Abstract
Mesenchymal cells are uniquely located at the interface between the epithelial lining and the stroma, allowing them to act as a signaling hub among diverse cellular compartments of the lung. During embryonic and postnatal lung development, mesenchyme-derived signals instruct epithelial budding, branching morphogenesis, and subsequent structural and functional maturation. Later during adult life, the mesenchyme plays divergent roles wherein its balanced activation promotes epithelial repair after injury while its aberrant activation can lead to pathological remodeling and fibrosis that are associated with multiple chronic pulmonary diseases, including bronchopulmonary dysplasia, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease. In this Review, we discuss the involvement of the lung mesenchyme in various morphogenic, neomorphogenic, and dysmorphogenic aspects of lung biology and health, with special emphasis on lung fibroblast subsets and smooth muscle cells, intercellular communication, and intrinsic mesenchymal mechanisms that drive such physiological and pathophysiological events throughout development, homeostasis, injury repair, regeneration, and aging.
Introduction
Over the past century, there has been a remarkable shift in disease burden from acute/subacute communicable diseases to more chronic noncommunicable diseases. The reasons for this shift are likely multifactorial, related to improved prevention and treatment of acute infectious diseases, increasing socialization and industrialization, lifestyle changes that contribute to metabolic disorders, and the aging of populations worldwide. Even with the recent viral pandemic, a notable proportion of survivors develop chronic, noncommunicable clinical syndromes following recovery from the acute infectious disease. Many of these chronic diseases of the 21st century can be attributed to loss of homeostatic maintenance and regeneration or inefficient/incomplete repair of affected tissues and organs. This often manifests as tissue fibrosis in diverse organ systems such as the liver, kidney, heart, and lungs. A fundamental organizing principle in the structural engineering of these tissues/organs during development is the close apposition of the epithelium with mesenchymal cells or fibroblasts that function as signaling hubs and stem cell niches to not only maintain homeostasis, but support the repair/regeneration of these tissues following injury.
The mammalian lung can be divided into two main compartments based on cellular composition and the surrounding tissue microenvironment: the epithelium, which constitutes the internal lining, and the stroma, which comprises the connective tissue and vasculature. The connective tissue predominantly consists of mesenchymal cells and extracellular matrix (ECM). While the lineage hierarchy and functional characteristics of epithelial cells have been well investigated during lung development and maturation, and to a certain extent in aging and disease, a similar understanding of the stromal compartment is still lagging. This is mainly due to a gap in our knowledge regarding mesenchymal cell heterogeneity and hierarchy, particularly in terms of cellular identity and plasticity, and how this translates to adaptive versus maladaptive tissue repair in physiological and pathophysiological settings, respectively. In this Review, we will focus our discussion on mesenchymal cell functions in support of epithelial repair/regeneration, and refer readers interested in a more expanded understanding of endothelial cell regeneration and vascular repair, the role of angiocrine-derived factors in epithelial regeneration, and mesenchymal-endothelial interactions to other recently published reviews as well as original research articles (1–6).
The epithelium of the lung is populated by specialized cell types along the proximo-distal axis, such as basal, club, and ciliated cells in the conducting airways and alveolar epithelial type 1 (AT1) and type 2 (AT2) cells in the smallest respiratory units of the lungs, the alveoli. Although the adult lung generally possesses a low cell turnover rate, specialized epithelial populations are engaged during repair after injury, including subsets of differentiated cells with stem/progenitor cell capacity such as basal cells, club cells, and AT2s, and cells in transitional states such as p63+ KRT5+ pods, KRT8+ basaloid cells, pre-AT1 transitional cell state (PATS), and interleukin-1 receptor–positive (IL-1R+) damage-associated transient progenitors (DATPs) (7–23). Interestingly, the mesenchymal counterpart that constitutes the niche for such epithelial subsets is poorly characterized at the spatial, cellular, and molecular levels. This is a major limitation given that the lung mesenchyme plays an indispensable role in instructing epithelial cell behavior not only during lung development but also during repair after injury, and — presumably — in dysmorphogenic events that lead to aberrant repair and chronic, predominantly progressive and fatal lung diseases.
During embryonic lung development, the interplay between the primitive endoderm and splanchnic mesoderm is pivotal for lung bud formation and subsequent branching morphogenesis, an event that marks the pseudoglandular stage of lung development (reviewed in refs. 24–27) (Figure 1, A and B). During later developmental stages, a similar mode of crosstalk is instrumental for the formation of mature differentiated epithelial cells such as AT1s and AT2s, as well as mesenchymal cells such as airway and vascular smooth muscle cells (ASMCs and VSMCs), alveolar myofibroblasts (AMFs), and lipofibroblasts (LIFs) (Figure 1C). The latter two populations have been studied mostly during the alveolar stage of lung development. Therefore, epithelial-mesenchymal interactions dictate and determine the morphogenic program during lung development, and most likely neomorphogenic programs during lung regeneration in adult life. In the next sections, we will discuss the involvement of mesenchymal cells in lung development as well as in normal (regeneration) versus aberrant repair (fibrosis) after injury in the adult, with a special emphasis on the function of the mesenchyme as a signaling hub for other cells, in particular, epithelial cells.
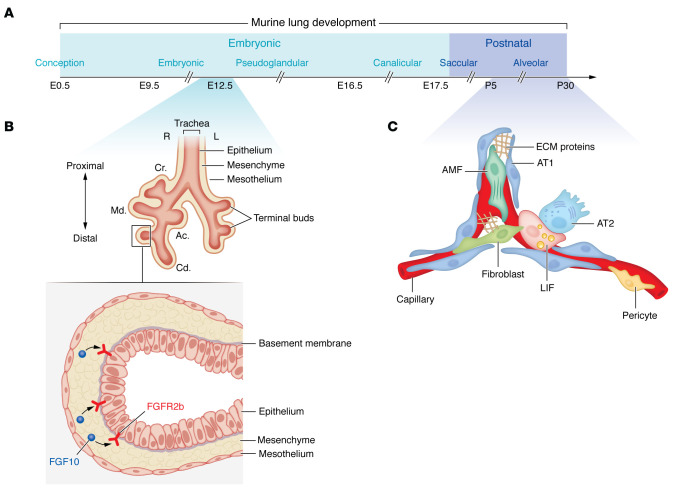
Figure 1. Overview of pre- and postnatal lung development.
(A) Lung development consists of an embryonic and postnatal phase. The different stages of mouse lung development are shown through P30. (B) A schematic representation of an embryonic lung at E12.5 and a corresponding sagittal section of a distal epithelial bud with the surrounding mesenchymal tissue. FGF10/FGFR2b signaling is highlighted. (C) A schematic representation of an alveolus with the constituent cell types during the alveolar stage of lung development. Cr., cranial lobe; Md., medial lobe; Cd., caudal lobe; Ac., accessory lobe; AMF, alveolar myofibroblast; AT1, alveolar epithelial type 1; AT2, alveolar epithelial type 2; LIF, lipofibroblast.
Mesenchymal cell hierarchy and heterogeneity in lung development
In contrast to the lung epithelium, the lineage hierarchy of lung mesenchymal cells is poorly understood. Clonal analysis of mesenchymal progenitors during embryonic lung development has shown that a single mesenchymal progenitor cell expressing the early lung mesenchymal marker T-box transcription factor 4 (Tbx4) undergoes clonal expansion, and its daughter cells migrate to occupy distinct stromal niches where they influence epithelial cell behavior and morphogenic programs (28). Another report revealed that pulmonary mesenchyme also derives from multipotent cardiopulmonary progenitors that originate from the heart and give rise to pulmonary ASMCs/VSMCs, proximal vascular endothelium, and pericyte-like cells (29). Fibroblast growth factor 10 (Fgf10), a downstream target of TBX4 in the embryonic lung, is characterized by a distinctive expression pattern in the distal mesenchyme, facing epithelial bud tips that express the epithelial receptor fibroblast growth factor receptor 2-IIIb (Fgfr2b) and undergoing branching (27, 30) (Figure 1B). Using grafting experiments, it was initially shown that this distal mesenchymal tissue contains the necessary information and signals to induce epithelial budding and subsequent branching morphogenesis (31). Such instructive signals are believed to be largely mediated by FGF10/FGFR2b signaling (Figure 1B). In fact, genetic deletion of Fgf10 or Fgfr2b leads to multiorgan agenesis, including the lung, as well as other developmental abnormalities (32–35).
FGF10 has long been believed to act as a chemoattractant, marking the future domain of epithelial tip outgrowth; therefore, its localized expression facing the growing epithelial buds has been assumed to be important for iterative branching and its associated stereotypy. However, this model has been challenged, as ubiquitous overexpression of Fgf10 in the background of Fgf10-knockout pups was shown to rescue lung agenesis, yielding a seemingly normal branching pattern in the embryonic lung (36). Another characteristic of distal FGF10+ cells is that they migrate proximally to fit the growing epithelial tubes like a sleeve and give rise to the surrounding ASMC layer (37). This observation was later confirmed using genetic lineage tracing, and it was shown that mesenchymal FGF10+ cells are progenitors not only for ASMCs but also for VSMCs during the early pseudoglandular stage of lung development, and additionally for LIFs during embryonic stages and postnatally (34, 38–40). It is worth mentioning that ASMC peristalsis might also promote branching morphogenesis by constricting bud tips and creating clefts at bifurcation sites, although this concept is still debatable (26, 41, 42). Moreover, lineage tracing of mesenchymal cells expressing transcription factor 21 (Tcf21), another gene that marks LIFs during development and adulthood, showed a similar progenitor profile to FGF10+ cells, with TCF21+ cells featuring an SMC differentiation program during early development and a LIF program during later stages (43).
During postnatal lung development, the two most prominent mesenchymal populations are AMFs and LIFs (44) (Figure 1C). AMFs are α-smooth muscle actin–positive (ACTA2+) mesenchymal cells located at the alveolar entry ring and are believed to drive secondary septation, a process by which primitive alveolar sacs are subdivided into alveoli. The concept that secondary septation occurs via protrusion of alveolar walls toward the airspace to form finger-like crests has been challenged by a study showing that such secondary septa are an artifact of 2D imaging of thin lung sections. Previous studies had shown that AMFs are detected at the tips of secondary septa during the alveolar stage of lung development. More recently, it was shown using 3D imaging of thick lung sections that such secondary septa are rather ridges that subdivide alveolar sacs into smaller alveoli (45–48). For a more comprehensive overview of alveologenesis, we refer readers to other recently published reviews (49–52).
AMFs seem to transiently appear in the developing lung, and previous studies have suggested that they undergo apoptotic clearance upon completion of alveologenesis (53, 54). Recent work has demonstrated strong interaction between AMFs and AT1s particularly via wingless-related integration site (WNT) and sonic hedgehog (Shh) signaling (48). AMFs also signal to AT2 progenitors and influence their proliferation (55). Platelet-derived growth factor receptor-α (PDGFRα) has been identified as a marker for AMFs (45, 56–62), and the progenitors for AMFs have been shown to be positive for glioma-associated oncogene 1 (Gli1) (63, 64). GLI1 is a downstream effector and readout for Shh signaling, although it has been shown that it can be activated in a noncanonical fashion, such as by MAPK (65). Interestingly, although AMFs emerge in the lung during the alveolar stage of lung development between postnatal day 5 (P5) and P30 (Figure 1C), their GLI1+ progenitors are specified very early on during embryonic lung development (63).
LIFs, on the other hand, are lipid droplet–containing mesenchymal cells that are closely associated with AT2s (44, 66, 67) (Figure 1C and Figure 2A). They store triglycerides and transfer them to adjacent AT2s to be used during the production of pulmonary surfactant. LIFs are also a source of important growth factors, such as FGF10. FGFR2b signaling has been demonstrated to be important for the maintenance of AT2 identity and progenitor state (68–71) (Figure 2A). The AT2-supportive potential of LIFs has also been demonstrated ex vivo using alveolar organoids (72, 73). In contrast to AMFs, LIFs persist in the lung following alveolar maturation, and their role in repair after injury will be discussed in subsequent sections. Although the presence of LIFs in the human lung has been questioned (74–78), recent studies, including those using single-cell transcriptomics, have confirmed it (79–82).
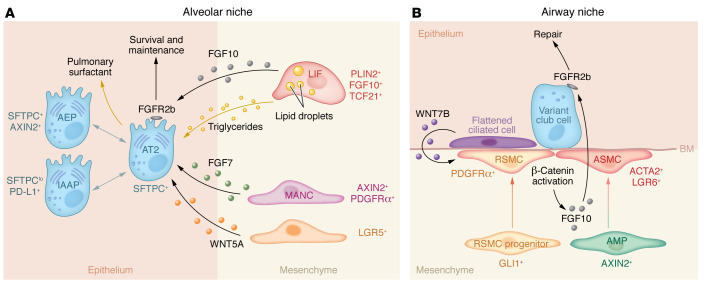
Figure 2. Mesenchymal-epithelial interactions in the alveolar and airway niches.
(A) AT2 and their so-far identified subclusters are shown. Among the identified niche cells are LIFs, MANCs, and LGR5+ cells. Cellular markers are also shown. (B) A WNT-FGF feedback loop mediates epithelial-mesenchymal communication during airway regeneration. AEP, alveolar epithelial progenitor; AMP, AXIN2+ myofibrogenic progenitor; ASMC, airway smooth muscle cell; AT2, alveolar epithelial type 2; BM, basement membrane; Epi, epithelium; IAAP, injury-activated alveolar progenitor; LGR5, leucine-rich repeat containing G protein–coupled receptor 5; LIF, lipofibroblast; MANC, mesenchymal alveolar niche cell; Mes, mesenchyme; RSMC, repair-supportive mesenchymal cell.
LIFs were initially identified in rodents by the expression of adipose differentiation–related protein (Adrp), also called perilipin 2 (Plin2), a protein involved in lipid droplet trafficking (83, 84). As mentioned above, lineage-tracing studies established Fgf10 and Tcf21 as LIF markers in the mouse lung (38, 40, 43). With the advent of single-cell transcriptomics, other markers have emerged as more specific than Plin2, including, apart from Fgf10 and Tcf21, LIM and calponin homolog domains 1 (Limch1), glycogenin (Gyg), microtubule-actin cross-linking factor 1 (Macf1), microfibril-associated protein 4 (Mfap4), nephronectin (Npnt), Wnt2, collagen type XIII α1 chain (Col13a1), and indolethylamine N-methyltransferase (Inmt) in mice and LIMCH1, α2-macroglobulin (A2M), regulator of cell cycle (RGCC), apolipoprotein E (APOE), and follistatin (FST) in humans (80, 81, 85, 86).
It is important to mention that the knowledge summarized above regarding branching morphogenesis, mesenchymal heterogeneity, and epithelial-mesenchymal crosstalk is predominantly based on research conducted in experimental rodent models, including transgenic mice. Corresponding data on human lung development are progressively emerging (87–94). For example, a comparative study highlighted differences in terms of expression patterns and biological activities of FGF ligands between mouse and human fetal lungs (90). Another study used distal tissues from prenatal human lungs to show that mesenchymal cells adjacent to bud tips express the WNT agonist R-SPONDIN 2 (RSPO2), which acts on its receptor leucine-rich repeat–containing G protein–coupled receptor 5 (LGR5) to maintain distal epithelial progenitors and their multipotency (91). Another study analyzed human terminal and respiratory bronchioles (TRBs) and fetal tissues to identity a novel population of bipotent alveolar type 0 (AT0) cells representing a transitional state as AT2s differentiate into AT1s or TRB secretory cells (94). This work also highlighted a population of LGR5+ fibroblasts enriched for WNT, PDGF, brain-derived neurotrophic factor (BDNF), and TGF-β signaling pathways, and serving as a source of FGF, bone morphogenetic protein (BMP), and WNT ligands that potentially signal to basal and secretory cells in the TRBs. The authors also identified the AT0 population in monkeys exposed to bleomycin (94). Human fetal lung atlases have also been recently published for 5 to 14 (87) and 5 to 22 weeks after conception (92).
Mesenchymal-epithelial interactions in the injured lung
In recent years, FGF signaling has emerged as an important mediator of stem cell activation and subsequent epithelial repair in the lung (reviewed in ref. 95). FGF ligands such as FGF7 and FGF10 are typically secreted by mesenchymal cells and act on epithelial stem and progenitor cells to initiate the repair/regenerative process. This mechanism has been demonstrated in the context of both alveolar (Figure 2A) and airway epithelial regeneration (Figure 2B). WNT ligands, particularly WNT5A and WNT7B, were also shown to be instrumental in driving regenerative mechanisms in the lung (Figure 2). In the next subsections, the mesenchymal niche and its secreted factors will be discussed in the context of airway and alveolar repair and regeneration.
The mesenchymal niche during airway epithelial regeneration
Club cells are dome-shaped, nonciliated secretory cells that are characterized by the expression of secretoglobin family 1A member 1 (SCGB1A1; also called Clara cell 10 kDa secretory protein CC10 or CCSP) and located in the conducting airways. These cells can give rise to both ciliated and secretory cells (96, 97). Because of their high expression of cytochrome P450 family 2 subfamily F polypeptide 2 (CYP2F2), club cells are selectively targeted and dramatically depleted by naphthalene (98–100). Interestingly, surviving variant club cells (CYP2F2lo) located at neuroepithelial bodies or bronchioalveolar duct junctions mediate the repair process in the bronchial epithelium (101–103) (Figure 2B). Accordingly, the naphthalene injury model is widely used to study mechanisms of club cell replenishment and airway regeneration in experimental mice.
Previous work has shown that following naphthalene injury, the majority of club cells are depleted, and ciliated cells flatten to cover the denuded epithelium and maintain barrier integrity (104, 105) (Figure 2B). These cells secrete the WNT ligand WNT7B, which acts on neighboring ASMCs to activate β-catenin signaling and FGF10 production. The latter acts on variant club cells expressing Fgfr2b to induce regeneration (105) (Figure 2B). It was also shown that Lgr6 expression identifies a subset of ASMCs that promotes epithelial repair after naphthalene injury in a similar WNT-FGF10–mediated mechanism (106) (Figure 2B). Another mesenchymal population that is relevant in this context is AXIN2+ myofibrogenic progenitors, which have been shown to contribute to the ASMC lineage during repair after naphthalene injury (107) (Figure 2B). It is also important to mention that basal cells in cartilaginous airways are also involved in airway regeneration through a WNT7B-FGF10 axis that is regulated by the Hippo pathway (108).
Recently, a novel population of repair-supportive mesenchymal cells (RSMCs) was identified (109). These cells are distinct from ASMCs and mostly appear after naphthalene injury (Figure 2B). RSMCs derive from ACTA2-negative progenitors that transit through the SMC lineage and gain Pdgfra expression. When compared with the SMC-enriched fraction, the RSMC-enriched fraction displays superior ability to support club cell growth in the context of the bronchiolosphere assay (109, 110). The cellular origin of RSMCs that appear around the injured airway epithelium was further investigated (111). Lineage tracing of preexisting versus de novo–formed ACTA2+ cells as well as GLI1+ cells in the context of naphthalene injury showed that preexisting GLI1+ cells are a source of RSMCs in the lung (111) (Figure 2B). Along this line of evidence, genetic deletion of Fgf10 in preexisting GLI1+ cells attenuates RSMC appearance and impairs club cell replenishment (111, 112). Bronchiolosphere assays confirmed the intrinsic ability of GLI1+ cells to support club cell growth (111). Further single-cell RNA sequencing (RNA-Seq) uncovered the cellular heterogeneity of GLI1+ cells in the healthy lung and suggested that alveolar fibroblasts might be an unexpected contributor to the airway mesenchymal niche (111). This finding is intriguing and goes against the dogma that mesenchymal niche cells are restricted to predefined anatomical locations in the adult lung; however, further studies are needed to determine whether these cells are capable of overcoming anatomical boundaries or whether they undergo reprogramming to resemble alveolar fibroblast–like cells that support epithelial regeneration.
The mesenchymal niche during alveolar repair and regeneration
Although AT2s have traditionally been regarded as a homogenous pool of progenitor/stem cells in the adult lung, emerging literature suggests that they also contain diverse subsets. Initial ablation experiments have shown that AT2s that escape ablation undergo clonal expansion and replenish the AT2 pool, thereby restoring homeostasis (72). These studies also established that PDGFRα+ stromal cells, a population that includes LIFs in the adult lung, represent an important mesenchymal niche for AT2s (72). Subsequently, it was shown that WNT-responsive AT2s are unique in their ability to repair the alveolar compartment (20, 23) (Figure 2A). Treatment of both mouse and human WNT-responsive alveolar epithelial progenitors with FGF7 or FGF10 significantly enhanced their growth in vitro (20). WNT ligands, such as WNT5A (23), also identify the mesenchymal niche for WNT-responsive AT2 stem cells (Figure 2A). Interestingly, the AT2 niche activity has recently been shown to be enriched within the FGF10+ LIF fraction of resident mesenchymal cells (73).
Recent work has also identified a subset of AT2s displaying low levels of the AT2 transcriptomic signature compared with classical AT2s (7). These SFTPClo cells, termed injury-activated alveolar progenitors (IAAPs), were preferentially amplified in response to pneumonectomy, upregulated the AT2 signature, and were positive for programmed cell death ligand 1 (PD-L1 or CD274) (7). These cells replenished the mature AT2 pool upon genetic deletion of Fgfr2b in AT2s (70). IAAPs were also activated in response to bleomycin-induced pulmonary fibrosis, and therapeutic intervention with recombinant FGF10 further boosted their response and improved repair (113).
Apart from LIFs, mesenchymal alveolar niche cells (MANCs) that are WNT-responsive (AXIN2+) mesenchymal cells residing in the alveolar regions have also been reported (107). These cells were PDGFRα+ and supported AT2s by producing IL-6 and FGF7 (107) (Figure 2A). MANCs also expressed tropomyosin receptor kinase B (TRKB) and therefore responded to BDNF secreted by AT2s undergoing differentiation into AT1s after acute lung injury (114). MANCs responded to AT2-derived BDNF by secreting FGF7 to promote regeneration (114). Another identified alveolar niche population is LGR5+ mesenchymal cells, which support alveolar epithelial differentiation by producing WNT ligands such as WNT5A (106) (Figure 2A). While LIFs, MANCs, and LGR5+ fibroblasts contribute to the mesenchymal niche for AT2s, the extent of overlap among these populations is still not clear. It also remains to be seen whether these niche cells equally support various AT2 subsets such as AXIN2+ AT2s and/or IAAPs, or whether there are specialized niche cell subsets dedicated to maintenance and expansion of each AT2 subset. It is worth mentioning that mesenchymal cells are not only involved in epithelial repair and regeneration after major injury but are also involved in compensatory growth following pneumonectomy. In this context, it was shown that mesenchymal contraction is critical for re-septation during compensatory regrowth in post-pneumonectomy (47).
In addition to AT2-mediated alveolar regeneration, airway epithelial cells can also be deployed when AT2s are exhausted as a result of extreme injury such as in the case of highly pathogenic influenza virus infection. Such airway cells include a rare population of intrapulmonary p63+ progenitor cells that mostly lead to dysplastic repair featuring bronchiolarization of the alveolar regions and persistence of KRT5+ basal cell–like clusters (pods) rather than give rise to AT1s and AT2s and effective regeneration (reviewed in ref. 115). In this context, genetic deletion of Fgfr2b in SOX2+ cells before bleomycin injury (Sox2 marks airway epithelial cells and not only intrapulmonary p63+ progenitors) inhibited the formation of KRT5+ pods and airway-derived AT2s (71). On the other hand, overexpression of Fgf10 in these cells favored the AT2 fate over the KRT5+ pod fate, thus promoting fibrosis resolution and alveolar regeneration (71). β-Catenin stabilization in preexisting SOX2+ cells decreased the number of traced KRT5+ cells while enhancing the number of traced AT2s, again establishing the notion that WNT signaling favors AT2 differentiation (18). However, precise analysis of the mesenchymal niche that sways such fate decisions is still lacking. In a similar context, influenza virus inhibited β-catenin–mediated Fgfr2b expression in epithelial stem/progenitor cells (EpiSPCs), and exogenously applied recombinant FGF10 activated noninfected EpiSPCs and improves outcomes in infected mice (11). It is therefore clear that mesenchyme-derived signals, such as FGF10, are important components/effectors of the niche that influences scar-free regeneration versus dysplastic remodeling after major alveolar injury.
Mesenchymal activation in the injured lung: complex outcomes
Orchestrated spatio-temporal activation of the mesenchyme is critical for adaptive repair and efficient regeneration of the lung, while a dysregulated mesenchymal response may lead to unremitting and uncontrolled repair responses that culminate in fibrosis. In most cases, it appears that the same developmental pathways that participate in normal repair are co-opted to promote pathological tissue responses. Identification of specific pathological mesenchymal cell populations that can be either reprogrammed or eliminated in concert with key signaling pathways and/or metabolic perturbations that drive fibrosis will be critical to developing novel and more effective therapies.
Shh/GLI1 axis in mesenchymal activation and airway regeneration.
Shh signaling is one of the most studied developmental pathways. During embryonic lung development, Shh is expressed at high levels by the distal epithelium, and it signals through its mesenchymal receptor patched 1 (Ptch1) to induce mesenchymal proliferation (116), although autocrine Shh signaling in the developing trachea has also been reported (117). Shh signaling is also important for mesenchymal differentiation such as toward SMCs during lung development (118). In strong contrast to the embryonic scenario, Shh was shown to maintain mesenchymal quiescence in the adult mouse lung (119). Loss of epithelial Shh due to naphthalene injury led to decreased mesenchymal Shh activation but increased GLI1+ mesenchymal cell expansion (119). During injury resolution and epithelial regeneration, there was enhanced Shh activation and decreased mesenchymal proliferation (119). Forced activation of Shh in the mesenchyme impaired epithelial regeneration (119). GLI1+ cells also serve as a source of RSMCs that produce FGF10 needed for club cell replenishment and epithelial regeneration in response to naphthalene injury (111). Therefore, activation of GLI1+ cells is an integral part of the airway repair machinery, and paracrine signaling between this population and airway epithelial progenitors largely mediates the regeneration process. Interestingly, long-term fate mapping also showed that RSMC descendants were not completely cleared from the lung following the completion of airway regeneration (109). Moreover, such descendants did not contribute to myofibroblast formation if the animals were re-exposed to bleomycin as a second hit (109). Further research is needed to elucidate the long-term function of these cells and whether they might contribute to other pathological events such as airway remodeling.
Aberrant mesenchymal activation in lung remodeling.
On the other hand, the literature clearly shows that aberrant mesenchymal activation, particularly that of the GLI1+ lineage, disrupts lung structure and can lead to fibrosis. GLI1+ cells, also regarded as perivascular mesenchymal stem cell–like (MSC-like) cells (120, 121), have been shown to be important contributors to fibrosis-associated myofibroblasts in multiple organs, including the lung (120, 121). Genetic ablation of these cells attenuated fibrosis in the kidney, heart, and bone marrow (120, 122). GLI1+ cells formed a pathological niche that skewed the differentiation of airway progenitors toward basal cell metaplasia instead of AT2 differentiation by antagonizing BMP signaling in the fibrotic lung (123). Ectopic hedgehog activation in distal fibroblasts led to loss of alveoli and airspace enlargement (124).
LIFs are another important contributor to fibrosis-associated myofibroblasts. Residing in close vicinity to AT2s, LIFs are naturally among the first responders to injury signals, which are largely profibrotic, released by AT2s and/or other alveolar cells. Earlier work had already shown that LIFs or LIF-like cells transdifferentiate into myofibroblasts in response to nicotine (125) or hyperoxia exposure (126). In studies using bleomycin to induce lung fibrosis in adult mice, LIFs were shown to give rise to fibrosis-associated myofibroblasts during fibrosis development (127). Interestingly, a recent report showed that, in addition to myofibroblasts, LIFs also display an augmented invasive, proliferative, contractile, and ECM-producing profile (128). Fibroblasts isolated from patients with idiopathic pulmonary fibrosis (IPF) exhibited an invasive phenotype that was dependent on hyaluronan synthase 2 (HAS2) and the hyaluronan receptor CD44 (129). This invasive IPF fibroblast phenotype is reminiscent of metastatic lung adenocarcinoma cancer cells (130). Importantly, the reverse differentiation trajectory (myofibroblast-to-LIF differentiation) occurs during fibrosis resolution (127). Fibrosis development and resolution are largely mediated by TGF-β1 and PPARγ signaling, respectively. In agreement with these findings, forced PPARγ activation in primary human lung fibroblasts attenuated TGF-β1–mediated fibrogenesis and promoted LIF formation (127). Therefore, myofibroblast-to-LIF transdifferentiation may represent an important route for myofibroblast deactivation and fibrosis resolution and could potentially be considered in future therapies to treat patients with progressive fibrotic disorders. Another recent study also described myofibroblast deactivation during the resolution phase (131). The authors identified aldehyde dehydrogenase 2 (Aldh2) and nuclear receptor subfamily 3 group C member 1 (Nr3c1) as potential antifibrotic genes that were downregulated at the peak of fibrosis and upregulated during fibrosis resolution (131). Apoptotic clearance is also a mechanism for fibrosis resolution in the lung, and it features the expression of proapoptotic markers such as the death receptor Fas (132, 133). The balance between myofibroblast deactivation and apoptosis during fibrosis resolution remains to be determined. It is also possible that both processes are critical to fibrosis resolution, as illustrated by suppression of the transcription factor Myo-D, which mediated myofibroblast dedifferentiation while also lowering the apoptosis threshold (134).
IPF is associated with metabolic disorders, and type 2 diabetes mellitus is a risk factor for developing this disease (134–137). Fibrosis-associated myofibroblasts displayed an altered bioenergetic profile where inactive adenosine monophosphate–activated protein kinase (AMPK) in these cells promoted their persistent activation by decreased autophagy, increased ECM production, mitochondrial dysfunction, and resistance to apoptosis (138). Restoring AMPK activity in such myofibroblasts improved mitochondrial biogenesis and enhanced autophagy, ECM turnover, and sensitivity to apoptosis, thus leading to myofibroblast deactivation (138). Notably, the first-line antidiabetic compound metformin, a known AMPK agonist, has proved to be effective in reversing lung fibrosis in the mouse bleomycin model via this mechanism (138). Interestingly, such a beneficial effect of metformin was independently validated when its administration accelerated fibrosis resolution by promoting myofibroblast-to-LIF transdifferentiation (139). The latter study also shed light on an additional AMPK-independent mechanism that leads to BMP2 release and PPARγ activation (139).
Heterogeneity of lung-resident mesenchymal cells in response to influenza virus infection has recently been investigated (140). The authors identified a subset of damage-responsive fibroblasts, expressing the ECM protease ADAMTS4, that aggravates the immune response and leads to structural and functional impairment of the lung (140). Although the immunomodulatory roles of mesenchymal cells, particularly those of MSCs, have already been reported (reviewed in ref. 141), niche–progenitor cell interactions in the context of influenza-induced acute respiratory distress syndrome or even SARS-CoV-2 are still largely unexplored.
Effects of mesenchymal aging on lung fibrosis.
Lung aging that may involve both cellular and noncellular components of the stem cell niche, particularly the ECM, adversely affects lung regenerative capacity, thus predisposing to chronic lung diseases such as IPF and chronic obstructive pulmonary disease. Previous studies have identified aging as a critical determinant of the lung’s ability to resolve fibrotic injury (142–145). While senescent cells have been shown to accumulate in aging tissues (146, 147), their role in age-related diseases has been debated. Considerable heterogeneity exists between senescent cells across tissues that may be related to their physiologically programmed, preexisting transcriptomic signatures and their unique cellular microenvironments; another important contribution to this heterogeneity lies in differences of the senescence-provoking stimuli. For example, oxidative stress–induced senescence in young mice is often transient and may even support physiological repair through a pro-regenerative senescence-associated secretory phenotype (SASP) profile (148), while senescence induced by the same stimulus in aged mice may confer a persistent/progressive, pathological response (142). Such differences highlight the importance of defining the heterogeneity and functional characteristics of cells that acquire a growth-arrested state with expression of the widely used senescence marker p16INK4a. Differences in the context and timing of elimination of p16INK4a-expressing cells may also explain differences in their (patho)physiological roles. For example, there are important differences in the outcome of the injury-repair process dependent on whether the intervention prevents the formation of senescent cells versus the elimination of senescent cells that accumulate during pathological disease states (149–151).
Studying the role of senescence and aging in lung diseases has further illuminated the importance of mesenchymal-epithelial crosstalk within the stem cell niche. The elimination of p16INK4a-expressing cells after established bronchopulmonary dysplasia in a murine hyperoxia model led to improved lung regeneration in association with increased numbers of LIFs and AT2s (151). In an ex vivo alveolosphere-organoid model, aging of the mesenchymal component was critical to AT2 proliferation and alveolosphere formation (152). This inability of mesenchymal cells to support AT2 cell proliferation and differentiation is linked to acquisition of senescence features and metabolic reprogramming, in part, related to elevated expression of the reactive oxygen species–generating enzyme NADPH oxidase 4 (NOX4). Epigenetic targeting of NOX4 with an inhibitor of bromodomain-containing protein 4 (BRD4) accelerated fibrosis resolution in an aging murine model of lung injury (153). In contrast to the pro-senescent, pro-oxidant, and profibrotic actions of NOX4 (154, 155), the augmentation of the antioxidant, antiinflammatory, and senolytic effects of the mitochondrial protein deacylase sirtuin-3 (SIRT3) on macrophages and fibroblasts was effective in restoring pro-regenerative effects in aged mice (145). Mitochondrial dysfunction has been implicated in both AT2 and mesenchymal cell senescence and aging (156, 157). Recent studies have implicated a role for uncoupling protein 2 (UCP2) in loss of mitochondrial bioenergetics, deficient fatty acid oxidation, and senescence of fibroblasts that may account for a nonresolving, persistent/progressive phenotype in aging (158). Thus, like lung development and homeostasis during adulthood, the metabolic and epigenetic programming of the mesenchyme during aging has a critical role in determining the outcome of repair/regenerative responses to lung injury.
Future directions and clinical implications
Recent and emerging studies highlight the importance of balanced mesenchymal activation and fate determination in the lung. Transient activation seems to initially occur in response to injury, and this event primes the lung to undergo regeneration and restore barrier integrity and respiratory function. On the other hand, dysregulated mesenchymal activation appears to be a driver of aberrant repair and fibrosis. The reversibility of injury and the robust reparative capacity observed in model systems have allowed the studying of “scarless” regenerative mechanisms in the mammalian lung.
One issue that urgently needs to be addressed by the scientific community is the integration of omics data published by various research groups and consortia, especially on single-cell RNA-Seq and spatial transcriptomics, into a comprehensive lung mesenchymal cell atlas. Such an atlas should not only list known and novel cell types during homeostasis, disease, and regeneration but also (a) deconvolute mesenchymal cell identity by demarcating stable versus transient cell states, (b) uncover the spatial, functional, and molecular overlap between published cell types and states, and (c) standardize/unify nomenclature. For example, the term “lipofibroblast” as we have used it in this Review is not consistently used in the literature; rather, these cells are often lumped into the designation of “alveolar fibroblasts” or “fibroblasts” as described in several single-cell RNA-Seq data sets (69, 88, 94, 148, 159–161). Although such efforts at standardization have already begun to gain interest within the community, a focused endeavor to develop consensus nomenclature is strongly warranted (also addressed in ref. 162). It is important to mention here that recent advancements in lineage-tracing tools such as the use of split-Cre, Cre/flippase, and Cre-ERT2/Dre-ERT2 dual recombinase approaches will certainly improve data interpretation in emerging research. These approaches, coupled with the increasing bioinformatics input into the field, will provide a platform to achieve better understanding of mesenchymal cell subsets and states under various settings.
Another issue is the transferring of knowledge from the mouse model system to the human context. Interestingly, recent work has highlighted some disparities between mouse and human lung cell biology and airway morphology. For example, it was shown that, unlike mouse AT2s, human AT2s give rise to basal cells during fibrotic remodeling (163). The use of novel tools such as induced pluripotent stem cell–derived cell lines and organoids, precision-cut lung slice cultures, and humanized injury models represents a step forward toward reconciling the concepts and paradigms established using mouse models in preclinical studies.
As discussed in this Review, the interdependence of the epithelium and mesenchyme in maintenance of homeostasis and repair of adult tissues has important clinical implications. The recognition that mesenchymal plasticity determines and drives epithelial repair and regenerative responses implies that health and resilience of tissues/organs will depend on assuring mesenchymal cell responses that are spatio-temporally regulated. Furthermore, in fibrotic diseases affecting diverse organs, therapeutic targeting of tissue-resident fibroblasts/mesenchymal cells to promote niche-supporting regenerative phenotypes, while reprogramming or eliminating fibrosis-perpetuating phenotypes, will lead to innovative new therapies for this recalcitrant, debilitating, and ultimately fatal group of disorders. The challenge remains to exploit advancing knowledge of mesenchymal plasticity and to innovate new therapeutic strategies that promote regeneration of the lung to prevent, retard, and even reverse fibrosis.
Acknowledgments
EEA acknowledges the support of the ILH, German Research Foundation (DFG; EL 931/5-1, EL 931/4-1, KFO309 284237345 P7, and SFB CRC1213 268555672 project A04), CPI, and DZL. VJT was supported by the US National Institutes of Health (grants P01-HL114470, R01-HL139617, R01-HL151702, and R01-HL152246) and a US Department of Veterans Affairs Merit Award (grant I01BX003056).
Version 1. 07/17/2023
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2023, El Agha et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2023;133(14):e170498. https://doi.org/10.1172/JCI170498.
References
- 1.Tsikis ST, et al. Targeting the lung endothelial niche to promote angiogenesis and regeneration: a review of applications. Front Mol Biosci. 2022;9:109336. doi: 10.3389/fmolb.2022.1093369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Millar FR, et al. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016;71(5):462–473. doi: 10.1136/thoraxjnl-2015-207461. [DOI] [PubMed] [Google Scholar]
- 3.Cao Z, et al. Targeting of the pulmonary capillary vascular niche promotes lung alveolar repair and ameliorates fibrosis. Nat Med. 2016;22(2):154–162. doi: 10.1038/nm.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding BS, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niethamer TK, et al. Defining the role of pulmonary endothelial cell heterogeneity in the response to acute lung injury. Elife. 2020;9:e53072. doi: 10.7554/eLife.53072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majka SM, et al. Mesenchymal regulation of the microvascular niche in chronic lung diseases. Compr Physiol. 2019;9(4):1431–1441. doi: 10.1002/cphy.c180043. [DOI] [PubMed] [Google Scholar]
- 7.Ahmadvand N, et al. Identification of a novel subset of alveolar type 2 cells enriched in PD-L1 and expanded following pneumonectomy. Eur Respir J. 2021;28(5):2004168. doi: 10.1183/13993003.04168-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi J, et al. Inflammatory signals induce AT2 cell-derived damage-associated transient progenitors that mediate alveolar regeneration. Cell Stem Cell. 2020;27(3):366–382. doi: 10.1016/j.stem.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobayashi Y, et al. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat Cell Biol. 2020;22(8):934–946. doi: 10.1038/s41556-020-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar PA, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147(3):525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quantius J, et al. Influenza virus infects epithelial stem/progenitor cells of the distal lung: impact on Fgfr2b-driven epithelial repair. PLoS Pathog. 2016;12(6):e1005544. doi: 10.1371/journal.ppat.1005544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray S, et al. Rare SOX2+ airway progenitor cells generate KRT5+ cells that repopulate damaged alveolar parenchyma following influenza virus infection. Stem Cell Reports. 2016;7(5):817–825. doi: 10.1016/j.stemcr.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salwig I, et al. Bronchioalveolar stem cells are a main source for regeneration of distal lung epithelia in vivo. EMBO J. 2019;38(12):1–16. doi: 10.15252/embj.2019102099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strunz M, et al. Alveolar regeneration through a Krt8+ transitional stem cell state that persists in human lung fibrosis. Nat Commun. 2020;11(1):3559. doi: 10.1038/s41467-020-17358-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaughan AE, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature. 2015;517(7536):621–625. doi: 10.1038/nature14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vazquez-Armendariz AI, et al. Multilineage murine stem cells generate complex organoids to model distal lung development and disease. EMBO J. 2020;39(21):e103476. doi: 10.15252/embj.2019103476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasnick RM, et al. Differential LysoTracker uptake defines two populations of distal epithelial cells in idiopathic pulmonary fibrosis. Cells. 2022;11(2):235. doi: 10.3390/cells11020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xi Y, et al. Local lung hypoxia determines epithelial fate decisions during alveolar regeneration. Nat Cell Biol. 2017;19(8):904–914. doi: 10.1038/ncb3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y, et al. Spatial-temporal lineage restrictions of embryonic p63+ progenitors establish distinct stem cell pools in adult airways. Dev Cell. 2018;44(6):752–761. doi: 10.1016/j.devcel.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zacharias WJ, et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature. 2018;555(7695):251–255. doi: 10.1038/nature25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng D, et al. Evidence for Scgb1a1(+) cells in the generation of p63(+) cells in the damaged lung parenchyma. Am J Respir Cell Mol Biol. 2014;50(3):595–604. doi: 10.1165/rcmb.2013-0327OC. [DOI] [PubMed] [Google Scholar]
- 22.Zuo W, et al. p63(+)Krt5(+) distal airway stem cells are essential for lung regeneration. Nature. 2015;517(7536):616–620. doi: 10.1038/nature13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nabhan AN, et al. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359(6380):1118–1123. doi: 10.1126/science.aam6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Agha E, Bellusci S. Walking along the fibroblast growth factor 10 route: a key pathway to understand the control and regulation of epithelial and mesenchymal cell-lineage formation during lung development and repair after injury. Scientifica. 2014;2014:538379. doi: 10.1155/2014/538379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan BLM, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15(2):123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kina YP, et al. The lung vasculature: a driver or passenger in lung branching morphogenesis? Front Cell Dev Biol. 2021;8:623868. doi: 10.3389/fcell.2020.623868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18(1):8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar ME et al. Mesenchymal cells. Defining a mesenchymal progenitor niche at single-cell resolution. Science. 2014;346(6211):1258810. doi: 10.1126/science.1258810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng T, et al. Coordination of heart and lung co-development by a multipotent cardiopulmonary progenitor. Nature. 2013;500(7464):589–592. doi: 10.1038/nature12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bellusci S, et al. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development. 1997;124(23):4867–4878. doi: 10.1242/dev.124.23.4867. [DOI] [PubMed] [Google Scholar]
- 31.Alescio T, Cassini A. Induction in vitro of tracheal buds by pulmonary mesenchyme grafted on tracheal epithelium. J Exp Zool. 1962;150(2):83–94. doi: 10.1002/jez.1401500202. [DOI] [PubMed] [Google Scholar]
- 32.Sekine K, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21(1):138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 33.Moerlooze LD, et al. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127(3):483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 34.El Agha E, et al. Characterization of a novel fibroblast growth factor 10 (Fgf10) knock-in mouse line to target mesenchymal progenitors during embryonic development. PLoS One. 2012;7(6):e38452. doi: 10.1371/journal.pone.0038452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chu X, et al. Validation of a novel Fgf10Cre–ERT2 knock-in mouse line targeting FGF10Pos cells postnatally. Front Cell Dev Biol. 2021;9:671841. doi: 10.3389/fcell.2021.671841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volckaert T, et al. Localized Fgf10 expression is not required for lung branching morphogenesis but prevents differentiation of epithelial progenitors. Development. 2013;140(18):3731–3742. doi: 10.1242/dev.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mailleux AA, et al. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132(9):2157–2166. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- 38.El Agha E, et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141(2):296–306. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El Agha E, et al. Ex vivo analysis of the contribution of FGF10+ cells to airway smooth muscle cell formation during early lung development. Dev Dyn. 2017;246(7):531–538. doi: 10.1002/dvdy.24504. [DOI] [PubMed] [Google Scholar]
- 40.Al Alam D, et al. Evidence for the involvement of fibroblast growth factor 10 in lipofibroblast formation during embryonic lung development. Development. 2015;142(23):4139–4150. doi: 10.1242/dev.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Young RE, et al. Smooth muscle differentiation is essential for airway size, tracheal cartilage segmentation, but dispensable for epithelial branching. Dev Cell. 2020;53(1):73–85. doi: 10.1016/j.devcel.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim HY, et al. Localized smooth muscle differentiation is essential for epithelial bifurcation during branching morphogenesis of the mammalian lung. Dev Cell. 2015;34(6):719–726. doi: 10.1016/j.devcel.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park J, et al. The Tcf21 lineage constitutes the lung lipofibroblast population. Am J Physiol Lung Cell Mol Physiol. 2019;316(5):L872–L885. doi: 10.1152/ajplung.00254.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaccaro C, Brody JS. Ultrastructure of developing alveoli. I. The role of the interstitial fibroblast. Anat Rec. 1978;192(4):467–479. doi: 10.1002/ar.1091920402. [DOI] [PubMed] [Google Scholar]
- 45.Branchfield K, et al. A three-dimensional study of alveologenesis in mouse lung. Dev Biol. 2016;409(2):429–441. doi: 10.1016/j.ydbio.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gouveia L, et al. PDGF-A signaling is required for secondary alveolar septation and controls epithelial proliferation in the developing lung. Development. 2018;145(7):dev161976. doi: 10.1242/dev.161976. [DOI] [PubMed] [Google Scholar]
- 47.Li R, et al. Myofibroblast contraction is essential for generating and regenerating the gas-exchange surface. J Clin Invest. 2020;130(6):2859–2871. doi: 10.1172/JCI132189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zepp JA, et al. Genomic, epigenomic, and biophysical cues controlling the emergence of the lung alveolus. Science. 2021;371(6534):eabc3172. doi: 10.1126/science.abc3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rippa AL, et al. Alveologenesis: what governs secondary septa formation. Int J Mol Sci. 2021;22(22):12107. doi: 10.3390/ijms222212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vila Ellis L, Chen J. A cell-centric view of lung alveologenesis. Dev Dyn. 2021;250(4):482–496. doi: 10.1002/dvdy.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lignelli E, et al. Recent advances in our understanding of the mechanisms of lung alveolarization and bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2019;317(6):L832–L887. doi: 10.1152/ajplung.00369.2019. [DOI] [PubMed] [Google Scholar]
- 52.Ushakumary MG, et al. Resident interstitial lung fibroblasts and their role in alveolar stem cell niche development, homeostasis, injury, and regeneration. Stem Cells Transl Med. 2021;10(7):1021–1032. doi: 10.1002/sctm.20-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hagan AS, et al. Identification of a FGF18-expressing alveolar myofibroblast that is developmentally cleared during alveologenesis. Development. 2020;147(2):dev181032. doi: 10.1242/dev.181032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Narvaez Del Pilar O, et al. Three-axis classification of mouse lung mesenchymal cells reveals two populations of myofibroblasts. Development. 2022;149(6):dev200081. doi: 10.1242/dev.200081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gao F, et al. Hedgehog-responsive PDGFRa(+) fibroblasts maintain a unique pool of alveolar epithelial progenitor cells during alveologenesis. Cell Rep. 2022;39(1):110608. doi: 10.1016/j.celrep.2022.110608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boström H, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85(6):863–873. doi: 10.1016/S0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 57.Lindahl P, et al. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124(20):3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 58.McGowan SE, et al. Platelet-derived growth factor receptor-alpha-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. Anat Rec (Hoboken) 2008;291(12):1649–1661. doi: 10.1002/ar.20764. [DOI] [PubMed] [Google Scholar]
- 59.Ntokou A, et al. Characterization of the platelet-derived growth factor receptor-α-positive cell lineage during murine late lung development. Am J Physiol Lung Cell Mol Physiol. 2015;309(9):L942–L958. doi: 10.1152/ajplung.00272.2014. [DOI] [PubMed] [Google Scholar]
- 60.Endale M, et al. Temporal, spatial, and phenotypical changes of PDGFRα expressing fibroblasts during late lung development. Dev Biol. 2017;425(2):161–175. doi: 10.1016/j.ydbio.2017.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gouveia L, et al. Expression analysis of platelet-derived growth factor receptor alpha and its ligands in the developing mouse lung. Physiol Rep. 2017;5(6):e13092. doi: 10.14814/phy2.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li R, et al. Pdgfra marks a cellular lineage with distinct contributions to myofibroblasts in lung maturation and injury response. Elife. 2018;7:e36865. doi: 10.7554/eLife.36865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li C, et al. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells. 2015;33(3):999–1012. doi: 10.1002/stem.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moiseenko A, et al. Origin and characterization of alpha smooth muscle actin-positive cells during murine lung development. Stem Cells. 2017;35(6):1566–1578. doi: 10.1002/stem.2615. [DOI] [PubMed] [Google Scholar]
- 65.Po A, et al. Noncanonical GLI1 signaling promotes stemness features and in vivo growth in lung adenocarcinoma. Oncogene. 2017;36(32):4641–4652. doi: 10.1038/onc.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGowan SE, Torday JS. The pulmonary lipofibroblast (lipid interstitial cell) and its contributions to alveolar development. Annu Rev Physiol. 1997;59:43–62. doi: 10.1146/annurev.physiol.59.1.43. [DOI] [PubMed] [Google Scholar]
- 67.O’Hare KH, Sheridan MN. Electron microscopic observations on the morphogenesis of the albino rat lung, with special reference to pulmonary epithelial cells. Am J Anat. 1970;127(2):181–205. doi: 10.1002/aja.1001270205. [DOI] [PubMed] [Google Scholar]
- 68.Liberti DC, et al. Alveolar epithelial cell fate is maintained in a spatially restricted manner to promote lung regeneration after acute injury. Cell Rep. 2021;35(6):109092. doi: 10.1016/j.celrep.2021.109092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brownfield DG, et al. Alveolar cell fate selection and lifelong maintenance of AT2 cells by FGF signaling. Nat Commun. 2022;13(1):7137. doi: 10.1038/s41467-022-34059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmadvand N, et al. Fgfr2b signaling is essential for the maintenance of the alveolar epithelial type 2 lineage during lung homeostasis in mice. Cell Mol Life Sci. 2022;79(6):302. doi: 10.1007/s00018-022-04327-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yuan T, et al. FGF10-FGFR2B signaling generates basal cells and drives alveolar epithelial regeneration by bronchial epithelial stem cells after lung injury. Stem Cell Reports. 2019;12(5):1041–1055. doi: 10.1016/j.stemcr.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barkauskas CE, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taghizadeh S, et al. Characterization in mice of the resident mesenchymal niche maintaining AT2 stem cell proliferation in homeostasis and disease. Stem Cells. 2021;39(10):1382–1394. doi: 10.1002/stem.3423. [DOI] [PubMed] [Google Scholar]
- 74.Rehan VK, et al. Evidence for the presence of lipofibroblasts in human lung. Exp Lung Res. 2006;32(8):379–393. doi: 10.1080/01902140600880257. [DOI] [PubMed] [Google Scholar]
- 75.Tahedl D, et al. How common is the lipid body-containing interstitial cell in the mammalian lung? Am J Physiol Lung Cell Mol Physiol. 2014;386(5):L386–L394. doi: 10.1152/ajplung.00131.2014. [DOI] [PubMed] [Google Scholar]
- 76.Ahlbrecht K, McGowan SE. In search of the elusive lipofibroblast in human lungs. Am J Physiol Lung Cell Mol Physiol. 2014;307(8):L605–L608. doi: 10.1152/ajplung.00230.2014. [DOI] [PubMed] [Google Scholar]
- 77.Schipke J, et al. Lipofibroblasts in structurally normal, fibrotic, and emphysematous human lungs. Am J Respir Crit Care Med. 2021;204(2):227–230. doi: 10.1164/rccm.202101-0043LE. [DOI] [PubMed] [Google Scholar]
- 78.Valenzi E, et al. Single-cell analysis reveals fibroblast heterogeneity and myofibroblasts in systemic sclerosis-associated interstitial lung disease. Ann Rheum Dis. 2019;78(10):1379–1387. doi: 10.1136/annrheumdis-2018-214865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Habermann AC, et al. Single-cell RNA sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1972. doi: 10.1126/sciadv.aba1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Travaglini KJ, et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature. 2020;1(7835):619–625. doi: 10.1038/s41586-020-2922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu X, et al. Categorization of lung mesenchymal cells in development and fibrosis. iScience. 2021;24(6):102551. doi: 10.1016/j.isci.2021.102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun X, et al. A census of the lung: CellCards from LungMAP. Dev Cell. 2022;57(1):112–145. doi: 10.1016/j.devcel.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schultz CJ, et al. Role of adipocyte differentiation-related protein in surfactant phospholipid synthesis by type II cells. Am J Physiol Lung Cell Mol Physiol. 2002;283(2):L288–L296. doi: 10.1152/ajplung.00204.2001. [DOI] [PubMed] [Google Scholar]
- 84.Ntokou A, et al. A novel mouse Cre-driver line targeting Perilipin 2-expressing cells in the neonatal lung. Genesis. 2017;55(12):e23080. doi: 10.1002/dvg.23080. [DOI] [PubMed] [Google Scholar]
- 85.Negretti NM, et al. A single-cell atlas of mouse lung development. Development. 2021;148(24):dev199512. doi: 10.1242/dev.199512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. El Agha E, Bellusci S. Evidence for the involvement of lipofibroblasts, airway smooth muscle cells and FGF10 signalling in lung repair. In: Nikolić MZ, Hogan BLM, eds. Lung Stem Cells in Development, Health and Disease. European Respiratory Society; 2021:99–113. [Google Scholar]
- 87.Sountoulidis A, et al. A topographic atlas defines developmental origins of cell heterogeneity in the human embryonic lung. Nat Cell Biol. 2023;1(2):351–365. doi: 10.1038/s41556-022-01064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lim K, et al. Organoid modeling of human fetal lung alveolar development reveals mechanisms of cell fate patterning and neonatal respiratory disease. Cell Stem Cell. 2023;30(1):20–37. doi: 10.1016/j.stem.2022.11.013. [DOI] [PubMed] [Google Scholar]
- 89.Danopoulos S, et al. Transcriptional characterisation of human lung cells identifies novel mesenchymal lineage markers. Eur Respir J. 2020;55(1):1900746. doi: 10.1183/13993003.00746-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Danopoulos S, et al. Discordant roles for FGF ligands in lung branching morphogenesis between human and mouse. J Pathol. 2019;247(2):254–265. doi: 10.1002/path.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hein RFC, et al. R-SPONDIN2+ mesenchymal cells form the bud tip progenitor niche during human lung development. Dev Cell. 2022;57(13):1598–1614. doi: 10.1016/j.devcel.2022.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He P, et al. A human fetal lung cell atlas uncovers proximal-distal gradients of differentiation and key regulators of epithelial fates. Cell. 2022;185(25):4841–4860. doi: 10.1016/j.cell.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Sun D, et al. SOX9 maintains human foetal lung tip progenitor state by enhancing WNT and RTK signalling. EMBO J. 2022;41(21):e111338. doi: 10.15252/embj.2022111338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kadur Lakshminarasimha Murthy P, et al. Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature. 2022;604(7904):111–119. doi: 10.1038/s41586-022-04541-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.El Agha E, et al. Therapeutic and pathological roles of fibroblast growth factors in pulmonary diseases. Dev Dyn. 2017;246(4):235–244. doi: 10.1002/dvdy.24468. [DOI] [PubMed] [Google Scholar]
- 96.Rawlins EL, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stripp BR, et al. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol. 1995;269(6 pt 1):L791–L799. doi: 10.1152/ajplung.1995.269.6.L791. [DOI] [PubMed] [Google Scholar]
- 98.Fanucchi MV, et al. Pulmonary cytochrome P450 monooxygenase and Clara cell differentiation in mice. Am J Respir Cell Mol Biol. 1997;17(3):302–314. doi: 10.1165/ajrcmb.17.3.2774. [DOI] [PubMed] [Google Scholar]
- 99.Boyd MR. Evidence for the Clara cell as a site of cytochrome P450-dependent mixed-function oxidase activity in lung. Nature. 1977;269(5630):713–715. doi: 10.1038/269713a0. [DOI] [PubMed] [Google Scholar]
- 100.Mahvi D, et al. Morphology of a naphthalene-induced bronchiolar lesion. Am J Pathol. 1977;86(3):558–572. [PMC free article] [PubMed] [Google Scholar]
- 101.Giangreco A, et al. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161(1):173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong KU, et al. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24(6):671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 103.Reynolds SD, et al. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156(1):269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rawlins EL, et al. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci U S A. 2007;104(2):410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volckaert T, et al. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121(11):4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee JH, et al. Anatomically and functionally distinct lung mesenchymal populations marked by Lgr5 and Lgr6. Cell. 2017;170(6):1149–1163. doi: 10.1016/j.cell.2017.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zepp JA, et al. Distinct mesenchymal lineages and niches promote epithelial self-renewal and myofibrogenesis in the lung. Cell. 2017;170(6):1134–1148. doi: 10.1016/j.cell.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Volckaert T, et al. Fgf10-Hippo epithelial-mesenchymal crosstalk maintains and recruits lung basal stem cells. Dev Cell. 2017;43(1):48–59. doi: 10.1016/j.devcel.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moiseenko A, et al. Identification of a repair-supportive mesenchymal cell population during airway epithelial regeneration. Cell Rep. 2020;33(12):108549. doi: 10.1016/j.celrep.2020.108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vazquez-Armendariz AI, et al. Protocol for the generation of murine bronchiolospheres. STAR Protoc. 2021;2(2):100594. doi: 10.1016/j.xpro.2021.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chu X, et al. GLI1+ cells are a source of repair-supportive mesenchymal cells (RSMCs) during airway epithelial regeneration. Cell Mol Life Sci. 2022;79(11):581. doi: 10.1007/s00018-022-04599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lyu H, et al. Niche-mediated repair of airways is directed in an occupant-dependent manner. Cell Rep. 2022;41(12):111863. doi: 10.1016/j.celrep.2022.111863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lv YQ, et al. FGF10 therapeutic administration promotes mobilization of injury-activated alveolar progenitors in a mouse fibrosis model. Cells. 2022;11(15):2396. doi: 10.3390/cells11152396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paris AJ, et al. STAT3-BDNF-TrkB signalling promotes alveolar epithelial regeneration after lung injury. Nat Cell Biol. 2020;22(10):1197–1210. doi: 10.1038/s41556-020-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fernanda de Mello Costa M, et al. Basal-like progenitor cells: a review of dysplastic alveolar regeneration and remodeling in lung repair. Stem Cell Reports. 2020;15(5):1015–1025. doi: 10.1016/j.stemcr.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bellusci S, et al. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development. 1997;124(1):53–63. doi: 10.1242/dev.124.1.53. [DOI] [PubMed] [Google Scholar]
- 117.Yin W, et al. An essential function for autocrine hedgehog signaling in epithelial proliferation and differentiation in the trachea. Development. 2022;149(3):dev199804. doi: 10.1242/dev.199804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Miller LAD, et al. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231(1):57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- 119.Peng T, et al. Hedgehog actively maintains adult lung quiescence and regulates repair and regeneration. Nature. 2015;526(7574):578–582. doi: 10.1038/nature14984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kramann R, et al. Perivascular Gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell Stem Cell. 2015;16(1):51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.El Agha E, et al. Mesenchymal stem cells in fibrotic disease. Cell Stem Cell. 2017;21(2):166–177. doi: 10.1016/j.stem.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 122.Schneider RK, et al. Gli1+ mesenchymal stromal cells are a key driver of bone marrow fibrosis and an important cellular therapeutic target. Cell Stem Cell. 2017;20(6):785–800.e8. doi: 10.1016/j.stem.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cassandras M, et al. Gli1+ mesenchymal stromal cells form a pathological niche to promote airway progenitor metaplasia in the fibrotic lung. Nat Cell Biol. 2020;22(11):1295–1306. doi: 10.1038/s41556-020-00591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang C, et al. Expansion of hedgehog disrupts mesenchymal identity and induces emphysema phenotype. J Clin Invest. 2018;128(10):4343–4358. doi: 10.1172/JCI99435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rehan VK, et al. Reversal of nicotine-induced alveolar lipofibroblast-to-myofibroblast transdifferentiation by stimulants of parathyroid hormone-related protein signaling. Lung. 2007;185(3):151–159. doi: 10.1007/s00408-007-9007-0. [DOI] [PubMed] [Google Scholar]
- 126.Rehan V, Torday J. Hyperoxia augments pulmonary lipofibroblast-to-myofibroblast transdifferentiation. Cell Biochem Biophys. 2003;38(3):239–250. doi: 10.1385/CBB:38:3:239. [DOI] [PubMed] [Google Scholar]
- 127.El Agha E, et al. Two-way conversion between lipogenic and myogenic fibroblastic phenotypes marks the progression and resolution of lung fibrosis. Cell Stem Cell. 2017;20(2):261–273. doi: 10.1016/j.stem.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Liu X, et al. Multiple fibroblast subtypes contribute to matrix deposition in pulmonary fibrosis. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2022-0292oc. [published online March 16, 2023]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y, et al. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208(7):1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu X, et al. HER2 drives lung fibrosis by activating a metastatic cancer signature in invasive lung fibroblasts. J Exp Med. 2022;219(10):e20220126. doi: 10.1084/jem.20220126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tan Q, et al. Spontaneous lung fibrosis resolution reveals novel antifibrotic regulators. Am J Respir Cell Mol Biol. 2021;64(4):453–464. doi: 10.1165/rcmb.2020-0396OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Redente EF, et al. Loss of Fas signaling in fibroblasts impairs homeostatic fibrosis resolution and promotes persistent pulmonary fibrosis. JCI Insight. 2021;6(1):e141618. doi: 10.1172/jci.insight.141618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pham TX, et al. Transcriptional analysis of lung fibroblasts identifies PIM1 signaling as a driver of aging-associated persistent fibrosis. JCI Insight. 2022;7(6):e153672. doi: 10.1172/jci.insight.153672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yan F, et al. Identification of the lipid biomarkers from plasma in idiopathic pulmonary fibrosis by Lipidomics. BMC Pulm Med. 2017;17(1):174. doi: 10.1186/s12890-017-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao YD, et al. Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study. BMJ Open Respir Res. 2017;4(1):e000183. doi: 10.1136/bmjresp-2017-000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Figueroa MCGS, et al. Risk factors for idiopathic pulmonary fibrosis in a Mexican population. A case-control study. Resp Med. 2010;104(2):305–309. doi: 10.1016/j.rmed.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 137.Bargagli E, et al. Metabolic dysregulation in idiopathic pulmonary fibrosis. Int J Mol Sci. 2020;21(16):5663. doi: 10.3390/ijms21165663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rangarajan S, et al. Metformin reverses established lung fibrosis in a bleomycin model. Nat Med. 2018;24(8):1121–1127. doi: 10.1038/s41591-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kheirollahi V, et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun. 2019;10(1):2987. doi: 10.1038/s41467-019-10839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Boyd DF, et al. Exuberant fibroblast activity compromises lung function via ADAMTS-4. Nature. 2020;587(7834):466–471. doi: 10.1038/s41586-020-2877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen L, et al. Mesenchymal stem cell-based treatments for COVID-19: status and future perspectives for clinical applications. Cell Mol Life Sci. 2022;79(3):142. doi: 10.1007/s00018-021-04096-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hecker L, et al. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci Transl Med. 2014;6(231):231ra47. doi: 10.1126/scitranslmed.3008182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Caporarello N, et al. Vascular dysfunction in aged mice contributes to persistent lung fibrosis. Aging Cell. 2020;19(8):e13196. doi: 10.1111/acel.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qu J, et al. Targeting mechanosensitive MDM4 promotes lung fibrosis resolution in aged mice. J Exp Med. 2021;218(5):e20202033. doi: 10.1084/jem.20202033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rehan M, et al. Restoration of SIRT3 gene expression by airway delivery resolves age-associated persistent lung fibrosis in mice. Nat Aging. 2021;1(2):205–217. doi: 10.1038/s43587-021-00027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Krishnamurthy J, et al. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114(9):1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yousefzadeh MJ, et al. Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell. 2020;19(3):e13094. doi: 10.1111/acel.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Reyes NS, et al. Sentinel p16INK4a+ cells in the basement membrane form a reparative niche in the lung. Science. 2022;378(6616):192–201. doi: 10.1126/science.abf3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sundar IK, et al. Genetic ablation of p16INK4a does not protect against cellular senescence in mouse models of chronic obstructive pulmonary disease/emphysema. Am J Respir Cell Mol Biol. 2018;59(2):189–199. doi: 10.1165/rcmb.2017-0390OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Antony VB, Thannickal VJ. Cellular senescence in chronic obstructive pulmonary disease: multifaceted and multifunctional. Am J Respir Cell Mol Biol. 2018;59(2):135–136. doi: 10.1165/rcmb.2018-0061ED. [DOI] [PubMed] [Google Scholar]
- 151.Zysman M, et al. Targeting p16INK4a promotes lipofibroblasts and alveolar regeneration after early-life injury. Am J Respir Crit Care Med. 2020;202(8):1088–1104. doi: 10.1164/rccm.201908-1573OC. [DOI] [PubMed] [Google Scholar]
- 152.Chanda D, et al. Mesenchymal stromal cell aging impairs the self-organizing capacity of lung alveolar epithelial stem cells. Elife. 2021;10:e68049. doi: 10.7554/eLife.68049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sanders YY, et al. Brd4-p300 inhibition downregulates Nox4 and accelerates lung fibrosis resolution in aged mice. JCI Insight. 2020;5(14):e137127. doi: 10.1172/jci.insight.137127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bernard K, et al. NADPH oxidase 4 (Nox4) suppresses mitochondrial biogenesis and bioenergetics in lung fibroblasts via a nuclear factor erythroid-derived 2-like 2 (Nrf2)-dependent pathway. J Biol Chem. 2017;292(7):3029–3038. doi: 10.1074/jbc.M116.752261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hecker L, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15(9):1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bueno M, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125(2):521–538. doi: 10.1172/JCI74942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rangarajan S, et al. Mitochondrial dysfunction in pulmonary fibrosis. Ann Am Thorac Soc. 2017;14(suppl 5):S383–S388. doi: 10.1513/AnnalsATS.201705-370AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rangarajan S, et al. Mitochondrial uncoupling protein-2 reprograms metabolism to induce oxidative stress and myofibroblast senescence in age-associated lung fibrosis. Aging Cell. 2022;21(9):e13674. doi: 10.1111/acel.13674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Adams TS, et al. Single-cell RNA-seq reveals ectopic and aberrant lung-resident cell populations in idiopathic pulmonary fibrosis. Sci Adv. 2020;6(28):eaba1983. doi: 10.1126/sciadv.aba1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tsukui T, et al. Collagen-producing lung cell atlas identifies multiple subsets with distinct localization and relevance to fibrosis. Nat Commun. 2020;11(1):1920. doi: 10.1038/s41467-020-15647-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Tsukui T, Sheppard D. Tracing the origin of pathologic pulmonary fibroblasts [preprint]. Posted on bioRxiv November 18, 2022. [DOI]
- 162.Riccetti M, et al. The elephant in the lung: integrating lineage-tracing, molecular markers, and single cell sequencing data to identify distinct fibroblast populations during lung development and regeneration. Matrix Biol. 2020;91:51–74. doi: 10.1016/j.matbio.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kathiriya JJ, et al. Human alveolar type 2 epithelium transdifferentiates into metaplastic KRT5+ basal cells. Nat Cell Biol. 2022;24(1):10–23. doi: 10.1038/s41556-021-00809-4. [DOI] [PMC free article] [PubMed] [Google Scholar]