Abstract
Background
We aimed to determine the cumulative burden of late (>five years from cancer diagnosis), major surgical intervention among childhood cancer survivors.
Methods
Self-reported late, major surgical interventions (anesthesia-requiring operations occurring >5 years after cancer diagnosis) were determined from the CCSS cohort and defined as the primary outcome. Cumulative burden was assessed using mean cumulative counts (MCC) of late, major surgical interventions. Piecewise exponential regression models with calculation of adjusted rate ratios (RR) evaluated associations between treatment exposures and late, major surgical interventions.
Findings
25,656 survivors diagnosed 1970–1999 (median follow-up 22.2 years, interquartile range [IQR]=16.5–29.7; median diagnosis age 6.1 years [IQR]=3.0–12.4) underwent 28,202 late, major surgical interventions. The 35-year MCC was 206.7 (95% confidence interval [CI]=202.7–210.8) per 100 survivors compared to 128.9 (95%CI=123.0–134.7) per 100 siblings. The RR of late, major surgical intervention was 1.8(95%CI=1.7–1.9) comparing survivors to siblings and 1.4 (95% CI 1.4–1.5) comparing female to male survivors. Survivors diagnosed in the 1990s (RR=1.4, 95%CI=1.3–1.5) experienced increased late surgery versus the 1970s. Survivors underwent late interventions at higher rates versus siblings in most anatomic regions/organ systems including central nervous system (RR=16.9, 95%CI=9.4–30.4), endocrine (RR=6.7, 95%CI=5.2–8.7), cardiovascular (RR=6.6, 95%CI=5.2–8.3), respiratory (RR=5.3, 95%CI=3.4–8.2), spine (RR=2.4, 95%CI=1.8–3.2), breast (RR=2.1, 95%CI=1.7–2.6), renal/urinary (RR=2.0, 95%CI=1.5–2.6), musculoskeletal (RR=1.5, 95%CI=1.4–1.7), gastrointestinal (RR=1.4, 95%CI=1.3–1.6), and head and neck (RR=1.2, 95%CI=1.1–1.4) interventions. Hodgkin lymphoma (35-year MCC=333.3 per 100 survivors, 95%CI=320.1–346.6), Ewing sarcoma (MCC=322.9, 95%CI=294.5–351.3), and osteosarcoma (MCC=269.6, 95%CI=250.1–289.2) survivors experienced the highest burden. Locoregional surgery and/or radiotherapy during cancer treatment were associated with undergoing late surgical intervention in the same body region or organ system.
Interpretation
Childhood cancer survivors have a significant burden of late, major surgical interventions, a late-effect that has previously been poorly quantified. Survivors would benefit from regular healthcare evaluations aiming to anticipate impending surgical issues and to intervene early when feasible.
INTRODUCTION
In the modern era, 85% of children diagnosed with cancer will become five-year survivors.(1) However, multimodal cancer therapy places childhood cancer survivors at increased risk for chronic health conditions, subsequent malignancies, and premature mortality as they age.(2–5) Surgery is a vital treatment for many of these late-onset health conditions and its high complexity and associated morbidity present a substantial burden for both individuals and health care systems. Although limited studies have evaluated rates and outcomes of undergoing specific operations among childhood cancer survivors, no study has assessed the burden of all surgical interventions that childhood cancer survivors undergo as they age.(6–10) An improved understanding of which subpopulations of survivors are more likely to need late surgical intervention, and which types of surgery are common could offer unique insight to inform current therapeutic choices to reduce risk for late surgical procedures.
To our knowledge, this is the first comprehensive study to ascertain the cumulative burden of late surgical intervention across the spectrum of childhood cancer survivors, utilizing a cohort of over 25,000 long-term survivors of childhood cancer with a median follow up of 22.2 years. The purpose of this study is to estimate the cumulative burden of late surgical intervention (>5 years after diagnosis of childhood cancer) among survivors as they age, compare it to siblings, and examine associations between specific childhood cancer treatments or diagnoses and burden of late surgical intervention.
METHODS
Study Populations
The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort study with longitudinal prospective follow-up of five-year survivors of childhood cancer treated among 31 North American institutions including a comparison group of nearest-age siblings of survivors selected by simple random sampling.(4, 11) All survivors were diagnosed with cancer before age 21 years between 1970–1999. Survivors were eligible at five years after original cancer diagnosis, and siblings entered on the same dates as their corresponding survivors. The institutional review board at each participating institution approved the CCSS protocol, and all participants or their legal guardians provided informed consent. This manuscript was prepared according to the STROBE guidelines checklist, which is included in the Supplementary Materials.
Late Surgical Intervention
The primary outcome was undergoing any late, major surgical intervention, defined as any surgical operation likely to require general anesthesia, or monitored anesthesia care, occurring five or more years after primary cancer diagnosis (Supplementary Data Files 1 and 2). Surgical interventions were self-reported by questionnaires that queried survivors and siblings about major surgical operations necessary for the treatment of significant acute or chronic conditions and their age at the time of the operations. A write-in option was included in the survey for surgical procedures performed if they did not correspond to specific questions (https://ccss.stjude.org/tools-documents/questionnaires/baseline-and-follow-up-questionnaires.html). To limit inclusion of interventions associated with routine screening or not associated with disease pathology, we excluded endoscopies and obstetric procedures from counts of major operative interventions. Non-obstetric gynecologic, genitourinary, and male reproductive interventions were included (Supplementary Data File 1).
All reported surgical interventions were centrally reviewed and assigned International Classification of Diseases, Ninth Revision (ICD-9) procedure codes. Any reported surgical intervention without a corresponding age was compared with the abstracted surgical records from the treatment of the primary cancer (within five years of diagnosis) and any potentially duplicate operation was excluded to minimize over-reporting. For time-dependent analyses, age at the time of the survey response was used when a corresponding age at surgery was not reported by the participant.
Explanatory Variables
Chemotherapy and radiotherapy for the original cancer treatment were systematically abstracted from the medical records of participating institutions and centrally reviewed. Radiotherapy dose to each body region was taken as the sum of the prescribed doses from all fields incident on a region as previously described.(12) There were no missing data values for age, sex, and race/ethnicity in the entire dataset. Health insurance status was missing from 2,155 of 25,656 (8.4%) survivors who completed the baseline survey, 78 of 9,301 (0.84%) who completed follow-up survey 2003, 65 of 8,007 (0.81%) who completed follow-up survey 2007, and 73 of 11,335 (0.64%) who completed follow-up survey 2015. The baseline survey was completed by all participants from the original (diagnosed 1970–86) and expansion (diagnosed 1987–1999) cohorts. The follow-up surveys 2003 and 2007 were for the original cohort only, while the follow-up survey 2015 was for both the original and expansion cohorts. For those with missing insurance status on the baseline survey, the status from the closest survey (i.e., follow-up 2003) was used.
Statistical Methods
The cumulative burden of late, major surgical interventions was defined as the mean cumulative count (MCC) of operations occurring greater than five years from diagnosis, which estimates the mean number of surgical operations by a given time point after 5-year survival in the presence of competing risk (mortality).(13) MCC, as opposed to cumulative incidence analysis in which a subject is censored at the time of the first event, allows for summarization of all events (multiple late, major surgical interventions) during the follow-up period, in order to measure the cumulative burden of late surgery in this cohort. MCC is represented per 100 individuals. In these analyses, the competing risk event (death) terminated the at-risk status for the event of interest (late, major surgical intervention). The follow-up period started five years from cancer diagnosis and ended with death, or most recent questionnaire completion, whichever occurred first. The MCC for all self-reported late, major surgical intervention was individually estimated and then summed to generate the MCC for grouped organ system categories. The bootstrap percentile method was used to estimate 95% confidence intervals (95%CI). The median age at first late, major surgical intervention was compared between survivors and siblings using the Wilcoxon signed-rank test. Unadjusted rates of late, major surgical intervention by organ system (per 1000 person-years of follow-up) were examined comparing all survivors, siblings, and survivors by cancer diagnosis to identify the distribution of surgical intervention within each group.
Multivariable piecewise exponential regression models estimated adjusted rate ratios (RR) of any late, major surgical intervention between survivors and siblings and between female and male survivors, adjusting for attained age as restricted cubic splines (knots at 15, 25, 30, 35, and 45), sex, race/ethnicity, and health insurance status. Similar models were constructed to estimate adjusted RRs of any, and subtype of, late, major surgical intervention (subtype according to organ system), with and without stratification by primary cancer diagnosis, relative to siblings. Adjusted RR for late, major surgical intervention according to diagnosis and surgery subtype were also calculated according to decade of diagnosis (1970s, 80s, 90s). For the decade of diagnosis analysis, only late, major surgical interventions occurring ≤ 20 years from diagnosis were included to control for inherent differential follow-up among survivors diagnosed during different decades.
A focused assessment of late, major surgical interventions less common in survivors than siblings compared crude rates (# interventions per 1000 person-years of follow-up) of all ICD-9-CM codes between survivors and siblings. Codes were selected with total event counts greater than 10 in siblings and with crude rates higher in siblings than in survivors. The above-mentioned piecewise exponential regression analysis was then performed using these codes.
All analyses, including reported percentages and means/medians, were weighted to account for under-sampling of acute lymphoblastic leukemia (ALL) survivors (1987–1999). Analyses were conducted with R Statistical Software v3.5.2 and SAS v9.4. All statistical inferences were two-sided, and p-values <0.05 were considered statistically significant.
Role of the Funding Source
The funding sources had no role in the study design, data analysis, collection, or interpretation. Furthermore, the funding sources had no role in the writing of the manuscript or the decision to submit the paper for publication.
RESULTS
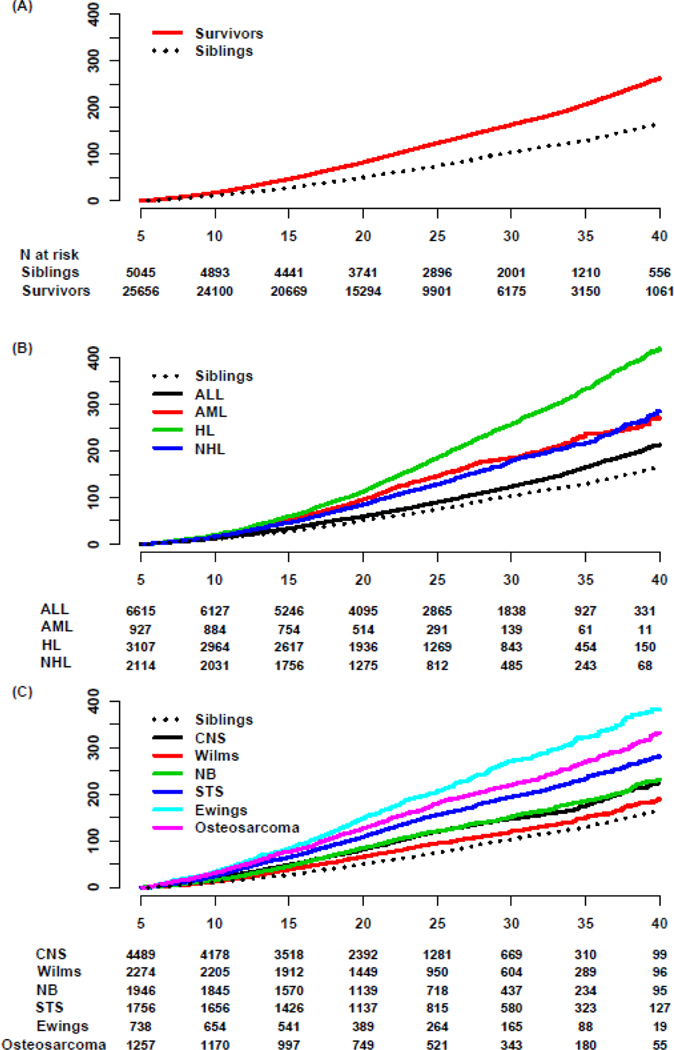
25,656 survivors diagnosed 1970–1999 (median follow-up 22.2 years, interquartile range [IQR]=16.5–29.7 underwent 28,202 late, major surgical interventions. The median age of survivors at cancer diagnosis was 6.1 years (interquartile range [IQR]=3.0–12.4) with median age at last follow-up of 29.4 years (IQR=22.9–37.1) and 35.2 years (IQR=26.8–43.9) for survivors and siblings, representing a median follow-up interval of 21.8 years (IQR=16.5–28.4) and 27.0 years (19.8–34.7), respectively (Table 1). The median age at first reported late, major surgical intervention was 23.5 years (IQR=16.5–30.5) for survivors and 25.4 (IQR=18.5–34.5) for siblings (p<0.0001, Supplementary Figure 1). The 35-year MCC of late, major surgical intervention (35 years after cancer diagnosis), was 206.7 (202.7–210.8) per 100 survivors and 128.9 (123.0–134.7) per 100 siblings (Figure 1; sex-stratified MCC are reported in Supplementary Figures 2-3). The adjusted rate ratio (RR) comparing late major surgical intervention in survivors to siblings was 1.8 (95% confidence interval [CI]=1.7–1.9, Table 2, Supplementary Table 1). Survivors of Hodgkin lymphoma (HL; 35-year-MCC=333.3/100 survivors, 95%CI=320.1–346.6), Ewing sarcoma (35-year-MCC=322.9/100, 95%CI=294.5–351.3), and osteosarcoma (35-year-MCC=269.6/100, 95%CI=250.1–289.2), experienced the highest cumulative burdens of late, major surgical intervention (Figure 1, Supplementary Table 1–2). Rates of death among survivors and siblings (competing event) are reported in Supplementary Table 3. A comprehensive list including percentages and frequencies of ICD-9-CM coded procedures is provided in Supplementary Data File 2 for survivors, siblings, and survivors according to their primary cancer diagnosis.
Table 1.
Demographic and treatment characteristics of childhood cancer survivors and siblings*
| Characteristic | Survivors (N = 25656) | Siblings (N = 5045) |
|---|---|---|
| Sex | ||
| Male | 13721 (53.5) | 2403 (47.6) |
| Female | 11935 (46.5) | 2642 (52.4) |
| Race/ethnicity | ||
| Non-Hispanic white | 20479 (79.8) | 4371 (86.6) |
| Non-Hispanic black | 1629 (6.3) | 149 (3.0) |
| Hispanic | 2045 (8.0) | 215 (4.3) |
| Other | 1503 (5.9) | 310 (6.1) |
| Health insurance status ** | ||
| Yes or Canadian resident | 20866 (87.9) | 4534 (90.7) |
| No | 2877 (12.1) | 467 (9.3) |
| Cancer diagnosis | ||
| Acute Lymphoblastic Leukemia | 6615 (25.8) | |
| Acute Myeloid Leukemia | 927 (3.6) | |
| Other Leukemia | 330 (1.3) | |
| CNS Tumor | 4489 (17.5) | |
| Astrocytomas | 2688 (10.5) | |
| Medulloblastoma, PNET | 1040 (4.1) | |
| Other CNS tumors | 761 (3.0) | |
| Hodgkin lymphoma | 3107 (12.1) | |
| Non-Hodgkin lymphoma | 2114 (8.2) | |
| Wilms Tumor | 2274 (8.9) | |
| Neuroblastoma | 1946 (7.6) | |
| Soft Tissue Sarcoma | 1756 (6.8) | |
| Ewing Sarcoma | 738 (2.9) | |
| Osteosarcoma | 1257 (4.9) | |
| Other bone tumors | 103 (0.4) | |
| Decade of diagnosis | ||
| 1970–1979 | 6612 (25.8) | |
| 1980–1989 | 10045 (39.2) | |
| 1990–1999 | 8999 (35.1) | |
| Age at diagnosis (years) | ||
| 0–3 | 8326 (32.5) | |
| 4–9 | 7645 (29.8) | |
| 10–14 | 5441 (21.2) | |
| 15+ | 4244 (16.5) | |
| Surgery as treatment for primary cancer | ||
| None | 6278 (27.6) | |
| 1–2 | 13039 (57.3) | |
| ≥3 | 3436 (15.1) | |
| Chemotherapy as treatment for primary cancer | ||
| Any | 19135 (81.4) | |
| Radiation therapy as treatment for primary cancer | ||
| Any radiotherapy | 13128 (55.7) | |
| Cranial radiotherapy | 6254 (27.3) | |
| Chest radiotherapy | 5200 (22.7) | |
| Abdominal/pelvic radiotherapy | 5265 (22.9) | |
| Total body irradiation | 605 (2.6) | |
| Any late, major surgical intervention | 12124 (47.3) | 2040 (40.6) |
| # of late, major surgical interventions | ||
| 1 | 5607 (46.2) | 1094 (53.6) |
| 2 | 2790 (23.0) | 462 (22.6) |
| 3–5 | 2914 (24.0) | 404 (19.8) |
| ≥6 | 813 (6.7) | 80 (3.9) |
While unweighted percentages are shown here for clarity, data used in analyses were weighted to account for undersampling of acute lymphoblastic leukemia (ALL) survivors (1987–1999).
from the most recent survey
Figure 1.
(A) Mean cumulative counts of major late surgical interventions in childhood cancer survivors compared to nearest-age sibling controls. (B) Mean cumulative counts of major late surgical procedures undergone by childhood cancer survivors of hematologic malignancies and (C) solid tumors compared to nearest-age sibling controls. ALL – acute lymphoblastic leukemia, AML – acute myeloid leukemia, HL – Hodgkin lymphoma, NHL – non-Hodgkin lymphoma, CNS – central nervous system tumors, Wilms – Wilms tumor, NB – neuroblastoma, STS – soft tissue sarcoma, Ewings – Ewing sarcoma, N – number. X-axis shows years from cancer diagnosis for A-C. Y-axis shows mean cumulative count per 100 individuals.
Table 2.
35-year MCC per 100 survivors, crude rates, and adjusted rate ratios comparing late, major surgical intervention in survivors versus siblings, including selected complex major surgical procedures.
| MCC, per 100 individuals Survivors | MCC, per 100 individuals Siblings | # of interventions per 1000 person-years of follow-up, Survivors | # of interventions per 1000 person-years of follow-up, Siblings | Adjusted rate ratio* (95% confidence interval) | |
|---|---|---|---|---|---|
| Any late major surgical procedure | 206.7 (202.7 – 210.8) | 128.9 (123.0 – 134.7) | 58.4 (57.8 – 59.1) | 36.8 (35.7 – 37.9) | 1.8 (1.7 – 1.9) |
| Surgery subtype | |||||
| Breast | 14.4 (13.1 – 15.7) | 7.6 (6.0 – 9.1) | 3.7 (3.4 – 3.9) | 2.5 (2.1 – 2.9) | 2.1 (1.7–2.6) |
| Mastectomy | 7.4 (6.4 – 8.3) | 1.5 (0.7 – 2.2) | 1.7 (1.6 – 1.9) | 0.5 (0.3 – 0.7) | 6.2 (3.8–10.2) |
| Cardiovascular | 19.6 (18.5 – 20.7) | 3.2 (2.4 – 4.0) | 5.5 (5.3 – 5.7) | 1.1 (0.9 – 1.3) | 6.6 (5.2 – 8.3) |
| Coronary artery bypass graft | 1.3 (1.0 – 1.6) | 0.3 (0.0 – 0.5) | 0.3 (0.2 – 0.3) | 0.1 (0.0 – 0.2) | 6.1 (2.4–14.5) |
| Heart valve replacement | 1.9 (1.5 – 2.3) | 0.3 (0.0 – 0.6) | 0.4 (0.4 – 0.5) | 0.1 (0.0 – 0.1) | 12.6 (4.8 – 33.1) |
| Central nervous system | 7.0 (6.4 – 7.7) | 0.3 (0.1 – 0.5) | 2.5 (2.3 – 2.6) | 0.1 (0.1 – 0.2) | 16.9 (9.4 – 30.4) |
| Ventriculoperitoneal shunt | 1.8 (1.5 – 2.0) | 0.1 (0.0 – 0.2) | 0.7 (0.6 – 0.7) | 0.0 (0.0 – 0.1) | 15.6 (4.7–51.6) |
| Endocrine | 14.3 (13.4 – 15.2) | 2.1 (1.5 – 2.8) | 3.7 (3.6 – 3.9) | 0.7 (0.6 – 0.9) | 6.7 (5.2 – 8.7) |
| Partial or total thyroidectomy | 13.3 (12.4 – 14.1) | 1.9 (1.3 – 2.5) | 3.5 (3.3 – 3.6) | 0.7 (0.5 – 0.8) | 6.9 (5.3–9.0) |
| Female gynecologic | 37.8 (35.7 – 39.9) | 36.0 (32.5 – 39.4) | 11.7 (11.3 – 12.2) | 13.5 (12.6 – 14.5) | 1.1 (1.0 – 1.2) |
| Hysterectomy | 17.2 (15.8 – 18.7) | 15.0 (12.7 – 17.4) | 4.1 (3.9 – 4.4) | 4.5 (4.0 – 5.2) | 1.3 (1.1–1.5) |
| Oophorectomy | 13.2 (12.0 – 14.4) | 9.1 (7.4 – 10.8) | 3.3 (3.1 – 3.6) | 3.0 (2.5 – 3.5) | 1.6 (1.3–1.9) |
| Gastrointestinal | 24.7 (23.5 – 25.9) | 18.4 (16.5 – 20.3) | 7.8 (7.6 – 8.1) | 5.9 (5.4 – 6.4) | 1.4 (1.3 – 1.6) |
| Colectomy or proctectomy | 2.3 (1.9 – 2.7) | 0.8 (0.4 – 1.3) | 0.7 (0.6 – 0.8) | 0.3 (0.2 – 0.5) | 2.9 (1.8–4.8) |
| Colostomy or ileostomy creation | 1.4 (1.1 – 1.7) | 0.4 (0.2 – 0.7) | 0.4 (0.3 – 0.5) | 0.1 (0.1 – 0.2) | 3.4 (2.0–5.7) |
| Surgery for intestinal obstruction | 2.8 (2.4 – 3.1) | 0.8 (0.4 – 1.1) | 0.9 (0.8 – 1.0) | 0.2 (0.1 – 0.3) | 4.8 (2.8–8.1) |
| Head and neck | 20.6 (19.6 – 21.6) | 18.5 (16.8 – 20.3) | 7.7 (7.5 – 7.9) | 6.4 (6.0 – 6.9) | 1.2 (1.1 – 1.4) |
| Cataract surgery | 2.7 (2.3 – 3.0) | 0.5 (0.2 – 0.8) | 0.9 (0.8 – 1.0) | 0.2 (0.2 – 0.3) | 4.9 (3.1–7.6) |
| Male reproductive | 5.4 (4.7 – 6.0) | 4.9 (3.8 – 5.9) | 2.7 (2.5 – 2.9) | 2.8 (2.3 – 3.3) | 1.0 (0.8 – 1.3) |
| Musculoskeletal | 32.3 (30.9 – 33.6) | 23.4 (21.1 – 25.6) | 11.8 (11.5 – 12.1) | 8.2 (7.6 – 8.7) | 1.5 (1.4 – 1.7) |
| Any limb amputation | 1.5 (1.3 – 1.7) | 0.5 (0.3 – 0.8) | 0.6 (0.5 – 0.6) | 0.2 (0.1 – 0.3) | 2.8 (1.7–4.5) |
| Any joint replacement | 5.4 (4.8 – 6.0) | 2.0 (1.3 – 2.7) | 1.8 (1.7 – 1.9) | 0.7 (0.6 – 0.9) | 2.8 (2.1–3.7) |
| Renal/urinary | 3.5 (3.1 – 3.9) | 1.9 (1.3 – 2.5) | 1.2 (1.1 – 1.3) | 0.6 (0.5 – 0.8) | 2.0 (1.5 – 2.6) |
| Respiratory | 3.7 (3.3 – 4.1) | 0.6 (0.3 – 0.9) | 1.4 (1.3 – 1.6) | 0.2 (0.2 – 0.3) | 5.3 (3.4–8.2) |
| Lung resection | 3.5 (3.1 – 3.9) | 0.6 (0.3 – 0.9) | 1.0 (0.9 – 1.1) | 0.1 (0.0 – 0.2) | 8.5 (4.6–15.6) |
| Spine | 5.1 (4.6 – 5.7) | 2.6 (1.9 – 3.4) | 1.9 (1.7 – 2.0) | 0.9 (0.7 – 1.0) | 2.4 (1.8–3.2) |
| Scoliosis correction | 2.6 (2.2 – 3.0) | 1.3 (0.7 – 1.9) | 1.0 (0.9 – 1.1) | 0.4 (0.3 – 0.5) | 2.9 (1.9–4.4) |
adjusted for attained age as cubic splines, sex, race/ethnicity, and health insurance status.
Survivors underwent central nervous system (RR=16.9, 95%CI=9.4–30.4), endocrine (RR=6.7, 95%CI=5.2–8.7), cardiovascular (RR=6.6, 95%CI=5.2–8.3), respiratory (RR=5.3, 95%CI=3.4–8.2), spine (RR=2.4, 95%CI=1.8–3.2), breast (RR=2.1, 95%CI=1.7–2.6), renal/urinary (RR=2.0, 95%CI=1.5–2.6), musculoskeletal (RR=1.5, 95%CI=1.4–1.7), gastrointestinal (RR=1.4, 95%CI=1.3–1.6), and head and neck (RR=1.2, 95%CI=1.1–1.4) interventions at higher rates relative to siblings (Table 2). Rates of male reproductive (RR=1.0, 95%CI=0.8–1.3) and female gynecologic (RR=1.1, 95%CI=1.0–1.2) surgical intervention were similar between survivors and siblings. In summary, survivors were more likely to have undergone surgical intervention in nearly every anatomic category and at an earlier age compared with siblings (Table 2, Supplementary Figure 1).
Female survivors underwent late, major surgical intervention at a higher rate than male survivors overall (RR=1.4, 95%CI=1.4–1.5) and for each cancer diagnosis (all p<0.0001 except neuroblastoma p=0.00064; Supplementary Table 4). This female predominance for late, major surgical intervention persisted even when sex-specific late, major surgical interventions (female gynecologic and male genitourinary procedures) were excluded from the analysis overall and for all cancer diagnoses except ALL, soft tissue sarcoma, and Ewing sarcoma (Supplementary Table 4).
Although no a priori anatomically defined categories of late, major surgical intervention were significantly less common in survivors versus siblings (Table 2), a focused analysis to query for individual procedure codes revealed that operations on the cornea (RR=0.5, 95%CI=0.3–0.8), teeth (RR=0.8, 95%CI=0.7–0.9), spleen and bone marrow (RR=0.5, 95%CI=0.2–0.9), anus (RR=0.4, 95%CI=0.2–0.7), spermatic cord, epididymis, and vas deferens (RR=0.5, 95% CI=0.4–0.6), fallopian tubes (RR=0.5–0.8, 95%CI=0.5–0.8), and cervix (RR=0.6, 95%CI=0.4–0.8) were significantly less common in survivors compared to siblings (all p<0.05; Supplementary Table 5).
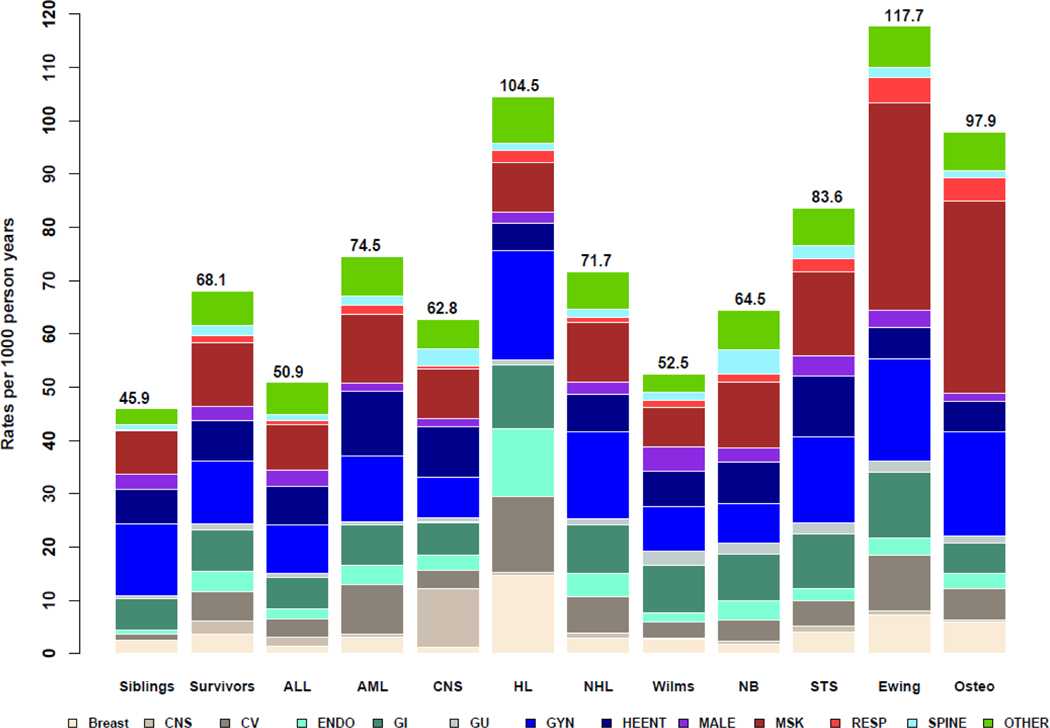
Unadjusted rates per 1000 person-years of follow-up of late, major surgical intervention in childhood cancer survivors, stratified by cancer diagnosis are shown in Figure 2 and are stratified by sex in Supplementary Figures 4-5. These results identify high rates of late central nervous system (CNS) surgeries among survivors of CNS malignancies, late cardiovascular, breast, and endocrine surgeries among HL survivors, late spine surgeries in neuroblastoma survivors, and late musculoskeletal surgeries among Ewing sarcoma and osteosarcoma survivors (Figure 2; Supplementary Data File 2). Rates of breast, cardiovascular, and endocrine interventions appeared particularly pronounced in female survivors of HL (Supplementary Figure 5).
Figure 2.
Rates of late, major surgical intervention among childhood cancer survivors (overall and by cancer diagnosis) and siblings, per 1000-person years of follow-up, stratified by organ system of surgery. Cancer types: ALL – acute lymphoblastic leukemia, AML – acute myeloid leukemia, CNS – central nervous system tumors, HL – Hodgkin lymphoma, NHL – non-Hodgkin lymphoma, Wilms – Wilms tumor, NB – neuroblastoma, STS – soft tissue sarcoma, Ewing – Ewing sarcoma. Organ systems: CNS – central nervous system, CV – cardiovascular, ENDO – endocrine, GI – gastrointestinal, GU – genitourinary, GYN – gynecologic, HEENT – head, eye, ears, nose and throat, Male – male reproductive surgery, MSK – musculoskeletal, RESP – respiratory
Treatment involving locoregional surgery and/or radiotherapy at the time of original cancer diagnosis was associated with undergoing late surgical intervention in the same body region or organ system (Table 3). Notably, locoregional surgery alone during initial cancer therapy was associated with a higher rate of late, major surgical intervention than radiotherapy alone in the CNS, respiratory, gastrointestinal, and musculoskeletal groups (Table 3).
Table 3.
Adjusted rate ratios* for late, major surgical intervention by organ system and based on treatment exposures within 5 years of cancer diagnosis
| Central Nervous System Treatment Exposures | Rate Ratio (95% CI) of Late Central Nervous System Surgery |
|---|---|
| No CNS surgery, no cranial RT | 1.0 (reference) |
| + CNS surgery, no cranial RT | 20.3 (15.3 – 26.8) |
| No CNS surgery, + cranial RT | 6.2 (4.7 – 8.3) |
| + CNS surgery + cranial RT | 21.9 (17.0 – 28.2) |
| Cardiovascular/Respiratory Treatment Exposures | Late Cardiovascular Surgery |
| No CV/Resp Surg, no chest RT | 1.0 (reference) |
| + CV/Resp Surg, no chest RT | 1.2 (1.0 – 1.6) |
| No CV/Resp Surg, + chest RT | 2.1 (1.8 – 2.3) |
| + CV/Resp Surg + chest RT | 2.5 (2.0 – 3.1) |
| Cardiovascular/Respiratory Treatment Exposures | Late Respiratory Surgery |
| No CV/Resp Surg, no chest RT | 1.0 (reference) |
| + CV/Resp Surg, no chest RT | 5.3 (3.9–7.4) |
| No CV/Resp Surg, + chest RT | 1.6 (1.1–2.1) |
| + CV/Resp Surg + chest RT | 4.6 (3.1–6.6) |
| Gastrointestinal Treatment Exposures | Late Gastrointestinal Surgery |
| No GI Surg, no abd/pelvis RT | 1.0 (reference) |
| + GI Surg, no abd/pelvis RT | 1.7 (1.4–1.9) |
| No GI Surg, + abd/pelvis RT | 1.3 (1.1–1.5) |
| + GI Surg + abd/pelvis RT | 1.9 (1.7–2.1) |
| Musculoskeletal Treatment Exposures | Late Musculoskeletal Surgery |
| No MSK surgery, no RT | 1.0 (reference) |
| + MSK surgery, no RT | 3.2 (2.8–3.6) |
| + RT, no MSK surgery | 1.0 (0.9–1.1) |
| + MSK surgery, + RT | 2.7 (2.4–3.2) |
Adjusted for attained age as cubic splines, sex, race/ethnicity, and health insurance status. CNS – central nervous system, CV – cardiovascular, Resp – respiratory, GI – gastrointestinal, MSK – musculoskeletal, RT – radiotherapy.
Respiratory surgeries comprise non-cardiac thoracic operations on the lung and airway.
When compared to survivors treated in the 1970s, survivors diagnosed in the 1990s (RR=1.4, 95%CI=1.3–1.5) experienced an increased rate of late, major surgery (Supplementary Table 6). This temporal trend was not seen in age-matched sibling controls who entered the cohort in the 1990s (RR=1.1, 95%CI=0.9–1.3) compared with the 1970s. A relative increase in the rate of late, major surgery was particularly evident for survivors of CNS malignancies (RR=1.8, 95%CI=1.5–2.2), neuroblastoma (RR=1.6, 95%CI=1.3–2.0), soft tissue sarcoma (RR=1.4, 95%CI=1.2–1.8), and osteosarcoma (RR=2.8, 95%CI=2.3–3.5) diagnosed during the 1990s compared with the 1970s (Supplementary Table 6). When specific surgery subtypes were evaluated, the increased rate of late, major surgery in survivors diagnosed in the 1990s was driven largely by CNS (RR=1.9, 95%CI=1.5–2.4) and musculoskeletal (RR=1.7, 95%CI=1.5–2.0) operations (Supplementary Table 7).
DISCUSSION
This study comprehensively describes the substantially increased cumulative burden of late, major surgical intervention experienced by childhood cancer survivors that occurs at increased rates and at a younger age when compared to siblings. Female survivors experience higher rates of late, major surgical intervention than males. The cumulative burden is highest among survivors of HL, Ewing sarcoma, and osteosarcoma. This burden is present across nearly all anatomically grouped procedure types. Late, major surgical intervention is associated with locoregional treatment exposures (surgery and radiotherapy) in the same organ system during primary cancer therapy. The need for late major surgical intervention in pediatric cancer survivors is increasing with time despite the well-established trend that chronic medical conditions are declining for more recent decades of diagnosis.(14)
Increased rates of late, major surgical intervention were present in nearly all anatomic systems when survivors were compared to siblings. Notably, male and female reproductive system operations were not found to be significantly different between survivors and siblings and no overall anatomic groups of procedures were found to be less common in survivors than siblings. However, when a focused analysis was performed to identify individual procedure codes within these anatomic groupings that were significantly less common in survivors compared to siblings, the resulting operations were notably less complex than those noted in survivors and included operations on the cornea, teeth, spleen, anus, spermatic cord, epididymis, vas deferens, fallopian tubes, and cervix. These results indicate that surgical care in siblings is more focused on routine procedures and reproductive health, while surgery in survivors is dominated by complex procedures resulting from late effects of primary cancer therapy.
The cumulative burden of late, major surgical intervention was noted to be highest in survivors of HL, Ewing sarcoma, and osteosarcoma. Among late surgical interventions in HL survivors, we found an increased proportion of breast, endocrine, and cardiovascular surgery that was most pronounced in females. Involved lymph nodes in HL are commonly treated with cervical and mediastinal radiation which is associated with subsequent thyroid and breast malignancies that require surgical intervention for local control.(15–17) A prior analysis showed that HL and Ewing sarcoma survivors had the highest excess absolute risk for the late development of subsequent malignancies associated with radiotherapy.(15) Furthermore, mediastinal radiation is associated with the development of coronary artery or cardiac valvular disease which can require percutaneous or open cardiac surgery.(18) Thus, it is clear that curative primary cancer therapy results in substantial surgical cost in the decades that follow.
Ewing sarcoma and osteosarcoma survivors have the highest cumulative burden of late, major surgical intervention among solid tumor survivors. A large component of this burden is the increased rate of additional late musculoskeletal surgeries. Late surgical intervention in primary bone sarcoma survivors involves arthroplasty, amputation, or prosthetic revision due to infection, device failure or associated fractures.(19–21) Furthermore, the 35-year cumulative incidence of Ewing sarcoma late recurrence is estimated to be 15.1%.(22) Late recurrence often requires surgical biopsy or local control. Appendicular skeletal osteosarcomas and Ewing sarcomas are treated with similar local control surgical approaches. However, axial Ewing sarcomas may be preferentially treated with high-dose radiotherapy as the solitary local control modality due to the morbidity associated with axial skeleton bony resection.(23) Furthermore, patients with pulmonary metastatic Ewing sarcoma typically receive whole-lung radiotherapy. Increased radiotherapy use in Ewing sarcoma compared to osteosarcoma may explain the increased cumulative burden of late, major surgical intervention among Ewing sarcoma survivors. An analysis of the CCSS Ewing sarcoma survivors demonstrated that musculoskeletal and cardiac chronic health conditions were most frequent and were associated with radiation exposure during cancer therapy.(22) High-dose musculoskeletal radiotherapy is associated with late complications including scoliosis, osteoarthritis, or subsequent malignant sarcomas, which may require late surgical intervention.(24) However, even without radiotherapy, the increased burden of late, major surgical intervention in osteosarcoma survivors demonstrates the profound impact that local control surgery can have on the need for late surgical intervention.
Female survivors of childhood cancer underwent late, major surgical intervention at higher rates versus males overall and for each cancer diagnosis included in the CCSS. This was particularly notable in, but not limited to, increased rates of breast, endocrine, and cardiovascular surgery in HL survivors. Oeffinger et. al. demonstrated that female survivors of childhood cancer were more likely than males to develop any grade 3–5 (severe) chronic health condition and specifically to develop multiple severe conditions.(2) Furthermore, female survivors of childhood cancer were previously shown to have a greater risk of several late-effects of cancer therapy including diminished overall health, subsequent malignancies, and anthracycline-related heart failure.(25–28) Our results confirm that these previously described late-effects of cancer therapy are more common in females also manifest in increased late, major surgical intervention.
This study firmly establishes a relationship between locoregional cancer therapy (surgery and/or radiation) and increased late surgical intervention in pediatric childhood cancer survivors. Despite prior studies showing a temporal trend of decreasing chronic health conditions in pediatric cancer survivors from more recent decades of diagnosis, our analysis shows that the rate of late, major surgery increased when survivors diagnosed in the 1990s were compared with the 1970s.(14) The increasing cumulative burden of late, major surgery is potentially due to an increased number of survivors of high-risk disease who underwent intense multimodality therapy including locoregional radiation and complex surgery in the case of CNS malignancies, neuroblastoma, and soft tissue sarcoma, and the increased implementation of limb-sparing procedures for primary therapy instead of amputation in bone sarcoma survivors such as osteosarcoma.
Counterintuitively, increased implementation of late, major surgical intervention was also noted for survivors of acute lymphoblastic leukemia, HL, and non-HL diagnosed in the 1990s versus the 1970s. Although the overall trend in therapy for these three malignancies has been de-intensification of therapy while preserving oncologic outcomes, therapies for specific moderate and high-risk groups have intensified during this time. Furthermore, technology and surgical advancements in recent decades may not have been available for survivors diagnosed in the 1970s and 1980s. Therefore, this study elucidates a formerly untold burden of late, major surgical intervention in childhood cancer survivors that is linked to locoregional therapy exposures, occurs at a younger age than sibling controls, is higher in females, and is increasing over time. The domain of late, major surgical intervention likely includes surgical procedures needed to treat chronic health conditions, but also includes treatment for the development of acute conditions such as a late fracture or prosthetic failure in a bone sarcoma survivor who underwent limb-sparing surgery during cancer treatment, a biopsy or tumor resection for late-relapse in a survivor of Ewing sarcoma, or cholecystitis requiring cholecystectomy or bowel obstruction exploratory laparotomy in survivors who underwent locoregional therapies for abdominal cancer.
There are limitations to this study to consider. First, the CCSS is not a population-based study. However, because the study contains participants who were treated at 31 North American institutions and diagnosed over three decades, the data are likely generalizable to the general pediatric cancer survivor population. Late surgical intervention was self-reported and thus omission or over-reporting may affect the estimated cumulative burden of late surgical intervention. Late, major surgical interventions were unable to be validated by comparison to hospital charts. Chemotherapy was not investigated in this pan-cancer CCSS analysis due to heterogeneous treatment agents and dosages utilized for each histology and the emphasis on anatomic/locoregional rather than systemic treatment exposures in the current study. As such, its role in influencing need for late surgery is unclear but could be further investigated in future studies focused on evaluating risk for late surgery among survivors of particular cancers. Because multiple factors contribute to the risk of undergoing surgery, including interaction of individual clinical performance status, comorbidities, surgical history, and inherent procedural complexities, we could not directly estimate the procedural risk for survivors compared with siblings. While the long-term follow-up of survivors diagnosed from 1970–1999 is a strength of this study, it does not contain information about survivors diagnosed in the last two decades.
In conclusion, this study demonstrates a newly understood chronic late-effect of pediatric cancer therapy comprising a significant burden of late, major surgical intervention. The need for late surgery should be anticipated and inform education of pediatric cancer patients at the time of diagnosis and treatment. Childhood cancer survivors should have regular healthcare evaluations which anticipate surgical issues and treat them early in the disease course.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We sought to determine whether childhood cancer survivors were undergoing higher rates of major surgical intervention greater than five years from their cancer diagnosis compared to the general population. We searched PubMed from the database origin to January 1, 2020 using the search terms “cancer survivors [MeSH Terms] AND (child [MeSH Terms] OR adolescent [MeSH Terms] OR infant [MeSH Terms]) AND Surgical Procedures, Operative [MeSH Terms]” for publications in all languages describing surgical procedures in survivors of childhood cancer. A second PubMed search was performed using the terms “‘childhood’ cancer survivors and ‘surgery.’” Previous studies demonstrated that childhood cancer survivors experience increased rates of specific late surgical procedures including cholecystectomy or limb amputation or specific domains of late surgical procedures including solid organ transplantation. However, the cumulative burden of late, major surgical intervention across procedure categories in childhood cancer survivors was poorly understood.
Added value of this study
To our knowledge, this is the first comprehensive study to detail the cumulative burden of late, major surgical interventions (anesthesia-requiring operations occurring greater than five years after cancer diagnosis) across the spectrum of childhood cancer survivors. Although a wide variety of chronic medical conditions and psychosocial late effects have been documented in childhood cancer survivors, the burden of late, major surgical intervention is particularly important to understand in this population because surgery and its associated complexity and possible morbidity present a substantial burden for both individuals and healthcare systems. This study also evaluates whether late, major surgical intervention is more common in subpopulations of childhood cancer survivors according to biological sex and cancer diagnosis. Furthermore, this study evaluates the temporal trend in late, major surgical intervention in childhood cancer survivors according to decade of diagnosis (1970s, 1980s, 1990s).
Implications of all the available evidence
Our study confirmed and expanded prior results that indicated childhood cancer survivors undergo increased rates of specific late surgical procedures or categories of procedures greater than five years from their original cancer diagnosis. In fact, our study shows that childhood cancer survivors undergo increased rates of late, major surgical intervention in nearly every organ system when compared to sibling controls. The burden of late, major surgical intervention is highest in female survivors and in survivors of Hodgkin lymphoma, Ewing sarcoma, and osteosarcoma. Late, major surgical intervention is increasing in more recently diagnosed pediatric cancer survivors. This could be due to increased survival in patients who underwent intense multimodality therapy or increased implementation of advancements in surgical technology. These data will influence informed consent to multimodality pediatric cancer treatment because, in addition to chronic medical conditions associated with therapy, we now know that survivors will undergo increased rates of major surgical intervention as they age when compared with the general population. This outcome should be anticipated and disclosed to patients and families, especially when complex local control interventions are planned for primary cancer therapy. Childhood cancer survivors would benefit from regular healthcare evaluations aiming to anticipate impending surgical issues and to intervene early in the disease course when feasible.
Acknowledgments of research support:
The Childhood Cancer Survivor Study is supported by the National Cancer Institute Grant No. CA55727 (G.T. Armstrong, PI) and by Cancer Center Support (CORE) Grant No. CA021765 (C. Roberts, PI) to St. Jude Children’s Research Hospital, as well as the American Lebanese Syrian Associated Charities.
The authors would like to thank the Childhood Cancer Survivor Study research coordinators and other health professionals who contributed to acquiring data utilized in this study. Finally, none of this would have been possible without the participation of childhood cancer survivors, their siblings, and their families – to them we are indebted.
Role of the funding source
This work was supported by the National Cancer Institute [Grant number CA55727], Cancer Center Support (CORE) [Grant number CA21765] to St. Jude Children’s Research Hospital, and the American Lebanese Syrian Associated Charities. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
National Institutes of Health/National Cancer Institute and American Lebanese Syrian Associated Charities/St. Jude Children’s Research Hospital
Footnotes
Declaration of interests
The authors have no relevant disclosures or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data sharing statement
Primary data from the Childhood Cancer Survivor Study are accessible to the public and available at: https://ccss.stjude.org/public-access-data/public-access-data-tables.html. These data include public access data tables from the baseline questionnaire, data on chronic conditions through follow-up survey six, and treatment data from medical record abstraction including chemotherapy, surgery, and radiotherapy. Mortality outcomes are available through 2017. CCSS questionnaires from which these data were obtained are available at: https://ccss.stjude.org/toolsdocuments/questionnaires/baseline-and-follow-up-questionnaires.html.
REFERENCES
- 1.Howlader N, Noone A, Krapcho M, Miller D, Bishop K, Kosary C, et al. SEER Cancer Statistics Review Bethesda, MD: National Cancer Institute; 1975-2014 [Available from: https://seer.cancer.gov/archive/csr/1975_2014/. [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. [DOI] [PubMed] [Google Scholar]
- 3.Bhakta N, Liu Q, Ness KK, Baassiri M, Eissa H, Yeo F, et al. The cumulative burden of surviving childhood cancer: an initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet. 2017;390(10112):2569–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diller L, Chow EJ, Gurney JG, Hudson MM, Kadin-Lottick NS, Kawashima TI, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. J Clin Oncol. 2009;27(14):2339–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suh E, Stratton KL, Leisenring WM, Nathan PC, Ford JS, Freyer DR, et al. Late mortality and chronic health conditions in long-term survivors of early-adolescent and young adult cancers: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2020;21(3):421–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dieffenbach BV, Li N, Madenci AL, Murphy AJ, Barnea D, Gibson TM, et al. Incidence of and risk factors for late cholecystectomy in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Eur J Cancer. 2020;133:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieffenbach BV, Liu Q, Murphy AJ, Stein DR, Wu N, Madenci AL, et al. Late-onset kidney failure in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Eur J Cancer. 2021;155:216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madenci AL, Fisher S, Diller LR, Goldsby RE, Leisenring WM, Oeffinger KC, et al. Intestinal Obstruction in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2015;33(26):2893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madenci AL, Dieffenbach BV, Liu Q, Yoneoka D, Knell J, Gibson TM, et al. Late-onset anorectal disease and psychosocial impact in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2019;125(21):3873–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dietz AC, Seidel K, Leisenring WM, Mulrooney DA, Tersak JM, Glick RD, et al. Solid organ transplantation after treatment for childhood cancer: a retrospective cohort analysis from the Childhood Cancer Survivor Study. Lancet Oncol. 2019;20(10):1420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howell RM, Smith SA, Weathers RE, Kry SF, Stovall M. Adaptations to a Generalized Radiation Dose Reconstruction Methodology for Use in Epidemiologic Studies: An Update from the MD Anderson Late Effect Group. Radiat Res. 2019;192(2):169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Robison LL, Leisenring WM, Martin LJ, Armstrong GT, Yasui Y. Estimating the burden of recurrent events in the presence of competing risks: the method of mean cumulative count. Am J Epidemiol. 2015;181(7):532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gibson TM, Mostoufi-Moab S, Stratton KL, Leisenring WM, Barnea D, Chow EJ, et al. Temporal patterns in the risk of chronic health conditions in survivors of childhood cancer diagnosed 1970–99: a report from the Childhood Cancer Survivor Study cohort. Lancet Oncol. 2018;19(12):1590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman DL, Whitton J, Leisenring W, Mertens AC, Hammond S, Stovall M, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Novetsky Friedman D, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32(21):2217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor AJ, Croft AP, Palace AM, Winter DL, Reulen RC, Stiller CA, et al. Risk of thyroid cancer in survivors of childhood cancer: results from the British Childhood Cancer Survivor Study. Int J Cancer. 2009;125(10):2400–5. [DOI] [PubMed] [Google Scholar]
- 18.Dunn AN, Donnellan E, Johnston DR, Alashi A, Reed GW, Jellis C, et al. Long-Term Outcomes of Patients With Mediastinal Radiation-Associated Coronary Artery Disease Undergoing Coronary Revascularization With Percutaneous Coronary Intervention and Coronary Artery Bypass Grafting. Circulation. 2020;142(14):1399–401. [DOI] [PubMed] [Google Scholar]
- 19.Jeys L, Grimer R. The long-term risks of infection and amputation with limb salvage surgery using endoprostheses. Recent Results Cancer Res. 2009;179:75–84. [DOI] [PubMed] [Google Scholar]
- 20.Toy PC, White JR, Scarborough MT, Enneking WF, Gibbs CP. Distal femoral osteoarticular allografts: long-term survival, but frequent complications. Clin Orthop Relat Res. 2010;468(11):2914–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiger EJ, Liu W, Srivastava DK, Bernthal NM, Weil BR, Yasui Y, et al. What Are Risk Factors for and Outcomes of Late Amputation After Treatment for Lower Extremity Sarcoma: A Childhood Cancer Survivor Study Report. Clin Orthop Relat Res. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marina NM, Liu Q, Donaldson SS, Sklar CA, Armstrong GT, Oeffinger KC, et al. Longitudinal follow-up of adult survivors of Ewing sarcoma: A report from the Childhood Cancer Survivor Study. Cancer. 2017;123(13):2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Indelicato DJ, Keole SR, Shahlaee AH, Shi W, Morris CG, Gibbs CP, et al. Impact of local management on long-term outcomes in Ewing tumors of the pelvis and sacral bones: the University of Florida experience. Int J Radiat Oncol Biol Phys. 2008;72(1):41–8. [DOI] [PubMed] [Google Scholar]
- 24.Kuttesch JF, Wexler LH, Marcus RB, Fairclough D, Weaver-McClure L, White M, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14(10):2818–25. [DOI] [PubMed] [Google Scholar]
- 25.Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–92. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia S, Yasui Y, Robison LL, Birch JM, Bogue MK, Diller L, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease: report from the Late Effects Study Group. J Clin Oncol. 2003;21(23):4386–94. [DOI] [PubMed] [Google Scholar]
- 27.Neglia JP, Friedman DL, Yasui Y, Mertens AC, Hammond S, Stovall M, et al. Second malignant neoplasms in five-year survivors of childhood cancer: childhood cancer survivor study. J Natl Cancer Inst. 2001;93(8):618–29. [DOI] [PubMed] [Google Scholar]
- 28.Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Primary data from the Childhood Cancer Survivor Study are accessible to the public and available at: https://ccss.stjude.org/public-access-data/public-access-data-tables.html. These data include public access data tables from the baseline questionnaire, data on chronic conditions through follow-up survey six, and treatment data from medical record abstraction including chemotherapy, surgery, and radiotherapy. Mortality outcomes are available through 2017. CCSS questionnaires from which these data were obtained are available at: https://ccss.stjude.org/toolsdocuments/questionnaires/baseline-and-follow-up-questionnaires.html.