BACKGROUND:
Patients with heart failure (HF) have a high burden of symptoms and physical limitations, regardless of ejection fraction (EF). Whether the benefits of SGLT2 (sodium-glucose cotransporter-2) inhibitors on these outcomes vary across the full range of EF remains unclear.
METHODS:
Patient-level data were pooled from the DEFINE-HF trial (Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction) of 263 participants with reduced EF (≤40%), and PRESERVED-HF trial (Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Preserved Ejection Fraction Heart Failure) of 324 participants with preserved EF (≥45%). Both were randomized, double-blind 12-week trials of dapagliflozin versus placebo, recruiting participants with New York Heart Association class II or higher and elevated natriuretic peptides. The effect of dapagliflozin on the change in the Kansas City Cardiomyopathy Questionnaire (KCCQ) Clinical Summary Score (CSS) at 12 weeks was tested with ANCOVA adjusted for sex, baseline KCCQ, EF, atrial fibrillation, estimated glomerular filtration rate, and type 2 diabetes. Interaction of dapagliflozin effects on KCCQ-CSS by EF was assessed using EF both categorically and continuously with restricted cubic spline. Responder analyses, examining proportions of patients with deterioration, and clinically meaningful improvements in KCCQ-CSS were conducted using logistic regression.
RESULTS:
Of 587 patients randomized (293 dapagliflozin, 294 placebo), EF was ≤40, >40-≤60, and >60% in 262 (45%), 199 (34%), and 126 (21%), respectively. Dapagliflozin improved KCCQ-CSS at 12 weeks (placebo-adjusted difference 5.0 points [95% CI, 2.6–7.5]; P<0.001). This was consistent in participants with EF≤40 (4.6 points [95% CI, 1.0–8.1]; P=0.01), >40 to ≤60 (4.9 points [95% CI, 0.8–9.0]; P=0.02) and >60% (6.8 points [95% CI, 1.5–12.1]; P=0.01; Pinteraction=0.79). Benefits of dapagliflozin on KCCQ-CSS were also consistent when analyzing EF continuously (Pinteraction=0.94). In responder analyses, fewer dapagliflozin-treated patients had deterioration and more had small, moderate, and large KCCQ-CSS improvements versus placebo; these results were also consistent regardless of EF (all Pinteractionvalues nonsignificant).
CONCLUSIONS:
In patients with HF, dapagliflozin significantly improves symptoms and physical limitations after 12 weeks of treatment, with consistent and clinically meaningful benefits across the full range of EF.
REGISTRATION:
URL: https://www.clinicaltrials.gov; Unique identifiers: NCT02653482 and NCT03030235.
Keywords: dapagliflozin, ejection fraction, health status, heart failure, Kansas City Cardiomyopathy Questionnaire, quality of life, SGLT2 inhibitors
WHAT IS NEW?
Reducing the burden of heart failure (HF)–related symptoms and physical limitations is a key goal of management.
Whether SGLT2 (sodium-glucose cotransporter-2) inhibitors improve these outcomes in individuals with HF across the full range of ejection fraction (EF) remains unclear.
Using a pooled analysis from 2 randomized trials of patients with HF, we demonstrate that SGLT2 inhibitor dapagliflozin produced significant and clinically meaningful improvements in symptoms and physical limitations across the full range of EF. There was no attenuation of these benefits among participants with EF in the normal range.
WHAT ARE THE CLINICAL IMPLICATIONS?
There is a dearth of efficacious therapies that improve health status (symptoms, physical limitations, and quality of life) in individuals with HF regardless of EF.
These findings address an important unmet clinical need, and provide a compelling additional rationale for using SGLT2 inhibitors in patients with HF across the full range of EF, in addition to their proven effects on reducing cardiovascular death and worsening HF events.
Patients with heart failure (HF) experience impaired health status, with a high burden of symptoms and physical limitations, and poor quality of life, regardless of whether they have reduced, mid-range, or preserved left ventricular ejection fraction (EF).1–3 Improving symptoms and physical limitations is a key goal of HF management across the full range of EF and is being increasingly emphasized by practice guidelines4 and regulatory agencies,5 and incorporated as a valuable outcome in clinical trials of HF therapies.6 Importantly, many HF treatments (including renin-angiotensin blockers, mineralocorticoid receptor antagonists, and angiotensin receptor–neprilysin inhibitors) do not improve symptoms and physical limitations in patients with HF with preserved EF (HFpEF),7,8 a cohort that fundamentally differs from patients with reduced EF (HFrEF),9 and in whom obesity and cardiometabolic stress are more strongly tied to health status.10 As such, development of therapies that have consistent benefits on symptoms, physical limitations, and quality of life in patients with HF across the full range of EF remains a critical unmet need.
SGLT2 (sodium-glucose cotransporter-2) inhibitors are now considered foundational therapy for individuals with HFrEF, where, in addition to reducing the risk of cardiovascular death and hospitalization for HF, they consistently improve HF-related health status.11–14 Although results from randomized clinical trials indicate that SGLT2 inhibitors improve HF-related symptoms and physical limitations in those with HFpEF,15–18 the magnitude of these estimated effects has varied across studies. Results from pooled patient-level data analyses from the EMPEROR program (Empagliflozin Outcome Trial in Patients With Chronic Heart Failure) suggest that the benefits of SGLT2 inhibitors on health status may be attenuated in patients with EF of 65% and above.19 However, the degree of symptomatic and functional impairment at baseline was relatively mild in those studies, especially among those with preserved EF, thus limiting the likelihood of detecting the benefits of any therapeutic intervention, including those from SGLT2 inhibitors. Consequently, it remains unclear whether these agents provide meaningful health status benefits among highly symptomatic and functionally impaired individuals with HF across the full range of EF.
In DEFINE-HF (Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction) and PRESERVED-HF (Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Preserved Ejection Fraction Heart Failure) trials, dapagliflozin has been previously reported to significantly improve symptoms, physical limitations and quality of life in individuals with HFrEF13 and HFpEF.17 Both trials included populations of patients with marked symptomatic and functional impairment due to HF. We performed post hoc, pooled patient-level analyses of the DEFINE-HF and PRESERVED-HF trials, enabling the opportunity to examine, in a granular fashion, the nature of the relationship between initiation of SGLT2 inhibitor dapagliflozin and its effects on HF-related health status across the full range of EF.
METHODS
The DEFINE-HF and PRESERVED-HF trials were investigator-initiated, multicenter, randomized, double-blind, placebo-controlled, parallel-group trials, with the concepts developed, and studies sponsored and executed by the national coordinating center at Saint Luke’s Mid America Heart Institute in collaboration with the respective Executive Committees. Both trials evaluated the efficacy of the SGLT2 inhibitor dapagliflozin versus placebo on HF-related health status over 12 weeks of treatment, as measured by the Kansas City Cardiomyopathy Questionnaire (KCCQ) in patients with HF. Both trials were carried out in the United States using similar eligibility criteria and similar case report forms. The data that support the findings of these studies are available from the corresponding author upon reasonable request.
Institutional review boards approved the study for all sites in both trials, and all patients provided written informed consent for research participation. Both trials were conducted in accordance with the ICH E6(R1) (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, topic E6, revision R1) Guidelines of Good Clinical Practice and the Declaration of Helsinki.
The primary difference between the 2 trials was the inclusion of patients with an EF of ≤40% in DEFINE-HF and ≥45% in PRESERVED-HF. The first and senior authors had unrestricted access to the patient-level data, prepared the first draft of the article, and edited it after review and input from all authors. The authors made the decision to submit the article for publication and assume full responsibility for the accuracy and completeness of the analyses.
Patient Populations
The inclusion and exclusion criteria for DEFINE-HF and PRESERVED-HF have been published.13,17 Both trials enrolled adult ambulatory patients (with or without type 2 diabetes [T2D]), clinical diagnosis of HF, and New York Heart Association (NYHA) class II-III (DEFINE-HF) or II-IV (PRESERVED-HF) symptoms. Patients were required to have elevated natriuretic peptides (NT-proBNP [N-terminal pro-B-type natriuretic peptide]) ≥400 or BNP (B-type natriuretic peptide) ≥100 pg/mL in DEFINE-HF; NT-proBNP ≥ 225 or BNP ≥ 75 pg/mL in PRESERVED-HF for patients in sinus rhythm). In the setting of atrial fibrillation (AF), NT-proBNP had to be ≥600 or BNP ≥400 pg/mL in DEFINE-HF and ≥375 or ≥100 pg/mL in PRESERVED-HF. In the latter trial, patients also had to be on diuretic therapy and have either HF hospitalization or urgent HF visit requiring intravenous diuretic treatment during the previous 12 months, documented elevated filling pressures on right or left heart catheterization, or echocardiographic evidence of structural heart abnormalities.
Key exclusion criteria were recent hospitalization for decompensated HF, estimated glomerular filtration rate (eGFR) <30 (DEFINE-HF) or <20 mL/min per 1.73m2 (PRESERVED-HF), type 1 diabetes, or history of diabetic ketoacidosis.
Trial Designs
For both trials, patients considered potentially eligible who agreed to participate and provided informed consent entered a 2-week screening phase, during which their eligibility was confirmed. Eligible patients were randomized in a double-blind fashion, 1:1 to oral dapagliflozin 10 mg or matching placebo once daily. Before administration of the first dose of dapagliflozin or placebo, patients underwent a physical exam, trial-related laboratory assessments, and completed the KCCQ. Patients then entered a 12-week treatment period, during which they were followed via 4 phone call visits, as well as 2 in-person study visits (at 6 and 12 weeks). Postrandomization assessments of KCCQ were obtained at 6- and 12-week visits in DEFINE-HF and at the 12-week visit in PRESERVED-HF. At week 12, study medication was discontinued, and patients were followed for 1 additional week to assess for any intercurrent safety events.
Outcomes
For the purposes of these post hoc, pooled patient-level analyses, the main outcome of interest was change in the KCCQ-CSS at 12 weeks, which was a secondary outcome in DEFINE-HF, and the primary outcome in PRESERVED-HF. The KCCQ is a standardized 23-item, self-administered instrument that quantifies HF-related symptoms (frequency, severity, and recent change), physical function, quality of life, and social function.20 For each domain, the validity, reproducibility, responsiveness, and interpretability have been independently established for both HFrEF and HFpEF populations.21 Scores are transformed to a range of 0 to 100, in which higher scores reflect better health status.22 KCCQ-CSS includes the symptom and physical function domains of the KCCQ (considered most likely to be modified by SGLT2 inhibitors).
Additional outcomes examined were individual components of KCCQ-CSS, including change in the KCCQ–Total Symptom Score (which summarizes symptom burden and frequency) and KCCQ–Physical Limitations Score (which summarizes physical function); as well as KCCQ–Overall Summary Score (KCCQ-OSS, which includes symptoms, physical limitations, quality of life and social function) at 12 weeks. Responder analyses were also performed, in which the proportions of patients with deterioration, as well as clinically meaningful improvement in KCCQ-CSS, were assessed.
Statistical Analysis
All outcomes were evaluated using the modified intention to treat data sets in both trials, which included all randomized patients who received at least 1 dose of study medication and had an evaluable KCCQ follow-up measurement. Continuous measures were summarized by mean±SD or median and interquartile range and compared using Student t test or Wilcoxon rank-sum test, as appropriate. Categorical variables were summarized by frequency and percent and compared using χ2 or Fisher exact tests, as appropriate.
For the main outcome of interest, an ANCOVA model was used to estimate the effect of dapagliflozin relative to placebo on the 12-week KCCQ-CSS, adjusting for baseline KCCQ-CSS value, sex, eGFR, T2D status, AF status, and left ventricular EF. Restricted cubic splines were included for continuous variables to accommodate nonlinear effects. The effects of dapagliflozin versus placebo on the KCCQ-CSS at 12 weeks were also examined across several key subgroups analyses, including baseline EF (stratified into 3 categories: ≤40%; >40 and <60%; and ≥60%); as well as age (<70, ≥70 years), sex (male, female), race, T2D status, body mass index (<median, ≥median), AF status, baseline KCCQ-OSS (<median, ≥median), baseline eGFR (<60, ≥60 mL/[min·1.73 m2]), baseline loop diuretic dose (furosemide equivalent mean daily dose: ≤40 mg, >40 mg), NYHA class, NT-proBNP (N-terminal pro-B-type natriuretic peptide; <median, ≥median), and baseline use of HF therapies (beta blockers, ACE (angiotensin-converting enzyme) inhibitors/angiotensin receptor blockers/angiotensin receptor–neprilysin inhibitors and mineralocorticoid receptor antagonists).
The relationship between the effects of dapagliflozin versus placebo on the 12-week KCCQ-CSS across the full range of baseline EF was examined in a more granular fashion, testing the dapagliflozin×EF interaction with EF modeled continuously with a restricted cubic spline, using the previously referenced ANCOVA model, and adjusting for baseline KCCQ-CSS value, sex, eGFR, T2D status, and AF status.
Finally, given the suggestion from the EMPEROR program that the benefits of SGLT2 inhibitors on health status may be attenuated in patients with EF of 65% and above,19 we performed an additional subgroup analysis, specifically examining the effects of dapagliflozin versus placebo on KCCQ-CSS at 12 weeks specifically in patients with EF of ≥65%.
The effects of dapagliflozin versus placebo on 12-week KCCQ–Total Symptom Score, KCCQ–Physical Limitations Score, and KCCQ-OSS were examined in a manner analogous to that used for KCCQ-CSS; this included evaluating these effects across the full range of baseline EF modeled as a continuous variable with a restricted cubic spline as previously described.
In the responder analyses, the proportions of dapagliflozin- and placebo-treated participants that had deterioration (greater than 5-point worsening), no change, as well as small-moderate (5–<10 point), moderate-large (10–<20 point), and very large (≥20 point) improvements in KCCQ-CSS at 12 weeks were calculated. Logistic regression models, adjusted for baseline KCCQ-CSS value, as well as sex, eGFR, T2D status, AF status, and EF, were used to compare the proportion of dapagliflozin- and placebo-treated patients who had deterioration, as well as at least small (≥5 point), at least moderate (≥10 point), and large (≥20 point) improvements in KCCQ-CSS. These analyses were then repeated to test whether these effects of dapagliflozin versus placebo on the proportion of patients with worsening versus various improvements in KCCQ-CSS at 12 weeks differed according to baseline EF (modeled in 3 categories: ≤40%; >40 and <60%; and ≥60%).
RESULTS
In total, 587 participants were included (263 from DEFINE-HF, 324 from PRESERVED-HF). Baseline characteristics of participants randomized to dapagliflozin or placebo are listed in the Table. Median age was 67 years (interquartile range [IQR], 58–74), 43% of participants were women, one-third (33%) were Black, most (66%) were previously hospitalized for HF, 59% had T2D, and 47% had AF. There was a high prevalence of obesity, with median body mass index of 33 kg/m2 (IQR, 29–39). Median NT-proBNP was elevated at baseline (844 pg/mL, IQR, 447–1692), whereas median eGFR was 59 mL/min per 1.73m2 (IQR, 46–77). Most patients were treated with loops diuretics (87%), ACE inhibitors–angiotensin receptor blocker/angiotensin receptor–neprilysin inhibitor (72%), and beta blockers (83%), whereas 47% were receiving mineralocorticoid receptor antagonists; 37% had NYHA class III to IV symptoms, and median baseline KCCQ-CSS was 67 points (IQR, 50–83).
Table.
Baseline Characteristics
Median EF was 50% (IQR, 29–60), with 262 (44.6%), 199 (33.9%), and 126 (21.5%) patients having EF ≤40%, >40 and <60%, and ≥60%, respectively.
Main Outcome of Interest
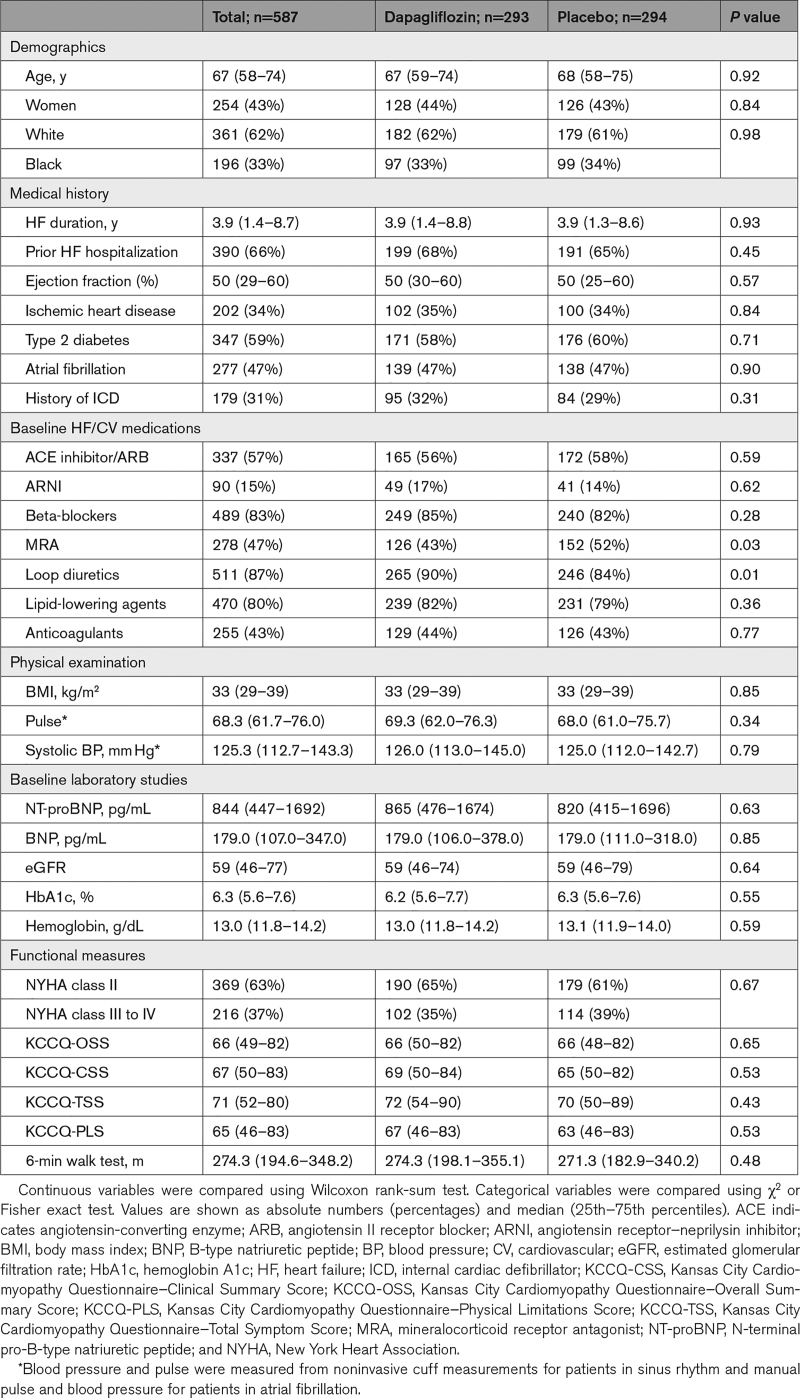
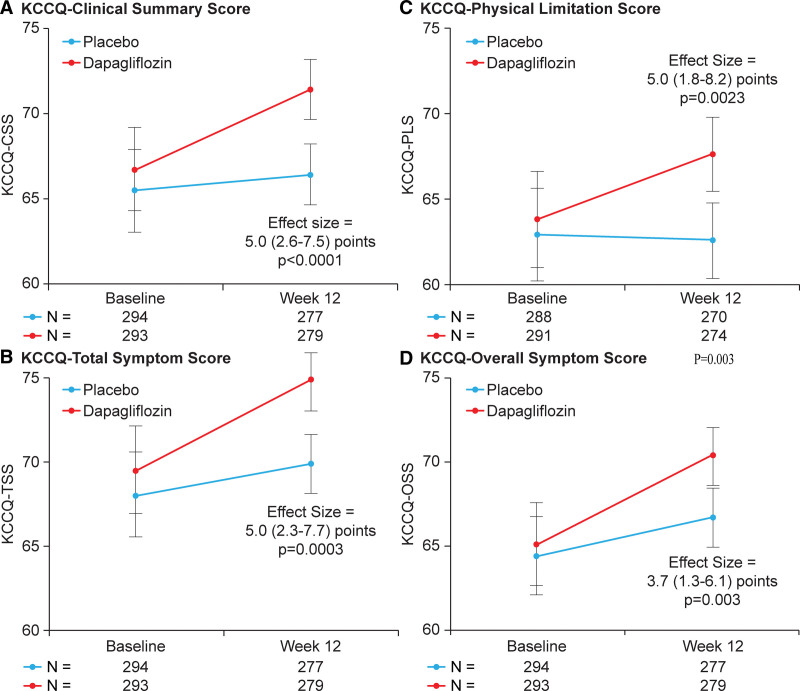
Dapagliflozin improved KCCQ-CSS at 12 weeks (placebo-adjusted difference 5.0 points [95% CI, 2.6–7.5]; P<0.001; Figure 1A). This was consistent in participants with EF ≤40 (4.6 points [95% CI, 1.0–8.1]; P=0.01), >40 to ≤60 (4.9 points [95% CI, 0.8–9.0]; P=0.02) and >60% (6.8 points [95% CI, 1.5–12.1]; P=0.01; Pinteraction=0.79; Figure 2A). The benefits of dapagliflozin on KCCQ-CSS were also consistent when analyzing EF as a continuous variable, with no evidence of treatment effect attenuation with higher EF values (Pinteraction=0.94; Figure 2B). There was no evidence of treatment effect heterogeneity when examining the effects of dapagliflozin on KCCQ-CSS at 12 weeks across all other demographic and clinical subgroups, with all Pinteraction values being nonsignificant (Figure 2A). Furthermore, dapagliflozin significantly improved KCCQ-CSS in patients with EF of 65% or greater (n=98) by 7.5 points (95% CI, 0.5–14.5; P=0.04).
Figure 1.
Effects of dapagliflozin vs placebo at 12 weeks on the Kansas City Cardiomyopathy Questionnaire (KCCQ) domains. KCCQ–Clinical Summary Score (KCCQ-CSS; A), KCCQ–Total Symptom Score (KCCQ-TSS; B), KCCQ–Physical Limitations Score (KCCQ-PLS; C), and KCCQ–Overall Summary Score (KCCQ-OSS; D). Data are presented as mean values with 95% CIs (adjusted for baseline KCCQ, sex, type 2 diabetes status, atrial fibrillation type, estimated glomerular filtration rate, and ejection fraction; models include patients with complete KCCQ data at baseline and 12 weeks).
Figure 2.
Effects of dapagliflozin vs placebo at 12 weeks on the Kansas City Cardiomyopathy Questionnaire–Clinical Summary Score (KCCQ-CSS) across demographic and clinical subgroups of interest. A, Effects on KCCQ-CSS across demographic and clinical subgroups of interest. B, Effects on KCCQ-CSS across the range of ejection fraction (ejection fraction modeled continuously with a restricted cubic spline; adjusted for baseline KCCQ-CSS, sex, type 2 diabetes status, atrial fibrillation [AF] type, and estimated glomerular filtration rate). LVEF indicates left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-B-type natriuretic peptide; and NYHA, New York Heart Association.
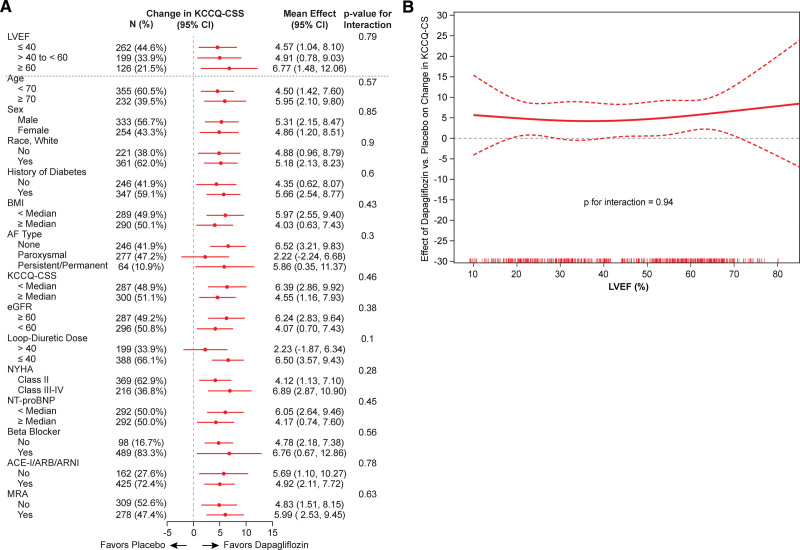
Dapagliflozin also significantly improved other KCCQ domains at 12 weeks, including Total Symptom Score (placebo-adjusted difference 5.0 points [95% CI, 2.3–7.7]; P<0.001; Figure 1B); Physical Limitations Score (placebo-adjusted difference, 5.0 points (95% CI, 1.8–8.2]; P=0.002; Figure 1C); and OSS (placebo-adjusted difference, 3.7 points [95% CI, 1.3–6.1]; P=0.003; Figure 1D). Similar to the effects on KCCQ-CSS, the benefits of dapagliflozin on these KCCQ domains were consistent across the entire range of EF, with no evidence of attenuation at higher EF values (Figure 3A through 3C for KCCQ–Total Symptom Score, KCCQ–Physical Limitations Score and KCCQ-OSS, respectively).
Figure 3.
Effects of dapagliflozin vs placebo on the 12-week Kansas City Cardiomyopathy Questionnaire (KCCQ) domains across the range of ejection fraction (ejection fraction modeled continuously with a restricted cubic spline; adjusted for baseline KCCQ, sex, type 2 diabetes status, atrial fibrillation [AF] type, and estimated glomerular filtration rate). KCCQ–Total Symptom Score (KCCQ-TSS; A), KCCQ–Physical Limitations Score (KCCQ-PLS; B), and KCCQ–Overall Summary Score (KCCQ-OSS; C). LVEF indicates left ventricular ejection fraction.
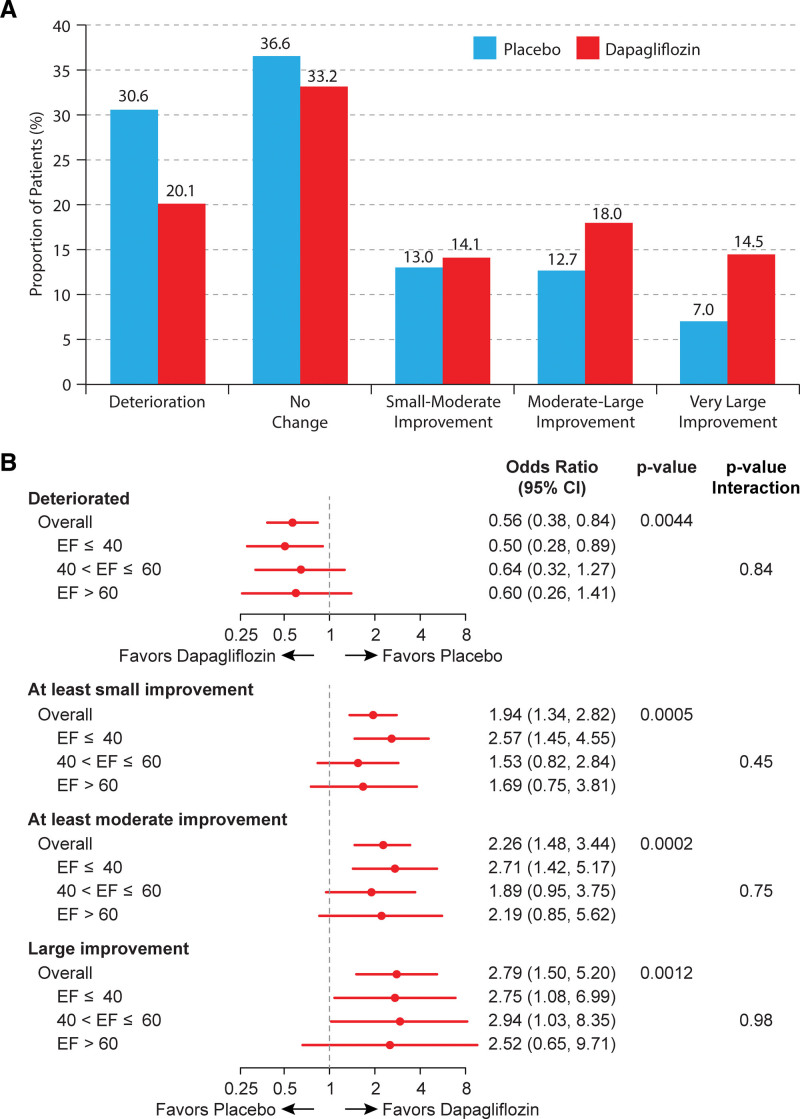
The results of the responder analyses are shown in Figure 4A and 4B. Fewer dapagliflozin- versus placebo-treated patients experienced worsening of KCCQ-CSS at 12 weeks (odds ratio, 0.56 [95% CI, 0.38–0.84]; P=0.004); and greater proportions of dapagliflozin-treated patients had at least small (odds ratio, 1.94 [95% CI, 1.34–2.82]; P<0.001), at least moderate (odds ratio, 2.26 [95% CI, 1.48–3.44]; P<0.001), and large improvements (odds ratio, 2.79 [95% CI, 1.50–5.20]; P=0.001). All of these results were consistent when stratified by EF categories, with all Pinteraction values being nonsignificant.
Figure 4.
Responder analyses. A, Proportions of dapagliflozin- and placebo-treated participants that had deterioration (>5-point worsening), no change, as well as small-moderate (5–<10 point), moderate-large (10–<20 point), and very large (≥20 point) improvements in Kansas City Cardiomyopathy Questionnaire-Clinical Summary Score (KCCQ-CSS) at 12 weeks. B, Forest plot demonstrating the results of logistic regression models (adjusted for baseline KCCQ-CSS value, sex, estimated glomerular filtration rate, type 2 diabetes status, atrial fibrillation status, and ejection fraction [EF]), which compare the proportion of dapagliflozin- and placebo-treated patients who had deterioration, as well as at least small (≥5 point), at least moderate (≥10 point), and large (≥20 point) improvements in KCCQ-CSS (overall and stratified by EF).
DISCUSSION
In these pooled patient-level analyses of the DEFINE-HF and PRESERVED-HF trials, the SGLT2 inhibitor dapagliflozin significantly improved symptoms and physical limitations in patients with chronic HF, with consistent benefits across the full range of EF, as well as across other demographic and clinical subgroups analyzed. There was no attenuation of the treatment benefit when EF was analyzed both continuously and categorically, including among those participants with EF of 60% and higher. Similar results were also seen with other key KCCQ domains. Dapagliflozin reduced the proportion of patients with deterioration and increased the proportion of patients with small, moderate, and large improvements in health status at 12 weeks, and these effects were also highly consistent across the full range of EF. Of note, the proportion of dapagliflozin-treated patients who had large KCCQ-CSS improvements was more than double when compared with placebo. Collectively, these data support treatment with dapagliflozin to improve symptoms and physical limitations in patients with HF regardless of baseline EF.
The present findings are of clinical relevance given that improving symptoms and physical limitations is a key goal of management in individuals with HF regardless of EF, and because other therapies previously evaluated in patients with HF across the range of EF have not demonstrated compelling benefits on these outcomes in those with preserved EF. Specifically, meta-analyses of prior trials show no significant effects of ACE inhibitors, angiotensin receptor blocker, or mineralocorticoid receptor antagonist therapies on symptoms and physical limitations in patients with HFpEF.9 Furthermore, while sacubitril-valsartan significantly improved KCCQ-OSS in patients with HFrEF,23 it had no effect on symptoms and physical limitations when tested in a large dedicated randomized trial of individuals with HFpEF.10 Thus, identifying treatments that can improve HF-related symptoms and physical limitations across the full range of EF remains a significant unmet clinical need.
The present results differ from a previously reported pooled patient-level analysis of the EMPEROR program (EMPEROR-REDUCED [Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction] and EMPEROR-PRESERVED [Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction]), which showed a modest, but statistically significant KCCQ improvements with empagliflozin versus placebo across the range of EF, except for individuals with EF ≥ 65%, in whom there was an attenuation of this effect, and no significant placebo-adjusted KCCQ increase was noted.19 A likely explanation for discrepancies between the EMPEROR program, several other SGLT2 inhibitor trial programs of similar size and duration as DEFINE-HF and PRESERVED-HF trials,24–26 and the present observations are differences in patient populations. These include a higher proportion of women and underrepresented minorities, and much higher body mass index in PRESERVED-HF, which enrolled patients exclusively in the United States, versus the global EMPEROR-PRESERVED study.15,17 Obesity is a stronger risk factor for HFpEF than HFrEF,10,27 and body mass is the strongest correlate of patient-reported health status in HFpEF. Importantly, patients in PRESERVED-HF were more symptomatic at baseline, with KCCQ-CSS values nearly 10 points lower, and the proportion of those with NYHA class III to IV being twice as high when compared with EMPEROR-PRESERVED. The higher burden of symptoms at baseline likely made the present patient population more likely to experience a large symptomatic improvement after a beneficial intervention, such as the initiation of SGLT2 inhibition. A recent analysis from the DELIVER (Dapagliflozin Evaluation to Improve the Lives of Patients With Preserved Ejection Fraction Heart Failure) trial also suggests that patients with HFpEF and a higher burden of symptomatic impairment at baseline (such as worse NYHA class) may experience a greater degree of symptomatic improvement with dapagliflozin versus placebo.28 However, we cannot conclude this definitively, and other potential explanations for the differences between the trial findings may exist.
The results of this study are highly complementary to the pooled analyses of DAPA-HF (Study to Evaluate the Effect of Dapagliflozin on the Incidence of Worsening Heart Failure or Cardiovascular Death in Patients With Chronic Heart Failure) and DELIVER trials,29 which showed highly consistent benefits of dapagliflozin on clinical events in individuals with HF across the entire range of EF. Collectively, these findings establish the efficacy of dapagliflozin on all key HF outcomes—cardiovascular death, worsening HF events, as well as patients’ symptoms, physical limitations, and quality of life—a conclusion of substantial importance to practicing clinicians.
The results of this study should be considered in the context of several potential limitations. First, although both DEFINE-HF and PRESERVED-HF trials had a similar design and were carried out using the same organizational infrastructure, these pooled patient-level analyses were planned post hoc. Second, the relatively short duration of follow-up (12 weeks) precludes conclusions regarding the durability of the observed benefit on HF disease-specific health status longer-term. Third, due to the inclusion and exclusion criteria of individual trials, those individuals with HF and EF between 41% and 44% were not included. Finally, the DEFINE-HF and PRESERVED-HF trials were not designed to evaluate clinical events (such as cardiovascular death, HF hospitalizations, and urgent visits), and this pooled analysis does not have the statistical power to examine these outcomes.
In conclusion, initiation of dapagliflozin in patients with HF significantly improved symptoms and physical limitations after 12 weeks of treatment, with consistent and clinically meaningful benefits across the full range of EF.
ARTICLE INFORMATION
Sources of Funding
The DEFINE-HF (Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction) and PRESERVED-HF (Effects of Dapagliflozin on Biomarkers, Symptoms and Functional Status in Patients With Preserved Ejection Fraction Heart Failure) studies were investigator-initiated trials funded by AstraZeneca and conducted by Saint Luke’s Mid America Heart Institute independent of the funding source.
Disclosures
Dr Nassif is a consultant to Vifor and has received research support from Cytokinetics. Dr Husain reports grant/research support and company relationship at AstraZeneca, Merck, and Novo Nordisk; is a consultant and reports a company relationship at AstraZeneca, Boehringer Ingleheim, Janssen, Merck, Novo Nordisk, and Roche; and has patent pending US61/721,819 (United States), patent US61/719,075 (United States), and patent pending EP2911686A1 (European Patent Office). Dr Borlaug reports grant/research support from the National Institutes of Health/National Heart, Lung, and Blood Institute, the US Department of Defense, Axon, AstraZeneca, Corvia, Medtronic, Novo Nordisk, and Tenax Therapeutics; and consulting/advisory board with Amgen, Aria, Boehringer Ingelheim, Edwards Lifesciences, Eli Lilly, Merck, Novo Nordisk, NGMBio, ShouTi, and VADovations. Dr Kitzman reports receiving honoraria as a consultant for Bayer, Merck, Medtronic, Relypsa, Corvia Medical, Boehringer Ingelheim, Novo Nordisk, AstraZeneca, and Novartis; grant funding from Novartis, Bayer, Novo Nordisk, and AstraZeneca; and has stock ownership in Gilead Sciences. Dr Inzucchi is an advisor/consultant for AstraZeneca, Bayer, Boehringer Ingelheim, Novo Nordisk, Merck, and Pfizer; and lectures for AstraZeneca and Boehringer Ingelheim. Dr McGuire reports research support for Clinical Trials Leadership from Boehringer Ingelheim, Sanofi, Merck & Co, Pfizer, AstraZeneca, Novo Nordisk, Esperion, Lilly USA, Lexicon, and CSL Behring; and honoraria for consultancy from Lilly USA, Boehringer Ingelheim, Merck & Co, Novo Nordisk, Applied Therapeutics, Metavant, Sanofi, Intercept Pharmaceuticals, Altimmune, CSL Behring, and Bayer. Dr Pitt reports consulting fees and company relationship with AstraZeneca, Boehringer Ingelheim, Bayer, Lexicon, Merck, and Phase Bio; consulting fees and/or stock options and company relationship with Vifor, KBP Biosciences, scPharmaceuticals, Cereno Scientific, Tricida, SQinnovation, G-# Pharmaceuticals, Protonintel, and Brainstorm Medical; and patent 9931412 (site-specific delivery of eplerenone to the myocardium; United States) patent pending 63/045,783 (histone-modulating agents for the protection and treatment of organ damage; United States). Dr Scirica reports institutional research grants to Brigham and Women’s Hospital from Better Therapeutics, Boehringer Ingelheim, Merck, Novo Nordisk, and Pfizer; consulting fees from Allergan, Boehringer Ingelheim, Better Therapeutics, Elsevier Practice Update Cardiology, Esperion, Hamni, Lexicon, Lexeo, and Novo Nordisk; and equity in Health [at] Scale and Doximity. Dr Shah reports research grants from the National Institutes of Health (R01 HL107577, R01 HL127028, R01 HL140731, and R01 HL149423), Actelion, AstraZeneca, Corvia, Novartis, and Pfizer; and consulting fees from Abbott, Actelion, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardiora, CVRx, Cytokinetics, Edwards Lifesciences, Eidos, Eisai, Imara, Impulse Dynamics, Intellia, Ionis, Ironwood, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sanofi, Shifamed, Tenax, Tenaya, and United Therapeutics. Dr Umpierrez reports research support to Emory University from AstraZeneca, Dexcom Inc, Baxter, and Bayer (since 2020). Dr Sharma is an advisory board member and consultant for Alleviant, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cytokinetics, Janssen, Novartis, Novo Nordisk, and RIVUS and receives honoraria. Dr Kosiborod reports grant/research support and company relationship at AstraZeneca, Boehringer Ingelheim, and Pfizer; honoraria and company relationship with AstraZeneca, Boehringer Ingelheim, and Novo Nordisk; consultant and company relationship with 35Pharma, Alnylam, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Dexcom, Eli Lilly, Esperion Therapeutics, Imbria Pharmaceuticals, Janssen, Lexicon, Merck (Diabetes and Cardiovascular), Novo Nordisk, Pharmacosmos, Pfizer, scPharmaceuticals, Structure Therapeutics, Vifor Pharma, Youngene Therapeutics; other research support and company relationship with AstraZeneca; and stock options and company relationship with Artera Health, and Saghmos Therapeutics.
Nonstandard Abbreviations and Acronyms
- ACE
- angiotensin-converting enzyme
- AF
- atrial fibrillation
- BNP
- B-type natriuretic peptide
- DEFINE-HF
- Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients With Heart Failure With Reduced Ejection Fraction
- EF
- ejection fraction
- eGFR
- estimated glomerular filtration rate
- HF
- heart failure
- HFpEF
- HF with preserved ejection fraction
- HFrEF
- HF with reduced ejection fraction
- IQR
- interquartile range
- KCCQ
- Kansas City Cardiomyopathy Questionnaire
- KCCQ-CSS
- KCCQ Clinical Summary Score
- KCCQ-OSS
- KCCQ Overall Summary Score
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
- NYHA
- New York Heart Association
- OR
- odds ratio
- PRESERVED-HF
- Effects of Dapagliflozin on Biomarkers, Symptoms, and Functional Status in Patients With Preserved Ejection Fraction Heart Failure
- SGLT2
- sodium-glucose cotransporter-2
- T2D
- type 2 diabetes
For Sources of Funding and Disclosures, see page 562.
Contributor Information
Michael E. Nassif, Email: mnassif@saint-lukes.org.
Sheryl L. Windsor, Email: swindsor@saint-lukes.org.
Kensey Gosch, Email: kgosch@saint-lukes.org.
Barry A. Borlaug, Email: borlaug.barry@mayo.edu.
Mansoor Husain, Email: mansoor.husain@uhn.ca.
Silvio E. Inzucchi, Email: silvio.inzucchi@yale.edu.
Dalane W. Kitzman, Email: dkitzman@wakehealth.edu.
Darren K. McGuire, Email: darren.mcguire@utsouthwestern.edu.
Bertram Pitt, Email: bpitt@umich.edu.
Benjamin M. Scirica, Email: bscirica@bwh.harvard.edu.
Sanjiv J. Shah, Email: Sanjiv.shah@northwestern.edu.
Guillermo Umpierrez, Email: geumpie@emory.edu.
Bethany A. Austin, Email: baustin@saint-lukes.org.
Sumant Lamba, Email: slamba@firstcoastcardio.com.
Taiyeb Khumri, Email: tkhumri@saint-lukes.org.
Kavita Sharma, Email: ksharma8@jhmi.edu.
REFERENCES
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14:591–602. doi: 10.1038/nrcardio.2017.65 [DOI] [PubMed] [Google Scholar]
- 3.Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124:1598–1617. doi: 10.1161/CIRCRESAHA.119.313572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e876–e894. doi: 10.1161/CIR.0000000000001062 [DOI] [PubMed] [Google Scholar]
- 5.US FDA. Treatment for Heart Failure: Endpoints for Drug Development Guidance for Industry. 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/treatment-heart-failure-endpoints-drug-development-guidance-industry
- 6.Tsevat J, Weeks JC, Guadagnoli E, Tosteson AN, Mangione CM, Pliskin JS, Weinstein MC, Cleary PD. Using health-related quality-of-life information: clinical encounters, clinical trials, and health policy. J Gen Intern Med. 1994;9:576–582. doi: 10.1007/BF02599287 [DOI] [PubMed] [Google Scholar]
- 7.Martin N, Manoharan K, Davies C, Lumbers RT. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2021;5:CD012721. doi: 10.1002/14651858.CD012721.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, Shi V, Zhao Z, Cowie MR; PARALLAX Investigators and Committee members. Effect of sacubitril/valsartan vs standard medical therapies on plasma NT-proBNP concentration and submaximal exercise capacity in patients with heart failure and preserved ejection fraction: the PARALLAX randomized clinical trial. JAMA. 2021;326:1919–1929. doi: 10.1001/jama.2021.18463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borlaug BA, Redfield MM. Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation. 2011;123:2006–13; discussion 2014. doi: 10.1161/CIRCULATIONAHA.110.954388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reddy YNV, Rikhi A, Obokata M, Shah SJ, Lewis GD, AbouEzzedine OF, Dunlay S, McNulty S, Chakraborty H, Stevenson LW, et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009–1018. doi: 10.1002/ejhf.1788 [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anand IS, Bělohlávek J, et al. ; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. doi: 10.1056/NEJMoa1911303 [DOI] [PubMed] [Google Scholar]
- 12.Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141:90–99. doi: 10.1161/CIRCULATIONAHA.119.044138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nassif ME, Windsor SL, Tang F, Khariton Y, Husain M, Inzucchi SE, McGuire DK, Pitt B, Scirica BM, Austin B, et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction: the DEFINE-HF trial. Circulation. 2019;140:1463–1476. doi: 10.1161/CIRCULATIONAHA.119.042929 [DOI] [PubMed] [Google Scholar]
- 14.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. ; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 15.Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, et al. ; EMPEROR-Preserved Trial Investigators. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021;385:1451–1461. doi: 10.1056/NEJMoa2107038 [DOI] [PubMed] [Google Scholar]
- 16.Butler J, Filippatos G, Jamal Siddiqi T, Brueckmann M, Böhm M, Chopra VK, Pedro Ferreira J, Januzzi JL, Kaul S, Piña IL, et al. Empagliflozin, health status, and quality of life in patients with heart failure and preserved ejection fraction: the EMPEROR-preserved trial. Circulation. 2022;145:184–193. doi: 10.1161/CIRCULATIONAHA.121.057812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, Khariton Y, Malik AO, Khumri T, Umpierrez G, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. 2021;27:1954–1960. doi: 10.1038/s41591-021-01536-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spertus JA, Birmingham MC, Nassif M, Damaraju CV, Abbate A, Butler J, Lanfear DE, Lingvay I, Kosiborod MN, Januzzi JL. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. 2022;28:809–813. doi: 10.1038/s41591-022-01703-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, Brueckmann M, Pocock SJ, Zannad F, Anker SD. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022;43:416–426. doi: 10.1093/eurheartj/ehab798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 21.Joseph SM, Novak E, Arnold SV, Jones PG, Khattak H, Platts AE, Dávila-Román VG, Mann DL, Spertus JA. Comparable performance of the Kansas City Cardiomyopathy Questionnaire in patients with heart failure with preserved and reduced ejection fraction. Circ Heart Fail. 2013;6:1139–1146. doi: 10.1161/CIRCHEARTFAILURE.113.000359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spertus JA, Jones PG, Sandhu AT, Arnold SV. Interpreting the Kansas City Cardiomyopathy Questionnaire in clinical trials and clinical care: JACC state-of-the-art review. J Am Coll Cardiol. 2020;76:2379–2390. doi: 10.1016/j.jacc.2020.09.542 [DOI] [PubMed] [Google Scholar]
- 23.Piña IL, Camacho A, Ibrahim NE, Felker GM, Butler J, Maisel AS, Prescott MF, Williamson KM, Claggett BL, Desai AS, et al. ; PROVE-HF Investigators. Improvement of health status following initiation of sacubitril/valsartan in heart failure and reduced ejection fraction. JACC Heart Fail. 2021;9:42–51. doi: 10.1016/j.jchf.2020.09.012 [DOI] [PubMed] [Google Scholar]
- 24.Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, Filippatos G, Gniot J, Fu M, Gullestad L, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. 2021;42:700–710. doi: 10.1093/eurheartj/ehaa943 [DOI] [PubMed] [Google Scholar]
- 25.DETERMINE-reduced – ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03877237?term=determine-reduced&draw=2&rank=1
- 26.DETERMINE-preserved – ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03877224?term=determine-preserved&draw=2&rank=1
- 27.Pandey A, LaMonte M, Klein L, Ayers C, Psaty BM, Eaton CB, Allen NB, de Lemos JA, Carnethon M, Greenland P, et al. Relationship between physical activity, body mass index, and risk of heart failure. J Am Coll Cardiol. 2017;69:1129–1142. doi: 10.1016/j.jacc.2016.11.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kosiborod MN, Bhatt AS, Claggett BL, Vaduganathan M, Kulac IJ, Lam CSP, Hernandez AF, Martinez FA, Inzucchi SE, Shah SJ, et al. Effect of dapagliflozin on health status in patients with preserved or mildly reduced ejection fraction. J Am Coll Cardiol. 2023;81:460–473. doi: 10.1016/j.jacc.2022.11.006 [DOI] [PubMed] [Google Scholar]
- 29.Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, Vaduganathan M, Gasparyan SB, Bengtsson O, Lindholm D, et al. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient-level, pooled meta-analysis of DAPA-HF and DELIVER. Nat Med. 2022;28:1956–1964. doi: 10.1038/s41591-022-01971-4 [DOI] [PMC free article] [PubMed] [Google Scholar]