Abstract
Recently, unconventional yeasts have become popular as fermentation starters in the brewing industry due to the growing consumer demand for aromatic diversity. Specifically, Schizosaccharomyces japonicus has been explored as a potential starter culture for beer and wine production because of its distinct brewing characteristics; however, its application in makgeolli fermentation has not been tested. Therefore, in the present study, two Sz. japonicus strains (SZJ-1 and SZJ-2) were isolated from natural sources, and their brewing characteristics for makgeolli fermentation were compared with those of commercial S. cerevisiae strain. Although the tested isolates showed a lower fermentation and carbon source consumption rate than control-, their overall alcohol fermentation characteristics were suitable for makgeolli production. Regarding flavor composition, Sz. japonicus-fermented makgeolli possessed more ester compounds (e.g., 2-phenylethyl acetate, ethyl acetate, and ethyl decanoate) than S. cerevisiae-fermented makgeolli. Therefore, Sz. japonicus can be used as an alternative culture starter in makgeolli fermentation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10068-023-01265-6.
Keywords: Alternative yeast starter, Brewing, Makgeolli, Malic acid, Schizosaccharomyces japonicus
Introduction
Many countries have their own conventional alcoholic beverages (Tamang and Kailasapathy, 2010), which are produced through alcoholic fermentation—an anaerobic biochemical metabolic reaction that releases energy from the breakdown of simple sugars, such as glucose and fructose, into ethanol and carbon dioxide. Makgeolli is one of the oldest fermented alcoholic beverages produced from rice in Korea. Typically, it is brewed using rice as the major fermentation substrate and nuruk containing various microorganisms and hydrolytic enzymes as a fermentation starter. Makgeolli research is largely divided into three fields. The first is related to the major starch ingredients, subsidiary materials, and manufacturing process of makgeolli. For instance, Cho et al. (2012) used purple sweet potato as raw material to produce purple makgeolli and studied its qualitative characteristics and antioxidant effects. In the second field, the functional activities of various ingredients contained in makgeolli are explored (Cho et al., 2012). Makgeolli has a relatively low alcohol content at 6–7% (%, v/v) and contains diverse proteins, sugars, lipids, dietary fibers, organic acids, and vitamins as well as various bioactive compounds (Kim et al., 2011; Park et al. 2018). Recently, various positive physiological effects of makgeolli, such as antioxidant, anticancer, antihypertensive, antidiabetic, skin whitening, and antibacterial activities, have been reported (Lee et al., 2013). Finally, the third field is related to the diversity and roles of microorganisms that are directly involved in the fermentation of makgeolli (Bal et al., 2016). Nuruk is a conventional Korean fermentation starter containing an array of microorganisms, including fungi, yeasts, and bacteria (Park and Chung, 2014). As these microorganisms are inevitably associated with makgeolli fermentation, their diversity and fermentation characteristics, particularly of fungi and yeasts, have been extensively studied in recent years. In particular, Aspergillus species, including Aspergillus oryzae, A. luchuensis, and A. kawachii, as well as S. cerevisiae have been comprehensively explored to improve the fermentation quality of makgeolli (Kim and Seo, 2021).
Furthermore, the potential of non-Saccharomyces fungi as alternative yeast starters for wine and beer fermentation has been documented (Benito et al., 2015). Thanks to these efforts, non-Saccharomyces yeasts are a useful tool for improving brewing characteristics, such as nutrient content, stability, and aromatic or bioactive profiles, regardless of their relatively lower fermentation potential than that of S. cerevisiae. With these recent scientific advances, major yeast manufacturers have started commercializing dry non-Saccharomyces species, such as Candida zemplinina, C. stellata, Hanseniaspora vineae, H. uvarum, Kazachstania aerobia, Kloeckera apiculata, and Schizosaccharomyces japonicus (Benito et al., 2019).
Among non-Saccharomyces yeasts, the utility of the genus Schizosaccharomyces in winemaking has been extensively studied. The most interesting brewing property of Schizosaccharomyces species for use as fermentation starters is their ability to decompose malic acid in alcoholic beverages via maloalcoholic fermentation (Benito et al., 2019). Maloalcoholic fermentation is an excellent approach to preserve fresh aromatic properties while reducing the harsh “green apple sourness” caused by malic acid in wine fermentation (Mylona et al., 2016). In addition, the use of Schizosaccharomyces as a fermentation starter may positively affect food safety. Specifically, Schizosaccharomyces could reduce the concentration of unwanted compounds, such as ochratoxin A (OTA) and biogenic amines, during alcohol fermentation (Cecchini et al., 2006). Typically, malic acid in alcoholic beverages is deacidified by lactic acid bacteria (LAB), such as Oenococcus oeni (Betteridge et al., 2015). However, the application of LAB for alcohol fermentation is associated with the risk of biogenic amine production. Meanwhile, the use of Schizosaccharomyces may reduce the malic acid content of alcoholic beverages without the risk of biogenic amine production (Benito et al., 2015). During makgeolli brewing, Aspergillus species and other fungi in nuruk are involved in fermentation. However, some Aspergillus species are major producers of mycotoxins, such as OTA, which is a potent nephrotoxin and carcinogen detected in various common foods and beverages. In a previous study, OTA was detected in makgeolli (Lee et al., 2012). Interestingly, Schizosaccharomyces species suppress OTA generation, which may positively affect the removal of this toxin during makgeolli fermentation. Another interesting feature of Schizosaccharomyces species is the release of large amounts of cell wall polysaccharides at the beginning of alcohol fermentation. Yeast cell wall-derived polysaccharides may improve the mouth feel of alcoholic beverages, induce a “fullness” sensation and enhance sweetness and roundness (Chalier et al., 2007). In addition, cell wall-derived mannoproteins may reduce the volatility of aromatic compounds, contributing to their stabilization in alcoholic beverages (Chalier et al., 2007).
In the present study, two Sz. japonicus strains (SZJ-1 and SZJ-2) were isolated from natural resources, and their applicability to the fermentation of alcoholic beverages, particularly makgeolli, was explored. In addition, overall alcoholic fermentation characteristics of makgeolli fermented using Sz. japonicus were compared with those of makgeolli fermented using a commercial S. cerevisiae strain CBS12555 (control). Additionally, fermentation kinetics and aroma profile of makgeolli fermented using SZJ-1 and SZJ-2 were investigated to confirm the possibility of using Sz. japonicus as an alternative starter yeast for makgeolli fermentation.
Materials and methods
Isolation and identification of Sz. japonicus strains
Different yeast strains have been isolated from natural resources, such as traditional fermented foods and flowers collected from various regions of Korea. Samples were dispensed in 0.89% sterilized phosphate-buffered saline (pH 7.2–7.4), and smashed using a sterilized glass rod to prepared dispersed samples. Supernatant (200 μL) from the dispersed samples was spread on YPD agar supplemented with ampicillin (50 µg mL−1). All isolates were subjected to preliminary microscopic investigation to verify their morphology. Species were identified through sequence analysis of the D1/D2 domains, as follows. Each strain was cultured, and genomic DNA was extracted as described previously (Harju et al., 2004). The yeast-specific universal primers NL1 (5′-TCCGTAGGTGAACCTGCGG-3′) and NL2 (5′-GCATATCAATAAGCGGAGGA-3′) were used to amplify the D1/D2 domain of eukaryotic 26S rRNA (Lee et al., 2011). PCR was performed under the following conditions: 95 °C for 5 min; 35 cycles of 95 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and 72 °C for 10 min. The PCR products were assessed for D1/D2 amplification by running 10% of the reaction volume on 0.8% (w/v) agarose gel at 100 V (560 bp expected product size). All amplicons were purified using a gel extraction kit (Nucleogen Inc., Siheung, Korea). Nucleotide sequences of the resulting PCR products were determined for species identification (Macrogen Inc., Seoul, Korea) using NL1 and NL4 primers, and the sequences of homology data were determined by searching databases using the BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST/). In the present study, sixty-two yeast strains were isolated from 31 samples and 2 Sz. japonicus strains were identified. The phylogenetic tree was constructed using MEGA 7 software.
Analysis of sugar assimilation using API 20C AUX
The API 20C AUX system (bioMérieux, Marcy l'Etoile, France) was used to evaluate sugar utilization by the isolated Sz. japonicus strains. API strips were prepared as recommended by the manufacturer and incubated at 30 °C for 72 h. Thereafter, sugar assimilation patterns were read from the test strips.
Scanning electron microscopy (SEM)
Yeast cells were fixed for SEM according to the method described by Murtey and Ramasamy (2016). Single colonies were obtained from YPD agar plates and grown in YPD broth at 25 °C for 24 h. Next, 1 mL of yeast culture was centrifuged at 19,000×g for 1 min, and the pellet was resuspended in 5% glutaraldehyde solution (Sigma-Aldrich, St. Louis, MO, USA) prepared in 0.1 M phosphate buffer (pH 7.2) for fixation. After 30 min, the samples were centrifuged, and the cell pellet was washed two times with 0.1 M phosphate buffer. The pellet was resuspended in 1% osmium tetroxide prepared in 0.1 M phosphate buffer. After 1 h, the cells were washed two times with 0.1 M phosphate buffer. The samples were dehydrated through an ethanol series of 35%, 50%, 75%, 95%, and absolute ethanol and hexamethyldisilazane (HDMS) for 30 min per step (the last two ethanol steps were performed two times) and centrifuged, and the supernatant was discarded at each change. Finally, the second HDMS was discarded, and the samples were dried overnight in a desiccator. The morphology of yeast cells was observed using FE-SEM (LEO SUPRA 55, Carl Zeiss, Germany) in the Central Laboratory for Instrumental Analysis at Kyung Hee University (Yongin, Korea). All samples were sputtered with a platinum ion beam for 300 s before observation.
Makgeolli brewing
The first mashing was initiated by soaking 1 kg of polished rice for 3 h. Thereafter, the soaked rice was steamed for 90 min and cooled for 1 h at 25 °C. The pitching rate was adjusted to 6.25 × 106 yeast cells mL−1 to ensure complete fermentation. In a 10 L fermenter, 1.2 L of distilled water and commercial nuruk (Korea Fermentation Co., Hwaseong, Korea, 1,800 SP) were mixed with rice. The first mashing was fermented at 25 °C for 48 h. The second mashing was performed by mixing the first-stage mash with 2 kg of streamed rice, 2.4 L of distilled water, and 28 g of commercial nuruk (Korea Fermentation Co.). The second mashing was fermented at 25 °C for 12 days. The pitching volume of yeast starters was 500 mL, and the yeast cell density was adjusted to 1.25 × 107 cells mL−1 using the LUNA-FX7™ Automated Cell Counter (Logos Biosystems, Inc., Anyang, Korea). After the second mashing, makgeolli were aseptically sampled at a volume of 150 mL every 24 h. The sampled makgeolli was filtered through Whatman No. 2 filter paper (GE Healthcare, Fairfield, CT, USA) before analysis.
Analysis of alcohol content, acidity, reducing sugars, and organic acids in makgeolli
To analyze the alcohol content, 100 mL of makgeolli was aseptically sampled in accordance with the Alcohol Analysis Regulations of the National Tax Service’s Technology Research Center. Next, three drops of an anti-foaming agent (DSA732, DYS Co., Seoul, Korea) were added to suppress bubble generation during the distillation of makgeolli samples (Seo et al., 2016a). After distillation, distilled water was added to 100 mL of the sample, and the collected liquid alcohol content was measured using a density meter (DMA 35 Portable Density Meter, Anton Paar, Austria).
Titratable acidity was evaluated as described previously (Kim et al., 2011). Two drops of the bromothymol blue (BTB)–neutral red (NR) mixed indicator (0.2 g BTB and 0.1 g NR in 300 mL of 95% ethyl alcohol) were applied to 10 mL of makgeolli filtrate. The sample was then titrated using 0.1N NaOH solution, and acidity was calculated based on the volume of NaOH solution required to neutralize the sample solution. The pH of each sample was measured using a pH meter (K6000-DO; Istek Inc., Seoul, Korea).
The reducing sugar content was determined using the dinitrosalicylic acid method with glucose as the standard molecule (Miller, 1959). Absorbance of the final reaction product was measured at 550 nm using a UV/VIS spectrophotometer (Beckman Coulter Inc., Brea, CA, USA).
Organic acids were determined using high performance liquid chromatography (HPLC) with the Agilent 1100 series HPLC system (Agilent, Palo Alto, CA, USA) coupled to a refractive index detector (Hewlett Packard HP-1047A, City, State, Country). All samples were filtrated through 0.45 μm nitrocellulose membranes and diluted in water to adjust the appropriate concentration. Next, each makgeolli sample (20 μL) was injected into the HPLC apparatus. Isocratic separation was performed at 75 °C on the Hi-Plex H (300 × 7.7 mm) column (Agilent). The mobile phase comprised 4 mM H2SO4 at a flow rate of 0.4 mL min−1. The compounds were identified and quantified based on comparison with external calibration curves for each compound.
Analysis of volatile compounds in makgeolli products
Samples (4 g) were transferred in 20 mL headspace screw vials (Agilent Technologies, Santa Clara, CA, USA) and subjected to solid phase microextraction (SPME). The vials were maintained at 30 °C for 30 min to reach the equilibrium state. Then, volatile compounds were adsorbed for 30 min using an SPME fiber coated with divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS, 75 μm, Supelco, Bellefonte, PA, USA). Volatile compounds were identified and quantified using a gas chromatograph (GC 7890 B, Agilent Technologies) and a mass selective detector (MS 5977 B, Agilent Technologies) equipped with the Stabilwax® column (30 m length × 0.25 mm ID, 25 μm film thickness; J&W Scientific, Folsom, CA, USA). The oven program was initially held at 40 °C for 5 min and ramped to 200 °C at the rate of 4 °C min−1 (10 min). Helium was used as the carrier gas at the flow rate of 0.8 mL min−1. Injector and detector temperatures were 220 °C and 250 °C, respectively. Mass spectral data were obtained at 70 eV through electron ionization (EI) in the mass scan range of 35–350 amu and at the rate of 4.5 scans per second. Identity was confirmed by comparing the mass spectral data with the Wiley9n.1 database and based on retention index (RI) values using the NIST Chemistry Webbook. The composition of the identified volatile compounds was determined as the ratio of peak area to the sum of total peak areas.
Statistical analysis
Statistical analysis was verified by analysis of variance (ANOVA) by SPSS program (Statistical Analysis Program, version 25, IBM Co., Armonk, NY, USA) and significance was confirmed at p < 0.05 using Tukey’s honestly significant difference test.
Result and discussion
Isolation and identification of Sz. japonicus
Traditionally, S. cerevisiae has been widely used for alcoholic fermentation; recently, however, many efforts have been devoted to apply non-conventional yeasts in this process. Canonico et al. examined 43 wild yeast strains, including Lachancea, Kluyveromyces, Torulaspora, Metschnikowia, Kazachstania, Brettanomyces, Pichia, Candida, Hanseniaspora, Rhodotorula, Rodosporidobolus, and Saccharomyces, to improve the aromatic characteristics and nutritional properties of craft beer (Canonico et al., 2021). According to the authors, some non-Saccharomyces yeasts may be suitable alternatives for fermenting premium craft beer with a promising sensory profile and improved nutritional and functional characteristics. In another study, Brettanomyces sp. was used to ferment Gueuze and Lambic beers and purposely inoculated into Trappist and sour ales for its typical sourness and unique flavor complexity (Schifferdecker et al., 2014). In addition, C. stellata, Pichia anomala, Sz. pombe, and H. uvarum have been used as fermentation starters in combination with S. cerevisiae for wine making in the form of co-fermentation (Andorrà et al., 2010).
Furthermore, Sz. pombe has several positive applications in the wine industry. Its ability to metabolize malic acid, facilitating the biological deacidification of juice and/or wine, is well known (Benito et al. 2015). Moreover, Benito et al. reported that the application of Sz. pombe and Lachancea thermotolerans in low-acidity musts reduced the risk of biogenic amine production associated with malolactic fermentation (Benito et al., 2015). A recent study showed that Sz. japonicus–S. cerevisiae mixed starter cultures modulated the volatile compound concentration and affected the glycerol and polysaccharide content, increasing the sweetness and color stability of Sangiovese wine (Portaro et al., 2022). From these reports, Schizosaccharomyces species can be applied in makgeolli fermentation to improve the quality of the product. Therefore, in the present study, Schizosaccharomyces strains suitable for makgeolli brewing were isolated from natural resources.
As such, 62 strains of putative wild yeasts were isolated from 31 natural samples, and their morphology and genetic variations were investigated. Genetic variation was identified based on sequencing of the D1/D2 domain of the eukaryotic 26S rRNA and phylogenetic tree analysis was performed (Fig. S1). Finally, two different strains, namely Sz. japonicus SZJ-1 and SZJ-2, were isolated from the 62 wild yeast strains (Table S1), and their alcohol production capacity and sugar utilization were evaluated (Table 2).
Table 2.
Volatile compounds in makgeolli fermented by Sz. japonicus SZJ-1, Sz. japonicus SZJ-2, and S. cerevisiae (control)
| Compound | RIa | Composition (%) | Odor descriptionb | ||
|---|---|---|---|---|---|
| Control | SZJ-1 | SZJ-2 | |||
| Acids | |||||
| Acetic acid | 1446 | 0.14 | 0.2 | 0.32 | Pungent, sour, vinegar |
| 3-Methylbutanoic acid | 1667 | ND | 0.1 | 0.07 | Sweaty, cheesy |
| Hexanoic acid | 1845 | ND | 0.16 | ND | Strong fatty, cheesy-sweaty odor and taste |
| Octanoic acid | 1903 | ND | 0.08 | ND | Fatty, rancid, animalic, sweaty odor; cheese-like taste in dilution |
| Nonanoic acid | 1904 | ND | 0.1 | ND | Mild fatty, dairy-cheese, nut-like odor; fatty-waxy-cheesy and nutty taste |
| Aldehydes | |||||
| Acetaldehyde | 900 | 0.08 | 0.01 | 0.01 | Pungent, breathtaking; a nutty alcoholic note in dilution; enhanced citrusy |
| Alcohols | |||||
| Ethanol | 941 | 34.46 | 26.23 | 26.16 | Sweet, ethereal (alcoholic) odor; primarily used as a solvent |
| 2-Methylpropan-1-ol | 1101 | 4.60 | 2.08 | 3.15 | Breathtaking, sweet, sweaty-chemical; fermented, whiskey-like in dilution |
| 3-Methylbutan-1-ol | 1218 | 35.70 | 31.98 | 31.37 | Breathtaking, alcoholic odor; a winey-brandy taste in dilution |
| 3-Ethoxypropan-1-ol | 1377 | ND | ND | 0.09 | Mild fruity |
| 2,6-Dimethylheptan-4-ol | 1539 | ND | 0.14 | ND | Mild sweet, fermented, yeasty, fruity, green odor with winey notes |
| Aromatic compounds | |||||
| Benzaldehyde | 1519 | 0.20 | ND | ND | Odor of bitter almond oil; characteristic sweet cherry taste |
| 1-Phenylethanone | 1651 | ND | 0.05 | 0.19 | Sweet, pungent, harsh, cherry-like odor and taste |
| Methyl 2-hydroxybenzoate | 1779 | 0.10 | 0.09 | 0.11 | Warm, sweet, wintergreen odor and taste |
| 2-Phenylethyl acetate | 1811 | 0.12 | 0.4 | 0.31 | Sweet, rose, fruity, honey-like odor; floral-honey taste |
| 2-Phenylethanol | 1900 | 6.56 | 7.45 | 8.53 | Floral, rose-like odor; floral taste |
| Benzoic acid | 1907 | ND | ND | 0.05 | Very weak balsamic odor and burning taste |
| Esters | |||||
| Ethyl acetate | 902 | 7.60 | 12.12 | 11.92 | Ethereal, sharp wine-brandy-like odor |
| 2-Methylpropyl acetate | 1009 | 0.27 | 0.42 | 0.00 | Fruity, banana-apple-pear-pineapple notes |
| Ethyl butanoate | 1027 | 0.22 | 0.61 | 0.61 | Ethereal, fruity odor; buttery-pineapple-banana, ripe fruit and juicy notes |
| Ethyl 2-methylbutanoate | 1041 | 0.28 | 0.63 | 0.63 | Strong, green, fruity, apple odor and taste |
| Ethyl 3-methylbutanoate | 1057 | ND | 0.21 | 0.19 | Sweet, fruity, banana, pear odor and taste |
| 3-Methylbutyl acetate | 1109 | 4.35 | 7.14 | 7.17 | Sweet, fruity, banana, pear odor and taste |
| Ethyl heptanoate | 1315 | 0.09 | 0.05 | 0.04 | Strong, fruity, winey, cognac-like odor and taste |
| Ethyl octanoate | 1417 | 3.20 | 4.64 | 3.74 | Fruity, winey, sweet odor; cognac-apricot taste |
| Ethyl nonanoate | 1525 | 0.26 | 0.66 | 0.55 | Fatty-waxy, oily, cognac, grape, nut-like odor |
| Ethyl decanoate | 1632 | 0.66 | 2.7 | 2.34 | Sweet, fatty, nut-like, winey-cognac odor |
| Diethyl butanedioate | 1674 | 0.34 | 0.43 | 0.33 | Weak, winey, ethereal, green, grape-like odor |
| Ethyl dodecanoate | 1842 | 0.07 | 0.1 | 0.25 | Mild fatty-waxy oily, floral fruity odor with fatty dairy fruity taste |
| Ethyl tetradecanoate | 1902 | ND | ND | 0.04 | Mild sweet, waxy-fatty odor and sweet waxy creamy taste |
| Ethyl hexadecanoate | 1906 | 0.03 | 0.02 | ND | Faint waxy, sweet odor; nearly tasteless; creamy mouthfeel |
| Hydrocarbons | |||||
| 2,4-Dimethylheptane | 901 | 0.02 | 0.01 | 0.01 | – |
| Phenols | |||||
| Phenol | 1901 | ND | ND | 0.03 | Phenolic medicinal odor and taste |
| 4-Ethenyl-2-methoxyphenol | 1905 | 0.02 | 0.26 | 0.22 | Sweet, spicy, clove-like, somewhat smoky odor; sweet taste |
| Sulfur-containing compounds | |||||
| 3-Methylsulfanylpropan-1-ol | 1737 | ND | 0.15 | 0.15 | Strong, meaty, mushroom, soup-like odor and taste in dilution |
ND not detected
aRetention indices (RI) were determined using n-paraffins C7–C30 as external standards
bFlavor-Base 2010 Professional, Leffingwell & Associates
Appearance of the two isolated Sz. japonicus strains was observed using SEM (Fig. S2). As expected, both Sz. japonicus strains showed a typical fission yeast morphology with elongated oval or spherical cells, which is rather distinct from the morphology of S. cerevisiae. There were no significant differences in morphology between SZJ-1 and SZJ-2. Regarding cell size, Sz. japonicus cells (5–10 μm rods) were relatively longer than brewer’s yeast cells (3–4 μm oval cells). Cell size is closely correlated with the total cell surface area, which determines the import and export rates of nutrients and fermentation products. Cell size can affect fermentation performance; therefore, it must be considered when determining the pitching rate.
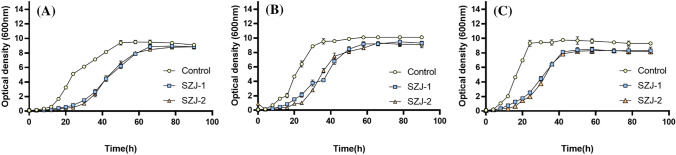
Because the general makgeolli fermentation temperature range is between 20 and 30 °C, growth patterns of Sz. japonicus strains were investigated at 20 °C, 25 °C, and 30 °C in YPD broth (Fig. 1). The growth patterns of Sz. japonicus differed from those of S. cerevisiae in terms of two aspects. First, Sz. japonicus presented a lower specific growth rate in the exponential phase, resulting in a longer growth duration (66 h) to reach the stationary phase, than S. cerevisiae (42 h). Second, Sz. japonicus showed a slightly lower cell density at the maximum optical density than S. cerevisiae. These growth patterns were confirmed at all temperatures (20 °C, 25 °C, and 30 °C). However, no significant strain-dependent differences in growth patterns were observed between SZJ-1 and SZJ-2. These results provide important information for determining the optimal makgeolli fermentation duration when using Sz. japonicus as the culture starter yeast.
Fig. 1.
Growth patterns of Schizosaccharomyces japonicus strains (SZJ-1 and SZJ-2) investigated at (A) 20 °C, (B) 25 °C, and (C) 30 °C in YPD broth
Sugar utilization and alcohol production
Sugar utilization by the two Sz. japonicus strains was examined using the API 20C AUX kits. Both SZJ-1 and SZJ-2 could utilize a narrow range of carbon substrates (Table 1). Both strains efficiently consumed glucose and sucrose among the 19 carbon substrates in the API AUX 20C kit. Additionally, SZJ-1 efficiently assimilated glycerol, whereas SZJ-2 weakly utilized raffinose. In a previous study (Vaughan-Martini and Martini, 2011), Sz. japonicus CBS354 isolated from strawberry wine in Japan could grow in a liquid medium containing glucose, sucrose, or raffinose as the sole carbon source but could not utilize glycerol. This information on sugar assimilation by Sz. japonicus strains provides a basis for the diversity of strain of this species and their applications in alcohol fermentation.
Table 1.
Sugar utilization by yeast strains as detected with the API AUX 20C kit
| Sugar | Control (S. cerevisiae CBS12555) | Sz. japonicus SZJ-1 | Sz. japonicus SZJ-2 |
|---|---|---|---|
| d-Glucose | + | + | + |
| Glycerol | − | w | − |
| Calcium 2-keto-gluconate | − | − | − |
| l-Arabinose | − | − | − |
| d-Xylose | − | − | − |
| Adonitol | − | − | − |
| Xylitol | − | − | − |
| d-Galactose | − | − | − |
| Inositol | − | − | − |
| d-Sorbitol | − | − | − |
| Methyl-α-d-glucopyranoside | + | − | − |
| N-Acetyl-glucosamine | − | − | − |
| d-Cellobiose | − | − | − |
| d-Lactose (bovine origin) | − | − | − |
| d-Maltose | + | − | − |
| d-Saccharose (sucrose) | + | + | + |
| d-Trehalose | w | − | − |
| d-Melezitose | w | − | − |
| d-Raffinose | w | − | w |
+ (positive), yeast strain could utilize the substrate; − (negative), yeast strain could not utilize the substrate; w (weak), yeast strain showed little turbidity with the API kit
Glucose is the most abundant free sugar in makgeolli wort, because the major substrate of makgeolli is rice starch. Lee et al. (2013) showed that glucose was the most abundant free sugar in rice makgeolli, with a concentration of 33.9 mg mL−1 on the first day of fermentation. The concentrations of other sugars, such as maltose (2.1 mg mL−1) and fructose (0.4 mg mL−1) were relatively low in makgeolli, while sucrose was not detected. Although both SZJ-1 and SZJ-2 exhibited a narrow range of sugar utilization characteristics, they could use glucose as a sole carbon substrate, indicating that these strains could be used as fermentation starters in makgeolli fermentation.
Fermentation characteristics in koji extract medium
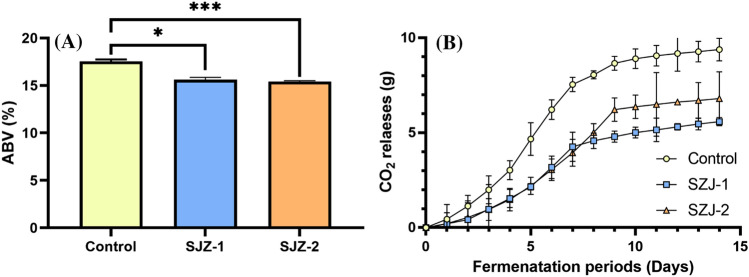
The alcohol production capacity of SZJ-1 and SZJ-2 was confirmed by growing them in koji extract medium. Historically, Schizosaccharomyces species, including Sz. japonicus and Sz. pombe, have been used in various alcohol fermentations. For instance, Sz. japonicus has been employed in wine making to improve its aroma, taste, and color stability (Domizio et al., 2017). However, Sz. japonicus, which uses rice starch as a substrate, has not been used for making makgeolli. To verify the alcohol fermentation characteristics of Sz. japonicus, fermentation was performed in koji extract medium. The koji extract medium was prepared as described in the Materials and methods section. Briefly, the yeast strains (SZJ-1, SZJ-2, or S. cerevisiae) were separately inoculated, and CO2 and alcohol production in the fermentation broth was evaluated. The commercial S. cerevisiae strain showed exponential CO2 release from the start up to day 7 of fermentation, followed by a plateau with a slightly upward trend until day 14 (Fig. 2). The two Sz. japonicus (SZJ-1 and SZJ-2) exhibited a similar pattern to S. cerevisiae, although the start day of the plateau and the amount of CO2 released from the fermentation broth were different (Fig. 2). The amount of CO2 released from the fermentation broth by the two Sz. japonicus was lower than by S. cerevisiae, indicating weaker alcohol fermentation by the two Sz. japonicus strains in koji extract medium. The alcohol content of fermented medium was respectively 17.4 ± 0.04%, 15.6 ± 0.03%, and 15.5 ± 0.01% for S. cerevisiae, SZJ-1, and SZJ-2. Even though the alcohol production ability of SZJ-1 and SZJ-2 in koji extract medium was 1.8–1.9% lower than that of the commercial S. cerevisiae strain, the alcohol content was sufficient for the production of makgeolli, since commercial makgeolli contains approximately 6% alcohol. Therefore, the two Sz. japonicus strains are suitable as fermentation starters for makgeolli production.
Fig. 2.
(A) Alcohol and (B) CO2 production by Schizosaccharomyces japonicus strains (SZJ-1 and SZJ-2) in the koji extract media
Stress tolerances of Sz. japonicus strains
In a high-gravity brewing environment, such as during makgeolli fermentation, yeast is exposed to high sugar concentration, requiring high osmotic resistance to and tolerance of ethanol generated during fermentation under high gravity (Puligundla et al., 2020). In addition, yeast are exposed to various acidic metabolites, including citric and lactic acid, derived from koji and fungi (Seo et al., 2016b). Therefore, acid tolerance comparable to that of commercial yeast is required for desirable makgeolli fermentation.
Therefore, stress tolerance of Sz. japonicus strains was investigated using ethanol, osmolytes (sorbitol), and acids. Specifically, stress tolerance was examined by determining the relative growth of Sz. japonicus according to the absence and presence of stress agents. Both Sz. japonicus strains (SZJ-1 and SZJ-2) were tolerant of ethanol concentration up to 5%, although their growth was inhibited at ethanol concentrations exceeding 10% (Fig. 3). Moreover, the growth of two Sz. japonicus was slower in the presence of 200 g L−1 sorbitol than in its absence. Regarding acidity, pH below 3 negatively affected the growth of both strains (SZJ-1 and SZJ-2) regardless of the type of acid used (strong or weak acid). Compared with S. cerevisiae, however, SZJ-1 and SZJ-2 exhibited a lower tolerance of both weak and strong acids. In particular, both strains couldn’t grow at a pH of 2.5 adjusted with malic acid, whereas S. cerevisiae could grow under the same conditions, albeit slowly. These results indicate that there may be no critical limitation to the application of Sz. japonicus for makgeolli fermentation.
Fig. 3.
Stress tolerances of Schizosaccharomyces japonicus strains (SZJ-1 and SZJ-2) by ethanol (A), osmolyte (sorbitol) (B), and acid [HCl (C), malic acid (D)]
Brewing characteristics of makgeolli fermented by Sz. japonicus
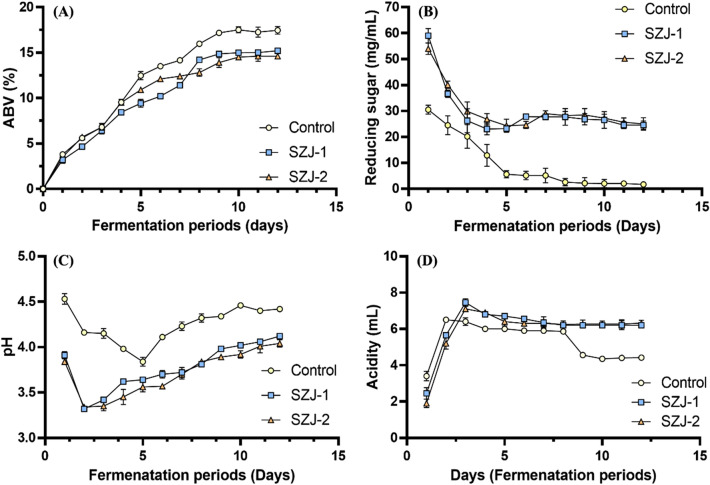
Makgeolli fermentation was realized using two Sz. japonicus strains, namely SZJ-1 and SZJ-2. The final alcohol content of makgeolli fermented by the two Sz. japonicus strains was approximately 15% (15.18 ± 0.01% in SZJ-1 makgeolli and 14.59 ± 0.04% in SZJ-2 makgeolli). The typical alcohol content of makgeolli fermented by S. cerevisiae is in the range of 16–18% (Kim et al., 2018) (Fig. 4A). Therefore, the two Sz. japonicus strains showed a lower alcohol fermentation capability than S. cerevisiae, although their fermentation times did not differ significantly. Because alcohol content was lower in makgeolli fermented by Sz. japonicus than by S. cerevisiae, more unfermented residual reducing sugars in the fermentation broth were expected. Accordingly, makgeolli fermented by Sz. japonicus contained 15-fold more reducing sugars than that fermented by S. cerevisiae (Fig. 4B). This observation may be explained by the low alcohol fermentation capability and/or polysaccharide over-production characteristic of Sz. japonicus (Domizio et al., 2017).
Fig. 4.
Patterns of makgeolli fermentation using two Schizosaccharomyces japonicus isolates (SZJ-1 and SZJ-2) and Saccharomyces cerevisiae CBS12555 (control). (A) Alcohol production, (B) reducing sugar content, (C) pH, and (D) total acidity
Changes in pH and total acidity during makgeolli fermentation by the two Sz. japonicus strains and S. cerevisiae are shown in Fig. 4C and D. The pattern of pH changes during makgeolli fermentation by the two Sz. japonicus strains was somewhat different from that by S. cerevisiae. During makgeolli fermentation by S. cerevisiae, pH gradually decreased until day 5 (pH 3.84 ± 0.05) and gradually increased from days 6 to 12, at the end of fermentation. Meanwhile, during makgeolli fermentation SZJ-1 and SZJ-2, the lowest pH (SZJ-1: 3.43 ± 0.02; SZJ-2: 3.52 ± 0.02) was recorded on day 2, and the value gradually increased from days 3 to 12. A similar decrease in pH at the early stages of makgeolli fermentation and its return to the baseline value by the end of fermentation have also been observed by Kim and Yi (2010). Interestingly, the pH of makgeolli fermented by Sz. japonicus was significantly lower than that of makgeolli fermented by S. cerevisiae.
Furthermore, the final acidity of makgeolli fermented by Sz. japonicus was 6.02 ± 0.03 mL for SZJ-1 and 6.25 ± 0.03 mL for SZJ-2, indicating significantly higher values than those for S. cerevisiae (4.42 ± 0.12 mL). Immediately after the start of makgeolli fermentation, the total acid content is mainly derived from the raw material or nuruk. However, as fermentation progresses, various organic acids are produced by yeasts involved in makgeolli fermentation, resulting in increased total acid content of the makgeolli mash (Song et al., 1997). The organic acid profiles of the final makgeolli product were analyzed using HPLC. Contents of organic acids, including malic, lactic, citric, succinic, and tartaric acid, were determined (Fig. S3), and two important observations were made. First, Sz. japonicus-fermented makgeolli contained more organic acids than S. cerevisiae-fermented makgeolli, and second, the lactic acid content of Sz. japonicus-fermented makgeolli (0.24% for SZJ-1 and 0.23% for SZJ-2) was significantly higher than that of S. cerevisiae-fermented makgeolli (0.03%). Lactic acid production during alcohol fermentation has been reported in a previous study using an alternative starter in LAB-free sour beer (Kara et al., 2017). To the best of our knowledge, the present study is the first to report that makgeolli brewed using an alternative yeast, Sz. japonicas, contained relatively high amounts of lactic acid. In addition, significant differences were noted in succinic acid content, with values for SZJ-1 (0.08%) and SZJ-2 (0.09%) being markedly higher than those for S. cerevisiae makgeolli (0.05%). Since the use of Sz. japonicus strains in makgeolli fermentation was the first attempt, to investigate fermentation metabolites, these strains warrant further studies in greater detail in the near future.
Volatile compounds in makgeolli brewed using Sz. japonicus
To verify differences in flavor between makgeolli brewed using Sz. japonicus and S. cerevisiae, volatile compounds in both types of makgeolli were analyzed (Table 2). As expected, ethanol content was clearly higher in S. cerevisiae-fermented makgeolli than in Sz. japonicus-fermented makgeolli. The characteristic volatile compounds were acetic acid, 3-methyl butanoic acid, 1-phenylethanone, 2-phenylethyl acetate, 2-phenylethanol, ethyl acetate, ethyl 3-methylbutanoate, ethyl 3-methylbutyl acetate, ethyl nonanoate, and ethyl decanoate.
The acetic acid content of SZJ-1- and SZJ-2-fermented makgeolli was slightly higher than that of S. cerevisiae-fermented makgeolli. Acetic acid production during alcoholic fermentation by yeasts belonging to the genus Schizosaccharomyces has been reported previously. A primary enological concern associated with the use of Schizosaccharomyces species is the significant production of acetic acid (> 1 g L−1) during lab-scale fermentation (Benito et al., 2015). Acetic acid negatively affects the flavor of alcoholic beverages; however, low levels of acetic acid generally produce better sensory profiles (Mylona et al., 2016). Therefore, the acetic acid content of Sz. japonicus-fermented makgeolli should not be a problem.
As an aromatic compound, 2-phenylethyl acetate is known as a volatile compound with a rose-like odor and honey flavor. The 2-phenylethyl acetate concentration of Sz. japonicus-fermented makgeolli was 2.5-fold higher than that of S. cerevisiae-fermented makgeolli. Additionally, ester compounds are more important than alcohols in terms of the flavor and taste of liquors. As a type of fatty acid ethyl esters, these compounds are considered a key aroma composite in Cheongju and beer (Yuda, 1976). Ethyl acetate was the major ester compound detected in SZJ-1- and SZJ-2-fermented makgeolli. Ethyl acetate is the most abundant ester compound in fermented alcoholic beverages, because it is a typical metabolite of yeast (Rosi and Bertuccioli, 1989). Moreover, ethyl acetate is a major volatile compound that imbues a fruity aroma to makgeolli, and it is the most abundant flavor compound in Schizosaccharomyces-fermented makgeolli. Ethyl butanoate is characterized by the aromas of passion fruit, mango, and pineapple. The ethyl butanoate content of Sz. japonicus-fermented makgeolli was threefold higher than that of S. cerevisiae-fermented makgeolli. Further, 3-methylbutyl acetate, or isoamyl acetate, is the key aroma component of Cheongju (Inoue et al., 2012) and makgeolli, and it is an important ester that produces banana and pear flavors. The peak area of 3-methylbutyl acetate was approximately two times higher for SZJ-1- and SZJ-2-fermented makgeolli than for S. cerevisiae-fermented makgeolli. Ethyl 3-methyl butanoate, which also has banana and pear-like aroma compounds, was detected only in Sz. japonicus-fermented makgeolli, but not in S. cerevisiae-fermented makgeolli. The content of ethyl octanoate, or ethyl caprylate, which contributes a sweet and apricot-like aroma to alcoholic beverages, was slightly higher in Sz. japonicus-fermented makgeolli than in S. cerevisiae-fermented makgeolli. Similarly, the content of ethyl nonanoate, which has a waxy and winey aroma, was approximately threefold higher in Sz. japonicus-fermented makgeolli than in S. cerevisiae-fermented makgeolli. Together, these results indicate that Sz. japonicus-fermented makgeolli presented completely different flavors compared with typical S. cerevisiae-fermented makgeolli. To confirm the sensory preference of consumers, analysis of flavor component composition and professional sensory evaluation of Schizosaccharomyces-fermented makgeolli are warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Institute of Biological Resources (Grant Numbers NIBR202102107 and NIBR202203112).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Juyong Choi and Sun-Young Park have contributed equally to this work.
Change history
12/11/2023
A Correction to this paper has been published: 10.1007/s10068-023-01498-5
References
- Andorrà I, Berradre M, Rozès N, Mas A, Guillamón JM, Esteve-Zarzoso B. Effect of pure and mixed cultures of the main wine yeast species on grape must fermentations. European Food Research and Technology. 2010;231:215–224. doi: 10.1007/s00217-010-1272-0. [DOI] [Google Scholar]
- Bal J, Yun SH, Yeo SH, Kim JM, Kim DH. Metagenomic analysis of fungal diversity in Korean traditional wheat-based fermentation starter nuruk. Food Microbiology. 2016;60:73–83. doi: 10.1016/j.fm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- Benito Á, Calderón F, Palomero F, Benito S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules. 2015;20:9510–9523. doi: 10.3390/molecules20069510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito S, Ruiz J, Belda I, Kiene F, Beisert B, Navascués E, Marquina D, Calderón F, Santos A, Rauhut D. Application of non-Saccharomyces yeasts in wine production. pp. 75–89. In: Non-conventional yeasts: From basic research to application. Sibirny A (ed). Springer, Cham, Switzerland (2019)
- Betteridge A, Grbin P, Jiranek V. Improving Oenococcus oeni to overcome challenges of wine malolactic fermentation. Trends in Biotechnology. 2015;33:547–553. doi: 10.1016/j.tibtech.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Canonico L, Zannini E, Ciani M, Comitini F. Assessment of non-conventional yeasts with potential probiotic for protein-fortified craft beer production. LWT. 2021;145:111361. doi: 10.1016/j.lwt.2021.111361. [DOI] [Google Scholar]
- Cecchini F, Morassut M, Moruno EG, Di Stefano R. Influence of yeast strain on ochratoxin A content during fermentation of white and red must. Food Microbiology. 2006;23:411–417. doi: 10.1016/j.fm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Chalier P, Angot B, Delteil D, Doco T, Gunata Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chemistry. 2007;100:22–30. doi: 10.1016/j.foodchem.2005.09.004. [DOI] [Google Scholar]
- Cho HK, Lee JY, Seo WT, Kim MK, Cho KM. Quality characteristics and antioxidant effects during makgeolli fermentation by purple sweet potato-rice nuruk. Korean Journal of Food Science and Technology. 2012;44:728–735. doi: 10.9721/KJFST.2012.44.6.728. [DOI] [Google Scholar]
- Quantification and characterization Domizio P, Liu Y, Bisson L, Barile D. Cell wall polysaccharides released during the alcoholic fermentation by Schizosaccharomyces pombe and S. japonicus. Food Microbiology. 2017;61:136–149. doi: 10.1016/j.fm.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harju S, Fedosyuk H, Peterson KR. Rapid isolation of yeast genomic DNA: Bust n'Grab. BMC Biotechnology. 2004;4:1–6. doi: 10.1186/1472-6750-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T, Iefuji H, Katsumata H. Characterization and isolation of mutants producing increased amounts of isoamyl acetate derived from hygromycin B-resistant sake yeast. Bioscience, Biotechnology, and Biochemistry. 2012;76:60–66. doi: 10.1271/bbb.110470. [DOI] [PubMed] [Google Scholar]
- Kara O, Justin A, Sara RM, David MN, Cody MR, Christopher S, Robert C, Justin M, Hongde L, Jason MT, Matthew LB. Primary souring: A novel bateria-free method for sour beer production. Food Microbiology. 2017;70:76–84. doi: 10.1016/j.fm.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Kim JY, Yi YH. pH, acidity, color, amino acids, reducing sugars, total sugars, and alcohol in puffed millet powder containing millet takju during fermentation. Korean Journal of Food Science and Technology. 2010;42:727–732. [Google Scholar]
- Kim M, Seo JA. Fermentation profiling of rice wine produced by Aspergillus oryzae KSS2 and Rhizopus oryzae KJJ39 newly isolated from Korean fermentation starter. Applied Biological Chemistry. 2021;64:1–7. doi: 10.1186/s13765-020-00582-2. [DOI] [Google Scholar]
- Kim JY, Kim D, Park P, Kang HI, Ryu EK, Kim SM. Effects of storage temperature and time on the biogenic amine content and microflora in Korean turbid rice wine. Makgeolli. Food Chemistry. 2011;128:87–92. doi: 10.1016/j.foodchem.2011.02.081. [DOI] [PubMed] [Google Scholar]
- Kim SH, Mun JY, Kim SY, Yeo SH. Quality characteristics of glutinous rice-Makgeolli fermented with Korean yeast (SC Y204 and Y283) isolated from Nuruk. Korean Journal of Food Preservation. 2018;25:874–884. doi: 10.11002/kjfp.2018.25.7.874. [DOI] [Google Scholar]
- Lee YJ, Choi YR, Lee SY, Park JT, Shim JH, Park KH, Kim JW. Screening wild yeast strains for alcohol fermentation from various fruits. Mycobiology. 2011;39:33–39. doi: 10.4489/MYCO.2011.39.1.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Kang YW, Jeong JH, Noh MJ, Ahn ES, Lee KH, Kim MH. Monitoring of ochratoxin in alcoholic beverages. Korean Journal of Food Science and Technology. 2012;44:235–239. doi: 10.9721/KJFST.2012.44.2.235. [DOI] [Google Scholar]
- Lee SJ, Kwon YY, Cho SW, Kwon HS, Shin WC. Effects of Ehwa Makgeolli containing oriental herbs on skin whitening and wrinkles. Journal of the Korean Society of Food Science and Nutrition. 2013;42:550–555. doi: 10.3746/jkfn.2013.42.4.550. [DOI] [Google Scholar]
- Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry. 1959;31:426–428. doi: 10.1021/ac60147a030. [DOI] [Google Scholar]
- Murtey MD, Ramasamy P. Sample preparations for scanning electron microscopy–Life sciences. pp. 161-185. In: Modern Electron Microscopy in Physical and Life Sciences. Janecek, M, Kral, R (Eds). IntechOpen, London, UK, (2016)
- Mylona A, Del Fresno J, Palomero F, Loira I, Bañuelos M, Morata A, Calderón F, Benito S, Suárez-Lepe JA. Use of Schizosaccharomyces strains for wine fermentation—Effect on the wine composition and food safety. International Journal of Food Microbiology. 2016;232:63–72. doi: 10.1016/j.ijfoodmicro.2016.05.023. [DOI] [PubMed] [Google Scholar]
- Park JH, Chung CH. Characteristics of takju (a cloudy Korean rice wine) prepared with nuruk (a traditional Korean rice wine fermentation starter), and identification of lactic acid bacteria in nuruk. Korean Journal of Food Science and Technology. 2014;46:153–164. doi: 10.9721/KJFST.2014.46.2.153. [DOI] [Google Scholar]
- Park N, Nguyen TTH, Lee GH, Jin SN, Kwak SH, Lee TK, Choi YH, Kim SB, Kimura A, Kim D. Composition and biochemical properties of L-carnitine fortified Makgeolli brewed by using fermented buckwheat. Food Science and Nutrition. 2018;6:2293–2300. doi: 10.1002/fsn3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaro L, Maioli F, Canuti V, Picchi M, Lencioni L, Mannazzu I, Domizio P. Schizosaccharomyces japonicus/Saccharomyces cerevisiae mixed starter cultures: New perspectives for the improvement of Sangiovese aroma, taste, and color stability. LWT. 2022;156:113009. doi: 10.1016/j.lwt.2021.113009. [DOI] [Google Scholar]
- Puligundla P, Smogrovicova D, Mok C, Obulam VSR. Recent developments in high gravity beer-brewing. Innovative Food Science and Emerging Technologies. 2020;64:102399. doi: 10.1016/j.ifset.2020.102399. [DOI] [Google Scholar]
- Rosi, I. and Bertuccioli, M. Esterase activity in wine yeasts. pp 206-211. In: Actualités Oenologiques 89. Dunod (ed). Technique and Documentation. Paris, France (1989)
- Schifferdecker AJ, Dashko S, Ishchuk OP, Piškur J. The wine and beer yeast Dekkera bruxellensis. Yeast. 2014;31:323–332. doi: 10.1002/yea.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo DH, Kim MS, Park CS, Choi HW, Sung JM, Park JD, Kum JS. Effect of millimeter waves on the microbiological and physicochemical properties of Korean rice wine Makgeolli. Food Science and Biotechnology. 2016;25:497–502. doi: 10.1007/s10068-016-0069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo SH, Park SE, Yoo SA, Lee KI, Na CS, Son HS. Metabolite profiling of Makgeolli for the understanding of yeast fermentation characteristics during fermentation and aging. Process Biochemistry. 2016;51:1363–1373. doi: 10.1016/j.procbio.2016.08.005. [DOI] [Google Scholar]
- Song JC, Park HJ, Shin WC. Changes of Takju qualities by addition of cyclodextrin during the brewing and aging. Korean Journal of Food Science and Technology. 1997;29:895–900. [Google Scholar]
- Tamang JP, Kailasapathy K. Fermented Foods and Beverages of the World. 1. Boca Raton, FL: CRC Press Inc; 2010. [Google Scholar]
- Vaughan-Martini A, Martini A. Schizosaccharomyces Lindner (1893). pp. 779-784. In: The Yeasts (5th ed). Kurtzman CP, Fell JW, and Boekhout T (eds). Elsevier, Amsterdam, Netherlands (2011)
- Yuda J. Volatile compounds from beer fermentation. Journal of Brewing Society of Japan. 1976;71:818–830. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.