Abstract
Objective
To evaluate mepolizumab's efficacy in eosinophilic granulomatosis with polyangiitis (EGPA) with and without a vasculitic phenotype.
Methods
The MIRRA study (NCT02020889/GSK ID: 115921) included adults with relapsing/refractory EGPA and 4 or more weeks of stable oral glucocorticoids (OG). Patients received mepolizumab (300 mg subcutaneously every 4 weeks) or placebo, plus standard of care for 52 weeks. This post hoc analysis assessed EGPA vasculitic phenotype using antineutrophil cytoplasmic antibody (ANCA) history, baseline Birmingham Vasculitis Activity Score (BVAS), and Vasculitis Damage Index (VDI) score. Coprimary endpoints included accrued remission over 52 weeks and proportion in remission at Week 36 and Week 48. Remission was defined as a BVAS equal to 0 and an OG dose of 4 or more mg/day of a prednisone equivalent. Types of relapses (vasculitis, asthma, and sino‐nasal) and EGPA vasculitic characteristics (by study remission status) were also assessed.
Results
A total of 136 patients were included (n = 68, mepolizumab and placebo). Irrespective of history of ANCA positivity status, baseline BVAS, or baseline VDI, the accrued remission duration and the proportion of patients in remission at Weeks 36 and 48 were greater with mepolizumab compared with placebo. With mepolizumab, remission at both Week 36 and Week 48 was achieved by 54% of patients with and 27% of patients without a history of ANCA positivity compared with 0% and 4%, respectively (placebo); 45% of patients with a BVAS of 0 and 22% of patients with BVAS of greater than 0 compared with 5% and 2%, respectively (placebo); and 29% of patients with a VDI score of less than 5 and 37% of patients with a VDI score of 5 or more compared with 6% and 0%, respectively (placebo). Mepolizumab reduced all types of relapses as compared with placebo. Baseline vasculitic characteristics (neuropathy, glomerulonephritis, alveolar hemorrhage, palpable purpura, and ANCA positivity) were generally similar among patients with and without remission.
Conclusion
Mepolizumab is associated with clinical benefits for patients with and without a vasculitic EGPA phenotype.
INTRODUCTION
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare systemic inflammatory disorder associated with blood and tissue eosinophilia, asthma, and necrotizing vasculitis and granulomatous inflammation (1, 2). These characteristics often contribute to organ injury, impairment, and life‐threatening conditions (3, 4). EGPA can be divided into predominantly vasculitic or eosinophilic phenotypes based on clusters of often overlapping clinical features (5, 6). Common features of the vasculitic phenotype include the presence of palpable purpura, peripheral neuropathy, and glomerulonephritis (6). In contrast, the eosinophilic phenotype is more frequently characterized by lung infiltrates and cardiomyopathy and is often associated with elevated blood and tissue eosinophil counts (5, 6). As some vasculitis‐associated manifestations such as glomerulonephritis or palpable purpura occur more frequently in patients with a positive antineutrophil cytoplasmic antibody (ANCA) status, the vasculitic and eosinophilic phenotypes can be often inferred by ANCA status (5, 6). However, typically, only 30% to 40% of patients with EGPA have ANCA positivity (6). Of those with ANCA positivity, the majority (≥90%) have ANCA specific for myeloperoxidase (MPO), and MPO‐ANCA‐positive EGPA may have a genetic contribution distinct from ANCA‐negative EGPA (6, 7). Moreover, only a small minority of patients have proteinase 3 (PR3)‐positive ANCA (6, 8).
The standard treatment for EGPA consists of combination treatment with systemic glucocorticoids, immunosuppressants, cytotoxic therapy, and biologic therapy (9, 10, 11). However, immunosuppressant, glucocorticoid, and cytotoxic therapies are associated with considerable toxicity, and relapses are common despite continuous therapy (9, 12, 13, 14, 15, 16, 17, 18). In particular, side effects of glucocorticoid treatment include osteoporosis, fractures, infections, type 2 diabetes, and cardiovascular events, highlighting the need for glucocorticoid‐sparing therapy (13, 19).
Mepolizumab is a humanized monoclonal antibody that binds to and inactivates interleukin‐5 (IL‐5), leading to the depletion of eosinophils within the blood and tissues (20, 21). Mepolizumab is approved for the treatment of severe eosinophilic asthma, EGPA, hypereosinophilic syndrome, and chronic rhinosinusitis with nasal polyps across multiple regions worldwide (22, 23, 24). Because eosinophils can contribute to manifestations of both EGPA phenotypes, eosinophil‐targeting biologic therapy may be beneficial for patients with vasculitic or eosinophilic EGPA (6, 25). IL‐5 is a key cytokine responsible for the proliferation, maturation, activation, recruitment, and survival of eosinophils and is implicated in EGPA pathogenesis (26, 27, 28). In the Phase III MIRRA study, patients with relapsing or refractory EGPA receiving mepolizumab had a greater duration of disease remission and reduced use of oral glucocorticoids (OG) versus placebo (29). However, although the MIRRA study enrolled both patients with vasculitic and/or eosinophilic phenotypes of EGPA (29), the primary analysis was not designed to investigate the clinical benefits of mepolizumab in specific EGPA phenotypes.
The objective of this post hoc analysis was to evaluate the clinical benefits of mepolizumab by examining disease control and OG use in patients with and without a vasculitic EGPA phenotype, using data from the MIRRA study.
PATIENTS AND METHODS
Study design and patients
The design and eligibility criteria of the MIRRA study have been published previously (29). Briefly, MIRRA (GSK ID: 115921; NCT02020889) was a randomized, double‐blind, Phase III multicenter trial in which patients randomly received (1:1) mepolizumab (300 mg subcutaneously every 4 weeks) or placebo for 52 weeks, in addition to standard of care including OG with or without immunosuppressive therapy. From 4 weeks after baseline, a patient's OG dose could be reduced using a recommended schedule at the investigator's discretion. Patients using immunosuppressive therapy were required to maintain a stable dose throughout the trial.
Key patient eligibility criteria included being 18 years of age or older, having an EGPA diagnosis for 6 months or longer based on the presence of asthma and eosinophilia (>1000 cells/μL and/or >10% of leucocytes), and having two or more of the following additional features of EGPA: i) biopsy showing histopathological evidence of eosinophilic vasculitis, or perivascular eosinophilic infiltration, or eosinophil‐rich granulomatous inflammation; ii) neuropathy; iii) nonfixed pulmonary infiltrates; iv) sino‐nasal abnormality; v) cardiomyopathy; vi) glomerulonephritis; vii) alveolar hemorrhage; viii) palpable purpura; or ix) ANCA (MPO or PR3) positivity. Patients were also required to have a history of relapsing or refractory disease and be receiving a stable dose (≥7.5 to 50 mg/day) of OG for 4 weeks or longer before baseline. Relapsing disease was defined by one or more confirmed EGPA relapses (requiring an OG dose increase, initiation or increased dose of immunosuppressant therapy or intravenous immunoglobulin, or hospitalization) that occurred within the previous 2 years and that occurred 12 weeks or longer prior to screening while receiving 7.5 mg/day or more of a prednisone equivalent. Refractory disease was defined according to European League Against Rheumatism criteria (19) as failure to attain remission (Birmingham Vasculitis Activity Score [BVAS] equal to 0 and OG dose 7.5 mg/day or less of a prednisone equivalent) within the last 6 months following induction treatment with a standard regimen administered for 3 months or longer or within the 6 months prior to screening or as the recurrence of EGPA symptoms while tapering OG dose (≥7.5 mg/day of a prednisone equivalent). Patients were excluded if they had granulomatosis with polyangiitis or microscopic polyangiitis at screening, or organ‐ or life‐threatening EGPA in the 3 months before screening.
The trial was conducted in accordance with the ethical principles of the Declaration of Helsinki, the International Conference on Harmonisation Good Clinical Practice guidelines, and the applicable country‐specific regulatory requirements, and all the patients provided written informed consent.
Identification of patients with and without a vasculitic EGPA phenotype
Patients were classified as likely to have a vasculitic phenotype based on three separate markers: 1) history of ANCA positivity, 2) baseline BVAS, or 3) baseline Vasculitis Damage Index (VDI) score. A baseline, or previous, positive test for MPO/PR3‐ANCA was used to define patients more likely to have a vasculitic EGPA phenotype (6, 7), whereas no history of ANCA positivity identified those less likely to have a vasculitic EGPA phenotype. Likelihood of vasculitic EGPA was also defined by a baseline BVAS of greater than zero (30) (vs. BVAS = 0 for patients less likely to have vasculitic EGPA). Finally, the likelihood of vasculitic EGPA was defined by a baseline VDI score of 5 or greater (vs. <5 for patients less likely to have vasculitic EGPA) as VDI scores of 5 or greater indicate more severe vasculitic disease. (31)
Endpoints and assessments
The coprimary endpoints of the MIRRA study were the total accrued duration of remission and proportion of patients in remission at both Weeks 36 and 48. Remission was defined as a BVAS (version 3) of 0 (scale: 0 to 63, with higher scores indicating greater disease activity) (32) and an OG dose of 4.0 mg/day or less of a prednisone equivalent. Accrued duration of remission was categorized in weeks (0, >0 to <12, 12 to <24, 24 to <36, and ≥36 weeks).
Endpoints included in this post hoc analysis were as follows: assessment of differences in patient characteristics (focusing on vasculitic components of EGPA diagnostic disease characteristics [neuropathy, glomerulonephritis, alveolar hemorrhage, palpable purpura, and ANCA‐positive status]) by patient remission status (achieved/did not achieve remission at any time and accrued remission ≥36 weeks); assessment of mepolizumab versus placebo treatment effect on the coprimary endpoints in subgroups by ANCA status (current or previous positive test for MPO/PR3‐ANCA or no history of a positive MPO/PR3‐ANCA test at baseline). Prespecified analyses included baseline BVAS (score = 0 or >0) and VDI scores (<5 or ≥5); the proportion of patients with a relapse and number of relapse events (relapses were classified according to categories of vasculitis only, asthma only, sino‐nasal only, or combinations of categories [vasculitis/asthma, vasculitis/sino‐nasal, asthma/sino‐nasal, vasculitis/asthma/sino‐nasal], all relapses (included relapses without another category defined and respective combinations of categories), and categories with or without another relapse category defined; cumulative incidence of relapse over time for all relapses and according to type of relapse (asthma only, vasculitis only, sino‐nasal only); and total accrued duration of BVAS equal to 0 over 52 weeks and proportion of patients achieving a BVAS equal to 0 at both Weeks 36 and 48.
Although not specifically developed for EGPA, BVAS is validated for the assessment of 66 active vasculitis manifestations across nine organ systems (32). The VDI measures features of vasculitis due to persistent damage across 11 organ systems in patients with or without current disease activity (31).
For relapse categorization, a vasculitis relapse was defined as a BVAS of more than 0, an asthma relapse was defined as active asthma symptoms and/or signs with a corresponding worsening in Asthma Control Questionnaire (ACQ)‐6 score (compared with the most recent previous score), and sino‐nasal relapse was defined as active nasal and/or sinus disease with corresponding worsening in one or more of the sino‐nasal symptom questions (compared with the most recent previous assessment).
Statistical analysis
Accrued duration of remission was categorized in weeks (0, >0 to <12, 12 to <24, 24 to <36 and ≥36) and analyzed using a proportional odds model with covariates of treatment, baseline prednisone daily dose, and baseline BVAS (excluding baseline BVAS analysis) and region (excluding analysis by historical ANCA status). The proportion of patients in remission at both Weeks 36 and 48 was analyzed using a logistic regression model with covariates of treatment, baseline prednisone daily dose, baseline BVAS (excluding baseline BVAS analysis), and region. Exploratory analyses of the interaction between baseline BVAS or baseline VDI score and mepolizumab treatment (vs. placebo) were performed, with baseline BVAS as a continuous and categorical variable (score = 0 or >0) and VDI score as a categorical (score <5 or ≥5) variable.
RESULTS
Patient characteristics by remission status
Of the 136 patients randomized to treatment (68 to mepolizumab and 68 to placebo), 49 achieved remission at any point during the 52‐week treatment period (36 mepolizumab, 13 placebo), and 11 patients accrued remission for 36 weeks or longer (9 mepolizumab, 2 placebo).
A similar proportion of patients (mepolizumab treated and placebo treated) who achieved and did not achieve remission had vasculitic manifestations of EGPA, as indicated by similar proportions of patients in each group who had a history or presence of neuropathy (39% and 43%), glomerulonephritis (0% and 1%), alveolar hemorrhage (4% and 2%), palpable purpura (12% and 13%) and a history of being ANCA positive (24% and 16%; Table 1). Of the seven patients in the overall population who were ANCA positive at screening, six were MPO‐ANCA positive, and one was PR3‐ANCA positive. For patients who did and did not accrue 36 weeks of remission or longer, results were generally similar, except that a higher proportion of patients who accrued 36 weeks of remission or longer had a history or presence of neuropathy compared with those who did not (64% and 39%; Table 1).
Table 1.
Patient characteristics by remission status
| Achieved remission at any time | Accrued ≥36 wk remission | |||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| (n = 49) | (n = 87) | (n = 11) | (n = 125) | |
| Age, y, mean (SD) | 47.7 (14.62) | 48.9 (12.64) | 51.6 (11.64) | 48.2 (13.49) |
| Female, n (%) | 28 (57) | 52 (60) | 7 (64) | 73 (58) |
| EGPA disease duration (y), mean (SD) | 5.9 (4.27) | 5.4 (4.83) | 4.8 (3.16) | 5.6 (4.74) |
| Patients with a history or presence of EGPA diagnostic features, n (%) | ||||
| Asthma with eosinophilia | 49 (100) | 87 (100) | 11 (100) | 125 (100) |
| Biopsy a | 18 (37) | 38 (44) | 3 (27) | 53 (42) |
| Neuropathy, mono or poly b , c | 19 (39) | 37 (43) | 7 (64) | 49 (39) |
| Pulmonary infiltrates, nonfixed | 32 (65) | 66 (76) | 8 (73) | 90 (72) |
| Sino‐nasal abnormality | 46 (94) | 82 (94) | 10 (91) | 118 (94) |
| Cardiomyopathy d | 6 (12) | 14 (16) | 1 (9) | 19 (15) |
| Glomerulonephritis d , e | 0 | 1 (1) | 0 | 1 (<1) |
| Alveolar hemorrhage e , f | 2 (4) | 2 (2) | 1 (9) | 3 (2) |
| Palpable purpura b | 6 (12) | 11 (13) | 2 (18) | 15 (12) |
| ANCA positive (MPO or PR3) b | 12 (24) | 14 (16) | 4 (36) | 22 (18) |
| History of relapsing or refractory disease, n (%) | ||||
| ≥1 relapse in prior 2 y | 36 (73) | 64 (74) | 10 (91) | 90 (72) |
| Refractory disease | 26 (53) | 48 (55) | 2 (18) | 72 (58) |
| Failed induction treatment | 1 (2) | 5 (6) | 0 | 6 (5) |
| Recurrence of EGPA symptoms while tapering oral glucocorticoid, n (%) | 25 (51) | 43 (49) | 2 (18) | 66 (53) |
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; EGPA, eosinophilic granulomatosis with polyangiitis; MPO, myeloperoxidase; MRI, magnetic resonance imaging; PR3, proteinase 3; SD, standard deviation.
A biopsy showing histopathological evidence of eosinophilic vasculitis, or perivascular eosinophilic infiltration, or eosinophil‐rich granulomatous inflammation.
Characteristics considered to be indicative of vasculitic EGPA.
Motor deficit or nerve conduction abnormality.
Established by echocardiography or MRI.
Hematuria, red cell casts, proteinuria.
Established by bronchoalveolar lavage.
Remission by historical ANCA status, baseline BVAS, and baseline VDI score
Of the 136 patients in the overall study population, 26 (19%) had a history of a positive ANCA test, 85 (63%) had a baseline BVAS of more than 0, and 62 (46%) had a baseline VDI score of 5 or greater, which were used as markers of vasculitic EGPA. Irrespective of history of ANCA positive status, baseline BVAS (=0 or >0), or baseline VDI score (<5 or ≥5), patients treated with mepolizumab accrued more time in remission versus those who received placebo (Figure 1; Table 2).
Figure 1.
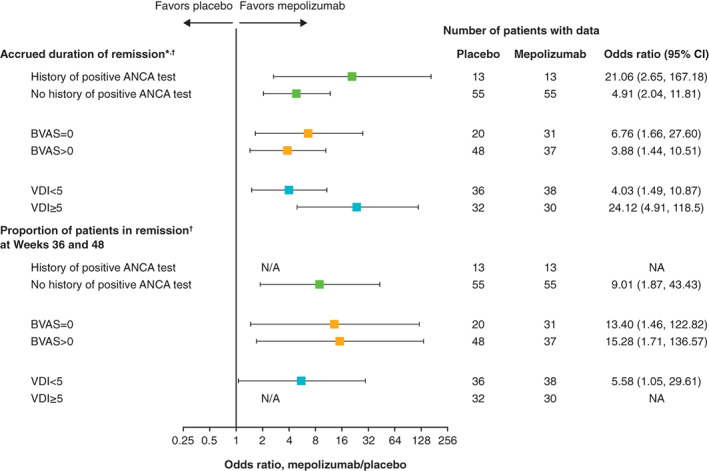
Accrued duration of remission and proportion of patients in remission at Weeks 36 and 48 by historical ANCA status, baseline BVAS, and baseline VDI score. *Accrued number of weeks of remission over the 52‐week study period categorized in weeks (0, >0 to <12, 12 to <24, 24 to <36, and ≥36 weeks). †Remission was defined as BVAS equal to 0 and OG dose 4 mg/day or more of a prednisolone equivalent. ANCA, antineutrophil cytoplasmic antibody; BVAS, Birmingham Vasculitis Activity Score; CI, confidence interval; NA, not available—estimate could not be calculated because no patients in the placebo group achieved remission at Weeks 36 and 48; VDI, Vasculitis Damage Index.
Table 2.
Analysis of remission in patients stratified by historical ANCA status, baseline BVAS, and baseline VDI score
| History of positive ANCA test | Baseline BVAS | Baseline VDI score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | BVAS = 0 | BVAS > 0 | VDI < 5 | VDI ≥ 5 | |||||||
| PBO | Mepo | PBO | Mepo | PBO | Mepo | PBO | Mepo | PBO | Mepo | PBO | Mepo | |
| (n = 13) | (n = 13) | (n = 55) | (n = 55) | (n = 20) | (n = 31) | (n = 48) | (n = 37) | (n = 36) | (n = 38) | (n = 32) | (n = 30) | |
| Accrued duration of remission a , b (wk), n (%) | ||||||||||||
| 0 | 10 (77) | 4 (31) | 45 (82) | 28 (51) | 16 (80) | 10 (32) | 39 (81) | 22 (59) | 27 (75) | 17 (45) | 28 (88) | 15 (50) |
| >0 to <12 | 2 (15) | 0 | 6 (11) | 8 (15) | 2 (10) | 3 (10) | 6 (13) | 5 (14) | 5 (14) | 6 (16) | 3 (9) | 2 (7) |
| 12 to <24 | 0 | 4 (31) | 3 (5) | 5 (9) | 0 | 8 (26) | 3 (6) | 1 (3) | 2 (6) | 6 (16) | 1 (3) | 3 (10) |
| 24 to <36 | 0 | 2 (15) | 0 | 8 (15) | 0 | 3 (10) | 0 | 7 (19) | 0 | 4 (11) | 0 | 6 (20) |
| ≥36 | 1 (8) | 3 (23) | 1 (2) | 6 (11) | 2 (10) | 7 (23) | 0 | 2 (5) | 2 (6) | 5 (13) | 0 | 4 (13) |
| OR (95% CI) c | 21.06 (2.65, 167.18) | 4.91 (2.04, 11.81) | 6.76 (1.66, 27.60) | 3.88 (1.44, 10.51) | 4.03 (1.49, 10.87) | 24.12 (4.91, 118.50) | ||||||
| Proportion of patients in remission b at Weeks 36 and 48, n (%) | 0 | 7 (54) | 2 (4) | 15 (27) | 1 (5) | 14 (45) | 1 (2) | 8 (22) | 2 (6) | 11 (29) | 0 | 11 (37) |
| OR (95% CI) c | NA | 9.01 (1.87, 43.43) | 13.40 (1.46, 122.82) | 15.28 (1.71, 136.57) | 5.58 (1.05, 29.61) | NA | ||||||
Abbreviations: ANCA, antineutrophil cytoplasmic antibody; BVAS, Birmingham Vasculitis Activity Score; CI, confidence interval; Mepo, mepolizumab; NA, not applicable—estimate could not be calculated owing to lack of patients in the placebo group achieving remission at Weeks 36 and 48; OR, odds ratio; PBO, placebo; VDI, Vasculitis Damage Index.
Accrued number of weeks of remission over the 52‐week study period categorized in weeks (0, >0 to <12, 12 to <24, 24 to <36, and ≥36 weeks).
Remission was defined as BVAS = 0 and oral glucocorticoid dose ≤4 mg/day of a prednisone equivalent.
Mepolizumab versus placebo.
Across all ANCA, BVAS, and VDI subgroups, a larger proportion of patients were in remission at both Weeks 36 and 48 with mepolizumab versus placebo (Figure 1; Table 2). These proportions (mepolizumab vs. placebo) were as follows: 54% (n = 7) versus 0% for patients with a history of ANCA positivity and 27% (n = 15) versus 4% (n = 2) for patients without a history of ANCA positivity; 45% (n = 14) versus 5% (n = 1) for patients with a BVAS equal to 0 and 22% (n = 8) versus 2% (n = 1) for patients with a BVAS of more than 0; and 29% (n = 11) versus 6% (n = 2) for patients with a VDI score of less than 5 and 37% (n = 11) versus 0% for patients with a VDI score of 5 or greater. Patients with ANCA positivity, a BVAS of more than 0, and a VDI score of 5 or greater are more likely to have a vasculitic EGPA phenotype.
Interaction between mepolizumab treatment effect and baseline variables
Baseline BVAS in the study population ranged from 0 to 22 with a median of 1.0, and the mean was 3.3 with a standard deviation (SD) of 4.70. There was no evidence that the mepolizumab treatment effect on accrued duration of remission differed by baseline BVAS when assessed as a continuous (P = 0.809) or categorical (defined as BVAS = 0 vs. BVAS > 0; P = 0.458) variable. Similar results were obtained for the proportion of patients in remission at both Weeks 36 and 48 (BVAS as a continuous variable: P = 0.736; BVAS as a categorical variable: P = 0.982).
Baseline VDI score in the study population ranged from 0 to 21 with a median of 4.0, and the mean was 4.6 with a SD of 3.10. There was no evidence that the mepolizumab treatment effect on accrued duration of remission differed by baseline VDI score, when assessed as a categorical variable (defined as VDI score <5 vs VDI score ≥5; P = 0.170). There was some evidence that the effect of mepolizumab treatment on the proportion of patients in remission at both Weeks 36 and 48 differed by baseline VDI score (P = 0.058); however, the treatment effect could not be estimated in patients with a baseline VDI score of 5 or greater because there were no patients in remission at Weeks 36 and 48 receiving a placebo within this subgroup.
Effect of mepolizumab on EGPA relapse, and by type of relapse
A lower proportion of patients experienced EGPA relapses with mepolizumab versus placebo; these relapses included vasculitic (BVAS > 0) only (no active asthma symptoms, no worsening in ACQ‐6 or sino‐nasal symptom questions scores [18% vs. 22%]) and vasculitis with or without another relapse category defined (43% vs. 65%), respectively. Similarly, patients receiving mepolizumab versus placebo also experienced a lower number of relapse events (Figure 2). Patients in the mepolizumab group had a lower cumulative number of all types of EGPA relapses than patients in the placebo group; differences between the mepolizumab and placebo groups according to individual relapse type (asthma only, vasculitis only, sino‐nasal only) were markedly smaller (Figure 3).
Figure 2.
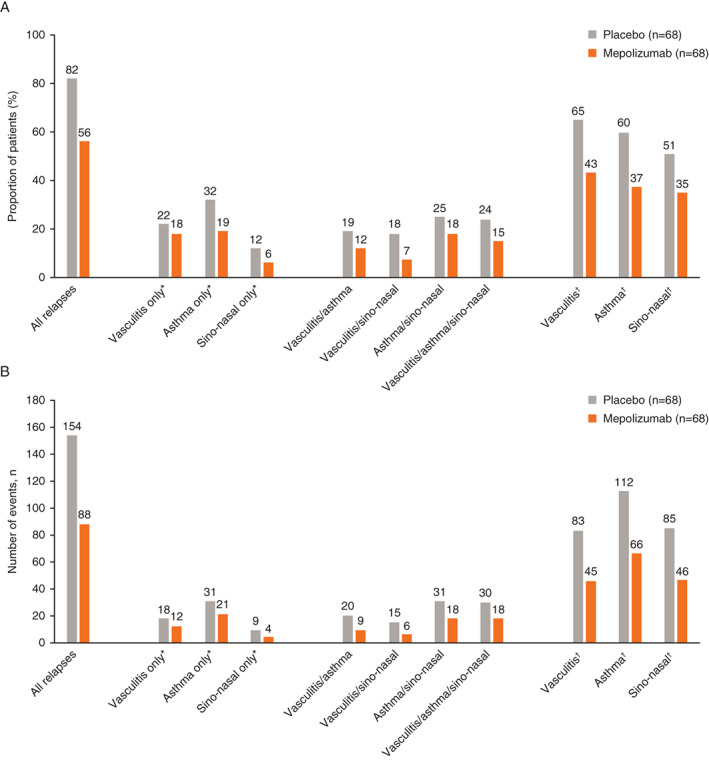
Proportion of patients experiencing a relapse (A) and number of relapse events (B) by type of relapse. *Relapse category selected without another relapse category defined. †Relapse category selected with or without another relapse category defined; vasculitis relapse defined as BVAS of more than 0; asthma relapse defined as active asthma symptoms and/or signs with a corresponding worsening in ACQ‐6 score (compared with the most recent previous score); sino‐nasal relapse defined as active nasal and/or sinus disease, with corresponding worsening in one or more of the sino‐nasal symptom questions (compared with the most recent previous assessment). ACQ‐6, Asthma Control Questionnaire 6; BVAS, Birmingham Vasculitis Activity Score.
Figure 3.
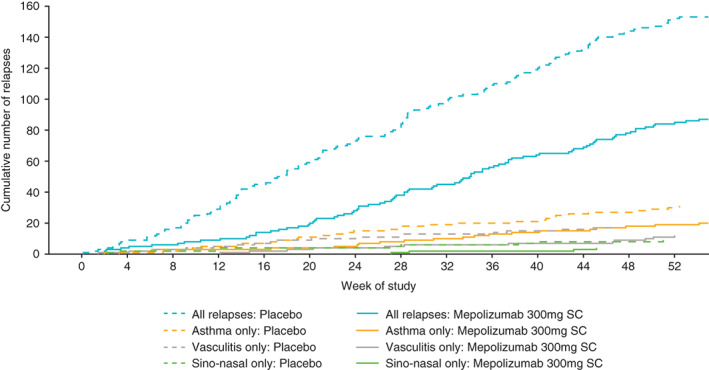
Cumulative number of EGPA relapses and by relapse type. For patients who withdrew from treatment, observed data following treatment discontinuation were used where available. Relapse categories are selected without another relapse category defined; asthma relapse defined as active asthma symptoms and/or signs with a corresponding worsening in ACQ‐6 score (compared with the most recent previous score); vasculitis relapse defined as BVAS of more than 0; sino‐nasal relapse defined as active nasal and/or sinus disease, with corresponding worsening in one or more of the sino‐nasal symptom questions (compared with the most recent previous assessment). ACQ‐6, Asthma Control Questionnaire 6; BVAS, Birmingham Vasculitis Activity Score; EGPA, eosinophilic granulomatosis with polyangiitis; SC, subcutaneous.
Patients receiving mepolizumab had accrued more time during which their BVAS was equal to 0 (odds ratio [95% confidence interval]: 3.71 [1.82‐7.60]; P < 0.001). In total, 50% of patients had a BVAS equal to 0 at Weeks 36 and 48 in the mepolizumab group versus 34% in the placebo group (P = 0.092; Table 3).
Table 3.
Accrued duration of BVAS = 0 and proportion of patients achieving BVAS = 0 during the treatment period
| PBO | Mepo | |
|---|---|---|
| (n = 68) | (n = 68) | |
| Accrued duration of BVAS = 0 over 52 wk, n (%) | ||
| 0 | 6 (9) | 3 (4) |
| >0 to <12 wk | 15 (22) | 13 (19) |
| 12 to <24 wk | 11 (16) | 5 (7) |
| 24 to <36 wk | 17 (25) | 2 (3) |
| ≥36 wk | 19 (28) | 45 (66) |
| OR (95% CI) a | 3.71 (1.82, 7.60); | |
| P value | P < 0.001 | |
| Proportion of patients with BVAS = 0 at Weeks 36 and 48, n (%) | 23 (34) | 34 (50) |
| OR (95% CI) a | 1.94 (0.90, 4.19); | |
| P value | P = 0.092 | |
Abbreviations: BVAS, Birmingham Vasculitis Activity Score; CI, confidence interval; Mepo, mepolizumab; OR, odds ratio; PBO, placebo.
Mepolizumab versus placebo.
DISCUSSION
Using data from the MIRRA study, this is the first analysis to evaluate the clinical benefit of mepolizumab in patients with a predominantly vasculitic EGPA phenotype (29). Irrespective of historical ANCA positive status, baseline BVAS, or baseline VDI score, patients treated with mepolizumab demonstrated a greater accrued duration of remission, and more patients treated with mepolizumab were in remission at both Weeks 36 and 48 than those who received placebo. Overall, the presence and/or history of vasculitic characteristics were generally similar in patients achieving remission and in those who did not achieve remission. Patients treated with mepolizumab also had fewer EGPA relapses including vasculitis events and more weeks without vasculitic disease activity. Together, these results suggest that both patients with and those without a vasculitic EGPA phenotype achieve clinical benefits with mepolizumab.
The results of this analysis expand on those previously demonstrated in the primary analysis of the MIRRA study, which demonstrated that patients with EGPA treated with mepolizumab had a greater accrued duration of remission versus placebo, with one‐third of mepolizumab‐treated patients in remission at both Weeks 36 and 48 (29). Additionally, increased duration of remission with mepolizumab over placebo has been demonstrated using alternative definitions of remission (30). Furthermore, in a subgroup of patients with EGPA and blood eosinophil counts of less than 150 cells/μL, mepolizumab increased the duration of remission (29, 30) although treatment benefits were more limited than in patients with counts of 150 cells/μL or more, potentially due to higher baseline OG doses in the former versus latter group (25, 28). In the current analysis, remission benefits with mepolizumab over placebo were observed in patients with and without features of a vasculitic phenotype including those with and without a history of ANCA positivity. This suggests that, although ANCA is often associated with the vasculitic EGPA phenotype (5, 6) and may directly contribute to disease pathology (6, 33), mepolizumab can facilitate remission via targeting eosinophils and their contribution to disease activity (4, 6). Accordingly, ANCA binding level does not always correlate with disease severity, ANCA can persist in disease remission, and some patients with vasculitic manifestations may test ANCA negative (34, 35, 36). In addition to the results by ANCA status, both patients with baseline BVAS greater than 0 and those with VDI scores of 5 or greater (features of vasculitic activity) also achieved a longer duration of accrued remission, and more of these patients were in remission at both Weeks 36 and 48 with mepolizumab compared with placebo. It should be noted that some vasculitic components were based on historical reports and may not represent active disease at the time mepolizumab was started. Furthermore, patients with organ‐ or life‐threatening manifestations in the 3 months before screening were not included in MIRRA. Nevertheless, taken together, these data support the use of mepolizumab in patients with EGPA with and without a vasculitic phenotype.
The proportion of patients with EGPA vasculitic disease components including neuropathy (39% to 43%), ANCA positivity (16% to 24%), palpable purpura (12% to 13%), alveolar hemorrhage (2% to 4%), and glomerulonephritis (0% to 1%) did not differ with remission status. Consistent with this, the proportion of patients who accrued 36 weeks of remission or longer was generally similar. Taken together these data suggest that the presence of the examined EGPA vasculitic components may not predict patient remission outcomes to mepolizumab. Additionally, the finding regarding ANCA status broadly supports the European EGPA study group consensus statement, which suggests that ANCA status by itself should not guide treatment decisions (34). The results are also informative for physicians when guiding treatment selection for remission induction in patients with active, nonsevere disease (ie, patients without life‐ or organ‐threatening manifestations, an area the American College of Rheumatology/Vasculitis Foundation guideline for ANCA‐associated vasculitis has outlined as requiring further investigation) (10).
Mepolizumab reduced the proportion of patients with all types of EGPA relapses, including vasculitis, asthma, and sino‐nasal relapses, compared with placebo, in addition to the cumulative number of all types of relapses. The events categorized into vasculitic only had concurrent asthma and sino‐nasal relapses removed; therefore, any influence by these events was likely excluded. Relapses are events that some patients continue to experience despite treatment with systemic glucocorticoids, immunosuppressants, or cytotoxic therapy (12, 14, 15, 18). Additionally, patients treated with mepolizumab also achieved a greater total accrued number of weeks with a BVAS of 0, indicating no vasculitic disease activity. This is consistent with previously reported results for patients with EGPA in Japan, including data reported for an interim analysis of a post‐marketing surveillance study, and these studies showed improvements in vasculitic symptoms based on BVAS following mepolizumab treatment (37, 38). These results are also in accordance with EGPA treatment goals to increase remission and reduce relapse rates, in addition to minimizing OG exposure (2). Another focus is on reducing organ damage, which can accumulate even several years after the EGPA diagnosis owing to continued disease activity and prolonged OG use (39). Organ damage can also lead to persistent symptoms, and patients can potentially become less responsive to further treatment (39).
This analysis has several limitations, which should be considered when interpreting results. Firstly, although a biopsy is recommended to support a diagnosis of vasculitis (9), biopsy criteria could not be used in the current analysis to support the identification of a vasculitic phenotype. This was because the historical biopsy eligibility criteria used in MIRRA included features that were not specific to vasculitic EGPA, including evidence of eosinophilic vasculitis, perivascular eosinophilic infiltration, and eosinophil‐rich granulomatous inflammation. Secondly, the most common criteria suggestive of vasculitic disease was neuropathy (41% of all MIRRA patients; mononeuropathy or polyneuropathy) (29). However, as neuropathy can be caused by either vasculitis or eosinophilic tissue infiltration (6), the possibility that patients had neuropathy of eosinophilic origin cannot be fully excluded. Thirdly, the BVAS tool has limitations, including a lack of specificity for vasculitis because asthma components such as wheezing could also be captured within the tool, which means that patients could have had a BVAS of greater than than 0 due to their asthma rather than vasculitis symptoms. BVAS also captures features that are more often attributed to tissue eosinophilia than vasculitis, such as cardiomyopathy (18, 40). Additionally, BVAS data were collected as total scores and not by individual components, and it was not possible to remove nonvasculitis components from BVAS data due to the lack of adjudication. Fourthly, although no evidence of interaction was seen between the baseline BVAS and the accrued duration of remission or proportion of patients in remission at both Weeks 36 and 48, there was some evidence that the mepolizumab treatment effect on the latter endpoint differed by baseline VDI score. However, given that no patients who were receiving placebo in the subgroup with a VDI score of 5 or greater achieved remission at both Weeks 36 and 48, results for this endpoint and subgroup should be interpreted with caution. Another limitation is that vasculitis features are most commonly present at the time of EGPA diagnosis, but the MIRRA eligibility criteria excluded any new EGPA diagnoses, which may have reduced the proportion of patients with vasculitis features. Finally, the study design encouraged recruitment of patients with established disease, particularly those with asthma and sino‐nasal disease, leading to small sample sizes for some analyses, including in the placebo group.
In this post hoc analysis of data from the MIRRA study, patients with EGPA and a predominantly vasculitic phenotype had similar mepolizumab treatment outcomes as those without a vasculitic phenotype. Moreover, patients achieving and not achieving remission were similar in terms of their history or presence of EGPA diagnostic features. These results suggest patients with EGPA and a vasculitic phenotype are likely to achieve clinical benefits with mepolizumab, thus providing valuable information for clinicians treating patients with EGPA with and without vasculitic phenotypes.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Acquisition of data
Terrier, Jayne, Hellmich, Akuthota, Khoury, Weschler.
Study conception and design
Steinfeld, Yancey.
Analysis and interpretation of data
Terrier, Jayne, Hellmich, Bentley, Steinfeld, Yancey, Kwon, Akuthota, Khoury, Baylis, Weschler.
Supporting information
Disclosure form
Appendix S1: Supplementary Information
ACKNOWLEDGMENTS
Professor Jayne is supported by the National Institute for Health and Care Research (NIHR) Cambridge Biomedical Research Centre. Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Sarah Farrar, PhD, at Fishawack Indicia Ltd, UK, part of Fishawack Health, with support provided by GSK.
This post hoc analysis and the parent study (GSK ID: 115921; NCT02020889) were funded by GSK; by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases (NIAID)/NIH; and by an investigator‐initiated clinical trial application (U01‐AI‐097073) through NIAID/NIH, by which five of eight clinical sites in the US were supported for participation in this trial.
Anonymized individual participant data and study documents can be requested from https://www.GSK-studyregister.com/en/ for further research.
Author disclosures are available online at https://onlinelibrary.wiley.com/doi/10.1002/acr2.11571.
REFERENCES
- 1. Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int 2019;68:430–6. [DOI] [PubMed] [Google Scholar]
- 2. Groh M, Pagnoux C, Baldini C, et al. Eosinophilic granulomatosis with polyangiitis (Churg‐Strauss) (EGPA) consensus task force recommendations for evaluation and management. Eur J Intern Med 2015;26:545–53. [DOI] [PubMed] [Google Scholar]
- 3. Greco A, Rizzo MI, De Virgilio A, et al. Churg‐Strauss syndrome. Autoimmun Rev 2015;14:341–8. [DOI] [PubMed] [Google Scholar]
- 4. Raffray L, Guillevin L. Treatment of eosinophilic granulomatosis with polyangiitis: a review. Drugs 2018;78:809–21. [DOI] [PubMed] [Google Scholar]
- 5. Latorre M, Novelli F, Baldini C, et al. Different disease subtypes in allergic eosinophilic granulomatosis with polyangiitis (EGPA). Eur Respir J 2013;42:1797. [Google Scholar]
- 6. Fagni F, Bello F, Emmi G. Eosinophilic granulomatosis with polyangiitis: dissecting the pathophysiology. Front Med (Lausanne) 2021;8:627776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lyons PA, Peters JE, Alberici F, et al. Genome‐wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat Commun 2019;10:5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sada KE, Amano K, Uehara R, et al. A nationwide survey on the epidemiology and clinical features of eosinophilic granulomatosis with polyangiitis (Churg‐Strauss) in Japan. Mod Rheumatol 2014;24:640–4. [DOI] [PubMed] [Google Scholar]
- 9. Yates M, Watts RA, Bajema IM, et al. EULAR/ERA‐EDTA recommendations for the management of ANCA‐associated vasculitis. Ann Rheum Dis 2016;75:1583–94. [DOI] [PubMed] [Google Scholar]
- 10. Chung SA, Langford CA, Maz M, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody‐associated vasculitis. Arthritis Rheumatol 2021;73:1366–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hellmich B, Sanchez‐Alamo B, Schirmer JH, et al. EULAR recommendations for the management of ANCA‐associated vasculitis: 2022 update. Ann Rheum Dis 2023:ard‐2022‐223764. [DOI] [PubMed] [Google Scholar]
- 12. Ribi C, Cohen P, Pagnoux C, et al. Treatment of Churg‐Strauss syndrome without poor‐prognosis factors: a multicenter, prospective, randomized, open‐label study of seventy‐two patients. Arthritis Rheum 2008;58:586–94. [DOI] [PubMed] [Google Scholar]
- 13. Sarnes E, Crofford L, Watson M, et al. Incidence and US costs of corticosteroid‐associated adverse events: a systematic literature review. Clin Ther 2011;33:1413–32. [DOI] [PubMed] [Google Scholar]
- 14. Durel CA, Berthiller J, Caboni S, et al. Long‐term followup of a multicenter cohort of 101 patients with eosinophilic granulomatosis with polyangiitis (Churg‐Strauss). Arthritis Care Res (Hoboken) 2016;68:374–87. [DOI] [PubMed] [Google Scholar]
- 15. Saku A, Furuta S, Hiraguri M, et al. Longterm outcomes of 188 Japanese patients with eosinophilic granulomatosis with polyangiitis. J Rheumatol 2018;45:1159–66. [DOI] [PubMed] [Google Scholar]
- 16. Gokhale M, Bell CF, Doyle S, et al. Prevalence of eosinophilic granulomatosis with polyangiitis and associated health care utilization among patients with concomitant asthma in US commercial claims database. J Clin Rheumatol 2021;27:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sada KE, Kojo Y, Fairburn‐Beech J, et al. The prevalence, burden of disease, and healthcare utilization of patients with eosinophilic granulomatosis with polyangiitis in Japan: a retrospective, descriptive cohort claims database study. Mod Rheumatol 2022;32:380–6. [DOI] [PubMed] [Google Scholar]
- 18. Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg‐Strauss): clinical characteristics and long‐term followup of the 383 patients enrolled in the French vasculitis study group cohort. Arthritis Rheum 2013;65:270–81. [DOI] [PubMed] [Google Scholar]
- 19. Strehl C, Bijlsma JW, De Wit M, et al. Defining conditions where long‐term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis 2016;75:952–7. [DOI] [PubMed] [Google Scholar]
- 20. Emma R, Morjaria JB, Fuochi V, et al. Mepolizumab in the management of severe eosinophilic asthma in adults: current evidence and practical experience. Ther Adv Respir Dis 2018;12:1753466618808490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flood‐Page P, Menzies‐Gow A, Phipps S, et al. Anti‐IL‐5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest 2003;112:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. European Medicines Agency . Mepolizumab summary of product characteristics. URL: https://www.ema.europa.eu/en/documents/product-information/nucala-epar-product-information_en.pdf.
- 23. GSK . Mepolizumab prescribing information. 2022. URL: https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Nucala/pdf/NUCALA‐PI‐PIL‐IFU‐COMBINED.PDF.
- 24. GSK . Mepolizumab prescribing information, Japan. 2020. URL: https://gskpro.com/ja-jp/products-info/nucala/index/.
- 25. Khoury P, Grayson PC, Klion AD. Eosinophils in vasculitis: characteristics and roles in pathogenesis. Nat Rev Rheumatol 2014;10:474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abdala‐Valencia H, Coden ME, Chiarella SE, et al. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J Leukoc Biol 2018;104:95–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stirling RG, van Rensen EL, Barnes PJ, et al. Interleukin‐5 induces CD34(+) eosinophil progenitor mobilization and eosinophil CCR3 expression in asthma. Am J Respir Crit Care Med 2001;164:1403–9. [DOI] [PubMed] [Google Scholar]
- 28. Yamaguchi Y, Suda T, Ohta S, et al. Analysis of the survival of mature human eosinophils: interleukin‐5 prevents apoptosis in mature human eosinophils. Blood 1991;78:2542–7. [PubMed] [Google Scholar]
- 29. Wechsler ME, Akuthota P, Jayne D, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med 2017;376:1921–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Steinfeld J, Bradford ES, Brown J, et al. Evaluation of clinical benefit from treatment with mepolizumab for patients with eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol 2019;143:2170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Exley AR, Bacon PA, Luqmani RA, et al. Examination of disease severity in systemic vasculitis from the novel perspective of damage using the vasculitis damage index (VDI). Br J Rheumatol 1998;37:57–63. [DOI] [PubMed] [Google Scholar]
- 32. Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham vasculitis activity score (version 3). Ann Rheum Dis 2009;68:1827–32. [DOI] [PubMed] [Google Scholar]
- 33. Shochet L, Holdsworth S, Kitching AR. Animal models of ANCA associated vasculitis. Front Immunol 2020;11:525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moiseev S, Bossuyt X, Arimura Y, et al. International consensus on ANCA testing in eosinophilic granulomatosis with polyangiitis. Am J Respir Crit Care Med 2020;10.1164. [DOI] [PubMed] [Google Scholar]
- 35. Tomasson G, Grayson PC, Mahr AD, et al. Value of ANCA measurements during remission to predict a relapse of ANCA‐associated vasculitis–a meta‐analysis. Rheumatology (Oxford) 2012;51:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cottin V, Bel E, Bottero P, et al. Revisiting the systemic vasculitis in eosinophilic granulomatosis with polyangiitis (Churg‐Strauss): a study of 157 patients by the groupe d'etudes et de recherche sur les maladies orphelines pulmonaires and the European respiratory society taskforce on eosinophilic granulomatosis with polyangiitis (Churg‐Strauss). Autoimmun Rev 2017;16:1–9. [DOI] [PubMed] [Google Scholar]
- 37. Ueno M, Miyagawa I, Nakano K, et al. Effectiveness and safety of mepolizumab in combination with corticosteroids in patients with eosinophilic granulomatosis with polyangiitis. Arthritis Res Ther 2021;23:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fujii T. Post–marketing surveillance of mepolizumab use in patients with eosinophilic granulomatosis with polyangiitis in Japan: interim analysis. Ther Res 2021;42:403–22. [Google Scholar]
- 39. Doubelt I, Cuthbertson D, Tomasson G, et al. 2019. ACR/ARP annual meeting abstract supplement [abstract]. Arthritis Rheumatol 2019; Suppl 10:1–5362. [Google Scholar]
- 40. Garcia‐Vives E, Rodriguez‐Palomares JF, Harty L, et al. Heart disease in eosinophilic granulomatosis with polyangiitis (EGPA) patients: a screening approach proposal. Rheumatology (Oxford) 2021;60:4538–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure form
Appendix S1: Supplementary Information


