Abstract
Background:
Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis and lower respiratory tract infections in children in their first year of life, disproportionately affecting infants in developing countries. Previous studies have found that the nasopharyngeal (NP) microbiome of infants with RSV infection has specific characteristics that correlate with disease severity, including lower biodiversity, perturbations of the microbiota and differences in relative abundance. These studies have focused on infants seen in clinical or hospital settings, predominantly in developed countries.
Methods:
We conducted a nested case control study within a random sample of 50 deceased RSV+ infants with age at death ranging from 4 days to 6 months and 50 matched deceased RSV− infants who were all previously enrolled in the Zambia Pertussis and RSV Infant Mortality Estimation (ZPRIME) study. All infants died within the community or within 48 hours of facility admittance. As part of the ZPRIME study procedures, all decedents underwent one-time, postmortem NP sampling. The current analysis explored the differences between the NP microbiome profiles of RSV+ and RSV− decedents using the 16S ribosomal DNA sequencing.
Results:
We found that Moraxella was more abundant in the NP microbiome of RSV+ decedents than in the RSV− decedents. Additionally, Gemella and Staphylococcus were less abundant in RSV+ decedents than in the RSV− decedents.
Conclusions:
These results support previously reported findings of the association between the NP microbiome and RSV and suggest that changes in the abundance of these microbes are likely specific to RSV and may correlate with mortality associated with the disease.
Keywords: nasopharyngeal microbiome, respiratory syncytial virus, infant, postmortem
Respiratory syncytial virus (RSV) infection is the most common cause of bronchiolitis and pneumonia in children in the first year of life.1,2 Symptoms of RSV range from mild upper respiratory infections to more severe infections such as bronchiolitis and pneumonia.3,4 RSV has been established as the leading global cause of lower respiratory tract infections in infants, with an etiological fraction for pneumonia 3 times higher than the next highest named pathogen.2 RSV continues to be a major health concern in both developed and developing nations.5,6 The developing world experiences the highest burden of RSV infection, accounting for 99% of world-wide deaths from RSV-related lower respiratory tract infections in children <5 years of age.3 We recently reported that in low-income countries, roughly two-thirds of infant RSV deaths occurred in the community.7
Several studies have looked at the associations between the nasopharyngeal (NP) microbiome profiles of infants and RSV disease severity. Previous studies have shown that the NP microbiome in RSV-infected infants has characteristics that changed with severity of disease.2,8–13 For instance, an overabundance of Achromobacter, Haemophilus, Moraxella and Streptococcus microbes and a loss of Corynebacterium, Staphylococcus and Veillonella have been associated with infants with more severe RSV.11–13 Studies have also shown that antibiotic use leads to decreased alpha diversity and dysbiosis in the NP microbiome.14,15
RSV infection may include indirect effects mediated by its impact on other members of the respiratory ecosystem.16–18 These effects seem to occur with bacteria such as H. influenzae and S. pneumoniae, suggesting that the impact of RSV on bacterial pathogenesis is relatively specific.19,20
Data from studies outside of the high-income countries is very limited, and yet most RSV-associated mortality is concentrated in low-income and middle-income countries.6 Until recently, the burden of RSV in low-income and middle-income countries was estimated with hospital-based surveillance data used to model RSV prevalence among community deaths. The Zambia Pertussis and RSV Infant Mortality Estimation (ZPRIME) study was a systematic, postmortem surveillance study designed to address this knowledge gap by directly measuring facility and community deaths in Lusaka, Zambia.7 The ZPRIME study found that RSV caused 2.8% of all infant deaths and 4.7% of all community deaths.
Our current analysis examines the NP microbiomes of a subset of RSV+ and RSV− deceased infants enrolled in the ZPRIME study. The 16S ribosomal DNA sequencing was conducted on postmortem infant NP samples with the goal of characterizing and comparing the NP microbiome profiles of RSV− and RSV+ decedents. Our study focuses on community deaths in a low-income county to address the gaps from previous studies.
MATERIALS AND METHODS
Study Population and Study Design
We identified a nested cross-sectional sample of RSV+ and RSV− deceased infants from the ZPRIME study.7 ZPRIME aimed to determine the proportion of infant deaths that could be attributed to Bordetella pertussis and RSV infections. Decedents were between 4 days and 6 months old and enrolled in the ZPRIME study between 2017 and 2019 within 48 hours of death. All decedents underwent one-time, postmortem NP sampling. NP samples were tested for RSV by reverse transcriptase quantitative polymerase chain reaction (PCR) following the testing protocol developed by the Centers for Disease Control and Surveillance.21 The ZPRIME study had a total of 2286 decedents, of which 779 community deaths with RSV PCR results were available at the time of this analysis. Of these 779 decedents, 78 decedents had a detectable RSV PCR result Ct<45, 62 decedents had an RSV PCR result Ct<35, and 701 decedents were RSV−. We selected a subset of decedents for our analysis from those decedents who were RSV+ with a PCR Ct<35. Demographic and clinical data, including sex, age at death, mother’s HIV status, location of death and cause of death, were collected for most decedents (Table 1). None of the decedents in our study were positive for B. pertussis.
TABLE 1.
Characteristics of Deceased RSV+ and RSV− Infants
| Characteristics of RSV+ and RSV− Decedents | ||||
|---|---|---|---|---|
| RSV+ Infants | RSV− Infants | All Subjects | P Value | |
| In study (n) | 50 | 50 | 100 | |
| Sex | 0.128 | |||
| Males % (n) | 46% (23/50) | 56% (28/50) | 51% (51/100) | |
| Females % (n) | 38% (19/50) | 40% (20/50) | 39% (39/100) | |
| Unknown sex % (n) | 16% (8/50) | 4% (2/50) | 10% (10/100) | |
| Age | 0.369 | |||
| Median age (days) | 41 | 39 | 40.5 | |
| 4–28 d % (n) 29 d |
38% (19/50) | 38% (19/50) | 38% (38/100) | |
| 29–61 d % (n) | 30% (15/50) | 28% (14/50) | 29% (29/100) | |
| 62–152 d % (n) | 24% (12/50) | 26% (13/50) | 25% (25/100) | |
| 153–182 d % (n) | 8% (4/50) | 8% (4/50) | 8% (8/100) | |
| Mother’s HIV status | 0.269 | |||
| HIV exposed % (n) | 4% (2/50) | 12% (6/50) | 8% (8/100) | |
| HIV unexposed % (n) | 96% (48/50) | 88% (44/50) | 92% (92/100) | |
| Location of death | 1.000 | |||
| Early facility death % (n) | 14% (7/50) | 16% (8/50) | 15% (15/100) | |
| Community death % (n) | 86% (43/50) | 84% (42/50) | 85% (85/100) | |
| Cause of death | <0.001 | |||
| Respiratory % (n) | 72% (36/50) | 26% (13/50) | 49% (49/100) | |
| Nonrespiratory % (n) | 22% (11/50) | 64% (32/50) | 43% (43/100) | |
| Unsure % (n) | 6% (3/50) | 10% (5/50) | 8% (8/100) | |
RSV+ infants were selected through random sampling and were matched to RSV− infants by age at death and date of death. The χ2 tests were performed for each group. Note that the infant’s sex was not reported in all cases.
These 62 RSV+ decedents were matched to one of the 701 RSV− decedents. RSV+ and RSV− infants were sorted into groups by age at death [4–28 days (0+ months), 29–61 days (1+ month), 62–152 days (2+ months) and 152–182 days (4+ months)] and date of death (±1.5 months) (Table 1) due to age-related and seasonality-related variations in RSV impact. Within each group, RSV+ samples were sorted by ID number and RSV− samples were assigned a random number and then sorted by this random number. RSV− samples with the lowest random number were matched to the RSV+ sample with the lowest ID number within each age and date of death group. From these 62 matched pairs, we selected 50 matched pairs via random sampling using PROC SURVEYSELECT in SAS.
Additional details about our study population are available in Text, Supplemental Digital Content 1, http://links.lww.com/INF/F29, as well as information about sample collection, processing, and storage, 16S ribosomal rDNA amplification and sequencing, and data processing.
Statistical Analysis
We analyzed microbe counts at both the genus and species levels, which is possible using PathoScope2.0.22–24 All samples had >25,000 reads. We performed χ2 tests to determine whether the population characteristics of our RSV+ and RSV− groups were evenly distributed. We calculated relative abundances based on the raw count data for the taxon grouped by disease state and constructed bar plots, boxplots, and heatmaps comparing microbe prevalence across the RSV+ and RSV− conditions.25,26 Samples in the bar chart are ordered by the top 4 most abundant samples found in RSV+ samples to enhance visual organization. Differential abundance analysis was conducted based on the relative abundance between genera using a nonparametric Wilcoxon rank-sum test.27–29 To correct for multiple testing, we used Benjamini-Hochberg P value adjustments.30 Significant results, defined as P < 0.05 and adjusted P < 0.25, were visualized with boxplots.
To analyze alpha diversity, we computed Shannon and Simpson indices and visualized each index as a boxplot.31,32 We compared indices across RSV state using Wilcoxon rank-sum test as the data were not normally distributed, as determined by the Shapiro-Wilk test for normality.27–29,33–35
To analyze beta diversity, we computed the Bray-Curtis dissimilarity indices to observe differences in microbial composition between the samples.36 The results were visualized using nonmetric dimensional scaling (NMDS) and principal coordinate analysis (PCoA).26,37,38 We performed NMDS with 20 stress test runs. Atchison distances were also computed and visualized via PCoA.39 Ellipses on the NMDS and PCoA plots represent a 95% confidence level for a multivariate t-distribution. PERMANOVA was performed to determine whether the centroids and dispersion were statistically significant between RSV states.40
We analyzed pathway abundances via a Wilcoxon rank-sum test with Benjamini-Hochberg corrections to determine whether there was a difference in inferred pathway abundances between RSV+ and RSV− samples.27–30,41–43 Significant results have an adjusted P < 0.05.
RESULTS
Data Filtering
Due to the heavy presence of taxa with relatively low abundances in the raw data, taxa with average relative abundances <0.1% were filtered from the dataset and regrouped as a single “other” category. This reduced our identified taxa assignments from 632 genera encompassing 1042 species to 36 genera encompassing 54 species, not including the “other” grouping. We used these data for all analyses expect for visualization of relative abundance, which used a data set including genera with average relative abundances <1%. This smaller group consisted of 15 genera and 1 “other” category.
Baseline Characteristics of the Study Population
Table 1 displays the baseline characteristics for the 100 decedents. The groups were roughly even across sex (51% male, 39% female, 10% unknown). The median age of death was 40.5 days. The majority (85%) of decedents were brought in dead from the community. A small proportion of deceased infants in both groups were known to be born to mothers infected with HIV (8%), although the final HIV status of the decedents themselves remains unknown. Among all decedents, there were 49 respiratory deaths, 43 nonrespiratory deaths and 8 inconclusive deaths. As expected, most RSV+ deaths were respiratory (72%) and most RSV− deaths were nonrespiratory (64%) in origin.
The χ2 tests were performed to determine whether the population characteristics were evenly distributed between the RSV+ and matched RSV− decedents. No significant difference was found for sex (P = 0.128), age (P = 0.369), HIV exposure status (P = 0.269) or location of death (P = 1.000) indicating an even decedent distribution among groups (Table 1). The cause of death (respiratory vs. nonrespiratory) differed significantly between RSV+ and RSV− deceased infants (P < 0.001).
Relative Abundance
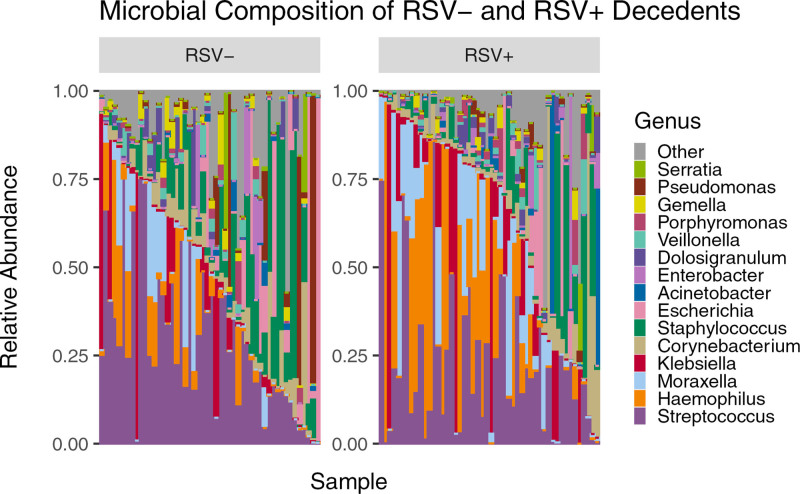
To compare the relative abundance of microbial taxa across RSV+ and RSV− samples, we generated a stacked bar plot composed of all genera with relative abundances >1% (Fig. 1). Figure 1 shows a total of 16 genera across all the samples with varying abundance levels. Each column represents the composite NP microbiome for a single participant and are ordered based on the abundance of the top 4 RSV+ microbes. When averaged across samples, the 5 most abundant microbes in RSV+ samples were Streptococcus (22.2%), Haemophilus (19.7%), Moraxella (11.0%), Klebsiella (10.2%) and Corynebacterium (6.8%). The 5 most abundant microbes in RSV− samples were Streptococcus (29.0%), Staphylococcus (13.5%), Escherichia (8.5%), Klebsiella (6.4%) and Haemophilus (5.7%).
FIGURE 1.
Microbial composition of RSV− and RSV+ samples showing microbe diversity within each sample. On average, the 5 most abundant microbes in RSV+ samples were Streptococcus (22.2%), Haemophilus (19.7%), Moraxella (11.0%), Klebsiella (10.2%) and Corynebacterium (6.8%). The 5 most abundant microbes in RSV− samples were Streptococcus (29.0%), Staphylococcus (13.5%), Escherichia (8.5%), Klebsiella (6.4%) and Haemophilus (5.7%).
Alpha Diversity
Shannon and Simpson Index
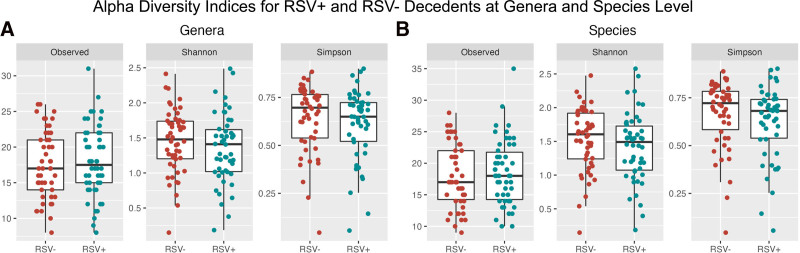
Shannon and Simpson indices were calculated, and a Shapiro-Wilk test was performed for each variable (Fig. 2A). The Shannon indices for genera did not follow a normal distribution (P = 0.985 for RSV+ and P = 0.860 for RSV−), whereas the Simpson indices did follow a normal distribution (P = 0.002 for RSV+ and P < 0.001 for RSV−). A Wilcoxon rank-sum test of significance was performed to determine significance of the differences in the Shannon and Simpson indices. We observed no significant difference in the alpha diversity between the RSV+ and RSV− decedents for genera (P = 0.621 for observed, P = 0.287 for Shannon and P = 0.206 for Simpson). No significant alpha diversity differences were found when comparing species level taxa in the same manner (P = 0.790 for observed, 0.173 for Shannon and 0.120 for Simpson) (Fig. 2B).
FIGURE 2.
Alpha diversity for RSV+ and RSV− Zambian decedents. No significant differences were found when conducting Wilcoxon rank-sum test for (A) genera (P = 0.621 for observed, 0.287 for Shannon and 0.206 for Simpson) or (B) species (P = 0.790, 0.173, 0.120, respectively).
Beta Diversity
Bray-Curtis Dissimilarity Index
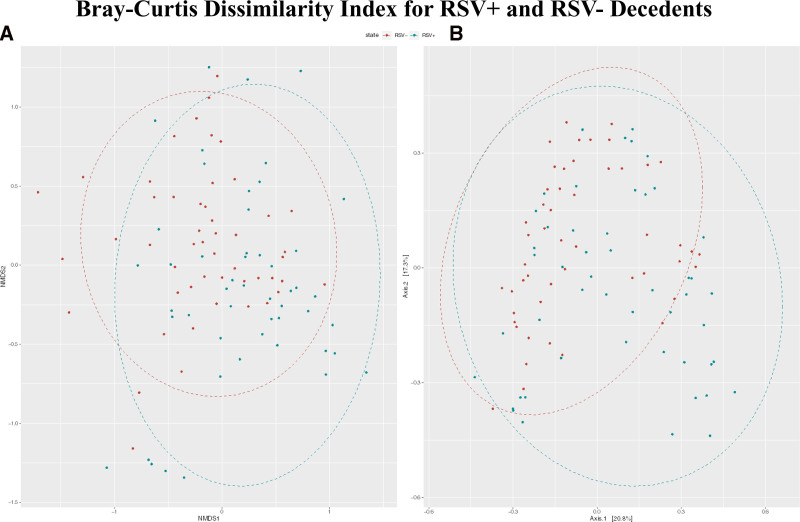
We computed Bray-Curtis dissimilarity indices and visualized the results using NMDS and PCoA (Fig. 3). Using PERMANOVA, we found that there was a significant difference in the centroids and dispersion between the RSV+ and RSV− groups (P < 0.001).
FIGURE 3.
A: NMDS displaying the Bray-Curtis dissimilarity index for genera comparison between RSV+ and RSV− deceased infants. The best solution stress value was 0.248. Some clustering of RSV+ decedents is visible to the bottom with some clustering of RSV− decedents to the top left. B: PCoA showing general clustering of RSV+ samples in the bottom right-hand corner without RSV− samples. However, otherwise there is substantial overlap between the 2 groups.
In the NMDS plot for genera, we observed clustering of RSV+ decedent samples to the bottom and RSV− decedent samples clustering to the upper left (Fig. 3A). However, there was little spatial separation of clusters, and the stress value was high at 0.248. This indicates some differentiation between groups, but a high degree of overlap. NMDS for species also showed very little clustering by RSV status with a stress value of 0.262 (Figure, Supplemental Digital Content 3A, http://links.lww.com/INF/F31).
From the PCoA plot for genera, we see that the first 2 axes account for 38% of the variability in the data (Fig. 3B). Some clustering is visible in the RSV+ samples, hedging away from the RSV− samples in the bottom right-hand corner with overlap between samples throughout the remainder. As with the NMDS analysis, signs of distinct clustering are largely absent. PCoA for species showed mostly overlap between RSV+ and RSV− samples with some individually clustered RSV+ samples in the bottom left (Figure, Supplemental Digital Content 3B, http://links.lww.com/INF/F31).
Differential Abundance Analysis
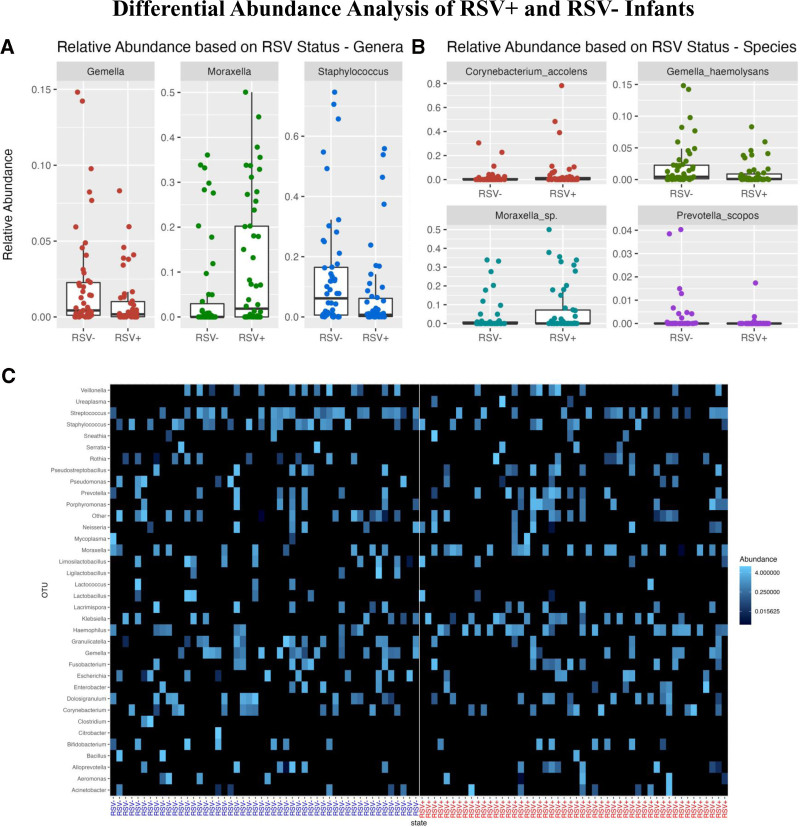
Differential abundance analysis via the nonparametric Wilcoxon rank-sum test was performed to compare the differences in relative microbial composition between RSV+ and RSV− samples. From this test, we determined that Gemella (P = 0.020), Moraxella (P = 0.006) and Staphylococcus (P = 0.018) all had marginally significant differences in median abundances between RSV+ and RSV− samples (Table, Supplemental Digital Content 2, http://links.lww.com/INF/F30).
We created box plots of genera abundances to visualize the intergroup differences. We observed that RSV+ decedents have lower abundances of Gemella and Staphylococcus and a higher abundance of Moraxella compared with RSV− decedents (Fig. 4A). We also created a heatmap based on the relative abundances. In the heatmap, we see subtle trends in abundance differences between the RSV+ and RSV− decedents consistent with our differential abundance results and box plots (Fig. 4C).
FIGURE 4.
Box plots of relative abundance indicating significant (A) intragenera or (B) intraspecies differences in RSV+ and RSV− samples. A: RSV+ samples show lower abundances in the genera of Gemella (P = 0.020) and Staphylococcus (P = 0.018) and a higher abundance of Moraxella (P = 0.006). B: RSV+ samples show higher abundances of the species Moraxella sp. (P = 0.016) and lower abundances of G. haemolysans (P = 0.006). Differences in Corynebacterium accolens (P = 0.049) and Prevotella scopos (P = 0.004) appear to be driven by a few outliers. C: Heatmap displaying the Bray-Curtis dissimilarity index for genera comparison between RSV+ and RSV− decedents. Higher abundances of Haemophilus and Moraxella are seen in RSV+ decedents. Lower abundances of Gemella, Staphylococcus and Streptococcus are present in RSV+ decedents.
Differential abundance analysis at the species level, which is possible using PathoScope2.0, indicated significant differences for C. accolens (P = 0.049), G. haemolysans (P = 0.006), Moraxella sp. (P = 0.016), and Prevotella scopos (P = 0.004).22–24 The box plots in Figure 4B illustrate higher abundances of Moraxella sp. in RSV+ decedents and lower abundances of G. haemolysans when compared with RSV− decedents. Differences in C. accolens (P = 0.049) and P. scopos appear to be driven by a few outliers. Heatmap visualization revealed similar visible differences (Figure, Supplemental Digital Content 4, http://links.lww.com/INF/F32).
DISCUSSION
Previous studies have shown that the microbial ecosystem in the nasopharynx, in which RSV acquisition occurs, may influence the host response to infection and certainly varies with disease severity.2,8–13 In contrast with prior studies, which focused mainly on infants who were newly diagnosed with RSV and were acutely ill, our study focused on deceased infants who died with RSV. This focuses our results on infants who had experienced the most extreme outcome of RSV infection, death. Previous studies have also shown that antibiotic use leads to decreased alpha diversity and dysbiosis in the NP microbiome.14,15 To avoid confounding by exposure to antibiotics, our analysis focused on comparing the microbiome profile of infants who died in the community. This includes infants who died in a facility with a stay <48 hours who would not be affected by nosocomial exposure due to limited time in the hospital.
We found no significant difference in genera or species richness or diversity in individual samples as measured by the Shannon and Simpson indexes. This indicates that the number of different species was roughly similar between the groups but does not provide insight into whether the specific compositions differed. To explore how the specific compositions of the NP microbiome differed, we turned to examine beta diversity via Bray-Curtis analysis. NMDS and PCoA plots showed high degrees of overlap, but PERMANOVA indicated a significant difference between the 2 groups (P < 0.001). To explore which microbes influenced the difference in beta diversity, we analyzed differences in the relative abundance of microbial taxa between conditions. RSV+ decedents had higher abundances of Moraxella (P = 0.006) and lower relative abundances of Gemella (P = 0.020) and Staphylococcus (P = 0.018) when compared with RSV− decedents. We also noticed increased relative abundances of Haemophilus in RSV+ decedents, although these were not significant. We identified significant differential abundance in 3 pathways associated with Haemophilus (Results in Text, Supplemental Digital Content 1, http://links.lww.com/INF/F29 and Table, Supplemental Digital Content 5, http://links.lww.com/INF/F33).44 This confirms findings reported previously by Ederveen et al, Rosas-Salazar et al and de Steenhuijsen Piters et al.11–13
Ederveen et al reported that an overabundance of Haemophilus and Achromobacter microbes and a loss of microbes such as Veillonella are associated with infants who had been hospitalized with RSV.12 They also indicated that infants who recover from RSV have higher Moraxella abundances. Additionally, Rosas-Salazar et al. observed that infants diagnosed with RSV had higher abundances of Haemophilus, Moraxella and Streptococcus, whereas healthy patients had high abundances of Corynebacterium and Staphylococcus.11 Also, de Steenhuijsen Piters et al reported that in infants hospitalized for RSV, H. influenzae and Streptococcus are seen in higher abundance and correlated to the disease severity.13 Our study found similar results in infants who died with RSV in Haemophilus, Moraxella and Staphylococcus. Our results confirm that higher abundances of Moraxella and decreases in Staphylococcus are correlated with severe RSV infection. Our results also show a trend in an increase in Haemophilus, although not significant, which may be due to our small sampling size.
Our study had several key limitations. For one, we did not have complete data on how the infants in either population died, and misclassifications are possible due to the limited descriptions of symptoms provided by the family member who often was not the primary care giver. Despite the adjudication process (Text, Supplemental Digital Content 1, http://links.lww.com/INF/F29), there is a chance that the RSV+ group may have died from issues unrelated to RSV or respiratory disease that could skew our results. We also do not have information on cause of the death beyond respiratory or nonrespiratory classification. We, therefore, have a heterogenous RSV− sample in terms of cause of death, which may impact our results. Compounding this, information about the final HIV status of the decedents is not available and HIV status of the mothers may not be accurately captured because mother’s HIV status was not always noted in medical charts or death records and was not inquired about during verbal autopsies. HIV exposure has been shown to cause NP microbiome dysbiosis and may affect our results.45,46 Furthermore, many of the decedents, particularly those who had died in a facility, may have been treated with antibiotics. Although our focus on community deaths was intended to reduce the confounding effect of antibiotics, we could not exclude the possibility that some decedents were exposed to antibiotics. We also do not have information regarding the use of antibiotics earlier in life, which may also disrupt the NP microbiome.15 Additionally, verbal autopsies did not yield accurate information about antibiotic exposure. Lastly, the impact of RSV on microbial ecology may not necessarily be confined to the acute RSV infection period but could occur at some time removed from the initial infection. The cross-sectional nature of our data set, focusing on time point shortly after death, could not detect those delayed effects.
With those caveats, our observations are consistent with what has been identified in previous research by Ederveen et al, Rosas-Salazar et al and de Steenhuijsen Piters et al.
CONCLUSIONS
Our findings support those findings previously published and indicate that RSV may be correlated with higher abundances of Moraxella and Haemophilus and lower abundances of Staphylococcus. Our study also points to decreased abundances of Gemella.
Further research should be done to confirm these findings and specifically to determine whether the change in microbiome proceeds RSV infection or is an effect of infection. Additional research looking at community-acquired RSV would also allow greater understanding of microbe differences without exposure to clinical settings or antibiotics. Better understanding may allow for better identification of at-risk infants and lead to better treatment and care.
Supplementary Material
Footnotes
Supported by the Bill and Melinda Gates Foundation. W.E.J. and J.M. were funded in part by NIH grant R01 GM127430. W.E.J. and A.R.O.M. were supported in part by the NIH under grant R21 AI154387. The other authors have no conflicts of interest to disclose.
C.J.G. and L.M. were the co-principal investigators for the Zambia Pertussis and RSV Infant Mortality Estimation (ZPRIME) study. W.B.M. was the ZPRIME study statistician and provided all needed data from the ZPRIME study for our analysis. A.I. oversaw the laboratory that generated the microbiome samples from ZPRIME for our study. R.P. was the ZPRIME project manager and aided in collaboration between our teams. R.L. contributed to the implementation of the ZPRIME study, including with the adjudication process. W.E.J. oversaw all data and statistical analysis for the current study and provided recommendations on best practices. N.K. completed initial data exploration and analysis. J.M. revised and completed the data visualization, data analysis and statistical analysis. She also took the lead on writing the article. A.R.O.M. provided support for data and statistical analysis, template code for sequencing, animalcules and PICRUSt analysis, and provided critical feedback on the article. J.M., A.R.O.M., C.J.G., W.B.M., R.P., R.L. and W.E.J. discussed the results, contributed to the ideas contained in the final article, and helped prepare the article. All authors reviewed the final article.
Raw data is available on the sequence read archive, accession number PRJNA913857. All processed data and code used for data preprocessing, statistical analysis, and figure generation is available on GitHub (https://github.com/jessmcc22/ZPRIME_RSV). Sample IDs are consistent across microbiome and demographic information on the sequence read achieve and GitHub. Full results for all analyses are also available on GitHub.
The ZPRIME study, where our data originates, was approved by the ethical review boards at Boston University Medical Center and the University of Zambia. Written informed consent was obtained from the decedents’ next of kin or guardian.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
Contributor Information
Jessica McClintock, Email: jessica.mcclintock@rutgers.edu.
Aubrey R. Odom-Mabey, Email: aodom@bu.edu.
Nitsueh Kebere, Email: nkebere@gmail.com.
Arshad Ismail, Email: arshadi@nicd.ac.za.
Lawrence Mwananyanda, Email: lawyanda@gmail.com.
Christopher J. Gill, Email: cgill@bu.edu.
William B. MacLeod, Email: wmacleod@bu.edu.
Rachel C. Pieciak, Email: rpieciak@bu.edu.
Rotem Lapidot, Email: rotem.lapidot@bmc.org.
REFERENCES
- 1.Navarro Alonso JA, Bont LJ, Bozzola E, et al. RSV: perspectives to strengthen the need for protection in all infants. Emerging Themes in Epidemiology. 2021;18:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Brien KL, Baggett HC, Brooks WA, et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. The Lancet. 2019;394:757–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet. 2010;375:1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Midulla F, Nenna R, Scagnolari C, et al. How respiratory syncytial virus genotypes influence the clinical course in infants hospitalized for bronchiolitis. J Infect Dis. 2019;219:526–534. [DOI] [PubMed] [Google Scholar]
- 5.Staadegaard L, Caini S, Wangchuk S, et al. The global epidemiology of rsv in community and hospitalized care: findings from 15 Countries. Open Forum Infect Dis. 2021;8:ofab159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi T, McAllister DA, O’Brien KL, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. The Lancet. 2017;390:946–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gill CJ, Mwananyanda L, MacLeod WB, et al. Infant deaths from respiratory syncytial virus in Lusaka, Zambia from the ZPRIME study: a 3-year, systematic, post-mortem surveillance project. Lancet Glob Health. 2022;10:e269–e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schippa S, Frassanito A, Marazzato M, et al. Nasal microbiota in RSV bronchiolitis. Microorganisms. 2020;8:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberger DM, Klugman KP, Steiner CA, et al. Association between Respiratory Syncytial Virus Activity and Pneumococcal Disease in Infants: a time series analysis of us hospitalization data. PLoS Med. 2015;12:e1001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Man WH, van Houten MA, Mérelle ME, et al. Bacterial and viral respiratory tract microbiota and host characteristics in children with lower respiratory tract infections: a matched case-control study. Lancet Respir Med. 2019;7:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosas-Salazar C, Shilts MH, Tovchigrechko A, et al. Nasopharyngeal microbiome in respiratory syncytial virus resembles profile associated with increased childhood asthma risk. Am J Respir Crit Care Med. 2016;193:1180–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ederveen THA, Ferwerda G, Ahout IM, et al. Haemophilus is overrepresented in the nasopharynx of infants hospitalized with RSV infection and associated with increased viral load and enhanced mucosal CXCL8 responses. Microbiome. 2018;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Steenhuijsen Piters WAA, Heinonen S, Hasrat R, et al. Nasopharyngeal microbiota, host transcriptome, and disease severity in children with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2016;194:1104–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn M, Dooley J. The microbiome of the nasopharynx. J Med Microbiol. 2021;70:001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raita Y, Toivonen L, Schuez-Havupalo L, et al. Maturation of nasal microbiota and antibiotic exposures during early childhood: a population-based cohort study. Clin Microbiol Infect. 2021;27:283-e1. [DOI] [PubMed] [Google Scholar]
- 16.Hament JM, Aerts PC, Fleer A, et al. Enhanced adherence of Streptococcus pneumoniae to human epithelial cells infected with respiratory synctial virus. Pediatr Res. 2004;55:972–978. [DOI] [PubMed] [Google Scholar]
- 17.Hament JM, Aerts PC, Fleer A, et al. Direct binding of respiratory syncytial virus to pneumococci: A phenomenon that enhances both pneumococcal adherence to human epithelial cells and pneumococcal invasiveness in a murine model. Pediatr Res. 2005;58:1198–1203. [DOI] [PubMed] [Google Scholar]
- 18.Sande CJ, Njunge JM, Mwongeli Ngoi J, et al. Airway response to respiratory syncytial virus has incidental antibacterial effects. Nat Commun. 2019;10:2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zar HJ, Nduru P, Stadler JAM, et al. Early-life respiratory syncytial virus lower respiratory tract infection in a South African birth cohort: epidemiology and effect on lung health. Lancet Glob Health. 2020;8:e1316–e1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhoeven D, Xu Q, Pichichero ME. Differential impact of respiratory syncytial virus and parainfluenza virus on the frequency of acute otitis media is explained by lower adaptive and innate immune responses in otitis-prone children. Clin Infect Dis. 2014;59:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Piedra PA, Avadhanula V, et al. Duplex real-time RT-PCR assay for detection and subgroup-specific identification of human respiratory syncytial virus. J Virol Methods. 2019;271:113676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faits T, Odom-Mabey AR, Castro-Nallar E, et al. Metagenomic profiling pipelines improve taxonomic classification for 16S amplicon sequencing data. bioRxiv. 2022. Available at: http://biorxiv.org/content/early/2022/07/29/2022.07.27.501757. preprint. doi:10.1101/2022.07.27.501757. [Internet]. [DOI] [PMC free article] [PubMed]
- 23.Byrd AL, Perez-Rogers JF, Manimaran S, et al. Clinical pathoscope: rapid alignment and filtration for accurate pathogen identification in clinical samples using unassembled sequencing data. BMC Bioinf. 2014;15:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong C, Manimaran S, Shen Y, et al. PathoScope 2.0: a complete computational framework for strain identification in environmental or clinical sequencing samples. Microbiome. 2014;2:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajaram S, Oono Y. NeatMap - non-clustering heat map alternatives in Rs. BMC Bioinformatic. 2010;11:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurdie PJ, Holmes S. Phyloseq: an r package for reproducible interactive analysis and graphics of microbiome census data. PLoS One. 2013;8:e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollander M, Wolfe DA, Chicken E. Nonparametric statistical methods. Nonparametric Statistical Methods. 2015. [Google Scholar]
- 28.Bauer DF. Constructing confidence sets using rank statistics. J Am Stat Assoc. 1972;67:687–690. [Google Scholar]
- 29.Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2021:201. [Google Scholar]
- 30.Shaffer JP. Multiple hypothesis testing. Annu Rev Psychol. 1995;46:561–584. [Google Scholar]
- 31.Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27:379–423. [Google Scholar]
- 32.Simpson EH. Measurement of diversity [16]. Nature. 1949;163:688–688. [Google Scholar]
- 33.Royston P. Remark AS R94: a remark on algorithm AS 181: The W-test for normality. Appl Stat. 1995;44:547–551. [Google Scholar]
- 34.Royston JP. Algorithm AS 181: the W test for normality. Appl Stat. 1982;31:176–180. [Google Scholar]
- 35.Royston JP. An extension of Shapiro and Wilk’s W test for normality to large samples. Appl Stat. 1982;31:115–124. [Google Scholar]
- 36.Bray JR, Curtis JT. An Ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr. 1957;27:325–349. [Google Scholar]
- 37.Minchin PR. An evaluation of the relative robustness of techniques for ecological ordination. Vegetatio. 1987;69:89–107. [Google Scholar]
- 38.Faith DP, Minchin PR, Belbin L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio. 1987;69:57–68. [Google Scholar]
- 39.Jones MC, Aitchison J. The statistical analysis of compositional data. J R Stat Soc Ser A. 1987;150:139–160. [Google Scholar]
- 40.McArdle BH, Anderson MJ. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology. 2001;82:290–297. [Google Scholar]
- 41.Fernandes AD, Macklaim JM, Linn TG, et al. ANOVA-Like differential expression (ALDEx) analysis for mixed population RNA-Seq. PLoS One. 2013;8:e67019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandes AD, Reid JN, Macklaim JM, et al. Unifying the analysis of high-throughput sequencing datasets: characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome. 2014;2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gloor GB, Macklaim JM, Fernandes AD. Displaying variation in large datasets: plotting a visual summary of effect sizes. J Comput Graph Stat. 2016;25:971–979. [Google Scholar]
- 44.Douglas GM, Maffei VJ, Zaneveld JR, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol. 2020;38:685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Odom-Mabey AR, Gill CJ, Pieciak R, et al. Characterization of longitudinal nasopharyngeal microbiome patterns in maternally HIV-exposed Zambian infants. Gates Open Res. 2022;6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bender JM, Li F, Martelly S, et al. Maternal HIV infection influences the microbiome of HIV-uninfected infants. Sci Transl Med. 2016;8:349ra100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.