Background and objective
Acute variceal bleeding (AVB) is a serious life-threatening complication of cirrhosis. This study aimed to validate the predictive value of Chronic Liver Failure-Consortium Acute Decompensation score (CLIF-C ADs) in the risk stratification of cirrhotic patients hospitalized with AVB.
Methods
A total of 235 cirrhotic patients with AVB and without acute-on-chronic liver failure (ACLF) were retrospectively enrolled. The discrimination, calibration, overall performance and clinical utility of CLIF-C AD were evaluated and compared with traditional prognostic scores.
Results
The area under the receiver operating characteristics curve of CLIF-C AD was significantly or numerically higher than that of Child-Turcotte-Pugh (CTP) (0.871 vs. 0.737, P = 0.03), Model for End-stage Liver Disease (MELD) (0.871 vs. 0.757, P = 0.1) and MELD-Sodium (MELD-Na) (0.871 vs. 0.822, P = 0.45). The calibration of CLIF-C AD was excellent and superior to that of CTP, MELD and MELD-Na. The brier score/R2 value for CLIF-C AD, CTP, MELD and MELD-Na were 0.045/0.278, 0.051/0.090, 0.050/0.123 and 0.046/0.207, respectively, suggesting a superior overall performance of CLIF-C AD to traditional scores. In decision curve analysis, the standardized net benefit of CLIF-C AD was higher to that of traditional scores. Patients with CLIF-C ADs ≤48, 49–59 and ≥60 were, respectively, stratified into low, moderate and high-risk groups (6-week mortality: 2.7% vs. 12.5% vs. 37.5%, P < 0.001).
Conclusion
The prediction performance and clinical utility of CLIF-C AD for 6-week mortality in cirrhotic patients with AVB and without ACLF are excellent and superior to traditional prognostic scores. The new risk stratification with CLIF-C ADs may be useful in guiding rational management of AVB.
Keywords: acute variceal bleeding, cirrhosis, Chronic Liver Failure-Consortium Acute Decompensation score, prognosis, risk stratification
Introduction
Acute variceal bleeding (AVB) is one of the most common and serious complications of cirrhosis. Despite recent improvements in therapy (medication, endoscopy, intervention), up to 10–15% of patients still have persistent bleeding or early rebleeding and the 6-week mortality at each episode of AVB is still approximately 15–25% [1–3]. Accurate and timely risk stratification can guide the rational management of AVB, such as providing intensive care or taking more effective treatments (e.g., preemptive transjugular intrahepatic portosystemic shunt, p-TIPS) for those with high risk of death [4–6]. Therefore, to improve the prognosis of this entity, multiple international consensuses recommended making a risk stratification in AVB patients [7–9].
So far, numerous studies have been carried out to identify the predictors of poor outcomes in cirrhotic patients with AVB, including the severity of cirrhosis, shock at admission, hepatic venous pressure gradient >20 mmHg, the concurrence of hepatic encephalopathy or hepatocellular carcinoma (HCC), renal failure and bacterial infection, etc [10–14]. Some prognostic scores, such as Child-Turcotte-Pugh (CTP) [15], Model for End-stage Liver Disease (MELD) [16] and MELD-Sodium (MELD-Na) [17] have also been proposed for predicting the prognosis of this entity. However, there are some limitations with these scores, such as subjective factors in CTP (hepatic encephalopathy and ascites), and underestimate of mortality in MELD [18] and MELD-Na [19], which reduced their predictive power in clinical practice.
AVB can not only lead to hemorrhagic shock but also acute-on-chronic liver failure (ACLF), a syndrome characterized by acute decompensation (i.e., ascites, hepatic encephalopathy, AVB and bacterial infection) of cirrhosis, multiple organ failures and high short-term mortality, which was first defined by European Association for the Study of the Liver-Chronic Liver Failure (EASL-CLIF) Consortium in the CANONIC study [20]. In this study, the 28-day mortality in patients with mere AD (acute decompensation) was much lower than that in those with ACLF (4.7% vs. 33.9%). However, the 90-day mortality increased to 12.6%, suggesting that some AD patients are also at high risk of short-term mortality. To specifically prognosticate the survival of AD patients, EASL-CLIF Consortium developed a prognostic score, Chronic Liver Failure-Consortium Acute Decompensation (CLIF-C AD) [21], in a large cohort of AD patients (1016 cases) from the CANONIC study. So far, the prediction performance of CLIF-C AD in cirrhotic patients complicated with AVB has never been exclusively investigated, even though Lv et al. [22] validated the prediction performance of CLIF-C AD in the prognosis of AVB patients with CTP grade B.
In this study, we aimed to (1) validate the prediction performance of CLIF-C AD in the prognosis of AD patients hospitalized with AVB and (2) compare the prediction performance of CLIF-C AD with traditional prognostic scores (CTP, MELD and MELD-Na).
Patients and methods
Patients
Consecutive cirrhotic patients with AVB treated in the Department of Gastroenterology of the second hospital of Hebei Medical University between 1 March 2021 and 1 March 2022 were retrospectively included in this study. Diagnosis of cirrhosis was based on previous liver biopsy or a combination of clinical, laboratory, endoscopic and imaging evidence. Diagnosis of AVB was based on endoscopy (Gastroesophageal varices-related bleeding). Exclusion criteria included the following: (1) endoscopy refused or intolerant; (2) endoscopy-confirmed non-variceal bleeding; (3) HCC out of Milan criterion; (4) incomplete medical records and (5) ACLF. ACLF at admission was retrospectively defined by EASL-CLIF Consortium [20] and diagnosed/graded according to chronic liver failure-organ failure (CLIF-OF) score [23]. This study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the second hospital of Hebei Medical University.
Data collection
Demographic characteristics, clinical features, laboratory parameters, imaging data, endoscopic findings/treatments, medication information and outcomes of each patient were obtained through the electronic medical record system. All baseline laboratory tests were performed within 24 h after admission. Ascites were detected using ultrasound and graded according to the definition of the International Ascites Club [24]. Hepatic encephalopathy was defined and graded according to the West Haven Criteria [25]. Bacterial infections were defined according to international guidelines [26,27]. CLIF-C AD score was calculated as: 10 × [0.03 × Age (years) + 0.66 × Ln (Creatinine{mg/dL}) + 1.71 × Ln (INR) + 0.88 × Ln (WBC {109 cells/L}) − 0.05 × Sodium (mmol/L) + 8] [21]. Scores for CTP [15], MELD [16] and MELD-Na [17] were calculated as described previously.
Treatments
After admission, patients were immediately treated with a combination of fluid resuscitation, vasoactive drugs (somatostatin 250–500 μg/h or octreotide 50–100 μg/h) and antibiotic prophylaxis (ceftriaxone 1 g/days or levofloxacin 500 mg/days for 5–7 days). Endoscopy was performed within 12 h of admission and once the patient is hemodynamically stable (without prior use of prokinetics). For simple esophageal varices or gastroesophageal varices type 1 (GOV1)-related bleeding, endoscopic variceal ligation (EVL) or endoscopic injection sclerotherapy (EIS) (lauromacrogol, if EVL was technically infeasible) was applied if necessary. For gastroesophageal varices type 2 (GOV2) or isolated gastric varices type 1 (IGV1)-related bleeding, gastric variceal obturation (GVO, ɑ-N-butyl cyanoacrylate), intensive EVL or a combination of both was applied if necessary. Patients receiving EVL were subsequently treated with proton pump inhibitor (omeprazole, 40 mg twice daily). Vasoactive drugs were continued for up to 5 days after the endoscopic intervention. Rescue TIPS or balloon retrograde transvenous occlusion (BRTO) was applied if medication plus endoscopic intervention failed. On day 6, continuous non-selective beta-blockers (propranolol 10 mg 2–3/d or carvedilol 6.25 mg 1–2/day) combined with EVL at 2–4 week intervals were initiated for prophylaxis of variceal rebleeding. Further variceal ligation sessions were conducted if rebleeding occurred.
Outcomes
The primary endpoint of this study was 6-week mortality according to Baveno VII Consensus Workshop [3]. The secondary endpoint was a composite outcome of death and rebleeding within 6 weeks, whichever occurred first. Rebleeding was defined as further bleeding from day 5 to day 42. Follow-up began on the day of admission and ended 42 days later or until death through an electronic medical record system or reserved phone number.
Statistical analysis
Continuous variables with normal or skew distribution were described as means (±SD) and medians (interquartile range), respectively. Categorical variables were described as numbers (percentage). Univariable and multivariable logistic regression analyses were performed to identify the risk factors for 6-week mortality in enrolled patients. Variables with P values <0.05 in univariable analysis were considered for multivariable analysis with backward stepwise method. To evaluate the performance of prognostic scores in predicting 6-week mortality, discrimination, calibration and overall performance of each score were studied. Discrimination was evaluated by area under the receiver operating characteristics curve (AUROC) and compared by the Delong test. Calibration was evaluated by the P value in Hosmer–Lemeshow goodness-of-fit test and the concordance between observed and predicted 6-week mortality in the calibration curve. Overall performance was assessed by Brier score and R2 value. A lower Brier score or higher R2 value indicates a better overall performance. Clinical utility was evaluated by the standardized net benefit in decision curve analysis (DCA) [28]. Cumulative survival curve was plotted by Kaplan–Meier method and cumulative survival rates were compared by log-rank test. Statistical analysis was performed with SPSS version 22.0, MedCalc version 19.0.4 and R version 4.2.0. A two-tailed P value <0.05 was considered to be statistically significant.
Results
Baseline characteristics
A total of 287 consecutive patients with cirrhosis and acute upper gastrointestinal bleeding (AUGIB) were screened, and 52 patients were excluded for the following reasons: endoscopy refused or intolerant (n = 6), nonvariceal AUGIB (n = 2), HCC out of Milan criteria (n = 11), incomplete medical records (n = 7) and ACLF (n = 26). Finally, 235 patients with cirrhosis and AVB who met the inclusion and exclusion criteria were included in the final analysis (Fig. 1). Their baseline characteristics and outcomes are shown in Table 1. Patients were predominantly male (61.7%), with a mean age of 56 years. The main etiology of cirrhosis was hepatitis B virus infection (HBV, 47.3%), followed by alcohol abuse (12.8%) and primary sclerosing cholangitis (11.0%). A total of 65.5%, 8.1%, 17.9% and 43.4% of patients had ascites, hepatic encephalopathy, bacterial infection and previous variceal bleeding at admission, respectively. One hundred twenty-one (51.4%) and 37 (15.7%) patients were categorized as CTP grades A, B (active bleeding: 15) and C (CTP < 14: 35), respectively. With regard to the source of bleeding, a total of 63 (26.8%), 18 (7.7%), 128 (54.5%), 2 (0.8%) and 24 (10.2%) of patients had esophageal varices, GOV1, GOV2, GOV1+GOV2 and IGV1, respectively. To control bleeding [active bleeding 62 (26.4%)], 195 (83.0%), 39 (16.6%) and 1 (0.4%) patients received endoscopic treatments (EVL, EIS or GVO), rescue TIPS and BRTO, respectively. Twenty-one (8.9%) and 179 patients (76.2%) received propranolol/carvedilol for primary and secondary prophylaxis of bleeding, respectively. 56 (23.8%) patients did not receive non selective beta blockers because of early death (n = 1), rescue TIPS (n = 39), non-compliance (n = 5) and unknown reasons (n = 11). A total of 13 (5.5%) and 36 patients (15.3%) patients died and met the composite outcome of death or rebleeding within 6 weeks, respectively. Causes of death are summarized in Table 1.
Fig. 1.
Flowchart of the study. AD, acute decompensation; ACLF, acute-on-chronic liver failure; AUGIB, acute upper gastrointestinal bleeding; AVB, acute variceal bleeding; HCC, hepatocellular carcinoma.
Table 1.
Baseline characteristics and outcomes of enrolled patients (N = 235)
| Baseline characteristics and outcome | Values |
|---|---|
| Age (years) | 56 ± 12 |
| Male, n (%) | 145 (61.7) |
| Etiology of cirrhosis, n (%) | |
| Hepatitis B virus | 111 (47.3) |
| Hepatitis C virus | 18 (7.6) |
| Alcohol | 30 (12.8) |
| Primary sclerosing cholangitis | 26 (11.0) |
| Others | 50 (21.3) |
| Decompensation at admission, n (%) | |
| Ascites | 154 (65.5) |
| Mild | 81 (34.4) |
| Moderate | 50 (21.2) |
| Massive | 23 (9.7) |
| Hepatic encephalopathy | 19 (8.1) |
| I–II | 8 (3.4) |
| III–IV | 11 (4.7) |
| Bacterial infection | 42 (17.9) |
| Respiratory infection | 19 (8.1) |
| Urinary tract infection | 6 (2.6) |
| Spontaneous peritonitis | 5 (2.1) |
| Others | 12 (5.1) |
| HCC within Milan criterion, n (%) | 20 (8.5) |
| Mean arterial pressure (mm Hg) | 85 ± 12 |
| Laboratory tests | |
| White blood cell (109/L) | 3.80 (2.54–6.37) |
| Hemoglobin (g/L) | 78.0 (69.0–91.0) |
| Platelet count (109/L) | 73.0 (49.0–105.0) |
| Bilirubin (mg/dL) | 18.9 (12.8–27.8) |
| Albumin (g/L) | 32.0 ± 5.3 |
| Alanine aminotransferase (U/L) | 19.1 (14.0–29.3) |
| Aspartate aminotransferase (U/L) | 26.8 (20.0–42.9) |
| International normalized ratio | 1.27 (1.16–1.41) |
| Prothrombin time (s) | 14.4 (13.3–16.1) |
| Serum creatinine (mg/dL) | 68.0 (58.0–79.0) |
| Serum sodium (mmol/L) | 139.1 (137.0–140.9) |
| Serum potassium (mmol/L) | 4.0 ± 0.5 |
| Previous variceal bleeding, n (%) | 102 (43.4) |
| Source of bleeding, n (%) | |
| EV | 63 (26.8) |
| GOV1 | 18 (7.7) |
| GOV2 | 128 (54.5) |
| GOV1+GOV2 | 2 (0.8) |
| IGV1 | 24 (10.2) |
| Size of EV (≥5 mm), n (%) | 183 (77.9) |
| Size of IGV1 (>10 mm), n (%) | 45 (19.1) |
| Acute bleeding at endoscopy, n (%) | 62 (26.4) |
| Number of band ligation | 12.6 ± 6.1 |
| Volume of sclerosing agent (mL) | 23.7 ± 6.9 |
| Volume of tissue glue (mL) | 2.9 ± 1.8 |
| Vasoactive drug therapy, n (%) | |
| Somatostatin | 189 (80.4) |
| Octreotide | 46 (19.6) |
| Prophylactic antibiotic therapy, n (%) | |
| Cefatriaxone | 180 (76.6) |
| Levofloxacin | 21 (8.9) |
| None | 34 (14.5) |
| Use of NSBB, n (%) | |
| Primary prophylaxis of bleeding | 21 (8.9) |
| Secondary prophylaxis of bleeding | 179 (76.2) |
| Prognostic scores at enrolment | |
| CLIF-C AD | 42 ± 8 |
| CTP | 7 (6–9) |
| MELD | 10 (9–12) |
| MELD-Na | 11 (9–13) |
| CTP class, n (%) | |
| A | 77 (32.7) |
| B | 121 (51.4) |
| C | 37 (15.7) |
| 6-week rebleeding, n (%) | 28 (11.9) |
| 6-week mortality, n (%) | 13 (5.5) |
| Cause of death, n (%) | |
| Hemorrhagic shock | 5 (2.1) |
| Multiple organ failure | 5 (2.1) |
| Liver failure | 2 (0.9) |
| Septic shock | 1 (0.4) |
Data were described as means (±SD), median (interquartile range) or number (percentage) of patients where appropriate.
CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation; EV, esophageal varices; GOV, gastroesophageal varices; HCC, hepatocellular carcinoma; IGV, isolated gastric varices; NSBB, non-selective beta blocker.
Distribution of Chronic Liver Failure-Consortium Acute Decompensation score
The mean value of CLIF-C AD score in enrolled 235 patients were 42 (range 16–69, SD = 8) (Fig. 2). The numbers (percent) of patients in the groups of low, moderate and high risk according to the original risk level in the CLIF-C ADs study [20] (low-risk level: CLIF-C ADs ≤45, moderate risk level: 45< CLIF-C ADs <60 and high-risk level: CLIF-C ADs ≥60) were 159 (67.6), 68 (28.9) and 8 (3.5), respectively.
Fig. 2.
Distribution of CLIF-C AD score in the 235 enrolled cirrhotic patients hospitalized with AVB. AVB, acute variceal bleeding; CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation.
Independent risk factors associated with the 6-week mortality in cirrhotic patients with acute variceal bleeding
In univariable logistic regression analysis, variables found statistically significant include age, white blood cell, total bilirubin, aspartate aminotransferase, serum creatinine, Serum potassium, serum sodium, HCC within Milan criteria, rebleeding and bacterial infection. In addition, all the prognostic scores were found statistically significant. Multivariable analysis showed that only age, serum sodium, and bacterial infection remained in the final model (Table 2).
Table 2.
Factors associated with 6-week mortality in cirrhotic patients with acute variceal bleeding
| Risk factors | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.086 (1.026–1.150) | 0.005 | 1.071 (1.005–1.141) | 0.035 |
| Female | 0.466 (0.125–1.739) | 0.256 | ||
| Etiology of cirrhosis (virus) | 1.063 (0.681–1.660) | 0.787 | ||
| HCC within Milan criterion | 8.625 (2.511–29.631) | 0.001 | 3.105 (1.005–1.141) | 0.244 |
| Decompensation at admission | ||||
| Ascites | 6.761 (0.863–52.954) | 0.069 | ||
| Hepatic encephalopathy | 2.701 (0.545–13.391) | 0.224 | ||
| Bacterial infection | 5.102 (1.601–16.261) | 0.006 | 2.360 (1.048–5.315) | 0.038 |
| Rebleeding | 3.659 (0.098–17.915) | 0.006 | 3.659 (0.098–17.915) | 0.098 |
| Laboratory tests | ||||
| White blood cell | 1.150 (1.111–1.280) | 0.032 | 1.009 (0.803–1.269) | 0.937 |
| Hemoglobin | 0.984 (0.954–1.016) | 0.323 | ||
| Platelet | 1.000 (0.991–1.010) | 0.919 | ||
| Albumin | 0.926 (0.825–1.038) | 0.185 | ||
| Total bilirubin | 1.016 (1.000–1.031) | 0.044 | 1.003 (0.979–1.028) | 0.810 |
| Alanine aminotransferase | 1.006 (0.998–1.016) | 0.156 | ||
| Aspartate aminotransferase | 1.015 (1.002–1.028) | 0.024 | 1.013 (0.995–1.031) | 0.148 |
| International normalized ratio | 4.542 (0.697–29.612) | 0.114 | ||
| Prothrombin time | 1.143 (0.975–1.341) | 0.099 | ||
| Serum creatinine | 1.031 (1.010–1.053) | 0.004 | 1.021 (0.998–1.046) | 0.079 |
| Serum sodium | 0.773 (0.669–0.892) | <0.001 | 0.817 (0.684–0.976) | 0.026 |
| Serum potassium | 4.906 (1.618–14.879) | 0.005 | 0.763 (0.140–4.160) | 0.754 |
| Prognosis scores | ||||
| CLIF-C AD | 1.192 (1.100–1.290) | <0.001 | ||
| CTP | 1.426 (1.113–1.827) | 0.005 | ||
| MELD | 1.298 (1.111–1.517) | 0.001 | ||
| MELD-Na | 1.246 (1.122–1.384) | <0.001 | ||
Bold values indicate statistical significance.
CI, confidence interval; CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation; CTP, Child-Turcotte-Pugh; HCC, hepatocellular carcinoma; MELD, Model for End-stage Liver Disease; MELD-Na, MELD-Sodium; OR, odds ratio.
Predictive performance of prognostic scores for 6-week mortality
Discrimination
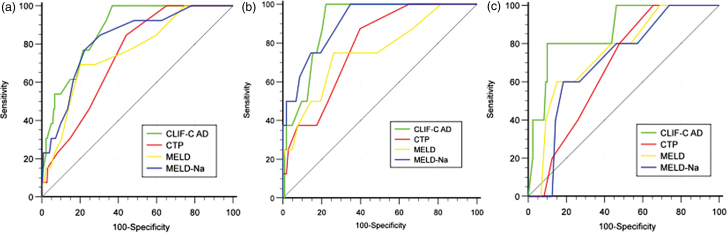
In all enrolled patients, the AUROC of CLIF-C AD was significantly or numerically higher than that of CTP (0.871 vs. 0.737, P = 0.03), MELD (0.871 vs. 0.757, P = 0.10) and MELD-Na (0.871 vs. 0.822, P = 0.45) (Fig. 3a). The cutoff value, Youden index, sensitivity, specificity, positive predictive value and negative predictive value for CLIF-C AD, CTP, MELD and MELD-Na were 43, 0.63, 100.0%, 63.1%, 13.7%, 100.0%; 7, 0.40, 84.6%, 55.9%, 10.1%, 98.4%; 0.49, 12, 69.2%, 79.7%, 16.7%, 97.8% and 0.54, 12, 76.9%, 77.5%, 16.7%, 98.3%, respectively (Table 3). In HBV patients, the AUROC of CLIF-C AD was significantly or numerically higher than that of CTP (0.900 vs. 0.781, P = 0.04) and MELD (0.900 vs. 0.755, P = 0.08) but numerically lower than that of MELD-Na (0.900 vs. 0.905, P = 0.87) (Fig. 3b). The cutoff value, Youden index, sensitivity, specificity, positive predictive value and negative predictive value for CLIF-C AD, CTP, MELD and MELD-Na were 44, 0.78, 100.0%, 77.7%, 25.8%, 100.0%; 7, 0.48, 87.5%, 60.2%, 14.6%, 98.4%; 12, 0.49, 75.0%, 73.8%, 18.2%, 97.4%; 11, 0.65, 100.0%, 65.1%, 18.2%, 100.0%. In non-HBV patients, the AUROC of CLIF-C AD was numerically higher than that of CTP (0.865 vs. 0.679, P = 0.20), MELD (0.865 vs. 0.753, P = 0.46) and MELD-Na (0.865 vs. 0.709, P = 0.46) (Fig. 3c). The cutoff value, Youden index, sensitivity, specificity, positive predictive value and negative predictive value for CLIF-C AD, CTP, MELD and MELD-Na were 52, 0.70, 80.0%, 89.9%, 25.0%, 99.1%; 6, 0.34, 100.0%, 34.5%, 6.0%, 100.0%; 12, 0.45, 60.0%, 84.9%, 14.3%, 98.1%; 12, 0.42, 60.0%, 81.5%, 12.0%, 98.0% (Table 3).
Fig. 3.
Discrimination of CTP, MELD, MELD-Na and CLIF-C AD for 6-week mortality in all enrolled patients (a), HBV patients (b) and non-HBV patients (c). CTP, Child-Turcotte-Pugh; CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation; HBV, hepatic B virus; MELD, Model for End-stage Liver Disease; MELD-Na, MELD-Sodium.
Table 3.
Predictive values of prognostic scores for 6-week mortality
| Scores | AUC | Youden index | Cutoff value | SEN (%) | SPE (%) | PPV (%) | NPV (%) | Brier | R2 | P in H-L test |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients | ||||||||||
| CLIF-C AD | 0.871 | 0.63 | 43 | 100.0 | 63.1 | 13.7 | 100.0 | 0.045 | 0.278 | 0.910 |
| CTP | 0.737 | 0.40 | 7 | 84.6 | 55.9 | 10.1 | 98.4 | 0.051 | 0.090 | 0.223 |
| MELD | 0.757 | 0.49 | 12 | 69.2 | 79.7 | 16.7 | 97.8 | 0.050 | 0.123 | 0.327 |
| MELD-Na | 0.822 | 0.54 | 12 | 76.9 | 77.5 | 16.7 | 98.3 | 0.046 | 0.207 | 0.269 |
| HBV patients | ||||||||||
| CLIF-C AD | 0.900 | 0.78 | 44 | 100.0 | 77.7 | 25.8 | 100.0 | 0.056 | 0.321 | 0.314 |
| CTP | 0.781 | 0.48 | 7 | 87.5 | 60.2 | 14.6 | 98.4 | 0.061 | 0.162 | 0.358 |
| MELD | 0.755 | 0.49 | 12 | 75.0 | 73.8 | 18.2 | 97.4 | 0.057 | 0.192 | 0.733 |
| MELD-Na | 0.905 | 0.65 | 11 | 100.0 | 65.1 | 18.2 | 100.0 | 0.041 | 0.470 | 0.798 |
| Non-HBV patients | ||||||||||
| CLIF-C AD | 0.865 | 0.70 | 52 | 80.0 | 89.9 | 25.0 | 99.1 | 0.034 | 0.280 | 0.804 |
| CTP | 0.679 | 0.34 | 6 | 100.0 | 34.5 | 6.0 | 100.0 | 0.039 | 0.023 | 0.742 |
| MELD | 0.753 | 0.45 | 12 | 60.0 | 84.9 | 14.3 | 98.1 | 0.039 | 0.047 | 0.108 |
| MELD-Na | 0.709 | 0.42 | 12 | 60.0 | 81.5 | 12.0 | 98.0 | 0.039 | 0.013 | 0.028 |
Bold values indicate statistical significance.
AUC, area under receiver operating characteristic curve; CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation; CTP, Child-Turcotte-Pugh; H-L test, Hosmer–Lemeshow goodness-of-fit test; MELD, Model for End-stage Liver Disease; MELD-Na, MELD-Sodium; NPV, negative predictive value; PPV, positive predictive value; SEN, sensitivity; SPE, specificity.
Calibration
In all enrolled patients, the P value in Hosmer–Lemeshow test for CLIF-C AD, CTP, MELD and MELD-Na were 0.910, 0.223, 0.327 and 0.269, respectively; the concordance between observed and predicted 6-week mortality in calibration curve for CLIF-C AD was excellent and superior to that for CTP, MELD and MELD-Na (Fig. 4a). In HBV patients, the P value in Hosmer–Lemeshow test for CLIF-C AD, CTP, MELD and MELD-Na were 0.314, 0.358, 0.733 and 0.798, respectively; the concordance between observed and predicted 6-week mortality in calibration curve for CLIF-C AD was satisfactory and similar to that for CTP and MELD but somewhat inferior to that for MELD-Na (Fig. 4b). In non-HBV patients, the P value in Hosmer–Lemeshow test for CLIF-C AD, CTP, MELD and MELD-Na were 0.804, 0.742, 0.108 and 0.028, respectively; the concordance between observed and predicted 6-week mortality in calibration curve for CLIF-C AD was satisfactory and superior to that for CTP, MELD and MELD-Na (Fig. 4c).
Fig. 4.
Calibration of CLIF-C AD, CTP, MELD and MELD-Na for 6-week mortality in all enrolled patients (a), HBV patients (b) and non-HBV patients (c). CTP, Child-Turcotte-Pugh; CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation score; HBV, hepatic B virus; MELD, Model for End-stage Liver Disease; MELD-Na, MELD-Sodium.
Overall performance
In all enrolled patients, the brier score/R2 value for CLIF-C AD, CTP, MELD and MELD-Na were 0.045/0.278, 0.051/0.090, 0.050/0.123 and 0.046/0.207, respectively, suggesting a superior overall performance of CLIF-C AD to traditional prognostic scores. In HBV patients, the brier score/R2 value for CLIF-C AD, CTP, MELD and MELD-Na were 0.056/0.321, 0.061/0.162, 0.057/0.192 and 0.041/0.470, respectively, suggesting a superior overall performance of MELD-Na to the other prognostic scores. In non-HBV patients, the brier score/R2 value for CLIF-C AD, CTP, MELD and MELD-Na was 0.034/0.280, 0.039/0.023, 0.039/0.047 and 0.039/0.013, respectively, suggesting a superior overall performance of CLIF-C AD to traditional prognostic scores (Table 3).
Decision curves analysis
In all enrolled patients, the standardized net benefit of CLIF-C AD was higher than that of CTP, MELD and MELD-Na in almost the entire range of threshold probabilities, indicating a superior clinical utility of CLIF-C AD to that of traditional prognostic scores (Fig. 5a). In HBV patients, the standardized net benefit of CLIF-C AD was lower than that of MELD-Na but higher than that of CTP and MELD (Fig. 5b). In non-HBV patients, the standardized net benefit of CLIF-C AD was higher than that of CTP, MELD and MELD-Na in almost the entire range of threshold probabilities (Fig. 5c) (Table 4).
Fig. 5.
Decision curves analysis for prognostic scores in all enrolled patients (a), HBV patients (b) and non-HBV patients (c). HBV, hepatic B virus.
Table 4.
Predictive values of prognostic scores for the composite outcome
| Scores | AUC | Youden index | Cutoff value | SEN (%) | SPE (%) | PPV (%) | NPV (%) | Brier | R2 | P in H-L test |
|---|---|---|---|---|---|---|---|---|---|---|
| All patients | ||||||||||
| CLIF-C AD | 0.741 | 0.63 | 43 | 100.0 | 63.1 | 13.7 | 100.0 | 0.119 | 0.141 | 0.395 |
| CTP | 0.730 | 0.40 | 7 | 84.6 | 55.9 | 10.1 | 98.4 | 0.121 | 0.126 | 0.064 |
| MELD | 0.681 | 0.49 | 12 | 69.2 | 79.7 | 16.7 | 97.8 | 0.050 | 0.123 | 0.237 |
| MELD-Na | 0.727 | 0.54 | 12 | 76.9 | 77.5 | 16.7 | 98.3 | 0.120 | 0.127 | 0.164 |
| HBV patients | ||||||||||
| CLIF-C AD | 0.721 | 0.40 | 44 | 61.1 | 78.5 | 35.5 | 91.2 | 0.121 | 0.149 | 0.912 |
| CTP | 0.724 | 0.32 | 6 | 94.4 | 37.6 | 22.7 | 97.2 | 0.122 | 0.152 | 0.619 |
| MELD | 0.688 | 0.37 | 12 | 61.1 | 76.3 | 33.3 | 91.0 | 0.122 | 0.094 | 0.433 |
| MELD-Na | 0.781 | 0.46 | 11 | 77.8 | 67.7 | 31.8 | 94.0 | 0.105 | 0.290 | 0.655 |
| Non-HBV patients | ||||||||||
| CLIF-C AD | 0.751 | 0.51 | 42 | 94.4 | 56.6 | 27.0 | 98.4 | 0.117 | 0.144 | 0.371 |
| CTP | 0.743 | 0.40 | 8 | 61.1 | 79.3 | 33.3 | 92.3 | 0.119 | 0.102 | 0.019 |
| MELD | 0.682 | 0.45 | 8 | 94.4 | 34.0 | 19.5 | 97.3 | 0.122 | 0.049 | 0.198 |
| MELD-Na | 0.684 | 0.29 | 8 | 100 | 29.3 | 19.4 | 100.0 | 0.123 | 0.031 | 0.057 |
AUC, area under receiver operating characteristic curve; CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation; CTP, Child-Turcotte-Pugh; H-L test, Hosmer–Lemeshow goodness-of-fit test; MELD, Model for End-stage Liver Disease; MELD-Na, MELD-Sodium; NPV, negative predictive value; PPV, positive predictive value; SEN, sensitivity; SPE, specificity.
Predictive performance of prognostic scores for secondary endpoint
In all patients, the AUROC of CLIF-C AD (0.741, 0.657–0.825) was numerically higher than that of CTP (0.730, 0.651–0.808, P = 0.84), MELD (0.681,0.588–0.773, P = 0.33) and MELD-Na (0.727, 0.640–0.814, P = 0.81) (Fig. 6a). The P value in Hosmer–Lemeshow test for CLIF-C AD, CTP, MELD and MELD-Na were 0.395, 0.064, 0.237 and 0.164, respectively; the concordance between observed and predicted 6-week composite outcome in calibration curve for CLIF-C AD was satisfactory and comparable to that for MELD but superior to that for CTP and MELD-Na (Fig. 6d). The brier score/R2 value for CLIF-C AD, CTP, MELD and MELD-Na was 0.119/0.141, 0.121/0.126, 0.122/0.094 and 0.120/0.127, respectively, suggesting a superior overall performance of CLIF-C AD to traditional prognostic scores (Table 4). The standardized net benefit of CLIF-C AD was higher than that of CTP, MELD and MELD-Na between the threshold of 0.1 and 0.3 (Fig. 6g). In HBV patients, the AUROC of MELD-Na (0.781, 0.657–0.825) was numerically higher than that of CTP (0.724, 0.631–0.804, P = 0.27), MELD (0.688, 0.593–0.772, P = 0.06) and CLIF-C AD (0.721, 0.628–0.802, P = 0.42) (Fig. 6b). The P value in Hosmer–Lemeshow test for CLIF-C AD, CTP, MELD and MELD-Na were 0.912, 0.619, 0.433 and 0.655, respectively. CLIF-C AD underestimated the composite outcome in the calibration curve. The concordance between observed and predicted composite outcomes for traditional prognostic scores were satisfactory and similar (Fig. 6e). The brier score/R2 value for CLIF-C AD, CTP, MELD and MELD-Na were 0.121/0.149, 0.122/0.152, 0.122/0.094 and 0.105/0.290, respectively, suggesting a superior overall performance of MELD-Na to the other prognostic scores. The standardized net benefit of CLIF-C AD was lower than that of MELD-Na but higher than that of CTP and MELD (Fig. 6h). In non-HBV patients, the AUROC of CLIF-C AD (0.751, 0.665–0.824) was numerically higher than that of CTP (0.743, 0.657–0.817, P = 0.91), MELD (0.682, 0.592–0.762, P = 0.37) and MELD-Na (0.684, 0.595–0.765, P = 0.38) (Fig. 6c). The P value in Hosmer–Lemeshow test for CLIF-C AD, CTP, MELD and MELD-Na were 0.371, 0.019, 0.198 and 0.057, respectively; the concordance between observed and predicted composite outcome in calibration curve for MELD-Na was satisfactory and superior to that for MELD, CTP and CLIF-C AD (Fig. 6f). The brier score/R2 value for CLIF-C AD, CTP, MELD and MELD-Na were 0.117/0.144, 0.119/0.102, 0.122/0.049 and 0.123/0.031, respectively, suggesting a superior overall performance of CLIF-C AD to traditional prognostic scores. The standardized net benefit of CLIF-C AD was comparable to that of CTP but higher than that of MELD and MELD-Na (Fig. 6i).
Fig. 6.
Discrimination (a–c), calibration (d–f) and decision curve analysis (g–i) of prognostic scores for 6-week composite outcome in all patients (a, d, g), HBV patients (b, e, h) and non-HBV patients (c, f, i). HBV, hepatic B virus.
Survival analysis according to Chronic Liver Failure-Consortium Acute Decompensation score
To make a risk stratification in AVB patients according to the CLIF-C AD score, we performed survival analysis on all enrolled patients according to the two optimal cutoff values of CLIF-C AD score (48 and 60) obtained through the X-tile software (version 3.6.1; Yale University, USA). Results showed that 5 (2.7%), 5 (12.5%) and 3 (37.5%) patients with CLIF-C AD score ≤48, 49–59 and ≥60 died within 6 weeks, respectively (P value in log-rank test <0.0001) (Fig. 7). Therefore, patients with CLIF-C AD score ≤48, 48–60 and ≥60 were correspondingly stratified into low, moderate and high death risk groups, respectively.
Fig. 7.
Survival analysis according to CLIF-C AD score in enrolled cirrhotic patients hospitalized with AVB. AVB, acute variceal bleeding; CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation.
Baseline characteristics of patients in different risk groups stratified by Chronic Liver Failure-Consortium Acute Decompensation score
As expected, patients in moderate or high-risk group were older, having higher WBC, INR and serum potassium, lower albumin and serum sodium, and more frequently presented with complications of cirrhosis (ascites, bacterial infection and hepatic encephalopathy) than those in low-risk group. Furthermore, patients in moderate and high-risk groups had significantly worse prognostic scores than those in low-risk group. There were no significant differences among the three groups regarding gender, etiology of cirrhosis, presence of HCC within Milan criteria, rebleeding, mean arterial pressure, hemoglobin, total bilirubin, ALT and AST at admission (Table 5).
Table 5.
Baseline characteristics of patients in different risk groups stratified according to Chronic Liver Failure-Consortium Acute Decompensation score
| Variables | Low-risk group (n = 187) | Moderate-risk group (n = 40) | High-risk group (n = 8) | P value |
|---|---|---|---|---|
| Age | 54.5 ± 11.5c | 60.4 ± 12.9 | 66.6 ± 8.8 | 0.001 |
| Male, n (%) | 114 (61.0) | 25 (62.5) | 6 (75.0) | 0.512 |
| Etiology of cirrhosis | 0.236 | |||
| HBV + HCV | 108 (57.8) | 17 (42.5) | 4 (50) | |
| Alcohol | 22 (11.8) | 8 (20) | 0 (0) | |
| Others | 57 (30.4) | 15 (37.5) | 4 (50) | |
| Ascites, n (%) | 115 (61.5)b,c | 32 (80)a,c | 7 (87.5)a,b | 0.034 |
| Bacterial infection | 25 (13.4) | 13 (32.5)a | 4 (50)a | 0.001 |
| Hepatic encephalopathy | 10 (5.3) | 7 (17.5)a | 2 (25)a | 0.008 |
| Rebleeding | 19 (10.2) | 8 (20) | 1 (12.5) | 0.218 |
| HCC within Milan criterion | 14 (7.5) | 4 (10) | 2 (25) | 0.206 |
| Mean arterial pressure | 85.1 ± 11.8 | 82.1 ± 13.1 | 88.4 ± 11.7 | 0.236 |
| Hemoglobin | 79.0 (69.0–92.8) | 74.5 (68.5–83.5) | 87.5 (74.0–95.0) | 0.316 |
| White blood cell (109/L) | 3.2 (2.3–4.6) | 7.7 (6.2–9.4)a | 11.5 (9.9–18.0)a | <0.001 |
| NLR | 3.57 (2.45–5.52) | 5.17 (3.27–7.93)a | 9.74 (6.95–12.94)a | <0.001 |
| Total bilirubin | 18.5 (13.1–27.0) | 22.0 (12.3–31.6) | 20.4 (14.0–84.0) | 0.371 |
| Albumin | 32.8 ± 5.1 | 29.0 ± 4.7a | 27.6 ± 5.6a | <0.001 |
| ALT | 19.6 (14.0–29.3) | 17.7 (14.4–28.0) | 27.8 (11.8–128.0) | 0.633 |
| AST | 26.6 (19.8–42.4) | 27.0 (20.2–42.1) | 67.3 (22.9–170.3) | 0.179 |
| INR | 1.25 (1.15–1.37)b | 1.33 (1.20–1.67) | 1.35 (1.17–1.68) | 0.016 |
| Serum creatinine | 66.0 (56.0–78.0) | 69.5 (63.5–84.0) | 84.0 (63.5–139.5) | 0.022* |
| Serum sodium | 139.7 (137.5–141.2) | 136.9 (134.2–138.4)a | 134.2 (130.1–135.4)a | <0.001 |
| Serum potassium | 3.9 ± 0.4c | 4.1 ± 0.4c | 4.8 ± 0.7 | <0.001 |
| CTP | 7 (6–8) | 9 (7.5–10.0)a | 8 (8–11)a | <0.001 |
| MELD | 10 (8–12) | 11 (9–15)a | 13.5 (11–18.5)a | <0.001 |
| MELD-Na | 10 (9–12) | 12 (9.5–16.5)a | 16.5 (12–24)a | <0.001 |
| CLIF-C AD | 39.0 ± 5.9b,c | 52.3 ± 3.2a,c | 62.0 ± 3.1a,b | <0.001 |
Data were described as means (±SD), median (interquartile range) or number (percentage) of patients where appropriate and compared by one-way analysis of variance (ANOVA), chi-square test and Kruskal-Wallis one-way ANOVA test, correspondingly. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count.
Bold values indicate statistical significance.
Significantly different from the low-risk group.
Significantly different from the moderate-risk group.
Significantly different from the high-risk group.
*No significant difference after adjustment for P value with Bonferroni method.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CLIF-C AD, Chronic Liver Failure-Consortium Acute Decompensation; CTP, Child-Turcotte-Pugh; HBV, hepatic B virus; HCV, hepatic C virus; HCC, hepatocellular carcinoma; H-L test, Hosmer–Lemeshow goodness-of-fit test; INR, international normalized ratio; MELD, Model for End-stage Liver Disease; MELD-Na, MELD-Sodium; NLR, neutrophil to lymphocyte ratio.
Discussion
AVB is a serious life-threatening complication of cirrhosis and an early and accurate risk stratification was crucial for its rational management. In this single-center and retrospective study, we found that the prediction performance of CLIF-C AD for 6-week mortality in cirrhotic patients hospitalized with AVB and without ACLF was excellent and superior to that of traditional prognostic scores. In addition, we specifically conducted a CLIF-C ADs-based risk stratification for AVB patients. To the best of our knowledge, this was the first study validating the prediction performance of CLIF-C AD in cirrhotic patients complicated with AVB, exclusively.
In this study, the 6-week mortality in enrolled patients was 5.5%, which seems far lower than 15–25% reported in recent literature [1–3]. One of the most important reasons might be that we only included patients without ACLF while previous studies included patients regardless of ACLF. On the other hand, our mortality coincided with most of the studies that stratified AD and ACLF [20,21,29]. In CANONIC [20] and CLIF-C AD study [21], the 28-day/90-day mortality in all AD patients was 4.7%/14% and 4.6%/12.6%, respectively. In a recent Korean study [29], the 28-day and 90-day mortality in AD patients with AVB alone were 3.4% and 5.3%, respectively. In contrast, our mortality was lower than that in the study by Trebicka et al [30] (6-week mortality in AD patients with AVB: 10.0%), which may be due to differences in patient’s conditions between the two studies, that is, Trebicka’s study included nearly one-third patients in the intensive care unit whereas we only included patients in the general ward.
An ideal prognostic score should contain the following assessments: discrimination, calibration and clinical utility, which respectively refer to the ability to stratify patients according to their risk of developing the outcome, concordance between predicted probability and actual outcome [31] and clinical benefit of patients [28]. In this study, CLIF-C AD exhibited superior discrimination to traditional prognostic scores, which might be due to its incorporation of age and serum sodium, totally or partly not included in CTP, MELD and MELD-Na. Advanced age is considered to be related to intolerance to various stresses and more organ dysfunction or failure [14,21,23,32]. Serum sodium was a well-recognized independent risk factor for the poor prognosis of cirrhotic patients [17,33]. Both of them proved to be independent risk factors for 6-week mortality in this study, supporting this explanation. In addition, the calibration of CLIF-C AD was excellent and superior to that of traditional scores, which was consistent with results in CLIF-C AD study and another prospective study [34]. This may be due to the distinct background in where these scores were developed. CLIF-C AD was specially developed in AD patients, whereas CTP and MELD were originally developed in patients (regardless of ACLF) who received surgery and TIPS therapy due to recurrent AVB and various complications of portal hypertension, respectively. Besides, CLIF-C AD was developed in 2015 when the standard care of AVB had been established already, whereas traditional scores were developed a long time ago (CTP in 1973, MELD in 2001, and MELD-Na in 2006) when more effective treatments currently used were not available at that time. Therefore, a superior calibration of CLIF-C AD to traditional scores could be expected. Furthermore, DCA showed that the clinical utility of CLIF-C AD was superior to that of traditional scores.
Since the main etiology of cirrhosis in this study was HBV whereas it was alcohol and HCV in CLIF-C AD study, we separately studied the prediction performance of CLIF-C AD in HBV and non-HBV patients. In HBV patients, the prediction performance of CLIF-C AD was slightly inferior to that of MELD-Na but superior to that of CTP and MELD. In non-HBV patients, the prediction performance of CLIF-C AD was superior to that of all traditional scores. Similarly, the clinical utility of CLIF-C AD was slightly inferior to that of MELD-Na but superior to that of CTP and MELD in HBV patients and superior to that of all traditional scores in non-HBV patients. There might be two reasons for this. First, the number of HBV patients was small (n = 111). Second, the different prognosis between patients with HBV and alcohol-related cirrhosis. Prospective studies with large samples may need to further validate this.
Up to now, there are no ideal prognostic scores for rebleeding, a well-recognized risk factor for 6-week mortality in AVB patients. Therefore, we validated the prediction performance of CLIF-C AD for the composite outcome of death and rebleeding within 6-weeks and found that its prediction accuracy declined but was still satisfactory. It might be explained that CLIF-C AD is a prognostic score aiming at predicting mortality in AD patients rather than rebleeding. The importance is that clinicians can use CLIF-C AD to identify patients with a high risk of mortality or rebleeding for more aggressive treatments such as P-TIPS, which is a well-recognized effective treatment to reduce mortality and rebleeding in AVB patients.
As CLIF-C AD was originally developed in cirrhotic patients with various ADs (without ACLF), the prediction accuracy of its original risk stratification may decline in the setting of AVB. Therefore, we specifically conducted a risk stratification for AVB patients according to CLIF-C AD score, that is, low risk ≤48 (6-week mortality 2.7%), moderate risk 49–59 (6-week mortality 12.5%) and high risk ≥ 60 (6-week mortality 37.5%). Compared with the study by Lv et al. [22] in which the 6-week mortality in CTP grade B of AVB patients with CLIF-C AD scores <48, 48–56 and >56 were 5.6%, 16.8% and 25.4%, our mortality was somewhat lower, which might be due to the inclusion of ACLF patients in that study. After this clear risk stratification by CLIF-C AD score, the low-risk patients can be considered to provide less intense therapy and advised for discharge safely to save medical resources and reduce the economic and psychological burden of patient’s family while the moderate and high-risk patients may need intensive care or emergency salvage treatment (e.g., p-TIPS) [4–6], respectively.
Furthermore, we compared the characteristics of patients in different risk groups according to CLIF-C AD score. Compared with low-risk group, moderate and high-risk groups exhibited a more intensive systemic inflammatory response, as indicated by a significantly higher WBC count and neutrophil-to-lymphocyte ratio level [35]. In addition, moderate or high-risk groups more frequently present complications of cirrhosis (ascites, hepatic encephalopathy and bacterial infections) and have significantly worse prognostic scores than patients in low-risk group. This was similar to findings in the PREDICTing Acute-on-Chronic Liver Failure (PREDICT)study [36] in which three courses of AD were first proposed, that is, stable decompensated cirrhosis (SDC), unstable decompensated cirrhosis (UDC) and pre-ACLF subgroup. Pre-ACLF is predominantly related to the rapid progression of systemic inflammation, ACLF development and an extremely high short-term mortality (53.7% in 90 days). UDC occurs in the context of rapid progression of portal hypertension and is associated with a less severe clinical course and lower short-term mortality (21.0% in 90 days). Both mechanisms progress slowly in SDC and patients follow a relatively benign course with longer survival (90-day mortality: 0%). Thus, we speculate that the low, moderate and high-risk group stratified by CLIF-C AD score in this study might be highly related to the SDC, UDC and Pre-ACLF course, respectively, although no significant difference was found between moderate and high-risk group, which might be due to the small number of patients in the high-risk group (n = 8). This not only proves the importance of risk stratification even in AD patients, but also validates the systemic inflammation hypothesis proposed in the PREDICT study.
Our study has some limitations. First, as this was a single center and observational study, selection, information and confounding biases were inevitable. For instance, our hospital did not routinely carry out p-TIPS due to the comprehensive effect of clinicians’ treatment concepts and patients’ actual condition. Second, due to the retrospective nature of this study, we could not calculate the exact prevalence of ACLF in enrolled patients, which might have some impact on the predictive performance of CLIF-C AD. Nonetheless, since the mortality in our cohort was consistent with that described in most prospective studies [20,21,30], we believe that our results are highly credible. Finally, due to the relatively small sample size and HBV-predominated etiology of cirrhosis, whether the prediction accuracy of risk stratification in AVB patients according to CLIF-C AD score can be applied to patients with different baseline characteristics or etiologies is still unknown, requiring more prospective studies to validate it.
Conclusion
The prediction performance and clinical utility of CLIF-C AD for 6-week mortality in cirrhotic patients hospitalized with AVB and without ACLF are excellent and superior to traditional prognostic scores. The new CLIF-C AD score-based risk stratification may be useful in guiding the rational management of AVB.
Acknowledgements
We gratefully recognize the patients who participated in this study.
This study was supported by grant from Health Care and Biomedicine Special Project Hebei Province Key R&D Program (182777117D).
Z.Z. collected and analyzed the data and wrote the article. H.J. designed the study and revised the article. All authors read and approved the final article.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Reverter E, Tandon P, Augustin S, Turon F, Casu S, Bastiampillai R, et al. A MELD-based model to determine risk of mortality among patients with acute variceal bleeding. Gastroenterology 2014; 146:412–19.e3. [DOI] [PubMed] [Google Scholar]
- 2.Fortune B, Garcia-Tsao G, Ciarleglio M, Deng Y, Fallon MB, Sigal S, et al. Child-Turcotte-Pugh class is best at stratifying risk in variceal hemorrhage: analysis of a U.S. multi-center prospective study. J Clin Gastroenterol 2017; 51:446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Franchis R, Bosch J, Garcia-Tsao G, et al. Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol 2022; 76:959–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, et al. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology 2004; 40:793–801. [DOI] [PubMed] [Google Scholar]
- 5.García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, et al. Early TIPS (Transjugular Intrahepatic Portosystemic Shunt) Cooperative Study Group. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med 2010; 362:2370–2379. [DOI] [PubMed] [Google Scholar]
- 6.Hernández-Gea V, Procopet B, Giráldez A, Amitrano L, Villanueva C, Thabut D, et al. International Variceal Bleeding Observational Study Group and Baveno Cooperation. Preemptive-TIPS improves outcome in high-risk variceal bleeding: an observational study. Hepatology 2019; 69:282–293. [DOI] [PubMed] [Google Scholar]
- 7.de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015; 63:743–752. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017; 65:310–335. [DOI] [PubMed] [Google Scholar]
- 9.Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, et al. Clinical Services and Standards Committee of the British Society of Gastroenterology. U.K. guidelines on the management of variceal hemorrhage in cirrhotic patients. Gut 2015; 64:1680–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augustin S, Muntaner L, Altamirano JT, González A, Saperas E, Dot J, et al. Predicting early mortality after acute variceal hemorrhage based on classification and regression tree analysis. Clin Gastroenterol Hepatol 2009; 7:1347–1354. [DOI] [PubMed] [Google Scholar]
- 11.Ripoll C, Banares R, Rincon D, Catalina MV, Lo Iacono O, Salcedo M, et al. Influence of hepatic venous pressure gradient on the prediction of survival of patients with cirrhosis in the MELD Era. Hepatology 2005; 42:793–801. [DOI] [PubMed] [Google Scholar]
- 12.Abraldes JG, Villanueva C, Bañares R, Aracil C, Catalina MV, Garci A-Pagán JC, et al. Spanish Cooperative Group for Portal Hypertension and Variceal Bleeding. Hepatic venous pressure gradient and prognosis in patients with acute variceal bleeding treated with pharmacologic and endoscopic therapy. J Hepatol 2008; 48:229–236. [DOI] [PubMed] [Google Scholar]
- 13.Rudler M, Bureau C, Carbonell N, Mathurin P, Saliba F, Mallat A, et al. French Club for the Study of Portal Hypertension (CFEHTP). Recalibrated MELD and hepatic encephalopathy are prognostic factors in cirrhotic patients with acute variceal bleeding. Liver Int 2018; 38:469–476. [DOI] [PubMed] [Google Scholar]
- 14.Martínez J, Hernández-Gea V, Rodríguez-de-Santiago E, Téllez L, Procopet B, Giráldez A, et al. International Variceal Bleeding Observational Study Group and Baveno Cooperation. Bacterial infections in patients with acute variceal bleeding in the era of antibiotic prophylaxis. J Hepatol 2021; 75:342–350. [DOI] [PubMed] [Google Scholar]
- 15.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. [DOI] [PubMed] [Google Scholar]
- 16.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology 2001; 33:464–470. [DOI] [PubMed] [Google Scholar]
- 17.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology 2006; 130:1652–1660. [DOI] [PubMed] [Google Scholar]
- 18.Somsouk M, Kornfield R, Vittinghoff E, Inadomi JM, Biggins SW. Moderate ascites identifies patients with low model for end-stage liver disease scores awaiting liver transplantation who have a high mortality risk. Liver Transpl 2011; 17:129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazumder NR, Atiemo K, Daud A, Kho A, Abecassis M, Levitsky J, Ladner DP. Patients with persistently low MELD-Na scores continue to be at risk of liver related death. Transplantation 2020; 104:1413–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreau R, Jalan R, Ginès P, Pavesi M, Angeli P, Cordoba J, et al. CANONIC Study Investigators of the EASL–CLIF Consortium. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144:1426–37, 1437.e1. [DOI] [PubMed] [Google Scholar]
- 21.Jalan R, Pavesi M, Saliba F, Amorós A, Fernandez J, Holland-Fischer P, et al. CANONIC Study Investigators; EASL-CLIF Consortium. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol 2015; 62:831–840. [DOI] [PubMed] [Google Scholar]
- 22.Lv Y, Wang Z, Li K, Wang Q, Bai W, Yuan X, et al. Risk stratification based on chronic liver failure consortium acute decompensation score in patients with child-Pugh B cirrhosis and acute variceal bleeding. Hepatology 2021; 73:1478–1493. [DOI] [PubMed] [Google Scholar]
- 23.Jalan R, Saliba F, Pavesi M, Amoros M, Moreau R, Gines P, et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol 2014; 61:1038–1047. [DOI] [PubMed] [Google Scholar]
- 24.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology 2003; 38:258–266. [DOI] [PubMed] [Google Scholar]
- 25.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the study of liver diseases and the European Association for the study of the Liver. Hepatology 2014; 60:715–735. [DOI] [PubMed] [Google Scholar]
- 26.,Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008; 36:309–332. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez J, Acevedo J, Wiest R, Gustot T, Amoros A, Deulofeu C, et al. European Foundation for the Study of Chronic Liver Failure. Bacterial and fungal infections in acute-on-chronic liver failure: prevalence, characteristics and impact on prognosis. Gut 2018; 67:1870–1880. [DOI] [PubMed] [Google Scholar]
- 28.Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Mak 2006; 26:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin J, Yu JH, Jin YJ, Yim HJ, Jung YK, Yang JM, et al. Acute-on-chronic liver failure as a major predictive factor for mortality in patients with variceal bleeding. Clin Mol Hepatol 2020; 26:540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trebicka J, Gu W, Ibáñez-Samaniego L, Hernández-Gea V, Pitarch C, Garcia E, et al. International Variceal Bleeding Observational Study Group and Baveno Cooperation. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J Hepatol 2020; 73:1082–1091. [DOI] [PubMed] [Google Scholar]
- 31.Kok B, Abraldes JG. Child-Pugh Classification: Time to Abandon? Semin Liver Dis 2019; 39:96–103. [DOI] [PubMed] [Google Scholar]
- 32.Saltzman JR, Tabak YP, Hyett BH, Sun X, Travis AC, Johannes RS. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc 2011; 74:1215–1224. [DOI] [PubMed] [Google Scholar]
- 33.Kim WR, Biggins SW, Kremers WK, Wiesner RH, Kamath PS, Benson JT, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med 2008; 359:1018–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexopoulou A, Vasilieva L, Mani I, Agiasotelli D, Pantelidaki H, Dourakis SP. Single center validation of mortality scores in patients with acute decompensation of cirrhosis with and without acute-on-chronic liver failure. Scand J Gastroenterol 2017; 52:1385–1390. [DOI] [PubMed] [Google Scholar]
- 35.Peng Y, Li Y, He Y, Wei Q, Xie Q, Zhang L, et al. The role of neutrophil to lymphocyte ratio for the assessment of liver fibrosis and cirrhosis: a systematic review. Expert Rev Gastroenterol Hepatol 2018; 12:503–513. [DOI] [PubMed] [Google Scholar]
- 36.Trebicka J, Fernandez J, Papp M, Caraceni P, Laleman W, Gambino C, et al. PREDICT STUDY group of the EASL-CLIF Consortium. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct distinct pathophysiology. J Hepatol 2020; 73:842–854. [DOI] [PubMed] [Google Scholar]