Abstract
Acute iliofemoral deep vein thrombosis and chronic iliofemoral venous obstruction cause substantial patient harm and are increasingly managed with endovascular venous interventions, including percutaneous mechanical thrombectomy and stent placement. However, studies of these treatment elements have not been designed and reported with sufficient rigor to support confident conclusions about their clinical utility. In this project, the Trustworthy consensus-based statement approach was utilized to develop, via a structured process, consensus-based statements to guide future investigators of venous interventions. Thirty statements were drafted to encompass major topics relevant to venous study description and design, safety outcome assessment, efficacy outcome assessment, and topics specific to evaluating percutaneous venous thrombectomy and stent placement. Using modified Delphi techniques for consensus achievement, a panel of physician experts in vascular disease voted on the statements and succeeded in reaching the predefined threshold of >80% consensus (agreement or strong agreement) on all 30 statements. It is hoped that the guidance from these statements will improve standardization, objectivity, and patient-centered relevance in the reporting of clinical outcomes of endovascular interventions for acute iliofemoral deep venous thrombosis and chronic iliofemoral venous obstruction in clinical studies and thereby enhance venous patient care.
Keywords: humans, physicians, stents, thrombectomy, thrombosis
Acute iliofemoral deep vein thrombosis (DVT) may be associated with substantial patient morbidity from pulmonary embolism (PE), early limb symptoms, recurrent venous thromboembolism (VTE), and the postthrombotic syndrome (PTS).1,2 Chronic iliofemoral venous obstruction from nonthrombotic iliac vein lesions (NIVLs) or previous DVT may cause profound limb symptoms that reduce a patient’s ambulatory capacity and quality of life (QOL).3,4 Because relief of venous obstruction has been hypothesized to decrease morbidity and improve QOL, endovascular therapies have been increasingly applied for these conditions. In recent years, endovascular therapy has been refined with the benefit of data from randomized controlled trials evaluating catheter-directed thrombolysis (CDT) and related methods for the management of acute DVT, improvements in antithrombotic drug therapy and venous imaging, and the introduction and US Food and Drug Administration clearance of new venous therapeutic devices.5–8 The use of endovascular mechanical thrombectomy (MT) and metallic stent implantation for venous disease has dramatically increased; however, clinical studies have not been designed with sufficient rigor to support confident conclusions regarding the biological effects, clinical efficacy, or overall risk-benefit ratio of these treatment elements.
Venous patient care can be enhanced by improving the rigor and impact of future studies. As such, the aims of this project were to (1) improve standardization, objectivity, and patient-centered relevance in reporting outcomes of endovascular interventions for acute iliofemoral DVT and chronic iliofemoral venous obstruction; (2) increase the alignment of regulatory and reimbursement policies with the actual impact of new devices on patient outcomes; and (3) accelerate innovation in venous care by enabling clearer insight into the true effectiveness of therapeutic interventions. To those ends, consensus-based statements were developed by physician experts in venous disease to guide the design, conduct, and reporting of future studies.
METHODS
In evidence-based guideline development, the Trustworthy consensus-based statement approach can yield unbiased, scientifically valid, and trustworthy guidelines through a transparent process that incorporates available scientific evidence and subject matter expert opinion.9–11 This process has been used to develop guidelines covering important clinical topics with relatively weak scientific evidence.12–16 Few studies have rigorously evaluated the utility of different venous study designs and outcome assessment methods, precluding meaningful quantitative analyses. Therefore, a version of the Trustworthy consensus-based statement process was used for this project. A professional guideline consultant (S.Z.L.) was contracted to advise on current standards and best practices. The consensus statement development process was led by 3 cochairs experienced in venous clinical practice, research, and guideline development who were free of substantial conflicts of interest (S.V., P.G., and T.L.C.). These individuals reviewed the published evidence, drafted the initial statements, provided oversight of revisions, and adjudicated key questions that arose. A list of potential panel members experienced in managing venous disease was developed by querying the physician database of the Vascular and Interventional Advances Foundation. Financial disclosures were obtained, and candidates with potential major conflicts of interest were removed from the list. The final panel included 5 vascular medicine specialists, 13 vascular surgeons, 6 interventional radiologists, and 3 cardiologists (Supplemental Material).
An initial list of key articles was identified from published guideline documents, and the panel members were invited to add additional papers of relevance. A final list of 75 publications was provided to the panel as reference materials. At a subsequent in-person meeting on April 29, 2022, the panelists were oriented to the project’s objectives and the planned processes for consensus statement development and voting.
Thirty statements addressing the topics included in this project were drafted by the cochairs and subsequently reviewed by the guideline consultant and 3 physician members of the Vascular and Interventional Advances Foundation Board of Directors (P.A.S., S.S.S., and R.K.). The revised versions were submitted for full panel review and voting. Using modified Delphi techniques for consensus achievement, the panelists reviewed each statement and anonymously voted in an online survey. The a priori rules called for up to 3 rounds of surveys to achieve consensus. Response rates of 85% were required, and consensus was defined as 80% of eligible panelists voting for agreement or strong agreement. Open fields solicited anonymous comments and suggestions from the panelists. The voting process was managed by the guideline consultant and staff from the Vascular and Interventional Advances Foundation.
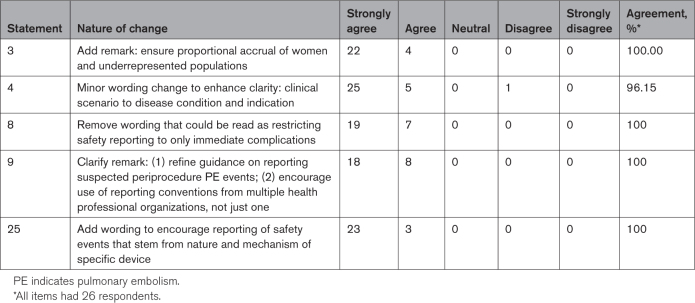
With 96% of panelists voting (26 of 27), all 30 statements achieved panel consensus on the first round, with agreement ranging from 81% to 100% (Table 1). All written comments from the survey were anonymously shared with the writing group. The cochairs had the option to revise any statements for which the comments would improve, but not significantly change, the statement’s meaning; however, any substantially revised statements were sent again through the modified Delphi survey process. After discussion, 5 statements were revised to incorporate the panel’s written comments. In a second round of voting, all 5 revised statements achieved panel consensus (Table 2). The consensus statements are summarized below, with introductory summaries that convey the rationale behind the recommendations.
Table 1.
First-Round Voting on Consensus-Based Statements: Modified Delphi Method

Table 2.
Second-Round Voting on Consensus-Based Statements: Modified Delphi Method
GENERAL STUDY DESCRIPTION AND DESIGN
In considering published studies that have evaluated endovascular venous therapies, the panel observed a number of common reporting issues that have clouded interpretation of study results. The panel recognized that because studies vary greatly in intent, scope, design, and resourcing, each study cannot be expected to fulfill every optimal design specification or best practice. However, investigators must still clearly delineate the methodological design that was used, indicate how the study was actually performed, draw scientifically valid inferences, summarize the findings in ways that encourage appropriate interpretation by clinicians, and be fully forthcoming about limitations and alternative interpretations. The panel’s recommendations in this section are aimed at ensuring that future studies avoid the reporting issues noted below.
Incomplete Description of Study Populations
For venous clinical practice to be informed by evidence from a clinical study, it must be reported in a manner that enables clear discernment of what study population was evaluated, facilitating comparison with other studies. Key elements that may influence treatment responses, complication rates, and the comparability of venous cohorts are summarized here.
First, the chronicity of the disease must be reported. At present, there is no widely available, inexpensive, and reliable blood test or noninvasive imaging method that can precisely date thrombus formation or the onset of other relevant pathological processes. The composition of intraluminal material, the inflammatory response to thrombosis (which may influence clinical presentation), the nature of standard therapy, and expectations for clinical change evolve substantially over time following the inciting event. Hence, notwithstanding its limitations, the duration of time since venous symptom onset remains a useful parameter in trial reporting. To date, published guidelines and randomized clinical trials have considered acute DVT as being associated with symptom duration of <14 to 21 days.1,5–7,17 The Society of Interventional Radiology Reporting Guidelines have considered subacute DVT and chronic DVT as being associated with symptom durations of 15 to 30 days and >30 days, respectively, but the actual use of these terms has varied significantly in the published literature.17 These definitions were largely derived from observations about the amenability of thrombus to dissolution or removal with fibrinolytic drugs and early thrombectomy devices. However, the progress of thrombus organization, inflammation, and fibrosis vary among patients; many patients have thrombus of mixed age; and new devices may be designed to treat a broader range of patients. Hence, to ensure clear description of study populations, investigators should specify the allowed duration of symptoms rather than relying on descriptive labels like acute, subacute, and chronic.
Direct visual inspection of material extracted from the venous system has also been used to define chronicity but has important limitations: (1) sampling error: when thrombus removal is incomplete, residual material can differ in composition from explanted material; (2) accuracy: categorizations based on gross visual inspection of explanted material by nonpathologists can be questioned; and (3) reporting bias: characterization of a population after the outcome of an attempted treatment is known creates strong potential for bias.18 The potential for miscategorization may be even greater when thrombus of multiple ages is found in a specimen. Hence, although such information may provide interesting supplementary insights, it should not be relied upon as the primary method of assigning thrombus chronicity for most study types.
Second, the anatomic extent of the disease must be reported. Patients with proximal DVT are at higher risk for PE and PTS than patients with distal DVT; patients with iliofemoral DVT (defined by the Society of Interventional Radiology and American Heart Association as DVT involving the iliac or common femoral vein, with or without involvement of additional veins) are at higher risk for PTS, severe PTS, and recurrent VTE than patients with femoral-popliteal or distal DVT.2–4,8,19 To avoid misperceptions about how patients were categorized, the source of information (eg, specific imaging test) for the anatomical extent should be stated. In chronic disease populations, the extent of valvular reflux at baseline should also be reported.
Third, the clinical manifestations of venous disease at baseline should be described. The presence, nature, and severity of symptoms and clinical signs are important for understanding the population studied and for establishing a baseline for posttreatment comparisons.
Fourth, the study eligibility criteria and population description should enable discernment of reasonable expectations for common safety outcomes such as bleeding and recurrent VTE.
Finally, it is important to describe the specific clinical indication for which treatment is being studied. It should be clear at what point in the disease course patients are being enrolled. Unselected all-comer populations are different from populations culled for responders or nonresponders. Precise descriptions are better than ambiguous terms; for example, the May-Thurner syndrome has been variably used to refer to left common iliac vein stenosis with iliofemoral DVT, iliac vein stenosis alone, or chronic iliac vein occlusion with a presumed pathogenesis. When venous stenosis is seen, it is often unknown whether it was caused by DVT, an intrinsic NIVL, external venous compression, or a combination of factors. Hence, rather than simply using the term, study reports should make clear what is actually known about a past history of DVT, external venous compression, or intrinsic venous stenosis in the patients.20 Previous treatments for venous disease and compliance with therapy should also be noted.
Inadequate Information on the Treatment or Treatment Strategy Being Investigated
For endovascular venous device studies, there should be clarity on what treatment elements were required, encouraged, allowed, discouraged, or prohibited during the study and the extent to which physician preference was accommodated. Endovascular care elements and standard therapy cointerventions that may influence the assessment of the efficacy or safety of the therapy under investigation should be delineated. When a device or treatment can be used in multiple ways (eg, thrombectomy device in aspiration mode or pulse-spray mode), the intended and allowed methods of use of the treatment being evaluated should be clarified. Because device use is operator dependent, the approach used to evaluate and ensure appropriate qualifications or training of the endovascular proceduralists for performance of venous interventions generally and use of the study device specifically should be presented. It should be made clear upfront whether the study is evaluating the 1-time use of a device, the 1-time use of an overall treatment strategy with multiple components (eg, pharmacomechanical CDT with adjunctive balloon angioplasty and stent placement), or the long-term use of an overall treatment strategy (eg, upfront stent placement with allowed repeat endovascular intervention during follow-up).
Inappropriate Attribution of Findings That May Reflect Natural History and Confounders
In the published literature, when a difference in outcome was observed before and after the use of an endovascular intervention, authors have often concluded that the intervention was responsible for the change. However, that inference may not be valid unless a difference from a properly chosen control group that did not receive the intervention is demonstrated. It is important to recognize that the clinical severity of acute DVT and chronic venous disease can exhibit improvement over time due to standard therapy or time-dependent natural healing, even without endovascular intervention.
An additional problem concerns the reporting of the outcomes of multicomponent endovascular interventions. When a change in patient outcomes is observed after such an intervention, it should be described as being associated with use of the overall treatment strategy, not to a particular component of therapy, unless this was specifically evaluated. For example, studies that report on use of recombinant tPA (tissue-type plasminogen activator) with other tools (eg, thrombectomy devices and drug delivery catheters) should not attribute thrombus reduction to a specific care component unless such assessment was specifically integrated into the study design. Investigators, reviewers, editors, and regulators should ensure that studies are designed to discern what treatment elements are effective and that all possibilities for what may have accounted for observed change are considered, with specific reference to any cointerventions.
Consensus-Based Statements 1 to 7
-
Studies of endovascular interventions for acute and chronic lower extremity venous conditions should be rigorously designed to enable clear discernment of the effects of the intervention being evaluated, with consideration of the natural history of the condition, cointerventions that may serve as confounding variables, and other potential sources of bias.
Remark: studies should distinguish between demonstrating the effects of (1) a specific device or subprocedure versus (2) an overall multimodality endovascular treatment or strategy.
-
In studies of endovascular interventions for acute and chronic lower extremity venous conditions, protocols should clearly describe the methodological design and, where feasible, standardize the study populations, methods of use of the interventions under study and any cointerventions, participant follow-up (including rules for crossover between treatment arms), outcome assessments, and other study processes.
Remark: the qualifications of the investigators and proceduralists should also be described. For prospective studies, the methodological design should be specified before study initiation, the study should be registered in a public repository (eg, www.clinicaltrials.gov), and the protocol should be posted in a publicly accessible location.
-
In studies of endovascular interventions for acute and chronic lower extremity venous conditions, study populations (eligibility criteria) should be described using updated venous disease categorization systems that have been endorsed by health professional organizations and should include the disease/symptom chronicity, anatomical extent (including what imaging method was used to characterize thrombus/disease extent, target vessel size, and other characteristics), and factors likely to influence the risk of bleeding or thrombosis.
Remark: to ensure generalizability of study results, efforts should be made to ensure that the study population is representative of the population with the condition under study. Special consideration should be given to ensuring proportional enrollment of women and minorities.
-
Studies of acute and chronic lower extremity venous conditions should clearly prespecify all procedures that will be counted within the definition of the endovascular intervention being evaluated and should identify the specific disease condition and indication for which treatment is being delivered.
Remark: treatment elements that influence participant risk or burden should be summarized, including but not limited to the type of anesthesia/sedation, sheath size, or use of fibrinolytic drugs.
-
In studies of endovascular interventions for acute and chronic lower extremity venous conditions, the use and monitoring of concomitant therapies should be described and standardized when feasible, including anticoagulant or antiplatelet therapies (drug, dose, timing of administration, and duration of therapy), compression (type, ankle pressure), venoactive agents, inferior vena cava filters, and venous ulcer treatments.
Remark: it should be clear what concomitant therapies were required, encouraged, allowed, discouraged, or prohibited and what level of discretion was granted to the site physicians.
-
In studies of endovascular interventions for acute and chronic lower extremity venous conditions, well-defined primary and major secondary outcomes should be prespecified.
Remark 1: in addition to safety and efficacy, high-quality evaluation of cost-effectiveness is encouraged, with guidance from specialized experts in economic analysis.
Remark 2: definitions of recurrences and reinterventions should also be prespecified.
For studies of endovascular interventions for acute and chronic lower extremity venous conditions, study conclusions in presentations, publications, regulatory submissions, and other communications should be closely aligned with what is supported by the study design.
SAFETY OUTCOME ASSESSMENT
Safety reporting is commonly focused on the requirements of applicable regulatory bodies.21,22 However, studies should also strive to report safety outcomes in a manner that reflects the scientific and clinical conventions for the applicable disease area, ideally with the use of society-endorsed standardized definitions and reporting guidelines that can enable meaningful comparisons across studies. Advance consideration should be given to prespecifying, defining, and reporting the full range of safety events that may be expected to occur with a particular intervention. When no events occurred, this fact should be explicitly stated in reporting results. Given the variability in devices and treatments, the duration of follow-up may vary; in general, longer follow-up should be considered for devices that are intended for long-term implantation.
Any venous interventional procedure may be complicated by the development of thrombosis or bleeding. Symptomatic thrombosis can manifest as DVT, PE, both, or neither. DVT can be limited to a treated vein segment or can extend to involve additional segments and can lead to PE, PTS, or neither; PE can lead to right heart strain, hemodynamic compromise, death, or none of these consequences. Blood loss can be overt or occult and can result from device-related aspiration, device-related hemolysis, venous access site hemorrhage, vascular perforation or rupture, or development of a distant site of bleeding; concomitant anticoagulant and fibrinolytic drugs can contribute to the onset and severity of these events. Some bleeding events require blood transfusion or surgical or endovascular therapy to address. In reporting events, such details should be provided to enable their overall clinical impact to be discerned. To enable standardization of event reporting, guidelines have been developed by the International Society of Thrombosis and Haemostasis, the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) investigators, and the Society of Interventional Radiology, among other bodies.23–25 The International Society of Thrombosis and Haemostasis scheme may be particularly applicable to VTE populations.
Safety events can be related to a specific device (eg, fracture of a stent), an overall endovascular treatment (eg, thrombosis of a venous segment treated with pharmacomechanical CDT followed by stent placement), associated treatments that were mandated by the protocol (eg, gastrointestinal bleed in a patient who was placed on antiplatelet therapy after stent placement), standard care (eg, subdural hematoma in a patient receiving anticoagulation for preexisting DVT), or none of these. Therefore, in reporting treatment-related events, investigators should provide the specific definition of relatedness that was used.
Consensus-Based Statements 8 and 9
-
8.
In evaluating endovascular devices/interventions for acute and chronic lower extremity venous conditions, demonstration of safety should include evidence of (1) successful device use/deployment/removal and index procedure completion without complications; (2) freedom from bleeding, thrombosis, vascular injury, death, or unplanned escalation of care (eg, transfer to intensive care unit or conversion to open surgery); (3) freedom from biological compatibility issues; and (4) freedom from late issues with device-related mechanical integrity (eg, fracture, migration, and embolization) or vascular injury.
-
9.
In studies of endovascular interventions for acute and chronic venous lower extremity conditions, suspected bleeding, suspected VTE (including DVT and PE), and effects on renal function should be routinely assessed and reported in a standardized way. Bleeding, VTE, device integrity, and deaths should be reported for the duration of the study.
Remark: Bleeding should be categorized by clinical severity (major or minor; involving a critical site; resulting in blood transfusion, embolization, or surgery). DVT events should be categorized by whether they represent thrombosis of a previously treated venous segment (device bearing or not), extension into new venous segments, or development in distant venous segments. PE events should be categorized by whether they are symptomatic or fatal and whether they are associated with right heart strain or acute clinical deterioration. Oxygen desaturation during or immediately after endovascular procedures should be reported and, in the appropriate clinical context, assessed as possible PE. The use of medications that influence thrombosis or hemostasis at the onset of safety events should be described. Events should be categorized by whether they are procedure or device related. To enable consistency and comparability across studies, use of standardized definitions of bleeding and recurrent VTE recommended by health professional organizations is suggested.
EFFICACY OUTCOME ASSESSMENT
A number of important challenges exist in achieving valid, objective, and clinically meaningful evaluations of efficacy for novel venous interventional tools and procedures. A central reality is that there does not exist any gold standard outcome measure that adequately captures the phenotypic diversity and clinical severity of venous disease with appropriate biological correlates. The relationships of inflammation, thrombophilia, and patterns of venous obstruction and valvular reflux to venous physiology, clinical phenotypes, and disease severity progression are complex.26–30
Assessment of Symptoms and Clinical Signs
Limb swelling can be assessed subjectively by patients and objectively by direct measurement of limb circumferences by study personnel.6,31 Estimates of limb volume can be derived from measurements of limb length and circumference.32 Limb pain can be reported by the patient or an assessor with information from the patient, using validated pain scales such as the visual analog scale, Likert scale, and others.33–37 These are useful metrics, but there can be significant overlaps between venous disease–attributable symptoms and those of other conditions such as cardiovascular diseases, lymphedema, skin disorders, and neurological and musculoskeletal diseases.
As a result, venous outcome assessment has evolved to rely largely on clinical scales that meld multiple symptoms and clinical signs of venous disease. Two measures that are commonly used in clinical studies, the Villalta scale and the venous clinical severity scale (VCSS), have been validated through studies showing correlations with other indicators of venous disease and venous disease severity and encompass a broad range of venous presentations.38–40 These 2 measures have different performance characteristics that render each well suited to serve specific types of clinical studies.41–43 The Villalta scale severity categories have been correlated with graded increases in ambulatory venous pressures.44 Both the Villalta scale and the VCSS have been correlated with health-related QOL; the Villalta scale has exhibited stronger correlations with QOL, presumably because 5 of its 11 items query symptoms.45,46 The VCSS has more items focused on skin manifestations and may, therefore, be more suitable to track disease severity at the more severe end of the chronic venous disease spectrum.47 However, both measures have drawbacks. They require in-person application by trained personnel, which impacts study feasibility and limits outcome assessments to just a few time points during follow-up. Like any measure that includes symptoms, they are susceptible to reporting bias, particularly in open-label studies (the VCSS may be less susceptible since it includes only 1 symptom). Correlations between these measures and anatomic and physiological assessments have tended to be of variable (often low to moderate) strength.47–49
In study populations, scores on some venous scales (including Villalta and VCSS) can be summarized as continuous variables or using binary or categorical cut points that correspond to escalating levels of disease severity. Because chronic venous disease occurs on a spectrum, the use of these measures as continuous outcomes may better reflect the disease burden in a study population and will often carry more statistical power to identify smaller differences between treatment groups. On the other hand, the demonstration of small differences on a continuous scale, even if statistically significant, may not impact clinical practice. The minimal clinically important difference has been retroactively estimated from clinical study data for some scales; however, minimal clinically important difference is a within-patient parameter, so even a highly effective intervention may not be expected to produce a mean change corresponding to the minimal clinically important difference across an entire study population.3,41,42,45 Hence, investigators should consider the specific study question, the anticipated prerequisites for changing clinical practice, and clinician tolerances for procedural risk in determining how to summarize and compare treatment outcomes.
Precautions Against Bias
The ability to accurately assess endovascular tools using clinical scales is contingent upon their use with rigorous precautions against bias (Table 3). When scoring of symptoms or QOL is patient reported, there is potential for reporting bias when patients know their treatment arm allocation. This may often be unavoidable, but at a minimum, patients should complete the measures unassisted and ideally without clinical or study personnel presence. For clinician-assessed parameters, bias may be most likely when assessment is performed by the endovascular operators but can occur with any assessor who is not blinded to treatment arm allocation. Hence, explicit precautions against bias (including blinding of clinicians, assessors, and adjudicators) should be taken and documented in the study protocol and in reports of the study results. This includes robust efforts to maximize the completeness of follow-up in patients in all study arms.
Table 3.
Strategies to Minimize Bias in Studies of Endovascular Therapy for Venous Disease
Endovascular venous interventions pose additional challenges to study in well-designed randomized controlled trials. The modest population frequency of some venous thrombotic conditions, the diffusion of venous patient care among diverse providers, the limitations of venous imaging, and the invasive nature of endovascular interventions combine to limit even the largest trials to no more than a few hundred patients. As such, achieving statistically precise treatment effect estimates is difficult, especially for secondary and subgroup outcomes. A double-blind design is usually not feasible since sham procedures are complex, risky, variably effective at achieving blinding, and have the potential to bias treatment arm comparisons by reducing the effectiveness of standard therapy in control arm cohorts (ie, by causing bleeding or thrombosis). Clinicians can harbor individual biases about specific treatments, so enrollment of a representative population often necessitates focused training on how to present the study to participants in a balanced way. The wide availability and insurance coverage for many devices reduces the impetus and ability to enroll patients in clinical trials.50 Standardization of cointerventions that can confound interpretation of results can be difficult given local variances in clinical practice, insurance coverage, and other health system realities.
Because a primary reason to treat venous disease is to reduce symptoms, assessments of clinical status will inevitably involve a subjective dimension. To maximize confidence in an intervention’s effects, investigators should configure studies to also document objective changes in anatomical or physiological venous parameters. This task also poses a number of challenges.
Venous Anatomic and Physiological Assessments
First, all available iliac vein imaging modalities have limitations.51–53 For this reason, it is valuable for clinical studies to utilize ≥1 imaging core laboratories that can ensure the qualifications of study site imaging facilities and personnel, develop protocols for standardized imaging assessments and data submission, deliver feedback to site personnel to ensure imaging quality control, and provide standardized interpretation and adjudication of imaging results. With Duplex ultrasound, the vein can be difficult to image in its entirety due to overlying bowel gas and other artifacts. With computed tomography venography and magnetic resonance venography, imaging must be timed to the arrival of the contrast bolus in the target vein, which can be challenging due to variation in body habitus, cardiac output, the degree of venous obstruction, and other factors. In patients who have had metallic devices implanted, magnetic resonance imaging artifacts can preclude evaluation of venous structures. Multiplanar venography with intravascular ultrasound (IVUS) was superior to multiplanar venography alone in assessing iliac vein disease in 1 prospective study and is likely the most accurate currently available iliac vein imaging strategy.54,55 Although venography/IVUS requires invasive catheter access into the venous system, its judicious use in clinical studies can be justified by the paramount importance to patients of obtaining accurate anatomic assessments of new endovascular tools. However, the need to avoid undue participant burden will usually preclude its repeated use during follow-up. Although the Marder score is a useful venographic thrombus scoring scale, it does not measure venous flow or characterize the degree of venous obstruction.56 At present, there does not exist a well-validated IVUS-based volumetric venous thrombus scoring system. IVUS may also overestimate venous stenosis due to patient volume status and positioning.57 Accordingly, quality venous assessment using venography and IVUS will benefit from active guidance from investigators to standardize the technique used.
Second, Duplex ultrasound is well suited to visualize thrombus in the common femoral, femoral, and popliteal veins. However, this modality is operator dependent, and reproducibility of assessments is a concern, especially given the length of the femoral vein and the varying distributions of thrombus and venous flow obstruction that may exist. In general, adherence to the technical standards for the performance and interpretation of venous Duplex ultrasound that were created by the International Accreditation Coalition and the American College of Radiology is likely to support consistent exam quality.58 A proactive plan for the core laboratory to review studies as they are submitted and retrain site technical staff as needed will also be helpful for many studies. The published literature currently suffers from a lack of standardization in venous ultrasound outcome reporting. For example, some studies have evaluated whether veins are completely compressible (free of thrombus) while others have evaluated for the presence of Doppler flow (which can exist even with substantial residual thrombus).59,60 While both parameters may be relevant, this variance precludes easy comparison of the results of different studies. In early VTE treatment studies, the development of thrombus involving >10 cm vein length or a change in residual vein diameter ≥4 mm during anteroposterior transducer compression correlated with symptomatic recurrent DVT events.61–65 In other studies, clinical patency constructs have been used in which patency was assumed if the patient did not present with recurrent symptoms. The panel strongly discourages assignment of patency to any vein that was not evaluated by imaging. Adjudication of imaging studies by blinded core laboratory personnel will help to minimize bias in outcome assessment.
Third, a central challenge is the absence of any well-validated, widely available venous physiological test. Although Duplex ultrasound is effective in evaluating the presence and duration of venous valvular reflux in individual veins, its ability to provide overall hemodynamic assessment of the limb is limited. Direct intravenous pressure measurements are rarely helpful because catheterized patients are generally supine, a position in which the hemodynamic and clinical alterations of venous disease are minimized. Although a resting supine mean venous pressure gradient of 4 mm is usually associated with clinically meaningful hemodynamic impairment, such patients almost always have clear venographic features of obstruction such as tight stenosis and robust filling of collateral veins.34,66–69 Pressure measurements are insensitive; many patients with symptomatic venous disease exhibit mean pressure gradients below 4 mm Hg. Plethysmography has historically been used to provide detailed physiological venous assessments, but few vascular laboratories now routinely conduct these studies.70–75 Development and validation of new tools that are easy to use and that enable accurate and reliable measurement of venous obstruction and overall venous function should be prioritized.
Remote Assessment
Finally, a key limitation is the lack of well-validated venous outcome measures that can be assessed remotely. A new patient-reported Villalta measure enables venous outcome assessment using the same 11 items as the original Villalta scale but with patient self-assessment of clinical signs.76 Early validation studies show good correlations for patient-reported Villalta and Villalta scores when patients are provided a visual aid to support self-assessment.77,78 Many QOL self-assessment measures can be completed remotely. However, in contemporary research on behavioral and neurological conditions, mobile technology–supported ecological momentary assessments are routinely used to enable more frequent and more convenient data capture from study participants.79 Venous disease symptoms fluctuate, so the reliance of pivotal clinical trials on just a few assessment time points creates a substantial potential for measurement imprecision and recall bias. Were ecological momentary assessment tools and strategies to be created and validated for venous outcome assessment, they might more fully capture the impact of venous disease upon patients’ daily lives over time and reduce the needed sample size of many studies by permitting more frequent and more convenient assessments of study enrollees.
Consensus-Based Statements 10 to 17
-
10.
Studies of endovascular interventions for acute and chronic lower extremity venous conditions should assess clinical efficacy by documenting treatment-associated change in clinical outcomes including (1) patient-centered sequelae of venous disease (using validated questionnaires or scales that document symptoms, ambulatory/functional capacity, or QOL) or (2) objective clinical signs of progressive venous disease (including but not limited to venous ulcers).
Remark: the strongest case for clinical efficacy would include evidence of treatment effect upon both patient-centered sequelae and objective clinical signs of venous disease, with supportive evidence that plausibly links improvement in anatomic and physiological outcomes to the intervention and the clinical outcome improvement observed.
-
11.
For acute and chronic lower extremity venous conditions, to attribute clinical benefit to an endovascular device/intervention, improvement in at least 1 clinical outcome including (1) patient-centered sequelae of venous disease (symptoms, ambulatory/functional capacity, or QOL) or (2) objective clinical signs of progressive venous disease (including but not limited to venous ulcers) should exceed that observed in nonintervened controls, with freedom from confounding and bias.
Remark: statement reflects evidence from prospective studies that in nonintervened patient populations, substantial clinical improvement is seen during the first year after DVT and that even in the chronic phase, clinical severity fluctuates over time in many patients. Hence, the clinical efficacy of a device/intervention cannot be readily distinguished absent nonintervened controls. However, immediate anatomic efficacy can be established without a control group when an intervention is shown to create anatomic change in a rapid time course that is inconsistent with the natural history of the disease with conservative management.
-
12.
For studies of endovascular interventions for acute and chronic lower extremity venous conditions, the use of validated venous disease assessment scoring systems and QOL measures is preferred over exclusive reliance on measurement of individual symptoms or signs.
Remark: the visual analog scale, Likert scale, and other scales are well validated for pain assessment, and direct measurement of limb circumferences (with or without calculation of estimated limb volume) can be performed to quantify swelling/edema. These measurements are useful, but since individual symptoms can have nonspecific (ie, nonvenous) pathogeneses and since venous disease is phenotypically diverse, exclusive reliance on such assessments may not permit a comprehensive assessment of venous disease burden in a study population.
-
13.
To enhance the validity and credibility of study results, venous assessment tools should be applied with explicit precautions to reduce bias: (1) studies using patient-reported outcomes should take precautions to reduce reporting bias, especially when patient blinding is not feasible and (2) assessors of clinical signs should be qualified clinical personnel who have had training on measurement tools and bias prevention and should be blinded to treatment arm.
Remark: In designing studies, investigators should consider ways to blind clinical providers, outcome event adjudicators, and outcome assessors to treatment arm allocation. Investigators and staff members should be trained on how to avoid conveying treatment bias to patients and should not be present when patient-reported questionnaires are completed. Participants should be informed in advance of follow-up visits to avoid conveying their treatment arm assignment to examiners. Examiners do not need to be told whether they are examining the index limb or the contralateral limb or whether the patient has completed treatment or not.
-
14.
In studies of endovascular interventions for acute and chronic lower extremity venous conditions, decisions on how to use venous assessment scoring systems should consider the specific clinical study question, anticipated prerequisites for changing clinical practice, the validation and previous use of the scales, and performance thresholds needed to see clinically important differences.
Remark: decisions include the choice of scale to use; whether to use a binary cut point or continuous scale scores; and what threshold to utilize. Chronic venous disease occurs on a spectrum of severity, so in general, the use of continuous measurements is encouraged when there is sufficient insight into what degree of difference would be considered clinically meaningful by clinical providers when weighed against an intervention’s risks and costs.
-
15.
For iliac vein assessment in studies of endovascular interventions for acute and chronic lower extremity venous conditions, we strongly suggest the use of multiplanar catheter venography along with IVUS.
Remark: IVUS is highly sensitive for venous disease assessment and can visualize stenosis and thrombus that are occult on venography. In 1 prospective study, baseline IVUS-determined stenosis and IVUS change in venous caliber with stent placement each correlated with subsequent clinical improvement. IVUS can accurately measure the diameter or area of a vein in a specific location at a single point in time; however, in making judgments about the presence of pathological stenosis, physicians should be mindful of the physiological factors that can alter venous caliber and lead to overdiagnosis. IVUS is likely to be most accurate with patient prehydration, positional maneuvers, visualization of dynamic changes in lumen caliber during the cardiorespiratory cycle, and venographic correlation. In addition, there currently exists no well-validated scale for IVUS quantification of thrombus volume. IVUS may not be needed if the status of the iliac vein is not relevant to the study.
-
16.
In studies of endovascular interventions for acute and chronic lower extremity venous conditions, we strongly suggest the use of Duplex ultrasound for noninvasive assessment of lower extremity venous caliber, patency, compressibility, thrombus extent, and valvular reflux. Reflux assessment should be performed with an automated cuff inflator or manual distal compression, with the patient standing; if physical limitations preclude standing, then use of a steep reverse Trendelenburg position is acceptable. Pathological valvular reflux is present in superficial veins if the valve closure time exceeds 0.5 s and in femoral and popliteal veins if the valve closure time exceeds 1.0 s.
Remark: Valsalva maneuvers may be substituted for distal compression when the common femoral vein or saphenofemoral junction is evaluated. Ultrasound laboratories should have processes for continuous quality control and should ideally follow updated standards of the International Accreditation Commission or American College of Radiology.
-
17.
When Duplex ultrasound is used to assess residual thrombus in studies of endovascular interventions for acute and chronic lower extremity venous conditions, documentation of venous compressibility and standardized measurement of the residual anteroposterior diameter of the compressed vein with imaging in the transverse plane are suggested.
Remark: complete venous compressibility connotes the absence of thrombus. The residual compressed vein diameter has been validated as a method of quantifying thrombus volume and identifying acute-on-chronic thrombus. Ultrasound challenges include operator variability, inability to reliably discern thrombus age, and challenges in depicting the longitudinal extent of thrombus over time (when important, the latter can be done by measuring and recording the distance from thrombus edge to fixed landmarks such as the saphenofemoral junction).
MT FOR ACUTE DVT
Currently, the clinical use of MT devices for acute DVT is largely based on shared anecdotal clinical observations, case series, and prospective single-arm studies that were not designed to clearly distinguish device-specific effects from those of other therapies. In considering the optimal design of future thrombectomy studies, the panel’s recommendations are strongly informed by key observations from the completed multicenter randomized controlled trials that have evaluated CDT and related therapies for the management of acute proximal DVT.
First, these studies provided important insights into the expected natural history of acute DVT managed without endovascular therapy. In particular, during the 2-year follow-up period, the ATTRACT trial (Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-
Directed Thrombolysis) observed substantial improvements from baseline in scores on the Villalta scale and VEINES (Venous Insufficiency Epidemiological and Economic Study) QOL venous disease-specific QOL measure in the control arm patients who were treated with anticoagulation and compression alone.80 Hence, clinical improvement observed after use of an endovascular procedure for acute DVT cannot be attributed to the procedure unless it exceeds that observed in a nonintervened control group, ideally in a randomized controlled trial that minimizes patient selection bias.
Second, although these studies confirmed previous observations that most PTS cases can be identified within 1 to 2 years, CAVENT (Catheter-Directed Venous Thrombolysis) documented evolution in the magnitude of the effects of CDT therapy upon PTS prevention from 2 to 5 years; specifically, the degree of benefit appeared to substantially increase over time.5,81 This finding suggests that while 1 to 2 years of follow-up may offer a pragmatic compromise that enables quality PTS assessment in a feasible manner, longer term assessments are still valuable to obtain when resources are available.
Third, in ATTRACT, the baseline ultrasound exam was used to designate those patients who met the above society-endorsed definition of acute iliofemoral DVT and the randomization was stratified accordingly, enabling quality subgroup analyses.1,17 Based on these analyses, which suggested that at least some outcomes of endovascular intervention may differ between patients with iliofemoral DVT versus isolated femoral-popliteal DVT, this key anatomic distinction has been incorporated into contemporary clinical practice guidelines.82–86 Investigators of future thrombectomy studies should present the baseline anatomic extent of DVT using these definitions and may be well advised to focus on patients with acute iliofemoral DVT since they are at higher risk for adverse outcomes and have shown greater potential to benefit from endovascular intervention than patients with less extensive DVT.81,87,88
Fourth, in patients with acute iliofemoral DVT in the ATTRACT trial, endovascular thrombus removal led to greater early (within 1 month) DVT symptom resolution, reduced PTS severity through 6 months, and venous QOL benefits that were most pronounced during the first 6 months of follow-up, compared with standard therapy alone.80,87 Although it is questionable whether early benefits alone are sufficient to justify routine assumption of the risks of fibrinolytic drug administration, decision-making might be different for endovascular strategies that involve a lower risk of serious complications. The panel believes that while the importance of long-term venous outcome data collection has not diminished, demonstration of shorter term symptom/QOL benefits in well-designed randomized trials could justify the clinical use of MT strategies if a favorable safety profile is also observed.
Fifth, an exploratory analysis of ATTRACT found that the baseline symptom severity as assessed by the Villalta scale was predictive of the treatment effects of pharmacomechanical CDT upon 2-year PTS severity and venous QOL.89 Hence, baseline characterization of study populations by some measure of presenting symptom severity may prove valuable in understanding the results of venous thrombectomy trials in a way that supports individualization of care to specific patients. Although the Villalta scale was not originally designed for this purpose and its 11 items may deter routine clinical use, the measure appears to be useful in stratifying patients in clinical studies. Simpler measures (eg, pain scores) might also be used for this purpose but have not been explicitly validated as being predictive of treatment effects.
Sixth, postprocedural rethrombosis is likely to be an important factor in defining long-term clinical outcomes after thrombectomy procedures. In the CAVA trial (Catheter-Directed Thrombolysis Versus Anticoagulation), which did not find benefits to adjunctive use of ultrasound-assisted CDT, early rethrombosis was frequent in CDT/stent recipients.6 In the ATTRACT and CAVENT trials, better status (common femoral vein compressibility in ATTRACT at 1 month, deep venous flow in CAVENT at 6 months) of the venous system on Duplex ultrasound predicted improved PTS-related outcomes at 2 years.60,90,91 In an exploratory analysis of ATTRACT, pharmacomechanical CDT that included use of the AngioJet Rheolytic Thrombectomy System (Boston Scientific Corporation, Marlborough, MA) did not provide long-term benefits and appeared to be associated with more recurrent DVT than standard therapy alone.92 Current and future venous thrombectomy devices may involve large sheaths placed in limbs with variable inflow vein status and cause varying degrees of mechanical trauma to the venous system. Hence, in addition to evaluating clinical outcomes, studies should report independent assessments of mid- to long-term patency rates in the access vein, the treated vein segments, and adjacent veins.
Finally, thrombus removal analysis in the above randomized trials was based on venograms performed after adjunctive endovascular procedures, sometimes including balloon venoplasty and stent placement, precluding determination of whether the thrombus removal strategy itself was effective. In future venous thrombectomy studies, venography should be documented immediately before and after use of the thrombectomy device to independently evaluate its effectiveness; an additional final venogram can be obtained to evaluate the overall endovascular strategy. Long-term evaluation of venous valvular function is also important to understanding the effects of MT. Study reports should clarify the timing of interventions and assessments.
Consensus-Based Statements 18 to 25
-
18.
For patients with acute iliofemoral DVT, we strongly encourage the design and conduct of multicenter randomized trials of venous thrombectomy.
Remark: Patients with acute iliofemoral DVT experience more severe presenting symptoms, more frequent recurrent DVT and PTS, and more severe PTS than other patients with DVT and appear to have the strongest potential to benefit from endovascular therapy. A randomized trial using standardized device/procedure protocol and assessment of both short-term (eg, early symptom relief) and long-term (eg, PTS severity) outcomes is most capable of credibly demonstrating any clinical benefit over conservative therapy alone.
-
19.
For studies of endovascular intervention for acute lower extremity DVT, the population description should include (1) characteristics of the index DVT—whether it was associated with presenting symptoms, PE, or a temporary provoking risk factor; (2) symptom duration, DVT history, and other indicators of DVT chronicity; (3) the most cephalad anatomic extent of the DVT, using accepted definitions; and (4) whether the study includes patients with initially presenting DVT, early treatment failure (eg, progression of symptoms or thrombus extent), or acute limb-threatening circulatory compromise.
Remark: patients with DVT with only temporary provoking risk factors are at lower risk for recurrence than patients with chronic provoking risk factors or unprovoked DVT. Per guidelines of the Society of Interventional Radiology and the American Heart Association, iliofemoral DVT should be used for DVT that involves the iliac vein or common femoral vein, with or without the involvement of other deep veins. Femoral-popliteal DVT should be used for femoral or popliteal DVT that does not extend into the common femoral vein or more cephalad veins.
-
20.
For studies of acute lower extremity DVT, the protocol and statistical analysis plan should prespecify the type of thrombus removal techniques used in the study, with particular reference to whether the study will assess stand-alone use of the device, its use along with fibrinolytic drug therapy, and its use along with other treatment methods. Any use of fibrinolytic drugs, the route of administration (eg, systemic or catheter directed), the sequence of use (eg, thrombectomy device first, drug first, simultaneous), the drug dosing scheme, and use of other thrombus removal methods should be reported to enable a clear understanding of the treatment delivered.
-
21.
In studies evaluating thrombectomy for acute lower extremity DVT, we suggest early patient follow-up at ≈7 to 10 days, 1 month, and 6 months to evaluate adverse events, early anatomic efficacy, and early clinical efficacy. To evaluate long-term clinical efficacy, follow-up of 1 to 2 years is sufficient to identify most cases of incident PTS and may optimize feasibility for many studies. Follow-up of 3 to 5 years, however, will enable the most definitive assessment of the PTS, the need for reintervention, and severe clinical manifestations (eg, ulcers).
-
22.
For acute lower extremity DVT treatments, demonstration of early clinical efficacy at 1 to 6 months compared with nonintervened controls can justify the use of a device/intervention in clinical practice provided the safety profile is appropriate and should include the demonstration of (1) early anatomic efficacy that supports the attribution of treatment effects to the intervention and (2) improvement in patient-centered sequelae of venous disease (eg, limb pain, ambulatory/functional capacity, and QOL) or objective clinical signs of venous disease (eg, swelling by measurement of limb circumference/volume), compared with a control group.
Remark: although studies to date have focused on prevention of PTS, many patients with acute iliofemoral DVT experience severe symptoms, functional impairment, and poor QOL during the early weeks and months. Independent of PTS reduction, acceleration of early clinical recovery from acute DVT would be worthwhile if achievable with acceptable safety. A control comparator is needed because the natural history of nonintervened (conservatively treated) acute DVT includes early clinical improvement that extends over the first year.
-
23.
For acute lower extremity DVT treatments, the primary assessment of early anatomic efficacy of the device/intervention should occur at least 1 month after use to enable confidence in both its immediate anatomic efficacy and the degree to which this is sustained beyond the initial use.
Remark: the medium-to-large bore venous access and catheter/device manipulations needed for venous thrombectomy may influence the risk of early rethrombosis after intervention. More aggressive interventions may demonstrate enhanced thrombus removal efficacy, while conversely increasing the risk of rethrombosis. Clinical trials have found the status of the venous system 1 to 6 months after DVT treatment initiation to correlate with 2-year outcomes. Hence, demonstration of success should require sustained patency for at least the first month.
-
24.
For a thrombectomy device to demonstrate immediate anatomic efficacy, demonstration of thrombus volume reduction by independent blinded assessment of venograms performed before and after the intervention, ideally using established assessment scales, is acceptable. For stand-alone device use to be considered effective, the posttreatment venogram must be performed before the use of other interventions that can influence thrombus removal or patency.
Remark: fibrinolytic drugs can dissolve venous thrombus, and venous angioplasty and stent placement can help restore venous patency. As such, when possible, studies should be designed to avoid confounding with the effects of venous thrombectomy devices.
-
25.
In acute lower extremity DVT thrombectomy studies, particular attention should be paid to risks of bleeding (device-related blood loss, access site bleeding, and distant bleeding), PE, early rethrombosis, vascular injury, bradycardia, renal failure, and any additional risks that stem from the nature and mechanism of action of any specific device that is used.
STENT PLACEMENT FOR CHRONIC ILIOFEMORAL VENOUS OBSTRUCTION
The Clinical-Etiological-Anatomical-Pathological (CEAP) classification system was developed to standardize the descriptive characterization of patients with chronic venous disorders and was subsequently revised into its current form.93–95 This tool is now widely used and has functioned well in enabling a degree of comparability of venous cohorts across studies. Because CEAP is a nonordinal scale with many static elements, this scale was not intended and is not suitable for longitudinal assessment of venous disease severity. In addition, patients in the same clinical class (especially class 3) can experience widely variable symptom severity and QOL impact.96 Hence, investigators should report CEAP at baseline but should use other tools to describe clinical severity at baseline and during follow-up. It is hoped that new methods of standardizing the reporting of functional disability will be validated.
Chronic venous disease symptoms often fluctuate within patients during the course of a day, reflecting their level of activity, the degree and duration of limb dependency, and the use of compression therapy. Hence, in clinical studies, it is ideal to standardize the time of day at which patients are examined and plans for managing compression before clinical assessments. In addition, chronic venous disease patients show dynamic changes in clinical severity over longer periods of time due to progression of venous pathophysiology, development of collateral veins, and changes in nonvenous contributors to symptoms. The VETO (Venous Thrombosis Outcomes) registry investigators observed that in patients who had a Villalta score documented 4 months after acute symptomatic DVT, about 20% of patients experienced a change in Villalta severity category (no, mild, moderate, or severe PTS) at their 12-month or 24-month assessments (2). This included 10% of patients who changed Villalta severity category from 12 to 24 months. These findings conform to clinical observations that PTS fluctuates over time within patients, even in the late chronic phase. For this reason, although it may be tempting to conclude that improvement in symptom severity after an endovascular intervention reflects a treatment effect, scientific reports should not attribute clinical change to a procedure without proof of benefit over nonintervened control patients.
Until recently, the prevailing understanding of venous stent placement outcomes was derived mainly from anecdotal clinical observations, pooled analyses of case series, and a single small pilot randomized trial35,97,98
The absence of large rigorously designed multicenter randomized trials has been observed by U.S. government agencies and private health care payors, and a National Institutes of Health funded trial (the C-TRACT trial [Chronic Venous Thrombosis: Relief With Adjunctive Catheter-Directed Therapy]) is currently underway.99–101 In recent years, prospective multicenter non-randomized studies resulted in Food and Drug Administration clearance of 4 new venous stents for the management of iliofemoral venous obstruction.102–105 The panel’s recommendations are informed by the accumulated clinical insights of endovascular practitioners over 30 years, by the above studies, and by postmarketing clinical events and observations. A few key considerations are summarized here.
First, in recent years, endovascular providers and investigators have clearly distinguished the use of iliac vein stent placement among patients with acute DVT, established PTS, or NIVLs.97,98,102–105 The panel supports continued reporting along these lines because while there is overlap in many assessment domains, these distinctions influence optimal treatment methods, anticipated results, and procedural outcome assessments.
Second, a number of factors appear to influence stent placement outcomes, including the patient’s history of thrombosis, the presence of thrombophilia, the quality of inflow veins, and the type of antithrombotic therapy delivered post-procedure.106 Hence, quality interpretation of future studies will be facilitated by routine reporting of these clinical characteristics. A new descriptive scheme for classifying inflow patterns has been developed and can be utilized.107
Third, in recent Food and Drug Administration–monitored studies, a 50% iliac vein diameter stenosis was used as a key threshold parameter for study inclusion. However, there are problems with using such a threshold: (1) with any imaging method, patient hypovolemia may result in over-estimation of the degree of stenosis; (2) the hemodynamic impact of a stenosis depends not just on the vein’s minimum diameter but also on its length, the presence of collateral veins, and other factors; and (3) venous diameter measurement is difficult to standardize and depends on the imaging method used. Measurement on computed tomography and magnetic resonance imaging scans is limited due to their poor resolution and variable venous contrast enhancement. Multiplanar venography can identify venous lumen boundaries but is susceptible to flow artifacts (eg, the full caliber of the target vein may not be apparent if there is inflow from nonopacified blood from venous tributaries or if there is poor axial inflow), the challenges of identifying the narrowest dimension of a 3-dimensional structure by imaging in 2 dimensions, and the inability to visualize subtle internal venous abnormalities. IVUS has excellent resolution, but its measurements may be influenced by patient position and the degree to which the catheter is centered in the vein lumen.57,108,109 These limitations are particularly apparent for NIVLs because the adjacent vein is not fibrosed, vein segments tend to exhibit greater dynamic change during the cardiorespiratory cycle; and (4) utilization of a percentage narrowing parameter requires a reference normal comparator vessel. However, unlike the arterial system, the venous system does not provide an easy comparator since veins distal or contralateral to an iliac vein obstructive lesion often exhibit compensatory dilatation. Even when the segment above the stenosis can be measured (not always the case for lesions below a major confluence), it is expected to be larger than the target vein, and there may be variable degrees of reverse taper.
Fourth, device migration can occur with any implanted stent brand and has been reported with greater-than-anticipated frequency for dedicated nitinol stents in the postmarketing phase, resulting in the permanent recall of 1 venous stent brand from the marketplace.110,111 Of the migrations, most seemed to occur in NIVL patients, and they may have been more frequent with shorter and smaller stents. Another stent brand experienced a temporary recall due to an issue with its delivery catheter.112,113 Accordingly, long-term follow-up is important for future devices to verify their long-term safety, stability, and mechanical integrity.
Finally, even with newer stent brands, patients with PTS remain highly prone to loss of patency.97,98,102–105 In addition to early events, prospective studies observed that patency rates continued to decline for at least some stent brands between 1 and 3 years after implantation.101,113 Hence, studies should report on baseline characteristics, treatment elements, and postprocedure care elements that may influence the likelihood of thrombosis. Definitions of patency and intended plans for allowed reinterventions should be prespecified. Because continued innovation in stent design is likely (eg, covered stents, drug-eluting stents, bioabsorbable scaffolds), study reporting should clearly specify study device characteristics. The type and duration of periprocedure and postprocedure antithrombotic therapy should be stated. Outcome reporting should distinguish objective anatomic findings (eg, stent restenosis or occlusion and how determined) from clinical sequelae (eg, recurrent symptoms, DVT event). Additional research into the mechanisms underlying stent thrombosis is of high priority.114
Consensus-Based Statements 26 to 30
-
26.
In studies evaluating stent placement for treatment of chronic iliofemoral venous obstruction, population description should include descriptive classification of chronic venous disease using the revised CEAP categorization system; delineation of whether the study included patients with a history of recent acute DVT, any prior DVT (ie, being treated for established PTS), or no previous DVT (ie, being treated for NIVL); the anatomic level (length, completeness) of venous obstruction and how determined (eg, imaging method); information on the quality of venous inflow and outflow; and the clinical severity of venous disease (presence and severity of symptoms, signs, and venous ulcer).
Remark: the revised CEAP categorization system is a useful way to descriptively classify a chronic venous disease population to enable comparison with other study cohorts. However, CEAP clinical class is not sufficient to characterize venous clinical severity. In particular, the spectrum of disease severity within CEAP clinical class 3 is quite broad, ranging from patients with mild (lifestyle inconsequential) ankle edema to those who are largely disabled by massive entire-leg edema and venous claudication. Hence, to categorize patients for study inclusion, studies should use at least 1 indicator of venous disease severity beyond CEAP clinical class. Validated venous scoring systems such as the VCSS and Villalta scale can support the description of baseline venous disease severity and are more suitable for longitudinal follow-up and outcome assessment.
-
27.
Studies of chronic iliofemoral venous obstruction treatments should transparently state all endovascular methods that were used or allowed, including fibrinolytic therapy, MT, balloon angioplasty (predilatation and poststent expansion), stent placement, and use of vascular crossing devices (traditional and power emitting). For stents, the type (brand), size, and the number of devices should be stated. If graft material, drugs, or biological agents are used to coat or elute within the stent, this should be stated.
-
28.
In studies of stent placement for treatment of chronic iliofemoral venous obstruction, the venous lesion being treated should be characterized as complete occlusion (no flow) or as stenosis. In describing the percentage stenosis, either an adjacent normal vein segment can be used as the reference standard or published norms (12-mm common femoral vein, 14-mm external iliac vein, and 16-mm common iliac vein) can be used. For comparisons of prestenting and poststenting lumen caliber, we suggest the use of the minimum diameter of a particular vein segment as a more reliably assessed measurement parameter than the percentage narrowing.
Remark: with venous obstruction, caudal ipsilateral and contralateral vein segments may enlarge due to venous congestion. Hence, it can be challenging to reliably use them as reference standards for measurement. Additional variability is derived from patient-specific factors such as native body size, venous inflow disease, and cardiovascular factors.
-
29.
In studies of stent placement for treatment of chronic iliofemoral venous obstruction, patients should be followed closely to evaluate and report device-related adverse events including but not limited to device fractures, migrations, embolizations, rethrombosis, and any needed reinterventions or other consequences. For metallic stents, we suggest clinical follow-up with radiographic imaging for at least 3 years, with further imaging or intervention if necessary.
Remark: the suggestion for extended follow-up reflects information from published studies on the median age of stent recipients to date, the timing of occurrence of adverse events, and the likelihood that some event types are susceptible to underreporting.101–104,109–113
-
30.
In studies of stent placement for treatment of chronic iliofemoral venous obstruction, the primary patency, primary assisted patency, and secondary patency of the target vein should be reported based upon prespecified imaging criteria. We strongly discourage the use of clinical patency constructs that do not include objective ascertainment of vein status. Change in venous clinical severity, ulcer healing, and ulcer recurrence should be assessed.
Remark: definitions of patency should clearly state whether there was flow throughout the target segment (occluded or not) and the minimum lumen diameter within the segment (to enable characterization of any stenosis). To minimize patient burden and expense, we suggest the use of Duplex ultrasound for most follow-up assessments of the iliac and lower extremity veins. However, it is reasonable to use venography with IVUS selectively (eg, once during follow-up) to obtain a more confident assessment of an iliac vein intervention. Venous clinical severity changes can be assessed with validated scoring systems (eg, VCSS, Villalta) and venous disease-specific QOL measures (eg, VEINES-QOL, CIVIQ). Venous ulcer healing can be characterized by the occurrence of complete skin closure at 24 weeks and by measurements of the wound with calculation of percentage of wound healing.
CONCLUSIONS
There exist myriad challenges in designing, conducting, and reporting clinical studies of endovascular venous interventions. A multidisciplinary panel used the Delphi consensus methodology to develop 30 statements to guide researchers in designing and reporting future studies evaluating MT for acute DVT and stent placement for chronic iliofemoral venous obstruction. It is hoped that this guidance will aid standardization and thereby increase the quality of reporting of clinical outcomes of endovascular interventions, enabling innovation and regulatory decision-making that optimally supports venous patient care.
ARTICLE INFORMATION
Acknowledgments
The authors thank the physicians listed in the Appendix for contributing their expertise to developing the consensus-based statements and staff from the Vascular and Interventional Advances Foundation (Christopher Ebbe and Julie Enichen) and EBQ Consulting, Inc (Melanie Golob), for their excellent administrative support.
Sources of Funding
This project was supported by the Vascular and Interventional Advances Foundation.
Disclosures
Dr Vedantham receives nonfinancial research support (donation of compression stockings to study patients) from Medi USA. Dr Zelman Lewis is the President of EBQ Consulting, LLC. Dr Schneider is a consultant for Cagent, Boston Scientific, Limflow, Medtronic, Philips, Silk Road, and Surmodics. Dr Sabri is a consultant for Alucent Biomedical, Boston Scientific, and Medtronic. Dr Kolluri receives honoraria from Medtronic and is a consultant for Abbott Vascular, Auxetics, Avail, Boston Scientific Corporation, Inari Medical, Medtronic, Penumbra Medical, Surmodics, Syntactyx, and Prairie Education and Research Cooperative (PERC). The other authors report no conflicts.
Supplemental Material
Appendix
Supplementary Material
Nonstandard Abbreviations and Acronyms
- ATTRACT
- Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-Directed Thrombolysis
- C-TRACT
- Chronic Venous Thrombosis: Relief With Adjunctive Catheter-Directed Therapy
- CAVA
- Catheter-Directed Thrombolysis Versus Anticoagulation
- CAVENT
- Catheter-Directed Venous Thrombolysis
- CDT
- catheter-directed thrombolysis
- CEAP
- Clinical-Etiological-Anatomic-Pathophysiologic
- DVT
- deep vein thrombosis
- GUSTO
- Global Use of Strategies to Open Occluded Coronary Arteries
- IVUS
- intravascular ultrasound
- MT
- mechanical thrombectomy
- NIVL
- nonthrombotic iliac vein lesion
- PE
- pulmonary embolism
- PTS
- postthrombotic syndrome
- QOL
- quality of life
- tPA
- tissue-type plasminogen activator
- VCSS
- venous clinical severity scale
- VEINES
- Venous Insufficiency Epidemiological and Economic Study
- VETO
- Venous Thrombosis Outcomes
- VTE
- venous thromboembolism
S. Vedantham and P. Gloviczki are co-first authors.
This manuscript was sent to Herbert D. Aronow, Guest Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 482.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCINTERVENTIONS.123.012894.
Contributor Information
Peter Gloviczki, Email: gloviczki.peter@mayo.edu.
Teresa L. Carman, Email: teresa.carman@uhhospitals.org.
Sandra Zelman Lewis, Email: ebqconsulting@gmail.com.
Peter A. Schneider, Email: peteraschneider@gmail.com.
Saher S. Sabri, Email: saherssabri@gmail.com.
Raghu Kolluri, Email: kolluri.raghu@gmail.com.
REFERENCES
- 1.Jaff MR, McMurtry MS, Archer SL, Cushman M, Goldenberg N, Goldhaber SZ, Jenkins JS, Kline JA, Michaels AD, Thistlethwaite P, et al. ; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123:1788–1830. doi: 10.1161/CIR.0b013e318214914f [DOI] [PubMed] [Google Scholar]
- 2.Kahn SR, Shrier I, Julian JA, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149:698–707. doi: 10.7326/0003-4819-149-10-200811180-00004 [DOI] [PubMed] [Google Scholar]
- 3.Delis KT, Bountouroglou D, Mansfield AO. Venous claudication in iliofemoral thrombosis: long-term effects on venous hemodynamics, clinical status, and quality of life. Ann Surg. 2004;239:118–126. doi: 10.1097/01.sla.0000103067.10695.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahn S, Shbaklo H, Lamping D, Holcroft C, Shrier I, Miron M, Roussin A, Desmarais S, Joyal F, Kassis J, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6:1105–1112. doi: 10.1111/j.1538-7836.2008.03002.x [DOI] [PubMed] [Google Scholar]
- 5.Enden T, Haig Y, Kløw NE, Slagsvold CE, Sandvik L, Ghanima W, Hafsahl G, Holme PA, Holmen LO, Njaastad AM, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379:31–38. doi: 10.1016/s0140-6736(11)61753-4 [DOI] [PubMed] [Google Scholar]
- 6.Notten P, ten Cate-Hoek AJ, Arnoldussen CW, Strijkers RH, de Smet AA, Tick LW, van de Poel MH, Wikkeling OR, Vleming LJ, Koster A. Ultrasound-accelerated catheter-directed thrombolysis versus anticoagulation for the prevention of post-thrombotic syndrome (CAVA): a single-blind, multicentre, randomised trial. Lancet Haematol. 2020;7:e40–e49. doi: 10.1016/S2352-3026(19)30209-1 [DOI] [PubMed] [Google Scholar]
- 7.Stevens SM, Woller SC, Kreuziger LB, Bounameaux H, Doerschug K, Geersing GJ, Huisman MV, Kearon C, King CS, Knighton AJ, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545–e608. doi: 10.1016/j.chest.2021.07.055 [DOI] [PubMed] [Google Scholar]
- 8.Vedantham S, Goldhaber SZ, Julian JA, Kahn SR, Jaff MR, Cohen DJ, Magnuson E, Razavi MK, Comerota AJ, Gornik HL, et al. ; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377:2240–2252. doi: 10.1056/NEJMoa1615066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis SZ, Diekemper R, Ornelas J, Casey KR. Methodologies for the development of CHEST guidelines and expert panel reports. Chest. 2014;146:182–192. doi: 10.1378/chest.14-0824 [DOI] [PubMed] [Google Scholar]
- 10.Neumann I, Schünemann HJ. Guideline groups should make recommendations even if the evidence is considered insufficient. CMAJ. 2020;192:E23–E24. doi: 10.1503/cmaj.190144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg E, Greenfield S, Wolman DM, Mancher M, Graham R. Clinical Practice Guidelines We Can Trust. National Academies Press; 2011. [PubMed] [Google Scholar]
- 12.Diekemper RL, Patel S, Mette SA, Ornelas J, Ouellette DR, Casey KR. Making the GRADE: CHEST updates its methodology. Chest. 2018;153:756–759. doi: 10.1016/j.chest.2016.04.018 [DOI] [PubMed] [Google Scholar]
- 13.Lewis SZ, Diekemper RL, French CT, Gold PM, Irwin RS, Adams TM, Altman KW, Barker AF, Birring SS, Bolser DC. Methodologies for the development of the management of cough: CHEST guideline and expert panel report. Chest. 2014;146:1395–1402. doi: 10.1378/chest.14-1484 [DOI] [PubMed] [Google Scholar]
- 14.Miller CRJ, Chrissian AA, Lee YCG, Rahman NM, Wahidi MM, Tremblay A, Hsia DW, Almeida FA, Shojaee S, Mudambi L, et al. Key highlights from the American Association for Bronchology and interventional pulmonology evidence-informed guidelines and expert panel report for the management of indwelling pleural catheters. Chest. 2021;159:920–923. doi: 10.1016/j.chest.2020.09.282 [DOI] [PubMed] [Google Scholar]
- 15.Miller RJ, Chrissian AA, Lee Y, Rahman NM, Wahidi MM, Tremblay A, Hsia DW, Almeida FA, Shojaee S, Mudambi L. AABIP evidence-informed guidelines and expert panel report for the management of indwelling pleural catheters. J Bronchol Interv Pulmonol. 2020;27:229–245. doi: 10.1097/LBR.0000000000000707 [DOI] [PubMed] [Google Scholar]
- 16.Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, Carcao M, Mahlangu J, Ragni MV, Windyga J, et al. ; WFH Guidelines for the Management of Hemophilia Panelists and Co-Authors. WFH guidelines for the management of hemophilia. Haemophilia. 2020;26:1–158. doi: 10.1111/hae.14046 [DOI] [PubMed] [Google Scholar]
- 17.Vedantham S, Grassi CJ, Ferral H, Patel NH, Thorpe PE, Antonacci VP, d’Othée BMJ, Hofmann LV, Cardella JF, Kundu S. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2006;17:417–434. doi: 10.1097/01.RVI.0000197359.26571.c2 [DOI] [PubMed] [Google Scholar]
- 18.Dexter DJ, Kado H, Schor J, Annambhotla S, Olivieri B, Mojibian H, Maldonado TS, Gandhi S, Paulisin J, Bunte MC, et al. ; CLOUT Investigators. Interim outcomes of mechanical thrombectomy for deep vein thrombosis from the All-Comer CLOUT registry. J Vasc Surg Venous Lymphat Disord. 2022;10:832–840.e2. doi: 10.1016/j.jvsv.2022.02.013 [DOI] [PubMed] [Google Scholar]
- 19.Douketis JD, Crowther MA, Foster GA, Ginsberg JS. Does the location of thrombosis determine the risk of disease recurrence in patients with proximal deep vein thrombosis?. Am J Med. 2001;110:515–519. doi: 10.1016/s0002-9343(01)00661-1 [DOI] [PubMed] [Google Scholar]
- 20.Birn J, Vedantham S. May–Thurner syndrome and other obstructive iliac vein lesions: Meaning, myth, and mystery. Vasc Med. 2015;20:74–83. doi: 10.1177/1358863X14560429 [DOI] [PubMed] [Google Scholar]
- 21.U.S. Department of Health and Human Services. Guidance for industry and investigators: safety reporting requirements for INDs and BA/BE studies. 2021. Accessed November 20, 2022. https://www.fda.gov/files/drugs/published/Safety-Reporting-Requirements-for-INDs-%28Investigational-New-Drug-Applications%29-and-BA-BE-%28Bioavailability-Bioequivalence%29-Studies.pdf
- 22.U.S. Department of Health and Human Services. Guidance for Industry: safety reporting for investigational drugs and devices. Draft guidance. 2021. Accessed November 20, 2022. https://www.fda.gov/media/152530/download
- 23.GUSTO Investigators. An international randomized trial comparing four thrombolytic strategies for acute myocardial infarction. N Engl J Med. 1993;329:673–682. doi: 10.1056/NEJM199309023291001 [DOI] [PubMed] [Google Scholar]
- 24.Khalilzadeh O, Baerlocher MO, Shyn PB, Connolly BL, Devane AM, Morris CS, Cohen AM, Midia M, Thornton RH, Gross K, et al. Proposal of a new adverse event classification by the Society of Interventional Radiology Standards of Practice Committee. J Vasc Interv Radiol. 2017;28:1432–1437.e3. doi: 10.1016/j.jvir.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 25.Schulman S, Kearon C; Scientific SoCoAot, Thrombosis SCotISo, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 26.Henke P, Sharma S, Wakefield T, Myers D, Obi A. Insights from experimental post-thrombotic syndrome and potential for novel therapies. Transl Res. 2020;225:95–104. doi: 10.1016/j.trsl.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahn SR, Comerota AJ, Cushman M, Evans NS, Ginsberg JS, Goldenberg NA, Gupta DK, Prandoni P, Vedantham S, Walsh ME, et al. ; American Heart Association Council on Peripheral Vascular Disease, Council on Clinical Cardiology, and Council on Cardiovascular and Stroke Nursing. The postthrombotic syndrome: evidence-based prevention, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2014;130:1636–1661. doi: 10.1161/CIR.0000000000000130 [DOI] [PubMed] [Google Scholar]
- 28.Labropoulos N, Volteas N, Leon M, Sowade O, Rulo A, Giannoukas AD, Nicolaides AN. The role of venous outflow obstruction in patients with chronic venous dysfunction. Arch Surg. 1997;132:46–51. doi: 10.1001/archsurg.1997.01430250048011 [DOI] [PubMed] [Google Scholar]
- 29.Nicolaides A, Hussein M, Szendro G, Christopoulos D, Vasdekis S, Clarke H. The relation of venous ulceration with ambulatory venous pressure measurements. J Vasc Surg. 1993;17:414–419. doi: 10.1016/0741-5214(93)90426-m [DOI] [PubMed] [Google Scholar]
- 30.Shull KC, Nicolaides AN, e Fernandes JF, Miles C, Homer J, Needham T, Cooke ED, Eastcott FH. Significance of popliteal reflux in relation to ambulatory venous pressure and ulceration. Arch Surg. 1979;114:1304–1306. doi: 10.1001/archsurg.1979.01370350106012 [DOI] [PubMed] [Google Scholar]
- 31.Bakar Y, Özdemir O, Sevim S, Duygu E, Tuğral A, Sürmeli M. Intra-observer and inter-observer reliability of leg circumference measurement among six observers: a single blinded randomized trial. J Med Life. 2017;10:176. [PMC free article] [PubMed] [Google Scholar]
- 32.Guex J, Perrin M. Edema and leg volume: methods of assessment. Angiology. 2000;51:9–12. doi: 10.1177/000331970005100103 [DOI] [PubMed] [Google Scholar]
- 33.Bernstein SL, Bijur PE, Gallagher EJ. Relationship between intensity and relief in patients with acute severe pain. Am J Emerg Med. 2006;24:162–166. doi: 10.1016/j.ajem.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 34.Herr KA, Mobily PR, Kohout FJ, Wagenaar D. Evaluation of the Faces Pain Scale for use with the elderly. Clin J Pain. 1998;14:29–38. doi: 10.1097/00002508-199803000-00005 [DOI] [PubMed] [Google Scholar]
- 35.Neglén P, Hollis KC, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46:979–990. doi: 10.1016/j.jvs.2007.06.046 [DOI] [PubMed] [Google Scholar]
- 36.Rossi FH, Kambara AM, Izukawa NM, Rodrigues TO, Rossi CB, Sousa AG, Metzger PB, Thorpe PE. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J Vasc Surg. 2018;6:183–191. doi: 10.1016/j.jvsv.2017.11.003 [DOI] [PubMed] [Google Scholar]
- 37.Scott J, Huskisson E. Graphic representation of pain. Pain. 1976;2:175–184. [PubMed] [Google Scholar]
- 38.Kahn SR, Partsch H, Vedantham S, Prandoni P, Kearon C; Scientific SoCoAot, Thrombosis SCotISo, Haemostasis. Definition of post-thrombotic syndrome of the leg for use in clinical investigations: a recommendation for standardization. J Thromb Haemost. 2009;7:879–883. doi: 10.1111/j.1538-7836.2009.03294.x [DOI] [PubMed] [Google Scholar]
- 39.Rutherford RB, Padberg FT, Jr, Comerota AJ, Kistner RL, Meissner MH, Moneta GL. Venous severity scoring: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–1312. doi: 10.1067/mva.2000.107094 [DOI] [PubMed] [Google Scholar]
- 40.Villalta S, Bagatella P, Piccioli A, Lensing A, Prins M, Prandoni P. Assessment of reliability and reproducibility of a clinical scale for the post-thrombotic syndrome. Haemostasis. 1994;24:158a. [Google Scholar]
- 41.Kahn S. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost. 2009;7:884–888. doi: 10.1111/j.1538-7836.2009.03339.x [DOI] [PubMed] [Google Scholar]
- 42.Meissner MH, Natiello C, Nicholls SC. Performance characteristics of the venous clinical severity score. J Vasc Surg. 2002;36:889–895. doi: 10.1067/mva.2002.128637 [DOI] [PubMed] [Google Scholar]
- 43.Vasquez MA, Rabe E, McLafferty RB, Shortell CK, Marston WA, Gillespie D, Meissner MH, Rutherford RB; American Venous Forum Ad Hoc Outcomes Working Group. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg. 2010;52:1387–1396. doi: 10.1016/j.jvs.2010.06.161 [DOI] [PubMed] [Google Scholar]
- 44.Villalta S, Prandoni P, Cogo A, Bagatella P, Piccioli A, Bernardi E, Simioni P, Scarano L, Girolami A. The utility of non-invasive tests for detection of previous proximal-vein thrombosis. Thromb Haemost. 1995;73:592–596. [PubMed] [Google Scholar]
- 45.Kahn SR, Lamping DL, Ducruet T, Arsenault L, Miron MJ, Roussin A, Desmarais S, Joyal F, Kassis J, Solymoss S, et al. VEINES-QOL/Sym questionnaire was a reliable and valid disease-specific quality of life measure for deep venous thrombosis. J Clin Epidemiol. 2006;59:1049–1056. doi: 10.1016/j.jclinepi.2005.10.016 [DOI] [PubMed] [Google Scholar]
- 46.Lee A, Gu C-S, Vedantham S, Kearon C, Blostein M, Kahn SR. Performance of two clinical scales to assess quality of life in patients with post-thrombotic syndrome. J Vasc Surg. 2021;9:1257–1265.e2. doi: 10.1016/j.jvsv.2021.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jayaraj A, Meissner MH. A comparison of Villalta-Prandoni scale and venous clinical severity score in the assessment of post thrombotic syndrome. Ann Vasc Surg. 2014;28:313–317. doi: 10.1016/j.avsg.2012.11.014 [DOI] [PubMed] [Google Scholar]
- 48.Kahn S, Desmarais S, Ducruet T, Arsenault L, Ginsberg J. Comparison of the Villalta and Ginsberg clinical scales to diagnose the post-thrombotic syndrome: correlation with patient-reported disease burden and venous valvular reflux. J Thromb Haemost. 2006;4:907–908. doi: 10.1111/j.1538-7836.2006.01824.x [DOI] [PubMed] [Google Scholar]
- 49.Prandoni P, Frulla M, Sartor D, Concolato A, Girolami A. Vein abnormalities and the post-thrombotic syndrome. J Thromb Haemost. 2005;3:401–402. doi: 10.1111/j.1538-7836.2004.01106.x [DOI] [PubMed] [Google Scholar]
- 50.Dhruva SS, Redberg RF. Coverage of transvenous pulmonary embolectomy - medicare’s missed opportunity for evidence generation. N Engl J Med. 2022;386:904–906. doi: 10.1056/NEJMp2114960 [DOI] [PubMed] [Google Scholar]
- 51.Forauer AR, Gemmete JJ, Dasika NL, Cho KJ, Williams DM. Intravascular ultrasound in the diagnosis and treatment of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2002;13:523–527. doi: 10.1016/s1051-0443(07)61535-8 [DOI] [PubMed] [Google Scholar]
- 52.Murphy EH, Broker HS, Johnson EJ, Modrall JG, Valentine RJ, Arko FR, 3rd. Device and imaging-specific volumetric analysis of clot lysis after percutaneous mechanical thrombectomy for iliofemoral DVT. J Endovasc Ther. 2010;17:423–433. doi: 10.1583/10-3088.1 [DOI] [PubMed] [Google Scholar]
- 53.Saleem T, Raju S. Comparison of intravascular ultrasound and multidimensional contrast imaging modalities for characterization of chronic occlusive iliofemoral venous disease: a systematic review. J Vasc Surg Venous Lymphat Disord. 2021;9:1545–1556.e2. doi: 10.1016/j.jvsv.2021.03.022 [DOI] [PubMed] [Google Scholar]
- 54.Gagne PJ, Gasparis A, Black S, Thorpe P, Passman M, Vedantham S, Marston W, Iafrati M. Analysis of threshold stenosis by multiplanar venogram and intravascular ultrasound examination for predicting clinical improvement after iliofemoral vein stenting in the VIDIO trial. J Vasc Surg Venous Lymphat Disord. 2018;6:48–56.e1. doi: 10.1016/j.jvsv.2017.07.009 [DOI] [PubMed] [Google Scholar]
- 55.Gagne PJ, Tahara RW, Fastabend CP, Dzieciuchowicz L, Marston W, Vedantham S, Ting W, Iafrati MD. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. J Vasc Surg Venous Lymphat Disord. 2017;5:678–687. doi: 10.1016/j.jvsv.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 56.Marder VJ, Soulen RL, Atichartakarn V, Budzynski AZ, Parulekar S, Kim JR, Edward N, Zahavi J, Algazy KM. Quantitative venographic assessment of deep vein thrombosis in the evaluation of streptokinase and heparin therapy. J Lab Clin Med. 1977;89:1018–1029. [PubMed] [Google Scholar]
- 57.Krzanowski M, Partyka L, Drelicharz L, Mielnik M, Frolow M, Malinowski KP, Sliwka A, Marciniak K, Aleksiejew-Kleszczynski T. Posture commonly and considerably modifies stenosis of left common iliac and left renal veins in women diagnosed with pelvic venous disorder. J Vasc Surg Venous Lymphat Disord. 2019;7:845–852.e2. doi: 10.1016/j.jvsv.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 58.American College of Cardiology Foundation Appropriate Use Criteria Task Force; American College of Radiology; American Institute of Ultrasound in Medicine; American Society of Echocardiography; Intersocietal Accreditation Commission; Society for Cardiovascular Angiography and Interventions; Society of Cardiovascular Computed Tomography; Society of Interventional Radiology; Society for Vascular Medicine; Society for Vascular Surgery; Society for Vascular Ultrasound. ACCF/ACR/AIUM/ASE/IAC/SCAI/SCVS/SIR/SVM/SVS/SVU 2013 appropriate use criteria for peripheral vascular ultrasound and physiological testing. Part II: Testing for venous disease and evaluation of hemodialysis access. Vasc Med. 2013;18:215–231. doi: 10.1177/1358863X1349763723897935 [Google Scholar]
- 59.Dexter DJ, Kado H, Schor J, Annambhotla S, Olivieri B, Mojibian H, Maldonado TS, Gandhi S, Paulisin J, Bunte MC. Interim outcomes of mechanical thrombectomy for deep vein thrombosis from the All-Comer CLOUT Registry. J Vasc Surg. 2022;10:832–840. [DOI] [PubMed] [Google Scholar]
- 60.Weinberg I, Vedantham S, Salter A, Hadley G, Al-Hammadi N, Kearon C, Julian JA, Razavi MK, Gornik HL, Goldhaber SZ, et al. ; ATTRACT Trial Investigators. Relationships between the use of pharmacomechanical catheter-directed thrombolysis, sonographic findings, and clinical outcomes in patients with acute proximal DVT: results from the ATTRACT multicenter randomized trial. Vasc Med. 2019;24:442–451. doi: 10.1177/1358863X19862043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kearon C, Ginsberg JS, Kovacs MJ, Anderson DR, Wells P, Julian JA, MacKinnon B, Weitz JI, Crowther MA, Dolan S, et al. ; Extended Low-Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med. 2003;349:631–639. doi: 10.1056/NEJMoa035422 [DOI] [PubMed] [Google Scholar]
- 62.Kearon C, Julian JA, Newman TE, Ginsberg JS. Noninvasive diagnosis of deep venous thrombosis. McMaster diagnostic imaging practice guidelines initiative. Ann Intern Med. 1998;128:663–677. doi: 10.7326/0003-4819-128-8-199804150-00011 [DOI] [PubMed] [Google Scholar]
- 63.Linkins LA, Pasquale P, Paterson S, Kearon C. Change in thrombus length on venous ultrasound and recurrent deep vein thrombosis. Arch Intern Med. 2004;164:1793–1796. doi: 10.1001/archinte.164.16.1793 [DOI] [PubMed] [Google Scholar]
- 64.Linkins LA, Stretton R, Probyn L, Kearon C. Interobserver agreement on ultrasound measurements of residual vein diameter, thrombus echogenicity and Doppler venous flow in patients with previous venous thrombosis. Thromb Res. 2006;117:241–247. doi: 10.1016/j.thromres.2005.02.011 [DOI] [PubMed] [Google Scholar]
- 65.Prandoni P, Cogo A, Bernardi E, Villalta S, Polistena P, Simioni P, Noventa F, Benedetti L, Girolami A. A simple ultrasound approach for detection of recurrent proximal-vein thrombosis. Circulation. 1993;88:1730–1735. doi: 10.1161/01.cir.88.4.1730 [DOI] [PubMed] [Google Scholar]
- 66.Albrechtsson U, Einarsson E, Eklof B. Femoral vein pressure measurements for evaluation of venous function in patients with postthrombotic iliac veins. Cardiovasc Intervent Radiol. 1981;4:43–50. doi: 10.1007/BF02552407 [DOI] [PubMed] [Google Scholar]
- 67.Nazarian GK, Bjarnason H, Dietz CA, Jr, Bernadas CA, Hunter DW. Iliofemoral venous stenoses: effectiveness of treatment with metallic endovascular stents. Radiology. 1996;200:193–199. doi: 10.1148/radiology.200.1.8657909 [DOI] [PubMed] [Google Scholar]
- 68.Neglen P, Raju S. Detection of outflow obstruction in chronic venous insufficiency. J Vasc Surg. 1993;17:583–589. doi: 10.1016/0741-5214(93)90159-j [DOI] [PubMed] [Google Scholar]
- 69.Negus D, Cockett FB. Femoral vein pressures in post-phlebitic iliac vein obstruction. Br J Surg. 1967;54:522–525. doi: 10.1002/bjs.1800540605 [DOI] [PubMed] [Google Scholar]
- 70.Kurstjens RL, de Wolf MA, Alsadah SA, Arnoldussen CW, Strijkers RH, Toonder IM, Wittens CH. The value of hemodynamic measurements by air plethysmography in diagnosing venous obstruction of the lower limb. J Vasc Surg Venous Lymphat Disord. 2016;4:313–319. doi: 10.1016/j.jvsv.2016.01.005 [DOI] [PubMed] [Google Scholar]
- 71.Lattimer C, Mendoza E. Which venous patients need to be investigated with air-plethysmography and why? Phlebolymphology. 2019;26:16–25. [Google Scholar]
- 72.Lattimer CR, Mendoza E, Kalodiki E. The current status of air-plethysmography in evaluating non-thrombotic iliac vein lesions. Phlebology. 2018;33:3–4. doi: 10.1177/0268355516687866 [DOI] [PubMed] [Google Scholar]
- 73.Nicolaides A, Christopoulos D. Quantification of venous reflux and outflow obstruction with air plethysmography. In: Bernstein E, ed. Vascular Diagnosis. Mosby-Year Book, Inc; 1993. [Google Scholar]
- 74.Raju S. New approaches to the diagnosis and treatment of venous obstruction. J Vasc Surg. 1986;4:42–54. [PubMed] [Google Scholar]
- 75.Raju S, Fredericks R. Venous obstruction: an analysis of one hundred thirty-seven cases with hemodynamic, venographic, and clinical correlations. J Vasc Surg. 1991;14:305–313. [PubMed] [Google Scholar]
- 76.Utne KK, Ghanima W, Foyn S, Kahn S, Sandset PM, Wik HS. Development and validation of a tool for patient reporting of symptoms and signs of the post-thrombotic syndrome. Thromb Haemost. 2016;115:361–367. doi: 10.1160/th15-04-0318 [DOI] [PubMed] [Google Scholar]
- 77.Ng S, Rodger MA, Ghanima W, Kovacs MJ, Shivakumar S, Kahn SR, Sandset PM, Kearon C, Mallick R, Delluc A. External validation of the patient-reported Villalta scale for the diagnosis of postthrombotic syndrome. Thromb Haemost. 2022;122:1379–1383. doi: 10.1055/a-1738-1313 [DOI] [PubMed] [Google Scholar]
- 78.Utne KK, Dahm A, Wik HS, Jelsness-Jorgensen LP, Sandset PM, Ghanima W. Rivaroxaban versus warfarin for the prevention of post-thrombotic syndrome. Thromb Res. 2018;163:6–11. doi: 10.1016/j.thromres.2018.01.013 [DOI] [PubMed] [Google Scholar]
- 79.Mofsen AM, Rodebaugh TL, Nicol GE, Depp CA, Miller JP, Lenze EJ. When all else fails, listen to the patient: a viewpoint on the use of ecological momentary assessment in clinical trials. JMIR Ment Health. 2019;6:e11845. doi: 10.2196/11845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kahn SR, Julian JA, Kearon C, Gu CS, Cohen DJ, Magnuson EA, Comerota AJ, Goldhaber SZ, Jaff MR, Razavi MK, et al. ; ATTRACT Trial Investigators. Quality of life after pharmacomechanical catheter-directed thrombolysis for proximal deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2020;8:8–23.e18. doi: 10.1016/j.jvsv.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Haig Y, Enden T, Grotta O, Klow NE, Slagsvold CE, Ghanima W, Sandvik L, Hafsahl G, Holme PA, Holmen LO, et al. ; CaVenT Study Group. Post-thrombotic syndrome after catheter-directed thrombolysis for deep vein thrombosis (CaVenT): 5-year follow-up results of an open-label, randomised controlled trial. Lancet Haematol. 2016;3:e64–e71. doi: 10.1016/S2352-3026(15)00248-3 [DOI] [PubMed] [Google Scholar]
- 82.National Institute for Health and Care Excellence. Percutaneous mechanical thrombectomy for acute deep vein thrombosis of the leg: interventional procedures guidance. NICE Guidance. 2019. Accessed May 14, 2023. www.nice.org.uk/guidance/ipg651 [Google Scholar]
- 83.National Institute for Health and Care Excellence. Venous thromboembolic diseases: diagnosis, management and thrombophilia testing. NICE Guidance. 2020. Accessed May 14, 2023. www.nice.org.uk/guidance/ng158
- 84.Kakkos SK, Gohel M, Baekgaard N, Bauersachs R, Bellmunt-Montoya S, Black SA, Ten Cate-Hoek AJ, Elalamy I, Enzmann FK, Geroulakos G, et al. Editor’s choice - European Society for Vascular Surgery (ESVS) 2021 clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61:9–82. doi: 10.1016/j.ejvs.2020.09.023 [DOI] [PubMed] [Google Scholar]
- 85.Ortel TL, Neumann I, Ageno W, Beyth R, Clark NP, Cuker A, Hutten BA, Jaff MR, Manja V, Schulman S, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693–4738. doi: 10.1182/bloodadvances.2020001830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vedantham S, Desai KR, Weinberg I, Marston W, Winokur R, Patel S, Kolli KP, Azene E, Nelson KJ. Society of interventional radiology position statement on the endovascular management of acute iliofemoral deep vein thrombosis. J Vasc Interv Radiol. 2023;34:284–299. doi: 10.1016/j.jvir.2022.10.038 [DOI] [PubMed] [Google Scholar]
- 87.Comerota AJ, Kearon C, Gu CS, Julian JA, Goldhaber SZ, Kahn SR, Jaff MR, Razavi MK, Kindzelski AL, Bashir R, et al. ; ATTRACT Trial Investigators. Endovascular thrombus removal for acute iliofemoral deep vein thrombosis. Circulation. 2019;139:1162–1173. doi: 10.1161/CIRCULATIONAHA.118.037425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kearon C, Gu CS, Julian JA, Goldhaber SZ, Comerota AJ, Gornik HL, Murphy TP, Lewis L, Kahn SR, Kindzelski AL, et al. Pharmacomechanical catheter-directed thrombolysis in acute femoral-popliteal deep vein thrombosis: analysis from a stratified randomized trial. Thromb Haemost. 2019;119:633–644. doi: 10.1055/s-0039-1677795 [DOI] [PubMed] [Google Scholar]
- 89.Thukral S, Salter A, Lancia S, Kahn SR, Vedantham S. Predictors of clinical outcomes of pharmacomechanical catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis: analysis of a multicenter randomized trial. J Vasc Interv Radiol. 2022;33:1161–1170.e11. doi: 10.1016/j.jvir.2022.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Haig Y, Enden T, Slagsvold CE, Sandvik L, Sandset PM, Klow NE. Determinants of early and long-term efficacy of catheter-directed thrombolysis in proximal deep vein thrombosis. J Vasc Interv Radiol. 2013;24:17–24; quiz 26. doi: 10.1016/j.jvir.2012.09.023 [DOI] [PubMed] [Google Scholar]
- 91.Haig Y, Enden T, Slagsvold CE, Sandvik L, Sandset PM, Klow NE. Residual rates of reflux and obstruction and their correlation to post-thrombotic syndrome in a randomized study on catheter-directed thrombolysis for deep vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2014;2:123–130. doi: 10.1016/j.jvsv.2013.10.054 [DOI] [PubMed] [Google Scholar]
- 92.Vedantham S, Salter A, Lancia S, Lewis L, Thukral S, Kahn SR. Clinical outcomes of a pharmacomechanical catheter-directed venous thrombolysis strategy that included rheolytic thrombectomy in a multicenter randomized trial. J Vasc Interv Radiol. 2021;32:1296–1309.e7. doi: 10.1016/j.jvir.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eklof B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, et al. ; American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. J Vasc Surg. 2004;40:1248–1252. doi: 10.1016/j.jvs.2004.09.027 [DOI] [PubMed] [Google Scholar]
- 94.Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H, Bush RL, Blebea J, Carpentier PH, De Maeseneer M, et al. The 2020 update of the CEAP classification system and reporting standards. J Vasc Surg Venous Lymphat Disord. 2020;8:342–352. doi: 10.1016/j.jvsv.2019.12.075 [DOI] [PubMed] [Google Scholar]
- 95.Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995;21:635–645. doi: 10.1016/s0741-5214(95)70195-8 [DOI] [PubMed] [Google Scholar]
- 96.Masuda E, Ozsvath K, Vossler J, Woo K, Kistner R, Lurie F, Monahan D, Brown W, Labropoulos N, Dalsing M, et al. The 2020 appropriate use criteria for chronic lower extremity venous disease of the American Venous Forum, the Society for Vascular Surgery, the American Vein and Lymphatic Society, and the Society of Interventional Radiology. J Vasc Surg Venous Lymphat Disord. 2020;8:505–525.e4. doi: 10.1016/j.jvsv.2020.02.001 [DOI] [PubMed] [Google Scholar]
- 97.Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8:e002772. doi: 10.1161/CIRCINTERVENTIONS.115.002772 [DOI] [PubMed] [Google Scholar]
- 98.Seager MJ, Busuttil A, Dharmarajah B, Davies AH. Editor’s choice-- a systematic review of endovenous stenting in chronic venous disease secondary to iliac vein obstruction. Eur J Vasc Endovasc Surg. 2016;51:100–120. doi: 10.1016/j.ejvs.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 99.Gloviczki P, Dalsing MC, Henke P, Lal BK, O’Donnell TF, Jr, Shortell CK, Huang Y, Markovic J, Wakefield TW. Report of the Society for Vascular Surgery and the American Venous Forum on the July 20, 2016 meeting of the Medicare Evidence Development and Coverage Advisory Committee panel on lower extremity chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2017;5:378–398. doi: 10.1016/j.jvsv.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 100.Vedantham S, Kahn SR, Goldhaber SZ, Comerota AJ, Parpia S, Meleth S, Earp D, Williams R, Sista AK, Marston W, et al. Endovascular therapy for advanced post-thrombotic syndrome: proceedings from a multidisciplinary consensus panel. Vasc Med. 2016;21:400–407. doi: 10.1177/1358863X16650747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vedantham S, Parpia S, Kahn SR. A clinical trial of venous stent placement for post-thrombotic syndrome: current status and pandemic-related changes. Vasc Endovasc Review. 2022;5:e06. doi: 10.15420/ver.2021.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dake MD, O’Sullivan G, Shammas NW, Lichtenberg M, Mwipatayi BP, Settlage RA, Investigators VT. Three-year results from the venovo venous stent study for the treatment of iliac and femoral vein obstruction. Cardiovasc Intervent Radiol. 2021;44:1918–1929. doi: 10.1007/s00270-021-02975-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Murphy E, Gibson K, Sapoval M, Dexter DJ, Kolluri R, Razavi M, Black S. Pivotal study evaluating the safety and effectiveness of the Abre venous self-expanding stent system in patients with symptomatic iliofemoral venous outflow obstruction. Circ Cardiovasc Interv. 2022;15:e010960. doi: 10.1161/CIRCINTERVENTIONS.121.010960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Razavi M, Marston W, Black S, Bentley D, Neglen P. The initial report on 1-year outcomes of the feasibility study of the VENITI VICI VENOUS STENT in symptomatic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2018;6:192–200. doi: 10.1016/j.jvsv.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 105.Razavi MK, Black S, Gagne P, Chiacchierini R, Nicolini P, Marston W; VIRTUS Investigators. Pivotal study of endovenous stent placement for symptomatic iliofemoral venous obstruction. Circ Cardiovasc Interv. 2019;12:e008268. doi: 10.1161/CIRCINTERVENTIONS.119.008268 [DOI] [PubMed] [Google Scholar]
- 106.Marston WA, Browder SE, Iles K, Griffith A, McGinigle KL. Early thrombosis after iliac stenting for venous outflow occlusion is related to disease severity and type of anticoagulation. J Vasc Surg Venous Lymphat Disord. 2021;9:1399–1407.e1. doi: 10.1016/j.jvsv.2021.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jalaie H. Validation of a chronic venous obstruction classification system. Paper/Poster presented at: VEITH Symposium; 2022. Accessed November 27, 2022. https://www.veithsymposium.org/index.php?pg=faculty-2022&letter=j [Google Scholar]
- 108.Jayaraj A, Buck W, Knight A, Johns B, Raju S. Impact of degree of stenosis in May-Thurner syndrome on iliac vein stenting. J Vasc Surg Venous Lymphat Disord. 2019;7:195–202. doi: 10.1016/j.jvsv.2018.10.001 [DOI] [PubMed] [Google Scholar]
- 109.Jayaraj A, Powell T, Raju S. Utility of the 50% stenosis criterion for patients undergoing stenting for chronic iliofemoral venous obstruction. J Vasc Surg Venous Lymphat Disord. 2021;9:1408–1415. doi: 10.1016/j.jvsv.2021.05.008 [DOI] [PubMed] [Google Scholar]
- 110.U.S. Food and Drug Administration. Boston Scientific Corporation recalls Vici venous stent system and Vici RDS venous stent system for potential of stent migration. 2021. Accessed November 20. https://www.fda.gov/medical-devices/medical-device-recalls/boston-scientific-corporation-recalls-vici-venous-stent-system-and-vici-rds-venous-stent-system
- 111.Badesha AS, Siddiqui MM, Bains BRS, Bains PRS, Khan T. A systematic review on the incidence of stent migration in the treatment of acute and chronic iliofemoral disease using dedicated venous stents. Ann Vasc Surg. 2022;83:328–348. doi: 10.1016/j.avsg.2021.12.084 [DOI] [PubMed] [Google Scholar]
- 112.U.S. Food and Drug Administration. Class 2 Device Recall VENOVO Venous Stent System. 2020. Accessed November 20. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?ID=187686
- 113.Bundy JJ, Shin DS, Meissner MH, Monroe EJ, Chick JFB. Maldeployment of the venovo stent: a series of 2 documented instances. J Vasc Interv Radiol. 2021;32:781–783. doi: 10.1016/j.jvir.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 114.Khaja MS, Obi AT, Sharma AM, Cuker A, McCann SS, Thukral S, Matson JT, Hofmann LV, Charalel R, Kanthi Y, et al. Optimal Medical Therapy Following Deep Venous Interventions: Proceedings from the Society of Interventional Radiology Foundation Research Consensus Panel. J Vasc Interv Radiol. 2022;33:78–85. doi: 10.1016/j.jvir.2021.09.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.