Abstract
Despite centuries of developing strategies to prevent food-associated illnesses, food safety remains a significant concern, even with multiple technological advancements. Consumers increasingly seek less processed and naturally preserved food options. One promising approach is food biopreservation, which uses natural antimicrobials found in food with a long history of safe consumption and can help reduce the reliance on chemically synthesized food preservatives. The hurdle technology method that combines multiple antimicrobial strategies is often used to improve the effectiveness of food biopreservation. This review attempts to provide a research summary on the utilization of lactic acid bacteria, bacteriocins, endolysins, bacteriophages, and biopolymers helps in the improvement of the shelf-life of food and lower the risk of food-borne pathogens throughout the food supply chain. This review also aims to evaluate current technologies that successfully employ the aforementioned preservatives to address obstacles in food biopreservation.
Keywords: Biopreservation, Bacteriophages, Bacteriocins, Endolysins, Encapsulation, Lactic acid bacteria
Introduction
The demand for food both high-quality and has a long shelf-life is increasing significantly, mainly due to changes in food habits and the growing global population. Astonishingly, one-third of the world's food production—equivalent to approximately 1.3 billion tons—goes to waste yearly. In the United States, for instance, a staggering 30% of food intended for human consumption has been wasted annually, with the bulk of the waste occurring in households, restaurants, and food service businesses. Similarly, according to Eurostat's 2006 data, Europe wastes 89 million tons of food each year during production and harvest stages. To combat this issue, the US Environmental Protection Agency has suggested the development of composting technology to aid in reducing food spoilage. The reduction of food waste is necessary to conserve food resources and make them available in regions that experience scarcity. To achieve this objective, food produced in one area must be preserved using cost-effective and sustainable techniques so that it can be transported and served in other locations at a later time.
Disinfectants play a crucial part in the food processing industry for effectively controlling the pathogenic microorganisms. The main purpose of disinfection is to remove the microorganism on the surface of the food contact, which prevents contamination in the food products by harmful pathogens and spoilage microorganisms (Nair et al., 2017). Traditional methods of food preservation such as cooking, drying, smoking, refrigeration, freezing, canning, pasteurizing, dehydrating, freeze-drying, salting, pickling, antioxidants, and fermentation also enhance the shelf-life of food while improving its nutritional value by converting complex compounds into biologically available ones. These methods offer benefits like slowing down or stopping bacterial growth at different temperatures, creating a sterile environment, and destroying microbes. However, these methods also have limitations, such as altering the texture of fruits, promoting the growth of anaerobic microbes in canning and bottling, cost-effectiveness, and decreasing the nutritional value of food during pasteurization.
Disinfection methods can be physical, chemical, or biological. Physical methods like sterilization, filtration, ultrasounds, ultraviolet light, pasteurization, freezing, irradiation, and drying remove visible waste, external matter, or ooze on instruments, among which heat is the most reliable and effective method for destroying microorganisms (Reda, 2019). Chemical methods involve the removal of unwanted chemical residues using electrolyzed oxidizing water, chlorine dioxide, ozone, organic acids, and direct and indirect antimicrobial preservatives. Chemical methods are currently used in many industries to control pathogens. Disinfectants typically contain various active chemical agents such as chlorine, alcohol, per oxygen, quaternary ammonium compounds, and aldehydes. Despite being flammable and damaging to metals, alcohol is inexpensive, easy to obtain, provides an immediate bactericidal impact, and does not have bacteriostatic activity or harmful effects (Song et al., 2019).
The chlorine chemical group consists of hypochlorite, chlorine dioxide, and chloramine-t-trihydrate, with hypochlorite being widely used for its quick action and low cost. It has a broad range of bactericidal effects without leaving harmful residues and is unaffected by water hardness. However, it can corrode metal, is naturally inactivated by organic matter, and causes irritation to the skin, eyes, and mucous membranes (Song et al., 2019). Different concentrations of disinfectants can disrupt the cell wall and membranes of target organisms, leading to growth inhibition or lethal activity (Song et al., 2019). Chemical preservatives effectively control surface-attached microbes, limit product contamination, prolong product shelf life, and reduce the risk of food-borne illness (Yuan et al., 2020). Multi-targeted activity methods are utilized, such as modifying the position of membrane cations in the gram-negative bacteria and passive diffusion in the gram-positive bacteria (Iñiguez-Moreno et al., 2017). However, the consumption of chemical additives can cause hyperactivity and neurophysiological problems in children.
The utilization of biopreservatives presents a potential solution to counteract the negative impacts of conventional preservatives. Table 1 listed out the foods that use bio-preservative over conventional preservatives. Biological methods are effective in eliminating pathogens and preventing food spoilage, utilizing a variety of techniques such as bacteriocins, enzymes, phytochemicals, bacteriophages, and protective cultures (Meireles et al., 2016). This strategy extends the food products' shelf life without degrading their quality (Mani Lopez et al., 2018). Several researchers have been developing novel bio-preservation strategies to overcome these obstacles and extension of the shelf life of food while preserving its natural quality. The numerous kinds of biopreservatives that are accessible as cutting-edge disinfection technologies for food preservation are covered in this review, along with methodology, procedures, targeted microorganisms, and applications. Many biopreservatives used to preserve food directly or through packaging are also highlighted. Overall, this review provides an extensive framework for understanding the developments in food disinfectants known as biopreservatives, which can help manufacturers produce foods with high levels of nutrients and extended shelf lives.
Table 1.
Foods that use bio-preservative over conventional preservative
| Food | Conventional preservative | Replaced with biopreservative |
|---|---|---|
| Dairy products | Sodium benzoate | Plant essential oils |
| Meat | Benzoic acid | Lactone parasorbic acid |
| Animal feed | Synthetic propionic salts | Fermented propionic acids |
| Pickle | Acetic acid | Rosemary extract |
| Biofilm formation on meat | Peptidomimetic Polyurethanes | Bacteriophages |
| Fish | Nitrites | Nisin |
Biopreservatives
People utilized salt, drying, and smoking to preserve food in ancient times. After some time, novel preservation methods were developed, including packaging, refrigeration, and chemical additions (Shajil et al., 2018). However, these methods lead to a loss of nutrients and natural flavor and can pose health risks. Advanced techniques are being explored to preserve food quality and texture to overcome these limitations. Amongst these techniques, biopreservation is highly effective in maintaining sterility and preserving food quality with minimal loss of nutrients and flavor (Singh, 2018). Biopreservatives are antimicrobial substances extracted from natural sources or produced by fermentation, enhancing food quality and safety (Mani-López et al., 2018). These natural additives are derived from microorganisms, plants, animals, and their metabolites are eco-friendly and have antimicrobial properties, which make them applicable to a broad range of food-borne microorganisms (Pisoschi et al., 2018).
Lactic acid bacteria (LAB), bacteriocins, yeast, bacteriophages, and endolysins are among the most common biopreservatives. Sourdough fermentation is a recent approach to creating an unfavorable environment for fungal growth by increasing food acidity. Saccharomyces cerevisiae and Pediococcus pentosaceus have been shown to exhibit antifungal activity against Aspergillus flavus by producing high levels of organic acids, particularly acetic and lactic acids (Jin et al., 2021). Adding natural compounds to food products is the best solution for improving their nutritional value. The technological advancements in this preservation technique are highly beneficial for the public and the food industry. Bio-preservation methods ensure the safety and quality of perishable food items by increasing their shelf life and sanitation. Biopreservation can be successfully achieved by combining various natural additives to restrict microbial growth and achieve food safety. It also supplements additional nutrition and protects food quality and nutritional properties (Gómez-Sala et al., 2016).
Biopreservatives serve as both starter cultures and protective cultures in food preservation. Starter cultures initiate fermentation and produce compounds that give fermented foods their unique texture and flavor, while protective cultures control antimicrobial activity and reduce the growth of microorganisms in food. These cultures are commonly utilized in the food sector. As a by-product of fermentation, organic acids serve as an antibacterial agent by lowering the pH of the immediate surroundings and establishing a barrier against non-acidophiles.
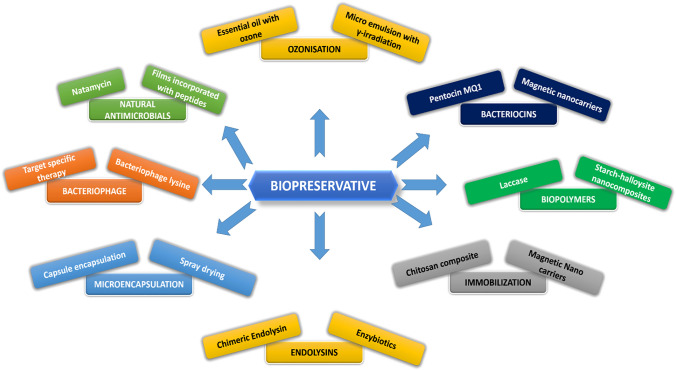
Lactic acid, in particular, kills bacteria by disrupting their cytoplasmic membrane and interfering with their membrane potential (Singh, 2018). For example, when stored in the refrigerator, olives fermented with lactic acid bacteria and packaged with a modified gas medium retained probiotic features for up to 6 months (Babich et al., 2019). Figure 1 illustrates recent advancements in biopreservatives for food products.
Fig. 1.
Recent advancements in bio-preservatives for food products
Natural antimicrobials for food preservation
Preventing food-borne illnesses caused by microbial pathogens is one of the growing concerns for consumers, the food sectors, and food safety authorities. Biopreservation involves using natural additives or secondary metabolites from microorganisms, algae, plants, and animals that possess antimicrobial properties, which can protect food from a wide range of pathogens. Additionally, the antioxidant properties of fruits, vegetables, seeds, leaves, herbs, spices, oil, and organic compounds can enhance the nutritional value of food products (Han et al., 2023). The demand for natural antimicrobial compounds is increasing as they offer a safer alternative to synthetic compounds that can pose health hazards.
Fish balls can be preserved naturally with liquid smoke without compromising their nutritional or aesthetic qualities. Liquid smoke is derived from palm kernel shells through pyrolysis at 340 °C, 360 °C, and 380 °C, followed by a two-stage distillation process at 200 °C to eliminate impurities. The preservation status of the fish balls is monitored by measuring total volatile bases, pH, and organoleptic test results. Aromatic and therapeutic plants contain a range of auxiliary metabolites, terpenoids, and alcoholic compounds that can be harnessed for food preservation. For instance, while refrigerating meat, Satureja essential oil can be coated with chitosan to combat Pseudomonas spp., molds, and yeasts (Noori et al., 2018). Organo essential oil can be microencapsulated with whey protein isolate to prevent mold and yeast growth and preserve grated cheese. The ginger essential oil can be nano-emulsified with sodium caseinate to protect chicken fillets from Listeria monocytogenes, Salmonella typhimurium, psychrophilic bacteria, molds, and yeasts (Kim et al., 2020).
Bacteriocins
Bacteriocins, antimicrobial proteins produced by bacteria, can prevent similar growth or closely associated bacteria and fungi from growing, thereby reducing microbiological spoilage (O’Connor et al., 2020). Bacteriocins have various applications in human, livestock, and aquaculture industries (Hammami et al., 2019). When combined with selected hurdles, such as pulsed electric field and modified atmospheric packaging, bacteriocins can be more effective by influencing the permeability of the external membrane and thus enhancing their efficacy against microorganisms. Nisin, pediocin, and enterocin are some bacteriocins used with hurdles (Pb and Gowda, 2021). Bacteriocins can be used in dairy products in two different ways: injecting the food along with a LAB that generates bacteriocins or simply introducing purified or semi-purified bacteriocins to the food. Using bacteriocin-releasing LAB cultures is preferred over semi-purified bacteriocins because bacteriocins can easily be adsorbed onto food matrices and degraded, leading to a lack of antibacterial activity (Silva et al., 2018).
Hydrocolloid biopolymers were commonly used as edible coatings and films on dairy products to improve their stability and shelf life. However, subsequent studies showed limited effectiveness in reducing pathogens such as Listeria monocytogenes, increased risk of food-borne illnesses, and decreased quality and acceptability (Pinnaduwa et al., 2020). Bacteriocins, which are powerful antimicrobial proteins produced by marine organisms, have become a valuable tool in reducing potential pathogens in the marine food industry (Ahmad et al., 2017). These proteins not only completely inhibit pathogenic microbes in water bodies but also enhance the production of inhibitory compounds and promote the nutritional status of species by synthesizing digestive proteins (Sharma et al., 2022). For instance, the growth of Aeromonas hydrophilia, Listeria monocytogenes, Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, and Salmonella typhimurium has been inhibited when the bacteriocin generated by Weissella hellenica has been integrated into poly-composite films and utilized with fish fillers. Additionally, spray treatments had no detrimental effects on salmon's sensory attributes on a commercial scale and were demonstrated to be efficient against Listeria monocytogenes (cold-smoked salmon) produced by the bacteriocin producer Carnobacterium divergens V41. (Wiernasz et al., 2017). Table 2 lists the available forms of bacteriocins, their sources, and the targeted microorganisms.
Table 2.
Various forms of bacteriocins and their target microorganism in various food types
| Bacteriocin | Source of bacteriocin | Target microorganism | Food application | Shelf life | References |
|---|---|---|---|---|---|
| Nisin & Natamycin | Lactococcus lacti | Listeria innocua | Cheese | > 28 days | Feichtmayer et al. (2017) |
| Nisin & Galactomannan | Lactococcus lacti | L. monocytogenes | Cheese | 35 days | Feichtmayer et al. (2017) |
| Nisin encapsulated in phosphatidyl choline nanoliposomes | Lactococcus lacti | L. monocytogenes | Cheese | 50 days | Fahim et al. (2016) |
| Pediocin & Zinc Oxide Nanoparticles | Pediococcus spp. | S. aureus and L. monocytogenes | Apple | 46 days | Brillet-Viel et al. (2016) |
| LJR1 | Pediococcus pentosaceus | L. monocytogenes | White leg Shrimp | 7 days | Ladha and Jeevaratnam (2020) |
| Divercin V41 | Carnobacterium divergens V41 | L. monocytogenes | Cold smoked Salmon | 7 days | Brandelli et al. (2017) |
| Bac23 & Silver Nanoparticles | Lactobacillus plantarum | Shigella flexneri, S. aureus and P. aeruginosa | Common food | 7 days | Kumar et al. (2009) |
| Divergicin M35 Chitosan Film | Carnobacterium divergens | Listeria sp. | Ready to eat foods | 21 days | Benabbou et al. (2020) |
| Nisin loaded Chitosan Nanoparticles | Lactococcus lacti | S. aureus, L. monocytogenes, E. coli O157:H7 and Salmonella typhimurium | Orange juice | 3 days | Lee et al. (2018) |
| Enterocin AS48 with EDTA | Enterococcus | L. monocytogenes | Apple | 7 days | López Aguayo et al. (2016) |
| Fermencin SA715 | Lactobacillus fermentum JA715 | L. monocytogenes, Bacillus cereus, S. aureus, Micrococcus luteus, P. aeruginosa & E. coli | Banana | 9 days | Wayah and Philip (2018) |
| BacBS2 | Bacillus velezensis BS2 | L. monocytogenes | Fermented foods | 54 days | Perumal et al. (2019) |
| Plantaricin JLA 9 | Lactobacillus plantarum JLA-9 | Bacillus spp. | Fermented food | 54 days | Zhao et al. (2016) |
The use of bacteriocins in fruit juices, raw vegetables, and fruits has been limited, with only a few bacteriocins being considered for this application. However, recent studies on Enterocin AS-48 have shown promising results, indicating that this bacteriocin can be used, when combined with other antimicrobials, to avoid the deterioration of fruit juices and fruit and vegetable decontamination. This approach can increase the effectiveness of treatments and broaden the range of inhibition against gram-negative bacteria. Moreover, recent research has explored the potential of other bacteriocins, such as colicins, microcins, and antimicrobial peptides from non-bacterial sources, that have been developed via combinatorial peptide design, for use on raw vegetables, fruits, and juices and against food borne pathogenic bacteria in pork (Dong et al., 2022).
The use of bacteriocins in fruit juice preservation has been shown to reduce bacterial load by 14–25% in apple and pomegranate juice. This suggests bacteriocins could be a safe and non-toxic solution for preserving natural fruit juice (Kraśniewska et al., 2020). Bacteriocin from B. methylotrophicus BM47, coated with carboxymethyl cellulose, has been employed to improve the shelf life of fresh strawberries. Using BM47 with carboxymethyl cellulose-based edibles improves fresh strawberries' quality, commercial appearance, and shelf life (Tumbarski et al., 2019). Although lactic acid bacteria can thrive at lower temperatures and provide desired properties, significant temperature changes can result in the freezing of bacteria. A combination of 2% lactic acid and nisin has significantly decreased the growth of Pseudomonas spp. and H2S production during chilled shrimp storage. Lactic acid bacteria culture can also provide specific advantages for seafood products, such as improving shelf life and reducing economic costs by minimizing product spoilage. LAB such as Enterococcus faecium and Lactococcus lactis produce large amounts of LAB, which can be used at low inhibitory concentrations as a bio-preservative for fruits, vegetables, and meat products against Listeria spp. and Staphylococcus aureus (Yépez et al., 2017). Recently, other bacteriocins like colicins, microcins, and antimicrobial peptides from non-bacterial sources developed through combinatorial peptide design have been tested for crude vegetables, fruits, and juices. Novel bacteriocin like peptides is characterized and studied against pathogenic bacteria, which showed inhibitory activity against Listeria monocytogenes, Staphylococcus aureus, Serratia marcescens, and Escherichia coli (Abitayeva et al., 2021).
Microencapsulation
Microencapsulation is a technique utilized in food industries, defined as packaging active materials in small compartments that gradually release their contents at controlled rates over an extended period. This process improves food additives’ the sensory, enzymatic, and nutritional quality with controlled bioactive delivery and enhances uniform coloration in food products (Shamloo et al., 2019). Microencapsulation shields active compounds from environmental conditions, such as oxygen, light, and temperature, and can also mask unpleasant flavors for various food applications (Rajam and Anandharamakrishnan, 2015). Polymers commonly used in microencapsulation techniques include cellulose, chitosan, alginate, hydrolyzed starches, pectin, proteins, polysaccharides, yeast cells, and vitamins (Shahbazi and Shavisi, 2019). The microencapsulation market has recently expanded to include probiotic encapsulation, multiple emulsions, cell electro-spinning, and spray chilling, all viewed as future trends in the food and beverage industry. Additionally, plant-derived food, animal-derived food, and its additives are used in microencapsulation (Alotaibi et al., 2019).
Microencapsulation has been used in plant-derived foods to enhance their nutritional value. Research suggested that adding the microencapsulated garden cress seed oil prevented the oxidation of α-linolenic acid and then enhanced the products’ shelf-life. Another study reported the use of microencapsulated Garcinia cowa fruit with whey protein in bread preparation. Alginate microbeads have also been found effective in reducing fermentation, preventing bacteriophage contamination, and enhancing the sensory properties of organic food varieties.
Microencapsulated clove oil presents an alternative antimicrobial additive for animal-derived food products. A low concentration of 0.70% microencapsulated clove oil and other additives were found to be effective in reducing mold and spore formation, making it suitable for use in cooked meat products. While nisin has antibacterial properties, it was not freely available after 28 days at 4 °C. In addition, microencapsulation technology has been widely employed in dairy products, particularly in yogurt, to enhance the viability of probiotics. This microencapsulation is achieved by lowering the pH, inhibiting gastric juice production, preventing post-acidification, and releasing microbial cells in the intestinal environment, which increases their bioavailability. According to a study, Lactobacillus plantarum microencapsulated along with whey protein and gum Arabic through complex coacervation demonstrated much greater viability in mimicking gastric juice and higher rates of survival over 60 days of the preservation at 4 °C than nano-encapsulated cells (Reineccius, 2017).
In the food and beverage organization, spray drying has been established technique for microencapsulating goods, including milk powder, starch, flavoring, oats, coffee, and tea. Spray drying, freeze drying, and spray chilling are the most often used procedures compared to other approaches. In this method, the active materials are suspended in water to form a slurry and then atomized at a high temperature. Initially, the slurry forms micelles, which transform into small droplets that eventually become solid shells, preserving all their natural properties (Tumbarski et al., 2019). The use of bacterial cellulose film containing Lactobacillus acidophilus and Bifidobacterium animalis, either free or microencapsulated with sodium alginate and pectin, has been shown to have antifungal properties that help preserve white brined cheese for up to 45 days. Of the microencapsulated options tested, Lactobacillus acidophilus encapsulated in sodium alginate was most efficient at preventing Aspergillus niger growth in white brined cheese over the 45 days. As a result, the bacterial cellulose film, which contains microcapsules of Lactobacillus acidophilus in alginate, was determined to be the better bioactive film for preserving white brined cheese, making it an appropriate and efficient bio preservative used to produce cheese (Motalebi Moghanjougi et al., 2020). Table 3 summarizes of LAB, its sources, target pathogens, and methods used in this study.
Table 3.
Lactic acid bacteria and their sources, target pathogens, and methods
| Sources | Lactic acid bacteria | Pathogens | Methods | References |
|---|---|---|---|---|
| Naturally-fermented Manzanilla, Cobrançosa table olives | Lactobacillus pentosus, Pediococcus parvulus & Leuconostoc pseudomesenteroides | S. aureus, L. monocytogenes, E. faecalis, B. cereus, Streptococcus mutans and Salmonella enterica | Agarose gel electrophoresis, REP-PCR fingerprinting | Reis et al. (2022) |
| Corn stover silage | L. plantarum, P. pentosaceus, E. mundtii, Weissella cibaria and L. pseudomesenteroides | S. enterica, M. luteus, and E. coli | Agar diffusion Assay method | Shamloo et al. (2019) |
| Traditional pickle of Himachal Pradesh | L. plantarum, L. brevis, L. mesenteroides and P. cerevisiae, P. pentosaceus and E. faecalis | E. coli, S. aureus, B. cereus and Shigella dysenteriae | Agar well diffusion method | Diez-Gutiérrez et al. (2022) |
| Traditional fermented Kenyan milk and maize products | L.plantarum, L. pentosus and L. paraplantarum | Aspergillus flavus | Spread plate method | Li et al. (2015) |
| Sliced apples and lamb's lettuce | L. plantarum CIT3 and V7B3 strains | Lactobacillus plantarum CIT3, Escherichia coli | Using MRS agar, on the matrix of the InstaGene Matrix kit | Siroli et al. (2015) |
| The rhizosphere of olive trees and desert truffles of Tunisia | Lactococcus, Pediococcus, Lactobacillus, Weissella, and Enterococcus | Aspergillus niger, Penicillium expansum, Botrytis cinerea, and Verticillium dahliae | agar-well-diffusion method | Houicher et al. (2021) |
| Wheat bread | Bacillus spp, Pseudomonas spp, Listeria spp and Escherichia spp | Fusarium culmorum, Penicillium chrysogenum, A. fumigatus, A. versicolor, Penicillium expansum, A.niger, Debaryomyces hansenii, and Candida parapsilosis | Antimicrobial activity testing by punched well technique | Cizeikiene et al. (2013) |
Immobilization
Immobilization of preservatives has found widespread use in various products, and modern techniques continue to be developed to create various immobilized proteins with superior efficiency and utility (Chourasia et al., 2020). Products that benefit from immobilized proteins such as confectioneries, syrups, flavors, fruit beverages, milk products, yeasts for baked goods, and whey lactose hydrolysates. The application of immobilized proteins in the dairy industry is particularly important, as many people are lactose intolerant and cannot digest milk. Lactose hydrolysis, which results in lactose-free milk, can be facilitated by immobilized lactase, which can be used to address this issue. In addition to being employed as biosensors to distinguish between different compounds and regulate product quality, immobilized enzymes are also utilized to manufacture fruit juice (Alotaibi et al., 2019). They are also used in food packaging, a newly developed method for increasing the quality and prolonging the life span of packaged foods.
The present investigation analyzes the advantages and requirements for using probiotic cultures as it explores the utilization of probiotic bacteria to produce innovative food items. This study also examines and assesses the potential future effects of immobilization methods used in the food sector to increase cell viability. The most frequently consumed probiotic-containing food items are fermented milk and cheese; however yogurt, beverages, and soy products are all becoming increasingly popular. The primary probiotics found in fermented dairy products are Bifidobacteria and Lactobacillus acidophilus. Other bacteria, including Enterococcus, Streptococcus, Propionibacterium, and yeasts, can also enhance human gut health, even though these strains are frequently utilized for human food. As immobilization supports, inorganic materials are not acceptable for intake by humans or animals. Consequently, the research emphasizes using substances with non-digestible sugars to manufacture probiotic food (Motalebi Moghanjougi et al., 2020).
The movement limitations of bacteriocins have restricted their use as bio-preservatives. Immobilization, however, is a promising solution to this issue, as it involves the confinement of these antimicrobials within food-grade materials, liposomes, and nanocarriers. One such approach which incorporating the bacteriocins into polymeric packaging systems, which provides a protective barrier and acts as a bio-preservative. Liposomes made from neutral phosphatidylcholine have been found to be more effective than those made from anionic phosphatidylglycerol. To ensure easy handling and lower costs, choline recovery from lecithin derived from soy, sunflower, and egg yolk is a viable option. In addition to that, the mutli-walled carbon nanotubes with nisin showed a sevenfold higher antimicrobial activity against Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, and Escherichia coli than the carbon nanotubes without nisin.
The positive outcomes observed in using immobilized bio-preservatives compared to free ones have led to a growing interest in the immobilization of bacteriocins produced by LAB as an innovative approach to quality improvement, natural food preservation, and safety (Huang et al., 2020). Recently, researchers studied the immobilization and freeze-drying of bacteriocin-producing Lactobacillus plantarum to explore its potential for bio-preservation of pineapple wine. Especially in the food business, carbohydrases are often utilized in industrial processes and products. These enzymes enable the production of various types of sugar syrups, serve as a sucrose substitute for improving the sensory properties in juices and wines and reducing lactose in milk.
Ahmad et al. (2017) demonstrated that soybean cotyledons immobilized with 2% NaOH, autoclaved at 125 °C for 15 min, and activated with 2.5% glutaraldehyde achieved an immobilization efficiency of 97%. In another study, a polylactic acid (PLA) film was produced using the extrusion-casting method. Plasma treatment was carried out to create carboxylic acid groups on the PLA film surface, followed by the covalent addition of the antibacterial agents, nisin or ε-polylysine, to the modified film surface. The quality and shelf life of fresh beef was determined to be improved by the antibacterial packing sheets that were produced. Therefore, covalently immobilized antimicrobial packaging technology has the potential to revolutionize the field of food preservation.
Likewise, the immobilization of soybean cotyledons was adjusted with 2% NaOH, autoclaved at 125 °C for 15 min, and actuated with 2.5% glutaraldehyde to provide immobilization effectiveness of 97%. The film made of polylactic acid (PLA) has been produced using the extrusion-casting technique. After plasma treatment to create carboxylic acid groups on the PLA film surface, the antibacterial agent nisin or ε-polylysine was covalently added to the modified film surface. The antibacterial packaging films with covalent immobilization positively impacted the shelf-life and quality of fresh beef. As a result, a covalently immobilized antimicrobial packaging technology could be a revolutionary means of food preservation.
Biopolymers
In ancient times, there has been a remarkable advancement in the application of biopolymers as intelligent and active polymer systems within the food industry. These biopolymers have been effective in encapsulating micronutrients and antioxidants within microparticles while also maintaining food quality. The use of smart and dynamic biopolymers in food quality monitoring systems and microparticles can potentially increase the nutritional content and shelf life of food items (Chiralt et al., 2020).
Producing biopolymers involves multiple steps that require expertise and a thorough understanding of their behavior during preparation. Depending on the processing pathway, biopolymers can be processed into a range of products, including packaging films, coated paper, plates, glasses, and cutlery. The first step in processing any biopolymer involves melting the biopolymer blend through casting, extrusion, or blow molding (Ataei et al., 2020). Biodegradable polyesters are currently the most dominant materials used in food packaging, which are readily available through standard procedures. These materials are used in monolayer and multi-layer applications within the food packaging industry. Among the most widely used thermoplastics are effective biopolymers, such as starch, polyhydroxyalkanoates, and polylactic acid, obtained from natural or genetically modified sources.
Fruit softening is a significant issue that requires attention. The degradation of pectin by enzymes such as pectin methylesterase and polygalacturonase leads to cell wall disintegration and methanol production. Thiol-containing substances, another group of active agents, are ineffective in restraining polyphenol oxidase enzymes (Ananda et al., 2017). Furthermore, silver nanoparticles have garnered a lot of interest in the production of biodegradable polymers. Coating dried silver nanoparticles have increased the shelf life of apples and sapota by 25 days. In another study, chitosan coating impregnated with nano silver showed antifungal properties against the significant pathogen Botrytis cinerea and was applied to strawberries, resulting in effective outcomes (Kraśniewska et al., 2020).
Biodegradable coatings have been developed to protect fruits and vegetables from spoilage caused by microorganisms. Chitosan-coated natural products exhibited only 10% contamination, while non-coated ones had a 90% contamination rate. Additionally, chitosan and silver nanoparticles coating on fresh-cut melons were effective in inhibiting the growth of various microorganisms such as mesophiles, psychrophiles, enterobacteria, yeasts, and molds (Kumari et al., 2021). Alginate coated with silver nanoparticles also increases in the shelf life of mushrooms by preventing the development of mesophilic, psychrophilic, pseudomonas, yeasts, and molds. Researchers have additionally observed how the microbiological condition of kinnow mandarin at various temperatures can be influenced by coatings derived from guar gum and carboxymethyl cellulose, both of which contain silver nanoparticles. Hence, modifying biopolymers with silver nanoparticles, chitosan, and alginate can alter the structure of the edible film and improve its physical and mechanical properties while also providing antimicrobial properties (Shankar and Rhim, 2018).
A study utilized Aspergillus niger to produce chitosan, which was subsequently used to generate nano chitosan (NCt) particles. The deacetylation rate of the chitosan was 88.7%, and its molecular weight of 24.5 kDa, with 98% soluble in dilute acetic acid. The NCt particles ranged in size from 35 to 65 nm. Edible coating films were created using chitosan, nano chitosan, pomegranate peel extract, and composites. These films were tested for their antifungal properties against mycotoxigenic fungi, such as Aspergillus ochraceus, Aspergillus flavus, and Fusarium moniliforme (Luong et al., 2021). The combination of NCt and antifungal films successfully controlled the fungal growth of date fruits. After 48 h of coating, the most effective films were found to be NCt+ extract and Cts+ extract-based films, which fully inhibited the growth of fungal spores on date fruits (Alotaibi et al., 2019).
Renewable edible films have become increasingly important in response to the environmental challenges posed by plastic materials. Sodium caseinate is a promising material for this purpose due to its excellent mechanical properties, strong air barrier properties, and high nutritional value. Listeria monocytogenes is a well-known food-borne pathogen that causes food spoilage and human illness. To combat this, Lactobacillus casei PTCC1608 was directly added to the film-forming solution to provide biopreservation. The bio-caseinate film was found to have the highest survival rate of Lactobacillus casei 1608 on the eighth day of culture, while the bio-methyl cellulose film had the highest inhibitory effect on the growth rate of Lactobacillus innocua 10,799 on the fourth day of the 12-day study period, according to data presented by (Li et al., 2015).
Bacteriophage
Bacteriophages have a wide range of potential uses in the food business, such as bio-preservatives, antibiotics for human beings, and instruments for detecting harmful microbes throughout the food chain. These viruses infect and lyse bacterial cells, offering several benefits for bio-preservation, such as safety, high specificity and activity, negligible impact on the intestinal microbiota, harmlessness to mammalian cells, auto-replication, and effectiveness against biofilms. Additionally, bacteriophages are genetically controllable and flexible for use across different environments, aiding pathogen detection. In the food industry, bacteriophages can be used to reduce colonization and illnesses in livestock, and sanitize carcasses and raw products like fruits and vegetables. As natural preservatives, clean equipment, and contact areas also help manufactured products endure longer shelf-life.
Bacteriophages have emerged as a potential solution to combat food-borne pathogens in the food industry, with applications in primary production, bio-sanitization, and biopreservation (Moye et al., 2018). Biopreservation involves the direct application of bacteriophages to food products to extend their shelf life. The European Food Safety Authority and the USFDA have approved using bacteriophage mixtures in the agri-food sector. Studies on food preservation using high hydrostatic pressure have shown promising results when combined with lysins. The use of lysine-secreting recombinant bacteria is another promising approach to introducing bacteriophages to food. Bacteriophages can be used pre-harvest or post-harvest, and there is growing interest in utilizing lytic phages as biocontrol agents. Some companies have already started contributing to phage innovation to combat pathogens in food. Bacteriophages offer a “green” technology to address food-borne microbes and have multiple potential applications in the food industry (Połaska and Sokołowska, 2019; Moye et al., 2018).
Phages have emerged as a promising and safe biopreservative for food products as they specifically target bacterial pathogens without affecting natural commensal microbes present in the gastrointestinal tract of humans and animals. Listeria monocytogenes is a significant cause of food-borne illness, commonly associated with fresh or minimally processed meals and processed foods stored at low temperatures. Due to its high fatality rate, Listeria spp. is considered an essential pathogen. A combination of bacteriocin nisin and a phage cocktail was used to control Listeria sp. on fresh, sliced apples and melon, demonstrating the potential for phages to be used as a biocontrol agent against this pathogen (Chang, 2020). Phages have also been successfully encapsulated in alginate capsules, allowing for extended storage periods at low temperatures without loss of potency (Gouvêa et al., 2016). In addition, using bacteriophages in refrigerator trays has been shown to reduce the availability of Salmonella typhimurium in the environment, thus extending the shelf life of refrigerated foods (Yan et al., 2021). Phages have generally shown potential in managing food-borne infectious agents and prolonging the shelf life of food goods, making them an essential tool in the food sector.
Bacteriophage lysins have gained attention as possible antibacterial drugs because they break down bacterial cell walls in recent years. Broad lytic spectra against Staphylococcus aureus, especially Methicillin-resistant Staphylococcus aureus (MRSA), have been observed for one of the lysines, LysGH15. It has been demonstrated to be effective against MRSA and six other S. aureus strains. Additionally, LysGH15 has shown potential biocontrol efficacy in foods high in salt, whereas CHAPLysGH15 has been reported to be efficient in meals low in salt. These lysins have specifically been employed in animal flesh and Chinese bacon, with CHAPLysGH15 being used for pork and LysGH15 for bacon. MRSA was significantly reduced by using these lysins, going from 104 to 0 CFU/cm2 (Gonzalez-Menendez et al., 2018) strengthening the potential in the food industry as biocontrol agents.
Endolysins
By focusing on bacterial pathogens and prolonging the shelf life of food items, bacteriophage-encoded endolysins have become effective antibacterial agents in the food sector. In order to develop variations with a more extensive activity range against common infectious agents, including Staphylococcus aureus, Salmonella sp., Escherichia coli, Listeria monocytogenes, and Clostridium spp., these kinds of enzymes were additionally employed in DNA shuffling and protein engineering techniques (Song et al., 2019). Endolysins can operate as a strong antibacterial agent by dissolving the peptidoglycan of gram-positive bacteria, and disruption of cell walls happens when administered to the bacterial cell. With the help of cytoplasm, the holin protein, which assists the endolysin to get to the cell wall, aids in the release of the phage offspring. Endolysins have been demonstrated to function as biocontrol agents in the food business, notably in the dairy sector. One illustration of the innovative theories and techniques being explored in this area involves the staphylococcal phage endolysin LysH5. Endolysins provide a novel method for preventing the development of antibiotic-resistant bacteria, which is a growing challenge in the food sector. Apart from acting as an antibacterial agent in food industries, endolysins can also remove biofilms on the surfaces of utensils. Biofilms are a collection of microbes that stick to moist surfaces and can reproduce, leading to food decay. Endolysins effectively break down the biofilm, destroying the targeted bacterial pathogens. The cell wall-binding domain, a functional domain of lysins from gram-positive backgrounds, guides endolysins to specific cell wall-associated ligands and identifies bacterial pathogens. Meanwhile, the enzymatically active domains provide the necessary enzymatic activity to cleave the peptidoglycan layer.
In food processing equipment, as well as in the food itself, biofilms can form and harbor pathogenic bacteria, which endolysis can effectively target. Endolysins typically contain one or two enzymatically active domains at their N-terminal, which cleave the bonds in the bacterial peptidoglycan. A cell wall-binding domain recognizes and binds to the host bacteria at the C-terminal end. The use of endolysins has become increasingly relevant due to the significant economic losses incurred by the food industry in the USA caused by food-borne pathogenic bacteria, which endolysins can effectively combat. As a result, endolysin-based techniques have gained recognition as novel food antimicrobials, effectively managing outbreaks of food-borne diseases (Shannon et al., 2020). When tested, carvacrol has been shown to have a synergistic effect with LysSA97, which can provide a broad application in the food industry when combined with other endolysins. This has been demonstrated by the inactivation of Staphylococcus aureus in skim milk through the synergistic effect of endolysin and carvacrol, which was more effective than whole milk (Huang et al., 2020).
Endolysin Lysdb has a muramidase domain and catalytic site, which shares homology with Chalaropsis-type lysozymes. Lysdb cleaves the 6-O-acetylated peptidoglycan found in the cell wall of Staphylococcus aureus due to its specific peptidoglycan hydrolytic bond specificity. The efficacy of Lysdb in lysing living cells of Staphylococcus aureus is demonstrated under mesothermal and acidic conditions, making it a widely used enzyme in the cheese manufacturing industry. In this context, the engineered strain Lactobacillus casei BL23 provides Lysdb to act against Staphylococcus aureus, reducing 105 folds compared to raw milk. Therefore, Lysdb has great potential to prevent Staphylococcus aureus and food-borne spoilage bacteria in cheese production, resolve dairy industry and animal health issues, and ensure milk safety and quality (Guo et al., 2016). Purified endolysins can either be produced and released by fermenting bacteria like Lactococcus lactis or Lactobacillus spp., or directly added to food items. Before employing endolysin in foods and food-processing plants, assessing their stability and safety is crucial.
In this review, the possible applications for endolysins in food processing have been discussed. A putative endolysin gene discovered from the Bacillus cereus phage BPS13 genome and expressed in E. coli was used to make one of these new endolysins such as LysBPS13. The higher lytic activity of LysBPS13 against B. cereus ATCC 10876 suggests that it may be used as a decontaminant in food processing applications. The dairy industry has also shown interest in using endolysins to manage the maturation of cheese. For instance, extracellular hemolysins and muramidase isolated from Lactobacillus delbrueckii phage can disturb the cell wall preparations of different subspecies of L. delbrueckii, providing activity against specific microbes. Despite facing certain legal and logistical challenges, the future of endolysin treatment looks promising with the introduction of engineered "next-generation" lysins.
Ozonization
Over the years, various food preservation technologies have emerged, but the focus has now shifted to ozone as a potent antibacterial agent that meets the needs of the food industry and is accepted by food regulatory bodies and consumers. Ozone can eliminate unwanted microorganisms, bacterial spores, and viruses at low concentrations and within a minimal time frame. As a result, it is regarded as one of the most potent compounds for water treatment and an efficient antibiotic and disinfection agent. Ozone has been considered an ideal method for the food processing sector, given such exceptional properties, capable of producing high-quality food products. The US FDA has granted the ozone technique GRAS status, as it is capable of destroying a broad range of microorganisms, such as bacteria, viruses, yeast, parasites, and fungi in the food sector due to its pro-oxidant properties (Abouloifa et al., 2022). The effectiveness of the ozone technique is expected to be higher against gram-negative bacteria. Ozone has various applications and can be beneficial for food preservation, sanitization, and product safety. The dose of ozone used varies depending on the product's chemical composition. For instance, fresh meat requires a higher amount of ozone due to its high-fat content, whereas fruits and vegetables require less due to their low-fat and high-carbohydrate content. The meat’s shelf life depends on the microbial population’s number and diversity. The meat contamination usually results from microorganisms such as Campylobacter spp., Salmonella spp., L. monocytogenes, and pathogenic E. coli during slaughtering, handling, storage, and distribution.
In the fresh food industry, acid-tolerant Salmonella spp., Listeria monocytogenes, and Escherichia coli, as well as microbes resistant to preservation factors, have been identified as a severe problem. Precut fruits and vegetables are especially prone to contamination by S. javiana, S. montevideo, and S. poona. Ozone has demonstrated the ability to inactivate various microorganisms, including bacteria, bacterial spores, molds, yeasts, protozoan cysts, and viruses, with low concentrations and brief exposure times. Ozone has been applied to almost every type of food to enhance safety and prolong shelf life. However, the effectiveness of ozone in disinfecting food contaminants varies depending on the type of microflora present. In some cases, ozone reduced food microflora by over 5 log units. The United States FDA has granted the ozone technique GRAS status, making it a safe and useful tool for the food industry.
Ozone has effectively reduced microbial pathogens and pesticide residues, such as azinphos-methyl, captan, formethanate-HC1, and ethylene thiourea, on fresh food products. This approach also helps to decrease the accumulation of inorganic waste in the environment. Ozone is a desirable, environmentally friendly sanitizer due to its rapid decomposition to oxygen and lack of toxic residues. Among the various techniques for food biopreservation, ozone treatment has distinct advantages, particularly for decontamination. This approach is characterized by short treatment times, the absence of residues, and reduced energy requirements, with no significant economic challenges except for the supply of power and filling of oxygen cylinders. In recent biochemical studies, it has been observed that ozone treatment has no adverse effect on the quality of seafood products and improves the shelf life of cauliflower under modified atmospheric conditions (Fasake et al., 2022). Intermittent ozonation has been identified as an efficient method of ozone delivery in freezing chambers. In addition, ozone pretreatment followed by pasteurization has effectively eliminated Cronobacter sakazakii from milk. In cheese production, ozone has been found to maintain the physicochemical properties of cheese, and ozonated water has been observed to partially disinfect microbes in mozzarella cheese (Brodowska et al., 2018).
Future prospects of bio-preservatives in food preservation
Despite the various advancements in the food industry, microbial spoilage continues to cause significant financial and food losses. Therefore, upgrading existing technologies is critical. In the future, research can focus on the direct application of bacteriocins infusion into food materials rather than just packaging. The bacteriocins’ shelf life and stability can be improved by using nanoencapsulation technology. Milk fermentate-derived powders could also be used as a cost-effective means of introducing bacteriocins into milk-based foods. Genetic research on bacteriocins will continue to pave the way for its applications. The ability to overproduce bacteriocins could make it a more cost-effective option. Further research into the prevalence of natural bacteriocin genetic strains in retail foodstuffs and toxicological considerations could provide more evidence for the safe utilization of bacteriocins in the food chain (Krivorotova et al., 2016).
In recent years, nanotechnology has been increasingly utilized in the food industry for various purposes, including tracking, tracing, and monitoring food quality, detecting toxic proteins, and developing packaging materials using carbon nanotubes (Bouarab Chibane et al., 2019). Additionally, there has been an increase in the desire for natural biopreservatives, and studies are currently being conducted to determine how well polyphenols and flavonoids are utilized to preserve food (Gutierrez-del-Rio et al., 2018). The combination of phages and endolysins has also shown great potential in food preservation, as they exhibit high specificity in targeting only undesirable food-spoiling pathogens. Additionally, endolysins can prevent the formation of biofilms in the food processing area (Afsah-Hejri et al., 2020). The bioengineering of altered or novel endolysins is another promising avenue for developing effective tools for killing or detecting germs, as these enzymes’ modular or globular structure presents unique engineering possibilities. Furthermore, the utilization of biopolymers in processed food packaging products has shown great potential for the food preservation industry. To meet the vast need for novel preservation methods in the food processing industry, further research requires funding from both public and private collaborations.
A significant conclusion drawn from this review is the need to move away from traditional disinfectant methods due to their harsh impact on food and the use of chemical preservatives with potential long-term consequences. New techniques such as bacteriocins, bacteriophages, biopolymers, LAB, ozonation, and endolysins have emerged as alternative preservative methods. In addition, using smart packaging and modified atmosphere packaging has expanded the range of preservation methods. Immobilization strategies have helped to overcome the limitations of bacteriocins and made them effective as alternative disinfectants. However, non-thermal methods have their limitations as food preservatives and are often combined. Bacteriophages have become popular due to their natural origin and multifunctional benefits in food preservation. Preservatives in packaging materials are also gaining momentum in the industry. The review provides a brief overview of various materials and approaches used in the inhibition of the microorganism growth that cause food spoilage. Endolysins have been found to be effective as biopreservative agents, particularly in eliminating biofilms. While preserving food is crucial, it is essential to identify and study sustainable and effective biopreservative agents.
Acknowledgements
The authors can acknowledge that the fund has not been received for this study.
Declarations
Conflict of interest
The author declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kirupa Sankar Muthuvelu, Email: krpasnkr@gmail.com.
Baranitharan Ethiraj, Email: baranitharanibt@gmail.com.
Shreyasi Pramnik, Email: shreyasip99@gmail.com.
N. Keerthish Raj, Email: keerthishnagraj1@gmail.com.
Swethaa Venkataraman, Email: swethaav96@gmail.com.
Devi Sri Rajendran, Email: r.devisri97@gmail.com.
Priyadharshini Bharathi, Email: priyanith2613@gmail.com.
Elakiya Palanisamy, Email: elakiya.bt18@bitsathy.ac.in.
Anusri Sathiya Narayanan, Email: anusri.bt18@bitsathy.ac.in.
Vinoth Kumar Vaidyanathan, Email: vinothkv@srmist.edu.in.
Shanmugaprakash Muthusamy, Email: shanmugaprakash.m.bt@kct.ac.in.
References
- Abitayeva GK, Urazova MS, Abilkhadirov AS, Sarmurzina ZS, Shaikhin SM. Characterization of a new bacteriocin-like inhibitory peptide produced by Lactobacillus sakei B-RKM 0559. Biotechnology Letters. 2021;43:2243–2257. doi: 10.1007/s10529-021-03193-z. [DOI] [PubMed] [Google Scholar]
- Abouloifa H, Rokni Y, Hasnaoui I, Bellaouchi R, Gaamouche S, Ghabbour N, Karboune S, Ben Salah R, Brasca M, D’hallewin G, Saalaoui E, Asehraou A. Characterization of antimicrobial compounds obtained from the potential probiotic Lactiplantibacillus plantarum S61 and their application as a biopreservative agent. Brazilian Journal of Microbiology. 2022;53:1501–1513. doi: 10.1007/s42770-022-00791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsah-Hejri L, Hajeb P, Ehsani RJ. Application of ozone for degradation of mycotoxins in food: A review. Comprehensive Reviews in Food Science and Food Safety. 2020;19:1777–1808. doi: 10.1111/1541-4337.12594. [DOI] [PubMed] [Google Scholar]
- Ahmad V, Khan MS, Jamal QMS, Alzohairy MA, Al Karaawi MA, Siddiqui MU. Antimicrobial potential of bacteriocins: in therapy, agriculture and food preservation. International Journal of Antimicrobial Agents. 2017;49:1–11. doi: 10.1016/j.ijantimicag.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Alotaibi MA, Tayel AA, Zidan NS, El Rabey HA. Bioactive coatings from nano-biopolymers/plant extract composites for complete protection from mycotoxigenic fungi in dates. Journal of the Science of Food and Agriculture. 2019;99:4338–4343. doi: 10.1002/jsfa.9667. [DOI] [PubMed] [Google Scholar]
- Ananda AP, Manukumar HM, Umesha S, Soumya G, Priyanka D, Mohan Kumar AS, Krishnamurthy NB, Savitha KR. A relook at food packaging for cost effective by incorporation of novel technologies. Journal of Packaging Technology and Research. 2017;1:67–85. [Google Scholar]
- Ataei S, Azari P, Hassan A, Pingguan-Murphy B, Yahya R, Muhamad F. Essential oils-loaded electrospun biopolymers: a future perspective for active food packaging. Advances in Polymer Technology. 2020;2020:1–21. [Google Scholar]
- Babich O, Dyshlyuk L, Sukhikh S, Prosekov A, Ivanova S, Pavsky V, Chaplygina T, Kriger O. Effects of biopreservatives combined with modified atmosphere packaging on the quality of apples and tomatoes. Polish Journal of Food and Nutrition Sciences. 2019;69:289–296. [Google Scholar]
- Benabbou R, Subirade M, Desbiens M, Fliss I. Divergicin M35-chitosan film: development and characterization. Probiotics and Antimicrobial Proteins. 2020;12:1562–1570. doi: 10.1007/s12602-020-09660-9. [DOI] [PubMed] [Google Scholar]
- Bouarab Chibane L, Degraeve P, Ferhout H, Bouajila J, Oulahal N. Plant antimicrobial polyphenols as potential natural food preservatives. Journal of the Science of Food and Agriculture. 2019;99:1457–1474. doi: 10.1002/jsfa.9357. [DOI] [PubMed] [Google Scholar]
- Brandelli A, Lopes NA and Boelter JF. Food applications of nanostructured antimicrobials. pp. 35–74. In: Food Preservation. Elsevier (2017).
- Brillet-Viel A, Pilet MF, Courcoux P, Prévost H, Leroi F. Optimization of growth and bacteriocin activity of the food bioprotective Carnobacterium divergens V41 in an animal origin protein free medium. Frontiers in Marine Science. 2016;3:128. [Google Scholar]
- Chang Y. Bacteriophage-derived endolysins applied as potent biocontrol agents to enhance food safety. Microorganisms. 2020;8:724. doi: 10.3390/microorganisms8050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiralt A, Menzel C, Hernandez-García E, Collazo S and Gonzalez-Martinez C. Use of by-products in edible coatings and biodegradable packaging materials for food preservation. pp. 101–127. In: Sustainability of the Food System: Sovereignty, Waste, and Nutrients Bioavailability. Elsevier Inc. (2020).
- Chourasia R, Phukon LC, Singh SP, Rai AK and Sahoo D. Role of enzymatic bioprocesses for the production of functional food and nutraceuticals. pp. 309–334. In: Biomass, Biofuels, Biochemicals. Elsevier (2020).
- Cizeikiene D, Juodeikiene G, Paskevicius A, Bartkiene E. Antimicrobial activity of lactic acid bacteria against pathogenic and spoilage microorganism isolated from food and their control in wheat bread. Food Control. 2013;31:539–545. [Google Scholar]
- Diez-Gutiérrez L, San Vicente L, Sáenz J, Barron LJR, Chávarri M. Characterisation of the probiotic potential of Lactiplantibacillus plantarum K16 and its ability to produce the postbiotic metabolite γ-aminobutyric acid. Journal of Functional Foods. 2022;97:105230. [Google Scholar]
- Dong B, Lin Y, Wang J, Du W, Sun C, Fu S, Wu T. Antibacterial activity of antimicrobial peptide gcDefb1 against food-borne pathogenic bacteria and its application in pork storage. Food Science and Biotechnology. 2022;31:597–605. doi: 10.1007/s10068-022-01060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim HA, Khairalla AS, El-Gendy AO. Nanotechnology: A valuable strategy to improve bacteriocin formulations. Frontiers in Microbiology. 2016;7:1385. doi: 10.3389/fmicb.2016.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasake V, Dash SK, Dhalsamant K, Sahoo NR, Pal US. Effect of ozone and antimicrobial treatments on the shelf life of cauliflower under modified atmosphere packaging. Journal of Food Science and Technology. 2022;59:2951–2961. doi: 10.1007/s13197-021-05326-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feichtmayer J, Deng L, Griebler C. Antagonistic microbial interactions: Contributions and potential applications for controlling pathogens in the aquatic systems. Frontiers in Microbiology. 2017;8:2192. doi: 10.3389/fmicb.2017.02192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Sala B, Herranz C, Díaz-Freitas B, Hernández PE, Sala A, Cintas LM. Strategies to increase the hygienic and economic value of fresh fish: Biopreservation using lactic acid bacteria of marine origin. International Journal of Food Microbiology. 2016;223:41–49. doi: 10.1016/j.ijfoodmicro.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Menendez E, Fernandez L, Gutierrez D, Rodríguez A, Martínez B, GarcíaI P. Comparative analysis of different preservation techniques for the storage of Staphylococcus phages aimed for the industrial development of phage-based antimicrobial products. PLoS ONE. 2018;13:e0205728. doi: 10.1371/journal.pone.0205728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvêa DM, Mendonça RCS, Lopez MES, Batalha LS. Absorbent food pads containing bacteriophages for potential antimicrobial use in refrigerated food products. LWT - Food Science and Technology. 2016;67:159–166. [Google Scholar]
- Guo T, Xin YP, Zhang C, Ouyang X, Kong J. The potential of the endolysin Lysdb from Lactobacillus delbrueckii phage for combating Staphylococcus aureus during cheese manufacture from raw milk. Applied Microbiology and Biotechnology. 2016;100:3545–3554. doi: 10.1007/s00253-015-7185-x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-del-Río I, Fernández J, Lombó F. Plant nutraceuticals as antimicrobial agents in food preservation: terpenoids, polyphenols and thiols. International Journal of Antimicrobial Agents. 2018;52:309–315. doi: 10.1016/j.ijantimicag.2018.04.024. [DOI] [PubMed] [Google Scholar]
- Hammami R, Fliss I, Corsetti A. Editorial: Application of protective cultures and bacteriocins for food biopreservation. Frontiers in Microbiology. 2019;10:1561. doi: 10.3389/fmicb.2019.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han A, Hwang JH, Lee SY. Antimicrobial activities of Asian plant extracts against pathogenic and spoilage bacteria. Food Science and Biotechnology. 2023;32:229–238. doi: 10.1007/s10068-022-01182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houicher A, Alkay Z, Bourahla M, Dertli E. Virulence factors, antibiotic resistance, and antimicrobial activity of Enterococcus spp. Isolated from different sources in Algeria. Journal of Microbiology, Biotechnology and Food Sciences. 2021;11:e1907–e1907. [Google Scholar]
- Huang Y, Wang Y, Li Y, Luo C, Yang C, Shi W, Li L. Covalent immobilization of polypeptides on polylactic acid films and their application to fresh beef preservation. Journal of Agricultural and Food Chemistry. 2020;68:10532–10541. doi: 10.1021/acs.jafc.0c03922. [DOI] [PubMed] [Google Scholar]
- Iñiguez-Moreno M, Avila-Novoa MG, Iñiguez-Moreno E, Guerrero-Medina PJ, Gutiérrez-Lomelí M. Antimicrobial activity of disinfectants commonly used in the food industry in Mexico. Journal of Global Antimicrobial Resistance. 2017;10:143–147. doi: 10.1016/j.jgar.2017.05.013. [DOI] [PubMed] [Google Scholar]
- Jin J, Nguyen TTH, Humayun S, Park SH, Oh H, Lim S, Mok IK, Li Y, Pal K, Kim D. Characteristics of sourdough bread fermented with Pediococcus pentosaceus and Saccharomyces cerevisiae and its bio-preservative effect against Aspergillus flavus. Food Chemistry. 2021;345:128787. doi: 10.1016/j.foodchem.2020.128787. [DOI] [PubMed] [Google Scholar]
- Pooja BK, Gowda TSH. Bacteriocins based strategies for food bio-preservation. Biotica Research Today. 2021;3:151–152. [Google Scholar]
- Kraśniewska K, Galus S, Gniewosz M. Biopolymers-based materials containing silver nanoparticles as active packaging for food applications-a review. International Journal of Molecular Sciences. 2020;21:698. doi: 10.3390/ijms21030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivorotova T, Cirkovas A, Maciulyte S, Staneviciene R, Budriene S, Serviene E, Sereikaite J. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocolloids. 2016;54:49–56. [Google Scholar]
- Kumar P, Barrett DM, Delwiche MJ, Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Industrial & Engineering Chemistry Research. 2009;48:3713–3729. [Google Scholar]
- Kumari A, Joshua R, Kumar R, Setlhoka MD. Biopreservation of pineapple wine using immobilized and freeze dried microcapsules of bacteriocin producing L. plantarum. Journal of Food Science and Technology. 2021;59:1–9. doi: 10.1007/s13197-021-05069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladha G, Jeevaratnam K. Characterization of purified antimicrobial peptide produced by Pediococcus pentosaceus LJR1, and its application in preservation of white leg shrimp. World Journal of Microbiology and Biotechnology. 2020 doi: 10.1007/s11274-020-02847-w. [DOI] [PubMed] [Google Scholar]
- Lee EH, Khan I, Oh DH. Evaluation of the efficacy of nisin-loaded chitosan nanoparticles against food-borne pathogens in orange juice. Journal of Food Science and Technology. 2018;55:1127–1133. doi: 10.1007/s13197-017-3028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Ni K, Pang H, Wang Y, Cai Y, Jin Q. Identification and antimicrobial activity detection of lactic acid bacteria isolated from corn stover silage. Asian-Australasian Journal of Animal Sciences. 2015;28:620–631. doi: 10.5713/ajas.14.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Aguayo MDC, Grande Burgos MJ, Pérez Pulido R, Gálvez A, Lucas López R. Effect of different activated coatings containing enterocin AS-48 against Listeria monocytogenes on apple cubes. Innovative Food Science and Emerging Technologies. 2016;35:177–183. [Google Scholar]
- Luong JH, Glennon JD and Malhotra BD. Nanocrystalline cellulose (NCC) composites with antimicrobial properties. Interfaces Between Nanomaterials and Microbes: 94–113 (2021).
- Mani-López E, Palou E and López-Malo A. Biopreservatives as agents to prevent food spoilage. pp. 235–270. In: Microbial Contamination and food degradation. Elsevier (2018).
- Meireles A, Giaouris E, Simões M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Research International. 2016;82:71–85. [Google Scholar]
- Motalebi Moghanjougi Z, Rezazadeh Bari M, Alizadeh Khaledabad M, Almasi H, Amiri S. Bio-preservation of white brined cheese (Feta) by using probiotic bacteria immobilized in bacterial cellulose: optimization by response surface method and characterization. LWT. 2020;117:108603. [Google Scholar]
- Moye ZD, Woolston J, Sulakvelidze A. Bacteriophage applications for food production and processing. Viruses. 2018;10:205. doi: 10.3390/v10040205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair MS, Upadhyaya I, Amalaradjou MAR and Venkitanarayanan K. Antimicrobial food additives and disinfectants: Mode of action and microbial resistance mechanisms. pp. 275–301. In: Food-borne Pathogens and Antibiotic Resistance. wiley (2017).
- Noori S, Zeynali F, Almasi H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control. 2018;84:312–320. [Google Scholar]
- O’Connor PM, Kuniyoshi TM, Oliveira RP, Hill C, Ross RP, Cotter PD. Antimicrobials for food and feed; a bacteriocin perspective. Current Opinion in Biotechnology. 2020;61:160–167. doi: 10.1016/j.copbio.2019.12.023. [DOI] [PubMed] [Google Scholar]
- Perumal V, Yao Z, Kim JA, Kim HJ, Kim JH. Purification and characterization of a bacteriocin, bacBS2, produced by bacillus velezensis BS2 isolated from Meongge jeotgal. Journal of Microbiology and Biotechnology. 2019;29:1033–1042. doi: 10.4014/jmb.1903.03065. [DOI] [PubMed] [Google Scholar]
- Pinnaduwa U, Mendis E and Kim S. Improvements in seafood products through recent technological advancements in seafood processing. pp. 2913–2938. In: Encyclopedia of Marine Biotechnology. Wiley (2020).
- Pisoschi AM, Pop A, Georgescu C, Turcuş V, Olah NK, Mathe E. An overview of natural antimicrobials role in food. European Journal of Medicinal Chemistry. 2018;143:922–935. doi: 10.1016/j.ejmech.2017.11.095. [DOI] [PubMed] [Google Scholar]
- Połaska M, Sokołowska B. Review bacteriophages—a new hope or a huge problem in the food industry. AIMS Microbiology. 2019;5:324–347. doi: 10.3934/microbiol.2019.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajam R, Anandharamakrishnan C. Spray freeze drying method for microencapsulation of Lactobacillus plantarum. Journal of Food Engineering. 2015;166:95–103. [Google Scholar]
- Reda FM. Antibacterial and anti-adhesive efficiency of Pediococcus acidilactici against food-borne biofilm producer Bacillus cereus attached on different food processing surfaces. Food Science and Biotechnology. 2019;28:841–850. doi: 10.1007/s10068-018-0518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineccius G. Aroma Encapsulation and controlled delivery. Vol. 12, pp. 33–34. In: Springer Handbooks. Springer (2017).
- Reis PJM, Tavares TG, Rocha JM, Malcata FX, Macedo AC. Cobranosa table olives: Characterization of processing method and lactic acid bacteria profile throughout spontaneous fermentation. Applied Sciences. 2022;12:9738. [Google Scholar]
- Shahbazi Y, Shavisi N. Effects of sodium alginate coating containing Mentha spicata essential oil and cellulose nanoparticles on extending the shelf life of raw silver carp (Hypophthalmichthys molitrix) fillets. Food Science and Biotechnology. 2019;28:433–440. doi: 10.1007/s10068-018-0486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shajil S, Mary A and Rani Juneius CE. Recent food preservation techniques employed in the food industry. Vol. 2, pp. 3–21. In: Microbial Biotechnology. Springer Singapore (2018).
- Shamloo E, Hosseini H, Moghadam AZ, Larsen HM, Haslberger A, Alebouyeh M. Importance of Listeria monocytogenes in food safety: A review of its prevalence, detection, and antibiotic resistance. Iranian Journal of Veterinary Research. 2019;20:241–254. [PMC free article] [PubMed] [Google Scholar]
- Shankar S and Rhim J-W. Bionanocomposite Films for Food Packaging Applications. In: Reference Module in Food Science. Elsevier (2018).
- Shannon R, Radford DR, Balamurugan S. Impacts of food matrix on bacteriophage and endolysin antimicrobial efficacy and performance. Critical Reviews in Food Science and Nutrition. 2020;60:1631–1640. doi: 10.1080/10408398.2019.1584874. [DOI] [PubMed] [Google Scholar]
- Sharma BR, Halami PM, Tamang JP. Novel pathways in bacteriocin synthesis by lactic acid bacteria with special reference to ethnic fermented foods. Food Science and Biotechnology. 2022;31:1–16. doi: 10.1007/s10068-021-00986-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CCG, Silva SPM, Ribeiro SC. Application of bacteriocins and protective cultures in dairy food preservation. Frontiers in Microbiology. 2018;9:594. doi: 10.3389/fmicb.2018.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP. Recent approaches in food bio-preservation-A review. Open Veterinary Journal. 2018;8:104–111. doi: 10.4314/ovj.v8i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroli L, Patrignani F, Serrazanetti DI, Tabanelli G, Montanari C, Gardini F, Lanciotti R. Lactic acid bacteria and natural antimicrobials to improve the safety and shelf-life of minimally processed sliced apples and lamb’s lettuce. Food Microbiology. 2015;47:74–84. doi: 10.1016/j.fm.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Song X, Vossebein L, Zille A. Efficacy of disinfectant-impregnated wipes used for surface disinfection in hospitals: A review. Antimicrobial Resistance and Infection Control. 2019;8:1–14. doi: 10.1186/s13756-019-0595-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarski Y, Nikolova R, Petkova N, Ivanov I, Lante A. Biopreservation of fresh strawberries by carboxymethyl cellulose edible coatings enriched with a bacteriocin from Bacillus methylotrophicus BM47. Food Technology and Biotechnology. 2019;57:230–237. doi: 10.17113/ftb.57.02.19.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayah SB, Philip K. Characterization, yield optimization, scale up and biopreservative potential of fermencin SA715, a novel bacteriocin from Lactobacillus fermentum GA715 of goat milk origin. Microbial Cell Factories. 2018;17:1–18. doi: 10.1186/s12934-018-0972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiernasz N, Cornet J, Cardinal M, Pilet MF, Passerini D, Leroi F. Lactic acid bacteria selection for biopreservation as a part of hurdle technology approach applied on seafood. Frontiers in Marine Science. 2017;4:119. [Google Scholar]
- Yan J, Yang R, Yu S, Zhao W. The strategy of biopreservation of meat product against MRSA using lytic domain of lysin from Staphylococcus aureus bacteriophage. Food Bioscience. 2021;41:100967. [Google Scholar]
- Yépez A, Luz C, Meca G, Vignolo G, Mañes J, Aznar R. Biopreservation potential of lactic acid bacteria from Andean fermented food of vegetal origin. Food Control. 2017;78:393–400. [Google Scholar]
- Yuan L, Sadiq FA, Wang N, Yang Z, He G. Recent advances in understanding the control of disinfectant-resistant biofilms by hurdle technology in the food industry. Critical Reviews in Food Science and Nutrition. 2020;61:3876–3891. doi: 10.1080/10408398.2020.1809345. [DOI] [PubMed] [Google Scholar]
- Zhao S, Han J, Bie X, Lu Z, Zhang C, Lv F. Purification and characterization of plantaricin JLA-9: A Novel bacteriocin against Bacillus spp. produced by Lactobacillus plantarum JLA-9 from Suan-Tsai, a traditional Chinese fermented cabbage. Journal of Agricultural and Food Chemistry. 2016;64:2754–2764. doi: 10.1021/acs.jafc.5b05717. [DOI] [PubMed] [Google Scholar]