Key message.
In this case of refractory polyarthritis, PCR from the joint and urine but not from the duodenal biopsy led to the diagnosis of Whipple’s disease.
Dear Editor, We report on a 51-year-old male patient who presented initially with symmetrical polyarthralgia, predominantly affecting large joints, that occurred intermittently for 20 years. On admission, laboratory diagnostics revealed a CRP of 37.1 mg/l (normal <5.0 mg/l), with slightly elevated ACPA and RF; the latter was normal in follow-up tests. Scintigraphy showed radionuclide enrichment in the right wrist and elbow and in both ankle joints. MRI of the right hand showed bone marrow oedema in the carpal joints and an erosion of the MCP joint III. Based on these findings, rheumatoid arthritis (RA) was diagnosed and therapy with prednisolone and MTX initiated.
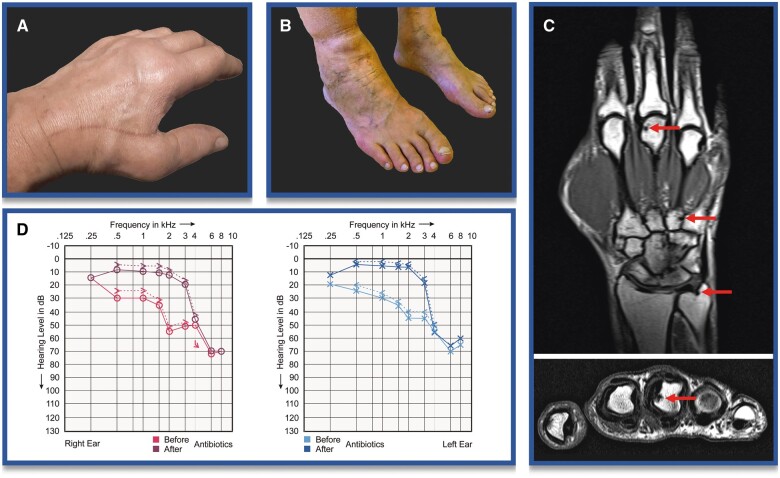
In the following months, the patient had continuing polyarthritis (Fig. 1A, B), mostly affecting the MCP, wrist and ankle joints. Radiography revealed initial erosions in the finger and toe joints (Fig. 1C). MTX, etanercept, baricitinib, upadacitinib, abatecept and local injection of glucocorticoids in the ankle joint were administered, without a therapeutic benefit. Only prednisolone doses >15 mg/day provided partial relief. CRP levels remained elevated 7- to 20-fold over 30 months.
Figure 1.
Clinical impressions. (A) MCP joint II/III swelling of the left hand. (B) Ankle swelling of the right leg. (C) T1-weighted MRI images of the right hand, showing erosions in coronary and axial planes. (D) Overlay of audiometry curves for the right and left ear, before and after 6 months of antibiotic administration
Owing to refractory arthritis, additional weight loss (4% within 1 year), pale skin coloration and epigastric discomfort, an initial reassessment of the working diagnosis was made, which was followed by oesophageal gastroscopy, leading to exclusion of Tropheryma whipplei by histology and PCR. Malignant disease was ruled out by PET-CT.
Subsequent use of tocilizumab, adalimumab and a radiosynovioorthesis of the ankle joint also had no effect. In addition to increasing pain, loss of function and reduced quality of life, the patient developed depression with suicidal ideation. Furthermore, he reported the onset of dizziness, nausea, headache, tinnitus and an increasing symmetrical hearing loss, which was objectified with audiometry (Fig. 1D), but otolaryngological evaluation revealed no probable causes. The patient was prescribed hearing aids.
Given that the refractory ankle arthritis threatened mobility and the wrist arthritis limited the ability to work, a synovectomy was discussed. Before surgical referral, a further joint puncture was performed to rule out infection with previously unconsidered pathogens, such as atypical mycobacteria, and to rule out T. whipplei again. Although PCR diagnostics from the synovial fluid of the right ankle did not reveal mycobacteria, the PCR for T. whipplei was positive. Subsequently, this ubiquitously occurring Gram-positive rod bacterium [1] was also detected by PCR in the urine and spinal fluid. Brain MRI and neuropsychological testing were normal.
The patient received ceftriaxone i.v. over 4 weeks (≤4 g/day), followed by oral sulfamethoxazole and trimethoprim (800 and 160 mg, respectively, twice daily) for 1 year. The initial administration of ceftriaxone resulted in a Jarisch–Herxheimer reaction, with chills and elevated blood pressure, which responded well to glucocorticoids. Polyarthritis, dizziness, nausea, headaches, tinnitus and hearing loss steadily receded and were almost completely resolved after 6 months (Fig. 1D). CRP normalized within 6 weeks after initiation of antibiotics. The patient was able to resume work and sports activities. Hearing aids were no longer needed.
We consider this case of general interest because the diagnosis of well-treatable Whipple’s disease is often challenging. In this case, initially slightly elevated ACPA and RF in association with elevated joint fluid cell count, symmetrical synovitis and erosions mimicked RA. According to the EULAR points to consider for difficult to treat RA, active reassessment of the diagnosis should take place before further cycling of therapies after an initial failure of two biologic/targeted synthetic DMARDs.
Initial diagnostic re-evaluation had included a duodenal biopsy to assess for Whipple’s disease, which was negative. Although small bowel biopsies allowed diagnosis in 95% of 191 cases of classic Whipple’s disease in one published cohort [2], it is important to note that duodenal biopsies do not have a high enough sensitivity to exclude an infection with T. whipplei in the context of non-classical Whipples’s disease and predominant joint affection [3, 4]. PCR from saliva and stool has been recommended as first-line screening [5]. Because the disease is rare, the described sensitivity of 62.5% led to a negative predictive value of 94.6% [5]. Importantly, both false-positive and false-negative results are possible [5, 6]. Interestingly, PCR from urine provided 75% sensitivity with 100% specificity in 12 Whipple’s disease patients and 110 healthy controls [6]. In our patient, earlier PCR diagnostics of the synovial fluid or urine for T. whipplei could have enabled earlier diagnosis and therapy.
Moreover, the clinical combination of arthritis and hearing loss is of special interest. Hearing loss has been described in individual patients with Whipple’s disease [7] and has even been discussed as a cause for Beethoven’s deafness [8]. Although neurological symptoms caused by Whipple’s disease are often irreversible, fortunately almost complete recovery could be achieved with antibiotic treatment (Fig. 1D). In summary, Whipple’s disease is an important differential diagnosis in refractory arthritis that should be tested by PCR from a focus of inflammation.
Acknowledgements
We are grateful to Heinz Biesen for technical assistance in creating Fig. 1.
Contributor Information
Robert Biesen, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Tobias Alexander, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Gerd-Rüdiger Burmester, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Fredrik N Albach, Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany.
Data availability
The data underlying this article are available from the corresponding author on reasonable request.
Author contributions
All authors contributed to acquisition, analysis and interpretation of the data and drafting the manuscript.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Disclosure statement: The authors have declared no conflicts of interest.
Consent: Informed consent has been obtained from the patient.
References
- 1. Marth T, Moos V, Müller C et al. Tropheryma whipplei infection and Whipple's disease. Lancet Infect Dis 2016;16:e13-22–e22. [DOI] [PubMed] [Google Scholar]
- 2. Günther U, Moos V, Offenmüller G et al. Gastrointestinal diagnosis of classical Whipple disease: clinical, endoscopic, and histopathologic features in 191 patients. Medicine (Baltimore) 2015;94:e714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tison A, Preuss P, Leleu C et al. Rheumatological features of Whipple disease. Sci Rep 2021;11:12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruffer N, Holzer M-T, Gkanatsas Y et al. Chronic Tropheryma whipplei infection: an important differential diagnosis of refractory polyarthritis. [In German] Z Rheumatol 2022. 10.1007/s00393-022-01194-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fenollar F, Laouira S, Lepidi H et al. Value of Tropheryma whipplei quantitative polymerase chain reaction assay for the diagnosis of Whipple disease: usefulness of saliva and stool specimens for first-line screening. Clin Infect Dis 2008;47:659–67. [DOI] [PubMed] [Google Scholar]
- 6. Moter A, Janneck M, Wolters M et al. Potential role for urine polymerase chain reaction in the diagnosis of Whipple‘s disease. Clin Infect Dis 2019;68:1089–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lo Monaco A, Govoni M, Zelante A et al. Whipple disease: unusual presentation of a protean and sometimes confusing disease. Semin Arthritis Rheum 2009;38:403–6. [DOI] [PubMed] [Google Scholar]
- 8. Sharma OP. Beethoven‘s illness: Whipple‘s disease rather than sarcoidosis? J R Soc Med 1994;87:283–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available from the corresponding author on reasonable request.