Abstract
Case investigation and contact tracing (CICT) is a longstanding cornerstone of public health disease control efforts for a wide array of communicable diseases, though the content of CICT varies substantially depending on the infection to which it is applied, the epidemiologic circumstances, and interventions available to control an epidemic. In this article, we discuss how CICT is currently used in public health communicable disease, sexually transmitted infection/human immunodeficiency virus, and tuberculosis control programs. We then review how CICT might be modernized, considering issues such as community and health care organization engagement, workforce development, public health program organizational structure, data information systems, case prioritization, and the content to CICT.
Case investigation and contact tracing (CICT), which is usually called partner notification when applied to human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs), refers to activities undertaken by public health authorities to collect clinical and epidemiologic data from persons with a communicable disease (case investigation), identify people who may have been exposed and infected as a result of contact with an infected person or environmental source (contact tracing), and ensure that infected cases and contacts receive treatment, counseling, and other services (case management). Health departments in the United States use CICT for a wide array of infectious diseases, including STIs, viral hepatitis, tuberculosis (TB), enteric pathogens, some vaccine preventable infections and, most recently, SARS-CoV-2.
The content of CICT and the meaning of the term varies depending on the infection to which it is applied. For the purpose of this review, we define CICT to include three major components—case investigation, contact tracing, and case management—although the extent to which each component is used varies depending on the epidemiologic characteristics of the infection and the public health activities proposed to address it (Table 1). Detailed case investigation is critical in identifying outbreaks of infection and plays an important role in developing public health communications, policy, and clinical guidance. The initial phase of the COVID-19 pandemic and the new and growing epidemic of syphilis among heterosexuals in the United States are recent examples of instances in which case investigation has been crucial.1,2 In contrast, case investigation plays a smaller role in CICT for infections for which the epidemiology is relatively stable and well defined (eg, syphilis in men who have sex with men [MSM]). Contract tracing can play a critical role in confronting infections that are more morbid, transmitted person-to-person or via a common source, when treatment or prophylaxis is an important part of disease control, when the serial interval allows adequate time to complete contact tracing before most transmission occurs and, in some instances, when the affected population is particularly vulnerable. Case management, which typically involves linking cases or contacts to services, is important when treatment is prolonged or complex and is often employed when working with highly vulnerable populations. Human immunodeficiency virus, TB, and syphilis are examples of infections for which case management is often important.
TABLE 1.
Factors Associated With the Need to Implement In-Depth Case Investigation, Contact Tracing, and Case Management*
| Factors Associated With the Need for in-Depth Case Investigation | Factors Associated With the Need to Implement Contact Tracing | Factors Associated With the Need to Implement Case Management | |
|---|---|---|---|
| Epidemiologic and clinical characteristics favoring implementation of in-depth case investigation, contact tracing, and/or case management | Effort to detect outbreaks or unusual clusters of cases (ie, pathogens commonly occurring in outbreaks; occurrence in atypical population, geographic, or seasonal distribution) Etiology or source of illness not defined Cases in vulnerable populations (long term care facilities and other institutional settings) Atypical pathogen (eg, viral hemorrhagic fever; toxigenic Corynebacterium diphtheriae). Illness is severe, communicable and/or associated with an environmental source or animal reservoir. |
Person-to-person transmission or common source outbreaks Infections concentrated in marginalized populations (syndemics common) that may require additional support services Number of contacts manageable using CICT staff or subset of cases/contacts can be prioritized Longer serial interval allowing for contact tracing to occur before most transmission occurs Evidence supports contact tracing as being effective More severe clinical outcomes |
Infections requiring longer or more complex treatment or prophylaxis Infections concentrated in marginalized populations (syndemics common) that may require additional support services Number of cases manageable using CICT staff or subset of cases can be prioritized Index cases have potential to benefit from intervention not directly related to infection for which investigation initiated (eg, HIV PrEP in index cases with syphilis) Evidence supports case management as being effective More severe clinical outcomes |
| Public health activities and interventions used to address problem | Facilitate treatment or PEP Communication to public and medical community Policy intervention or clinical guidance Informs development of new disease control strategy |
CICT to identify contacts of cases and persons exposed to potential common source and follow-up with those persons. Quarantine of contacts is an important part of disease control. Case management (linkage of cases and contacts to care and support services) Treatment of cases or prophylaxis in contacts decreases morbidity and/or transmission |
Linkage of cases to clinical or support services, adherence support Case isolation is an important part of disease control. |
| Examples | Emerging pathogens Severe communicable diseases (SARS-CoV-2; meningococcal disease, rabies, legionellosis) Certain foodborne illness (STEC, hepatitis A, shellfish-associated illness). Nosocomial Infections (MDRO) Outbreaks of syphilis and HIV in heterosexuals |
Syphilis† HIV† TB Measles Meningococcus |
Syphilis HIV TB Meningococcus |
*Activities are not mutually exclusive and, in most instances, CICT involves varying amounts of each activity.
†Contemporary case-finding through contact tracing in MSM is very low.
STEC, Shiga toxin-producing E. coli; MDRO, Multidrug-resistant organism.
The COVID-19 pandemic highlighted the critical role of CICT in public health outbreak response and the need for a larger, more versatile CICT workforce. It also illustrated the limitations contact tracing in controlling a highly contagious respiratory infection with asymptomatic transmission.3 However, COVID-19 is not the only factor prompting a reconsideration of when and how to use CICT. The Ending the HIV Epidemic initiative4; the growing epidemic of congenital syphilis2; and the conversation of HCV from a chronic, difficult to treat infection to a readily curable infectious disease possibly amenable to elimination5 all challenge public health authorities to rethink how to optimize CICT.
In this article, we discuss the evolving role of CICT and the disease investigators who do this work. We start by separately discussing how CICT is currently employed for HIV/STI, TB, and other communicable diseases, including the current focus of work and opportunities for change, and end with thoughts on how CICT might evolve in the wake of 2 years of COVID-19, taking a broader view that spans disease-specific areas.
THE ROLE OF CICT IN COMMUNICABLE DISEASE CONTROL
Larger health departments typically have communicable disease programs charged with investigating dozens of reportable diseases, including foodborne and waterborne illnesses; respiratory, nosocomial, vaccine preventable, zoonotic, and travel-related infections; viral hepatitis; diseases of undetermined cause; emerging infections; and suspected cases of biological terrorism. Case investigations, which are initiated after medical providers or laboratories report infections to public health departments, provide the foundation for much of the surveillance and epidemiological work central to communicable disease programs.
For diseases that do not transmit from person-to-person, CICT is often limited to case investigations alone. However, identifying other potentially exposed persons among the contacts of cases of noncommunicable diseases is important when there are potential common exposure sources (eg, legionellosis). For diseases that transmit person-to-person, including some vaccine preventable diseases (eg, measles, meningococcal disease), contact tracing is essential to limit the spread of infection and ensure that infection control measures are implemented and that exposed and infected persons have timely access to preventive therapy and/or treatment. Health care-associated investigations, such as those resulting from contaminated medical devices, can result in large numbers of exposures that require follow-up investigations, risk assessment, counseling and, in some instances, treatment.
Key factors that determine if and how CICT is implemented include the severity of the disease, mode of transmission (airborne, droplet, blood borne, fecal-oral), disease incubation period and serial interval, whether the disease is communicable prior to the onset of symptoms, and what post-exposure prophylaxis or treatments are available. For certain high-consequence communicable diseases, exposed persons are monitored for the duration of their incubation period for the development of symptoms and to ensure compliance with infection control guidance.
The value of CICT is supported by the many instances in which such efforts have been instrumental in the identification of new and emerging pathogens, such as legionella,6 HIV,7 hantavirus8 and SARS-CoV-2.1 However, the evidence supporting the intervention's effectiveness in containing the transmission of infectious diseases is more limited, and in many instances the evaluation of CICT is probably not amenable to controlled studies.9 Most data on CICT effectiveness for communicable disease come from recent studies of COVID-19. Quasi-experimental,10 pre-post,11 and retrospective cohort studies12,13 suggest that CICT was effective in decreasing SARS-CoV-2 transmission in the United Kingdom and South Korea, at least before the emergence of more transmissible variants, like Omicron. This conclusion is also supported by a study in which persons interviewed for CICT reported that they notified more contacts as a result of speaking with contact tracers,14 as well as a CDC modeling study.15 However, other studies, including an evaluation of the temporal association of implementation of nonpharmacologic disease control measures and the reproductive number for SARS-CoV-2 in 130 countries, have not supported contact tracing's effectiveness.16,17 Data on the effectiveness of digital CICT for COVID are mixed.13,18 Case investigation and contact tracing for SARS-CoV-2 has also been used to selectively provide support services, such as groceries and rental assistance, to persons and communities disproportionately affected by COVID-19 with the goal of diminishing the economic toll and disparate impacts of the infection.14,19
THE ROLE OF CICT IN HIV AND STI
Case investigation and contact tracing is a core component of public health disease control for HIV and syphilis and, in some instances, has been used to attempt to control gonorrhea and, to a much lesser extent, chlamydial infection. However, there is increasing recognition that our approach to CICT for HIV/STI is outmoded and in need of significant modernization.20,21
The data supporting the efficacy of traditional CICT for HIV/STI in high-income nations is meager and of uncertain contemporary significance. Two randomized controlled trials conducted in the 1970 to 1980s among male STD clinic patients—only one of which was published—found that contact tracing undertaken by public health staff (ie, assisted partner notification services [APS]) was superior to leaving partner notification to patients themselves (ie, patient referral),22–24 a finding also supported by a small randomized controlled trial performed in the late 1980s among patients with HIV.25 Nonrandomized experimental, quasi-experimental, and case-control studies, mostly from the 1970s to 1980s, reported mixed results.26–30,31s More recent studies from sub-Saharan Africa support the efficacy of APS,32s–35s but the applicability of these studies to the United States is uncertain. Moreover, several studies suggest that the effectiveness of traditional partner services has eroded over time, and that many of the partners defined as treated through APS would have been treated in the absence of any intervention.36s–38s Summarizing data from US studies of syphilis partner notification published between 1995 and 2003, Brewer reported a median brought-to-treatment index (infected partners treated through per index case) of 0.22, whereas a recent study of syphilis APS in 7 US jurisdictions from 2015 to 2017 reported an index of 0.15,36s,39s a 32% decline. Human immunodeficiency virus APS effectiveness also appears to have declined in both the United States and United Kingdom (Fig. 1), with a recent study summarizing data from 14 US health departments in 2019 estimating that the median number of new HIV cases identified annually per fulltime DIS providing HIV APS was only 2.39s–43s Taken together, these data suggest that public health partner notification can be effective, but that the case-finding effectiveness of APS as currently provided by US health departments is low and, in and of itself, may not justify the county's current investment in partner notification.
Figure 1.
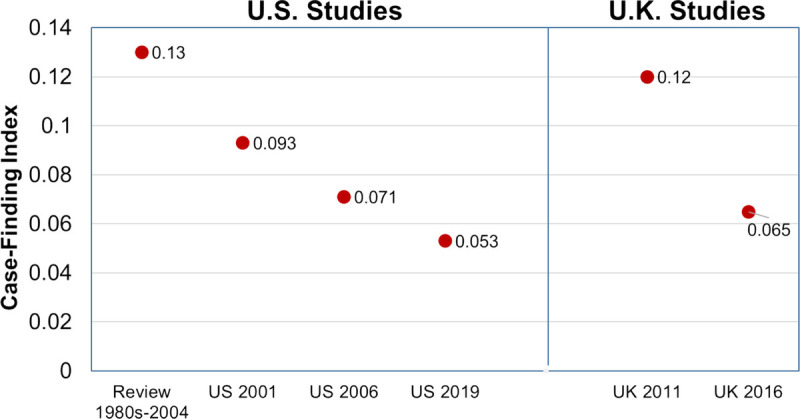
HIV partner services case-finding indices in larger published reports, United States and United Kingdom.39s–41s,43s,76s,77s
In contrast with these discouraging findings, several studies suggest that APS is or could be effective in advancing a wider array of public health objectives. As with CICT programs for other communicable diseases, APS programs have been important in identifying and characterizing outbreaks of HIV and syphilis.44s But a growing body of evidence also supports the intervention's effectiveness in promoting outcomes such as HIV testing among persons with bacterial STI,45s,46s linkage to HIV pre-exposure prophylaxis (PrEP),47s–49s and linkage and relinkage to HIV care50s–53s (Table 2). Studies from King County, WA, Chicago, and Iowa found that 25% to 44% of non-PrEP using HIV-negative MSM receiving APS will start PrEP after receiving APS that includes PrEP navigation. Meanwhile, a multistate study reported that 26% of HIV positive persons reported to health departments with bacterial STIs in 2016 to 2017 were out of care or unsuppressed.53s Whether APS can successfully promote relinkage to care and achievement of viral suppression in this population is unknown. Cost-effectiveness analyses suggest that integrating these outcomes into existing APS activities is likely cost-effective.54s,55s Whether a focus on these expanded outcomes warrants extending APS to populations that do not currently receive them is uncertain.
TABLE 2.
Studies Evaluating Expanded APS Outcomes for HIV in the United States
| Author | Population and Year | Design | Outcomes |
|---|---|---|---|
| Integrating HIV testing into APS for bacterial STI | |||
| Katz45s | MSM recipients of APS for bacterial STI in WA State 2010–2014 | Comparison of HIV testing and case-finding before and during a period when HIV testing was embedded into STD PS as an outcome | –Increase HIV testing from 63% to 91% –Relative risk HIV diagnosis = 1.34 (P = 0.07) |
| Samoff46s | 1646 index cases and 2181 contacts receiving APS for syphilis in North Carolina, 2015 | Program evaluation | –NNTI for syphilis to find a case of HIV was 43 |
| Avoundjan38s | 1535 index cases with 226 partners receiving APS in MS, 2014–16 | Program evaluation | –745 (56%) of 1321 without prior HIV dx tested for HIV, or whom 24 were newly diagnosed with HIV –NNTI HIV = 64 (38 for index cases with previously diagnosed HIV and 97 for HIV negative index cases) |
| Ronen74s | 8236 MSM receiving PS for bacterial STI in King County, WA, 2013–2018 | Program evaluation of offering SMS reminders to prompt HIV/STI testing | –13% of MSM accepted SMS HIV testing reminders. –Most men already had a system to prompt HIV repeat testing/ –Accepting reminders not associated with increased diagnosis asymptomatic STI |
| Integrating PrEP referral into APS for bacterial STI | |||
| Katz75s | 2055 HIV negative MSM with bacterial STI receiving APS in King County, WA, 2014–17 | Program evaluation | –49% MSM already on PrEP –73% of men off PrEP offered referrals, of whom 54% accepted. 47% of men who accepted referrals started PrEP. –15% of all MSM receiving APS, and 32% of high-risk men, initiated PrEP through APS referral |
| Howren49s | 189 persons receiving HIV testing and PS in Iowa, 2018–19. | Prospective cohort study with follow-up 30 days after receipt of APS | –15 (13%) of 117 PS enrolled PS recipients with follow-up data initiated PrEP –12 (44%) of 27 MSM initiated PrEP |
| Tex Da Silva48s | 146 Black MSM and trans women (TW) recruited from STI clinic PS program or social network strategy, Chicago, 2015–17 | Pilot randomized controlled trial of PrEP referral from a network intervention | –24% vs. 11% of intervention participants linked to PrEP P = 0.04 |
| Integrating linkage to care into HIV APS | |||
| Bocour50s | Persons with newly diagnosed HIV infection in New York City, 2006 | Observational study linking HIV APS data to HIV surveillance | Linkage to HIV care was higher in APS recipients than nonrecipients at 90 days (79% vs. 66%, P < 0.0001) |
| Hood51s | Persons with newly diagnosed HIV in King County, WA, 2010–15 | Observational study linking HIV APS data to HIV surveillance | Linkage to HIV care was higher in APS recipients than nonrecipients at 90 days (90% vs. 81%, P < 0.001) |
| Integrating relinkage to HIV care in APS for bacterial STI | |||
| Udeagu52s | 6315 persons interviewed for HIV APS 2015–18 | Program evaluation | Index cases named 858 previously diagnosed partners, of whom 366 were out of care or viremic. |
| Norkin53s | 20,780 persons reported with bacterial STI in 6 US states, 2016–17 | Matching of state STI and HIV surveillance data | 39% of ES, 27% LS, and 6% of GC cases were HIV+ 26–33% of HIV+ persons with STIs were out of care or not suppressed |
NNTI, number needed to interview.
THE ROLE OF CICT IN TB MANAGEMENT AND CONTROL
Case investigation and contact tracing is a core component of disease control activities for TB, often employing an approach that integrates CICT with the medical management of the index case (ie, case management). In the United States, CICT for TB is often called TB contact investigation (TBCI).56s The current scope of TBCI includes the following steps: (1) estimating the infectious period and assessing the likelihood of transmission from the index case, (2) identifying persons exposed during the infectious period and assigning priorities for evaluation based on the level of exposure and likely risk of progression to TB disease, (3) clinical evaluation of contacts to identify additional cases and persons with latent TB infection (LTBI) (eg, TB symptoms, a tuberculin skin test or an interferon gamma release assay), (4) treatment of contacts for TB disease and LTBI, and (5) assessment of whether TBCI should be expanded following the concentric circle approach based on the results of priority contacts.
Most people infected with Mycobacterium tuberculosis do not progress to active TB disease. The risk of progression from LTBI to TB disease varies based on age and the presence or absence of specific medical conditions, but is estimated to be 5% to 10% over the course of a lifetime, with half of TB disease occurring within the first 2 years of acquisition of M. tuberculosis.57s Because of this natural history, TBCI seeks to identify and treat both contacts with TB disease and those with LTBI. Few controlled studies have evaluated the efficacy or effectiveness of CICT in high-income nations, though a larger body of literature supports its efficacy in low- and middle-income countries.9 A study from Portugal comparing two periods of CICT, one during which index cases were interviewed to identify contacts and one that include interviews plus home and workplace visits, found that the more intensive approach identified more contacts with active TB and LTBI.58s In the absence of more controlled data, evidence supporting the effectiveness of CICT for TB relies on observational data and program evaluations. A study in the United States and Canada showed that TB disease was newly diagnosed in 2.1% of the contacts identified through TBCI.59s From 2003 to 2012, 65,195 (57%) of 114,003 TB cases in the United States received CICT leading to the evaluation of 857,807 contacts, 6235 (0.7%) of whom had TB disease (TB diagnoses per index [eg, case-finding index] 0.11), and 169,332 of whom had LTBI (case-finding index 2.6); 119,150 contacts initiated LTBI treatment (70%), of whom 77,264 (65%) completed that treatment.60s Although uncontrolled, these data strongly suggest that TBCI can be effective in identifying secondary cases of TB and LTBI and in preventing transmission, as well as TB disease.
Despite evidence supporting the effectiveness of TBCI, important challenges limit the intervention's success. Tuberculosis contact investigation requires multiple encounters with public health or clinical staff for clinical evaluation, testing, a chest x-ray and LTBI (or TB disease) treatment and monitoring. Consistent with the data cited above, a prospective study of close contacts to TB cases diagnosed with LTBI in the United States and Canada 2002 to 2006 found that only 53% completed preventive treatment, with lower treatment completion associated with receipt of 6 to 9 months INH treatment compared with shorter course regimens containing rifampin.61s
The challenge of ensuring index cases' and contacts' sustained engagement with care is sometimes compounded by the economic circumstances of affected populations, the diverse languages spoken by cases and contacts, and cultural barriers. National Health and Nutrition Examination Survey data from 2011 to 2012 reported point estimates for interferon gamma release assay positivity prevalence in US-born and non–US-born persons of 2.8% and 15.9%, respectively,62s and in 2021, 71% of TB cases in the United States occurred among non–US-born persons.
While the barriers to successful TBCI are formidable, there are also important opportunities driven by scientific and technical advances. New treatment regimens have the potential to increase LTBI treatment completion while decreasing demands on TB staff. Recent CDC guidelines on LTBI treatment recommend 3 to 4 months of rifamycin-based regimens, replacing 6 to 9 months of isoniazid as the preferred regimen.63s Currently, the CDC-sponsored TB Trials Consortium is conducting a randomized control trial to evaluate the efficacy and safety of 6 weeks of daily rifapentine, which could further shorten LTBI therapy.64s Electronic directly observed therapy, which can augment adherence to LTBI treatment, represents another opportunity to improve program effectiveness and efficiency.65s
DEVELOPING CICT CAPACITY TO CONFRONT CONTEMPORARY INFECTIOUS DISEASE CHALLENGES
CICT is a longstanding, core public health strategy in the control of a wide spectrum of infectious diseases. Existing data suggest that CICT can be effective in controlling infectious diseases,9 but the intervention's objectives, content, and impact is heterogeneous. The COVID-19 pandemic, changes in the epidemiology of infections such as syphilis, and the availability of new prevention and treatment interventions for infections like HIV, TB, and HCV all challenge public health authorities to rethink CICT with the goal of developing CICT capacity that is more versatile and better poised to advance diverse public health outcomes. At present, the relative investment health departments make in CICT for different infections is ill-defined and gaining a better understanding of this baseline would aid in developing a more effective system. Of note, the absence of virtually any public health investment in using disease surveillance and CICT to promote HCV treatment is an important oversight in current scope of CICT and should be an area of active research and new program development in the coming years. In the remainder of this article, we consider how health departments might develop CICT capacity to meet the needs of the 21st century (Table 3). Some of the subjects we address are discussed at greater length elsewhere in this issue of Sexually Transmitted Diseases.
TABLE 3.
Program Activities and Characteristics Related to Modernizing CICT
| Programmatic Activities and Characteristics | Considerations |
|---|---|
| Engaging community and health care organizations | • Staff should be reflective of communities receiving CICT • Community engagement to shape process and promote CICT acceptance • Engagement with HCOs to access data, develop and promulgate clinical guidance, and coordinate clinical services |
| Workforce development and retention | • Staff diversity—diverse clinical, language, and cultural skills to accomplish diverse CICT objectives • Training ○ Combination of federally and state/locally developed trainings ○ Varied modality—online/didactic, skills-based, mentored work ○ Core elements for all DIS + program specific training ○ Need to balance desire to impart comprehensive skills/knowledge with need to rapidly engage staff in work that promotes the public's health • Retention through competitive compensation and a clear career development path • Contingency plans to rapidly expand workforce to respond to emerging threats |
| Organizational structure | • Dilemma—vertical vs horizontal program structure—need to ensure collaboration between disease investigators, epidemiology, and clinical staff |
| Data Information Systems | • Dilemma—large, multipurpose data systems vs small, project specific data systems ○ Balance large scale capacity and functionality with need for versatility and programmatic control • Need to engage diverse stakeholders |
| Prioritization | • Clearly define hierarchy of priorities ○ Occurs at both the departmental and program level ○ Priorities defined by disease, population, and content of intervention • Which cases are worked varies over time reflecting hierarchy of priorities, population-level morbidity, and staffing |
| Content of CICT | • Varies depending on health condition, epidemiologic context, and resources • Whenever possible, the goal is to integrate epidemiologic data collection, disease prevention, and activities that benefit index cases and partners directly • Expanding measurable goals beyond data collection and contact tracing for a single specific infection can improve the impact and cost-effectiveness of CICT |
| Surge capacity | • Staff should be prepared to shift work to address public health emergencies |
HCO, health care organization.
Engaging the Clinical Health Care Delivery System and Communities
The success of CICT is highly dependent on the ability of health departments to foster collaborative relationships with the communities they serve and the health care organizations in their area. This collaboration is critical at every stage of the CICT process and should inform the development, staffing, and provision of CICT. Health departments should seek to educate health care providers about disease reporting and CICT and, whenever possible, seek to reach agreements with health care organizations to gain remote access to electronic medical records to aid in case investigations and to help coordinate cases' and contacts' medical care.
Workforce Development
The public health workforce employed in CICT varies based on the infection being investigated and the community affected, with nurses employed in some programs and disease investigators—often called disease intervention specialists (DIS) or public health investigators (PHIs)—acting as primary investigators in many others. Differences in program staffing typically reflect how much medical care is integrated into management of the cases CICT and whether it is provided by the public health agency or community-based health care providers. Regardless of the staffing model, building a successful CICT team requires recruitment of a diverse workforce with the community relationships and cultural and language skills needed to engage the populations affected by the health conditions public health seeks to investigate and control.
In response to the COVID-19 pandemic, the United States now seeks to vastly expand the cadre of DIS.66s,67s To be successful, this effort will require developing new training programs (including ongoing training and supervisor training); competitive compensation; and conscious efforts to retain the best staff, including a model of career advancement that rewards competence and creativity.
Major challenges in DIS training include defining core versus disease-specific specialty skills, determining the length of training prior to the initiation of independent work, identifying what trainings and disease-specific protocols can be developed nationally and what needs to be developed and provided at the state or local level, and building training capacity. Ideally, DIS training should include core elements imparting knowledge and skills—including soft skills—that all DIS need, as well as training to prepare staff for specific bodies of work (eg, HIV/STD, communicable disease, TB). Core elements include things like interviewing and communication skills, privacy and confidentiality, cultural competence, basic surveillance and epidemiology, medical record extraction, computer literacy, use of online resources for case investigation, field investigation, outbreak response, and emergency preparedness. Program-specific trainings focus on individual infections; interventions related to those infections; use of local databases; and how to engage people, communities, and key stakeholders (eg, medical providers) important in the public health response to a specific problem. Trainings have typically included face-to-face and online components followed by mentored work. For at least some infections, the CDC supports prevention training centers and online resources to train DIS, and it is currently developing a DIS certification program.68s Many state and local health departments develop trainings for their own staff, and CDC and private foundations have put forth broader plans related to training the public health workforce that are germane to the development of CICT capacity.69s,70s
The issue of compensation and retention is critical. At present, DIS salaries are highly variable, and so is retention. In some programs, attrition approaches 50% per year (Dan George, personal communication). Because salaries are set at the state and local level, this problem is not easily addressed nationally. This topic is addressed at greater length in another article in this issue.
Organizational Structure: Creating Interdisciplinary Teams
Building effective CICT capacity may require reorganizing CICT teams and reconfiguring the way public health infectious disease programs are administered, which is often driven by siloed categorical funding. Broadly speaking, public health departments or programs can be organized into units defined by a discipline (eg, clinical care, epidemiology, disease investigation) or by problem (eg, syphilis, TB, newly diagnosed HIV). Defining teams based on discipline can promote consistency and perhaps efficiencies as staff from the same discipline learn from peers and work across programs. But such an organizational structure has the potential to create unproductive silos as DIS, clinicians, and epidemiologists work in relative isolation. In general, science advances through interdisciplinary collaboration. Insofar as programs seek to create more refined hierarchies of disease investigation priorities (eg, selectively working syphilis cases based on HIV status, PrEP use, or viral suppression) and integrate new outcomes, they will need strong epidemiologic and program evaluation support. Meanwhile, with highly integrated teams, surveillance systems can benefit from bidirectional sharing of information obtained through CICT,51s something that is common when CICT focuses primarily on case investigation, but less common when DIS focus primarily on contact tracing and case management, often with minimal epidemiologic support. Different organizational structures can probably be successful as long as they promote regular communication and collaboration between epidemiologic, investigatory, clinical and, at times, social support service staff.
Data Management
Case investigation and contact tracing programs require data management systems. A huge array of systems exist, many of which proved unequal to the challenge of COVID-19. Choosing public health data systems presents fundamental dilemmas which have the potential to divide key stakeholders. Perhaps the most important of these is whether it is better a have a large multi-purpose system versus a series of smaller databases, sometimes developed on a common platform (eg, REDCap), designed to address specific public health problems. Large, multipurpose systems allow data related to different medical conditions to be maintained on a single data management platform. Such platforms often have broad functionality, interoperability with laboratory reporting and electronic health records, built-in reports, and have the potential to facilitate analyses addressing multiple health problems (eg, HCV in people with HIV). They are often favored by information technology programs, which prefer to minimize the number of platforms they support, and are potentially well aligned with national efforts to modernize public health data information systems.71s However, these large systems often come at a cost at the program level: flexibility and practicality. People charged with implementing public health programs often prioritize things like the user interface, the ability to rapidly create or modify data collection instruments without the support of IT programs or outside vendors, the impact of data entry on staff time and not on work satisfaction, and the ability to easily extract data at the local program level. This flexibility is critical when implementing new public health programs, which often require serial changes in data collection over a short period of time. There is no easy answer for how to confront this issue, but decision-makers should have this at the forefront of their minds as they engage stakeholders in selecting data management systems. The Association of State and Territorial Health Officers maintains a list of data systems used during the COVID-19 pandemic.72s
Defining CICT Priorities
Developing a contemporary and more effective approach to CICT requires an explicit effort to define priorities for which infections or problems should be investigated in which populations and with what CICT objectives. This would ideally happen at the national, local departmental, and the program level. For example, national guidance might establish broad standards, a health department would define local priorities and a plan for redeploying staff to confront major epidemics, and an HIV/STI program would establish a priority hierarchy of which cases to investigate that might place pregnant women with syphilis or unsuppressed HIV at the top.20,21 Ordering priorities allows programs to adjust which cases are assigned depending on population-level morbidity and available staffing, though in some instances this flexibility is limited by mandates created by categorical funding. This is particularly important when DIS need to be reassigned to confront an emergency, as occurred with COVID-19, when HIV/STD and TB DIS were reassigned early in the epidemic and often became trainers for rapidly growing DIS teams.14
Redefining the Objectives of CICT
As discussed above, an effective CICT program needs to think broadly about the objectives and content of CICT for different infections and different populations and how they will be realized given available resources. Case investigation and contact tracing is not a single intervention. For bacterial STIs—particularly among MSM and transgender persons—this will mean integrating outcomes like HIV testing and linkage to HIV care and PrEP into CICT. In some instances, these outcomes are likely to be more important than partner treatment. In confronting COVID-19, focusing limited CICT capacity on specific vulnerable or disproportionately affected population was justified, at least in part, to provide those communities with economic support.14,73s
Surge Capacity
The COVID-19 pandemic highlighted the inadequacy of the United States's public health infrastructure, including its CICT capacity. The nation is now making a major investment to expand that capacity.66s Part of that investment needs to be development of nimbler programs with greater capacity to rapidly move staff into new work in response to public health emergencies.
CONCLUSION
Strengthening health departments' workforce and capacity for CICT is a critical step in building a more effective public health system. Achieving that goal will require new efforts to engage communities and key stakeholders, build a diverse and well-trained professional workforce, and reimagine the organization and objectives CICT programs to take a more holistic approach that addresses the diverse needs of affected populations. In some instances, it will also require additional efforts to integrate CICT with disease surveillance and program evaluation. With sufficient political commitment and funding, we can establish more effective disease intervention systems and a workforce that can capitalize on new interventions to better control the infections that have long plagued the population, respond to the current COVID-19 pandemic, and be better prepared for inevitable future pandemics.
For further references, please see “Supplemental References,” http://links.lww.com/OLQ/A854.
Footnotes
Research reported in this publication was supported by the University of Washington/Fred Hutch Center for AIDS Research, an NIH-funded program under award number AI027757 which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, NIDDK.
Conflict of Interest and Sources of Funding: None declared.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (http://www.stdjournal.com).
Contributor Information
Masahiro Narita, Email: masa.narita@kingcounty.gov.
Lucretia Jones, Email: ljones@health.nyc.gov.
Peter Kerndt, Email: pkerndt@usaid.gov.
Jeffery Duchin, Email: Jeff.Duchin@kingcounty.gov.
REFERENCES
- 1.Holshue ML DeBolt C Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowen VB McDonald R Grey JA, et al. High congenital syphilis case counts among U.S. Infants born in 2020. N Engl J Med 2021; 385:1144–1145. [DOI] [PubMed] [Google Scholar]
- 3.National Association of County Health Officials (NACCHO). Joint Statement: Public Health Agencies Transitioning Away from Universal Case Investigation and Contact Tracing for Individual Cases of COVID-19. 2022. Available at: https://www.naccho.org/blog/articles/joint-statement-public-health-agencies-transitioning-away-from-universal-case-investigation-and-contact-tracing-for-individual-cases-of-covid-19. Accessed September 13, 2022.
- 4.Fauci AS Redfield RR Sigounas G, et al. Ending the HIV epidemic: A plan for the United States. JAMA 2019; 321:844–845. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services . Viral Hepatitis National Strategic Plan for the United States: A Roadmap to Elimination (2021–2025). Washington, DC, 2020. Available at: https://www.hhs.gov/sites/default/files/Viral-Hepatitis-National-Strategic-Plan-2021-2025.pdf. Accessed September 13, 2022. [Google Scholar]
- 6.Fraser DW Tsai TR Orenstein W, et al. Legionnaires' disease: Description of an epidemic of pneumonia. N Engl J Med 1977; 297:1189–1197. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control (CDC) . Pneumocystis pneumonia—Los Angeles. MMWR Morb Mortal Wkly Rep 1981; 30:250–252. [PubMed] [Google Scholar]
- 8.Duchin JS Koster FT Peters CJ, et al. Hantavirus pulmonary syndrome: A clinical description of 17 patients with a newly recognized disease. The hantavirus study group. N Engl J Med 1994; 330:949–955. [DOI] [PubMed] [Google Scholar]
- 9.Hossain AD Jarolimova J Elnaiem A, et al. Effectiveness of contact tracing in the control of infectious diseases: A systematic review. Lancet Public Health 2022; 7:e259–e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fetzer T, Graeber T. Measuring the scientific effectiveness of contact tracing: Evidence from a natural experiment. Proc Natl Acad Sci U S A 2021; 118:e2100814118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park Y Huh IS Lee J, et al. Seoul Metropolitan Government COVID-19 Rapid Response (SCoRR) Team . Application of testing-tracing-treatment strategy in response to the COVID-19 outbreak in Seoul, Korea. J Korean Med Sci 2020; 35:e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendall M Milsom L Abeler-Dorner L, et al. Epidemiological changes on the Isle of Wight after the launch of the NHS test and trace programme: A preliminary analysis. Lancet Digit Health 2020; 2:e658–e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wymant C Ferretti L Tsallis D, et al. The epidemiological impact of the NHS COVID-19 app. Nature 2021; 594:408–412. [DOI] [PubMed] [Google Scholar]
- 14.Hood JE Kubiak RW Avoundjian T, et al. A multifaceted evaluation of a COVID-19 contact tracing program in King County, Washington. J Public Health Manag Pract 2022; 28:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rainisch G Jeon S Pappas D, et al. Estimated COVID-19 cases and hospitalizations averted by case investigation and contact tracing in the US. JAMA Netw Open 2022; 5:e224042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malheiro R Figueiredo AL Magalhaes JP, et al. Effectiveness of contact tracing and quarantine on reducing COVID-19 transmission: A retrospective cohort study. Public Health 2020; 189:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y Morgenstern C Kelly J Lowe R, CMMID COVID-19 Working Group, Jit M. The impact of non-pharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories. BMC Med 2021;19:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogt F Haire B Selvey L, et al. Effectiveness evaluation of digital contact tracing for COVID-19 in New South Wales, Australia. Lancet Public Health 2022; 7:e250–e258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid M Enanoria W Stoltey J, et al. The SARS-CoV-2 pandemic: The race to trace: Contact tracing scale-up in San Francisco-early lessons learned. J Public Health Policy 2021; 42:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden MR, Katz DA, Dombrowski JC. Modernizing field services for human immunodeficiency virus and sexually transmitted infections in the United States. Sex Transm Dis 2017; 44:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden MR Gibbs J Woodward C, et al. Priorities in the implementation of partner services for HIV/STIs in high-income nations: a narrative review of evidence and recommendations. Sex Health 2022; 19:309–318. [DOI] [PubMed] [Google Scholar]
- 22.Cleveland JQ. A cost-effective study of alternative methods for gonorrhea contact referral and rescreening [unpublished report]. Dade County, FL: Dade County Department of Public Health. [Google Scholar]
- 23.Katz BP Danos CS Quinn TS, et al. Efficiency and cost-effectiveness of field follow-up for patients with Chlamydia trachomatis infection in a sexually transmitted diseases clinic. Sex Transm Dis 1988; 15:11–16. [DOI] [PubMed] [Google Scholar]
- 24.Golden MR, Faxelid E, Low N. Partner notification for sexually transmitted infections including HIV infection: An evidence-based assessment. In: Holmes KK Sparling PF Stamm WE, et al., eds. Sexually Transmitted Diseases, 4th ed. New York: McGraw Hill, 2008. [Google Scholar]
- 25.Landis SE Schoenbach VJ Weber DJ, et al. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med 1992; 326:101–106. [DOI] [PubMed] [Google Scholar]
- 26.Hammar H, Ljungberg L. Factors affecting contact tracing of gonorrhoea. Acta Derm Venereol 1972; 52:233–240. [PubMed] [Google Scholar]
- 27.Potterat JJ, Rothenberg R. The case-finding effectiveness of self-referral system for gonorrhea: A preliminary report. Am J Public Health 1977; 67:174–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Judson FN, Wolf FC. Tracing and treating contacts of gonorrhea patients in a clinic for sexually transmitted diseases. Public Health Rep 1978; 93:460–463. [PMC free article] [PubMed] [Google Scholar]
- 29.Woodhouse DE Potterat JJ Muth JB, et al. A civilian-military partnership to reduce the incidence of gonorrhea. Public Health Rep 1985; 100:61–65. [PMC free article] [PubMed] [Google Scholar]
- 30.Montesinos L Frisch LE Greene BF, et al. An analysis of and intervention in the sexual transmission of disease. J Appl Behav Anal 1990; 23:275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]


