Version Changes
Revised. Amendments from Version 3
This version contains a revised Table S1 highlighting the parasitemia and DNA concentrations of the artificial DNA mixture used as sequencing controls. Table S4 shows the occurrence of all microhaplotypes across all participants, while Table S5 shows the occurrence of rare microhaplotypes. The term 'aliquot' has since been used to designate the multiple portions taken from the control samples. Similarly, the term 'duplicate' refers to the repeated measurements or observations taken from these control aliquots and samples to ensure consistency and reliability in our results. The methods have been revised to provide more clarity, such as thresholds for read-depths used.
Abstract
Introduction
Antimalarial therapeutic efficacy studies are routinely conducted in malaria-endemic countries to assess the effectiveness of antimalarial treatment strategies. Targeted amplicon sequencing (AmpSeq) uniquely identifies and quantifies genetically distinct parasites within an infection. In this study, AmpSeq of Plasmodium falciparum apical membrane antigen 1 ( ama1), and multidrug resistance gene 1 ( mdr1), were used to characterise the complexity of infection (COI) and drug-resistance genotypes, respectively.
Methods
P. falciparum-positive samples were obtained from a triple artemisinin combination therapy clinical trial conducted in 30 children under 13 years of age between 2018 and 2019 in Kilifi, Kenya. Nine of the 30 participants presented with recurrent parasitemia from day 26 (624h) onwards. The ama1 and mdr1 genes were amplified and sequenced, while msp1, msp2 and glurp data were obtained from the original clinical study.
Results
The COI was comparable between ama1 and msp1, msp2 and glurp; overall, ama1 detected more microhaplotypes. Based on ama1, a stable number of microhaplotypes were detected throughout treatment until day 3. Additionally, a recrudescent infection was identified with an ama1 microhaplotype initially observed at 30h and later in an unscheduled follow-up visit. Using the relative frequencies of ama1 microhaplotypes and parasitemia, we identified a fast (<1h) and slow (>5h) clearing microhaplotype. As expected, only two mdr1 microhaplotypes (NF and NY) were identified based on the combination of amino acid polymorphisms at codons 86 and 184.
Conclusions
This study highlights AmpSeq as a tool for highly-resolution tracking of parasite microhaplotypes throughout treatment and can detect variation in microhaplotype clearance estimates. AmpSeq can also identify slow-clearing microhaplotypes, a potential early sign of selection during treatment. Consequently, AmpSeq has the capability of improving the discriminatory power to distinguish recrudescences from reinfections accurately.
Keywords: Artemisinin-based combination therapy, pfama1, Pfmdr1, artemisinin resistance, antimalarial resistance, targeted deep sequencing, deep sequencing, msp1, msp2, glurp
Introduction
Artemisinin-based combination therapies (ACTs) have led to high cure rates for P. falciparum malaria ( Bhatt et al., 2015). Still, artemisinin (ART) resistance emerged and spread in Southeast (SE) Asia, evidenced by delayed parasite clearance following ACT treatment ( Ashley et al., 2014; Dondorp et al., 2009; Noedl et al., 2008; van der Pluijm et al., 2019). Two recent studies have identified early signs of ART partial resistance in Rwanda ( Uwimana et al., 2021) and Uganda ( Balikagala et al., 2021), and this looming threat of widespread ACT resistance would be catastrophic in sub-Saharan Africa, where the burden of malaria is the most significant ( WHO, 2021a).
To minimise the development of drug-resistant parasites and rescue a regimen with an already failing component of ACTs, novel chemotherapeutic strategies involving the roll-out of triple artemisinin-based combination therapies (TACTs) are being evaluated ( van der Pluijm et al., 2021). TACTs combine an established ACT with a second, slowly eliminated partner drug for additional antimalarial activity and protection of partner drug resistance. The potential advantage of TACTs is supported by evidence from Cambodia that artemisinin partner drugs may exert opposing selection pressures making it difficult to adapt to multiple partner drugs simultaneously ( Parobek et al., 2017). The safety and efficacy of this approach have been shown in clinical trials ( Hamaluba et al., 2021; van der Pluijm et al., 2020), and the antimalarial therapeutic outcomes are assessed for a maximum of 42 days. Based on molecular methods, recurrent parasitemia during this period is classified as a new (reinfection) or recrudescent infection. The former is determined when genotyping methods find the recurrent parasites are distinguishable from those in the pre-treatment infection, and the latter when the parasites are indistinguishable. The standard genotyping method termed PCR correction examines three-length polymorphic markers in parasites, namely merozoite surface protein 1 ( msp1), msp2 and glutamate-rich protein ( glurp) ( Snounou & Beck, 1998). The amplicon sizes of these three markers are compared between pre-treatment and post-treatment parasites by either gel or capillary electrophoresis ( Liljander et al., 2009; WHO, 2003). However, the use of msp1/ msp2/ glurp genotyping is met with challenges such as the reliance on gel electrophoresis being limited to discriminating alleles of similar sizes (those with size differences less than 20 bp) and the inability to detect low-density parasite clones ( Felger et al., 2020; Jones et al., 2021). Therefore, studies that rely on these markers may underestimate parasite diversity, are insensitive to low-abundant variants and are not quantitative for relative proportions of circulating parasite clones. Targeted amplicon sequencing, referred to as amplicon sequencing (AmpSeq) from here henceforth, offers high sensitivity in detecting minority parasite variants, quantifying the number of variants and their relative frequencies. AmpSeq also offers high-throughput sequencing of P. falciparum diversity and drug-resistance markers ( Gruenberg et al., 2019; Miller et al., 2017). Apical membrane antigen 1 ( ama1) is a highly polymorphic merozoite surface antigen ( Polley & Conway, 2001) and serves as an excellent marker to explore parasite diversity within infections. On the other hand, mutations at codons N86Y and F184Y of the multidrug resistance gene ( mdr1) modulate parasite susceptibility to ACT partner drugs such as amodiaquine, lumefantrine, piperaquine and mefloquine ( Veiga et al., 2016). Additionally, the rollout of ACTs has led to an increase in the mdr1-NFD microhaplotype (based on the combination of amino acid polymorphisms at codons 86, 184 and 1246) across several African studies, possibly due to ACT selection pressure ( Okell et al., 2018).
In this study, we examined samples from a TACT efficacy study in Kilifi ( Hamaluba et al., 2021). The administration of drugs was done under observation, and the patients were monitored in the hospital for three days of treatment. Frequent blood samples were obtained for pharmacokinetic analyses that were subsequently used for AmpSeq. The purpose of this study was to examine the utility of a genetic diversity marker ( ama1) combined in a single deep sequencing run with a drug resistance marker ( mdr1) to identify and track changes in the complexity of infections (COI), i.e., the number of ama1 genotypes per infection as well as the mdr1 wild-type and mutant genotypes throughout treatment.
Methods
Study design
P. falciparum positive samples were obtained from the TACT Kenya clinical trial conducted from 2018 to 2019 (ClinicalTrials.gov Identifier: NCT03452475) described in Hamaluba et al. (2021). The three-drug arms were arterolane-piperaquine (ART-PQ), arterolane-piperaquine + mefloquine (ART-PQ+MF) and artemether-lumefantrine (AL). A random sample of 30 individuals was selected, including all their sampling time-points: 0 hours (h), 0.5h, 1h, 2h, 3h, 4h, 6h, 8h, 12h, 18h, 24h, 30h, 36h, 42h, 48h, 72h (day 3), 168h (day 7), 336h (day 14), 504h (day 21), 672h (day 28), 840h (day 35), 1008h (day 42) and the hour of recurrent infection (REC, any sample taken during an unscheduled visit by the study participant, Table 1). 9/30 participants presented with recurrent parasitemia based on microscopy from 624h onwards, making a total of 609 individual samples. This study was approved by the Oxford Tropical Research Ethics Committee in the United Kingdom and the Kenya Medical Research Institute (KEMRI) -Scientific and Ethics Review Unit (SERU).
Table 1. Characteristics of the study participants.
| Characteristic | Antimalarial regimen | p-value | ||
|---|---|---|---|---|
| ART+PQ | ART+PQ+MF | AL | ||
| Number of Participants
[n=30] |
11 | 11 | 8 | 0.41 |
| Median age in years
[Range] |
6.7 | 5.7 | 10.65 | 0.51 |
| [2.7 – 10.3] | [2.1 – 11.9] | [6.0 - 12.6] | ||
| Gender [Females] | 3 | 7 | 3 | 0.12 |
| Median Parasitemia per
μl [Range] |
142,236 | 78,274 | 90,158.5 | 0.7 |
| [8560 – 571,530] | [15,232 – 326,020] | [25,328 – 266,146] | ||
ART-PQ - arterolane-piperaquine, ART-PQ+MF arterolane-piperaquine + mefloquine and AL - artemether-lumefantrine.
DNA preparation and PCR from sequencing controls and clinical samples
Henceforth, we use the term “microhaplotype” to refer to the set of amino acid polymorphisms found on a single DNA amplicon. DNA was extracted from culture-adapted laboratory P. falciparum isolates, 3D7 and Dd2 (BEI Resources), and from 200μl of frozen patient blood samples using the QIAamp DNA Blood Mini Kit (Qiagen) according to the manufacturer’s instructions. The DNA from 3D7 and Dd2 were mixed in the following ratios to generate artificial mixtures of sequencing controls: 1:1, 0.75:0.25, 0.85:0.15, 0.95:0.05 and 1:0 to determine the lowest limit of a microhaplotype detection. The level of parasitemia for the P. falciparum cultures from which 3D7 and Dd2 DNA were extracted is typically above 1% ( www.beiresources.org/Catalog/BEIParasiticProtozoa/MRA-102.aspx and www.beiresources.org/Catalog/BEIParasiticProtozoa/MRA-150.aspx). These levels correspond to an approximate density of 50,000 parasites/µl, while the specific parasitemia for each isolate in the final mixture is presented in Table S1. The sequencing controls allowed for the detection of two ama1 (3D7 and Dd2) and three mdr1 (one from 3D7 and two from Dd2) microhaplotypes. Dd2 contained two mdr1 gene copies generated due to adaptation to in vitro culture. Amplicons spanning ama1 (PF3D7_1133400, nucleotides 441–946) and mdr1 (PF3D7_0523000, nucleotides 183–719) were generated in duplicate from each control and sample. This process was applied to each of the six aliquots prepared for every control, and to a single aliquot from each sample using primers designed in this study (Table S2) as follows: 1µl of template DNA (final amount <50ng), 0.2µl of Q5 ® High-Fidelity DNA Polymerase (final concentration 0.02U/µl, New England BioLabs), 1µl (10mM) forward primers tagged with Roche ® molecular identifiers (MIDs, Table S2), and reverse primers, 0.4µl of 10mM dNTPs, 4µl of 5X Q5 reaction buffer, and 12.4µl of nuclease-free water. For both ama1 and mdr1, the cycling conditions were: initial denaturation (98°C - 30 sec), followed by 30 cycles of denaturation (98°C - 10 sec), annealing (60°C - 30 sec), extension (72°C - 30 sec), and final extension (72°C - 2 min). PCR products were visualised on 1% agarose gels stained with RedSafe™ Nucleic Acid Staining Solution (iNtRON Biotechnology DR). Amplicon failures were repeated with 1.5µl of template DNA.
Amplicon Library Preparation and Sequencing
PCR amplicons were purified using the Zymo ZR-96 DNA Clean & Concentrator-5 Kit (Zymo Research) and quantified using Quant-iT™ dsDNA Assay Kit, High Sensitivity (Invitrogen). Both procedures were done following the manufacturer’s instructions. Subsequently, the PCR amplicons were normalised to equal amounts of 1ng each using EB Buffer (Qiagen) and mixed to create amplicon pools of non-overlapping 26 MIDs. The KAPA Dual-Indexed Adapter Kit and the KAPA Hyper Prep Kit (Roche) were used for library preparation, and the Agilent High Sensitivity D1000 ScreenTape System (5067-5584) confirmed adapter ligation. Eventually, the ama1 and mdr1 amplicon libraries were mixed to generate the final pool for paired-end sequencing (2x300bp chemistry) using MiSeq Reagent Kit v3 (Illumina).
Sequence data analysis
Data extraction, quality control processing, and microhaplotype clustering were performed using SeekDeep v3.0.0 ( Hathaway et al., 2018). We implemented SeekDeep's default threshold of 250 reads as the minimum required read-depth for each individual PCR replicate. Additionally, for a sample to be included in the analysis, it needed to have a combined total of at least 500 reads, summing the read counts from all its replicates. For samples that met this criterion, microhaplotypes were discarded if they did not occur in the two PCR duplicates and if their relative frequency was <5% (or less than 25 reads). A conservative cut-off of 5% was set based on the isolate with the least proportion in one of the artificial mixtures of sequencing controls (ratio of 0.95 3D7 to 0.05 Dd2) unless the microhaplotype was independently detected in other samples at >5%. Chimeric reads were considered PCR artefacts and discarded. The relative microhaplotype frequency in a sample was calculated as the number of reads of each microhaplotype over the total number of reads per sample. COI was defined as the number of distinct ama1 microhaplotypes (varying at the nucleotide level) in each sample, while codons 86 and 184 defined the mdr1 microhaplotypes. ama1 expected heterozygosity ( H e ) was calculated using the formula below, where n is the sample size and p i is the relative frequency of the i th microhaplotype in the population ( Nei, 1978):
msp1/msp2/glurp genotyping was performed according to the WHO-recommended method of gel electrophoresis ( WHO, 2008). These data were obtained from the original study, and the following was ensured during the analysis: Each PCR product had to have well-defined and easy to visualise, bands had to be bright and sharp to be of sufficient quality for scoring, PCRs were repeated if bands appeared in the negative control, the interpretation of results did not include products with less than 100 bp and did not account for faint bands or bands that formed smile-shaped patterns on the gel ( Hamaluba et al., 2021). All statistical analyses were carried out in R v4.0.2, and all plots were generated using the R packages ggplot2 v3.3.1 and ggpubr v0.3.0 ( Kassambara, 2020; Wickham, 2016).
Based on simulation studies of amplicon sequencing data analysis ( Jones et al., 2021), we set the lower sampling limit for a parasite (blood sampling limit) at ten parasites/μl. Finally, using the complexity of infection for each sample, we back-calculated each parasite isolate’s parasitemia to determine which isolates were at risk of falling below the sampling limit.
Parasite clearance estimation
One of the early signs of slow clearing parasites is a clearance half-life greater than five hours ( Ashley et al., 2014). Therefore, parasite clearance half-lives were calculated using the Worldwide Antimalarial Resistance Network’s (WWARN) parasite clearance estimator ( Flegg et al., 2011). This was done for the 30 participants by extrapolating the total parasitemia to each ama1 microhaplotype per infection. An estimate of the parasitaemia for each ama1 microhaplotype was calculated by multiplying the parasitaemia (based on microscopy) at each time point by the frequency of each microhaplotype ( Mideo et al., 2016) based on the number of reads per microhaplotype over the total number of reads per sample. These estimates were plotted as histograms.
Results
AmpSeq in artificial mixtures of sequencing controls
The expected microhaplotypes were successfully detected from the sequencing controls (two from ama1 and three from mdr1). Additionally, the 3D7 and Dd2 ama1 microhaplotypes were detected consistently across all mixtures and in the expected proportions. This provided evidence that the assay could detect mixed infections in clinical samples, but only when the minor microhaplotype was at a relative frequency of ≥5% (Figure S1).
AmpSeq of pre-and post-treatment samples
From the 30 individuals sampled, 11 were in both the ART+PQ and ART+PQ+MF drug arms, respectively, while 8 were in the AL drug arm. There was no difference in the baseline median parasitaemia between the drug arms ( Table 1). From the available 608 samples (timepoints 0h–1008h), ama1 and mdr1 sequence data were successfully obtained from 330 and 233 samples, respectively (Figure S2A and B). Samples were grouped into three categories based on parasitemia: high (>5,000), moderate (100–5,000) and low (<100 parasites/μl). Many samples collected between 0h–12h had high parasitemia, those collected between 18h–30h had moderate parasitemia, while those collected after 30h were primarily low parasitemia (Figure S2C). The median read depth was 11,147 reads (range 580 –33,714) for ama1 and 11,548 reads (range 1,022 – 55,664) for mdr1, consistent with decreasing parasitemia, post-treatment samples had lower sequencing success.
Pfama1 genetic diversity during and after treatment
Throughout treatment, the mean COI (Figure S3) and number of ama1 microhaplotypes ( Figure 1) were relatively stable and the expected heterozygosity for ama1 was high, 0.96. The mean COI and 95% confidence interval (CI) at 0h, 0.5h – 72h (during treatment) and post-treatment (after 72h) was 2.44 [2.13-1.75], 2.37 [2.27-2.48] and 2.0 [1.28-2.7], respectively. Overall, 33 ama1 microhaplotypes were detected from the 330 successfully sequenced samples (Table S3), and only 10 of these microhaplotypes were detected at frequencies >5%.
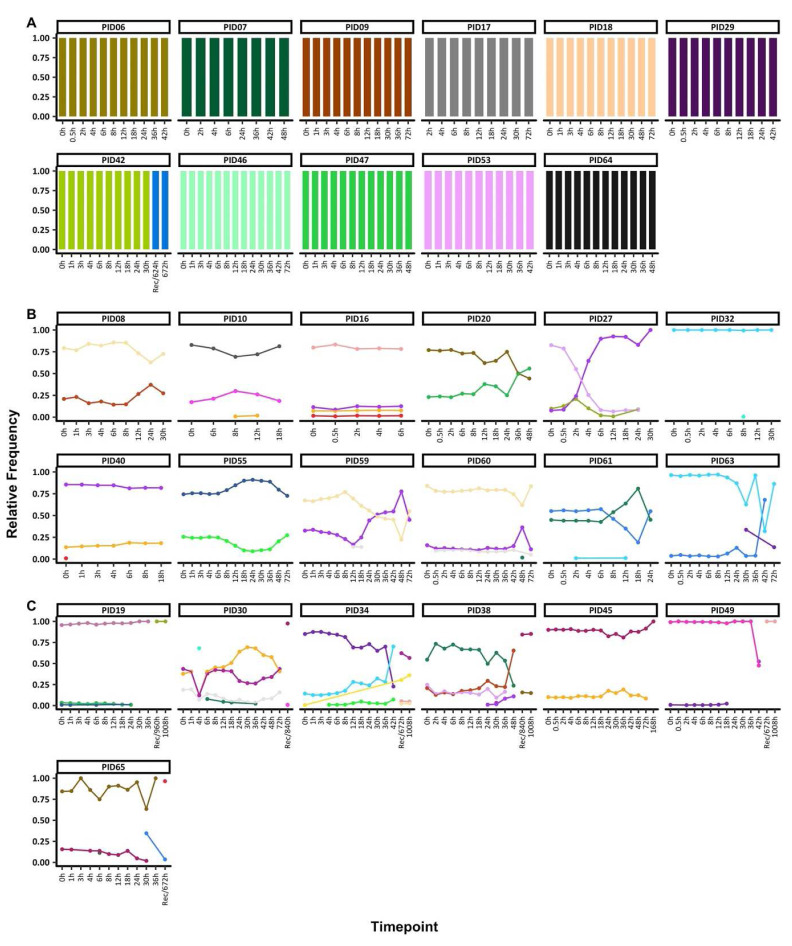
Figure 1. Temporal changes in ama1 microhaplotypes throughout treatment.
The figure highlights the individuals with A) monoclonal infections, if they had only one ama1 microhaplotype throughout treatment, B) polyclonal infections, if they had more than one ama1 microhaplotype throughout treatment and sampled up to 72h and C) polyclonal infections, if they more than one ama1 microhaplotype throughout treatment and sampled beyond 72h. Each coloured barplot represents a unique ama1 microhaplotype, and matching colours represent the same ama1 microhaplotype. Above each plot is the respective participant ID. The x-axis displays time points at which samples were collected, measured in hours. 'Rec' denotes samples taken during unscheduled visits due to the recurrence of parasites, although parasitemia data at these recurrence timepoints are not available, hence the line plot does not extend to these points. The primary y-axis corresponds to the bar charts, quantifying the relative distribution of ama1 microhaplotypes. The secondary y-axis (left) aligns with the dashed line graph, indicating the parasitemia levels on a log10 scale per microliter of blood. Logarithmic values of zero in the log10 parasitemia levels indicate samples that were determined to have zero parasitemia when examined using microscopy.
Two groups of individuals were identified based on COI, 11 individuals with monoclonal infections compared to 19 polyclonal infections. For participants with monoclonal infections and with sequence data only up to 72h, the same ama1 microhaplotype was seen throughout. Only one exception, individual PID42 had data post-72h and a change in the ama1 microhaplotype was detected before and after 72h ( Figure 1A, Table S4).
Participants with polyclonal infections harboured more than one ama1 microhaplotype at any one-time point. In individuals with sequence data up to 72h (n=11), the ama1 microhaplotypes detected maintained relatively stable frequencies up to <24h, with most changes occurring after 24h. In five individuals (PIDs 10, 30, 32, 40, 59, 60 and 61), there were sporadic detections of rare ama1 microhaplotypes ( Figure 1B, Table S4), including one microhaplotype each in PID10 at 8h and 12h, PID30 at 4h, PID32 at 8h, PID38 at 30h, PID40 at 0h, PID49 at 42h, PID60 at 48h, PID63 at 30h and 72h, PID61 at 12h and PID65 at 6h. Rare microhaplotypes, except for one detected exclusively in PID30, were identified in multiple samples, often at relative frequencies above 5% (Table S5). Additionally, except for PID60, all such sporadic microhaplotypes were detected above the sampling limit of 10 parasites/μl (see Table S6). The analysis revealed no significant difference in read counts between samples harboring either monoclonal or polyclonal infections ( p = 0.092, Wilcoxon signed-rank test).
Participant PID27 experienced a drastic change in the relative microhaplotype frequencies by 4h post-treatment. The least dominant microhaplotype at 0h had become dominant by the 4h and remained this way up to the last timepoint with sequencing data (30h). All the remaining seven individuals with post-treatment data cleared their infections. However, the recurrent sample (672h) for PID65 contained an ama1 microhaplotype present in the 30h sample, likely from a minor microhaplotype not detected at 0h ( Figure 1C, Table S4). All participants with polyclonal infections also had polyclonal baseline samples (0h), except for PID32 who had only one polyclonal sample at 8h.
AmpSeq compared to msp1/msp2/glurp genotyping of recurrent infections
Based on microscopy data, all the nine individuals recurrences (two in ART-PPQ, two in ART-PPQ+MF, and five in the AL arm) were categorised as new infections based on msp1/ msp2/ glurp data ( Hamaluba et al., 2021). msp1/msp2/glurp identified a total of 13, 19 and 12 microhaplotypes, respectively, in the recurrent samples of these nine participants. Of these nine participants, ama1 deep sequencing data were available for six participants and were also classified as new infections. In contrast to msp1/msp2/glurp genotyping, AmpSeq identified 21 ama1 microhaplotypes, slightly more than the other markers. For the six individuals, paired msp1/msp2/glurp and AmpSeq data revealed that the mean COI and 95% CI was highest in msp1 = 2.5 [0.98 – 3.5], msp2 = 2.5 [0.99 – 3.7], ama1 = 2.3 [0.81 – 3.3] and lowest in glurp = 1.2) during treatment (0h-72h), with the same trend post-treatment (>72h): msp1 COI = 2.2, msp2 (COI = 2), ama1 (COI = 1.8) and glurp (COI = 1.2), and no significant difference was observed ( p=0.2).
Parasite clearance estimates
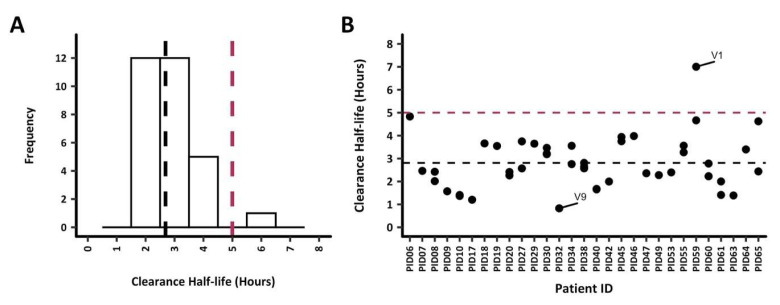
Parasite clearance half-lives among all 30 study participants were below 5h except for PID59, with a parasite clearance half-life of 5.7h ( Figure 2A). Nonetheless, the mean parasite clearance half-life was 2.8h for all participants. The extrapolation of the clearance rates to each ama1 microhaplotype (based on the number of reads to quantify each microhaplotype) per infection demonstrated a similar clearance rate across most microhaplotypes. PID16 was excluded from any subsequent analysis since data was available for 6 hours only ( Figure 2B). Participant PID32 exhibited rapid clearance of the sole detected ama1 microhaplotype V9, with a clearance half-life of less than 1 hour. On the other hand, PID06, PID59, and PID65 each presented with at least one microhaplotype with a slower clearance half-life, ranging from 4.5 to 5 hours. Notably, PID59 harboured the V1 ama1 microhaplotype with the longest clearance half-life in the study, recorded at 7 hours. There was no significant difference in the mean clearance half-lives when the microhaplotypes were grouped as major and minor (<5%) microhaplotypes (Welch two-sample t-test, p = 0.61).
Figure 2. Parasite clearance estimates (PCE) for all study participants.
Parasite clearance estimates for the 30 study participants are shown. The dotted lines represent the 5h clearance cut-off (red) and median clearance half-life, 2.6h (black). A) PCEs were calculated based on total parasitemia for each participant, the median clearance half-life was 2.7 hours. All participants had clearance half-lives <5h, however, PID59 had a clearance half-life of 5.7h. B) PCEs were calculated by extrapolating the clearance rates to each ama1 microhaplotype (based on the parasitemia and number of reads to quantify each microhaplotype). The ama1 microhaplotype V1 with the highest clearance half-life of 7h was from PID59. On the other hand, PID32 had the fastest clearing (0.8h) ama1 microhaplotype V9.
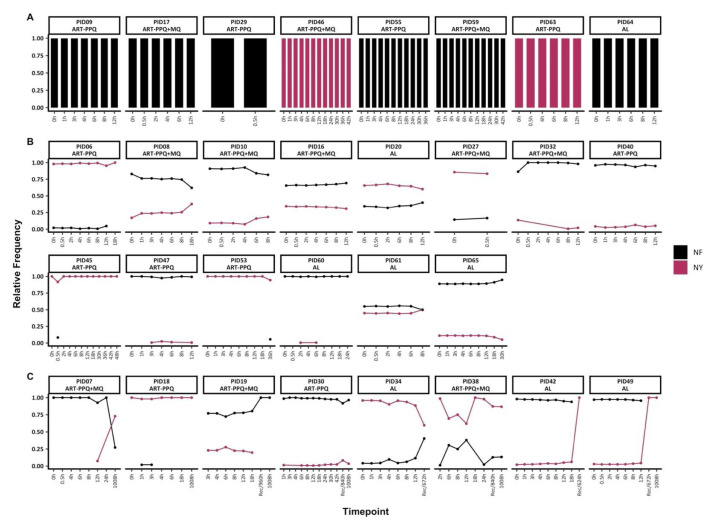
Pfmdr1 genetic diversity pre-and post-treatment
Based on the combination of amino acid polymorphisms at codons N86Y and F184Y, only two microhaplotypes (NY and NF) were detected. Of the 30 individuals with baseline data, 22 had mixed infections with both mdr1 microhaplotypes, while 8 had monoclonal mdr1 infections (either NF or NY) throughout treatment ( Figure 3A). Of the individuals with mixed mdr1 infections, 15/22 had sequence data up to 72h only, and they maintained mdr1 microhaplotypes at stable frequencies, similar to ama1. However, in two individuals (PIDs 45 and 53), there were sporadic detections of rare mdr1 microhaplotypes ( Figure 3B).
Figure 3. Temporal changes in mdr1 microhaplotypes throughout treatment.
The figure highlights the individuals with A) monoclonal infections, if they had only one mdr1 at any timepoint B) polyclonal infections, if they had more than one mdr1 microhaplotype throughout treatment and sampled up to 72h and C) polyclonal infections, if they more than one mdr1 microhaplotype throughout treatment and sampled beyond 72h. Each coloured barplot/point/line represents a unique mdr1 microhaplotype (black-NF and maroon-NY). Above each plot is the respective participant id. The x-axis represents the sampling timepoints at different hours and the recurrence time with the prefix "Rec" while the y-axis represents the relative proportions of mdr1 microhaplotypes.
In the remaining 8/22 individuals with mixed mdr1 infections and with post-treatment (>72h) sequence data, three of these individuals switched from a predominance of the NF microhaplotype to the NY microhaplotype during follow-up either during recurrence infection (unscheduled visit) or on day 42 (1008h). Only PID18 maintained the same dominant microhaplotype (NY) throughout the treatment and follow-up period on day 42 (1008h) ( Figure 3C). There was no significant difference in the frequencies of Y184 and 184F microhaplotypes in baseline samples vs samples collected from 12h onward (Chi-square test, p = 0.52).
Discussion
Children in this moderate-high malaria transmission setting in Kilifi maintained a stable homogeneous microhaplotype population throughout treatment until day 3 (72h). This is not surprising since febrile infections tend to occur with low COI even with high parasite densities ( Beck et al., 1997) and treatment reduces the establishment of new infections. Thus, the genetic homogeneity improves the confidence in determining reinfections post-treatment (day 28 onwards), such as the one individual identified with a microhaplotype at 30h and later in their recurrent infection (for an unscheduled visit at 672h). The sporadic observation of rare microhaplotypes at only a single time point potentially highlights the changes in parasite density, sequestration of parasites, lingering genetic material from dead parasites or the presence of parasites below the blood sampling limit ( Jones et al., 2021). Notably, PID27 had a COI of three at 0h, and while the relative frequencies of two ama1 microhaplotypes decreased over time, one microhaplotype continued to increase in frequency. This patient did not experience a recurrence and had no microscopically detectable parasites by 72h. Therefore, the increase in one ama1 microhaplotype may have originated from the nucleic material of dead parasites. Most of these distinct changes in ama1 microhaplotype frequencies occurred post-treatment from day 7 onwards, when reinfections are likely as the levels of drugs in the body continue to decrease. Importantly, our analysis of rare microhaplotypes was limited to a 5% cut-off based on sequencing controls to increase our confidence in calling mixed infections.
The clearance rates were similar within and between individuals irrespective of clonality, suggesting that the three antimalarial treatments were equally effective in clearing microhaplotypes and parasitaemia. Any significant deviations in microhaplotype clearance rates would signal emerging resistance during treatment if a microhaplotype was consistently cleared at a slower rate within and between individuals. This analysis identified one such individual with an estimated slow clearance of 5.7h. A closer examination of the two main microhaplotypes in this participant identified a slow clearing microhaplotype with an estimated clearance half-life of 7h. Thus, a drug-resistant microhaplotype circulating at a low frequency before treatment may survive and rapidly expand following treatment ( Ecker et al., 2012; Jafari et al., 2004). In addition to the slow clearing microhaplotype, the sole fast and three slower clearing microhaplotypes indicate the variation in an individual’s ability to clear an infection. Such infections should be interrogated to determine additional factors contributing to the slow clearing of parasites.
The mdr1 genotype in codon 86 was 100% wild type (N86). This is consistent with previous findings ( Wamae et al., 2019) in the study area of a shift from 86Y to N86 by 2018 when this study began, and thus only two microhaplotypes were observed based on codon 184. Though the sample size was small, as expected, there was no selection of codon 184 with these drugs. This is in contrast to a study conducted in Tanzania between 2002 and 2004, when there was a higher frequency of mdr1 mutant genotypes, that observed more N86 and 184F in post-treatment samples following artemether-lumefantrine treatment ( Humphreys et al., 2007).
Limitations of this study include the small sample size across the three treatment arms, which may have led to biases. For example, all but one of the recurrent infections were new infections with entirely different microhaplotypes from the pre-treatment sample by ama1 AmpSeq. Therefore, in more extensive studies, it remains to be seen how sensitive AmpSeq will be in distinguishing new vs recurrent infections compared to msp1/mps2/glurp genotyping. The observations made from a single individual with a slow clearing microhaplotype require further validation as this was based on data from only one individual. Moreover, since this study was set up to provide a proof of concept, it is a scalable assay that allows for additional drug resistance markers, such as k13, to be monitored. The low parasitaemia following treatment minimised the generation of good quality AmpSeq data, impacting the sample size. Consequently, there were limited samples to examine the changes in microhaplotypes throughout the study period. However, the findings were similar to several drug trials that predominantly identified new rather than recrudescent infections when efficacious drugs are tested ( Adegbite et al., 2019; Davlantes et al., 2018; Kakolwa et al., 2018). Additionally, immunity may play a role in clearing infections; thus, new microhaplotypes are likely present in subsequent infections. Microhaplotypes detected post-treatment might have originated from circulating gametocytes, dormant or dead parasites. However, all participants were gametocyte negative throughout treatment except PID38 who had five gametocytes/μl at 72h but was negative thereafter, so it is unlikely that gametocytaemia biased our findings. As for genetic material originating from dormant or dead parasites, parasite mRNA can also be detected up to two weeks after successful treatment in microscopy-negative individuals ( Mahamar et al., 2021). However, additional work is needed to determine whether these originate from viable infections. Finally, future studies should include artificial sequencing controls of decreasing parasitaemia from, e.g., dilutions ranging from 10,000 to 1 parasite/μl to reliably determine the limit of detection, especially in post-treatment with low parasitemia.
Improved accuracy in distinguishing infections post-treatment is essential when considering the WHO recommendation of abandoning a drug if failure rates are >10% ( WHO, 2008), determined by the number of recrudescent infections that is likely to vary based on genotyping methods used and genetic markers examined. The additional analyses of tracking variants throughout treatment improve the ability to identify dominant pre-treatment variants that appear as a new infection (in the follow-up period) and are misclassified as a recrudescent infection. Ama1 yielded comparable COI to msp1 and msp2, hence, the ama1 locus genotyped in this study is a highly discriminatory marker with a larger number of variants at a prevalence of <5% and with a high expected heterozygosity value (0.96). Coupled with the high sensitivity of amplicon sequencing to capture minor variants, this assay provides a higher resolution to better distinguish reinfections from recrudescent infections in moderate to high transmission settings where polyclonal infections are common.
The assessment of genotypes throughout treatment follows the trajectory of infection, identifies the number of infecting microhaplotypes and rare variants per individual. This allows for the early detection of emerging resistant variants by identifying slow-clearing variants. Furthermore, examining drug resistance mutation frequencies during treatment can identify distinct shifts in occurrences likely to indicate directional selection of a rapidly rising variant ( Henriques et al., 2014; Mideo et al., 2013). Given the high disease burden and high levels of reinfection in sub-Saharan Africa, there is a need for improved tools for conducting molecular assays to improve the interpretation of the genotyping outputs. In fact, a recent WHO consultation meeting concluded that AmpSeq provides the most robust and reliable genotyping method ( WHO, 2021b). The AmpSeq assay, presented in this study, highlights a potential genotyping tool for PCR correction and monitoring drug resistance markers. The need for more genotyping reference labs in Africa remains to support this endeavour.
While this study serves as a proof of concept, the sample size employed was not aimed at establishing the clear superiority of AmpSeq over msp1/msp2/glurp genotyping for tasks such as PCR correction and determining infection complexity. Moreover, the adoption of AmpSeq in low and middle-income countries (LMICs) is challenged by its current cost and the requisite molecular biology and bioinformatics expertise needed for effective implementation. Nevertheless, the constantly declining costs of next-generation sequencing, coupled with emerging technologies like the Oxford Nanopore platform that offer reduced buy-in and maintenance expenses, hint at a potentially more accessible AmpSeq in the future.
Regarding its performance, our study illustrates that AmpSeq yields results comparable to those obtained from msp1/msp2/glurp genotyping. However, AmpSeq boasts additional benefits over msp1/mps2/glurp genotyping. It demonstrates scalability advantages and heightened resolution based on sequence identity, surpassing the limitations of msp1/msp2/glurp genotyping, such as the reliance on labour-intensive gel electrophoresis-based band size analysis or fragment analysis that can be subject to interpretation errors and the inability to detect low-density parasite clones. An additional AmpSeq's strength lies in its potential for multiplexing, enabling the simultaneous identification of drug resistance markers alongside genotyping. This multiplexing capability enhances the assay's versatility and could offer valuable insights into the parasite's drug resistance profiles.
In light of these considerations, while the current prerequisites of expertise and cost might impede the immediate integration of AmpSeq in LMICs, the evolving landscape of next-generation sequencing technologies and cost reductions inspire optimism regarding AmpSeq's future accessibility. Therefore, this study recognises the prospective value and adaptability of AmpSeq for tasks like PCR correction and determining infection complexity, even though broader adoption may necessitate ongoing advancements in affordability and the availability of expertise.
Acknowledgements
We thank the staff of the Department of Molecular Tropical Medicine and Genetics, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand, for their contribution to the PCR correction of recurrent infections. Additionally, the following reagents were obtained through BEI Resources, NIAID, NIH: Genomic DNA from Plasmodium falciparum, Strain 3D7, MRA-102G, contributed by Daniel J. Carucci and genomic DNA from Plasmodium falciparum, Strain Dd2, MRA-150G, contributed by David Walliker.
Funding Statement
This work was supported by Developing Excellence in Leadership, Training and Science (DELTAS) Africa Initiative (grant number: DEL-15-003) and a Wellcome Trust Intermediate Fellowship awarded to L.I.O.-O (grant no. 107568/Z/15/Z). J.A.B and J.J.J received support from the National Institutes of Health (R01AI121558 to JAB and K24AI13499 and R01AI121558 to JJJ). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (107769/Z/10/Z) and the UK government. The views expressed in this publication are those of the authors and not necessarily those of AAS, NEPAD Agency, Wellcome Trust, or the UK government.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 4; peer review: 2 approved, 2 approved with reservations]
Data availability
Underlying data
The raw fastq files have been deposited in Zenodo under: (Fastq Files) Amplicon sequencing of ama1 and mdr1 to track within-host P. falciparum diversity in Kilifi, KENYA (Version 1) [Data set]. Zenodo. https://doi.org/10.5281/zenodo.6243929 ( Wamae et al., 2022a)
The nucleotide sequence data reported in this paper are available in the GenBank database under the accession numbers: ama1 (MZ593448 - MZ593480) and mdr1 (MZ593481 - MZ593484).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Extended data
Extended data tables and figures have been deposited in Zenodo under: (Extended Data) Amplicon sequencing of ama1 and mdr1 to track within-host P. falciparum diversity throughout treatment in a clinical drug trial (Version 1) [Data set]. DOI: https://doi.org/10.5281/zenodo.10801586 ( Wamae et al., 2022b).
This collection contains the following extended data:
Table S1: Concentration ratios and resulting parasitemia in artificial DNA mixtures of P. falciparum Lab Isolates 3D7 and Dd2. This table presents the parasitemia for the artificial mixtures of P. falciparum lab isolates 3D7 and Dd2. Each mixture was prepared at varying ratios of 3D7 to Dd2, starting from equal proportions to a complete presence of only 3D7. The original concentration of each isolate was approximately 50,000 parasites per microliter (pf/μl), and the table displays the proportion of each strain in the mixture and the resulting total parasitemia concentration.
Table S2. List of PCR and deep sequencing primers. This table shows the list of forward and reverse primers used for deep sequencing. In boldface are the MID tags, while in the regular face are the forward primers
Table S3. The relative frequencies of each ama1 variant and the number of samples with each variant. The relative frequencies (%) of the 33 AMA1 variants in pre- and post-treatment samples (n = 330) are shown as a 33 amino acid sequence. The frequencies were calculated by dividing the number of reads of each microhaplotype by the total number of reads obtained per sample (116,187,131).
Table S4. Distribution of microhaplotypes among samples. This table shows the occurrence of microhaplotypes across all participants, both with monoclonal and multiclonal ama1 infections. It presents the ama1 clonality – monoclonal or multiclonal (column 1) - participant IDs (column 2), microhaplotype IDs (column 3), and the relative frequencies of these microhaplotypes across timepoints from 0 to 1008 hours (day 42) (column 3). Dashes represent time points where microhaplotypes were missing or were not detected.
Table S5. Distribution of rare microhaplotypes among samples. This table shows the occurrence of rare microhaplotypes in various samples. It presents participant IDs (column 1), microhaplotype IDs (column 2), and the relative frequencies of these microhaplotypes across time points from 0 to 1008 hours (day 42) (column 3). Samples containing rare microhaplotypes - specifically from PID10, PID32, PID38, PID40, PID49, PID60, PID63, and PID65 - are shown in orange, along with the corresponding rare microhaplotypes and their time points of occurrence. Furthermore, participants are categorised by shared microhaplotypes to indicate instances of rarity and commonality. Except for one microhaplotype unique to PID30, rare microhaplotypes were detected in several samples, frequently exceeding a 5% relative frequency. Dashes represent time points where microhaplotypes were missing or were not detected.
Table S6. The parasitemia levels associated with each ama1 microhaplotype per timepoint. This table shows the parasitemia for each ama1 microhaplotype per timepoint and each participant. “Patient ID” represents the patient ID, “AMA1 COI at 0h” represents the complexity of infection (COI) for each participant at baseline, based on ama1 while subsequent columns represent the parasitemia for each ama1 microhaplotype from timepoint 0h to 1008h. Parasitemia was back-calculated using the COI and total parasitemia for each time point. For time points with a COI > 1, parasitemia for the respective ama1 microhaplotypes are separated by commas, cells in red indicate timepoints without sequencing data (ND = not determined). In contrast, cells in grey indicate time points where microhaplotypes were detected below 10 parasites/μl, hence at risk of falling below the sampling limit.
Figure S1. Performance of AmpSeq in the sequencing controls. Six aliquots were prepared for each control set to ensure sufficient control data in case of PCR or sequencing failure. The median read depth in the lab controls was 5,658 (range 4,310 – 12,603) and 704 (291 – 1,676). The x-axis represents the aliquot identifier across the five mixtures, starting from 1 to 6, while the y-axis represents the proportions of each variant across all aliquots. For ama1 (A), two variants (3D7 and Dd2) were detected, whereas in mdr1 (B), two variants were detected YY, FY and NY following amplification of Dd2 Copy I, Dd2 Copy II and 3D7, respectively. For ama1, sequencing failed for aliquot 6 of control set 1, while for mdr1, sequencing failed for aliquot 2 and 6 of control set 3, aliquots 1 and 6 of control set 4 and aliquots 1 and 5 of control set 5. Under the mdr1 control set 4, the Dd2 copy II (86F, 184Y) was not identified, possibly due to having very low concentrations that were not picked up in this aliquot. Based on our control mixtures, the minimum variant frequency we could detect was 5%.
Figure S2. Heatmaps of the successfully PCR amplified and sequenced samples for ama1 (A) and mdr1 (B). The rows represent the study participants, while the columns represent time in hours. Successfully sequenced samples are shown in blue, those that failed PCR are shown in red and those that failed sequencing are in black. The timepoint “ Rec” represents unscheduled visits where a recurrent sample was collected. The unshaded areas with "-" are time points where samples were not collected. For each time point, the number of samples successfully sequenced (n Successful) is indicated in the last row of each panel. The table in panel C shows the groupings of samples based on parasitemia, high (> 5,000), moderate (100-5,000) and low (< 100 parasites per microlitre). Many samples collected between 0h-12h had high parasitemia, samples collected between 18h–30h had moderate parasitemia, while samples collected after 30h were primarily of low parasitemia.
Figure S3. The mean complexity of infection (COI) by AMA1 throughout treatment. The mean COI (red diamonds) appeared to be stable (between 1.5 - 2) from baseline (0h) up to 72h and thereafter fluctuated due to the small sample sizes (<5) in the post-treatment samples. The black dots represent the COI per sample.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
References
- Adegbite BR, Edoa JR, Honkpehedji YJ, et al. : Monitoring of efficacy, tolerability and safety of artemether-lumefantrine and artesunate-amodiaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Lambaréné, Gabon: an open-label clinical trial. Malar J. 2019;18(1): 424. 10.1186/s12936-019-3015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley EA, Dhorda M, Fairhurst RM, et al. : Spread of Artemisinin Resistance in Plasmodium falciparum Malaria. N Engl J Med. 2014;371(5):411–423. 10.1056/NEJMoa1314981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balikagala B, Fukuda N, Ikeda M, et al. : Evidence of Artemisinin-Resistant Malaria in Africa. N Engl J Med. 2021;385(13):1163–1171. 10.1056/NEJMoa2101746 [DOI] [PubMed] [Google Scholar]
- Beck HP, Felger I, Huber W, et al. : Analysis of Multiple Plasmodium falciparum Infections in Tanzanian Children during the Phase III Trial of the Malaria Vaccine SPf66. J Infect Dis. 1997;175(4):921–926. 10.1086/513991 [DOI] [PubMed] [Google Scholar]
- Bhatt S, Weiss DJ, Cameron E, et al. : The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526(7572):207–211. 10.1038/nature15535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davlantes E, Dimbu PR, Ferreira CM, et al. : Efficacy and safety of artemether-lumefantrine, artesunate-amodiaquine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in three provinces in Angola, 2017. Malar J. 2018;17(1): 144. 10.1186/s12936-018-2290-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Nosten F, Yi P, et al. : Artemisinin Resistance in Plasmodium falciparum Malaria. N Engl J Med. 2009;361(5):455–467. 10.1056/NEJMoa0808859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker A, Lehane AM, Clain J, et al. : PfCRT and its role in antimalarial drug resistance. Trends Parasitol. 2012;28(11):504–514. 10.1016/j.pt.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger I, Snounou G, Hastings I, et al. : PCR correction strategies for malaria drug trials: updates and clarifications. Lancet Infect Dis. 2020;20(1):e20–e25. 10.1016/S1473-3099(19)30426-8 [DOI] [PubMed] [Google Scholar]
- Flegg JA, Guerin PJ, White NJ, et al. : Standardizing the measurement of parasite clearance in falciparum malaria: the parasite clearance estimator. Malar J. 2011;10: 339. 10.1186/1475-2875-10-339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenberg M, Lerch A, Beck HP, et al. : Amplicon deep sequencing improves Plasmodium falciparum genotyping in clinical trials of antimalarial drugs. Sci Rep. 2019;9(1): 17790. 10.1038/s41598-019-54203-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaluba M, van der Pluijm RW, Weya J, et al. : Arterolane-piperaquine-mefloquine versus arterolane-piperaquine and artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a single-centre, open-label, randomised, non-inferiority trial. Lancet Infect Dis. 2021;21(10):1395–1406. 10.1016/S1473-3099(20)30929-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway NJ, Parobek CM, Juliano JJ, et al. : SeekDeep: single-base resolution de novo clustering for amplicon deep sequencing. Nucleic Acids Res. 2018;46(4):e21. 10.1093/nar/gkx1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques G, Hallett RL, Beshir KB, et al. : Directional selection at the pfmdr1, pfcrt, pfubp1, and pfap2mu loci of Plasmodium falciparum in Kenyan children treated with ACT. J Infect Dis. 2014;210(12):2001–2008. 10.1093/infdis/jiu358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys GS, Merinopoulos I, Ahmed J, et al. : Amodiaquine and Artemether-Lumefantrine Select Distinct Alleles of the Plasmodium falciparum mdr1 Gene in Tanzanian Children Treated for Uncomplicated Malaria. Antimicrob Agents Chemother. 2007;51(3):991–997. 10.1128/AAC.00875-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari S, Le Bras J, Bouchaud O, et al. : Plasmodium falciparum clonal population dynamics during malaria treatment. J Infect Dis. 2004;189(2):195–203. 10.1086/380910 [DOI] [PubMed] [Google Scholar]
- Jones S, Kay K, Hodel EM, et al. : Should Deep-Sequenced Amplicons Become the New Gold Standard for Analyzing Malaria Drug Clinical Trials? Antimicrob Agents Chemother. 2021;65(10): e0043721. 10.1128/AAC.00437-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakolwa MA, Mahende MK, Ishengoma DS, et al. : Efficacy and safety of artemisinin-based combination therapy, and molecular markers for artemisinin and piperaquine resistance in Mainland Tanzania. Malar J. 2018;17(1): 369. 10.1186/s12936-018-2524-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassambara A: ggpubr: 'ggplot2' Based Publication Ready Plots.2020. Reference Source
- Liljander A, Wiklund L, Falk N, et al. : Optimization and validation of multi-coloured capillary electrophoresis for genotyping of Plasmodium falciparum merozoite surface proteins ( msp1 and 2). Malar J. 2009;8: 78. 10.1186/1475-2875-8-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamar A, Lanke K, Graumans W, et al. : Persistence of mRNA indicative of Plasmodium falciparum ring-stage parasites 42 days after artemisinin and non-artemisinin combination therapy in naturally infected Malians. Malar J. 2021;20(1): 34. 10.1186/s12936-020-03576-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mideo N, Bailey JA, Hathaway NJ, et al. : A deep sequencing tool for partitioning clearance rates following antimalarial treatment in polyclonal infections. Evol Med Public Health. 2016;2016(1):21–36. 10.1093/emph/eov036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mideo N, Kennedy DA, Carlton JM, et al. : Ahead of the curve: next generation estimators of drug resistance in malaria infections. Trends Parasitol. 2013;29(7):321–8. 10.1016/j.pt.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Hathaway NJ, Kharabora O, et al. : A deep sequencing approach to estimate Plasmodium falciparum complexity of infection (COI) and explore apical membrane antigen 1 diversity. Malar J. 2017;16(1): 490. 10.1186/s12936-017-2137-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M: Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978;89(3):583–590. 10.1093/genetics/89.3.583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noedl H, Se Y, Schaecher K, et al. : Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359(24):2619–20. 10.1056/NEJMc0805011 [DOI] [PubMed] [Google Scholar]
- Okell LC, Reiter LM, Ebbe LS, et al. : Emerging implications of policies on malaria treatment: genetic changes in the Pfmdr-1 gene affecting susceptibility to artemether–lumefantrine and artesunate–amodiaquine in Africa. BMJ Glob Health. 2018;3(5): e000999. 10.1136/bmjgh-2018-000999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parobek CM, Parr JB, Brazeau NF, et al. : Partner-drug resistance and population substructuring of artemisinin-resistant plasmodium falciparum in Cambodia. Genome Biol Evol. 2017;9(6):1673–1686. 10.1093/gbe/evx126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polley SD, Conway DJ: Strong diversifying selection on domains of the Plasmodium falciparum apical membrane antigen 1 gene. Genetics. 2001;158(4):1505–12. 10.1093/genetics/158.4.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snounou G, Beck HP: The use of PCR genotyping in the assessment of recrudescence or reinfection after antimalarial drug treatment. Parasitol Today. 1998;14(11):462–467. 10.1016/s0169-4758(98)01340-4 [DOI] [PubMed] [Google Scholar]
- Uwimana A, Umulisa N, Venkatesan M, et al. : Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis. 2021;21(8):1120–1128. 10.1016/S1473-3099(21)00142-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pluijm RW, Amaratunga C, Dhorda M, et al. : Triple Artemisinin-Based Combination Therapies for Malaria - A New Paradigm? Trends Parasitol. 2021;37(1):15–24. 10.1016/j.pt.2020.09.011 [DOI] [PubMed] [Google Scholar]
- van der Pluijm RW, Imwong M, Chau NH, et al. : Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019;19(9):952–961. 10.1016/S1473-3099(19)30391-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pluijm RW, Tripura R, Hoglund RM, et al. : Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial. Lancet. 2020;395(10233):1345–1360. 10.1016/S0140-6736(20)30552-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga MI, Dhingra SK, Henrich PP, et al. : Globally prevalent PfMDR1 mutations modulate Plasmodium falciparum susceptibility to artemisinin-based combination therapies. Nat Commun. 2016;7: 11553. 10.1038/ncomms11553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamae K, Ndwiga L, Kharabora O, et al. : (Fastq Files) Amplicon sequencing of ama1 and mdr1 to track within-host P. falciparum diversity in Kilifi, KENYA (Version 1). [Data set]. Zenodo.2022a. 10.5281/zenodo.6243929 [DOI] [PMC free article] [PubMed]
- Wamae K, Ndwiga L, Kharabora O, et al. : (Extended Data) Amplicon deep sequencing of ama1 and mdr1 to track within-host P. falciparum diversity throughout treatment in a clinical drug trial (Version 1).[Data set]. Zenodo.2022b. 10.5281/zenodo.6253570 [DOI] [PMC free article] [PubMed]
- Wamae K, Okanda D, Ndwiga L, et al. : No evidence of P. falciparum K13 artemisinin conferring mutations over a 24-year analysis in Coastal Kenya, but a near complete reversion to chloroquine wild type parasites. Antimicrob Agents Chemother. 2019;63(12):e01067–19. 10.1128/AAC.01067-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO: Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria.World Health Organization (Ref.No# WHO/HTM/RBM/2003.50),2003. Reference Source
- WHO: Informal consultation on methodology to distinguish reinfection from recrudescence in high malaria transmission areas.2021b. Reference Source
- WHO: Methods and techniques for clinical trials on antimalarial drug efficacy: Genotyping to identify parasite populations.World Health Organization,2008. Reference Source
- WHO: World Malaria Report 2021.2021a. Reference Source
- Wickham H: ggplot2: Elegant Graphics for Data Analysis.2016. Reference Source [Google Scholar]