Abstract
To learn to read, the brain must repurpose neural systems for oral language and visual processing to mediate written language. We begin with a description of computational models for how alphabetic written language is processed. Next, we explain the roles of a dorsal sublexical system in the brain that relates print and speech, a ventral lexical system that develops the visual expertise for rapid orthographic processing at the word level, and the role of cognitive control networks that regulate attentional processes as children read. We then use studies of children, adult illiterates learning to read, and studies of poor readers involved in intervention, to demonstrate the plasticity of these neural networks in development and in relation to instruction. We provide a brief overview of the rapid increase in the field’s understanding and technology for assessing genetic influence on reading. Family studies of twins have shown that reading skills are heritable, and molecular genetic studies have identified numerous regions of the genome that may harbor candidate genes for the heritability of reading. In selected families, reading impairment has been associated with major genetic effects, despite individual gene contributions across the broader population that appear to be small. Neural and genetic studies do not prescribe how children should be taught to read, but these studies have underscored the critical role of early intervention and ongoing support. These studies also have highlighted how structured instruction that facilitates access to the sublexical components of words is a critical part of training the brain to read.
We read because we and others write. We read because only about 4,000 years ago, it became important to make meaning out of purposeful scribbles. Through those early scribbles, human communication changed so it did not have to be face-to-face (Wolf, 2007). Language and sensory processing (usually visual, although Braille is a tactile example; visual processing is our focus here) are key components of the reading process. The capacity for language and visual processing are considered experience-expectant aspects of the developing brain that any baby born with typical, healthy sensory organs and brain will develop. Literacy, in contrast, is considered an experience-dependent brain process because without a substantial amount of exposure and instruction within a culture, the brain will not typically develop this ability.
Unlike spoken language, reading is acquired. The brain must forge novel connections between language and visual systems for successful reading, repurposing evolutionarily determined centers. The acquisition of literacy rewrites the organization of the brain (Dehaene, 2009). Thus, it not surprising that this process can be difficult for many children, and illiteracy is a worldwide problem; although many children come to school primed to learn to read and engage the brain in the necessary reorganization, approximately 40% of people struggle with reading even with sufficient instruction and opportunity (Seidenberg, 2017). Further, reading difficulties often run in families, speaking to the critical role of genes in the reading process. Genetic processes contribute to individual differences in learning to read most likely because of genes driving the structure and function of the brain and its malleability. Thus, those with particular genetic profiles, resulting in particular structural and functional properties of the brain, may be at increased risk for reading difficulties.
In this article, we review what is known about reading in the brain, how changes in reading ability impact the brain, the heritability and genetic etiology of literacy, and the importance of early experiences and education for developing literacy. The development and improvement of literacy in a person offers a remarkable opportunity to study the brain’s plasticity for a novel skill, and the impact of genes and environmental factors on that development. This prospect is true in typically developing readers, struggling readers, and those identified with the term specific reading disability (SRD), encompassing both word- and text-level difficulties in reading (American Psychiatric Association, 2013).
There are multiple ways different cultures have represented language through writing. Writing systems generally fit into one of three classes: alphabetic (a mapping of symbols to sublexical units, such as phonemes), syllabic (a mapping of symbols to syllable), or logographic (a mapping of symbol to word or morpheme), although in some languages, the symbol can map to single or multiple phonemes depending on the context (e.g., akshara units; Nag, 2014; Perfetti & Dunlap, 2008). Because the bulk of neurobiological research has addressed alphabetic writing systems, in the literature review, we focus on those research findings. The reader is urged to consider the growing number of studies of nonalphabetic written languages and to not presume that all results discussed here hold across different writing systems (Share, 2014).
Models of the Reading Process
Multiple influential models of reading have been proposed based on intensive and careful studies of reading performance in both humans and artificial (computer) systems (Dehaene, 2009; Seidenberg, Cooper Borkenhagen, & Kearns, 2020). One is the dual-route model, which proposes that every word is processed simultaneously by orthographic (lexical; word recognition) and phonological (sublexical; sound-it-out) processing streams, with fluent reading relying more on rapid orthographic processing and early reading relying more on the phonological processing stream (Church, Coalson, Lugar, Petersen, & Schlaggar, 2008; Coltheart, Rastle, Perry, Langdon, & Ziegler, 2001; Pugh et al., 2001). This model was heavily influenced by behavioral studies of people who sustained strokes and lost specific capacities for reading.
Another influential set of models, which are derived from computational modeling and statistical learning theory, are connectionist models. Often referred to as the triangle model, connectionist models explicitly add semantic processing to the orthographic and phonologic pathways (Plaut, McClelland, Seidenberg, & Patterson, 1996; Seidenberg, 2017; Seidenberg et al., 2020). As Figure 1 shows, dual-route and connectionist models converge on the idea of brain pathways for lexical and sublexical processing. However, in connectionist models, the underlying mechanism is not dual but singular. Word processing occurs as simultaneous, parallel processes based on the weighted connections of different components of the word. As neural networks are trained through exposure and feedback, different orthographic units (words) activate different brain patterns. Training and feedback lead to the development of computational rules that become implicit and automatic. The relation of orthography and phonology is learned because the brain is exposed to a visual representation and encodes pronunciation of the word. Feedback is received on the correct pronunciation. This iterative process continues as the strength of connections grows among the different units of the word: Orthographic, phonological, and semantic weights for each word are strengthened and become more implicit and automatic (J.S.H. Taylor, Rastle, & Davis, 2013).
FIGURE 1. Two Proposed Paths of Reading in the Brain: the Dorsal, Phonographic Route and the Ventral, Orthographic Route (Dark Arrows).
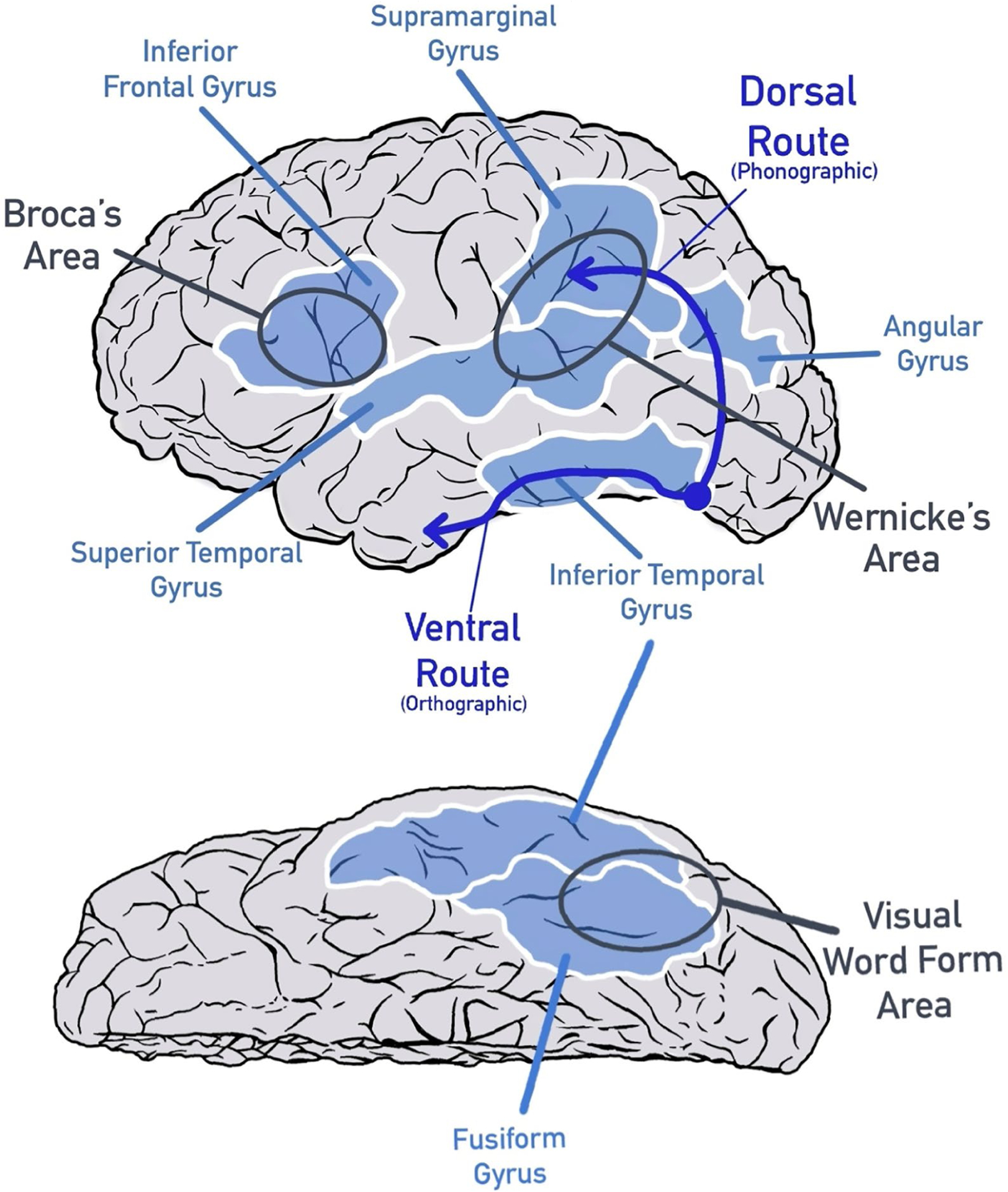
Note. Key regions of the reading brain are indicated on the left hemisphere.
Reading and the Brain
As understanding of the brain has grown, theoretical models of reading have been related to engagement of specific brain regions during the reading process. Brain regions that are important for reading are known because of studies of reading problems after brain damage (e.g., stroke) and, in the last quarter century, because of noninvasive neuroimaging tools. Neuroimaging tools such as functional magnetic resonance imaging (fMRI) allow unprecedented access to populations that do not typically experience stroke or other types of brain damage (i.e., children, young adults). From these studies of changes in the oxygenated blood flow in the brain during reading-related tasks in English and other alphabetic languages, the field has learned that reading engages the left hemisphere of the brain more than the right hemisphere, which is similar to other aspects of language processing.
When learning to read, a student must understand that symbols represent particular units of a language. Early readers of alphabetic written languages must make the association to the phonological structure of speech explicit and apply it to print: first at a sublexical level and then in larger and larger units; this is known as the alphabetic principle (Liberman, 1996). This is easier (and faster) to learn for orthographies in which symbols map consistently onto particular sounds (e.g., Italian) than in English, in which letter symbols frequently represent different sounds depending on a word’s context (e.g., the a in want vs. wait). Experience with reading eventually leads to the rapid lexical processing and immediate orthographic access to meaning common to dual-route and connectionist models. As Figure 1 shows, this early, effortful sublexical processing takes a dorsal, or higher, route through the brain from the back of the brain (where visual processing occurs) forward. The sublexical system traveling through the middle temporal and inferior parietal regions is indirect and inefficient for accessing meaning at any kind of speed; still, it is the only way a nonskilled reader can make sense of print as words. The new reader has to explicitly connect what words look like with how they sound. The lexical, ventral, or lower, system for reading (see Figure 1) develops almost simultaneously in the left occipitotemporal region of the brain and takes advantage of an existing evolutionary system for visual processing, including faces and objects (Vogel, Miezin, Petersen, & Schlaggar, 2012).
As soon as the emerging reader begins to make sense of words as forms, this system begins to organize as a rapid orthographic processor based in part on the statistical properties by which letters and letter combinations occur, consistent with connectionist models (Dehaene, Cohen, Morais, & Kolinsky, 2015). The ventral system requires considerable exposure to print in order to reorganize for reading. If access to print is delayed, as is the case for many children at risk for reading difficulties, these systems do not receive the early experience needed to optimally create the expertise for rapid processing of print. These two different sets of brain regions align well with the dual-route and connectionist models of reading, with the triangle model adding a set of semantic-related brain regions (generally also located in the temporoparietal cortex but shown to be widely distributed; Huth, de Heer, Griffiths, Theunissen, & Gallant, 2016; Seidenberg, 2017).
These models and the corresponding brain systems make specific predictions about where differences in an alphabetic writing system should be seen in struggling readers relative to proficient readers. Some neuroimaging studies have focused on structural differences in the brains of different groups of people (i.e., struggling and typical readers). In structural analyses, researchers have compared brain region size, thickness, or the white matter fiber bundles that connect regions to one another. Many of the studies found differences in the structure of reading-related brain regions in those who struggle to read (e.g., Black et al., 2012; Qi et al., 2016; Richlan, Kronbichler, & Wimmer, 2013; Williams, Juranek, Cirino, & Fletcher, 2018).
Early in the use of fMRI, functional brain analyses of reading in older children and adults established that struggling readers engage these reading-related brain regions less than their nonstruggling peers do (Eden et al., 2004; B.A. Shaywitz et al., 2002; S.E. Shaywitz et al., 1998). Much work has subsequently expanded on this initial finding of decreased engagement, studying functional brain activity in children in different types of reading tasks, such as reading comprehension (Meyler et al., 2007; Roe et al., 2018), or activity related to change from reading interventions (Barquero, Davis, & Cutting, 2014; Hoeft et al., 2011; Nugiel et al., 2019; Odegard, Ring, Smith, Biggan, & Black, 2008; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003). Thus, in recent years, research has focused less on if or where reading difficulties are seen and more on complicated questions such as how the reading brain changes over age or with skill change, the impact of learning gains or loss, and the impact of genetic risk or instructional approach on the reading brain.
Changes in Literacy and Changes in the Brain
Struggling Readers Over Time
There are three primary findings from studies of neural changes in struggling readers related to time or intervention. First, in over 20 studies, when reading improves, differences seen in reading-related brain regions (often in the left hemisphere) relative to nonstruggling readers seem to decrease, with brain activity related to reading improvement relates to normalization of previously decreased activity (for a summary, see Fletcher, Lyon, Fuchs, & Barnes, 2019). Relatedly, stronger readers appear to have more consistent engagement of reading-related regions from trial to trial (Malins et al., 2018). Second, some studies have found evidence of right-hemisphere engagement increasing in struggling readers who go on to experience improvement (Hoeft et al., 2011; Nugiel et al., 2019; B.A. Shaywitz et al., 2004). This greater engagement of the right hemisphere in the reading process is most often interpreted as a potential compensatory or alternate mechanism by which the brain attempts to improve reading, when the original pathways are not optimal.
Third, some studies have begun to expand beyond a focus on reading-related regions of the brain to other brain networks (e.g., those brain regions related to cognitive control, or brain regions known as default regions because they typically show negative signal during tasks when other regions exhibit positive signal). These studies have found that reading-related change over time involves changes in these nonreading networks as well, possibly related to the maturation of controlled attention, or reflecting change in effort or motivation over time (Aboud, Barquero, & Cutting, 2018; Hoeft et al., 2011; Nugiel et al., 2019). Engagement of these additional brain regions in the reading process may depend on the type of reading task being studied; reading comprehension tasks may be more likely to engage the brain’s cognitive control regions related to reading ability (Aboud, Bailey, Petrill, & Cutting, 2016; Meyler et al., 2007; Patael et al., 2018; Roe et al., 2018). Interestingly, differences in the engagement of these nonreading networks between struggling and nonstruggling readers, or in struggling readers over time, are apparent only during reading tasks; cognitive control tasks that do not require reading do not differentiate good and poor readers (Nugiel et al., 2019; Roe et al., 2018).
Prereaders
Studies of brain structure have tested young children who are at familial risk for reading problems or have delayed language onset, prior to the start of reading instruction (prekindergarten), and found brain differences even in very young children (Myers et al., 2014; Ozernov-Palchik et al., 2019; Raschle, Chang, & Gaab, 2011; Saygin et al., 2013; Vandermosten, Hoeft, & Norton, 2016). These types of studies are important because they can begin to separate more causal brain mechanisms from compensatory mechanisms, as both are likely present in older readers. For example, one study reported altered sulcal (grooves in the brain) patterns in prereaders at risk for reading problems (Im, Raschle, Smith, Grant, & Gaab, 2016). There have also been findings relating the brain’s white matter organization in young children to their later reading success (e.g., Zuk et al., 2020). Further, studies have shown that the brain’s white matter undergoes ongoing changes related to reading skill acquisition over time (Travis, Adams, Kovachy, Ben-Shachar, & Feldman, 2017; Vandermosten et al., 2015).
Analysis of brain structure of very young children with MRI is possible because children can watch a movie or otherwise relax (even sleep) during the MRI. It remains challenging to study brain function using MRI in very young children (ages 0–6 years) because of the simultaneous demands of holding still and responding to a task. However, researchers have already been working on this scientific frontier with increasing success (e.g., Centanni et al., 2019; Kersey, Wakim, Li, & Cantlon, 2019; Turesky, Vanderauwera, & Gaab, 2021; Wang, Joanisse, & Booth, 2018; Weiss, Cweigenberg, & Booth, 2018; Yamada et al., 2011). These studies of brain function in young prereaders and early readers have been able to capture how parts of the brain’s reading network develop in tandem with reading ability and reshape the field’s understanding of brain specialization and plasticity over age and experience (Centanni et al., 2018; Dehaene-Lambertz, Monzalvo, & Dehaene, 2018; Yamada et al., 2011). Further, there are other techniques that have long been used to study functional brain activity in young children. Some fMRI studies have complemented ongoing electroencephalography studies using event-related potentials of prereaders and young readers (Bach, Richardson, Brandeis, Martin, & Brem, 2013; Brem et al., 2010; Hämäläinen et al., 2013; Hong et al., 2018; Stevens et al., 2013), showing that certain early sound and letter processing-related activity relates to later reading abilities.
Adult Illiteracy
What happens in the brains of individuals who do not learn to read until later in life? A number of studies have researched brain activity related to illiteracy or literacy abilities in adults (Cachia et al., 2018; Pegado et al., 2014; Resende et al., 2018). Adults who, for a variety of reasons, did not have the opportunity or ability to learn to read as children can be invaluable participants in research that improves the field’s understanding of plasticity in the mature brain (López-Barroso et al., 2020). Dehaene and colleagues (2015) reported that the neural network that emerges with instruction for children and adolescents also emerges in adults acquiring reading skills for the first time. Due to reduced and late exposure, automaticity does not emerge as quickly because print exposure is insufficient to adapt the ventral systems. However, engagement of the dorsal system is apparent with the onset of instruction. The ventral system also begins to reorganize. The left fusiform gyrus becomes increasingly specialized for reading, as is seen in younger readers. There is no loss of other capacities, and acquiring literacy actually enhances other skills, possibly due to the development of the neural circuitry mediating reading (Dehaene et al., 2010). Many studies have focused on the role of the ventral system via the fusiform gyrus (the bottom cortex traveling from the back, visual areas of the brain forward toward the temporal, or side, areas; see Figure 1) as an interface between vision and language. Better literacy in adults is typically characterized by faster visual discrimination of letter and word stimuli, as well as a growing specialization of the left fusiform cortex for word and letter stimuli with gains in literacy (López-Barroso et al., 2020; Pegado et al., 2014). Again, the picture of reading as an acquired skill that reorganizes the brain is apparent in any learner exposed to formal reading instruction, regardless of age.
Learning to read has been characterized as learning a second language (Seidenberg et al., 2020). In this respect, another approach to understanding literacy-related changes is to ask adults to learn to read a new language (J.S.H. Taylor, Davis, & Rastle, 2017; Younger & Booth, 2018; Zhao, Li, Elliott, & Rueckl, 2018). Notably, adults learn novel written languages best through a print-to-sound teaching strategy, consistent with child learning studies (J.S.H. Taylor et al., 2017). Further, novel language learning can capture and explore differences seen in cross-cultural studies of language and reading processing across different age groups (e.g., syllabic vs. letter-level symbolism; Hirshorn, Wrencher, Durisko, Moore, & Fiez, 2016).
The Role of Individual Differences
Although we have described how the brain reorganizes to support literacy, not all brains are equally adept at learning. Studies of individual differences in reading using neuroimaging have related out-of-scanner (e.g., school-based, standardized) reading measures of each participant to a brain area’s size (e.g., Torre, Matejko, & Eden, 2020), white matter robustness (e.g., De Vos et al., 2020), or the level of activity during a given fMRI task (e.g., Roe et al., 2018). For these analyses, it is important to carefully consider both sampling size and variability within the measures being compared, as both meaningfully impact sensitivity to detect statistically significant and reliable effects. It is also critical to not evaluate individual differences within a region already shown to have a statistically significant group effect (i.e., this is a statistical error of double-dipping; Button, 2019; Kriegeskorte, Simmons, Bellgowan, & Baker, 2009).
Understanding of individual differences in reading via neuroimaging has also benefited from multivariate techniques such as multivariate pattern analysis, which can test for patterns across single or multiple data types to predict reading (e.g., Kristanto, Liu, Liu, Sommer, & Zhou, 2020), or categorize or even identify individuals or their family members from a group (e.g., Demeter et al., 2020). More mobile neuroimaging technologies, such as functional near-infrared spectroscopy, may make acquiring more diverse, longitudinal developmental samples easier, although each technology has its own set of limitations (e.g., functional near-infrared spectroscopy cannot currently analyze subcortical structures).
Critically, child engagement of reading-related brain regions has been associated with parental reading ability, pointing to the heritable nature of many reading components and the importance of the home reading environment (Horowitz-Kraus, Hutton, Phelan, & Holland, 2018). These biological and environmental constraints lead to individual differences in the success people experience in responding to reading instruction, and highlight the need to consider learners individually, especially in relation to the type and intensity of reading instruction. However, these individual differences are quantitative, not qualitative, and exist on a continuum. They are a matter of degree, not kind. The nature of these constraints and how individual differences manifest becomes clearer when considering genetic influences on learning to read.
The Heritability of Learning to Read and Reading Disabilities
A Brief History of the Research
The moment modern culture began to require literacy as a means of inclusion in the labor market, it became important to understand that not all people are able to become proficient readers with the same success; not all people’s experience with learning how to read is the same (Kerr, 1897). Teachers cannot guarantee that their method of delivering reading instruction will reach each student equally, because students’ reading abilities vary. Early studies about such a variation in reading abilities brought up the idea that gaps in ability could be innate (J.H. Fisher, 1905, 1910; Hinshelwood, 1900, 1902, 1907; Stephenson, 1904, 1907; Thomas, 1905). Later, positions on familiality, heredity, and reading ability surfaced (Hallgren, 1950) based on evidence highlighting the brain’s involvement in reading difficulties (Orton, 1939).
Since the late 20th century, scientists have utilized Hallgren’s (1950) ideas surrounding the genetic factors that relate to and potentially influence reading difficulties. This scientific journey has been as structured as possible, given various limitations, such as the lack of diagnostic materials surrounding genetics and reading difficulties, the price and availability of necessary associated (molecular genetic) technologies, and the availability of analytical methods necessary to appraise the role of the genome in reading-related difficulties. By the early 1980s, pioneering articles surfaced, allowing for heightened development within the field. It is important to note the following influential publications.
Major gene models—recessive (Lewitter, DeFries, & Elston, 1980), dominant (Gilger, Borecki, DeFries, & Pennington, 1994; Pennington et al., 1991), and additive (Pennington et al., 1991)—and polygenic models (Pennington et al., 1991) are noteworthy because of their alignment with varying sets of family data. Although not maintained within the modern findings, these ideas are important to this line of research for multiple reasons. For example, this research defined reading difficulties through two key lenses: qualitative phenotypes and quantitative phenotypes, a notable approach. Additionally, the varied results generated by this research have illuminated the diversity of the genetic mechanisms of reading and reading disability. Currently, new analytical approaches to this research highlight the involvement of multiple genes most likely of moderate to low effect sizes (Hsu, Wijsman, Berninger, Thomson, & Raskind, 2002; Naples, Chang, Katz, & Grigorenko, 2009; Wijsman et al., 2000). Finally, because varied degree-of-genetic relationships samples from the field are available, estimates of relative risk (Ziegler et al., 2005) consistent with the heritability estimates for (a)typical reading and related traits have been generated.
The numerous genetically informed samples acquired have allowed the field to grow significantly (i.e., twins or other siblings, nuclear and extended families). This has provided key information on various issues surrounding the topic. With that, more accurate estimates of heritability are now available for reading performance and reading-related skills. Importantly, the vastly available literature can and has been meta-analyzed, and results reveal that when error variance is noted, heritability estimates are between 41% and 74% for reading ability and up to 90% for reading-related skills (Grigorenko, 2004). Estimates of heritability are not controlled by sex (Hawke, Wadsworth, Olson, & DeFries, 2007), but other factors, such as age (Byrne et al., 2009) and ethnicity (Grigorenko, Ngorosho, Jukes, & Bundy, 2006), come into play. Furthermore, heritability estimates differ in relation to the severity of difficulties, in that it is more sizable in groups with major deficits (Hawke et al., 2007) and higher IQ (Wadsworth, Olson, & DeFries, 2010) and increases nonlinearly as children age (Byrne et al., 2005; Kovas et al., 2013; Samuelsson et al., 2007; Wadsworth, Corley, Hewitt, & DeFries, 2001).
The foundation of quantitative genetics indicated that heritability is most influential when the environment is uniform and beneficially primed for people to acquire reading and reading-related sills. Alternatively, environments that lack such support do not allow people to convey their genetic potential for reading. This has been highlighted in literature associated with teaching instruction (J. Taylor, Roehrig, Soden Hensler, Connor, & Schatschneider, 2010), parental education (Friend et al., 2009), and socioeconomic status (SES; Hart, Soden, Johnson, Schatschneider, & Taylor, 2013).
Finally, Smith, Kimberling, Pennington, and Lubs (1983) highlighted genetic factors and interpreted the heritability of reading and reading-related skills into specific molecular mechanisms. This one-of-a-kind report instigated ongoing research pertaining to the discovery of specific molecules and their pathways that set the standard for sociocultural construction of the reading brain.
Molecular Genetic Aspects of Learning to Read and Reading Difficulties
Similar to many other complex disorders, the field of molecular genetic research into (a)typical reading has been dominated by two major models of the overall genetic architecture of complex human traits. The elemental difference between these models lies in the identification of the disability and the interpretation of normal variation in reading performance. Genetic studies of SRD have recently been dominated by the common disorder–common variant (CDCV) hypothesis that SRD arises on the polygenic background: The inheritance of multiple common genetic risk variants are individually characterized by small effect sizes but collectively represent a certain liability threshold above which the disability is manifested. It is also assumed that some of these risk variants are general to all facets of SRD and, perhaps, to SRD and other learning difficulties, such as with math skills (Plomin & Kovas, 2005), whereas others are SRD-specific and even reading component–specific (Naples et al., 2009). These partial overlaps of risk variants can explain substantial, but far from 1.0, genetic correlations between different reading-related componential processes, both in typical reading and SRD. These partial overlaps can also explain differential heritability estimates for different reading-related componential processes and their fluctuations when other sources of variance (e.g., age, ethnicity, SES, quality of teaching) are considered. Finally, the CDCV hypothesis assumes the continuity between typical and atypical states (i.e., a single underlying trait that defines various states), as common risk variants are present in the general population at levels below the liability threshold, and this presence guarantees a progression of reading performance.
The common disorder–rare variant (CDRV) hypothesis, on the contrary, assumes that almost each case is caused by a single rare variant of large effect size and that these variants can materialize in different genes in different families/individuals. This hypothesis can explain the powerful findings of the genetic foundation for specific families and the lack of generalizability of these findings to heterogeneous SRD samples. Although both hypotheses and their multiple versions are implied in the literature on SRD, they have not been methodically tested. This is true for the CDRV hypothesis, the evidence in support of which in the field of SRD is, although strong, rather circumstantial than systematic. To describe the research noting both hypotheses, we provide a brief introduction on the terminology, conceptual apparatus, and common grounds on which the corresponding studies have been generated.
The first draft of the human genome sparked a decade of studies surrounding the genetic makeup of common human traits/disorders and has resulted in a number of understandings. For example, it is not apparent that the statistical power of genome-wide association studies of complex traits/diseases, even with sample sizes that were a great deal larger than previously employed (e.g., thousands of cases and controls), remains low (Manolio, Brooks, & Collins, 2008; Rodriguez-Murillo & Greenberg, 2008; Tenesa et al., 2008; Wellcome Trust Case Control Consortium, 2007).
To address this issue, more robust samples have been requested (Manolio, 2010), although disagreements regarding the potential yield of these calls have arisen (Stein & Elston, 2009). The attempt to comprehend the heterogeneity has resulted in a growing interest in the role of rare genetic variants in the etiology of complex disorders (Ahituv et al., 2007; Cohen et al., 2004, 2006; Ji et al., 2008; Romeo et al., 2007, 2009; Zhu, Feng, Li, Lu, & Elston, 2010). Often, the effect of a rare variant is limited to specific isolated families or individuals, thus assuming the presence of many heterogeneous rare variants, both transmitted in families and arising de novo, the large-effect (Gorlov, Gorlova, Sunyaev, Spitz, & Amos, 2008) impact of which prompts homogeneous (or semihomogeneous) behavior manifestations.
Upon the completion of the first draft of the human genome (International Human Genome Sequencing Consortium, 2001), the assumption in the field was that delineating the main type of genomic/genetic variation between two individual human beings would be captured primarily (although not exclusively) by their complement of single nucleotide polymorphisms (SNPs; a common variation in each of the nucleotides—adenine, thymine, cytosine, or guanine—of the DNA sequence). This type of variation was expected to affect approximately .1% of the total genomic sequence. Yet, ensuing research has not confirmed that delineation and, instead, has revealed copy number variation and structural variation (CNV/SV1) to be the main sources of variation among humans. The essence of this variation is complicated: In addition to SNPs, each genome contains an abundance of tiny insertions and deletions (indels: the insertion or deletion of bases in the DNA sequence longer than one nucleotide) and a plethora of CNV/SV, where entire blocks of the DNA sequence, ranging in size from just 1 KB to several MB, have been inserted, deleted, inverted, or somehow translocated (Conrad et al., 2010).
Similar to this basic realization depicting the nature of normal variation in the human genome, more studies have linked not only point mutations and functional SNPs but also events such as CNV/SV to phenotypic effects, both in fit and unfit individuals. Yet, technology has only recently become available that may permit the linking of comprehensively mapped genotypes consisting of all classes/sizes of variation events to clinical phenotypes. Normal human genomic variation must be considered when attempting to interpret complex traits/disorders, if indeed a complex trait/disorder is caused not by a single genetic event of strong effect but by a combination of variants, each of small effect, or by a rare variant of medium strong effect embedded in a background of modifying normal variants (i.e., the merger of the CDCV and CDRV hypotheses).
We hope that our explanations have explained the stockpile available to the genome to control both typical and atypical development of a skill. Reading development is obviously not an exception, and the genome has recruited many tricks that demonstrate the diversity of human brain structure and function. This is reflected, in turn, in the diversity of human development in general and reading development in particular. Next, we present a brief overview to illustrate how the two hypotheses, CDCV and CDRV, may be exemplified in the modern literature on the molecular genetic bases of typical and atypical reading.
Recent Developments in Genetic Studies of Reading and Reading Difficulties
CDCV has leveraged the frequent manifestation of reading difficulties in the general population, whereas CDRC has capitalized on the infrequent manifestation of severe reading difficulties existing within extended families highly dense with SRD. These studies (see Grigorenko, 2005) can be broken down into a number of key overlapping categories, by the type of samples they engaged (i.e., genetically unrelated cases/probands and matched controls vs. family units such as siblings or nuclear and extended families) and by the type of genetic units they targeted (i.e., specific genes or specific genetic regions vs. the whole genome). For all approaches combined, there are currently references to approximately 20 (Schumacher, Hoffmann, Schmäl, Schulte-Körne, & Nöthen, 2007) potential genetic susceptibility loci (i.e., regions of the genome that have demonstrated a statistically significant linkage to SRD; in most cases, these regions consist of more than one and often hundreds of genes) and six or more official (Grigorenko & Naples, 2009; Peterson & Pennington, 2012) and many unofficial, but recognized, candidate genes (i.e., genes located within susceptibility loci that have been statistically associated with SRD). None of these loci or genes have been either entirely welcomed or rebuffed by the field. Moreover, new regions and candidate genes are being submitted regularly, and both catalogs are likely to continue growing (Rubenstein, Matsushita, Berninger, Raskind, & Wijsman, 2011).
To date, multiple genome-wide screens for SRD and reading-related difficulties (e.g., Brkanac et al., 2008; de Kovel et al., 2004; Eicher et al., 2013; Fagerheim et al., 1999; Field et al., 2013; S.E. Fisher et al., 2002; Gialluisi et al., 2014; Igo et al., 2006; Kaminen et al., 2003; Luciano et al., 2013; Meaburn, Harlaar, Craig, Schalkwyk, & Plomin, 2008; Nopola-Hemmi et al., 2002; Raskind et al., 2005; Roeske et al., 2011; Svensson, 2011) have been reported. These studies, driven by the CDCV hypothesis, utilized hundreds of thousands of SNPs, as technology and cost allowed. Studies also have focused on select areas of the genome. The selection of these regions is usually determined either by a previous whole-genome scan or by a theoretical hypothesis capitalizing on SRD and its componential processes (Skiba, Landi, Wagner, & Grigorenko, 2011).
Nevertheless, some researchers have decided on candidate regions through different methods, such as through a known chromosomal aberration (i.e., through the verification of the CDRV hypothesis). For instance, Denmark evaluates all newborns for macrochromosomal changes (e.g., large rearrangements). With those data, researchers have evaluated those who have such macrochromosomal changes for the presence of SRD as they participate in early schooling (Buonincontri et al., 2011). The conjecture is that a gene affected by such a chromosomal abnormality is associated with SRD in some way. ROBO1 (Hannula-Jouppi et al., 2005), DYX1C1 (Taipale et al., 2003), and SEMA6D2 (Ercan-Sencicek et al., 2012) are candidate genes for reading difficulties that have been illuminated by this process. All of these genes have been detected through studies of isolated families (Taipale et al., 2003) or even individual cases (Ercan-Sencicek et al., 20123).
It is important to stress that such large variations are relatively rare (e.g., <1% of the general population), and the underlying assumption here is that the identification of such rare variants will provide a clue for future studies of the gene(s) affected by this structural alteration, or the pathway in which this gene(s) is(are) involved. The idea is that once a rare variant is identified and associated with a particular trait (e.g., reading), there is a need to investigate common variance in the gene/region that was impacted by this rare variant. In the field of reading, an example of such a transition from a rare variant to a continuous trait is the research on the ROBO1 gene (Bates, Luciano, Montgomery, Wright, & Martin, 2011).
Nine candidate regions of the human genome have been involved in SRD. These regions are recognized as SRD candidate regions; they are abbreviated as DYX1–DYX9 (DYX for dyslexia, a term often used to refer to SRD) and refer to the regions on chromosomes 15q, 6p, 2p, 6q, 3cen, 18p, 11p, 1p, and Xq, respectively. Each of these regions harbors dozens of genes, and some of them have been named as risk genes for SRD: two for the 15q region, DYX1C1 and SEMA6D; two for the 6p region, KIAA0319 and DCDC2; two for the 2p region, C2Orf3 and MRPL19; and one for the 3cen region, ROBO1. Although the field has not yet converged on firm candidates, it is remarkable, and of scientific interest, that of the current candidate genes for SRD, five genes (DYX1C1, SEMA6D, KIAA0319, DCDC2, and ROBO1) are involved in biological functions of neuronal migration and axonal crossing. Thus, all of these genes are plausible candidates for understanding the pattern of brain functioning in those with reading difficulty.
Importantly, more genes have been reported as presumed additions to this list (e.g., Buonincontri et al., 2011; Ercan-Sencicek et al., 2012; Gialluisi et al., 2019; Newbury et al., 2011; Scerri et al., 2010). At this point, the field contains both support and lack of support for the involvement of each of these genes; thus, the findings are somewhat difficult to interpret. In addition, there is an ongoing debate regarding the specificity of the impact of SRD-related genes. Some of the genes identified as candidate genes for SRD have been featured in other learning disabilities (e.g., Mascheretti et al., 2014), as well as in other developmental disorders and unrelated health difficulties, such as attention deficit hyperactivity disorder and autism spectrum disorders (e.g., Price et al., 2020). Finally, other genes known to be associated with a variety of cognitive processes have been shown to be associated with reading and other cognitive functions, thus questioning the specificity of the genetic sources of individual differences for variation in reading and in related brain functions (Jasińska et al., 2016; Landi et al., 2013).
Implications for Reading Instruction and Practice: Some Controversy
There is controversy over the implications of neuroimaging and genetic research for education. We think that the primary contribution thus far of these advances is at the level of mechanism. Bowers (2016) was critical of applications of cognitive neuroscience to education, because there were no clear implications in the brain data for instruction. He argued that claims for the value of educational neuroscience were exaggerated and that there was little about instruction that could not be explained at a behavioral level. Bowers also argued that demonstrations of the malleability of the brain in response to reading instruction were irrelevant because all that was needed was the evidence from standard reading tests that improvement had occurred.
In responding to Bowers (2016), Gabrieli (2016) noted that expecting basic neuroscience research to directly inform classroom teaching was a high bar. Instead, neuroscience and genetic research helps in understanding mechanisms that affect vulnerable youth, and contribute to a more rounded understanding of how reading develops at different levels of explanation. Gabrieli also argued that brain and genetic markers could facilitate early intervention because they may identify risk at earlier ages than behavioral measures would, and may be more precise because the error variance is smaller.
In a different vein, Protopapas and Parrila (2018) questioned whether neuroimaging studies can show that SRD is a neurodevelopmental disorder or not. Because neuroimaging studies largely have demonstrated that differences in brain structure and function are quantitative in nature, perhaps they should not be regarded as atypical or abnormal and, as such, do not justify labeling SRD, or dyslexia, as a disorder per se. In response, Fraga González, Karipidis, and Tijms (2018) argued that SRD should be considered a disorder because it has adverse consequences for adjustment and long-term consequences for quality of life. In addition, the researchers noted that the neural networks mediating reading in people identified with SRD were atypical, which in turn supported the use of the term disorder when referring to SRD. Like Gabrieli (2016), Fraga González and colleagues argued that neuroimaging studies help demonstrate that reading difficulties have a biological basis in the brain and that brain disorders can exist on a spectrum of (dis)ability.
Many developmental and medical disorders represent dimensional phenomena in which the defining attributes are normally distributed (Ellis, 1985). SRD is the lower end of an unbroken continuum of reading ability. The threshold for determining SRD is arbitrary and dependent on demonstrations of adverse effects on outcomes (see Fletcher et al., 2019). However, the same can be said for other heritable neurodevelopmental disorders, such as attention deficit hyperactivity disorder, where the key attributes of inattention and hyperactivity-impulsivity are normally distributed in the population. Similarly, medical disorders such as obesity and hypertension are defined by normally distributed attributes and are considered disorders when health consequences emerge in relation to objective indices of maladaptation (e.g., stroke, diabetes).
Dimensionality does not mean that reading is not heritable or brain based, and does not mean that environmental factors, such as the quality of the home environment for language, literacy, and instruction, are not contributory. There is malleability in both the neural and environmental contributions that can be manipulated to shift the reading continuum upward in performance so lower levels of reading ability are less maladaptive for individuals in our society. Literacy and SRD should not be considered as a skill or disorder that represents categories that are static and where the goal is to identify abnormalities for treatment. Rather, the goal should be to shift the entire continuum so outcomes for lower end readers are less adverse. This means that in order to shift the reading continuum of abilities, efforts should focus on the entire spectrum of instruction, from general education to remedial intervention.
These demonstrations of the continuity of reading ability should alter traditional, static conceptualizations of SRD from a disorder of constitutional origin (Critchley, 1970) to a more contemporary account. Certain heritable gene combinations put the brain at risk, and certain environmental factors, such as high-quality instruction, reduce this risk. It is helpful to examine the dorsal and ventral reading systems in relation to connectionist models of the reading process. Paralleling the increasing involvement of the ventral, lexical system in the brain, as exposure increases, implicit learning and self-teaching (Share, 1995) lead to increasing automaticity and the capacity for directly accessing meaning from orthography (Seidenberg et al., 2020). However, reading is a developmental process in which phonological processing is essential in the initial phases. Behavioral research has supported a need for explicit instruction in sound–symbol relations to access sublexical components of words through phonics, morphology, and spelling instruction (Seidenberg, 2017). We argue that neuroscience and genetic research has identified mechanisms and concurrent evidence to support this instructional approach.
Another example of the relevance of neuroimaging research for reading instruction concerns the importance of early intervention. As discussed earlier, reading involves knowledge of both the form and meaning of a word, which are processed parallel to the act of reading (Perfetti, 2007). A young child or an adult who has had no formal reading instruction cannot recognize the form of the word. The child or adult can match letters, but the word is not an entity in the person’s visual field unless he or she can link the sublexical components of the word to their pronunciations. At a neural level, this means accessing and utilizing the person’s capacity for oral language, which means teaching the dorsal system to learn to link print and sound. Once this learning begins, the ventral system begins almost immediately to reorganize into a rapid orthographic processor. Without sufficient exposure to print, children fall behind in their development of a sight word vocabulary; thus, remediation at older ages is slow, and many do not develop the automaticity needed to make reading enjoyable and effortless. Comparisons of the efficacy of reading instruction with struggling readers have shown much more effectiveness in or before grade 3 (Connor et al., 2013; Lovett et al., 2017). Neuroimaging studies have informed our understanding of this process and provided some ability to measure change and timing that supplement and inform behavioral studies, including that some children improve in reading by generating or giving weight to alternate neural pathways (Hoeft et al., 2011; Nugiel et al., 2019). Neuroimaging studies thus have illuminated the idea of behavioral phenocopy, or the idea that different brain organizations can result in similar behavioral output; these alternate pathways are not apparent when only behavioral measures are used (Church, Petersen, & Schlaggar, 2010).
Neuroimaging also can identify supportive cognitive processes critical for the reading process that may contrast to behavioral findings. For example, measures of cognitive control (sometimes referred to as executive functions) have weak behavioral relations with reading despite the evidence of self-regulation difficulties in many children who struggle (Cirino et al., 2019). In neuroimaging studies, there has been clear involvement of regions of the brain that involve cognitive control in both decoding (Aboud et al., 2018; Horowitz-Kraus, Toro-Serey, & DiFrancesco, 2015) and comprehension tasks (Nugiel et al., 2019). Further, engagement of nonreading brain regions related to subsequent reading change when behavioral measures of reading did not yet differentiate between individuals (Nugiel et al., 2019).
Similarly, genetic research can identify specific genetic pathways or systems whose function might be responsive to feedback, globally defined. What this means mechanically is that there could be some environmental agents (e.g., repeated practice) that can change biology through processes such as epigenetic regulation of the genome. These patterns of expression in the identified pathways and systems, in turn, will influence the structure and function of the brain regions that are engaged in reading. Clearly, being able to manipulate these mechanisms requires the identification of the components of these mechanisms (i.e., regions of the genome and brain) and the dynamics of their interaction (e.g., the role of epigenetic processes).
One commonly cited justification for neuroimaging and genetic studies is the earlier identification of SRD risk, another high bar (Gabrieli, 2009). This possibility is apparent with genetic studies, where sequencing of the genotype could identify genetic variations as early as birth that might establish SRD risk. Yet, because reading is experience dependent and acquired, only risk can be established, and the environment would play a major role in any actual manifestation of SRD. It is not likely that single-gene defect models will arise that account for a large number of SRD cases. Thus, genetic research is not at a point where clear manifestation or risk can be established. It has also been suggested (Gabrieli, 2009) that neuroimaging research could be more precise in establishing SRD risk in comparison with behavioral studies, but the cost-effectiveness of such an approach is not apparent. In addition, it has not yet been established that large-scale screening based on neuroimaging studies is more precise than behavioral screening of risk, even if able to be done earlier than much behavioral testing.
As a final example, consider the long-observed tendency for young children to reverse letters and write words backward. A developmental phenomenon, this tendency recedes as children grow older but has also (mistakenly) been seen as a marker for SRD because it seems to persist in some children. Using neuroimaging, Dehaene and colleagues (2010) showed that in literate adults, the ventral pathway for reading, and specifically the left fusiform, is activated when viewing pictures that are mirrored, but not for mirrored words. In illiterate adults, however, both mirrored pictures and words engaged this region. As an adult learns to read, the activation for reading mirrored images in the left fusiform gyrus diminishes; it becomes specialized for graphemic processing. From this adult work, we can surmise what is happening in young children, where the left fusiform is not yet specialized; mirrored writing and reversals are common until the left fusiform becomes specialized for word processing and are signs of the remarkable rewiring process that occurs as the brain learns to read. Neuroimaging and genetic work across different ages and different skills thus all have the potential to inform understanding of the learning process from un-skilled to skilled reading, and can potentially explain commonly observed but misunderstood phenomena.
Future Challenges
As this review has hopefully made clear, neuroimaging and genetic approaches provide a biological level with which to understand the mechanisms underlying academic difficulties. This level is complementary to cognitive (i.e., neuropsychological testing) and behavioral (i.e., observation) approaches (Frith, 2001). Biological approaches struggle with similar difficulties as in cognitive and behavioral approaches (no objective cut point, multiple sources of heterogeneity) but can also provide additional information that could ultimately help parse some of the diverse profiles inherent in reading problems.
One future challenge is to explain sources of heterogeneity in neural and genetic data, to identify differences of behavioral relevance from those due to other sources (e.g., measurement noise, artifactual contamination). Another future challenge is to increase sample sizes and the diversity of biological samples, both within a study to be representative of the community (in terms of race, gender, SES, and other important sources of variance) and across international studies, to understand which genetic and neural indicators of reading difficulties may transcend writing system, and age, and which are more culturally or developmentally specific.
Conclusions
Neuroimaging and genetic studies of reading and reading difficulties have contributed novel and important windows into understanding the reading process and reading development. Neuroimaging data support dual, parallel processes of reading through the brain that depend first on sublexical linking of language sound to written symbol. Genetic research has identified many potential candidate genes that convey genetic risk for SRD, but are also taking genome-wide approaches to understand how subtle differences among people lead to variability in reading ability. Neuroimaging and genetic research tools are rapidly developing. As researchers learn more about genetic risk factors and early brain markers, the field grows more able to identify children at risk for SRD earlier and thus more able to intervene earlier. The field also gains insights into the biological and environmental factors that may allow variability reduction in reading ability and boost the effectiveness, type, and timing of reading instruction.
Acknowledgments
Some of this work was supported in part by a grant (P50HD052117) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) to the University of Houston for the Texas Center for Learning Disabilities (principal investigator: Jack M. Fletcher) and by a grant (18-18-00451) from the Russian Science Foundation (principal investigator: Elena L. Grigorenko). The content is solely our responsibility and does not necessarily reflect the views of the National Institutes of Health or the NICHD. We thank Tehila Nugiel for thoughtful comments on an early version of this manuscript, Alice Aizza and AnnaCarolina Garza for figure assistance, and Nicole Guha and Mei Tan for editorial support.
Biography
JESSICA A. CHURCH (corresponding author) is an associate professor in the Department of Psychology at The University of Texas at Austin, USA; email church@austin.utexas.edu. Her research focuses on the developmental cognitive neuroscience of cognitive control and academic skills, with a particular emphasis on change over time.
ELENA L. GRIGORENKO is the Hugh Roy and Lillie Cranz Cullen Distinguished Professor of Psychology at the University of Houston, Texas, USA; a research-certified professor in the Department of Molecular and Human Genetics at Baylor College of Medicine, Houston, Texas, USA; and a leading research scientist at St. Peterburg University, Russia; email elena.grigorenko@times.uh.edu. Her research focuses on human development in diverse contexts across multiple levels, using methods that integrate behavior, neuroimaging, and molecular genetic analyses.
JACK M. FLETCHER is the principal investigator of the Texas Center for Learning Disabilities and the Hugh Roy and Lillie Cranz Cullen Distinguished University Chair of Psychology at the University of Houston, Texas, USA; email jackfletcher@uh.edu. His research spans disability definition, classification, neurobiological correlates, and intervention in multiple disorders, including learning disabilities and pediatric brain injury.
Footnotes
SVs are comprised of CNVs such as deletions and insertions, as well as copy number neutral events such as inversions and balanced translocations.
Semaphorins are a large family of proteins, including both secreted and membrane-associated proteins, many of which have been implicated as inhibitors or chemorepellents in axon pathfinding, fasciculation and branching, and target selection (National Center for Biotechnology Information, 2021).
In this particular case, the proband was originally referred at the age of 3, as a child with developmental language disorders, but later, at the age of 12, he presented with a reading comprehension disorder with intact decoding skills.
Contributor Information
Jessica A. Church, The University of Texas at Austin, USA
Elena L. Grigorenko, University of Houston, Texas, USA; Baylor College of Medicine, Houston, Texas, USA; and St. Petersburg State University, Russia
Jack M. Fletcher, University of Houston, Texas, USA
REFERENCES
- Aboud KS, Bailey SK, Petrill SA, & Cutting LE (2016). Comprehending text versus reading words in young readers with varying reading ability: Distinct patterns of functional connectivity from common processing hubs. Developmental Science, 19(4), 632–656. 10.1111/desc.12422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboud KS, Barquero LA, & Cutting LE (2018). Prefrontal mediation of the reading network predicts intervention response in dyslexia. Cortex, 101, 96–106. 10.1016/j.cortex.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahituv N, Kavaslar N, Schackwitz W, Ustaszewska A, Martin J, Hébert S, … Pennacchio LA (2007). Medical sequencing at the extremes of human body mass. American Journal of Human Genetics, 80(4), 779–791. 10.1086/513471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Arlington, VA: Author. [Google Scholar]
- Bach S, Richardson U, Brandeis D, Martin E, & Brem S (2013). Print-specific multimodal brain activation in kindergarten improves prediction of reading skills in second grade. NeuroImage, 82, 605–615. 10.1016/j.neuroimage.2013.05.062 [DOI] [PubMed] [Google Scholar]
- Barquero LA, Davis N, & Cutting LE (2014). Neuroimaging of reading intervention: A systematic review and activation likelihood estimate meta-analysis. PLoS One, 9(1), Article e83668. 10.1371/journal.pone.0083668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TC, Luciano M, Montgomery GW, Wright MJ, & Martin NG (2011). Genes for a component of the language acquisition mechanism: ROBO1 polymorphisms associated with phonological buffer deficits. Behavior Genetics, 41, 50–57. 10.1007/s10519-010-9402-9 [DOI] [PubMed] [Google Scholar]
- Black JM, Tanaka H, Stanley L, Nagamine M, Zakerani N, Thurston A, … Hoeft F (2012). Maternal history of reading difficulty is associated with reduced language-related gray matter in beginning readers. NeuroImage, 59(3), 3021–3032. 10.1016/j.neuroimage.2011.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JS (2016). The practical and principled problems with educational neuroscience. Psychological Review, 123(5), 600–612. 10.1037/rev0000025 [DOI] [PubMed] [Google Scholar]
- Brem S, Bach S, Kucian K, Kujala JV, Guttorm TK, Martin E, … Richardson U (2010). Brain sensitivity to print emerges when children learn letter–speech sound correspondences. Proceedings of the National Academy of Sciences of the United States of America, 107(17), 7939–7944. 10.1073/pnas.0904402107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brkanac Z, Chapman NH, Igo RP Jr., Matsushita MM, Nielsen K, Berninger VW, … Raskind WH (2008). Genome scan of a non-word repetition phenotype in families with dyslexia: Evidence for multiple loci. Behavior Genetics, 38, 462–475. 10.1007/s10519-008-9215-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonincontri R, Bache I, Silahtaroglu A, Elbro C, Veber Nielsen A-M, Ullmann R, … Tommerup N (2011). A cohort of balanced reciprocal translocations associated with dyslexia: Identification of two putative candidate genes at DYX1. Behavior Genetics, 41, 125–133. 10.1007/s10519-010-9389-2 [DOI] [PubMed] [Google Scholar]
- Button KS (2019). Double-dipping revisited. Nature Neuroscience, 22, 688–690. 10.1038/s41593-019-0398-z [DOI] [PubMed] [Google Scholar]
- Byrne B, Coventry WL, Olson RK, Samuelsson S, Corley R, Willcutt EG, … DeFries JC (2009). Genetic and environmental influences on aspects of literacy and language in early childhood: Continuity and change from preschool to grade 2. Journal of Neurolinguistics, 22(3), 219–236. 10.1016/j.jneuroling.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B, Wadsworth S, Corley R, Samuelsson S, Quain P, DeFries JC, … Olson RK (2005). Longitudinal twin study of early literacy development: Preschool and kindergarten phases. Scientific Studies of Reading, 9(3), 219–235. 10.1207/s1532799xssr0903_3 [DOI] [Google Scholar]
- Cachia A, Roell M, Mangin J-F, Sun ZY, Jobert A, Braga LW, … Borst G (2018). How interindividual differences in brain anatomy shape reading accuracy. Brain Structure & Function, 223(2), 701–712. 10.1007/s00429-017-1516-x [DOI] [PubMed] [Google Scholar]
- Centanni TM, Norton ES, Ozernov-Palchik O, Park A, Beach SD, Halverson K, … Gabrieli JDE (2019). Disrupted left fusiform response to print in beginning kindergartners is associated with subsequent reading. NeuroImage: Clinical, 22, Article 101715. 10.1016/j.nicl.2019.101715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centanni TM, Norton ES, Park A, Beach SD, Halverson K, Ozernov-Palchik O, … Gabrieli JDE (2018). Early development of letter specialization in left fusiform is associated with better word reading and smaller fusiform face area. Developmental Science, 21(5), Article e12658. 10.1111/desc.12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Coalson RS, Lugar HM, Petersen SE, & Schlaggar BL (2008). A developmental fMRI study of reading and repetition reveals changes in phonological and visual mechanisms over age. Cerebral Cortex, 18(9), 2054–2065. 10.1093/cercor/bhm228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JA, Petersen SE, & Schlaggar BL (2010). The “task B problem” and other considerations in developmental functional neuroimaging. Human Brain Mapping, 31(6), 852–862. 10.1002/hbm.21036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirino PT, Miciak J, Ahmed Y, Barnes MA, Taylor WP, & Gerst EH (2019). Executive function: Association with multiple reading skills. Reading and Writing, 32, 1819–1846. 10.1007/s11145-018-9923-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JC, Kiss RS, Pertsemlidis A, Marcel YL, McPherson R, & Hobbs HH (2004). Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science, 305(5685), 869–872. 10.1126/science.1099870 [DOI] [PubMed] [Google Scholar]
- Cohen JC, Pertsemlidis A, Fahmi S, Esmail S, Vega GL, Grundy SM, & Hobbs HH (2006). Multiple rare variants in NPC1L1 associated with reduced sterol absorption and plasma low-density lipo-protein levels. Proceedings of the National Academy of Sciences of the United States of America, 103(6), 1810–1815. 10.1073/pnas.0508483103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, & Ziegler JC (2001). DRC: A dual route cascaded model of visual word recognition and reading aloud. Psychological Review, 108(1), 204–256. 10.1037/0033-295X.108.1.204 [DOI] [PubMed] [Google Scholar]
- Connor CM, Morrison FJ, Fishman B, Crowe EC, Al Otaiba S, & Schatschneider C (2013). A longitudinal cluster-randomized controlled study on the accumulating effects of individualized literacy instruction on students’ reading from first through third grade. Psychological Science, 24(8), 1408–1419. 10.1177/0956797612472204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad DF, Pinto D, Redon R, Feuk L, Gokcumen O, Zhang Y, … Hurles ME (2010). Origins and functional impact of copy number variation in the human genome. Nature, 464, 704–712. 10.1038/nature08516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley M (1970). The dyslexic child (2nd ed.). London, UK: Heinemann Medical. [Google Scholar]
- Dehaene S (2009). Reading in the brain: The new science of how we read. New York, NY: Penguin. [Google Scholar]
- Dehaene S, Cohen L, Morais J, & Kolinsky R (2015). Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nature Reviews Neuroscience, 16(4), 234–244. 10.1038/nrn3924 [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, … Cohen L (2010). How learning to read changes the cortical networks for vision and language. Science, 330(6009), 1359–1364. 10.1126/science.1194140 [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Monzalvo K, & Dehaene S (2018). The emergence of the visual word form: Longitudinal evolution of category-specific ventral visual areas during reading acquisition. PLoS Biology, 16(3), Article e2004103. 10.1371/journal.pbio.2004103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kovel CGF, Hol FA, Heister JGAM, Willemen JJHT, Sand-kuijl LA, Franke B, & Padberg GW (2004). Genomewide scan identifies susceptibility locus for dyslexia on Xq27 in an extended Dutch family. Journal of Medical Genetics, 41(9), 652–657. 10.1136/jmg.2003.012294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter DV, Engelhardt LE, Mallett R, Gordon EM, Nugiel T, Harden KP, … Church JA (2020). Functional connectivity fingerprints at rest are similar across youths and adults and vary with genetic similarity. iScience, 23(1), Article 100801. 10.1016/j.isci.2019.100801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos A, Vanderauwera J, Vanvooren S, Vandermosten M, Ghesquière P, & Wouters J (2020). The relation between neurofunctional and neurostructural determinants of phonological processing in pre-readers. Developmental Cognitive Neuroscience, 46, Article 100874. 10.1016/j.dcn.2020.100874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden GF, Jones KM, Cappell K, Gareau L, Wood FB, Zeffiro TA, … Flowers DL (2004). Neural changes following remediation in adult developmental dyslexia. Neuron, 44(3), 411–422. 10.1016/j.neuron.2004.10.019 [DOI] [PubMed] [Google Scholar]
- Eicher JD, Powers NR, Miller LL, Akshoomoff N, Amaral DG, Bloss CS, … Gruen JR (2013). Genome-wide association study of shared components of reading disability and language impairment. Genes, Brain and Behavior, 12(8), 792–801. 10.1111/gbb.12085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis AW (1985). The cognitive neuropsychology of developmental (and acquired) dyslexia: A critical survey. Cognitive Neuropsychology, 2(2), 169–205. 10.1080/02643298508252865 [DOI] [Google Scholar]
- Ercan-Sencicek AG, Davis Wright NR, Sanders SS, Oakman N, Valdes L, Bakkaloglu B, … Grigorenko EL (2012). A balanced t(10;15) translocation in a male patient with developmental language disorder. European Journal of Medical Genetics, 55(2), 128–131. 10.1016/j.ejmg.2011.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerheim T, Raeymaekers P, Tønnessen FE, Pedersen M, Tranebjærg L, & Lubs HA (1999). A new gene (DYX3) for dyslexia is located on chromosome 2. Journal of Medical Genetics, 36(9), 664–669. [PMC free article] [PubMed] [Google Scholar]
- Field LL, Shumansky K, Ryan J, Truong D, Swiergala E, & Kaplan BJ (2013). Dense-map genome scan for dyslexia supports loci at 4q13, 16p12, 17q22; suggests novel locus at 7q36. Genes, Brain and Behavior, 12(1), 56–69. 10.1111/gbb.12003 [DOI] [PubMed] [Google Scholar]
- Fisher JH (1905). Case of congenital word-blindness (inability to learn to read). Ophthalmic Review, 24, 315–318. [Google Scholar]
- Fisher JH (1910). Congenital word blindness (inability to learn to read). Transactions of the Ophthalmological Societies of the United Kingdom, 30, 216–225. [Google Scholar]
- Fisher SE, Francks C, Marlow AJ, MacPhie IL, Newbury DF, Cardon LR, … Monaco AP (2002). Independent genome-wide scans identify a chromosome 18 quantitative-trait locus influencing dyslexia. Nature Genetics, 30, 86–91. 10.1038/ng792 [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lyon GR, Fuchs LS, & Barnes MA (2019). Learning disabilities: From identification to intervention (2nd ed.). New York, NY: Guilford. [Google Scholar]
- Fraga González G, Karipidis II, & Tijms J (2018). Dyslexia as a neurodevelopmental disorder and what makes it different from a chess disorder. Brain Sciences, 8(10), Article 189. 10.3390/brainsci8100189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friend A, DeFries J, Olson R, Pennington B, Harlaar N, Byrne B, … Keenan J (2009). Heritability of high reading ability and its interaction with parental education. Behavior Genetics, 39, 427–436. 10.1007/s10519-009-9263-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U (2001). What framework should we use for understanding developmental disorders? Developmental Neuropsychology, 20(2), 555–563. 10.1207/S15326942DN2002_6 [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE (2009). Dyslexia: A new synergy between education and cognitive neuroscience. Science, 325(5938), 280–283. 10.1126/science.1171999 [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE (2016). The promise of educational neuroscience: Comment on Bowers (2016). Psychological Review, 123(5), 613–619. 10.1037/rev0000034 [DOI] [PubMed] [Google Scholar]
- Gialluisi A, Andlauer TFM, Mirza-Schreiber N, Moll K, Becker J, Hoffmann P, … Schulte-Körne G (2019). Genome-wide association scan identifies new variants associated with a cognitive predictor of dyslexia. Translational Psychiatry, 9, Article 77. 10.1038/s41398-019-0402-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gialluisi A, Newbury DF, Wilcutt EG, Olson RK, DeFries JC, Brandler WM, … Fisher SE (2014). Genome-wide screening for DNA variants associated with reading and language traits. Genes, Brain and Behavior, 13(7), 686–701. 10.1111/gbb.12158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilger JW, Borecki IB, DeFries JC, & Pennington BF (1994). Com-mingling and segregation analysis of reading performance in families of normal reading probands. Behavior Genetics, 24, 345–355. 10.1007/BF01067536 [DOI] [PubMed] [Google Scholar]
- Gorlov IP, Gorlova OY, Sunyaev SR, Spitz MR, & Amos CI (2008). Shifting paradigm of association studies: Value of rare single-nucleotide polymorphisms. American Journal of Human Genetics, 82(1), 100–112. 10.1016/j.ajhg.2007.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorenko EL (2004). Genetic bases of developmental dyslexia: A capsule review of heritability estimates. Enfance, 56(3), 273–288. 10.3917/enf.563.0273 [DOI] [Google Scholar]
- Grigorenko EL (2005). A conservative meta-analysis of linkage and linkage-association studies of developmental dyslexia. Scientific Studies of Reading, 9(3), 285–316. 10.1207/s1532799xssr0903_6 [DOI] [Google Scholar]
- Grigorenko EL, & Naples AJ (2009). The devil is in the details: Decoding the genetics of reading. In Pugh K & McCardle P (Eds.), Helping children learn to read: Current issues and new directions in the integration of cognition, neurobiology and genetics of reading and dyslexia research and practice (pp. 133–148). New York, NY: Psychological. [Google Scholar]
- Grigorenko EL, Ngorosho D, Jukes M, & Bundy D (2006). Reading in able and disabled readers from around the world: Same or different? An illustration from a study of reading-related processes in a Swahili sample of siblings. Journal of Research in Reading, 29(1), 104–123. 10.1111/j.1467-9817.2006.00295.x [DOI] [Google Scholar]
- Hallgren B (1950). Specific dyslexia (“congenital word-blindness”): A clinical and genetic study (E. Odelberg, Trans.). Acta Psychiatrica et Neurologica, 65(Suppl.), 1–287. [PubMed] [Google Scholar]
- Hämäläinen JA, Guttorm TK, Richardson U, Alku P, Lyytinen H, & Leppänen PHT (2013). Auditory event-related potoentials measured in kindergarten predict later reading problems at school age. Developmental Neuropsychology, 38(8), 550–566. 10.1080/87565641.2012.718817 [DOI] [PubMed] [Google Scholar]
- Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, Kääriäinen H, & Kere J (2005). The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genetics, 1(4), Article e50. 10.1371/journal.pgen.0010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Soden B, Johnson W, Schatschneider C, & Taylor J (2013). Expanding the environment: Gene × School-Level SES interaction on reading comprehension. Journal of Child Psychology and Psychiatry, 54(10), 1047–1055. 10.1111/jcpp.12083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke JL, Wadsworth SJ, Olson RK, & DeFries JC (2007). Etiology of reading difficulties as a function of gender and severity. Reading and Writing, 20, 13–25. 10.1007/s11145-006-9016-z [DOI] [Google Scholar]
- Hinshelwood J (1900). Congenital word-blindness. Lancet, 155(4004), 1506–1508. 10.1016/S0140-6736(01)99645-X [DOI] [Google Scholar]
- Hinshelwood J (1902). Congenital word-blindness, with reports of two cases. Ophthalmic Review, 21(246), 91–99. [Google Scholar]
- Hinshelwood J (1907). Four cases of congenital word-blindness occurring in the same family. The British Medical Journal, 2(2444), 1229–1232. [Google Scholar]
- Hirshorn EA, Wrencher A, Durisko C, Moore MW, & Fiez JA (2016). Fusiform gyrus laterality in writing systems with different mapping principles: An artificial orthography training study. Journal of Cognitive Neuroscience, 28(6), 882–894. 10.1162/jocn_a_00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, McCandliss BD, Black JM, Gantman A, Zakerani N, Hulme C, … Gabrieli JDE (2011). Neural systems predicting long-term outcome in dyslexia. Proceedings of the National Academy of Sciences of the United States of America, 108(1), 361–366. 10.1073/pnas.1008950108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T, Shuai L, Frost SJ, Landi N, Pugh KR, & Shu H (2018). Cortical responses to Chinese phonemes in preschoolers predict their literacy skills at school age. Developmental Neuropsychology, 43(4), 356–369. 10.1080/87565641.2018.1439946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Hutton JS, Phelan K, & Holland SK (2018). Maternal reading fluency is positively associated with greater functional connectivity between the child’s future reading network and regions related to executive functions and language processing in preschool-age children. Brain and Cognition, 121, 17–23. 10.1016/j.bandc.2018.01.003 [DOI] [PubMed] [Google Scholar]
- Horowitz-Kraus T, Toro-Serey C, & DiFrancesco M (2015). Increased resting-state functional connectivity in the cingulo-opercular cognitive-control network after intervention in children with reading difficulties. PLoS One, 10(7), Article e0133762. 10.1371/journal.pone.0133762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L, Wijsman EM, Berninger VW, Thomson JB, & Raskind WH (2002). Familial aggregation of dyslexia phenotypes. II: Paired correlated measures. American Journal of Medical Genetics, 114(4), 471–478. 10.1002/ajmg.10523 [DOI] [PubMed] [Google Scholar]
- Huth AG, de Heer WA, Griffiths TL, Theunissen FE, & Gallant JL (2016). Natural speech reveals the semantic maps that tile human cerebral cortex. Nature, 532, 453–458. 10.1038/nature17637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo RP Jr., Chapman NH, Berninger VW, Matsushita M, Brkanac Z, Rothstein JH, … Wijsman EM (2006). Genomewide scan for real-word reading subphenotypes of dyslexia: Novel chromosome 13 locus and genetic complexity. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 141B(1), 15–27. 10.1002/ajmg.b.30245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im K, Raschle NM, Smith SA, Grant PE, & Gaab N (2016). Atypical sulcal pattern in children with developmental dyslexia and at-risk kindergarteners. Cerebral Cortex, 26(3), 1138–1148. 10.1093/cercor/bhu305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium. (2001). Initial sequencing and analysis of the human genome. Nature, 409(6822), 860–921. 10.1038/35057062 [DOI] [PubMed] [Google Scholar]
- Jasińska KK, Molfese PJ, Kornilov SA, Mencl WE, Frost SJ, Lee M, … Landi N (2016). The BDNF Val66Met polymorphism influences reading ability and patterns of neural activation in children. PLoS One, 11(8), Article e0157449. 10.1371/journal.pone.0157449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, … Lifton RP (2008). Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nature Genetics, 40, 592–599. 10.1038/ng.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminen N, Hannula-Jouppi K, Kestilä M, Lahermo P, Muller K, Kaaranen M, … Kere J (2003). A genome scan for developmental dyslexia confirms linkage to chromosome 2p11 and suggests a new locus on 7q32. Journal of Medical Genetics, 40(5), 340–345. 10.1136/jmg.40.5.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr J (1897). School hygiene, in its mental, moral, and physical aspects. Journal of the Royal Statistical Society, 60(3), 613–680. 10.2307/2979713 [DOI] [Google Scholar]
- Kersey AJ, Wakim K-M, Li R, & Cantlon JF (2019). Developing, mature, and unique functions of the child’s brain in reading and mathematics. Developmental Cognitive Neuroscience, 39, Article 100684. 10.1016/j.dcn.2019.100684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas Y, Voronin I, Kaydalov A, Malykh SB, Dale PS, & Plomin R (2013). Literacy and numeracy are more heritable than intelligence in primary school. Psychological Science, 24(10), 2048–2056. 10.1177/0956797613486982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, & Baker CI (2009). Circular analysis in systems neuroscience: The dangers of double dipping. Nature Neuroscience, 12(5), 535–540. 10.1038/nn.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristanto D, Liu M, Liu X, Sommer W, & Zhou C (2020). Predicting reading ability from brain anatomy and function: From areas to connections. NeuroImage, 218, Article 116966. 10.1016/j.neuroimage.2020.116966 [DOI] [PubMed] [Google Scholar]
- Landi N, Frost SJ, Mencl WE, Preston JL, Jacobsen LK, Lee M, … Grigorenko EL (2013). The COMT Val/Met polymorphism is associated with reading-related skills and consistent patterns of functional neural activation. Developmental Science, 16(1), 13–23. 10.1111/j.1467-7687.2012.01180.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitter FI, DeFries JC, & Elston RC (1980). Genetic models of reading disability. Behavior Genetics, 10, 9–30. 10.1007/BF01067316 [DOI] [PubMed] [Google Scholar]
- Liberman AM (1996). Speech: A special code. Cambridge, MA: MIT Press. [Google Scholar]
- López-Barroso D, Thiebaut de Schotten M, Morais J, Kolinsky R, Braga LW, Guerreiro-Tauil A, … Cohen L (2020). Impact of literacy on the functional connectivity of vision and language related networks. NeuroImage, 213, Article 116722. 10.1016/j.neuroimage.2020.116722 [DOI] [PubMed] [Google Scholar]
- Lovett MW, Frijters JC, Wolf M, Steinbach KA, Sevcik RA, & Morris RD (2017). Early intervention for children at risk for reading disabilities: The impact of grade at intervention and individual differences on intervention outcomes. Journal of Educational Psychology, 109(7), 889–914. 10.1037/edu0000181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Evans DM, Hansell NK, Medland SE, Montgomery GW, Martin NG, … Bates TC (2013). A genome-wide association study for reading and language abilities in two population cohorts. Genes, Brain and Behavior, 12(6), 645–652. 10.1111/gbb.12053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malins JG, Pugh KR, Buis B, Frost SJ, Hoeft F, Landi N, … Morris R (2018). Individual differences in reading skill are related to trial-by-trial neural activation variability in the reading network. The Journal of Neuroscience, 38(12), 2981–2989. 10.1523/JNEUROSCI.0907-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolio TA (2010). Genomewide association studies and assessment of the risk of disease. The New England Journal of Medicine, 363, 166–176. 10.1056/NEJMra0905980 [DOI] [PubMed] [Google Scholar]
- Manolio TA, Brooks LD, & Collins FS (2008). A HapMap harvest of insights into the genetics of common disease. The Journal of Clinical Investigation, 118(5), 1590–1605. 10.1172/JCI34772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascheretti S, Riva V, Giorda R, Beri S, Lanzoni LFE, Cellino MR, & Marino C (2014). KIAA0319 and ROBO1: Evidence on association with reading and pleiotropic effects on language and mathematics abilities in developmental dyslexia. Journal of Human Genetics, 59, 189–197. 10.1038/jhg.2013.141 [DOI] [PubMed] [Google Scholar]
- Meaburn EL, Harlaar N, Craig IW, Schalkwyk LC, & Plomin R (2008). Quantitative trait locus association scan of early reading disability and ability using pooled DNA and 100K SNP microarrays in a sample of 5760 children. Molecular Psychiatry, 13, 729–740. 10.1038/sj.mp.4002063 [DOI] [PubMed] [Google Scholar]
- Meyler A, Keller TA, Cherkassky VL, Lee D, Hoeft F, Whitfield-Gabrieli S, … Just MA (2007). Brain activation during sentence comprehension among good and poor readers. Cerebral Cortex, 17(12), 2780–2787. 10.1093/cercor/bhm006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CA, Vandermosten M, Farris EA, Hancock R, Gimenez P, Black JM, … Hoeft F (2014). White matter morphometric changes uniquely predict children’s reading acquisition. Psychological Science, 25(10), 1870–1883. 10.1177/0956797614544511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nag S (2014). Alphabetism and the science of reading: From the perspective of the akshara languages [Peer commentary on the paper “Alphabetism in reading science” by D.L. Share]. Frontiers in Psychology, 5, Article 866. 10.3389/fpsyg.2014.00866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naples AJ, Chang JT, Katz L, & Grigorenko EL (2009). Same or different? Insights into the etiology of phonological awareness and rapid naming. Biological Psychology, 80(2), 226–239. 10.1016/j.biopsycho.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Biotechnology Information. (2021). SEMA6D (Rev. ed.). Bethesda, MD: Author. Retrieved from https://www.ncbi.nlm.nih.gov/gene/80031 [Google Scholar]
- Newbury DF, Paracchini S, Scerri TS, Winchester L, Addis L, Walter J, … Monaco AP (2011). Investigation of dyslexia and SLI risk variants in reading- and language-impaired subjects. Behavior Genetics, 41, 90–104. 10.1007/s10519-010-9424-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopola-Hemmi J, Myllyluoma B, Voutilainen A, Leinonen S, Kere J, & Ahonen T (2002). Familial dyslexia: Neurocognitive and genetic correlation in a large Finnish family. Developmental Medicine & Child Neurology, 44(9), 580–586. 10.1111/j.1469-8749.2002.tb00842.x [DOI] [PubMed] [Google Scholar]
- Nugiel T, Roe MA, Taylor WP, Cirino PT, Vaughn SR, Fletcher JM, … Church JA (2019). Brain activity in struggling readers before intervention relates to future reading gains. Cortex, 111, 286–302. 10.1016/j.cortex.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard TN, Ring J, Smith S, Biggan J, & Black J (2008). Differentiating the neural response to intervention in children with developmental dyslexia. Annals of Dyslexia, 58, Article 1. 10.1007/s11881-008-0014-5 [DOI] [PubMed] [Google Scholar]
- Orton ST (1939). A neurological explanation of the reading disability. Educational Record, 20(Suppl. 12), 58–68. [Google Scholar]
- Ozernov-Palchik O, Norton ES, Wang Y, Beach SD, Zuk J, Wolf M, … Gaab N (2019). The relationship between socioeconomic status and white matter microstructure in pre-reading children: A longitudinal investigation. Human Brain Mapping, 40(3), 741–754. 10.1002/hbm.24407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patael SZ, Farris EA, Black JM, Hancock R, Gabrieli JDE, Cutting LE, & Hoeft F (2018). Brain basis of cognitive resilience: Prefrontal cortex predicts better reading comprehension in relation to decoding. PLoS One, 13(6), Article e0198791. 10.1371/journal.pone.0198791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegado F, Comerlato E, Ventura F, Jobert A, Nakamura K, Buiatti M, … Dehaene S (2014). Timing the impact of literacy on visual processing. Proceedings of the National Academy of Sciences of the United States of America, 111(49), E5233–E5242. 10.1073/pnas.1417347111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington BF, Gilger JW, Pauls D, Smith SA, Smith S, & DeFries JC (1991). Evidence for major gene transmission of developmental dyslexia. JAMA, 266(11), 1527–1534. 10.1001/jama.1991.03470110073036 [DOI] [PubMed] [Google Scholar]
- Perfetti C (2007). Reading ability: Lexical quality to comprehension. Scientific Studies of Reading, 11(4), 357–383. 10.1080/10888430701530730 [DOI] [Google Scholar]
- Perfetti CA, & Dunlap S (2008). Learning to read: General principles and writing system variations. In Koda K & Zehler AM (Eds.), Learning to read across languages: Cross-linguistic relationships in first-and second-language literacy development (pp. 13–38). New York, NY: Routledge. [Google Scholar]
- Peterson RL, & Pennington BF (2012). Developmental dyslexia. The Lancet, 379(9830), 1997–2007. 10.1016/S0140-6736(12)60198-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, & Patterson K (1996). Understanding normal and impaired word reading: Computational principles in quasi-regular domains. Psychological Review, 103(1), 56–115. 10.1037/0033-295X.103.1.56 [DOI] [PubMed] [Google Scholar]
- Plomin R, & Kovas Y (2005). Generalist genes and learning disabilities. Psychological Bulletin, 131(4), 592–617. 10.1037/0033-2909.131.4.592 [DOI] [PubMed] [Google Scholar]
- Price KM, Wigg KG, Feng Y, Blokland K, Wilkinson M, He G, … Barr CL (2020). Genome-wide association study of word reading: Overlap with risk genes for neurodevelopmental disorders. Genes, Brain and Behavior, 19(6), Article e12648. 10.1111/gbb.12648 [DOI] [PubMed] [Google Scholar]
- Protopapas A, & Parrila R (2018). Is dyslexia a brain disorder? Brain Sciences, 8(4), Article 61. 10.3390/brainsci8040061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, … Shaywitz BA (2001). Neurobiological studies of reading and reading disability. Journal of Communication Disorders, 34(6), 479–492. 10.1016/S0021-9924(01)00060-0 [DOI] [PubMed] [Google Scholar]
- Qi T, Gu B, Ding G, Gong G, Lu C, Peng D, … Liu L (2016). More bilateral, more anterior: Alterations of brain organization in the large-scale structural network in Chinese dyslexia. NeuroImage, 124(Pt. A), 63–74. 10.1016/j.neuroimage.2015.09.011 [DOI] [PubMed] [Google Scholar]
- Raschle NM, Chang M, & Gaab N (2011). Structural brain alterations associated with dyslexia predate reading onset. NeuroImage, 57(3), 742–749. 10.1016/j.neuroimage.2010.09.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind WH, Igo RP Jr., Chapman NH, Berninger VW, Thomson JB, Matsushita M, … Wijsman EM (2005). A genome scan in multigenerational families with dyslexia: Identification of a novel locus on chromosome 2q that contributes to phonological decoding efficiency. Molecular Psychiatry, 10, 699–711. 10.1038/sj.mp.4001657 [DOI] [PubMed] [Google Scholar]
- Resende EPF, Tovar-Moll FF, Ferreira FM, Bramati I, de Souza LC, Carmona KC, … Caramelli P (2018). White matter microstructure in illiterate and low-literate elderly Brazilians: Preliminary findings. Cognitive and Behavioral Neurology, 31(4), 193–200. 10.1097/WNN.0000000000000173 [DOI] [PubMed] [Google Scholar]
- Richlan F, Kronbichler M, & Wimmer H (2013). Structural abnormalities in the dyslexic brain: A meta-analysis of voxel-based morphometry studies. Human Brain Mapping, 34(11), 3055–3065. 10.1002/hbm.22127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Murillo L, & Greenberg DA (2008). Genetic association analysis: A primer on how it works, its strengths and its weaknesses. International Journal of Andrology, 31(6), 546–556. 10.1111/j.1365-2605.2008.00896.x [DOI] [PubMed] [Google Scholar]
- Roe MA, Martinez JE, Mumford JA, Taylor WP, Cirino PT, Fletcher JM, … Church JA (2018). Control engagement during sentence and inhibition fMRI tasks in children with reading difficulties. Cerebral Cortex, 28(10), 3697–3710. 10.1093/cercor/bhy170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeske D, Ludwig KU, Neuhoff N, Becker J, Bartling J, Bruder J, … Schulte-Körne G (2011). First genome-wide association scan on neurophysiological endophenotypes points to trans-regulation effects on SLC2A3 in dyslexic children. Molecular Psychiatry, 16, 97–107. 10.1038/mp.2009.102 [DOI] [PubMed] [Google Scholar]
- Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, & Cohen JC (2007). Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nature Genetics, 39, 513–516. 10.1038/ng1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Yin W, Kozlitina J, Pennacchio LA, Boerwinkle E, Hobbs HH, & Cohen JC (2009). Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. The Journal of Clinical Investigation, 119(1), 70–79. 10.1172/JCI37118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein K, Matsushita M, Berninger VW, Raskind WH, & Wijsman EM (2011). Genome scan for spelling deficits: Effects of verbal IQ on models of transmission and trait gene localization. Behavior Genetics, 41, 31–42. 10.1007/s10519-010-9390-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson S, Olson R, Wadsworth S, Corley R, DeFries JC, Willcutt E, … Byrne B (2007). Genetic and environmental influences on prereading skills and early reading and spelling development in the United States, Australia, and Scandinavia. Reading and Writing, 20, 51–75. 10.1007/s11145-006-9018-x [DOI] [Google Scholar]
- Saygin ZM, Norton ES, Osher DE, Beach SD, Cyr AB, Ozernov-Palchik O, … Gabrieli JDE (2013). Tracking the roots of reading ability: White matter volume and integrity correlate with phonological awareness in prereading and early-reading kindergarten children. The Journal of Neuroscience, 33(33), 13251–13258. 10.1523/JNEUROSCI.4383-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerri TS, Paracchini S, Morris A, MacPhie IL, Talcott J, Stein J, … Richardson AJ (2010). Identification of candidate genes for dyslexia susceptibility on chromosome 18. PLoS One, 5(10), Article e13712. 10.1371/journal.pone.0013712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher J, Hoffmann P, Schmäl C, Schulte-Körne G, & Nöthen MM (2007). Genetics of dyslexia: The evolving landscape. Journal of Medical Genetics, 44(5), 289–297. 10.1136/jmg.2006.046516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg M (2017). Language at the speed of sight: How we read, why so many can’t, and what can be done about it. New York, NY: Basic. [Google Scholar]
- Seidenberg MS, Cooper Borkenhagen M, & Kearns DM (2020). Lost in translation? Challenges in connecting reading science and educational practice. Reading Research Quarterly, 55(S1), S119–S130. 10.1002/rrq.341 [DOI] [Google Scholar]
- Share DL (1995). Phonological recoding and self-teaching: Sine qua non of reading acquisition. Cognition, 55(2), 151–218. 10.1016/0010-0277(94)00645-2 [DOI] [PubMed] [Google Scholar]
- Share DL (2014). Alphabetism in reading science. Frontiers in Psychology, 5, Article 752. 10.3389/fpsyg.2014.00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Blachman BA, Pugh KR, Fulbright RK, Skudlarski P, … Gore JC (2004). Development of left occipitotemporal systems for skilled reading in children after a phonologically-based intervention. Biological Psychiatry, 55(9), 926–933. 10.1016/j.biopsych.2003.12.019 [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Skudlarski P, … Gore JC (2002). Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychiatry, 52(2), 101–110. 10.1016/S0006-3223(02)01365-3 [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Constable RT, Mencl WE, … Gore JC (1998). Functional disruption in the organization of the brain for reading in dyslexia. Proceedings of the National Academy of Sciences of the United States of America, 95(5), 2636–2641. 10.1073/pnas.95.5.2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiba T, Landi N, Wagner R, & Grigorenko EL (2011). In search of the perfect phenotype: An analysis of linkage and association studies of reading and reading-related processes. Behavior Genetics, 41, 6–30. 10.1007/s10519-011-9444-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SD, Kimberling WJ, Pennington BF, & Lubs HA (1983). Specific reading disability: Identification of an inherited form through linkage analyses. Science, 219(4590), 1345–1347. 10.1126/science.6828864 [DOI] [PubMed] [Google Scholar]
- Stein CM, & Elston RC (2009). Finding genes underlying human disease. Clinical Genetics, 75(2), 101–106. 10.1111/j.1399-0004.2008.01083.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson S (1904). Congenital word-blindness. The Lancet, 164(4229), 827–828. 10.1016/S0140-6736(01)31885-8 [DOI] [Google Scholar]
- Stephenson S (1907). Six cases of congenital word-blindness affecting three generations of one family. Ophthalmoscope, 5, 482–484. [Google Scholar]
- Stevens C, Harn B, Chard DJ, Currin J, Parisi D, & Nelville H (2013). Examining the role of attention and instruction in at-risk kindergarteners: Electrophysiological measures of selective auditory attention before and after an early literacy intervention. Journal of Learning Disabilities, 46(1), 73–86. 10.1177/0022219411417877 [DOI] [PubMed] [Google Scholar]
- Svensson I (2011). Reading and writing disabilities among inmates in correctional settings: A Swedish perspective. Learning and Individual Differences, 21(1), 19–29. 10.1016/j.lindif.2010.08.002 [DOI] [Google Scholar]
- Taipale M, Kaminen N, Nopola-Hemmi J, Haltia T, Myllyluoma B, Lyytinen H, … Kere J (2003). A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proceedings of the National Academy of Sciences of the United States of America, 100(20), 11553–11558. 10.1073/pnas.1833911100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J, Roehrig AD, Soden Hensler B, Connor CM, & Schatschneider C (2010). Teacher quality moderates the genetic effects on early reading. Science, 328(5977), 512–514. 10.1126/science.1186149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JSH, Davis MH, & Rastle K (2017). Comparing and validating methods of reading instruction using behavioural and neural findings in an artificial orthography. Journal of Experimental Psychology: General, 146(6), 826–858. 10.1037/xge0000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JSH, Rastle K, & Davis MH (2013). Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychological Bulletin, 139(4), 766–791. 10.1037/a0030266 [DOI] [PubMed] [Google Scholar]
- Tenesa A, Farrington SM, Prendergast JGD, Porteous ME, Walker M, Haq N, … Dunlop MG (2008). Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nature Genetics, 40, 631–637. 10.1038/ng.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CJ (1905). Congenital “word-blindness” and its treatment. Ophthalmoscope, 3, 380–385. [Google Scholar]
- Torre GA, Matejko AA, & Eden GF (2020). The relationship between brain structure and proficiency in reading and mathematics in children, adolescents, and emerging adults. Developmental Cognitive Neuroscience, 45, Article 100856. 10.1016/j.dcn.2020.100856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis KE, Adams JN, Kovachy VN, Ben-Shachar M, & Feldman HM (2017). White matter properties differ in 6-year old readers and pre-readers. Brain Structure and Function, 222(4), 1685–1703. 10.1007/s00429-016-1302-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesky TK, Vanderauwera J, & Gaab N (2021). Imaging the rapidly developing brain: Current challenges for MRI studies in the first five years of life. Developmental Cognitive Neuroscience, 47, Article 100893. 10.1016/j.dcn.2020.100893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, & Eden GF (2003). Development of neural mechanisms for reading. Nature Neuroscience, 6(7), 767–773. 10.1038/nn1065 [DOI] [PubMed] [Google Scholar]
- Vandermosten M, Hoeft F, & Norton ES (2016). Integrating MRI brain imaging studies of pre-reading children with current theories of developmental dyslexia: A review and quantitative meta-analysis. Current Opinion in Behavioral Sciences, 10, 155–161. 10.1016/j.cobeha.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandermosten M, Vanderauwera J, Theys C, De Vos A, Vanvooren S, Sunaert S, … Ghesquière P (2015). A DTI tractography study in pre-readers at risk for dyslexia. Developmental Cognitive Neuroscience, 14, 8–15. 10.1016/j.dcn.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel AC, Miezin FM, Petersen SE, & Schlaggar BL (2012). The putative visual word form area is functionally connected to the dorsal attention network. Cerebral Cortex, 22(3), 537–549. 10.1093/cercor/bhr100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth SJ, Corley RP, Hewitt JK, & DeFries JC (2001). Stability of genetic and environmental influences on reading performance at 7, 12, and 16 years of age in the Colorado Adoption Project. Behavior Genetics, 31, 353–359. 10.1023/A:1012218301437 [DOI] [PubMed] [Google Scholar]
- Wadsworth SJ, Olson RK, & DeFries JC (2010). Differential genetic etiology of reading difficulties as a function of IQ: An update. Behavior Genetics, 40, 751–758. 10.1007/s10519-010-9349-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Joanisse MF, & Booth JR (2018). Reading skill related to left ventral occipitotemporal cortex during a phonological awareness task in 5–6-year old children. Developmental Cognitive Neuroscience, 30, 116–122. 10.1016/j.dcn.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss Y, Cweigenberg HG, & Booth JR (2018). Neural specialization of phonological and semantic processing in young children. Human Brain Mapping, 39(11), 4334–4348. 10.1002/hbm.24274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellcome Trust Case Control Consortium. (2007). Genome-wide association study of 14,000 cases of seven common diseases and 3,000 controls. Nature, 447, 661–678. 10.1038/nature05911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman EM, Peterson D, Leutenegger A-L, Thomson JB, Goddard KAB, Hsu L, … Raskind WH (2000). Segregation analysis of phenotypic components of learning disabilities. I: Nonword memory and digit span. The American Journal of Human Genetics, 67(3), 631–646. 10.1086/303044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams VJ, Juranek J, Cirino P, & Fletcher JM (2018). Cortical thickness and local gyrification in children with developmental dyslexia. Cerebral Cortex, 28(3), 963–973. 10.1093/cercor/bhx001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M (2007). Proust and the squid: The story and science of the reading brain. New York, NY: Harper. [Google Scholar]
- Yamada Y, Stevens C, Dow M, Harn BA, Chard DJ, & Neville HJ (2011). Emergence of the neural network for reading in five-year-old beginning readers of different levels of pre-literacy abilities: An fMRI study. NeuroImage, 57(3), 704–713. 10.1016/j.neuroimage.2010.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younger JW, & Booth JR (2018). Parietotemporal stimulation affects acquisition of novel grapheme-phoneme mappings in adult readers. Frontiers in Human Neuroscience, 12, Article 109. 10.3389/fnhum.2018.00109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li T, Elliott MA, & Rueckl JG (2018). Statistical and cooperative learning in reading: An artificial orthography learning study. Scientific Studies of Reading, 22(3), 191–208. 10.1080/10888438.2017.1414219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Feng T, Li Y, Lu Q, & Elston RC (2010). Detecting rare variants for complex traits using family and unrelated data. Genetic Epidemiology, 34(2), 171–187. 10.1002/gepi.20449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A, König IR, Deimel W, Plume E, Nöthen MM, Propping P, … Schulte-Körne G (2005). Developmental dyslexia—recurrence risk estimates from a German bi-center study using the single proband sib pair design. Human Heredity, 59, 136–143. 10.1159/000085572 [DOI] [PubMed] [Google Scholar]
- Zuk J, Dunstan J, Norton E, Yu X, Ozernov-Palchik O, Wang Y, … Gaab N (2020). Multifactorial pathways facilitate resilience among kindergarteners at risk for dyslexia: A longitudinal behavioral and neuroimaging study. Developmental Science, 24(1), Article e12983. 10.1111/desc.12983 [DOI] [PMC free article] [PubMed] [Google Scholar]


