Abstract
Phosphoglycosyl transferases (PGTs) are among the first membrane-bound enzymes involved in the biosynthesis of bacterial glycoconjugates. Robust expression and purification protocols for an abundant subfamily of PGTs remains lacking. Recent advancements in detergent-free methods for membrane protein solubilization open the door for purification of difficult membrane proteins directly from cell membranes into native-like liponanoparticles. By leveraging autoinduction, in vivo SUMO tag cleavage, styrene maleic acid co-polymer liponanoparticles (SMALPs), and Strep-Tag purification, we have established a robust workflow for expression and purification of previously unobtainable PGTs. The material generated from this workflow is extremely pure and can be directly visualized by Cryogenic Electron Microscopy (CryoEM). The methods presented here promise to be generalizable to additional membrane proteins recombinantly expressed in E. coli and should be of interest to the greater membrane proteomics community.
Keywords: Glycoconjugate biosynthesis, membrane protein, styrene maleic acid copolymer, cryogenic electron microscopy, UDP-Sugar
Introduction
The presence of a heterogenous coat of cell surface and extracellular glycoconjugates is a common theme across all domains of life.[1] Notable examples of glycoconjugates in prokaryotes include lipopolysaccharide (LPS), capsular polysaccharide (CPS), exopolysaccharide (EPS), and wall teichoic acid (WTA). Biosynthesis of glycoconjugates in prokaryotes can be broadly classified into the wzx/wzy-dependent or wzx/wzxy-independent pathways.[2, 3] In bacteria, including many human pathogens, genetic deletion or small molecule-based inhibition of the biosynthetic machinery involved in the assembly of the complex glycoconjugates reduces viability and impacts virulence.[4, 5] As such, the enzymes involved in glycoconjugate biosynthesis represent attractive targets for detailed studies.
Among the enzymes involved in the early steps of en bloc glycoconjugate biosynthesis in bacteria are phosphoglycosyl transferases (PGTs).[6, 7] These enzymes catalyze the transfer of a phospho-sugar from a nucleotide sugar donor to an acceptor polyprenol phosphate – most commonly undecaprenyl phosphate (UndP).[8] PGTs are integral membrane proteins and can be classified into two superfamilies based on the topologies of the functional domains – polytopic (polyPGTs) wherein the catalytic domain comprises several transmembrane helices (TMH) and monotopic (monoPGTs), with a catalytic domain including a single reentrant membrane helix (RMH).[6, 9] Initial sequence and hydropathy analysis of monoPGTs misannotated the RMH as a TMH, confounding early topological assessment of these enzymes.[10, 11] Landmark studies culminating with the crystal structure of PglC from Campylobacter concisus, unequivocally established the topology of the monoPGT catalytic domain and identified a distinct sequence fingerprint for the RMH and catalytic motif.[12–14]
Recently, the PGT sequence fingerprint was utilized to establish a sequence similarity network (SSN) comprising 38,000 nonredundant PGT sequences.[15] Analysis of this network demonstrated that monotopic PGTs broadly cluster into three sub-families: small (Sm-PGT), containing only the catalytic domain, bifunctional (Bi-PGT) containing the catalytic PGT domain and an additional functional domain, and large (Lg-PGT) containing a putative four-transmembrane helix bundle and a domain of unknown function (DUF) fused to the N-terminus of the catalytic domain. A comparison of the domain structure of the Sm- and Lg-PGTs is illustrated in Figure 1. The Lg-PGT subfamily is predominant and represents 47% of all non-redundant PGT sequences within the SSN. However, biochemical characterization of these proteins remains limited as recombinant expression of the full-length integral membrane Lg-PGTs has represented a major hurdle to progress in understanding the extensive enzyme family. Additionally, the establishment of the PGT catalytic domain topology warrants careful re-examination of early studies, as the proper orientation of Lg-PGTs places the DUF found N-terminal to the catalytic domain in the cytoplasm as opposed to the periplasm.[10, 11, 14, 16]
Figure 1.

Comparison of topology between the Sm- and Lg-PGTs. Left: PglC from Campylobacter concisus (PDBID: 5W7L) comprises only the PGT catalytic domain, enters and exits the membrane on the same leaflet, by way of the N-terminal re-entrant membrane helix (RMH). Right: Schematic of a LG-PGT. The N-terminus of Lg-PGT contains four putative transmembrane helices, followed by a domain of unknown function, and finally a catalytic domain resembling PglC.
Methodologies for stabilizing membrane proteins on extraction from cell membrane are rapidly evolving.[17] Historically, detergents such as n-dodecyl-β-D-maltoside (DDM) or N,N-dimethyl-n-dodecylamine N-oxide (LDAO) have been used to solubilize membrane-bound proteins into detergent micelles, often at the expense of protein stability.[18] Recently, techniques for isolating target proteins within liponanoparticles have been described. Of these, the styrene maleic acid copolymer (SMA), stands out for its ability to extract proteins directly into liponanoparticles known as SMALPs.[19] SMALPs are compatible with common purification techniques,[20, 21] often stabilize the targets they encapsulate,[22] and have been used as a platform for a wide variety of studies.[23–26]
Here, we describe an optimized expression system, for recombinant production of full-length Lg-PGTs from various bacteria (Fig. 2). Using radioactivity-based assays, we investigate the substrate specificity of successfully expressed targets in cell envelope fractions. Then, we explore the application of the SMALP approach for solubilizing Lg-PGTs and identify optimal conditions for producing highly purified liponanoparticles which can be directly visualized via cryogenic electron microscopy (CryoEM). These studies establish SMALP as powerful approach for Lg-PGT purification, highlight the substrate specificity of recombinantly expressed Lg-PGTs, and establish the groundwork for future biophysical and structural biology studies of this major subfamily of monoPGTs. Significantly, the overall strategy may prove advantageous in applications towards other classes of integral membrane proteins.
Figure 2.

Top: Lg-PGT expression and purification strategy. Left: The expression vector encodes an N-terminal SUMO fusion, a dual-strep tag for purification, a TEV cleavage site for tag removal, followed by Lg-PGTs of interest. Right: The expression and purification strategy utilizes co-expression of the Ulp1 protease for in vivo SUMO tag removal. Downstream chromatography utilizing StreptactinXT resin provides highly pure material. Optional clean-up can be achieved using size exclusion chromatography, and the dual-strep tag can be cleaved via incubation with TEV protease.
Materials and Methods:
Synthetic genes encoding WbaP from S. enterica (Uniprot: P26406), WbaP from T. thermophilus (Uniprot: A0A510HWX9), WecP from Aeromonas hydrophila (Uniprot: B3FN88), CpsE from Streptococcus pneumoniae (Uniprot: Q8KW9P) and WcaJ from Escherichia coli (Uniprot: P71241) were codon optimized for expression in E. coli. An expression vector was derived from pE-SUMOpro (LifeSensors) to encode a dual-strep tag followed by a TEV protease site between the SUMO tag and MCS (His-SUMO-dual strep-TEV-PGT). Fragments were assembled using Gibson assembly. All plasmid sequences were verified via sanger sequencing. Plasmids were transformed into E. coli C43 harboring pAM174,[27] which encodes Ulp1 under an arabinose promotor. Proteins were expressed using autoinduction[28] in 0.5 L cultures supplemented with 150 μg/mL kanamycin and 25 μg/mL chloramphenicol at 37°C. One gram of powdered L-arabinose was added to cultures after OD600 reached ~1.5 and the temperature was adjusted to 18° C. After 18 hr expression, cells were harvested via centrifugation, transferred to 1-gallon Ziplock® freezer bags, spread to a thin layer, and frozen at −80° C.
To express SUMO-tagged CpsE and WcaJ, the above protocol was followed using BL21 DE3 E. coli lacking the pAM174 plasmid.
Protein Purification:
Isolation of Cell membranes
All purification steps were performed on ice unless otherwise noted. Frozen cell pellets were manually broken in bags and resuspended in buffer A (50 mM HEPES pH 8.0, 300 mM NaCl) at 4 mL per g pellet. Resuspended pellets were supplemented with 2 mM MgCl2, 0.06 mg/mL lysozyme (RPI), and 0.5 mg/mL DNase I (Millipore Sigma) and incubated for 30 minutes. Cells were disrupted via sonication (2x, 50% amplitude, 1s on 2 s off), and cell membrane was isolated via differential centrifugation (Ti-45 rotor, 9,000g 45 min, reserve supernatant, 140,000g 65 min). Cell membranes were resuspended to 50 mg/mL (assessed by raw UV signal at 280 nm) in buffer A, a 1 mL aliquot was reserved for activity assay, flash frozen in liquid N2, and stored at −80° C.
Catalytic variants of E. coli WcaJ and S. pneumoniae CpsE
Site-directed mutagenesis was done via PCR. Primers were designed via Quikchange primer design tool (Agilent). Designed primer pairs are shown below. Sequences of all mutagenized plasmids were confirmed by Sanger sequencing.
| Primers used for QuikChange (5’ →3’) | |
|---|---|
| WcaJ D362N_f | CGCACGAGTCTGAACGAACTCCCGCAG |
| WcaJ D362N_r | CTGCGGGAGTTCGTTCAGACTCGTGCG |
| CpsE D360N_f | CAAGACCAGTCTCAACGAGCTCCCGCAATTC |
| CpsE D360N_r | GAATTGCGGGAGCTCGTTGAGACTGGTCTTG |
SMA solubilization and purification
The frozen cell membrane fraction was thawed and mixed 1:1 (v/v) with a 2% stock solution of styrene maleic acid copolymer (Polyscope) or diisobutylene maleic acid copolymer (BASF) in buffer A and rotated at room temperature for one hour. Soluble SMA liponanoparticles (SMALPs) or DIBMA liponanoparticles (DIBMALPs) were isolated by centrifugation (Ti45 rotor, 160,000g 65 min). The supernatant was flowed over 1 mL StreptactinXT 4flow resin (IBA Biosciences) pre-equilibrated with buffer A. The flow-through was re-run over the column bed, and the column was washed with 5 CV buffer A. Protein was eluted using 3 CV Buffer A + 50 mM Biotin (buffer B). Protein containing fractions were pooled and loaded onto 3x tandem 5 mL HiTrap desalting columns (Cytiva) equilibrated with 25 mM HEPES pH 8.0, 150 mM NaCl (buffer C) to remove biotin. Protein purity was assessed via SDS-PAGE. For SMALP samples, protein concentration was determined via BCA assay (Pierce). Protein was concentrated to 2 mg/mL, and flash frozen in liquid N2, then stored at −80° C.
Activity assay:
The PGT radioactivity-based activity assay followed previously published protocols.[29] Assay buffer comprised 20 μM UndP, 0.1% Triton X-100, 10% DMSO, 50 mM HEPES pH 7.5, 100 mM NaCl, 5 mM MgCL2, 20 μM UndP, 2 μL [3H] labeled UDP-sugar substrate (Table S1). A 4 μL aliquot of PGT-containing CEF was added to 46 μL reaction buffer and incubated at room temperature. A series of 12 μL aliquots were taken at 0.5, 1, 3, 6 min, and quenched by addition to a vial containing 1 mL 2:1 chloroform:methanol and 500 μL pure solvent upper phase (PSUP, 15 mL chloroform, 240 mL methanol, 1.83 g KCl, 235 mL H2O). Quenched reactions were vortexed, aqueous/organic phases were allowed to separate, and the aqueous phase was removed. Reactions were re-extracted an additional 2x with 500 μL PSUP. A 5 mL aliquot of Opti-Fluor™ O scintillation fluid (Perkin Elmer) was added to each reaction, and tubes were counted on a liquid scintillation counter (Beckman LS6500). Readings were taken in triplicate, and figures were generated using GraphPad Prism (Version 9.4.1 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com”).
To measure activity of SUMO-tagged CpsE and WcaJ, the above protocol was modified to include 1 μM non-radiolabeled UDP-Glc in the assay buffer.
Cryo-Electron Microscopy of S. enterica WbaP in SMALP
Quantafoil 1.2/1.3 300 mesh Cu grids were glow discharged using an EMITech K100 glow discharger at 15 mA for 60 seconds. Grids were prepared using a Vitrobot Mark IV (Thermo Scientific) following standard protocols. Briefly, 2.5 μL of S. enterica WbaP in SMA30 at 2.0 mg/mL was deposited on a glow-discharged grid. After 5 seconds, grids were blotted at blot force 0 for 7 seconds and plunged into liquid ethane. Grids were clipped and stored in liquid N2.
Movies were collected on a 200 kV Talos Arctica equipped with a Falcon 3EC camera at 73,000x magnification, nominal pixel size of 2.007 Å, with a spherical aberration of 2.7 mm. Total dose on the sample was 56.75 e/Å2. A total of 544 movies were collected, and data were processed using Cryosparc V 3.0.1.[30]
Figures generated using Biorender.com
Results
Expression and purification of full-length dual-strep tagged Lg-PGTs in styrene maleic acid liponanoparticles (SMALP)
Initial attempts at the expression and solubilization of full-length Lg-PGTs using DDM yielded only poor yields of truncated protein (Fig S1). Previously, our group had successfully applied detergent-free methods to study Sm-PGTs.[23, 24] Accordingly, we hypothesized that isolating target Lg-PGTs in liponanoparticles, as applied to Sm-PGT could provide an alternative approach for stabilization and purification. Owing to the capability to directly extract membrane proteins from lipids in a bacterial cell envelope fraction, we turned to SMA and related DIBMA polymers.[20, 31] As these polymers have only recently been described, robust protocols to produce material suitable for applications such as structural and functional studies continue to be explored. We developed the expression and purification scheme based on several criteria: 1: Compatibility with a SUMO tag, which promotes improved expression yields and the stability of membrane proteins,[32] 2: Compatibility with autoinduction,[28] which has previously been used to express robust levels of PGTs,[8, 12] 3: Use of a purification strategy that avoids immobilized metal ion affinity chromatography (IMAC), because the SMA and DIBMA polymers interact with divalent cations such as Ni2+ hindering purification.[25, 33, 34]
An expression plasmid was constructed that encoded an N-terminal SUMO-tag, a short linker followed by a dual-strep tag, a short linker preceding a tobacco etch virus (TEV) protease cleavage site, and the genes of interest (Figure 2). Additionally, a modified C43-based E. coli expression system was chosen which facilitated in vivo cleavage of the SUMO tag by co-expression of Ulp1 under an orthogonal promotor (pBAD).[27]
A small panel of SMA polymers was chosen to screen for optimal solubilization of Lg-PGTs. The SMA polymers differ from one another in terms of the syrene:maleic acid ratio, while DIBMA contains a diisobutylene moiety in place of the styrene found in the SMA polymers. Solubilized material was then purified using StreptactinXT resin, and SDS-PAGE was used to assess solubilization and purification efficiency. As is often the case with SMA-solubilization, each of the PGTs tested displayed differing solubilization profiles (Fig. 3). At least one condition for solubilization and purification was identified for each of the proteins assessed, with SMA-30, in general, performing well for all Lg-PGTs.
Figure 3.

SDS-PAGE gels of elution fractions from small-scale SMALP purification screen. Arrows denote bands corresponding to purified target proteins. A small set of SMA polymers were screened, with SMA30 demonstrating optimal solubilization for each of the targets. Owing to their high isoelectric points, each PGT runs at a lower-than-expected molecular weight. Predicted molecular weights (kDa): Se WbaP: 61.2, Tt WbaP: 58.7, Ah WecP 54.0, Ec WcaJ: 57.5, Spn CpsE: 57.2.
Activity and substrate specificity of Lg-PGTs in cell membranes
The ability to heterologously express full-length Lg-PGTs from various organisms in E. coli provided enzymes to assess NDP-sugar substrate selectivity. A radioactivity-based assay was used to monitor transfer of labeled phospho-sugars onto UndP.[29] Recombinantly expressed WbaP from S. enterica exhibited high specificity for UDP-Gal, as did WbaP from T. thermophilus (Fig. 4). WecP from A. hydrophila was found to utilize UDP-GalNAc as its preferred substrate (Fig. 4). In initial analyses, both E. coli WcaJ and S. pneumoniae CpsE expressed in C43 E. coli did not show turnover for any of the substrates paneled, despite reports of both of these enzymes utilizing UDP-Glc (Fig. S3).[35, 36]
Figure 4.
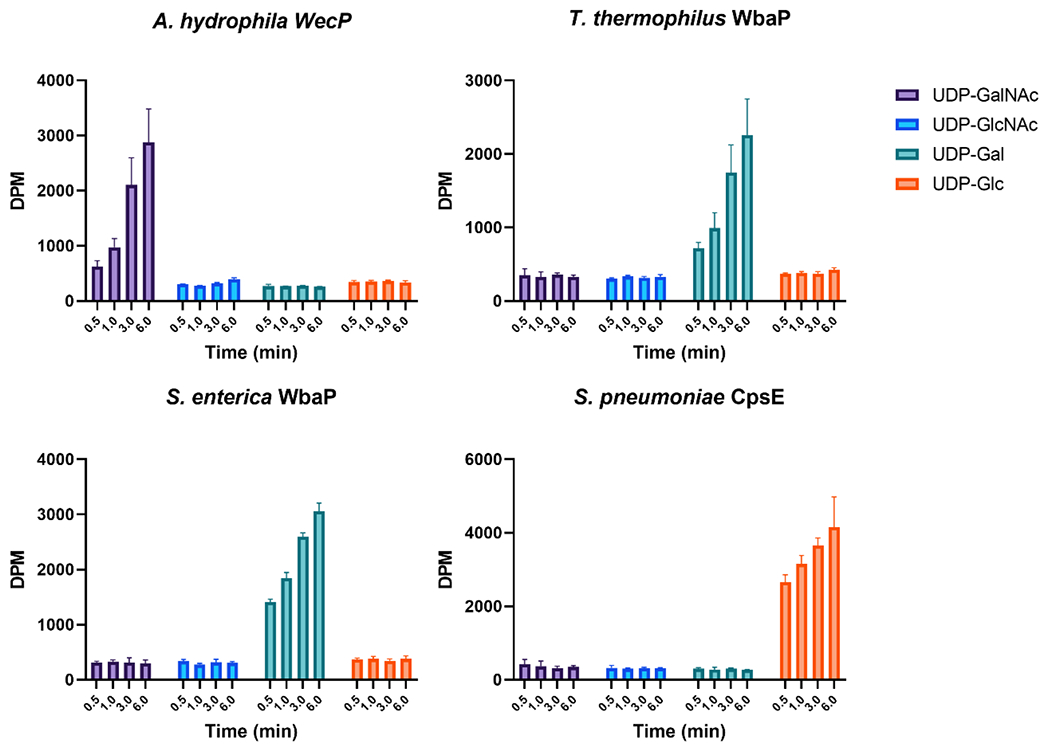
Substrate Panel of full-length LG-PGTs in cell membranes. S. enterica and T. thermophilus WbaP in cell membranes display high specificity for UDP-Gal. A. hydrophila WecP displays high specificity for UDP-GalNAc. S. pneumoniae CpsE displays no activity in C43 cell membranes, but readily transfers UDP-Glc when expressed as a SUMO-fusion in BL21 DE3.
In effort to identify conditions in which active WcaJ and CpsE could be produced, we expressed these plasmids in BL21 DE3 E. coli, which would leave the potentially stabilizing SUMO-tag in place. This led to the identification of an unusual phenotype. When overexpressed in BL21 DE3 E. coli, both SUMO-WcaJ and SUMO-CpsE caused production of a viscous, opaque slime visible after cells were pelleted (Fig. S2). This behavior was not observed when variants lacking the catalytic aspartic acid in the conserved DE dyad were overexpressed.[8, 13] Owing to the striking mucoid phenotype in cell culture, we isolated BL21 DE3 cell membranes containing SUMO-CpsE and SUMO-WcaJ and assessed activity using UDP-Glc as a substrate. Transfer of [3H]-labeled UDP-Glc to UndP was observed in both experiments, although WcaJ had reduced activity compared to the other Lg-PGTs assayed (Fig. 4, S3). The ability to monitor activity of WcaJ and CpsE using OD of spent media will greatly enhance future inhibitor development by providing a simple and straightforward in vivo activity readout. Retention of the SUMO-tag during expression provides a route to recombinantly express Lg-PGTs that may otherwise prove inactive or unstable.
Cryo-Electron Microscopy (CryoEM) of Lg-PGTs in SMALP
Although the molecular weights of the Lg-PGTs expressed using the presented Strep-tag/SMALP system are relatively small for CryoEM (~60 kDa), we reasoned that the additional mass from the stabilizing SMA polymer and the phospholipid annulus around the proteins in SMALP may facilitate direct visualization of Lg-PGTs. As S. enterica WbaP solubilized in SMA30 gave highest yields, this protein was chosen for CryoEM analysis. Particles were readily visible in micrographs collected, and 2D classes generated from a small dataset showed intriguing features, including putative C2 symmetry (Fig. 5). A preliminary low resolution 3D reconstruction is shown in the supporting information (Fig. S4). On-going efforts to solve a high-resolution reconstruction of S. enterica WbaP in SMALP will be reported in due course. These results will yield critical information on the positioning of Lg-PGTs within the lipid bilayer, as well as the overall architecture of these complex multi-domain enzymes.
Figure 5.

Cryogenic Electron microscopy of purified S. enterica WbaP in SMA30 liponanoparticles. Top: Example micrograph lowpass filtered to 5Å and particles picked in cryosparc. Bottom: Selected 2D class averages from 189,401 extracted particles
Discussion
Bacterial glycoconjugates play critical roles in survival, virulence, antibiotic resistance, and host immunomodulation. However, understanding their biosynthesis is stymied by the non-templated nature of their assembly. Furthermore, linking substrate specificity to sequence of enzymes involved in glycoconjugate assembly has proven a significant challenge. As such, direct characterization of the enzymes involved in glycoconjugate biosynthesis remains a requirement to fully understand pathway logic. Compounding the above issues is the localization of many of the glycoconjugate assembly enzymes to the membrane. Methodologies to improve expression and purification of membrane-bound glycoconjugate biosynthesis enzymes will advance studies connecting enzyme sequence, to structure, function, and final glycoconjugate identity.
Previous attempts to recombinantly overexpress Lg-PGTs has been met with limited success.[35, 37–40] Here we present an improved method of producing full-length Lg-PGTs in C43 E. coli by incorporating a SUMO-tag for stabilization and improved yield, utilizing autoinduction, and in vivo Ulp1 cleavage to remove the SUMO tag from mature protein prior to extraction of PGTs from cell membranes (Figs. 2, 3). Cell membranes isolated from E. coli overexpressing S. enterica WbaP, and A. hydrophila WecP contained active full-length enzymes whose substrate preference matches that reported previously (Fig. 4). T. thermophilus WbaP was also active in cell membranes, and displayed strong preference for UDP-Gal, implying that the sugar at the reducing end of LPS O-Antigen repeat unit for strain ATCC 27634 is Gal. Both E. coli WcaJ and S. pneumoniae CpsE were inactive in membranes isolated from C43 E. coli (Figs. 4, S3). Intriguingly, when expressed in BL21 DE3 lacking co-expressed Ulp1, both plasmids caused the appearance of a turbid mucoid slime in the media. Membranes isolated from these cells readily transferred UDP-Glc onto UndP in vitro (Fig. 4, S3). The observed phenotype is reminiscent of cells producing colanic acid.[41] Additionally, when the signature active site aspartic acid residue in either WcaJ or CpsE were mutated to asparagine, the spent media remained optically transparent after cells were removed via centrifugation. As Und-PP-Glc is the initial lipid-linked glycan in colanic acid biosynthesis,[42] it is tempting to speculate that the mucoid phenotype may be a result of colanic acid overproduction in effort to process UndP and Und-PP-Glc through the pathway to recycle the essential UndP.
Although detergent solubilization has been a mainstay for membrane protein extraction and stabilization, the application of detergents comes with significant downsides. Namely, excess detergent above the critical micelle concentration is required in all buffers after protein extraction, detergent micelles often destabilize solubilized proteins in part due to the depletion of the native phopholiplipid context, and some membrane proteins remain recalcitrant to detergent solubilization. Indeed, in the case of each of the Lg-PGTs tested here, DDM failed to extract full-length material from cell membranes. SMA offers an excellent alternative to canonical detergents, as these polymers directly extract membrane proteins and protein complexes from cell membranes inside a nanoparticle of annular lipids.[19, 21] Previous experiments in our group demonstrated that SMALPs were excellent vehicles for solubilization of Sm-PGTs.[23, 24] Recombinantly expressed Lg-PGTs from S. enterica, T. thermophilus, E. coli, and S. pneumoniae were readily solubilized in SMALP directly from isolated E. coli cell membranes. By utilizing the highly specific dual-Strep Tag for purification, PGT-containing SMALPs were purified in a single step. Purity and final yield of PGTs varied depending on the type of SMA polymer used for purification, with the combination of SMA30 and S. enterica WbaP demonstrating optimal yield and purity as judged by SDS-PAGE (Fig. 3). The advantage of SMALPs for purification of the Lg-PGTs described here is striking as shown by the comparison of SMA30 and DDM solubilization, side-by-side, on identical cell membrane extracts. None of the PGTs were stable or soluble in DDM micelles, while each PGT tested was successfully isolated in SMALP (Fig S1). Although all the proteins produced here displayed activity in isolated cell membranes, none of the Lg-PGTs were active in SMALP. SMALP-mediated influences on protein function are not unprecedented and may indicate the rigidity of the bilayer stabilized by the SMA prevents catalysis.[43] Alternatively, as PGTs require Mg2+ as a cofactor, the chelating nature of the maleic acid moiety found on SMA polymers may strip the Lg-PGTs of sufficient Mg2+ for catalysis.[25, 33, 34] Despite these limitations, SMALPs may still be used as a “shuttle” membrane mimetic to stably transfer Lg-PGTs from cell membrane to other systems such as proteoliposomes.[44]
Although S. enterica WbaP in SMA30 appeared highly pure by SDS-PAGE, the quality of the protein in SMALPs in solution remained unclear. As CryoEM optics, sample preparation techniques, and data processing strategies have continued to improve, characterization of small membrane proteins both in detergent or SMALP has become more feasible.[45, 46] A small screening dataset collected on a Talos Arctica microscope revealed S. enterica WbaP particles were readily distinguishable. Particles had a diameter ~120 Å, similar to that reported for SMALPs previously.[20] 2D classification of 189,401 particles extracted from 475 micrographs revealed classes with distinct features, including apparent C2 symmetry (Fig 5). The simplicity of the expression and purification protocol described here and the apparent high-quality of the protein produced are ideally suited for Cryo-EM of difficult membrane proteins. Future work will aim to resolve the structure of a Lg-PGT in SMALP to high resolution to better understand the molecular architecture of these enzymes in a native-like environment.
Supplementary Material
Highlights:
Expression and purification of full-length Lg-PGTs has proven challenging.
Autoinduction and in vivo Ulp1 cleavage produces active full-length Lg-PGTs.
SMA and DIBMA are vastly superior to DDM for Lg-PGT solubilization.
Strep-tag purification yields SMALPs suitable for CryoEM characterization.
Funding:
This work was funded by National Institutes of Health R01 GM131627 and GM039334 (to B.I), and F32 GM134576 (G.J.D.)
Abbreviations:
- PGT
phosphoglycosyl transferase
- SMALP
styrene maleic acid co-polymer liponanoparticle
- CryoEM
cryogenic electron microscopy
- LPS
lipopolysaccharide
- CPS
capsular polysaccharide
- EPS
exopolysaccharide
- WTA
Wall teichoic acid
- UndP
undecaprenol phosphate
- Poly
polytopic
- Mono
monotopic
- TMH
transmembrane helix
- RMH
re-entrant membrane helix
- Sm
small
- Bi
bifunctional
- Lg
large
- DUF
domain of unknown function
- SSN
sequence similarity network
- DDM
n-dodecyl-β-D-maltoside
- LDAO
N.N-dimethyl-N-dodecylamine-N-oxide
- DIBMA
di-isobutylene maleic acid
- PSUP
pure solvent upper phase
- IMAC
immobilized metal affinity chromatography
- TEV
tobacco etch virus
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
Footnotes
CRediT authorship contribution statement:
Greg J. Dodge: Conceptualized and carried out experiments, prepared samples and collected data for electron microscopy, analyzed and visualized data, generated figures, Writing – original draft, review & editing. Hannah M. Bernstein: Methodology, carried out experiments, analyzed and visualized data, Writing – review & editing. Barbara Imperiali: Conceptualization, Writing – review & editing, supervision, and funding acquisition.
Declaration of Competing Interest:
The authors declare that they have no competing personal or financial relationships that could appear to influence the work reported herein.
Data Availability:
Any data, plasmids, or protocols will be made available upon request to authors.
References:
- [1].Tytgat HL, Lebeer S, The sweet tooth of bacteria: common themes in bacterial glycoconjugates. Microbiology and Molecular Biology Reviews 78 (2014) 372–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Whitfield C, Williams DM, Kelly SD, Lipopolysaccharide O-antigens—bacterial glycans made to measure. Journal of Biological Chemistry 295 (2020) 10593–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Whitfield C, Trent MS, Biosynthesis and export of bacterial lipopolysaccharides. Annual review of biochemistry 83 (2014) 99–128. [DOI] [PubMed] [Google Scholar]
- [4].Day CJ, Semchenko EA, Korolik V, Glycoconjugates play a key role in Campylobacter jejuni infection: interactions between host and pathogen. Frontiers in Cellular and Infection Microbiology 2 (2012) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kong Q, Yang J, Liu Q, Alamuri P, Roland KL, Curtiss III R, Effect of deletion of genes involved in lipopolysaccharide core and O-antigen synthesis on virulence and immunogenicity of Salmonella enterica serovar Typhimurium. Infection and immunity 79 (2011) 4227–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Allen KN, Imperiali B, Structural and mechanistic themes in glycoconjugate biosynthesis at membrane interfaces. Current opinion in structural biology 59 (2019) 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lukose V, Walvoort MT, Imperiali B, Bacterial phosphoglycosyl transferases: initiators of glycan biosynthesis at the membrane interface. Glycobiology (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Das D, Kuzmic P, Imperiali B, Analysis of a dual domain phosphoglycosyl transferase reveals a ping-pong mechanism with a covalent enzyme intermediate. Proceedings of the National Academy of Sciences 114 (2017) 7019–7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Allen KN, Entova S, Ray LC, Imperiali B, Monotopic membrane proteins join the fold. Trends in biochemical sciences 44 (2019) 7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].James DB, Gupta K, Hauser JR, Yother J, Biochemical activities of Streptococcus pneumoniae serotype 2 capsular glycosyltransferases and significance of suppressor mutations affecting the initiating glycosyltransferase Cps2E. Journal of bacteriology 195 (2013) 5469–5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang L, Liu D, Reeves PR, C-terminal half of Salmonella enterica WbaP (RfbP) is the galactosyl-1-phosphate transferase domain catalyzing the first step of O-antigen synthesis. Journal of Bacteriology 178 (1996) 2598–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lukose V, Luo L, Kozakov D, Vajda S, Allen KN, Imperiali B, Conservation and covariance in small bacterial phosphoglycosyltransferases identify the functional catalytic core. Biochemistry 54 (2015) 7326–7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ray LC, Das D, Entova S, Lukose V, Lynch AJ, Imperiali B, Allen KN, Membrane association of monotopic phosphoglycosyl transferase underpins function. Nature chemical biology 14 (2018) 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Furlong SE, Ford A, Albarnez-Rodriguez L, Valvano MA, Topological analysis of the Escherichia coli WcaJ protein reveals a new conserved configuration for the polyisoprenyl-phosphate hexose-1-phosphate transferase family. Scientific reports 5 (2015) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].O’Toole KH, Imperiali B, Allen KN, Glycoconjugate pathway connections revealed by sequence similarity network analysis of the monotopic phosphoglycosyl transferases. Proceedings of the National Academy of Sciences 118 (2021) e2018289118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Entova S, Billod J-M, Swiecicki J-M, Martín-Santamaría S, Imperiali B, Insights into the key determinants of membrane protein topology enable the identification of new monotopic folds. Elife 7 (2018) e40889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pandey A, Shin K, Patterson RE, Liu X-Q, Rainey JK, Current strategies for protein production and purification enabling membrane protein structural biology. Biochemistry and Cell Biology 94 (2016) 507–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Orwick-Rydmark M, Arnold T, Linke D, The use of detergents to purify membrane proteins. Current Protocols in Protein Science 84 (2016) 4.8. 1–4.8. 35. [DOI] [PubMed] [Google Scholar]
- [19].Knowles TJ, Finka R, Smith C, Lin Y-P, Dafforn T, Overduin M, Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. Journal of the American Chemical Society 131 (2009) 7484–7485. [DOI] [PubMed] [Google Scholar]
- [20].Gulamhussein AA, Uddin R, Tighe BJ, Poyner DR, Rothnie AJ, A comparison of SMA (styrene maleic acid) and DIBMA (di-isobutylene maleic acid) for membrane protein purification. Biochimica et Biophysica Acta (BBA)-Biomembranes 1862 (2020) 183281. [DOI] [PubMed] [Google Scholar]
- [21].Morrison KA, Akram A, Mathews A, Khan ZA, Patel JH, Zhou C, Hardy DJ, Moore-Kelly C, Patel R, Odiba V, Membrane protein extraction and purification using styrene–maleic acid (SMA) copolymer: effect of variations in polymer structure. Biochemical Journal 473 (2016) 4349–4360. [DOI] [PubMed] [Google Scholar]
- [22].Jamshad M, Charlton J, Lin Y-P, Routledge SJ, Bawa Z, Knowles TJ, Overduin M, Dekker N, Dafforn TR, Bill RM, G-protein coupled receptor solubilization and purification for biophysical analysis and functional studies, in the total absence of detergent. Bioscience reports 35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Entova S, Guan Z, Imperiali B, Investigation of the conserved reentrant membrane helix in the monotopic phosphoglycosyl transferase superfamily supports key molecular interactions with polyprenol phosphate substrates. Archives of biochemistry and biophysics 675 (2019) 108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Swiecicki J-M, Santana JT, Imperiali B, A Strategic Approach for Fluorescence Imaging of Membrane Proteins in a Native-like Environment. Cell chemical biology 27 (2020) 245–251. e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Broecker J, Eger BT, Ernst OP, Crystallogenesis of membrane proteins mediated by polymer-bounded lipid nanodiscs. Structure 25 (2017) 384–392. [DOI] [PubMed] [Google Scholar]
- [26].Qiu W, Fu Z, Xu GG, Grassucci RA, Zhang Y, Frank J, Hendrickson WA, Guo Y, Structure and activity of lipid bilayer within a membrane-protein transporter. Proceedings of the National Academy of Sciences 115 (2018) 12985–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sjodt M, Brock K, Dobihal G, Rohs PD, Green AG, Hopf TA, Meeske AJ, Srisuknimit V, Kahne D, Walker S, Structure of the peptidoglycan polymerase RodA resolved by evolutionary coupling analysis. Nature 556 (2018) 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Studier FW, Protein production by auto-induction in high-density shaking cultures. Protein expression and purification 41 (2005) 207–234. [DOI] [PubMed] [Google Scholar]
- [29].Glover KJ, Weerapana E, Chen MM, Imperiali B, Direct biochemical evidence for the utilization of UDP-bacillosamine by PglC, an essential glycosyl-1-phosphate transferase in the Campylobacter jejuni N-linked glycosylation pathway. Biochemistry 45 (2006) 5343–5350. [DOI] [PubMed] [Google Scholar]
- [30].Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA, cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nature methods 14 (2017) 290–296. [DOI] [PubMed] [Google Scholar]
- [31].Dörr JM, Scheidelaar S, Koorengevel MC, Dominguez JJ, Schäfer M, van Walree CA, Killian JA, The styrene–maleic acid copolymer: a versatile tool in membrane research. European Biophysics Journal 45 (2016) 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zuo X, Li S, Hall J, Mattern MR, Tran H, Shoo J, Tan R, Weiss SR, Butt TR, Enhanced expression and purification of membrane proteins by SUMO fusion in Escherichia coli. Journal of structural and functional genomics 6 (2005) 103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dörr JM, Koorengevel MC, Schäfer M, Prokofyev AV, Scheidelaar S, Van Der Cruijsen EA, Dafforn TR, Baldus M, Killian JA, Detergent-free isolation, characterization, and functional reconstitution of a tetrameric K+ channel: the power of native nanodiscs. Proceedings of the National Academy of Sciences 111 (2014) 18607–18612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gulati S, Jamshad M, Knowles TJ, Morrison KA, Downing R, Cant N, Collins R, Koenderink JB, Ford RC, Overduin M, Detergent-free purification of ABC (ATP-binding-cassette) transporters. Biochemical Journal 461 (2014) 269–278. [DOI] [PubMed] [Google Scholar]
- [35].Cartee RT, Forsee WT, Bender MH, Ambrose KD, Yother J, CpsE from type 2 Streptococcus pneumoniae catalyzes the reversible addition of glucose-1-phosphate to a polyprenyl phosphate acceptor, initiating type 2 capsule repeat unit formation. Journal of bacteriology 187 (2005) 7425–7433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Patel KB, Toh E, Fernandez XB, Hanuszkiewicz A, Hardy GG, Brun YV, Bernards MA, Valvano MA, Functional characterization of UDP-glucose: undecaprenyl-phosphate glucose-1-phosphate transferases of Escherichia coli and Caulobacter crescentus. Journal of bacteriology 194 (2012) 2646–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Patel KB, Ciepichal E, Swiezewska E, Valvano MA, The C-terminal domain of the Salmonella enterica WbaP (UDP-galactose: Und-P galactose-1-phosphate transferase) is sufficient for catalytic activity and specificity for undecaprenyl monophosphate. Glycobiology 22 (2012) 116–122. [DOI] [PubMed] [Google Scholar]
- [38].Patel KB, Furlong SE, Valvano MA, Functional analysis of the C-terminal domain of the WbaP protein that mediates initiation of O antigen synthesis in Salmonella enterica. Glycobiology 20 (2010) 1389–1401. [DOI] [PubMed] [Google Scholar]
- [39].Saldías MS, Patel K, Marolda CL, Bittner M, Contreras I, Valvano MA, Distinct functional domains of the Salmonella enterica WbaP transferase that is involved in the initiation reaction for synthesis of the O antigen subunit. Microbiology 154 (2008) 440–453. [DOI] [PubMed] [Google Scholar]
- [40].Merino S, Jimenez N, Molero R, Bouamama L, Regué M, Tomás JM, A UDP-HexNAc: polyprenol-P GalNAc-1-P transferase (WecP) representing a new subgroup of the enzyme family. Journal of bacteriology 193 (2011) 1943–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nassrf X, Honore N, Vasselon T, Cole S, Sansonetti P, Positive control of colanic acid synthesis in Escherichia coli by rmpA and rmpB, two virulence-plasmid genes of Kiebsiella pneumoniae. Molecular microbiology 3 (1989) 1349–1359. [DOI] [PubMed] [Google Scholar]
- [42].Reid AJ, Eade CR, Jones KJ, Jorgenson MA, Troutman JM, Tracking colanic acid repeat unit formation from stepwise biosynthesis inactivation in Escherichia coli. Biochemistry 60 (2021) 2221–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Morrison KA, Wood L, Edler KJ, Doutch J, Price GJ, Koumanov F, Whitley P, Membrane extraction with styrene-maleic acid copolymer results in insulin receptor autophosphorylation in the absence of ligand. Scientific Reports 12 (2022) 3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hesketh SJ, Klebl DP, Higgins AJ, Thomsen M, Pickles IB, Sobott F, Sivaprasadarao A, Postis VL, Muench SP, Styrene maleic-acid lipid particles (SMALPs) into detergent or amphipols: an exchange protocol for membrane protein characterisation. Biochimica et Biophysica Acta (BBA)-Biomembranes 1862 (2020) 183192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Nygaard R, Kim J, Mancia F, Cryo-electron microscopy analysis of small membrane proteins. Current opinion in structural biology 64 (2020) 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Piper SJ, Johnson RM, Wootten D, Sexton PM, Membranes under the Magnetic Lens: A Dive into the Diverse World of Membrane Protein Structures Using Cryo-EM. Chemical reviews 122 (2022) 13989–14017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any data, plasmids, or protocols will be made available upon request to authors.


