000 and chlamydia was no longer statistically significant (IRR, 1.25; 95% CI, 1.00–1.56; P = 0.054; Table 1). Within the multivariable model for COVID-19 cases, increased unemployment and number of individuals below the poverty line were associated with an increased average number of chlamydia cases per 100,000, as was an increased percentage of Black or African American and American Indian or Alaska Native individuals relative to White individuals. Rural counties had a lower average number of chlamydia cases per 100,000 than urban counties, but the difference between suburban and urban counties was not significant.
TABLE 1.
Multivariable Association Between COVID-19 Cases and Deaths and Chlamydia Cases Per 100,000
| Parameter | COVID-19 Case Model | COVID-19 Death Model | ||
|---|---|---|---|---|
| IRR (95% CI) | P* | IRR (95% CI) | P † | |
| COVID-19 cases (1000 cases/100,000 people) | 1.02 (1.02–1.03) | <0.001 | — | — |
| COVID-19 deaths (1000 deaths/100,000 people) | — | — | 1.25 (1.00–1.56) | 0.054 |
| Unemployment (5 percentage points) | 1.18 (1.08–1.28) | <0.001 | 1.12 (1.03–1.22) | 0.007 |
| Below poverty line (5 percentage points) | 1.11 (1.08–1.15) | <0.001 | 1.11 (1.08–1.15) | <0.001 |
| Education (reference: high school diploma, 5 percentage points) | ||||
| Less than high school diploma | 0.94 (0.91–0.97) | <0.001 | 0.94 (0.91–0.97) | <0.001 |
| Bachelor's degree and higher | 1.00 (0.98–1.02) | 0.948 | 1.00 (0.98–1.01) | 0.608 |
| Race (reference: White, 5 percentage points) | ||||
| Black or African American | 1.09 (1.09–1.10) | <0.001 | 1.09 (10.9–1.10) | <0.001 |
| American Indian or Alaska Native | 1.06 (1.04–1.08) | <0.001 | 1.06 (1.05–1.08) | <0.001 |
| Asian | 1.02 (0.98–1.05) | 0.338 | 1.01 (0.97–1.05) | 0.683 |
| Native Hawaiian or other Pacific Islander | 0.96 (0.84–1.10) | 0.538 | 0.95 (0.83–1.09) | 0.475 |
| Some other race | 1.04 (1.02–1.06) | <0.001 | 1.04 (1.02–1.06) | <0.001 |
| Age (reference: 20–64 y, 5 percentage points), y | ||||
| ≤19 | 1.01 (0.97–1.04) | 0.734 | 1.01 (0.98–1.05) | 0.367 |
| ≥65 | 0.85 (0.83–0.87) | <0.001 | 0.84 (0.82–0.86) | <0.001 |
| Rurality (reference: urban) | ||||
| Suburban | 0.98 (0.94–1.02) | 0.255 | 0.99 (0.95–1.02) | 0.459 |
| Rural | 0.77 (0.74–0.81) | <0.001 | 0.79 (0.75–0.83) | <0.001 |
Note that IRR values for COVID-19 cases and deaths are expressed for every 1000 new cases or deaths per 100,000 individuals, whereas IRR values for demographic variables are expressed for every 5-additional-percentage-point increase in that variable relative to a 5-percentage-point decrease in the reference category.
*P values are derived from a multivariable quasi-Poisson model with an outcome of county-level chlamydia cases per 100,000, a primary exposure of COVID-19 cases, and adjustments for the enumerated covariates.
†P values are derived from a multivariable quasi-Poisson model with an outcome of county-level chlamydia cases per 100,000, a primary exposure of COVID-19 deaths, and adjustments for the enumerated covariates.
Multivariable Association Between Gonorrhea and COVID-19 Cases or Deaths
After adjusting for covariates, every 1000 additional COVID-19 cases per 100,000 was associated with a 5.00% increase (IRR, 1.05; 95% CI, 1.04–1.06; P < 0.001) in the average number of gonorrhea cases per 100,000 within a county in 2020 (Table 2). In addition, after adjusting for covariates, every 1000 additional COVID-19 deaths was associated with a 57.9% increase (IRR, 1.58; 95% CI, 1.10–2.27; P < 0.001; Table 2) in the average number of gonorrhea cases. Counties with a higher unemployment rate and percentage below the poverty line also experienced a greater average number of gonorrhea cases per 100,000, as did counties with a higher proportion of Black or African American and American Indian or Alaska Native individuals relative to White individuals. Suburban and rural counties both experienced a lower average number of gonorrhea cases on average compared with urban counties.
TABLE 2.
Multivariable Association Between COVID-19 Cases and Deaths and Gonorrhea Cases Per 100,000
| Parameter | COVID-19 Case Model | COVID-19 Death Model | ||
|---|---|---|---|---|
| IRR (95% CI) | P* | IRR (95% CI) | P † | |
| COVID-19 cases (1000 cases/100,000 people) | 1.05 (1.04–1.06) | <0.001 | — | — |
| COVID-19 deaths (1000 deaths/100,000 people) | — | — | 1.58 (1.10–2.27) | 0.013 |
| Unemployment (5 percentage points) | 1.15 (0.98–1.35) | 0.078 | 1.05 (0.91–1.23) | 0.510 |
| Below poverty line (5 percentage points) | 1.19 (1.13–1.25) | <0.001 | 1.19 (1.13–1.25) | <0.001 |
| Education (reference: high school diploma, 5 percentage points) | ||||
| Less than high school diploma | 0.94 (0.89–0.99) | 0.017 | 0.94 (0.89–1.00) | 0.034 |
| Bachelor's degree and higher | 0.99 (0.96–1.02) | 0.602 | 0.98 (0.96–1.01) | 0.283 |
| Race (reference: White, 5 percentage points) | ||||
| Black or African American | 1.12 (1.11–1.13) | <0.001 | 1.12 (1.10–1.13) | <0.001 |
| American Indian or Alaska Native | 1.08 (1.05–1.11) | <0.001 | 1.09 (1.06–1.11) | <0.001 |
| Asian | 1.06 (1.00–1.12) | 0.060 | 1.03 (0.97–1.09) | 0.340 |
| Native Hawaiian or other Pacific Islander | 0.91 (0.79–1.04) | 0.177 | 0.90 (0.79–1.04) | 0.141 |
| Some other race | 0.99 (0.96–1.03) | 0.708 | 1.00 (0.96–1.03) | 0.903 |
| Age (reference: 20–64 y, 5 percentage points), y | ||||
| ≤19 | 1.00 (0.95–1.05) | 0.952 | 1.02 (0.97–1.08) | 0.410 |
| ≥65 | 0.85 (0.82–0.89) | <0.001 | 0.83 (0.79–0.86) | <0.001 |
| Rurality (reference: urban) | ||||
| Suburban | 0.86 (0.81–0.91) | <0.001 | 0.87 (0.82–0.92) | <0.001 |
| Rural | 0.63 (0.58–0.68) | <0.001 | 0.65 (0.60–0.71) | <0.001 |
Note that IRR values for COVID-19 cases and deaths are expressed for every 1000 new cases or deaths per 100,000 individuals, whereas IRR values for demographic variables are expressed for every 5-additional-percentage-point increase in that variable relative to a 5-percentage-point decrease in the reference category.
*P values are derived from a multivariable quasi-Poisson model with an outcome of county-level gonorrhea cases per 100,000, a primary exposure of COVID-19 cases, and adjustments for the enumerated covariates.
†P values are derived from a multivariable quasi-Poisson model with an outcome of county-level gonorrhea cases per 100,000, a primary exposure of COVID-19 deaths, and adjustments for the enumerated covariates.
Multivariable Association Between Syphilis and COVID-19 Cases and Deaths
The relationship between COVID-19 cases and syphilis cases remained statistically insignificant after adjusting for covariates (IRR, 1.00; 95% CI, 0.97–1.02; P = 0.760). However, the relationship between COVID-19 deaths and syphilis cases became significant after adjustment. In particular, for every additional 1000 COVID-19 deaths per 100,000, the average number of syphilis cases per 100,000 in 2020 decreased by 74.2% (IRR, 0.26; 95% CI, 0.10–0.64; P = 0.004; Supplemental Digital Content 2, http://links.lww.com/OLQ/A934). Within the multivariable model, increased unemployment and percent above poverty line were associated with an increased average number of syphilis cases per 100,000, as was an increased percent of Black or African American, Asian, and American Indian or Alaska Native individuals relative to White individuals. Both suburban and rural counties experienced a lower average number of syphilis cases per 100,000 compared with urban counties.
DISCUSSION
This study is among the first to evaluate the association between COVID-19 cases and deaths and rates of 3 STIs (chlamydia, gonorrhea, and syphilis) at a county-level in the United States in 2020. Despite the decline in chlamydia cases across the United States, counties with more COVID-19 cases experienced a significantly higher average number of chlamydia cases. Counties with more COVID-19 cases also had significantly more gonorrhea cases compared with counties with lower COVID-19 cases, but the relationship with syphilis was not significant. In contrast, counties with more COVID-19 deaths experienced a significantly higher average number of gonorrhea cases, a significantly lower average number of syphilis cases, and no significant relationship with chlamydia cases. These between-county findings demonstrate a complex interplay between multiple contagious diseases that provide valuable insights into the impact a response to one disease may have on other diseases at varying geographic levels.
Similar studies around the world are inconsistent with respect to the change in gonorrhea and syphilis during the pandemic. A study in Denmark found that gonorrhea and syphilis cases were not significantly different between 2017 and 2020; one study in Italy found that gonorrhea and syphilis cases increased between 2019 and 2020 among men who have sex with men; a separate Italian study found that syphilis cases decreased during the early phase of a national lockdown in 2020; a study in 3 Scandinavian countries observed a decrease in gonorrhea cases in Sweden and Norway (but an increase in Denmark) between 2019 and 2020; a study in the United Kingdom observed a decrease in gonorrhea cases between 2019 and 2020; and a study in Australia found a decrease in gonorrhea between 2020 and 2021 but an increase in syphilis.14–19 In contrast, countries have consistent findings for chlamydia; Australia, Italy, Sweden, Denmark, and Norway all experienced a decrease in chlamydia reporting, which parallels the national results found in this study.15,17,19
The national trend for chlamydia, gonorrhea, and syphilis observed within this study may be explained in part by pathophysiological differences between the diseases. A majority of chlamydia cases are asymptomatic for both men (50%) and women (70%).20 In contrast, more than 90% of men infected by urethral gonorrhea will develop symptoms (although most women will be asymptomatic, as are pharyngeal and rectal infections), and syphilis can also be highly symptomatic depending on the stage.21,22 This difference in symptom presentation directly contributes to how cases of each STI are detected, which was impacted during the COVID-19 pandemic. A study of sexual health clinics in Australia found that a majority of surveyed clinics suspended asymptomatic screening for certain patient populations, experienced delays or other limitations in testing, and faced staffing shortages as staff were “redeployed” to COVID-19–related tasks.23 This limitation was not unique to Australia, as clinics in the United States also reduced their services or closed entirely and shifted staff to focus on COVID-19.2 For example, almost all US STD programs funded by the CDC had staff reassigned to work on COVID-19, and a majority of those programs experienced disruptions in both laboratory testing and access to select STD tests.24 Given that a majority of chlamydia cases are transmitted from asymptomatically infected individuals (whereas gonorrhea and syphilis are typically detected in symptomatic individuals), the suspension of asymptomatic screening may directly contribute to the observed decrease in national chlamydia cases that was not observed for gonorrhea or syphilis.
However, this framework is challenged by the county-level analysis, which suggests that counties with a higher number of COVID-19 cases experienced a higher average number of chlamydia cases, and those with a higher number of COVID-19 deaths experienced a higher average number of gonorrhea cases. This discrepancy may be related to differential access to public health resources (including sexual health services) at a county level. For example, counties with substantial public health resources may have had the capacity to both continue asymptomatic or preventive STI testing (including that delivered via telemedicine) and maintain COVID-19 testing capacity. This could lead to more detected COVID-19 and chlamydia cases, hence the direct relationship. In contrast, counties with worse access to public health resources may have still detected gonorrhea through their symptomatic testing programs that were not suspended. Concurrently, these programs would not have the same ability to deal with surging COVID-19 cases that initially disproportionately impacted underrepresented and underprivileged communities, thereby contributing to an increase in deaths. Thus, the relationship between COVID-19 deaths and gonorrhea would seem to be a direct relationship. This is further exemplified by the protective relationship between rurality and STIs observed within this study, which may be demonstrative of disparities in healthcare access between urban and rural communities that were exacerbated by the COVID-19 pandemic despite increases in telemedicine opportunities that were meant to expand access.25,26
However, this hypothesis may be challenged by the near-universal shift in resources that occurred within the United States to refocus STI personnel (including disease intervention specialists) to COVID-19.24 Alternatively, these variations may be result of regional differences in how individuals changed their sexual behavior in response to lockdowns and other physical distancing policies.27 For instance, a study in Australia found that suburban areas with a smaller gay population reported fewer partners and less HIV testing compared with larger populations. Thus, the higher rates of chlamydia in areas with more COVID-19 cases and deaths may correlate with areas with higher populations and thus more opportunities to meet casual partners. However, the true underlying reason for these associations cannot be determined by the present study, and future work should explore the relationship between COVID-19, STIs, and public health infrastructure in more detail.
The relationship between STIs and COVID-19 observed within this study has direct implications for public health and emergency preparedness, as it demonstrates how shifting resources to almost exclusively focus on one disease may have unanticipated consequences for other diseases. In some cases, these “consequences” may be positive. For instance, respiratory diseases, injection drug use–associated diseases, and food-water-and-vector-borne diseases all significantly decreased between 2019 and 2020, and the 2020–2021 influenza season was one of the most mild on record.1,26 All of this may be directly due to behavioral and policy changes that occurred during the first phase of the pandemic (including masking, hand hygiene, and physical distancing), and it is possible that these policies also impacted sexual health behaviors by decreasing the number and frequency of contact with casual partners.6,27–29,31s,32s However, not all changes are necessarily positive. For example, disruptions in healthcare service may have exacerbated chronic illnesses, social isolation brought on physical distancing compounded depression, and a possible decline in preexposure prophylaxis usage.27,33s,34s Furthermore, behavioral changes made during the first phase of the pandemic were not always sustainable, and some studies suggest a rebound of STI cases that correspond with the end to lockdown policies.6,27,31s,32s,35s It is important to note that the heterogeneity of resources within and demographic makeup of each state and county means that policy shifts at all levels of government may have highly variable impacts across the country. Taken together with the findings presented here, this indicates that diseases oftentimes influence one another in unanticipated ways, and it is imperative to consider both synergistic and antagonist effects that may arise when applying policies at the local, state, and federal levels to respond to a single threat.
This study presents a comprehensive analysis of the impact that the COVID-19 pandemic had on chlamydia, gonorrhea, and syphilis cases, and it is the first to present these results at a county level. However, there are several limitations that are important to consider. First, this work does not account for the impact of diverse policy decisions, such as mandatory quarantining, masking requirements, restrictions on entertainment venues (such as bars, nightclubs, or concert venues), or changes in sexual networks due to lockdowns, travel restrictions, and people moving their primary residences. Second, data were only available in aggregate for a single year, and thus, we were unable to conduct temporal analysis to understand the nuances behind how chlamydia, gonorrhea, and syphilis changed on a weekly or monthly level. We also did not have access to the breakdown of STI cases by gender identity, sex assigned at birth, or sexual orientation. Future work could repeat this analysis stratified by these variables and apply interrupted time series models to pinpoint when the most drastic changes in chlamydia, gonorrhea, and syphilis occurred and whether they corresponded temporally with COVID-19 cases and deaths. Third, lags in data reporting may have misattributed STI cases to one year when they should be attributed to another; however, this is expected to have occurred infrequently, so any resulting misclassification is expected to be negligible. Fourth, given the ecological nature of the study, we are unable to establish patient-level links between STIs and COVID-19, including the behaviors or attitudes (such as a preponderance for masking or physical distancing or access to sexual health clinics with testing services) that may influence it. Future work could conduct patient-level analysis using a combination of medical records and surveys or interviews.
Sexually transmitted infections are heterogeneous infectious diseases that require constant surveillance across the population and access to frequent diagnostic testing. The results presented here demonstrated that seemingly unrelated diseases (such as COVID-19) can have a profound impact on the rates of STIs. COVID-19 cases were associated with a significant county-level increase in chlamydia and gonorrhea cases, whereas COVID-19 deaths were associated with a significant increase in gonorrhea cases but a significant decrease in syphilis cases. The distinct differences between counties further emphasizes the need to consider how broader public health decisions made on a state or federal level may manifest differently within individual counties. Ongoing and future pandemic preparedness efforts should consider the impact that such decisions made to reduce the burden of one disease may have on other infectious diseases of public health importance. This should include strengthening public health funding that might be uneven across states and counties, which would provide a strategic advantage that would ensure the resiliency of public health infrastructure against novel and emerging infections.
Supplementary Material
ORCID iDs
Catherine Pollack https://orcid.org/0000-0002-7434-5306
Yukari C. Manabe https://orcid.org/0000-0001-8619-5598
Lea E. Widdice https://orcid.org/0000-0002-3866-9776
Charlotte A. Gaydos https://orcid.org/0000-0002-1021-3195
Susan A. Tuddenham https://orcid.org/0000-0001-5910-956X
Anne M. Rompalo https://orcid.org/0000-0001-6398-9061
Joany Jackman https://orcid.org/0000-0003-3316-4071
Collin M. Timm https://orcid.org/0000-0002-7804-5044
For further references, please see “Supplemental References,” http://links.lww.com/OLQ/A936.
Footnotes
C.P. and J.B. contributed equally as co-first authors. J.B. is an undergraduate student at the University of Washington.
Acknowledgments: The authors would like to acknowledge Dr Kaitlin Lovett, Dr Mark Panaggio, Dr Luke Mullany, Damon Duquaine, Dr Jane Valentine, and Dr Amanda Galante of the Johns Hopkins University Applied Physics Laboratory for their thoughtful suggestions on the analysis and presentation of the article.
Conflict of Interest and Sources of Funding: The authors declare no conflict of interest. Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number U54EB007958. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: J.B., C.P., Y.C.M., N.K.L., and C.M.T. formulated the research question and designed the analysis; J.B. and C.P. conducted the analysis; all authors analyzed the data, drafted the initial manuscript, and revised and approved the final manuscript.
Ethics Statement: This effort has not been previously published and is not under consideration for publication elsewhere. All research was performed with adherence to ethical standards.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Catherine C. Pollack, Email: catherine.pollack@jhuapl.edu.
Jordan Bradburne, Email: jordan.bradburne@jhuapl.edu.
Natalie K. Lee, Email: natalie.lee@jhuapl.edu.
Yukari C. Manabe, Email: ymanabe@jhmi.edu.
Lea E. Widdice, Email: Lea.Widdice@cchmc.org.
Charlotte A. Gaydos, Email: cgaydos@jhmi.edu.
Susan A. Tuddenham, Email: studden1@jhmi.edu.
Anne M. Rompalo, Email: arompalo@jhmi.edu.
Joany Jackman, Email: joany.jackman@jhuapl.edu.
REFERENCES
- 1.Crane MA Popovic A Panaparambil R, et al. Reporting of infectious diseases in the United States during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis 2022; 74:901–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance, 2020. Washington, DC: US Department of Health and Human Services; 2022. [Google Scholar]
- 3.Banerjee T, Nayak A. U.S. county level analysis to determine If social distancing slowed the spread of COVID-19. Rev Panam Salud Publica 2020; 44:e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinto CN Niles JK Kaufman HW, et al. Impact of the COVID-19 pandemic on chlamydia and gonorrhea screening in the U.S. Am J Prev Med 2021; 61:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi M Yuan Y Miranda W, et al. A peek into the future: How a pandemic resulted in the creation of models to predict the impact on sexually transmitted infection(s) in New York State (excluding New York City). Sex Transm Dis 2021; 48:381–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berzkalns A Thibault CS Barbee LA, et al. Decreases in reported sexually transmitted infections during the time of COVID-19 in King County, WA: Decreased transmission or screening? Sex Transm Dis 2021; 48(8S):S44–S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers B Tao J Murphy M, et al. The COVID-19 pandemic and sexually transmitted infections: Where do we go from here? Sex Transm Dis 2021; 48:e94–e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li D Gaynor SM Quick C, et al. Identifying US county-level characteristics associated with high COVID-19 burden. BMC Public Health 2021; 21:1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Detailed Methodology and Sources: COVID-19 Data. USAFacts. Available at: https://usafacts.org/articles/detailed-methodology-covid-19-data/. Accessed October 5, 2022. [Google Scholar]
- 10.US Census Bureau . American Community Survey (ACS). Census.gov. Available at: https://www.census.gov/programs-surveys/acs. Accessed October 5, 2022.
- 11.USDA ERS—Download Data. Available at: https://www.ers.usda.gov/data-products/county-level-data-sets/download-data/. Accessed November 16, 2022.
- 12.USDA ERS—Documentation. Available at: https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/documentation/#background. Accessed November 16, 2022.
- 13.Ver Hoef JM, Boveng PL. Quasi-Poisson vs. negative binomial regression: How should we model overdispersed count data? Ecology 2007; 88:2766–2772. [DOI] [PubMed] [Google Scholar]
- 14.Heerfordt IM. STIs during the first and second wave of COVID-19 in Denmark. Sex Transm Infect 2022; 98:150–151. [DOI] [PubMed] [Google Scholar]
- 15.Cusini M Benardon S Vidoni G, et al. Trend of main STIs during COVID-19 pandemic in Milan, Italy. Sex Transm Infect 2021; 97:99. [DOI] [PubMed] [Google Scholar]
- 16.Latini A Magri F Donà MG, et al. Is COVID-19 affecting the epidemiology of STIs? The experience of syphilis in Rome. Sex Transm Infect 2021; 97:78–78. [DOI] [PubMed] [Google Scholar]
- 17.Ivarsson L De Arriba Sánchez de la Campa M Elfving K, et al. Changes in testing and incidence of Chlamydia trachomatis and Neisseria gonorrhoeae—The possible impact of the COVID-19 pandemic in the three Scandinavian countries. Infect Dis (Lond) 2022; 54:623–631. [DOI] [PubMed] [Google Scholar]
- 18.Whitlock GG, McOwan A, Nugent D. Gonorrhoea during COVID-19 in London, UK. Sex Transm Infect 2021; 97:622–623. [DOI] [PubMed] [Google Scholar]
- 19.King J McManus H Kwon A, et al. HIV, viral hepatitis and sexually transmissible infections in Australia: Annual Surveillance Report 2022. UNSW Sydney: The Kirby Institute, 2022. Available at: https://kirby.unsw.edu.au/report/asr2022. Accessed December 13, 2022. [Google Scholar]
- 20.European Centre for Disease Prevention and Control . Facts about chlamydia. Available at: https://www.ecdc.europa.eu/en/chlamydia/facts. Accessed October 5, 2022.
- 21.Fairley CK Hocking JS Zhang L, et al. Frequent transmission of gonorrhea in men who have sex with men. Emerg Infect Dis 2017; 23:102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.STD Facts—Syphilis & MSM. Available at: https://www.cdc.gov/std/syphilis/stdfact-msm-syphilis.htm. Published June 28, 2022. Accessed March 20, 2023.
- 23.Phillips TR Fairley CK Donovan B, et al. Sexual health service adaptations to the coronavirus disease 2019 (COVID-19) pandemic in Australia: A nationwide online survey. Aust N Z J Public Health 2021; 45:622–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright SS Kreisel KM Hitt JC, et al. Impact of the COVID-19 pandemic on Centers for Disease Control and Prevention–funded sexually transmitted disease programs. Sex Transm Dis 2022; 49:e61–e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anderson TJ Saman DM Lipsky MS, et al. A cross-sectional study on health differences between rural and non-rural U.S. counties using the County Health Rankings. BMC Health Serv Res 2015; 15:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poeran J Cho LD Wilson L, et al. Pre-existing disparities and potential implications for the rapid expansion of telemedicine in response to the coronavirus disease 2019 pandemic. Med Care 2021; 59:694–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holt M Chan C Broady TR, et al. Adjusting behavioural surveillance and assessing disparities in the impact of COVID-19 on gay and bisexual men's HIV-related behaviour in Australia. AIDS Behav 2023; 27:518–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamill MM Yu T Armington GS, et al. Factors associated with new sexual partnerships during the COVID-19 pandemic: A survey of online sexually transmitted infection testing platform users. Sex Transm Dis 2022; 49:695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chow EPF Hocking JS Ong JJ, et al. Brief report: Changes in PrEP use, sexual practice, and use of face mask during sex among MSM during the second wave of COVID-19 in Melbourne, Australia. J Acquir Immune Defic Syndr 2021; 86:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]


