Abstract
NLRP3 inflammasome is a multiprotein complex expressed in a variety of cells to stimulate the production of inflammatory factors. Activation of NLRP3 inflammasome depends on a complex regulatory mechanism, and its pro-inflammatory function plays an important role in pancreatic diseases. In this literature review, we summarize the activation mechanism of NLRP3 and analyze its role in each of the four typical pancreatic diseases. Through this article, we provide a relatively comprehensive summary to the researchers in this field, and provide some targeted therapy routes.
Subject terms: Inflammasome, Mechanisms of disease
Facts
Activation of NLRP3 inflammasome is thought to occur by three different pathways: the canonical pathway, the non-canonical and the alternative pathway.
NLRP3 inflammasome converts pro-IL-1β and pro-IL-18 into biologically active IL-1β and IL-18 by activating caspase-1 activity, which plays a pro-inflammatory role.
The pro-inflammatory mechanism of NLRP3 inflammatories is related to pyroptosis.
In the course of diabetes, NLRP3 inflammasome plays a pro-inflammatory role by disrupting β cell function.
The search for effective new drugs to inhibit the action of NLRP3 inflammasome is a promising direction for the future treatment of pancreatic diseases.
Open questions
What signals regulate the priming and activation of NLRP3 inflammasome?
What role does NLRP3 inflammasome play in acute and chronic pancreatitis?
Whether NLRP3 inflammasome plays a different role in pancreatic cancer than in inflammation?
Introduction
Since the 21st century, people have undoubtedly made significant progress in the field of pancreatic disease in pathophysiology, diagnosis, and treatment. However, the mortality rate of pancreatic disease has barely decreased over the past few decades, and its expensive consumption is also unacceptable [1]. With the development of molecular biology, more and more molecular mechanisms related to the pathogenesis of pancreatic disease have been studied.
The inflammasome is multiprotein complexes assembled by intracytoplasmic pattern recognition receptors (PRRs). After these pattern recognition receptors recognizing corresponding ligand, the downstream signaling pathway will be activated and promote the upregulation of inflammatory cytokines and chemokines, thereby regulating the inflammatory response. Among them, the leucine-rich repeat NOD-like receptors (NLRs), mainly expressed by myeloid cells, are the most common PRRs. Activation of the NLRP3 inflammasome in the NLRs family can trigger an inflammatory response.
The NLRP3 inflammasome has been found to express in various cells such as monocytes, neutrophils, dendritic cells, lymphocytes, osteoblasts, and epithelial cells. In immune cells, strong expression of NLRP3 was detected in neutrophils, monocytes, and dendritic cell, which are thought to be high producers of IL-1β. Interestingly, the expression of NLRP3 in neutrophils requires stimulation. NLRP3 was well expressed in B lymphocytes and T lymphocytes. The NLRP3 inflammasome plays a key role in the first line of defense against invading pathogens and possible allergens.
In recent years, researchers have found that there is a particular relationship between the occurrence and development of pancreatic diseases and inflammasomes [2]. Although there are more than one inflammasome associated with pancreatic diseases, such as absent in melanoma 2 (AIM2) inflammasome, which can also promote the release of IL-1β and IL-18 and lead to pancreatic inflammatory response. NLRP3, as a more widely studied inflammasome, is the main driving force of inflammation. Meanwhile, no article summarizes the relationship between pancreatic diseases and NLRP3 inflammasomes. We will review the effects of NLRP3 inflammasome on pancreas-related diseases (acute pancreatitis, diabetes, chronic pancreatitis, and pancreatic cancer), in order to provide insights into the pathogenesis of pancreatic diseases for researchers.
The structure and effect mechanism of NLRP3 inflammasome
Structure of NLRP3 inflammasome
NLRs generally consist of three parts: an N-terminal effector domain, a central nucleotide-binding domain (NBD/NOD/NACHT), and C-terminal leucine-rich repeats (LRR). Among NLRs, NACHT and LRR domains are the common features of all NLR proteins except NLRP10, but the N-terminal effector domains are variable. Therefore, the type and outcome of the regulation of NLRs activation can be influenced by the N-terminal effector domain. In all NLRs, N-terminal effector domains include pyrin domain (PYD) domains, caspase activation and recruitment domain (CARD) domains, and baculovirus inhibitory repeat domains. We can further subdivide NLR proteins based on various N-terminal effector domains.
The NLRP3 inflammasome is thought to be composed of an upstream receptor NLR family protein, an adapter protein named ASC (apoptosis-associated speck-like protein containing a CARD domain) acting as a bridge, and a downstream effector protein pro-caspase-1. The N-terminal effector domain of NLRP3 is the PYD domain, which is a typical PYD receptor protein. The N-terminus of the ASC interact with that of the NLR through the PYD domain to bind with each other and form a homotypic PYD: PYD. Furthermore, the C-terminal CARD is released from its self-inhibited conformation to recruit pro-caspase-1 through the homotypic CARD-CARD interaction, and aggregation of pro-caspase-1 results in autoproteolysis and generation of enzymatically active caspase-1 [3]. When pathogen-associated molecular patterns (PAMPs) or damage-associated molecular patterns (DAMPs) stimulate the receptor protein, it assembles and exerts its effect through the LRR-NACHT-PYD: PYD-CARD-CARD-pro-caspase 1 axis. High concentrations of pro-caspase 1 facilitates its heterodimerization, self-cleavage, and activation, thereby activating caspase-1 [4–6]. Active caspase-1 converts the cytokines pro-IL-1β and pro-IL-18 into the mature bioactive forms IL-1β and IL-18 [7].
The mechanism of action of NLRP3 inflammasome
The canonical pathway
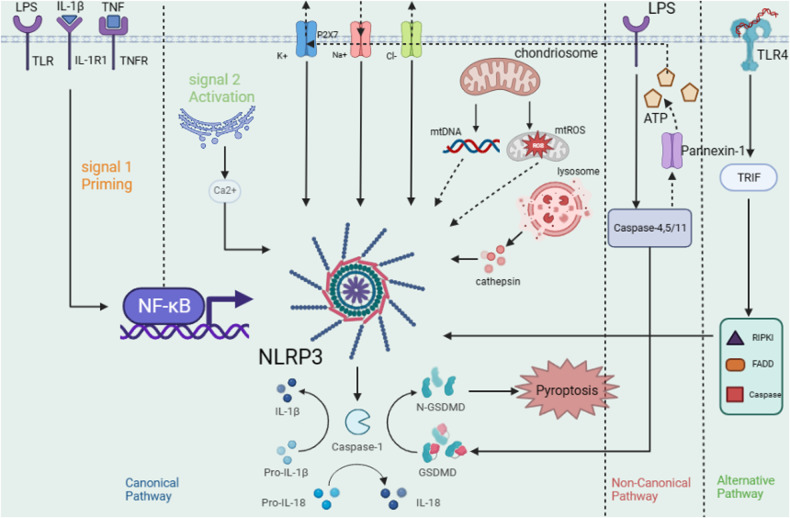
As Fig. 1 shows, the canonical pathway is generally divided into priming (signal 1) and activation (signal 2). The amount of NLRP3 in cells in a quiescent state is insufficient to activate the production of inflammasomes directly, and the levels of ASC and caspase-1 are stable. The canonical pathway holds that inflammasome cannot be produced by stimulating the body activation signal without the priming signal [8]. Typical priming signals (signal 1) receptors are interleukin 1 receptor type I (IL-1R1), Toll-like receptors (TLRs), and tumor necrosis factor receptor (TNFR). These receptor signals can lead to activation of the transcription factor NF-κB and upregulation of NF-κB-dependent expression levels of NLRP3 and pro-IL-1β [9].
Fig. 1. Activation pathway of NLRP3 inflammasome: generally, it can be divided into the canonical pathway; the non-canonical pathways and the alternative pathway.
In canonical pathway,there are two steps which can activate NLRP3 inflammasome. The ultimate goal of these three pathways is to promote the secretion of IL-18 and IL-1β from NLRP3 inflammasome.
NLRP3 can be indirectly activated by many pathogenic and sterile inflammatory signals. These signals include bacterial and viral PAMPs such as LPS and pathogen RNA [10]; pore-forming bacterial toxins such as melanin and gramin; extracellular adenosine triphosphate (ATP), reactive oxygen species, heme [11], and various metabolic crystals [12]. These agonists trigger specific activation of NLRP3, assembly of the inflammasome complex, and ultimately caspase-1 activation. Three significant signals that have been proven to activate the NLRP3 inflammasome include four ion fluxes, mitochondrial dysfunction and reactive oxygen species (ROS) production, and lysosomal damage.
K+ efflux is the earliest known and widely recognized NLRP3 activator [13]. Some familiar NLPR3 activating substances such as melanin, gramin, valinomycin, ATP, granule molecules, and cry can promote K+ efflux [14]. However, substances such as CL097 and imiquimod can promote the activation of NLRP3 inflammasomes by inducing the production of ROS rather than the potassium efflux mechanism [15]. This shows that K+ efflux is not necessary for NLRP3 activation but is still important. Increased cytosolic Ca2+ is especially significant for NLRP3 inflammasome activation, as inhibition of endoplasmic reticulum (ER) or plasma membrane Ca2+ channels attenuates caspase-1 activation and IL-1β secretion in response to NLRP3 stimulation. At the same time, the increase of Ca2+ can promote the interaction between NLRP3 and ASC and may affect the mitochondrial Ca2+ load, leading to mitochondrial dysfunction, generating mitochondrial ROS (mtROS), and thus affecting the activation of NLRP3 [16]. We believe that these two channels are important, but neither of them is indispensable in the activation of the NLRP3 inflammasome.
Na+ influx and Cl- efflux: Since Na+ influx alone cannot activate the NLRP3 inflammasome, therefore, the relevant literature is less descriptive. However, it has been shown that increased Na+ influx could regulate NLRP3 inflammasome activation by reducing the threshold of K+ efflux [14]. Chloride channels, including the volume-regulated anion channel (VRAC) and the chloride intracellular channel (CLIC), are thought to regulate NLRP3 inflammasome activation by promoting NLRP3-Nek7 interaction [17]. Among these, CLIC-dependent chloride ion efflux is a downstream event of the potassium efflux-mitochondrial ROS axis, and CLIC-mediated chloride efflux can promote NEK7–NLRP3 interaction and subsequent ASC oligomerization. Nek7 is thought to be involved in forming or providing a common signal that acts upstream of NLRP3 and transmits that signal to its adaptation protein ASC. It may also mediate NLRP3 polymerization and point-like ASC aggregation [18]. On the other hand, through its function as a microtubule dynamics regulator, Nek7 may also facilitate the interaction of NLRP3 and ASC [19]. At the same time, decreased concentration of extracellular Cl- can enhance ATP-induced caspase-1 activation and IL-1β maturation and secretion [20].
Mitochondria are generally thought to influence inflammasome activation either through ROS generation or through interaction with NLRP3 inflammasome components [21]. Although mtROS and oxidized mtDNA (mitochondrial DNA) produced by dysfunctional mitochondria are widely believed to be essential activators of NLRP3 inflammasome activation. Iyer and associates found ROS production induces NLRP3 inflammasome activation in a manner dependent on mitochondrial dysfunction [22], suggesting that ROS production is not a requirement for inflammasome activation.
Some particulate or crystalline substances, such as silica, calcium crystals, and cholesterol crystals, are engulfed by cells through the destruction of lysosomes, resulting in the release of lysosomal contents, such as lysosomal cathepsin B, together with lysosomal calcium signaling to regulates the release of IL-1β, which induces the activation of NLRP3 inflammasome [23]. At the same time, the lysosomal damage-mediated release of cathepsins and other factors can also activate the NLRP3 inflammasome by acting on the cell membrane to cause K+ efflux.
Influence of pyroptosis on canonical pathways
Pyroptosis is characterized by dependence on inflammatory caspase (primarily caspase-1, 4, 5, 11), accompanied by the release of a large number of pro-inflammatory factors. Involves the formation of cell membrane pores mediated by gasdermin proteins, causing ion transport, accompanied by inflammation and immune responses. The ion imbalance causes cells to swell, dissolve, and release pro-inflammatory factors, including interleukin-1β (IL-1β), interleukin-18 (IL-18), ATP, and the high mobility group box 1 (HMGB1) protein.
NLRP3 inflammasome mediates pyroptosis through cleaving gasdermin (GSDM) proteins, while GSDMD is the main substrate of NLRP3 inflammasome-induced pyroptosis. GSDMD has an N-terminal prereforming domain and a C-terminal self-suppression domain [24]. After PAMPs and DAMPs trigger NLRP3, NLRP3 promotes self-cleavage of pro-caspase-1 to produce activated caspase-1, thereby transforming pro-IL-1β and pro-IL-18 into the mature bioactive forms of IL-1β and IL-18 [25]. The activated caspase‐1 splits the GSDMD protein molecule and initiates oligomerization of the 31 kDa amino terminal portion (GSDMD-N) to form the membrane pore. This promotes the secretion of inflammatory factors and cell infiltration.
Non-canonical and alternative pathways
Non-canonical activation of the NLRP3 inflammasome is primarily initiated by the LPS of Gram-negative bacteria, which can be recognized by caspase-11 in mice (or caspase-4/5 in humans) through direct interaction, leading to caspase -11 autoproteolysis and activation. Activated caspase-11 also cleaves GSDMD to induce membrane pore formation and pyroptosis. The GSDMD protein is cut into two independent domain fragments at the N-terminus and C-terminus [26]. This leads to changes in cell osmotic pressure that lead to cell membrane rupture and cell death. Unlike canonical pathways, caspase-1 can process pro-IL-1β and pro-IL-18, but caspase-11 cannot [27]. In addition to GSDMD, it can also activate pannexin-1 through caspase-11 to release ATP to activate the P2X7 receptor (P2X7R) [28]. P2X7R, acting as an ATP-gated cation-selective channel, opens a pore that triggers K+ efflux and Induces K+ efflux, which drives NLRP3 inflammasome assembly and IL-1β release.
The alternative pathway is a newly proposed concept of inflammasome activation, which is considered having no concern with K+ efflux and induction of apoptosis. A recent study suggested that this NLRP3 activation is induced through the RIPK1, FADD, and caspase-8 pathways downstream of TLR4-TRIF signaling [29]. Similarly, Gaidt also proposed that TLR4-TRIF-RIPK1 - FADD-CASP8 signaling is involved in this alternative pathway to promote NLRP3 inflammasome activation, so they speculate that caspase-8-mediated activation of an unknown intermediate protein is indispensable for alternative inflammasome activation [30].
The role of NLRP3 inflammasome in acute pancreatitis
The pro-inflammatory mechanism of NLRP3 inflammasome in acute pancreatitis
The mechanism of acute pancreatitis (AP) is the self-digestion of pancreatic tissue caused by the increase in the level of pancreatic enzymes or premature activation caused by various reasons, which leads to pancreatic acinar cells damaged. IL-1β and IL-18 are typical cytokines that promote pancreatic inflammation, but because they are synthesized as precursor proteins, they depend on the proteolytic activity of caspase-1 to produce their bioactive forms. Here again, NLRP3 is required to convert caspase-1 into an activated state. The mechanism of NLRP3 inflammasome in pancreatitis can be regulated by the central transcription factor NF-κB on the expression of known pancreatitis-related genes such as pro-inflammatory factors IL1β, IL6, IL8, IL18, tumor necrosis factor α (TNFα) and chemokine MCP-1 [31].
IL1β, produced in response to activation of the NLRP3 inflammasome, can be transcribed by monocytes, macrophages, and dendritic cells, and is considered the main cytokine that mediates early inflammation and proliferation to extrapancreatic tissues during AP. IL-1β induces autophagy and reduces cell viability of pancreatic acinar cells by affecting calcium homeostasis and inducing trypsinogen activation in pancreatic acinar cells [32]. Similarly, IL18, expressed by macrophages, epithelial cells, and dendritic cells and stored in the cytoplasm, also plays a vital role in acute pancreatitis. In addition to being cleaved by caspase-1 to exert anti-inflammatory effects, IL18 can also function by linking IL18 receptor (IL18R) α and β, recruiting MyD88 and leading to the activation of NF-κB and mitogen-activated protein kinase (MAPK) [33]. Dual roles of IL-18 in T cell differentiation, IL-18 together with IL-12 enhances Th1 differentiation, but in the absence of IL-12, it induces Th2 cell responses. IL-18 can induce neutrophil migration and maturation during pancreatitis and plays a crucial role in activating endothelial cells [34].
Different from IL-1β and IL-18, although IL-33 is also involved in the pathway of inflammasome-induced pancreatitis, it does not require the activation of the inflammasome because caspase-1 will deactivate IL-33 [35]. Interestingly, similar to IL-18, IL-33 signaling activates the MyD88/Interleukin-1 receptor-associated kinases (IRAK)/TNF receptor-associated factor 6 (TRAF6) axis, activating downstream NF-κB and MAPK signaling pathways to promote NLRP3 production [36]. Nevertheless, some data show that IL-33 has a protective effect in AP [37, 38]. Therefore, its anti-inflammatory effect is not yet apparent, and further experiments are needed to confirm it.
Factors affecting the effect of NLRP3 in acute pancreatitis
Related DAMP signals
Associated DAMP signals, which includes HMGB1, heatshockprotein70 (HSP70), ATP, and mtDNA, induce acute pancreatitis by affecting the NLRP3 inflammasome. HMGB1 is a widely distributed and highly conserved nuclear protein that may function as a DAMP to exacerbate pancreatitis damage through TLR4 and TLR9. Among them, TLR4 leads to the release of mtDNA during pancreatic injury, thereby accelerating the activation of NLRP3 inflammasome [39], which in turn induces acute pancreatitis. In recent years, studies have also found that HMGB1 can contribute to pancreatic damage by activating the neutrophil extracellular trap and then inducing the processing and expression of IL-1β in the pancreas [40].
As a typical protein, HSP70 also affects the inflammasome pathway. Asea found that extracellular HSP70 interacts with TLR and CD14 and regulates the immune response through the MyD88/IRAK/NF-κB signal transduction pathway which is similar to pathway of NLRP3-induced pancreatitis [41]. Song has found that recombinant HSP70 can aggravate AP in a TLR4-dependent manner in a mouse model [42].
Extracellular ATP released from damaged cells interacts with P2X7 to induce mitochondrial dysfunction, and P2X7 acts as an activator of the NLRP3 inflammasome, thus leading to inflammasome production, caspase-1 activation and secretion of IL1β and IL18 [3]. Intracellular and extracellular mtDNA play different roles. Intracellular mtDNA is easily oxidized and transferred to the cytoplasm, where it directly binds to NLRP3 to activate the NLRP3 inflammasome; extracellular mtDNA acts as a DAMP to participate in the initiation and activation of the NLRP3 inflammasome [43].
Cathepsins
Cathepsins are thought to induce acute pancreatitis by affecting the NLRP3 inflammasome. Lysosome-released cathepsin B (CTSB) is a cysteine protease localized in lysosomes [44]. Increaseing expression of activated CTSB aggravates injury and inflammation of pancreatic tissue. During the development of AP, CTSB not only activates the NLRP3 inflammasome pathway, which in turn induces caspase-1 activation and subsequent IL-1β, and IL-18 secretion, leading to inflammation, but also induces acinar cell death through pyroptosis, aggravating pancreatitis [45]. Furthermore, the release of cathepsins and other factors mediated by lysosomal damage can also activate the NLRP3 inflammasome by acting on the cell membrane to cause potassium efflux.
Inflammasome pathway inhibitors in experimental acute pancreatitis
Since inflammasomes plays an essential role in the occurrence and development of acute pancreatitis, inhibiting the formation of inflammasomes and their pathways and pro-inflammatory effector molecules has become a hotspot in the treatment of experimental acute pancreatitis (Table 1).
Table 1.
NLRP3 inflammasome pathway therapy for experimental acute pancreatitis.
| DRUG | TYPE | THERAPY PATHWAY | PMID |
|---|---|---|---|
| Annakinra | Recombinant IL1 receptor antagonist anakinra | Inhibit IL-1 | 24912987 |
| Rilonacept | Soluble IL-1β receptor | Weaken the function of IL-1β | 20074281 |
| Canakinumab | IL-1β monocolonal antibody | Weaken the function of IL-1β | 26284424 |
| MCC950 | Sulfonylureas | Eliminate the oligomerization of ASC | 31593700 |
| Emodin | Natural plant extract | Inhibit P2X7/NLRP3 pathway | 20572302 |
| Danshensu | Natural plant extract | Inhibit NLRP3 inflammasome, NF-κB pathway, and STAT3 pathway | 30425719 |
| Fraxinellone | Natural plant extract | Inhibit caspase-1, IL-1β and NLRP3 | 30716587 |
| Withferin A | Natural plant extract | Inhibit NF-κB and the activation of NLRP3 inflammasome | 27418337 |
| Rutin | Natural plant extract | Decrease the expression levels of ASC, TNF-α, and IL-1β, and inhibit the activation of caspase-1 | 25060908 |
| Cordycepin | Natural plant extract | Inhibit the activation of NF-κB and NLRP3 inflammasome | 32268154 |
| Apocynin | Natural plant extract | Inhibit the activation of NLRP3, pro-caspase-1, and IL-1β | 31437787 |
| Indomethacin | Inhibitor of COX-2 | Inhibit the activation of NLRP3 inflammasome | 28867183 |
| Iguratimod | Inhibitor of COX-2 | Inhibit NF-κB pathway and activation of NLRP3 inflammasome | 31541854 |
| INT-777 | Agonist of bile acid receptor | Block out ROS/NLRP3 pathway | 29859191 |
| Butyrate | Butyrate | Inhibit the activation of NLRP3 inflammasome | 31347703 |
| β-hydroxubutyric | Water-soluble ketone body | Decrease the expression levels of caspase-1, IL-1β, and NLRP3 | 34528884 |
| Vancomycin, neomycin, and polymyxin-b | Antibiotic | Inhibiting the pancreatic NLRP3 pathway | 31615312 |
| 3,4-DAA | O-aminobenzoic acid derivatives | Inactivating NF-κB and NLRP3 inflammasome signaling pathway | 33319359 |
IL1 antagonists
Three typical IL-1 antagonists have been used clinically, namely: the recombinant IL-1 receptor antagonist Anakinra, the soluble decoy IL-1β receptor Rilonacept, and the neutralizing IL-1β antibody Canakinumab. These drugs can inhibit inflammation by inhibiting inflammasome generate an active IL family. The most typical drug is Annakinra, which can significantly reduce coronary protein-related pancreatic tissue damage and pancreatic apoptosis in rats [46]. This was the first IL-1 inhibitor designed and was approved by the Food and Drug Administration (FDA) for the treatment of patients in 2001 [47], but it has not been documented for the treatment of acute pancreatitis. The use of such drugs to prevent pancreatitis is still in the animal testing stage.
MCC950
MCC950, as the most effective selective inhibitor of NLRP3 inflammasome [48], reduces the formation of NLRP3 inflammasome mainly by eliminating ASC oligomerization. MCC950 has the ability to block NLRP3 inflammasome activation and IL-1β production by abolishing ASC oligomerization. And has also been proven to not only block local and systemic immune responses but also alleviate the severity of the disease [34].
Natural plant extracts
Some natural plant extracts have also been applied to block NLRP3 inflammasomes, among which the typical one is Emodin, which can delay the progression of AP by inhibiting P2X7/NLRP3 signaling pathway, thereby ameliorating the associated systemic inflammation [49]. As a flavonoid with anti-inflammatory, anti-allergic, anti-viral, and antioxidant properties, Rutin inhibits the autoactivation of trypsin and inhibits caspase-1 by attenuating the expression of ASC, TNF-α and IL-1β activation of NLRP3 inflammasomes, which can reduce pancreatic inflammation [50]. Danshensu, a Chinese herb, can inhibit the inflammatory response of AP by inhibiting the activation of NF-κB, STAT3, and NLRP3 inflammasome [51].
Cyclooxygenase-2 inhibitors
In recent years, it has been found that cyclooxygenase-2 (COX-2) inhibitor is one of the effective regulators of inflammatory response and NLRP3 inflammasome activation [52]. Indomethacin, the most common non-steroidal anti-inflammatory drug (NSAIDs) and cyclooxygenase-2 (Cox-2) inhibitor, can inhibit the activation of NLRP3 inflammasome and inflammatory response, and reduce the expression of IL-1β to protect the pancreas from damage [53]. Another COX-2 inhibitor, Iguratimod (T-614), was confirmed to inhibit the NF-κB signaling pathway and NLRP3 inflammasome activity [54].
Other substances that affect the formation of NLRP3 inflammasome
Some other substances can also affect the formation of NLRP3 inflammasome in pancreatitis. For example, the bile acid receptor agonist INT-777 effectively relieves inflammation and pancreatic acinar cell damage by blocking the ROS/NLRP3 pathway; ABX combination therapy (vancomycin, neomycin, and polymyxin b) attenuated the activation of TLR4 and NLRP3 by inhibiting the translocation of gut bacteria to the pancreas, thereby suppressing uncontrolled diffuse inflammation in the pancreas and adjacent organs during AP; β-hydroxybutyric acid can reduce the activity of caspase-1 and inhibit the maturation of IL-1β, thereby inhibiting NLRP3 inflammasome [55]; 3,4-DAA and Butyrate have also been found to affect the formation of NLRP3 inflammasomes through different pathways [56, 57].
The role of NLRP3 inflammasome in diabetes
Although different types of diabetes have different etiologies, islet β cells damage is a common feature [58]. Recent studies have reported that the NLRP3 inflammasome is the critical substance in β cells dysfunction and death in type 1 and type 2 diabetes [59, 60]. NLRP3 can be activated by various substances such as glucose, uric acid, and cholesterol crystals and subsequently produce caspase-1, IL-18, and IL-1β, which promote islet inflammation, damage islet β cells, and reduce insulin secretion [61].
TXNIP/NLRP3/IL-1β signaling pathway mediates β cell apoptosis
It is well known that the dysfunction of pancreatic β cells in T2D patients is closely related to the increase of β cells apoptosis. It is a relatively recognized mechanism that the thioredoxin-interacting protein (TXNIP)/NLRP3/IL-1β signaling pathway mediates the apoptosis of β cells for NLRP3 inflammasome to induce T2DM diabetes. TXNIP is an important factor expressed in β cells and regulates β cells apoptosis, inflammation, and oxidative stress. β cells themselves are capable of producing IL-1β independently of any viral infection or immune-mediated process, and elevated glucose concentrations induce islet beta cells to secrete more IL-1β. IL-1β triggers apoptosis by reducing I-κB expression and activating the transcription factor NF-κB, inhibiting β cell function and promoting Fas receptor upregulation. In addition to acting as a mediator of glucose-induced β cell apoptosis, IL-1β may also be involved in controlling pancreatic insulin reserve [62]. In type 2 diabetes, when β cells fail to compensate, it decreases insulin signaling and insulin-dependent glucose uptake, resulting in persistent hyperglycemia. After that, islets trigger the induction of ROS through the NADPH oxidase system, glucose induces the expression of TXNIP, and ROS triggers the dissociation of TXNIP from thioredoxin (TXN), thereby triggering the TXNIP-dependent activation of the NLRP3 inflammasome, and finally leads to the secretion of mature IL1β. Elevated IL-1β is an important cause of β cell death and dysfunction and decreased insulin secretion, contributing to decreased insulin secretion and aggravating hyperglycemia [63].
Recent research showed that another NLRP3-related cytokine, IL-18, was also positively associated with insulin resistance and increased risk of T2DM. However, the role of IL-18 in T1D is controversial. Some studies have shown that IL-18 may promote T1D development by inducing diabetic T-cell expansion in mice [64]. Other studies have proved that T1D development does not require IL-18 [65].
Other NLRP3-related causes mediating β-cell apoptosis
In addition to TXNIP, the activation of NLRP3 inflammasome due to the increase of reactive oxygen species (ROS) production caused by other reasons is also one of the causes of diabetes. For example, in diabetic conditions, pancreatic β cell autophagic flux is blocked, resulting in intracellular accumulation of impaired organelles, generating a large amount of ROS, which in turn activates the NLRP3 inflammasome [66]. Donath found that advanced glycation end-products (AGEs) upregulate the protein expression level of the AGE pattern recognition receptor (RAGE), which can also increase ROS production, stimulate the activation of NLRP3 inflammasome, and lead to the activation of IL-1β. Subsequently, IL-1β secreted in the microenvironment exacerbates the chronic inflammatory response of islets [67]. Interestingly, administration of AGEs is also thought to significantly increase superoxide anion levels through upregulation of NADPH oxidase 2 (NOX2) protein, which generates increased expression levels of TXNIP and NLRP3 inflammasome components and protein interactions between TXNIP and NLRP3 [68], so we speculate that the two pathways leading to diabetes may be carried out together.
We found that there are a number of clinical factors affecting NLRP3 that have led to the study of diabetes. Youm’s study showed that pancreatic β cells in obese NLRP3-deficient mice were protected from inflammatory damage induced by a high-fat diet and were able to compensate by increasing insulin levels when blood sugar levels were higher than normal. At the same time, mtROS increases during obesity, and reactive oxygen species participate in the assembly of NLRP3 inflammasome. Mitochondrial dysfunction may affect the activation of NLRP3 inflammasome, leading to pancreatic injury in obese patients. Elimination of the NLRP3 inflammasome protects pancreatic β cells from cell death caused by prolonged high-fat feeding during obesity, and activation of the NLRP3 inflammasome in diet-induced obesity is a key trigger for pancreatic damage and an important mechanism for the progression of type 2 diabetes [69]. In addition, plasma IL-18 induced by NLRP3 was also positively associated with insulin resistance and an increased risk of type 2 diabetes [70]. Some substances, such as IAPP [71] and crystalline substances [72], were identified as possible triggers of inflammasome activation in type 2 diabetes.
Other points of view
Although most experiments have shown that NLRP3 plays an important role in the development of diabetes, some believe that the absence of NLRP3 does not affect blood glucose levels or the apoptotis of β cells, suggesting that the NLRP3 inflammasome does not contribute to β cell function loss [73]. Experiments by Gurzov showed that mouse islets incubated with IL-1β alone did not exhibit β cell apoptosis [74]. Therefore, whether NLRP3 comes into play in the course of diabetes needs to be further studied in the future.
The role of NLRP3 inflammasome in the treatment of diabetes
In treating diabetes, some substances related to NLRP3 inflammasome can inhibit the occurrence and development of diabetes in different ways (Table 2). We divide them into four categories.
Table 2.
Drugs that affect the development of diabetes by interfering with the NLRP3 inflammasome and its associated molecules.
| DRUG | TYPE | THERAPY PATHWAY | PMID |
|---|---|---|---|
| Arglabin | Natural compound | Inhibit NLRP3 by autophagy | 27044804 |
| Urolithin A | Natural metabolite | Activate autophagy to inhibit TXNIP/NLRP3 IL-1β inflammatory signal | 34656886 |
| Ginsenoside Rg1 | Traditional Chinese medicine | Reduce inflammatory factors IL-1β and IL-18, and weaken the function of NLRP3 | 31738935 |
| MCC950 | Sulfonylureas | Inhibit the activation of NLRP3 inflammasome and Ang-II, decrease of IL-1β, and decrease islet β cell apoptosis | 30939192 |
| Glyburide | Sulfonylureas | Inhibit the activation of NLRP3 inflammasome | 28703484 |
| Astragaloside-IV (AS-IV) | Traditional Chinese medicine | Inhibit the activation of NLRP3 inflammasome | 31558153 |
| Grape seed procyanidinB2 (GSPB2) | Natural plant extract | Regulating IL-1β and NLRP3 | 26207855 |
| Wu-Min-Wan | Traditional Chinese medicine | Decrease the expression levels of NLRP3, the adapter protein ASC, and caspase-1 | 30704457 |
| Oridonin (Ori) | Natural plant extract | Block the interaction between NLRP3 and NEK7, inhibit the activation of NLRP3 inflammasome | 33388733 |
TXNIP Inhibitors
TXNIP inhibitors are effective therapeutic agents for diabetes. Urolithin A, as a natural metabolite, significantly inhibited TXNIP expression, NLRP3 inflammasome activation, and IL-1β levels in β cells. Moreover, it inhibited glycolipid toxicity through the AMPK pathway and autophagy activation [63].
Inflammasome activation inhibitors
Arglabin, a natural product isolated from Artemisia glabella, controls inflammasome activation by inducing autophagy and inhibits the conversion of pro-IL-1β to bioactive IL-1β in a concentration-dependent manner in T2DM development Maturation pathway [70]. Astragalus (AS-IV) can suppress NLRP3 inflammasome activation in gestational diabetes pancreas by inhibiting the NF-κB pathway [75]. Wu-Mei-Wan, reduces the protein expression in NLRP3 as well reduces that of other inflammasome components, such as ASC and caspase-1 [76]. Oridonin (Ori) directly interacts with NLRP3 molecular binding, antagonism inhibits the function of the interaction between NLRP3 and NEK7, affects the activation of NLRP3 inflammasome [77]. In addition, the NLRP3 inflammasome inhibitor MCC950 is deemed to inhibit not only inflammasome activation but also Ang II-induced IL-1β elevation and apoptosis [78].
Inflammatory factor inhibitors
Some drugs improve the level of diabetes by lowering the concentration of inflammatory factors: Ginsenoside Rg1 was found to reduce the level of inflammatory factors IL-1β and IL-18 in the mouse T1DM model, promote insulin secretion, and weaken the function of NLRP3 in mouse liver and pancreas, is a potential drug for preventing the development and progression of T1DM [79].
Glyburide
Glyburide, also known as glibenclamide, is an NLRP3 inhibitor of sulfonylurea drugs, widely used in treating type 2 diabetes. It blocks potassium K+ channels on the membrane of pancreatic beta cells, preventing the efflux of K+ from cells [80]. At present, this drug has been widely used in the United States to treat T2D [81].
Role of the NLRP3 inflammasome in chronic pancreatitis and pancreatic ductal adenocarcinoma
In addition to acute pancreatitis and diabetes, NLRP3 inflammasome has also become the object of attention in the research of chronic pancreatitis (CP) and pancreatic cancer recently.
The role of NLRP3 in chronic pancreatitis
Similar to acute pancreatitis, Kanak demonstrated for the first time the role of the inflammasome in the pathogenesis of CP. Typical DAMPs such as HMGB-1 or ATP released by damaged acinar cells might lead to the assembly and activation of NLRP3 inflammasome, thereby exacerbating inflammation during CP. At the same time, it was proved that when the NF-κB pathway is suppressed, the inflammasome expression will be reduced, thereby reducing the severity of chronic pancreatitis [82].
Several drug studies targeting NLRP3 inflammasome therapy have been conducted in animal trials (Table 3). Similar to acute pancreatitis, Withaferin A (WA) acts as an inhibitor of NF-κB, blocking ER stress and NLRP3 inflammasome [82]. Total flavonoids from Psidium guajava leaves (TFPGL) significantly reduce inflammatory cell invasion and fibrosis. The expression of NLRP3 and caspase-1 was significantly decreased at both gene and protein levels. The expression of IL-1β and IL-18 was decreased [83]. P2X7R antagonist, Xiao Chai Hu Tang (XCHT) and Morus alba root bark (MEMARB) also inhibit chronic pancreatitis by decreasing the expression of NLRP3 in pancreas [84–86].
Table 3.
Drugs that affect the development of chronic pancreatitis by interfering with the NLRP3 inflammasome and its associated molecules.
| DRUG | TYPE | THERAPY PATHWAY | PMID |
|---|---|---|---|
| Withferin A | Natural plant extract | Block ER stress and NLRP3 inflammasome | 27418337 |
| Total flavonoids from Psidium guajava leaves (TFPGL) | Traditional medicinal plant | Decreasing the expression of NLRP3 and caspase-1 | 34961420 |
| Oxidized ATP (OxATP) | P2X7R antagonist | Decreasing the expression of NLRP3 and caspase-1 | 28930866 |
| Brilliant blue G (BBG) | P2X7R antagonist | Decreasing the expression of NLRP3 and caspase-1 | 28930866 |
| Xiao Chai Hu Tang (XCHT) | Traditional Chinese medicine | Decreasing the expression of NLRP3 | 36096349 |
| Morus alba root bark (MEMARB) | Traditional Asian medicine | Decreasing the expression of HSP70 and NLRP3-ASC | 30335608 |
The role of NLRP3 in pancreatic ductal adenocarcinoma
The role of NLRP3 in various cancers has been widely studied. Kantono found that the inflammasome is responsible for the complex biological mechanism of mediating inflammation in cancer cells [87]. NLRP3 is highly expressed in pancreatic ductal adenocarcinoma (PDA) cells and tissues, and knockdown of NLRP3 can reduce the proliferation, invasion and EMT (epithelial–mesenchymal transition) of cancer cells in vitro [88]. PDA may be related to NLRP3 polymorphism [89]. PDA is caused by chronic inflammation and driven by persistent inflammation associated with immunosuppressive CD4+ T cells. NLRP3 signaling drives CD4+ T cell differentiation into tumor-promoting type 2 helper T cells (Th2 cells), Th17 cells, and regulatory T cell populations, while inhibiting Th1 cell polarization and cytotoxic CD8+ T cell activation. Hu’s study showed that down-regulating NLRP3 inhibits PDA progression and reduces cell invasion induced by epithelial mesenchymal transformation [88]. An immunohistochemical and survival analyses showing that high NLRP3 expression was associated with lower survival and poorer prognosis in these patients, possibly due to an ineffective immune system response and increased tumor-promoted inflammation [90]. Research shows that inhibition of NLRP3 inflammasome activation by the specific NLRP3 antagonist MCC950 reduces cell viability in pancreatic cancer cells; however, the efficacy of MCC950 varies by cell type [91].
Das has confirmed that the TLR4/NLRP3/IL-1β inflammasome signaling axis might be the pathway through which it affects early pancreatic tumors. Tumor-derived IL-1β established an immunosuppressive cell population mediated by M2 macrophages, myeloid-derived suppressor cells, CD1dhiCD5+ regulatory B cells, and Th17 cells. When the absence of tumor cell-derived IL-1β signal in the tumor stroma enables CD8+ cytotoxic T cells to infiltrate and activate in the tumor, reducing the growth of pancreatic tumors, playing a significant part in the development and invasiveness of pancreatic ductal adenocarcinoma [88, 92]. IL-1β production by PDA-associated myeloid cells may also support tumor progression by promoting immune tolerance [92]. IL-18 can enhance the Th1 type immune response, thus producing a large amount of INF-γ and TNF-α, promoting chronic inflammation and forming the basis of tumor occurrence [93, 94]. Carbone conducted a study on 58 patients with pancreatic cancer and found that the expression of IL-18 in tumor tissues and serum was up-regulated, and the increase of serum IL-18 was associated with poor prognosis [95].
Conclusion
As important diseases in the digestive system, pancreas-related diseases have brought many troubles to human beings in treatment. The gradual in-depth research on the NLRP3 inflammasome has made the corresponding pathway and the molecules on the pathway therapeutic targets for pancreatic diseases. This work comprehensively reviews the research status, mechanism of action, and drugs used in related treatments of NLRP3 inflammasome in four specific pancreas-related diseases, hoping to provide a logical and comprehensive summary article for relevant personnel.
Through summarizing the existing literature, we found that although the role of NLRP3 inflammasome in acute pancreatitis and diabetes is still controversial, the general mechanism is precise. We found that NLRP3 studies in pancreatic cancer and chronic pancreatitis are still at the basic stage. This may be the hotspot of future research in this field. NLRP3 may also be a key factor in the transformation of inflammation and cancer in the pancreas.
Acknowledgements
We are indebted to our colleagues and mentors, past and present, for continuous discussions. Figure 1 was created with BioRender.com, so we are grateful for the site.
Author contributions
TL and QW wrote the manuscript. ZD improved the content of the article. LY designed the tables and JL designed the pictures. DX and XM revised the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82270598, 82170654) and Heilongjiang Province Education Science ' 14th Five-Year Plan 2022 Key Topics (GJB1422769).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tianming Liu, Qiang Wang.
Contributor Information
Xianzhi Meng, Email: mengxianzhi@hrbmu.edu.cn.
Dongbo Xue, Email: xuedongbo@hrbmu.edu.cn.
References
- 1.McNamara D. Pancreatic diseases. Aliment Pharmacol Ther. 2003;18:60–5. doi: 10.1046/j.0953-0673.2003.01731.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferrero-Andrés A, Panisello-Roselló A, Roselló-Catafau J, Folch-Puy E. NLRP3 inflammasome-mediated inflammation in acute pancreatitis. Int J Mol Sci. 2020;21:5386. doi: 10.3390/ijms21155386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoque R, Sohail M, Malik A, Sarwar S, Luo Y, Shah A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141:358–69. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broz P, von Moltke J, Jones JW, Vance RE, Monack DM. Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe. 2010;8:471–83. doi: 10.1016/j.chom.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chavarría-Smith J, Mitchell PS, Ho AM, Daugherty MD, Vance RE. Functional and evolutionary analyses identify proteolysis as a general mechanism for NLRP1 inflammasome activation. PLoS Pathog. 2016;12:e1006052. doi: 10.1371/journal.ppat.1006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Chen S, Ruan J, Wu J, Tong AB, Yin Q, et al. Cryo-EM structure of the activated NAIP2-NLRC4 inflammasome reveals nucleated polymerization. Science. 2015;350:404–9. doi: 10.1126/science.aac5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchi L, Warner N, Viani K, Nuñez G. Function of Nod-like receptors in microbial recognition and host defense. Immunol Rev. 2009;227:106–28. doi: 10.1111/j.1600-065X.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 9.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eigenbrod T, Dalpke AH. Bacterial RNA: an underestimated stimulus for innate immune responses. J Immunol. 2015;195:411–8. doi: 10.4049/jimmunol.1500530. [DOI] [PubMed] [Google Scholar]
- 11.Erdei J, Tóth A, Balogh E, Nyakundi BB, Bányai E, Ryffel B, et al. Induction of NLRP3 inflammasome activation by heme in human endothelial cells. Oxid Med Cell Longev. 2018;2018:4310816. doi: 10.1155/2018/4310816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Hauenstein AV. The NLRP3 inflammasome: mechanism of action, role in disease and therapies. Mol Asp Med. 2020;76:100889. doi: 10.1016/j.mam.2020.100889. [DOI] [PubMed] [Google Scholar]
- 13.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–9. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–53. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Groß CJ, Mishra R, Schneider KS, Médard G, Wettmarshausen J, Dittlein DC, et al. K(+) efflux-independent NLRP3 inflammasome activation by small molecules targeting mitochondria. Immunity. 2016;45:761–73. doi: 10.1016/j.immuni.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Camello-Almaraz C, Gomez-Pinilla PJ, Pozo MJ, Camello PJ. Mitochondrial reactive oxygen species and Ca2+ signaling. Am J Physiol Cell Physiol. 2006;291:C1082–1088. doi: 10.1152/ajpcell.00217.2006. [DOI] [PubMed] [Google Scholar]
- 17.Tang T, Lang X, Xu C, Wang X, Gong T, Yang Y, et al. CLICs-dependent chloride efflux is an essential and proximal upstream event for NLRP3 inflammasome activation. Nat Commun. 2017;8:202. doi: 10.1038/s41467-017-00227-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai H, Wang P, Zhang B, Dong X. Expression of the NEK7/NLRP3 inflammasome pathway in patients with diabetic lower extremity arterial disease. BMJ Open Diabetes Res Care. 2020;8:e001808. doi: 10.1136/bmjdrc-2020-001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmid-Burgk JL, Chauhan D, Schmidt T, Ebert TS, Reinhardt J, Endl E, et al. A genome-wide CRISPR (clustered regularly interspaced short palindromic repeats) screen identifies NEK7 as an essential component of NLRP3 inflammasome activation. J Biol Chem. 2016;291:103–9. doi: 10.1074/jbc.C115.700492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verhoef PA, Kertesy SB, Lundberg K, Kahlenberg JM, Dubyak GR. Inhibitory effects of chloride on the activation of caspase-1, IL-1beta secretion, and cytolysis by the P2X7 receptor. J Immunol. 2005;175:7623–34. doi: 10.4049/jimmunol.175.11.7623. [DOI] [PubMed] [Google Scholar]
- 21.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–5. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 22.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–23. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weber K, Schilling JD. Lysosomes integrate metabolic-inflammatory cross-talk in primary macrophage inflammasome activation. J Biol Chem. 2014;289:9158–71. doi: 10.1074/jbc.M113.531202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kayagaki N, Stowe IB, Lee BL, O'Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 26.Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Erratum: Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;540:150. doi: 10.1038/nature20106. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez MLG, Poreba M, Snipas SJ, Groborz K, Drag M, Salvesen GS. Extensive peptide and natural protein substrate screens reveal that mouse caspase-11 has much narrower substrate specificity than caspase-1. J Biol Chem. 2018;293:7058–67. doi: 10.1074/jbc.RA117.001329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccini A, Carta S, Tassi S, Lasiglié D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci USA. 2008;105:8067–72. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He Y, Franchi L, Núñez G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190:334–9. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–46. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Yu J, Ni L, Zhang X, Zhang J, Abdel-Razek O, Wang G. Surfactant protein D dampens lung injury by suppressing NLRP3 inflammasome activation and NF-κB signaling in acute pancreatitis. Shock. 2019;51:557–68. doi: 10.1097/SHK.0000000000001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu B, Bai B, Sha S, Yu P, An Y, Wang S, et al. Interleukin-1β induces autophagy by affecting calcium homeostasis and trypsinogen activation in pancreatic acinar cells. Int J Clin Exp Pathol. 2014;7:3620–31. [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee S, Bond JS. Prointerleukin-18 is activated by meprin beta in vitro and in vivo in intestinal inflammation. J Biol Chem. 2008;283:31371–7. doi: 10.1074/jbc.M802814200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sendler M, van den Brandt C, Glaubitz J, Wilden A, Golchert J, Weiss FU, et al. NLRP3 inflammasome regulates development of systemic inflammatory response and compensatory anti-inflammatory response syndromes in mice with acute pancreatitis. Gastroenterology. 2020;158:253–69.e214. doi: 10.1053/j.gastro.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 35.Lüthi AU, Cullen SP, McNeela EA, Duriez PJ, Afonina IS, Sheridan C, et al. Suppression of interleukin-33 bioactivity through proteolysis by apoptotic caspases. Immunity. 2009;31:84–98. doi: 10.1016/j.immuni.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Pinto SM, Subbannayya Y, Rex DAB, Raju R, Chatterjee O, Advani J, et al. A network map of IL-33 signaling pathway. J cell Commun Signal. 2018;12:615–24. doi: 10.1007/s12079-018-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouziel R, Gustot T, Moreno C, Arvanitakis M, Degré D, Trépo E, et al. The ST2 pathway is involved in acute pancreatitis: a translational study in humans and mice. Am J Pathol. 2012;180:2330–9. doi: 10.1016/j.ajpath.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sesti-Costa R, Silva GK, Proença-Módena JL, Carlos D, Silva ML, Alves-Filho JC, et al. The IL-33/ST2 pathway controls coxsackievirus B5-induced experimental pancreatitis. J Immunol. 2013;191:283–92. doi: 10.4049/jimmunol.1202806. [DOI] [PubMed] [Google Scholar]
- 39.Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763–74. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu X, Yang Z, Wang H, Zhao Y, Gao X, Zang B. High-mobility group box protein-1 induces acute pancreatitis through activation of neutrophil extracellular trap and subsequent production of IL-1β. Life Sci. 2021;286:119231. doi: 10.1016/j.lfs.2021.119231. [DOI] [PubMed] [Google Scholar]
- 41.Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–42. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 42.Song JM, Liu HX, Li Y, Zeng YJ, Zhou ZG, Liu HY, et al. Extracellular heat-shock protein 70 aggravates cerulein-induced pancreatitis through toll-like receptor-4 in mice. Chin Med J. 2008;121:1420–5. doi: 10.1097/00029330-200808010-00016. [DOI] [PubMed] [Google Scholar]
- 43.Qiu Y, Huang Y, Chen M, Yang Y, Li X, Zhang W. Mitochondrial DNA in NLRP3 inflammasome activation. Int Immunopharmacol. 2022;108:108719. doi: 10.1016/j.intimp.2022.108719. [DOI] [PubMed] [Google Scholar]
- 44.Mayerle J, Dummer A, Sendler M, Malla SR, van den Brandt C, Teller S, et al. Differential roles of inflammatory cells in pancreatitis. J Gastroenterol Hepatol. 2012;27:47–51. doi: 10.1111/j.1440-1746.2011.07011.x. [DOI] [PubMed] [Google Scholar]
- 45.Wang J, Wang L, Zhang X, Xu Y, Chen L, Zhang W, et al. Cathepsin B aggravates acute pancreatitis by activating the NLRP3 inflammasome and promoting the caspase-1-induced pyroptosis. Int Immunopharmacol. 2021;94:107496. doi: 10.1016/j.intimp.2021.107496. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan M, Yazgan Y, Tanoglu A, Berber U, Oncu K, Kara M, et al. Effectiveness of interleukin-1 receptor antagonist (Anakinra) on cerulein-induced experimental acute pancreatitis in rats. Scand J Gastroenterol. 2014;49:1124–30. doi: 10.3109/00365521.2014.926983. [DOI] [PubMed] [Google Scholar]
- 47.Mitroulis I, Skendros P, Ritis K. Targeting IL-1beta in disease; the expanding role of NLRP3 inflammasome. Eur J Intern Med. 2010;21:157–63. doi: 10.1016/j.ejim.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–55. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xia XM, Wang FY, Wang ZK, Wan HJ, Xu WA, Lu H. Emodin enhances alveolar epithelial barrier function in rats with experimental acute pancreatitis. World J Gastroenterol. 2010;16:2994–3001. doi: 10.3748/wjg.v16.i24.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aruna R, Geetha A, Suguna P. Rutin modulates ASC expression in NLRP3 inflammasome: a study in alcohol and cerulein-induced rat model of pancreatitis. Mol Cell Biochem. 2014;396:269–80. doi: 10.1007/s11010-014-2162-8. [DOI] [PubMed] [Google Scholar]
- 51.Ren Z, Li H, Zhang M, Zhao Y, Fang X, Li X, et al. A novel derivative of the natural product danshensu suppresses inflammatory responses to alleviate caerulein-induced acute pancreatitis. Front Immunol. 2018;9:2513. doi: 10.3389/fimmu.2018.02513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hua KF, Chou JC, Ka SM, Tasi YL, Chen A, Wu SH, et al. Cyclooxygenase-2 regulates NLRP3 inflammasome-derived IL-1β production. J Cell Physiol. 2015;230:863–74. doi: 10.1002/jcp.24815. [DOI] [PubMed] [Google Scholar]
- 53.Lu G, Pan Y, Kayoumu A, Zhang L, Yin T, Tong Z, et al. Indomethacin inhabits the NLRP3 inflammasome pathway and protects severe acute pancreatitis in mice. Biochem Biophys Res Commun. 2017;493:827–32. doi: 10.1016/j.bbrc.2017.08.060. [DOI] [PubMed] [Google Scholar]
- 54.Hou C, Zhu X, Shi C, Peng Y, Huang D, Li Q, et al. Iguratimod (T-614) attenuates severe acute pancreatitis by inhibiting the NLRP3 inflammasome and NF-κB pathway. Biomed Pharmacother. 2019;119:109455. doi: 10.1016/j.biopha.2019.109455. [DOI] [PubMed] [Google Scholar]
- 55.Şahin E, Bektur Aykanat NE, Kacar S, Bagci R, Sahinturk V. β-Hydroxybutyrate, one of the three main ketone bodies, ameliorates acute pancreatitis in rats by suppressing the NLRP3 inflammasome pathway. Turkish J Gastroenterol. 2021;32:702–11. doi: 10.5152/tjg.2021.191062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Z, Lu L, Li W. N-(3',4'-dimethoxycinnamonyl) anthranilic acid alleviates severe acute pancreatitis by inhibiting intestinal barrier dysfunction and NF-κB activation. Drug Dev Res. 2021;82:458–64. doi: 10.1002/ddr.21769. [DOI] [PubMed] [Google Scholar]
- 57.Pan X, Fang X, Wang F, Li H, Niu W, Liang W, et al. Butyrate ameliorates caerulein-induced acute pancreatitis and associated intestinal injury by tissue-specific mechanisms. Br J Pharm. 2019;176:4446–61. doi: 10.1111/bph.14806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:S97–107. doi: 10.2337/diabetes.54.suppl_2.S97. [DOI] [PubMed] [Google Scholar]
- 59.Lebreton F, Berishvili E, Parnaud G, Rouget C, Bosco D, Berney T, et al. NLRP3 inflammasome is expressed and regulated in human islets. Cell Death Dis. 2018;9:726. doi: 10.1038/s41419-018-0764-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hui Q, Asadi A, Park YJ, Kieffer TJ, Ao Z, Warnock GL, et al. Amyloid formation disrupts the balance between interleukin-1β and interleukin-1 receptor antagonist in human islets. Mol Metab. 2017;6:833–44. doi: 10.1016/j.molmet.2017.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill JR, Coll RC, Sue N, Reid JC, Dou J, Holley CL, et al. Sulfonylureas as concomitant insulin secretagogues and NLRP3 inflammasome inhibitors. ChemMedChem. 2017;12:1449–57. doi: 10.1002/cmdc.201700270. [DOI] [PubMed] [Google Scholar]
- 62.Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110:851–60. doi: 10.1172/JCI200215318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Aisker G, Dong H, Halemahebai G, Zhang Y, Tian L. Urolithin A suppresses glucolipotoxicity-induced ER stress and TXNIP/NLRP3/IL-1β inflammation signal in pancreatic β cells by regulating AMPK and autophagy. Phytomedicine. 2021;93:153741. doi: 10.1016/j.phymed.2021.153741. [DOI] [PubMed] [Google Scholar]
- 64.Oikawa Y, Shimada A, Kasuga A, Morimoto J, Osaki T, Tahara H, et al. Systemic administration of IL-18 promotes diabetes development in young nonobese diabetic mice. J Immunol. 2003;171:5865–75. doi: 10.4049/jimmunol.171.11.5865. [DOI] [PubMed] [Google Scholar]
- 65.Wen L, Green EA, Stratmann T, Panosa A, Gomis R, Eynon EE, et al. In vivo diabetogenic action of CD4 + T lymphocytes requires Fas expression and is independent of IL-1 and IL-18. Eur J Immunol. 2011;41:1344–51. doi: 10.1002/eji.201041216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Li YB, Yin JJ, Wang Y, Zhu LB, Xie GY, et al. Autophagy regulates inflammation following oxidative injury in diabetes. Autophagy. 2013;9:272–7. doi: 10.4161/auto.23628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Donath MY, Dalmas É, Sauter NS, Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 2013;17:860–72. doi: 10.1016/j.cmet.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Kong X, Lu AL, Yao XM, Hua Q, Li XY, Qin L, et al. Activation of NLRP3 inflammasome by advanced glycation end products promotes pancreatic islet damage. Oxid Med Cell Longev. 2017;2017:9692546. doi: 10.1155/2017/9692546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Youm YH, Adijiang A, Vandanmagsar B, Burk D, Ravussin A, Dixit VD. Elimination of the NLRP3-ASC inflammasome protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152:4039–45. doi: 10.1210/en.2011-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abderrazak A, El Hadri K, Bosc E, Blondeau B, Slimane MN, Büchele B, et al. Inhibition of the inflammasome NLRP3 by arglabin attenuates inflammation, protects pancreatic β-cells from apoptosis, and prevents type 2 diabetes mellitus development in ApoE2Ki mice on a chronic high-fat diet. J Pharmacol Exp Ther. 2016;357:487–94. doi: 10.1124/jpet.116.232934. [DOI] [PubMed] [Google Scholar]
- 71.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heller A, Jarvis K, Coffman SS. Association of type 2 diabetes with submicron titanium dioxide crystals in the pancreas. Chem Res Toxicol. 2018;31:506–9. doi: 10.1021/acs.chemrestox.8b00047. [DOI] [PubMed] [Google Scholar]
- 73.Wang J, Song MY, Lee JY, Kwon KS, Park BH. The NLRP3 inflammasome is dispensable for ER stress-induced pancreatic β-cell damage in Akita mice. Biochem Biophys Res Commun. 2015;466:300–5. doi: 10.1016/j.bbrc.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Gurzov EN, Germano CM, Cunha DA, Ortis F, Vanderwinden JM, Marchetti P, et al. p53 up-regulated modulator of apoptosis (PUMA) activation contributes to pancreatic beta-cell apoptosis induced by proinflammatory cytokines and endoplasmic reticulum stress. J Biol Chem. 2010;285:19910–20. doi: 10.1074/jbc.M110.122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang R, Zhang X, Xing B, Zhao J, Zhang P, Shi D, et al. Astragaloside IV attenuates gestational diabetes mellitus via targeting NLRP3 inflammasome in genetic mice. Reprod Biol Endocrinol. 2019;17:77. doi: 10.1186/s12958-019-0522-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang X, Lu F, Li L, Li J, Luo J, Zhang S, et al. Wu-Mei-wan protects pancreatic β cells by inhibiting NLRP3 Inflammasome activation in diabetic mice. BMC Complementary Altern Med. 2019;19:35. doi: 10.1186/s12906-019-2443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liang L, Zheng Y, Xie Y, Xiao L, Wang G. Oridonin ameliorates insulin resistance partially through inhibition of inflammatory response in rats subjected to chronic unpredictable mild stress. Int Immunopharmacol. 2021;91:107298. doi: 10.1016/j.intimp.2020.107298. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Feng Y, Huo H, Zhang X, Yue J, Zhang W, et al. NLRP3 inflammasome mediates angiotensin II-induced islet β cell apoptosis. Acta Biochim Biophys Sin. 2019;51:501–8. doi: 10.1093/abbs/gmz032. [DOI] [PubMed] [Google Scholar]
- 79.Gao Y, Li J, Chu S, Zhang Z, Chen N, Li L, et al. Ginsenoside Rg1 protects mice against streptozotocin-induced type 1 diabetic by modulating the NLRP3 and Keap1/Nrf2/HO-1 pathways. Eur J Pharm. 2020;866:172801. doi: 10.1016/j.ejphar.2019.172801. [DOI] [PubMed] [Google Scholar]
- 80.Zahid A, Li B, Kombe AJK, Jin T, Tao J. Pharmacological Inhibitors of the NLRP3 Inflammasome. Front Immunol. 2019;10:2538. doi: 10.3389/fimmu.2019.02538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riddle MC. Editorial: sulfonylureas differ in effects on ischemic preconditioning-is it time to retire glyburide? J Clin Endocrinol Metab. 2003;88:528–30. doi: 10.1210/jc.2002-021971. [DOI] [PubMed] [Google Scholar]
- 82.Kanak MA, Shahbazov R, Yoshimatsu G, Levy MF, Lawrence MC, Naziruddin B. A small molecule inhibitor of NFκB blocks ER stress and the NLRP3 inflammasome and prevents progression of pancreatitis. J Gastroenterol. 2017;52:352–65. doi: 10.1007/s00535-016-1238-5. [DOI] [PubMed] [Google Scholar]
- 83.Zhang G, Tang L, Liu H, Liu D, Wang M, Cai J, et al. Psidium guajava flavonoids prevent NLRP3 inflammasome activation and alleviate the pancreatic fibrosis in a chronic pancreatitis mouse model. Am J Chin Med. 2021;49:2001–15. doi: 10.1142/S0192415X21500944. [DOI] [PubMed] [Google Scholar]
- 84.Zhang GX, Wang MX, Nie W, Liu DW, Zhang Y, Liu HB. P2X7R blockade prevents NLRP3 inflammasome activation and pancreatic fibrosis in a mouse model of chronic pancreatitis. Pancreas. 2017;46:1327–35. doi: 10.1097/MPA.0000000000000928. [DOI] [PubMed] [Google Scholar]
- 85.Zhang G, Zhao X, Cai J, Li S, Li X, Li W, et al. XCHT alleviates the pancreatic fibrosis via VDR/NLRP3 signaling pathway in a mouse model of CP. J Ethnopharmacol. 2023;300:115689. doi: 10.1016/j.jep.2022.115689. [DOI] [PubMed] [Google Scholar]
- 86.Yuvaraj K, Geetha A. Effect of Morus alba root bark extract on gene-level expression of inflammatory markers in rats subjected to ethanol and cerulein induced pancreatitis-influence of heat shock protein 70. J Complement Integr Med. 2018;16. [DOI] [PubMed]
- 87.Kantono M, Guo B. Inflammasomes and cancer: the dynamic role of the inflammasome in tumor development. Front Immunol. 2017;8:1132. doi: 10.3389/fimmu.2017.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hu H, Wang Y, Ding X, He Y, Lu Z, Wu P, et al. Long non-coding RNA XLOC_000647 suppresses progression of pancreatic cancer and decreases epithelial-mesenchymal transition-induced cell invasion by down-regulating NLRP3. Mol Cancer. 2018;17:18. doi: 10.1186/s12943-018-0761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miskiewicz A, Szparecki G, Durlik M, Rydzewska G, Ziobrowski I, Górska R. The Q705K and F359L single-nucleotide polymorphisms of NOD-like receptor signaling pathway: association with chronic pancreatitis, pancreatic cancer, and periodontitis. Arch Immunol Ther Exp. 2015;63:485–94. doi: 10.1007/s00005-015-0355-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fraile-Martinez O, García-Montero C, Pekarek L, Saz JV, Alvarez-Mon M, Barrena-Blázquez S, et al. Decreased survival in patients with pancreatic cancer may be associated with an increase in histopathological expression of inflammasome marker NLRP3. Histol Histopathology. 2023;18617. [DOI] [PubMed]
- 91.Yaw ACK, Chan EWL, Yap JKY, Mai CW. The effects of NLRP3 inflammasome inhibition by MCC950 on LPS-induced pancreatic adenocarcinoma inflammation. J Cancer Res Clin Oncol. 2020;146:2219–29. doi: 10.1007/s00432-020-03274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Daley D, Mani VR, Mohan N, Akkad N, Pandian G, Savadkar S, et al. NLRP3 signaling drives macrophage-induced adaptive immune suppression in pancreatic carcinoma. J Exp Med. 2017;214:1711–24. doi: 10.1084/jem.20161707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mojtahedi Z. Interleukin (IL)-18 may enhance Th1 response in early cancer but aggravate malignant disease in its later stages. Med Hypotheses. 2005;65:995–6. doi: 10.1016/j.mehy.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 94.Rovina N, Hillas G, Dima E, Vlastos F, Loukides S, Veldekis D, et al. VEGF and IL-18 in induced sputum of lung cancer patients. Cytokine. 2011;54:277–81. doi: 10.1016/j.cyto.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 95.Carbone A, Vizio B, Novarino A, Mauri FA, Geuna M, Robino C, et al. IL-18 paradox in pancreatic carcinoma: elevated serum levels of free IL-18 are correlated with poor survival. J Immunother. 2009;32:920–31. doi: 10.1097/CJI.0b013e3181b29168. [DOI] [PubMed] [Google Scholar]