Abstract
Insulin signaling plays a critical role in regulating various aspects of insect biology, including development, reproduction, and the formation of wing polyphenism. This leads to differentiation among insect populations at different levels. The insulin family exhibits functional variation, resulting in diverse functional pathways. Aphis gossypii Glover, commonly known as the cotton‐melon aphid, is a highly adaptable aphid species that has evolved into multiple biotypes. To understand the genetic structure of the insulin family and its evolutionary diversification and expression patterns in A. gossypii, we conducted studies using genome annotation files and RNA‐sequencing data. Consequently, we identified 11 insulin receptor protein (IRP) genes in the genomes of the examined biotypes. Among these, eight AgosIRPs were dispersed across the X chromosome, while two were found in tandem on the A1 chromosome. Notably, AgosIRP2 exhibited alternative splicing, resulting in the formation of two isoforms. The AgosIRP genes displayed a high degree of conservation between Hap1 and Hap3, although some variations were observed between their genomes. For instance, a transposon was present in the coding regions of AgosIRP3 and AgosIRP9 in the Hap3 genome but not in the Hap1 genome. RNA‐sequencing data revealed that four AgosIRPs were expressed ubiquitously across different morphs of A. gossypii, while others showed specific expression patterns in adult gynopara and adult males. Furthermore, the expression levels of most AgosIRPs decreased upon treatment with the pesticide acetamiprid. These findings demonstrate the evolutionary diversification of AgosIRPs between the genomes of the two biotypes and provide insights into their expression profiles across different morphs, developmental stages, and biotypes. Overall, this study contributes valuable information for investigating aphid genome evolution and the functions of insulin receptor proteins.
Keywords: aphid, gene variation, genome, population evolution, RNA‐sequencing, transposon
Eleven IRPs were identified in Aphis gossypii. AgosIRP2 exists as an alternative splicing site. Most AgosIRP expression levels decreased when treated with pesticide.
1. INTRODUCTION
Aphis gossypii Glover, commonly known as the cotton‐melon aphid, is a significant agricultural pest that causes substantial economic losses. It poses a threat by aggregating in large numbers to feed on plant sap and transmitting viral diseases to host plants (Blackman & Eastop, 2000). A. gossypii is highly polyphagous, capable of infesting over 600 plant species, including important crops like cotton and cucumber (Ebert & Cartwright, 1997). The ability of A. gossypii biotypes to adapt to different host plants is clearly distinct (Carletto et al., 2009; Zhang et al., 2018). In a study using the mitochondrial cytb gene region, researchers identified 57 haplotypes from 1046 A. gossypii individuals in northern China. Among these, Hap1 and Hap4 were the most prevalent biotypes, predominantly found on cotton plants, while Hap3 was the most common biotype associated with cucumber plants (Zhang et al., 2018).
Aphis gossypii exhibits a wide distribution across various geographical regions, and its ability to adapt to different host plants has contributed to its widespread prevalence. This adaptability is facilitated by its rapid evolutionary processes. A. gossypii has evolved into complex life cycles, reproductive strategies, host biotypes, wing polyphenism, and pesticide resistance to cope with different climates, hosts, and environments (Kwon & Kim, 2017; Loxdale & Balog, 2018; Shi et al., 2010; Wang et al., 2016; Zeng et al., 2021). Additionally, the body size and fertility of A. gossypii can be influenced by the nutritional conditions provided by the host plants (Nevo & Coll, 2001).
Hormones play a crucial role in mediating alternative phenotypes in insects (Nijhout, 1999). Juvenile hormones, ecdysteroid, and insulin (insulin‐related peptides, IRPs) are the three most important hormones (Ogawa & Miura, 2014; Simon et al., 2010). The insulin signaling pathway, which operates through a highly conserved signaling transduction pathway, is involved in determining and regulating various phenotypes in insects. In aphids, the insulin signaling pathway has been extensively investigated in Acyrthosiphon pisum for its role in wing dimorphism (Grantham et al., 2020; Shang et al., 2020), control of aphid life cycle during long‐day seasons (Barberà et al., 2019), and regulating embryo development (Guo et al., 2016). Additionally, the insulin signaling pathway has been reported as essential for the successful transition from nymph to the adult stage in Aphis (Toxoptera) citricidus (Ding et al., 2017).
Due to the functional diversity of the insulin signaling pathway, the number and structure of IRPs differ significantly between insect species. The number of IRP genes is ranged between 3 and 15 among aphids (Huygens et al., 2022; Nässel & Broeck, 2016). However, the functions of most aphid IRPs remain poorly characterized, as the annotation of IRPs in aphid genomes is still incomplete (Huybrechts et al., 2010). In a previous study using the A. gossypii genome (ASM401081v1), 10 IRPs were annotated (Huygens et al., 2022). Recently, chromosome‐level genome assemblies of two biotypes of A. gossypii were released (Zhang et al., 2022). By reannotating the IRPs in these two A. gossypii biotypes and utilizing RNA‐sequencing data, we aimed to investigate their genomic structures, gene variations, and transcriptional expression patterns. This analysis could provide new insights into the evolution and function of insulin signaling in aphids.
2. MATERIALS AND METHODS
2.1. IRP identification
A Hidden Markov Model (HMM) pattern analysis, based on the insulin protein family database (Pfam, PF00049 of insect), was used to identify the IRPs in Hap1 and Hap3 genome data of A. gossypii (Majoros et al., 2004; Zhang et al., 2022). Meanwhile, amino acid sequences of insect IRPs from GenBank (http://www.ncbi.nlm.nih.gov) were selected as template sequences and homology‐based searches were carried out in Hap1 and Hap3 genomes. Finally, putative IRPs of A. gossypii were manually corrected and compared to previously reported IRP sequences of the insect (Huygens et al., 2022).
2.2. Genome‐level variation analysis of IRPs between Hap1 and Hap3
Two methods were used to determine the position on the genome and to find the possible duplication of AgosIRPs genes; first, extraction of the position of all genes from the Hap1 and Hap3 genome annotation files were carried out (Zhang et al., 2022) and second, the putative positions of all genes were obtained by BLAST‐aligned AgosIRPs genes to the genome assemblies. Finally, the putative AgosIRPs position on the genomes was manually amended. To check the genome‐level variation of AgosIRPs, the gene fragments between upstream of gene stop and downstream of gene start were obtained from Hap1 and Hap3, respectively. Based on the data, the coding exon/intron structures were analyzed. In order to compare the IRPs genome sequences, deletions, insertions, and substitutions in Hap1 and Hap3 were carried out on MAFFT (version 7) with default parameters (Katoh et al., 2019). The CENSOR software tool was used to select the insect database to identify the type of transposon in the sequence (Kohany et al., 2006).
2.3. Insect samples and RNA‐sequences
Wingless individuals of A. gossypii were collected from cotton and cucumber plants sown in Jiangsu province, China (32.392° N, 119.422° E). The biotypes were determined as previously reported (Zhang et al., 2016, 2018), and single mother‐generated populations were obtained by rearing on the host plant leaves. Four populations of each biotype, Hap1, and Hap3, were chosen and reared on cotton and cucumber leaves under controlled conditions at 26 ± 1°C temperature, 65 ± 5% relative humidity with a photoperiod of 14 h light and 10 h dark. For RNA‐sequencing, one sample per population (Hap1 and Hap3 populations), each sample containing 50 wingless aphids of mixed ages, was selected. The whole body of the insect was used to extract total RNA, purified the mRNA for library generation, and RNA‐sequencing and raw reads were processed by FASTQC and Trimmomatic softwares (Bolger et al., 2014).
2.4. IRP gene structure and variation analysis
To determine the predicted transcripts from the genome and discover new transcripts, de novo assembly was performed by using TRINITY software (Grabherr et al., 2011). RNA‐sequencing data, including Hap1, Hap3, and 50 Sequence Read Archive (SRA) files, were downloaded from the NCBI public database. The SRA files correspond to four morphs of an adult aphid, A. gossypii, including alate parthenogenetic females, apterous parthenogenetic females, gynoparae, and male, different development stages of male and neonicotinoid insecticides treated samples (Table S1). Both mitochondrial cytb and AgosIRPs sequences were selected from de novo transcript sequences by homology searches using BLASTn. Mitochondrial cytb sequences were used to determine the biotypes of A. gossypii, according to a previous report (Zhang et al., 2018). Selected AgosIRPs sequences were manually adjusted for further structural analysis. The nucleic acid sequence and protein sequence of AgosIRPs were examined using the Vector NTI software. Alternative splicing of AgosIRPs was revealed by aligned RNA‐sequencing read to AgosIRPs coding region, using the trial version of Geneious software. Signal peptides were identified in all AgosIRPs using the SignalP prediction software. Potential cleavage sites of insulin were predicted at either specific single or pairs of basic residues of the general formula (R/K)–Xn–(R/K) (Seidah & Chrétien, 1997).
2.5. Phylogenetic tree construction
The IRP transcript sequences of aphids were searched using AgosIRPs in the NCBI website using both BLASTn and BLASTp tools. Whole genome annotation data of Aphis glycines, Eriosoma lanigerum, Pentalonia nigronervosa, and Rhopalosiphum padi were downloaded from AphidBase (https://bipaa.genouest.org/is/aphidbase/), the IRP transcript sequences were obtained performing local BLAST search. After manual processing, the transcript sequences of the IRPs were used to construct a phylogenetic tree using the PhyloSuite software (Zhang et al., 2020). Graphical representation of the tree was performed with iTOL (https://itol.embl.de/).
2.6. Expression analyses of IRPs in A. gossypii
Quantification of the AgosIRPs was carried out at the transcript level with Salmon (v0.12.0). The transcript per million (TPM) value was used to reads counts by normalizing the gene length first and then by stabilizing the sequencing depth. In this study, we analyzed the expression profile of AgosIRPs in several RNA‐sequencing libraries corresponding to different A. gossypii morphs (males, sexual females, parthenogenetic females, and winged females) are publicly available in the NCBI SRA database (Table S1). The expression cutoff of at least two fragments per library was mapped. SPSS (version 20.0) was used to evaluate the differences in TPM values. To analyze the differences between the data with normal distribution, ANOVA was performed, while to analyze the data without normal distribution, the Mann–Whitney U test was carried out.
3. RESULTS AND DISCUSSION
3.1. IRPs identification in A. gossypii
To date, there has been limited in‐depth research on IRPs in A. pisum. The exact numbers of IRPs in other aphid species have not been conclusively reported yet (Cuti et al., 2021; Gronke et al., 2010; Guo et al., 2016; Huygens et al., 2022; Shang et al., 2012). In this study, we identified a total of 11 insulin‐related peptide genes (AgosIRP1‐AgosIRP11) in two biotypes of A.gossypii. Among these 11 AgosIRPs, 10 genes were already present in the NCBI‐annotated database, which were based on the genomes previously published by our research group (Zhang et al., 2022). AgosIRP11 was a novel IRP identified in A. gossypii, belonging to the insulin‐like growth factor superfamily. The IRPs number varied from 3 to 15 among aphids, and this variability could be attributed to the division of aphid species and the limited availability of comprehensive genomic information for all aphid species. Different aphid species may possess different numbers of IRPs due to evolutionary adaptations and species‐specific requirements (Huygens et al., 2022). Genomic data have proven to be valuable in improving the annotation of insect genes, including those encoding IRPs. By comparing the IRP gene sequences obtained from annotated genomes, such as those of A. pisum, Myzus persicae, and Rhopalosiphum maidis, to the sequences in the NCBI database, significant alignments were observed. These alignments were mostly generated from high‐quality genome assemblies. However, to determine the exact number of IRPs in each aphid species, further investigations are necessary, particularly through the analysis of aphid genomic databases. Continued research and exploration of aphid genomes will provide valuable insights into the diversity and characteristics of IRPs in different aphid species.
3.2. IRPs in Hap1 and Hap3 A. gossypii genomes
In A. gossypii, the species under investigation, the genome consists of four chromosomes, including one sex chromosome (X) and three autosomes. Among the insulin‐related peptide (AgosIRP) genes, eight genes were found scattered across the X chromosome. The distance between AgosIRP5 and AgosIRP6 on the X chromosome was found to be the shortest, measuring 65 kb. On the other hand, the remaining three AgosIRPs were located on the A1 chromosome, with two of them arranged in a tandem fashion (Figure 1). Interestingly, in aphids, it appears that majority of IRP genes are situated on the X chromosome. For instance, in the case of A. pisum, four ApisIRPs were located on the X chromosome, while three ApisIRPs were found on autosomes (Huygens et al., 2022). However, the distribution pattern of IRP genes differs in other insect species, such as Diptera. For example, in Anopheles gambiae, a species of mosquito, out of the seven insulin‐like peptide genes, five were located on autosomes, while two were found on the X chromosome (Krieger et al., 2004). Similarly, in D. melanogaster, five DILP genes were situated on autosomes, and two were present on the X chromosome. The region surrounding the DILP genes in Drosophila has provided evidence of ongoing adaptive processes, as suggested by patterns of genetic variation in proximity to these genes (Guirao‐Rico & Aguade, 2013).
FIGURE 1.

Schematic of the AgosIRPs located on Aphis gossypii chromosomes.
The comparison of coding and non‐coding regions can provide insights into the reported differences in host ranges and life cycle diversity between Hap1 and Hap3 in A. gossypii (Andolfatto, 2005). The coding exon number in AgosIRPs were ranged from 2 to 4; however, no variation in coding exon numbers were observed among Hap1 and Hap3. The length of the coding regions varied, with the longest region measuring 3.4 kb and the shortest region measuring 0.5 kb. Four AgosIRP coding regions were conserved between Hap1 and Hap3, with only one having single‐nucleotide polymorphism (SNP) in each gene. However, two AgosIRP coding regions, AgosIRP3 and AgosIRP9, showed difference of more than 2 kb length between Hap1 and Hap3. Further analysis showed that that these regions contained transposon insertions, specifically the piggyBac transposon in AgosIRP3 and the SINE2 transposon in AgosIRP9 (Figure 1).
It should be noted that changes in the number of repetitive DNA elements, including transposons, contribute to the variation in genome size between Hap1 and Hap3 (Zhang et al., 2022). Transposons are repetitive DNA elements capable of moving within the genome and are known to play a significant role in insect adaptation. For example, transposon insertions in the genome of M. persicae have been associated with potent insecticide resistance (Panini et al., 2021). However, the specific functions of the transposons inserted in the coding regions of AgosIRPs remain unidentified. It is worth mentioning that the piggyBac transposon system is widely used for genetic manipulation in insects and has been reported in A. gossypii, with nine piggyBac transposons identified in a previous study (Luo et al., 2011; Yusa, 2015). These transposons exhibit high sequence similarity with each other.
3.3. IRPs diversity among aphids
In our study, we performed a comparison of IRPs in aphids to investigate gene evolution. We utilized aligned amino acid sequences and constructed a phylogenetic tree based on nucleic acid sequences (Figures 2 and 3). Among the IRPs identified in A gossypii, AgosIRP6 and AgosIRP7 represented two typical insulin‐like peptides. Similarly, in pea aphids, four IRPs were found, while other aphid species from different genera also exhibited two typical insulin‐like peptides, except for R. maidis, which had only one typical insulin‐like peptide (Huygens et al., 2022). The conservation of these two IRPs across aphids was evident, with an overall minimum range of 74% amino acid identity.
FIGURE 2.

Predicted structure and gene variation of IRPs between aphids. Sequence names are indicated by a prefix formed from the abbreviated species name (Aphis craccivora, Acra; Aphis glycines, Agly; Acyrthosiphon pisum, Apis; Daktulosphaira vitifoliae, Dv; Diuraphis noxia, Dnox; Melanaphis sacchari, Msac; Myzus persicae, Mper; and Rhopalosiphum maidis, Rmai), followed by the IRP protein sequences accession number.
FIGURE 3.

Phylogenetic tree based on nuclear acid sequences of IRP transcripts from various aphid species. IRP transcripts sequence was aligned with MAFFT v7.505 using “‐‐auto” strategy and codon alignment mode. Gap sites were removed with trimAI v1.2rev57 using “‐automated1” command. ModelFinder v2.2.0 was used to select the best‐fit model using BIC criterion. Maximum likelihood phylogenies were inferred using IQ‐TREE v2.2.0 under the model automatically selected by IQ‐TREE (“Auto” option in IQ‐TREE) for 20,000 ultrafast bootstraps, as well as the Shimodaira–Hasegawa–like approximate likelihood‐ratio test. The IRPs from different species are highlighted by various colors. The labels include the species name and the corresponding IRP transcript accession number.
Furthermore, we identified homologous amino acid sequences of AgosIRP3 in eight other aphid species. All of these genes exhibited a characteristic pattern of 12 amino acid residues between two cysteine residues in the B chain, but variations were observed in the A chain. A. gossypii, M. persicae, and Aphis craccivora possessed an additional residue in the A chain, resulting in a CC(X)4C(X)8C motif (Figure 2). This motif has also been observed in DILPs of Drosophila melanogaster and ILPs of mosquitoes but differs from the IRPs found in aphids. It is worth noting that these genes with 11 amino acid residues between two cysteine residues in the B chain have been implicated in various physiological processes such as adult fat body function, lifespan extension, and determination of adult body size in previous studies (Bai et al., 2012; Okamoto et al., 2009; Sharma et al., 2019).
AgosIRP2 and AgosIRP8 belong to the highly conserved IRP family, with a higher amino acid identity (>88% and >84%, respectively). The AgosIRP2 family contains seven aphid species, while the AgosIRP8 family contains three aphid species belonging to the Aphis genus. Interestingly, the AgosIRP2 family genes were located on two distinct branches of phylogenetic tree. Among seven aphids, ApisIRP6 showed >74% full lengths amino acid identity. AgosIRP9 represents another gene that exhibits a structural similarity to insulin‐like growth factors, showing >62% amino acid identity across its full length among the five aphid IRPs (Figures 2 and 3).
On the other hand, AgosIRP4, AgosIRP5, and AgosIRP11 display an additional residue in the A chain, resulting in a CC(X)3C(X)9C motif (Figure 2). The AgosIRP10 homologous genes are conserved among four aphid species, with almost identical amino acid sequences at the C‐terminal. In contrast, the AgosIRP4 and AgosIRP5 homologous genes are conserved across aphid species at the N‐terminal, suggesting that these genes may have evolved, diverged, and duplicated from a common ancestor (Figure 2; Irwin, 2021).
3.4. IRPs diversity between two biotypes A. gossypii
The divergence between Hap1 and Hap3 of A. gossypii occurred approximately 15.72 M.Y.B.P. (Zhang et al., 2022). Despite this divergence, the IRPs in these two biotypes remain highly conserved. Only three IRPs have been identified as exhibiting diversity, and among them, AgosIRP3 and AgosIRP9 have a single SNP each, resulting in one amino acid difference between Hap1 and Hap3, respectively. Notably, these variant amino acids are not located on any functional chain (Figure 4). It is worth mentioning that variations in IRP genes within species are rarely reported. However, in Anopheles gambiae, a SNP was detected in the gene encoding insulin‐like peptide, which was suggested to be associated with parasite infection (Horton et al., 2010).
FIGURE 4.

Predicted structure and gene variation of AgosIRPs between Hap1 and Hap3. (a) AgosIRP2 exists in one alternative splicing site in ORF region, and is present in two isoforms (AgosIRP2a and AgosIRP2b); amino acid different between Hap1 and Hap3 of AgosIRP3 (b) and AgosIRP9 (c). Disulfide bonds between conserved cysteines are indicated by yellow color; the black dot below the sequence indicates the signal peptide; the solid black line below the sequence indicates the A chain or B chain; and black five‐pointed star denote canonical prohormone convertase or furin cleavage sites.
In insects, alternative splicing is a common feature that leads to the creation of multiple protein isoforms, which increases diversity of proteome (Zhao et al., 2021). Insulin signaling pathway regulates several genes through alternative mRNA splicing, even the insulin receptor has known splice variants (Västermark et al., 2013). Sexual differentiation in the Bombyx mori is regulated by sex‐specific splicing of the protein that binds to the mRNA of insulin‐like growth factor II (Suzuki et al., 2014). In this study, we reported a novel mRNA alternative splicing of A. gossypii. Specifically, AgosIRP2 was found to undergo alternative splicing, resulting in two isoforms: AgosIRP2a and AgosIRP2b (Figures 4 and 5). This splice variant resulted in the short isoform, AgosIRP2b, because it lacks the third coding exons and creates mRNA with premature stop codon at beginning of fourth coding exons. AgosIRP2 showed features similar to insulin‐like growth factor, and the portion that is absent, corresponds to entire E peptides (Figure 4). In vertebrates such as humans, mice, and sheep, insulin‐like growth factor genes undergo alternative splicing to produce multiple isoforms, and different transcripts contain distinct E peptides in these isoforms (Dai et al., 2010; Song et al., 2021). However, complete absence of the entire E peptide region, as observed in AgosIRP2b, has not been reported in insect‐based IRPs before. The E peptides may function either alone or in conjunction with mature insulin‐like growth factor (Brisson & Barton, 2012; Hede et al., 2012). Further studies are required to investigate the biological role of E peptides in A. gossypii.
FIGURE 5.

Mapped RNA‐seq reads were used to show novel mRNA alternative splicing in AgosIRP2.
3.5. Expression profile of IRPs in polymorphism A. gossypii
It is observed that A. gossypii primarily reproduces parthenogenetically for most of the year, with alate or apterous parthenogenetic females being produced. However, in the fall, A. gossypii is capable to produce alate gynoparae and males. The gynoparae migrate to primary host plants, where they give rise to sexual females that mate with alate males. The mated females then lay fertilized eggs, which undergo overwintering (Kwon & Kim, 2017; Liu et al., 2014; Miura et al., 2003). In the case of A. pisum, both sexual females and males are produced by gynoparae (Miura et al., 2003). Based on RNA‐sequencing data, it was found that four AgosIRPs (AgosIRP2, AgosIRP3, AgosIRP6, and AgosIRP7) are ubiquitously expressed in different morphs of A. gossypii. All four genes are located on the A1 chromosome, except for AgosIRP2, which is located on the X chromosome. These four AgosIRPs show uniform and high expression values, except for AgosIRP7, which exhibits the highest expression in adult gynoparae. Interestingly, the remaining seven AgosIRPs show specific expression patterns, being expressed only in adult males with low TPM values (Table 1). It is important to note that most of the RNA‐sequencing libraries used in this study were constructed using adult aphids. However, since AgosIRPs may play specific roles in different developmental stages, tissues, or cells, further studies are needed with additional samples from various developmental stages and tissues to confirm the expression patterns of IRPs in A. gossypii.
TABLE 1.
Expression profile of AgosIRPs in Aphis gossypii polymorphism.
| Genes | Wing parthenogenetical adult | Wingless parthenogenetical adult | Gynopara adult | Male | Sexual female adult |
|---|---|---|---|---|---|
| AgosIRP1 | ND | ND | ++ | + | ND |
| AgosIRP2 | +++ | ++++ | ++++ | +++ | ++++ |
| AgosIRP3 | +++ | +++ | ++++ | +++ | +++ |
| AgosIRP4 | ND | ND | ++ | ++ | ND |
| AgosIRP5 | ND | ND | ++ | ++ | ND |
| AgosIRP 6 | ++ | ++ | +++ | +++ | ++ |
| AgosIRP7 | + | + | ++ | + | + |
| AgosIRP8 | ND | ND | ++ | ++ | ND |
| AgosIRP9 | ND | ND | ++ | + | ND |
| AgosIRP10 | ND | ND | ++ | + | ND |
| AgosIRP11 | ND | ND | + | + | ND |
Note: Sequence Read Archive (SRA) files were downloaded from the NCBI public database, more than three SRA files in each polymorphism except sexual female adult, which only have one SRA file. +, TPM (transcript per million) value <1; ++, 1 ≤ TPM value <50; +++, 50 ≤ TPM value <100; ++++, 100 ≤ TPM value.
Abbreviation: ND, not determined.
3.6. Gene structure and expression patterns of IRPs in A. gossypii
In this study, the expression levels of AgosIRPs were analyzed in four morphs of adult aphid (alate parthenogenetic females, apterous parthenogenetic females, gynoparae, and males), different development stages of male, two biotypes (Hap1 and Hap3) and neonicotinoid insecticides treated samples (Hirata et al., 2017). The RNA‐sequencing data were analyzed with Kallisto, mostly used to align more distinct transcripts with very low TPM values (Ajaykumar & Yang, 2022). A. gossypii was found to contain four AgosIRPs that are ubiquitously expressed. Among these, AgosIRP6 and AgosIRP7 show structural similarities to genes that produce insulin, AgosIRP2 shows structural similarities to insulin‐like growth factors, and AgosIRP3 is a structurally divergent IRP similar to ApisIRP11 (Huygens et al., 2022).
Two spliced isoforms, AgosIRP2a and AgosIRP2b, were detected in all RNA‐sequenced samples. Generally, AgosIRP2b exhibited higher expression levels than AgosIRP2a in the four morphs of adult aphids. For example, in alate and apterous parthenogenetic females, the expression levels of AgosIRP2b were 2.4‐ and 3.2‐fold higher than AgosIRP2a, respectively (Figure 6). Both spliced isoforms produce the same mature IRP, but AgosIRP2a includes an additional enzymatic cleavage that generates an E peptide. The presence of this additional enzymatic cleavage in AgosIRP2a suggests that it may produce mature IRP at a slower pace compared to AgosIRP2b. The fact that the pathway utilizing AgosIRP2a to produce mature IRP was not abandoned indicates that the E peptide may play a role in A. gossypii.
FIGURE 6.
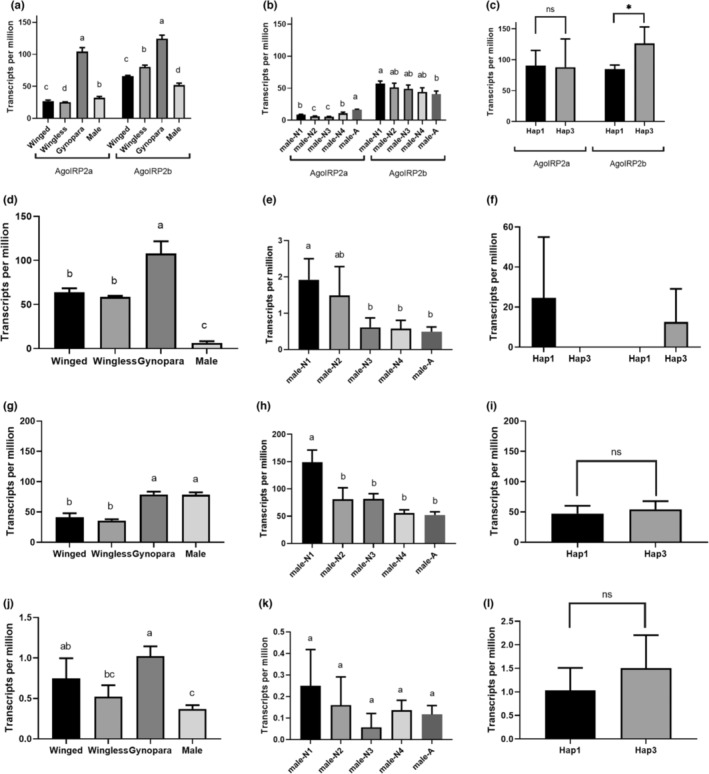
Relative expression quantity of AgosIRPs in polymorphism Aphis gossypii. AgosIRP2 in four morphs of A. gossypii adult aphid (including alate parthenogenetic females, apterous parthenogenetic females, gynoparae, and male) (a), different development stages of male (b), biotypes Hap1 and Hap3 (c); AgosIRP3 in four morphs of A. gossypii adult aphid (including alate parthenogenetic females, apterous parthenogenetic females, gynoparae, and male) (d), different development stages of male (e), biotypes Hap1 and Hap3 (f); AgosIRP6 in four morphs of A. gossypii adult aphid (including alate parthenogenetic females, apterous parthenogenetic females, gynoparae, and male) (g), different development stages of male (h), biotypes Hap1 and Hap3 (i); AgosIRP7 in four morphs of A. gossypii adult aphid (including alate parthenogenetic females, apterous parthenogenetic females, gynoparae, and male) (j), different development stages of male (k), biotypes Hap1 and Hap3 (l). First instar nymph male (male‐N1), second instar nymph male (male‐N2), third instar nymph male (male‐N3), fourth instar nymph male (male‐N4), and adult male (male‐A). The Y‐axis was the transcript per million (TPM) value (mean ± standard error of the mean). Different lowercase letters (a–d) indicate significant differences between treatments (p < .05) according to the post hoc Tukey's HSD method; *p < .05, ns, no significant difference, data were analyzed by Mann–Whitney U test.
Gynoparae, which are produced under short‐day conditions and give rise to sexual females, showed the highest expression levels of both AgosIRP2a and AgosIRP2b compared to the smallest samples of apterous parthenogenetic females and males. The expression levels of AgosIRP2a and AgosIRP2b in adult gynoparae were 4.1‐ and 2.4‐fold higher, respectively, than in apterous parthenogenetic females and males (Figure 6). The presence of the female embryo in the adult gynoparae samples suggests that further research is needed to understand the reasons behind the high expression of AgosIRP2 in gynoparae.
Among the four morphs, gynoparae expressed AgosIRP2 at the highest levels, along with the other three widely expressed AgosIRPs. All four of the AgosIRPs are ubiquitously expressed, with the exception of AgosIRP6 having the lowest levels of expression in male. Male was a special morph of aphid that only appeared once a year, and possesses only one X chromosome. In all male developmental stages, AgosIRP2a has lower expression levels than AgosIRP2b, and this difference is just 0.4‐fold at adult stage (Figure 6). The TPM values of AgosIRP2a ranged from 5.6 to 10.8 during the nymph stages while exhibiting highest expression levels at adult stage, with TPM value of 16.5. The expression level of AgosIRP2b was decreased with the maturation of male, the TPM value was 57.5 in first instar nymph and 40.5 in mature male (Figure 6).
Apterous viviparous parthenogenetic females were the main morph during aphid outbreaks in the growing season, while alate parthenogenetic females develop when aphid densities increase or the quality of the host plant deteriorates (Brisson, 2010). Apterous females are larger and produce more offspring than alate morphs. Several A. pisum insulin‐related peptide genes reported with significant difference in third instar, winged, and wingless nymphs (Guo et al., 2016). In this study, four ubiquitously expressed AgosIRPs showed no significant differences between apterous parthenogenetic adult females and alate parthenogenetic adult females. This suggests that AgosIRPs may play roles at the stage when winged and wingless nymphs show significant expression level differences.
Transposable element (TE) insertions can affect the up‐ or down‐regulation of immune‐related genes (Rech et al., 2022). In the study, a TE insertion was found in the AgosIRP3 and AgosIRP9 genomic regions, which was not present in Hap3 A. gossypii. AgosIRP9 was exclusively found in samples from gynoparae and males, indicating a potential involvement in aphid sexual reproduction stages. However, further analysis revealed that the sexual samples came from an uncharacterized biotype, Hap4 A. gossypii, suggesting the need for further study to understand the function of TE insertions in AgosIRP9 in Hap3 A. gossypii.
AgosIRP3, which is a ubiquitously expressed gene, had a piggyBac insertion in the CDS region of the Hap3 genome. The TPM value of AgosIRP3 in Hap3 was 24.7, higher than in Hap1 (TPM value 12.6), suggesting that this TE insertion is associated with upregulated genes in Hap3 (Figure 6). Additionally, there was one nucleotide difference in the AgosIRP3 sequence between Hap1 and Hap3. Interestingly, Hap4 had a similar AgosIRP3 sequence to Hap1, while Hap3 contained both types of AgosIRP3 in Japanese aphids but only one form in Chinese aphids.
The insecticides have been reported to regulate the expression of insulin‐like peptides in the insects (Chowański et al., 2019; Li et al., 2016). In the study, acetamiprid‐treated samples showed decreased expression levels of AgosIRPs, except for AgosIRP3 and AgosIRP7 in Miyazaki susceptible (MS) clones. The expression levels of AgosIRP2 showed variation between insecticide‐resistant and insecticide‐susceptible samples, but there was no correlation between TPM values and insecticide resistance levels. The lowest and highest TPM values were found in two insecticide‐susceptible samples, while the neonicotinoid‐resistant samples exhibited intermediate TPM values. Acetamiprid treatment reduced the expression levels of AgosIRP2 in all samples (Figure 7).
FIGURE 7.

Relative expression quantity of AgosIRPs in Aphis gossypii treated with pesticide acetamiprid. (a): AgosIRP2; (b): AgosIRP3; (c): AgosIRP6; (d): AgosIRP7. The Y‐axis was the transcript per million (TPM) value (mean ± standard error of the mean). NS and MS were two susceptible clones, KR was one resistant clone, (T) means with acetamiprid treatment (Hirata et al., 2017). *p < .05, ns, no significant difference, data were analyzed by Mann–Whitney U test.
4. CONCLUSIONS
In conclusion, this study sheds light on the diversity and expression patterns of insulin‐related peptides (IRPs) in A. gossypii, a polyphagous aphid species. The analysis revealed the presence of multiple IRPs, indicating the specialization of insulin function in different biological processes. The genomic analysis identified evolutionary diversification in the aphid genome, attributed to transposon insertions and alternative splicing events. Furthermore, specific expression profiles of IRPs were observed in different morphs and developmental stages of aphids, suggesting their involvement in regulating aphid life cycles, reproductive strategies, host plant preferences, wing polyphenism, and pesticide resistance. These findings provide a valuable resource for further investigations into the evolution of the aphid genome and the functional roles of IRPs. Understanding the intricate mechanisms of insulin signaling in aphids can contribute to our knowledge of aphid biology, adaptation, and potential targets for pest management strategies. The comprehensive analysis of IRPs in A. gossypii presented in this study adds to our understanding of the complex regulatory networks governing aphid physiology and opens up new avenues for future research.
AUTHOR CONTRIBUTIONS
Weili Jiang: Data curation (lead); formal analysis (lead); funding acquisition (lead); investigation (lead); writing – original draft (equal). Muhammad Nasir: Writing – original draft (equal); writing – review and editing (equal). Chenchen Zhao: Conceptualization (lead); supervision (lead); writing – review and editing (equal).
CONFLICT OF INTEREST STATEMENT
The authors wish to declare no competing interests.
Supporting information
Table S1
ACKNOWLEDGMENTS
This study was supported by a grant from the National Natural Science Foundation of China (32272521).
Jiang, W. , Nasir, M. , & Zhao, C. (2023). Variation of insulin‐related peptides accompanying the differentiation of Aphis gossypii biotypes and their expression profiles. Ecology and Evolution, 13, e10306. 10.1002/ece3.10306
Contributor Information
Weili Jiang, Email: jiangweili@yzu.edu.cn.
Chenchen Zhao, Email: zhaochen06166@163.com.
DATA AVAILABILITY STATEMENT
The data are available as in the National Center for Biotechnology Information under BioProject accession no. PRJNA936785 and BioSample Accession no. SAMN33368520 and SAMN33368521.
REFERENCES
- Ajaykumar, A. , & Yang, J. J. (2022). Integrative comparison of burrows‐wheeler transform‐based mapping algorithm with de Bruijn graph for identification of lung/liver cancer‐specific gene. Journal of Microbiology and Biotechnology, 32, 149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfatto, P. (2005). Adaptive evolution of non‐coding DNA in Drosophila . Nature, 437, 1149–1152. [DOI] [PubMed] [Google Scholar]
- Bai, H. , Kang, P. , & Tatar, M. (2012). Drosophila insulin‐like peptide‐6 (dilp6) expression from fat body extends lifespan and represses secretion of Drosophila insulin‐like peptide‐2 from the brain. Aging Cell, 11, 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberà, M. , Cañas‐Cañas, R. , & Martínez‐Torres, D. (2019). Insulin‐like peptides involved in photoperiodism in the aphid Acyrthosiphon pisum . Insect Biochemistry and Molecular Biology, 112, 103185. [DOI] [PubMed] [Google Scholar]
- Blackman, R. L. , & Eastop, V. F. (2000). Aphids on the world's crops. An identification guide. Wiley‐Interscience. [Google Scholar]
- Bolger, A. M. , Lohse, M. , & Usadel, B. (2014). Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30, 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson, B. K. , & Barton, E. R. (2012). Insulin‐like growth factor‐I E‐peptide activity is dependent on the IGF‐I receptor. PLoS One, 7, e45588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson, J. A. (2010). Aphid wing dimorphisms: Linking environmental and genetic control of trait variation. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 365, 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletto, J. , Lombaert, E. , Chavigny, P. , Brevault, T. , Lapchin, L. , & Vanlerberghe‐Masutti, F. (2009). Ecological specialization of the aphid Aphis gossypii glover on cultivated host plants. Molecular Ecology, 18, 2198–2212. [DOI] [PubMed] [Google Scholar]
- Chowański, S. , Pacholska‐Bogalska, J. , & Rosiński, G. (2019). Cholinergic agonists and antagonists have an effect on the metabolism of the beetle Tenebrio molitor . Molecules, 24, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuti, P. , Barberà, M. , Veenstra, J. A. , & Martínez‐Torres, D. (2021). Progress in the characterization of insulin‐like peptides in aphids: Immunohistochemical mapping of ILP4. Insect Biochemistry and Molecular Biology, 136, 103623. [DOI] [PubMed] [Google Scholar]
- Dai, Z. , Wu, F. , Yeung, E. W. , & Li, Y. (2010). IGF‐IEc expression, regulation and biological function in different tissues. Growth Hormone & IGF Research, 20, 275–281. [DOI] [PubMed] [Google Scholar]
- Ding, B.‐Y. , Shang, F. , Zhang, Q. , Xiong, Y. , Yang, Q. , Niu, J.‐Z. , Smagghe, G. , & Wang, J.‐J. (2017). Silencing of two insulin receptor genes disrupts nymph‐adult transition of alate brown citrus aphid. International Journal of Molecular Sciences, 18, 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert, T. A. , & Cartwright, B. (1997). Biology and ecology of Aphis gossypii glover (Homoptera: Aphididae). Southwestern Entomologist, 22, 116. [Google Scholar]
- Grabherr, M. G. , Haas, B. J. , Yassour, M. , Levin, J. Z. , Thompson, D. A. , Amit, I. , Adiconis, X. , Fan, L. , Raychowdhury, R. , Zeng, Q. , Chen, Z. , Mauceli, E. , Hacohen, N. , Gnirke, A. , Rhind, N. , di Palma, F. , Birren, B. W. , Nusbaum, C. , Lindblad‐Toh, K. , … Regev, A. (2011). Full‐length transcriptome assembly from RNA‐Seq data without a reference genome. Nature Biotechnology, 29, 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham, M. E. , Shingleton, A. W. , Dudley, E. , & Brisson, J. A. (2020). Expression profiling of winged‐ and wingless‐destined pea aphid embryos implicates insulin/insulin growth factor signaling in morph differences. Evolution & Development, 22, 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke, S. , Clarke, D. F. , Broughton, S. , Andrews, T. D. , & Partridge, L. (2010). Molecular evolution and functional characterization of Drosophila insulin‐like peptides. PLoS Genetics, 6, e1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guirao‐Rico, S. , & Aguade, M. (2013). Patterns of nucleotide diversity at the regions encompassing the Drosophila insulin‐like peptide (dilp) genes: Demography vs. positive selection in Drosophila melanogaster . PLoS One, 8, e53593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, S.‐S. , Zhang, M. , & Liu, T.‐X. (2016). Insulin‐related peptide 5 is involved in regulating embryo development and biochemical composition in pea aphid with wing polyphenism. Frontiers in Physiology, 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hede, M. S. , Salimova, E. , Piszczek, A. , Perlas, E. , Winn, N. , Nastasi, T. , & Rosenthal, N. (2012). E‐peptides control bioavailability of IGF‐1. PLoS One, 7, e51152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, K. , Jouraku, A. , Kuwazaki, S. , Shimomura, H. , & Iwasa, T. (2017). Studies on Aphis gossypii cytochrome P450s CYP6CY22 and CYP6CY13 using an in vitro system. Journal of Pesticide Science, 42, 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton, A. A. , Lee, Y. , Coulibaly, C. A. , Rashbrook, V. K. , Cornel, A. J. , Lanzaro, G. C. , & Luckhart, S. (2010). Identification of three single nucleotide polymorphisms in Anopheles gambiae immune signaling genes that are associated with natural Plasmodium falciparum infection. Malaria Journal, 9, 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts, J. , Bonhomme, J. , Minoli, S. , Prunier‐Leterme, N. , Dombrovsky, A. , Abdel‐Latief, M. , Robichon, A. , Veenstra, J. A. , & Tagu, D. (2010). Neuropeptide and neurohormone precursors in the pea aphid, Acyrthosiphon pisum . Insect Molecular Biology, 19, 87–95. [DOI] [PubMed] [Google Scholar]
- Huygens, C. , Ribeiro Lopes, M. , Gaget, K. , Duport, G. , Peignier, S. , De Groef, S. , Parisot, N. , Calevro, F. , & Callaerts, P. (2022). Evolutionary diversification of insulin‐related peptides (IRPs) in aphids and spatiotemporal distribution in Acyrthosiphon pisum . Insect Biochemistry and Molecular Biology, 141, 103670. [DOI] [PubMed] [Google Scholar]
- Irwin, D. M. (2021). Evolution of the insulin gene: Changes in gene number, sequence, and processing. Frontiers in Endocrinology, 12, 649255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh, K. , Rozewicki, J. , & Yamada, K. D. (2019). MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Briefings in Bioinformatics, 20, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohany, O. , Gentles, A. J. , Hankus, L. , & Jurka, J. (2006). Annotation, submission and screening of repetitive elements in Repbase: RepbaseSubmitter and Censor. BMC Bioinformatics, 7, 474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger, M. J. B. , Jahan, N. , Riehle, M. A. , Cao, C. , & Brown, M. R. (2004). Molecular characterization of insulin‐like peptide genes and their expression in the African malaria mosquito, Anopheles gambiae . Insect Molecular Biology, 13, 305–315. [DOI] [PubMed] [Google Scholar]
- Kwon, S. H. , & Kim, D. S. (2017). Effects of temperature and photoperiod on the production of sexual morphs of Aphis gossypii (Hemiptera: Aphididae) in Jeju, Korea. Journal of Asia‐Pacific Entomology, 20, 53–56. [Google Scholar]
- Li, F. C. , Hu, J. S. , Tian, J. H. , Xu, K. Z. , Ni, M. , Wang, B. B. , Shen, W. D. , & Li, B. (2016). Effects of phoxim on nutrient metabolism and insulin signaling pathway in silkworm midgut. Chemosphere, 146, 478–485. [DOI] [PubMed] [Google Scholar]
- Liu, L. J. , Zheng, H. Y. , Jiang, F. , Guo, W. , & Zhou, S. T. (2014). Comparative transcriptional analysis of asexual and sexual morphs reveals possible mechanisms in reproductive polyphenism of the cotton aphid. PLoS One, 9, e99506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loxdale, H. D. , & Balog, A. (2018). Aphid specialism as an example of ecological–evolutionary divergence. Biological Reviews, 93, 642–657. [DOI] [PubMed] [Google Scholar]
- Luo, G. H. , Wu, M. , Wang, X. F. , Zhang, W. , & Han, Z. J. (2011). A new active piggyBac‐like element in Aphis gossypii . Insect Sci., 18, 652–662. [Google Scholar]
- Majoros, W. H. , Pertea, M. , & Salzberg, S. L. (2004). TigrScan and GlimmerHMM: Two open source ab initio eukaryotic gene‐finders. Bioinformatics, 20, 2878–2879. [DOI] [PubMed] [Google Scholar]
- Miura, T. , Braendle, C. , Shingleton, A. , Sisk, G. , Kambhampati, S. , & Stern, D. L. (2003). A comparison of parthenogenetic and sexual embryogenesis of the pea aphid Acyrthosiphon pisum (Hemiptera: Aphidoidea). Journal of Experimental Zoology Part B‐Molecular and Developmental Evolution, 295b, 59–81. [DOI] [PubMed] [Google Scholar]
- Nässel, D. R. , & Broeck, J. V. (2016). Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post‐release action of the insulin‐like peptides. Cellular and Molecular Life Sciences, 73, 271–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo, E. , & Coll, M. (2001). Effect of nitrogen fertilization on Aphis gossypii (Homoptera: Aphididae): Variation in size, color, and reproduction. Journal of Economic Entomology, 94, 27–32. [DOI] [PubMed] [Google Scholar]
- Nijhout, H. F. (1999). Control mechanisms of polyphenic development in insects: In polyphenic development, environmental factors alter some aspects of development in an orderly and predictable way. Bioscience, 49, 181–192. [Google Scholar]
- Ogawa, K. , & Miura, T. (2014). Aphid polyphenisms: Trans‐generational developmental regulation through viviparity. Frontiers in Physiology, 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, N. , Yamanaka, N. , Yagi, Y. , Nishida, Y. , Kataoka, H. , O'Connor, M. B. , & Mizoguchi, A. (2009). A fat body‐derived IGF‐like peptide regulates postfeeding growth in Drosophila . Developmental Cell, 17, 885–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panini, M. , Chiesa, O. , Troczka, B. J. , Mallott, M. , Manicardi, G. C. , Cassanelli, S. , Cominelli, F. , Hayward, A. , Mazzoni, E. , & Bass, C. (2021). Transposon‐mediated insertional mutagenesis unmasks recessive insecticide resistance in the aphid Myzus persicae . Proceedings of the National Academy of Sciences of the United States of America, 118, e2100559118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech, G. E. , Radio, S. , Guirao‐Rico, S. , Aguilera, L. , Horvath, V. , Green, L. , Lindstadt, H. , Jamilloux, V. , Quesneville, H. , & Gonzalez, J. (2022). Population‐scale long‐read sequencing uncovers transposable elements associated with gene expression variation and adaptive signatures in Drosophila . Nature Communications, 13, 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidah, N. G. , & Chrétien, M. (1997). Eukaryotic protein processing: Endoproteolysis of precursor proteins. Current Opinion in Biotechnology, 8, 602–607. [DOI] [PubMed] [Google Scholar]
- Shang, F. , Niu, J. , Ding, B.‐Y. , Zhang, W. , Wei, D.‐D. , Wei, D. , Jiang, H.‐B. , & Wang, J.‐J. (2020). The miR‐9b microRNA mediates dimorphism and development of wing in aphids. Proceedings of the National Academy of Sciences of the United States of America, 117, 8404–8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, Q. , Pan, Y. , Fang, K. , Xi, J. , & Brennan, J. A. (2012). Biochemical characterization of acetylcholinesterase, cytochrome P450 and cross‐resistance in an omethoate‐resistant strain of Aphis gossypii Glover. Crop Protection, 31, 15–20. [Google Scholar]
- Sharma, A. , Nuss, A. B. , & Gulia‐Nuss, M. (2019). Insulin‐like peptide signaling in mosquitoes: The road behind and the road ahead. Frontiers in Endocrinology, 10, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, S. L. , Liu, X. X. , Zhang, Q. W. , & Zhao, Z. W. (2010). Morph‐specific differences in metabolism related to flight in the wing‐dimorphic Aphis gossypii . Insect Sci., 17, 527–534. [Google Scholar]
- Simon, J.‐C. , Stoeckel, S. , & Tagu, D. (2010). Evolutionary and functional insights into reproductive strategies of aphids. Comptes Rendus Biologies, 333, 488–496. [DOI] [PubMed] [Google Scholar]
- Song, X.‐T. , Zhang, J.‐N. , Zhao, D.‐W. , Zhai, Y.‐F. , Lu, Q. , Qi, M.‐Y. , Lu, M.‐H. , Deng, S.‐L. , Han, H.‐B. , Yang, X.‐Q. , & Yao, Y.‐C. (2021). Molecular cloning, expression, and functional features of IGF1 splice variants in sheep. Endocrine Connections, 10, 980–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki, M. G. , Kobayashi, S. , & Aoki, F. (2014). Male‐specific splicing of the silkworm imp gene is maintained by an autoregulatory mechanism. Mechanisms of Development, 131, 47–56. [DOI] [PubMed] [Google Scholar]
- Västermark, Å. , Rask‐Andersen, M. , Sawant, R. S. , Reiter, J. L. , Schiöth, H. B. , & Williams, M. J. (2013). Insulin receptor‐like ectodomain genes and splice variants are found in both arthropods and human brain cDNA. Journal of Systematics and Evolution, 51, 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Zhang, S. , Luo, J. Y. , Wang, C. Y. , Lv, L. M. , Zhu, X. Z. , Li, C. H. , & Cui, J. J. (2016). Identification of Aphis gossypii glover (Hemiptera: Aphididae) biotypes from different host plants in North China. PLoS One, 11, e0146345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa, K. (2015). piggyBac transposon. Microbiology Spectrum, 3, MDNA3‐0028‐2014. [DOI] [PubMed]
- Zeng, X. , Pan, Y. , Song, J. , Li, J. , Lv, Y. , Gao, X. , Tian, F. , Peng, T. , Xu, H. , & Shang, Q. (2021). Resistance risk assessment of the Ryanoid anthranilic diamide insecticide Cyantraniliprole in Aphis gossypii glover. Journal of Agricultural and Food Chemistry, 69, 5849–5857. [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Gao, F. , Jakovlić, I. , Zou, H. , Zhang, J. , Li, W. X. , & Wang, G. T. (2020). PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Molecular Ecology Resources, 20, 348–355. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Gao, X. , Wang, L. , Jiang, W. , Su, H. , Jing, T. , Cui, J. , Zhang, L. , & Yang, Y. (2022). Chromosome‐level genome assemblies of two cotton‐melon aphid Aphis gossypii biotypes unveil mechanisms of host adaption. Molecular Ecology Resources, 22, 1120–1134. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Luo, J. Y. , Wang, C. Y. , Lv, L. M. , Li, C. H. , Jiang, W. L. , Cui, J. J. , & Rajput, L. B. (2016). Complete mitochondrial genome of Aphis gossypii glover (Hemiptera: Aphididae). Mitochondrial DNA, 27, 854–855. [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Luo, J. Y. , Wang, L. , Wang, C. Y. , Lu, L. M. , Zhang, L. J. , Zhu, X. Z. , & Cui, J. J. (2018). The biotypes and host shifts of cotton‐melon aphids Aphis gossypii in northern China. Journal of Integrative Agriculture, 17, 2066–2073. [Google Scholar]
- Zhao, Z. , Elsik, C. G. , Hibbard, B. E. , & Shelby, K. S. (2021). Detection of alternative splicing in western corn rootworm (Diabrotica virgifera virgifera LeConte) in association with eCry3.1Ab resistance using RNA‐seq and PacBio Iso‐Seq. Insect Molecular Biology, 30, 436–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data are available as in the National Center for Biotechnology Information under BioProject accession no. PRJNA936785 and BioSample Accession no. SAMN33368520 and SAMN33368521.


