Abstract
One novel option for treating metastatic castration resistant prostate cancer is radionuclide therapy targeting prostate-specific membrane antigen (PSMA), e.g. [177Lu]Lu-PSMA-617. Overexpression of HER2 has been found in 80% of metastatic cases of prostate cancer. Previous research showed that HER2 is elevated post irradiation in PC-3 prostate cancer cells. Co-treating with anti-HER2 antibody Trastuzumab gave less proliferation of irradiated tumor cells in vitro, and when using radionuclide therapy, also in vivo. The aim of this study is to determine whether the same holds true in PSMA-expressing PC-3 PIP cells using [177Lu]Lu-PSMA-617 radionuclide therapy. PC-3 PIP and 22Rv1 prostate cancer cells were tested in vitro, treated with 6 Gy of x-rays with or without Trastuzumab incubation. We measured uptake of HER2-targeting affibody [68Ga]Ga-ABY-025 and cell survival, e.g. using the WST-1 assay. Three groups (n=10 each) of male nude Balb/c mice were inoculated with PC-3 PIP xenograft tumors and treated with just [177Lu]Lu-PSMA-617 (20 MBq), [177Lu]Lu-PSMA-617 (20 MBq) and Trastuzumab (4 × 5 mg/kg), or left untreated. Tumor sizes and animal survival was observed. In vitro, x-ray irradiation did reduce survival in 22Rv1 but not PC-3 PIP cells, and there was no significant effect of Trastuzumab treatment. Cells expressed HER2 but not significantly elevated post irradiation. In vivo, mice co-treated with Trastuzumab had significantly longer survival than untreated mice, but not than only [177Lu]Lu-PSMA-617. Staining of tumor sections showed similar HER2 and PSMA expression across groups. In conclusion, these results give no support for any benefit from co-treatment with anti-HER2 antibody for PSMA-targeted radioligand therapy.
Keywords: PSMA, PSMA-617, prostate cancer, HER2, Trastuzumab, PC-3, PC-3 PIP, radionuclide therapy
Introduction
Metastatic prostate cancer (PCa) poses a significant clinical challenge. In the STAMPEDE trial, the median failure free survival for patients with metastatic androgen sensitive PCa treated with androgen deprivation therapy alone was 11.2 months [1]. PCa that develops resistance to androgen deprivation therapy has even fewer options available for therapy [2]. One promising therapy target is the prostate-specific membrane antigen (PSMA). Radionuclide therapy with lutetium-177 PSMA-617 delivers beta-particle radiation to PSMA-expressing cells and poses a new treatment option with a good safety profile.
As the first international multicenter phase III study, the VISION trial [3] examined therapy with [177Lu]Lu-PSMA-617 and randomized 813 patients with metastatic castration-resistant prostate cancer and positive PSMA-PET scans. Compared with standard of care alone, patients who received [177Lu]Lu-PSMA-617 therapy plus standard of care exhibited prolonged progression-free survival (median, 8.7 vs 3.4 months) and overall survival (median, 15.3 vs 11.3 months). Despite increased adverse events, quality of life was not adversely affected. The radiopharmaceutical [177Lu]Lu-PSMA-617 was approved by both the FDA and EMA in 2022 for treatment of metastatic castration-resistant PCa.
A 2001 study showed that in untreated primary PCa tumors, HER-2/neu protein (HER2) expression was only 20%. But overexpression was observed in 80% of metastatic cases and 67% of primary tumors surviving after androgen deprivation therapy [4]. It is speculated that elevated HER2 expression could be associated with prior androgen depravation therapy, allowing for hormone-independent growth of the tumor [4,5]. HER2 expression is also known to have a role in radiation resistance [6]. In a 2015 study, Andersson et al. showed that HER2 expression in androgen-independent PC-3 PCa cells significantly increased after irradiation leading to resistance to therapy [7]. Subsequent post irradiation treatment with the anti-HER2 antibody Trastuzumab resulted in a twofold decrease in the PC-3 cell survival in vitro. And in a follow up 2019 study, co-administration of Trastuzumab led to a significantly increased survival in mice with PC-3 xenografts treated with the GRPR-antagonist PEG2-RM26 labeled with 177Lu [8].
Affibody molecules are small (7 kDa) affinity scaffold proteins which can be engineered to bind to desired molecular targets [9]. ABY-025 is a synthetically produced highly stable affibody molecule that binds to HER2 with high affinity [10]. It has been shown, when radiolabeled, to visualize even lower (HER2+) expression levels in a clinical setting [11] and has passed the phase I stage of clinical trials [12].
The aim of this study is to determine whether co-administration of Trastuzumab antibody could improve the survival of mice undergoing [177Lu]Lu-PSMA-617 treatment. PC-3 cells do not express PSMA and are not a good target for this treatment, but a genetically modified variant, PC-3 PIP, does [13], so it will be employed in vitro and in vivo to test this combination therapy.
Materials and methods
Cell lines
Human prostate cancer cell line 22Rv1 were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). PC-3 PIP cells were obtained from Dr. Warren Heston, Cleveland Clinic. The cells were cultured as monolayer using RPMI-1640 medium with L-glutamine (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% Fetal Bovine Serum (Thermo Fisher Scientific) and 1% penicillin-streptomycin (Thermo Fisher Scientific). The cells were grown in incubators with 37°C, 5% CO2, and in a humidified atmosphere. The cultures got fresh medium twice per week. The cells were sub-cultured when they reach confluence, by detaching them using trypsin/EDTA 0.25% 1X (Thermo Fisher Scientific) and transferring them to a new vessel with a fresh medium. Cell counting was carried out using The Countess™ Automated Cell Counter (Thermo Fisher Scientific) which utilizes a trypan-blue staining method and image analysis program to calculate the live, dead and total cells. Screening the cells was done in regular time using the mycoplasma check (Eurofins Genomics, Ebersberg, Germany) to avoid the negative effects of possible mycoplasma cell contamination.
ABY-025 radiolabeling
The HER2-targeting affibody ABY-025 was radiolabeled with gallium-68 by adding 12 MBq of freshly eluted gallium-68 to 15 µg of ABY-025 in sodium acetate buffer (1.25 M, pH 3.6), the mixture was incubated at 75°C for 10 minutes, the radiochemical yield was determined by instant thin-layer chromatography (iTLC) using 0.2 M citric acid as the solvent, the phosphor image used was Cyclone® Plus Storage Phosphor System (PerkinElmer, Waltham, MA, USA). [68Ga]Ga-ABY-025 was purified using size exclusion NAP-5 cartridges and the radiochemical purity was determined by iTLC.
Cell irradiation and Trastuzumab treatment
Cells were seeded in 6-well plates (n=5 PC-3 PIP, n=5 22Rv1) at 106 cells per well. One day post seeding, 3 plates per cell line were irradiated with a 225 kVp X-ray source (Xstrahl Ltd., Brownhills West Midlands, UK) until an absorbed dose of 6 Gy had been reached. One day post irradiation, one plate per cell line had Trastuzumab (MedChemExpress, Monmouth Junction, NJ, USA) added to the cell medium so that the concentration reached 0.05 mg/ml in each well. One irradiated and one untreated plate for each cell line had cell media changed to fresh medium. These two plates per cell lines were then set aside for HER2 expression measurement. The remaining 3 plates per cell line had cell media changed to fresh medium at two days post irradiation.
HER2 expression
At two days post irradiation, HER2 expression on PC-3 PIP and 22Rv1 cells was evaluated. Half the wells in the irradiated and untreated plates were treated by incubating 500 nM of ABY-025 affibody per well for 10 minutes at room temperature to saturate HER2. After incubation, 6 nM/well of [68Ga]Ga-ABY-025 was added to the incubated wells and unsaturated cells. The plates were incubated for 1 hour at 37°C in a humidified incubator at 5% CO2. The cells were then washed, detached, collected, and measured for their radioactivity content on a 2480 Wizard2 automatic gamma counter (PerkinElmer, Waltham, MA, USA).
Cell survival
At 3 days post irradiation, three wells per group were treated with trypsin-EDTA to detach the cells. After cell detachment, 10 µl of the cell suspension was added to 10 µl of trypan blue, the mixture was resuspended, and the cells were counted using the automated cell counter.
Cell viability
At 7 days post irradiation, three wells per group were incubated for 1 hour with 1 ml of medium and 200 µl WST-1 (Premix WST-1, Takara Bio Europe, Saint-Germain-en-Laye, France) at 37°C. Medium from each well was then transferred to 5 wells on a 96-well plate and the 440 nm absorbance (600 nm reference) of each well was read out on a microplate reader (SpectraMax iD3, Molecular Device, San Jose, CA, USA).
PSMA-617 radiolabeling
The radiolabeling of PSMA-617 was performed by adding 580 MBq of lutetium-177 to 11 µg of PSMA-617 (MedChemExpress) in ammonium acetate buffer (0.2 M, pH 5.5). The mixture was incubated at 85°C for 30 minutes. The radiochemical yield was determined by iTLC.
In vivo therapy study
All experiments were conducted under permit 04350-2020 with the addition 5.8.18-07300/2021 in accordance with the directions given by the regional ethical committee for animal trials. All animals used in the study were male BALB/c nude mice (Janvier Labs, Le Genest-Saint-Isle, France) of 7-8 weeks of age at time of tumor inoculation.
PC-3 PIP cells were inoculated subcutaneously in the right flank of 30 male Balb/c nude mice. Cells were counted and resuspended in a concentration of 5-6 × 10^6 cells/200 μl for each mouse. Cell suspension was prepared using Cultrex BME, Type 3 (R&D Systems, Minneapolis, MN, USA) in concentration of 8 mg/ml diluted with RPMI-1640 medium with L-glutamine (Fisher Scientific). Cultrex BME provides basement membranes of thin extracellular matrices to support cell growing in vivo. Tumor sizes were measured by caliper twice per week until they reached the limit of 1000 mm3 and the animal was sacrificed.
At 13 days post inoculation, some animals (n=20) were injected i.v. with 20.2±0.4 MBq of [177Lu]Lu-PSMA-617. Half of these animals (n=10) were simultaneously injected with 5 mg/kg body weight Trastuzumab i.v. and then injected with the same amount of Trastuzumab i.p. at 2, 4 and 6 days post the first injection. The PSMA-only group also had PBS injected at the same time points, of the same volume and route of administration as the other group had Trastuzumab injected except for 4 days post the first injection. Of the untreated animals, half (n=5) were inoculated with tumors in advance of the main study to follow tumor growth in order to aid main study planning and that data was integrated with that from the untreated animals during the main study (n=5).
At 24 hours post injection, some animals from the PSMA-only group (n=4) and the PSMA-plus-Trastuzumab group (n=4) were imaged by SPECT (NanoSPECT/CT Plus, Mediso; Budapest, Hungary) equipped with the NSP-106 multipinhole mouse collimator and SPECT data was reconstructed using HiSPECT software (SciVis, Göttingen, Germany) and the “Standard” preset. Volumes of interest were drawn by hand around tumors in the images and the activity quantified in VivoQuant 3.0 software (inviCRO, Boston, MA, USA).
Histology, immunohistochemistry and autoradiography
When the animals had been sacrificed, the tumor was excised and was embedded in OCT cryomount (HistoLab Products AB, Gothenburg, Sweden), then frozen on dry ice. 5 tumors per group were cryosectioned at 10 µm thickness, with adjacent tumor sections on 3 different microscope slides, with 3-4 sections on each.
Three slides with adjacent sections were stained with hematoxylin and eosin (HE), one set was immunolabeled for PSMA and one set was immunolabeled for HER2.
For two animals from the PSMA-only group, sacrificed at 12 and 15 days post the first injection, the sections that were to be stained with HE were initially imaged by autoradiography. Autoradiography was performed using a Biomolex 700 Imager (Biomolex AS, Oslo, Norway) having an intrinsic spatial resolution of 50 µm [14]. Data was exported from the instrument in list-mode format and software was developed in IDL 8.5 (Exelis VIS, Harris Corporation) to reconstruct images from list-mode data and apply corrections for dead or miscalilbrated detector strips.
As pre-treatment of sections for immunolabeling of HER2 and PSMA sections were post-fixed in paraformaldehyde (4%, pH 7.4), rinsed in phosphate buffer saline (PBS, 0.1 M, pH 7.4) and heat induced epitope retrieval was performed in citrate buffer (pH 6.0). Following rinses in PBS, sections were incubated with H2O2 (0.3% in PBS as quenching) for 10 minutes (min) at room temperature (RT) and were then rinsed in PBS containing Triton X-100 (0.05%, PBS-TX). Sections were then incubated with bovine serum albumin (BSA 1% in PBS-TX) for 30 min at RT.
Sections were incubated with rabbit anti-PSMA antibodies (1:500, ab133579, Abcam, Cambridge, UK) or with FITC conjugated (ab102884, Abcam) Trastuzumab (1:100, HY-P9907, MedChemExpress) for 90 min, at RT. Sections were then rinsed in PBS.
In one series of slides, sections were incubated with anti-PSMA, rinsed in PBS and incubated with goat anti-rabbit horse-radish peroxidase (HRP) conjugated secondary antibodies (1:1, MP-7451, Vector, Newark, USA), for 30 min at RT. Sections incubated with FITC conjugated Trastuzumab were rinsed in PBS followed by incubated with antibodies against FITC (1:400, sheep IgG, #64001, AbD Serotec Bio-Rad, Solna, Sweden), for 30 min at RT, followed by rinses in PBS and incubation with HRP conjugated antibodies against sheep IgG (donkey anti-sheep, 713-036-147, Jackson Immunoresearch, Cambridgeshire, UK) for 30 min at RT.
Following rinses in PBS, slides were immersed in a solution of di-aminobenzidine (DAB, 0.5 mg/ml) and H2O2, and let to react for 10 min. Following rinses in PBS and H2O, slides were immersed in Mayers hematoxylin (Histolab, Gothenborg, Sweden) for 1 min, were rinsed in H2O and were then dehydrated in a graded alcohol series ended with Xylen (100%). Slides were coverslipped and mounted in Pertex (Histolab).
Analyses of immunolabeled and HE stained sections were performed in a bright-field microscope (Olympus IX73, Shinjuku, Tokyo, Japan) and in scanned digital images of whole sections using the Axio scan Z1 imager (Zeiss, Oberkochen, Germany).
Statistical analysis
Statistical tests were performed using non-parametric methods due to the small sample size. A p-value of <0.05 was considered statistically significant. Calculations were performed using SPSS Statistics version 27 (IBM, Armonk, NY, USA).
Results
Radiolabeling of ABY-025 and PSMA-617
ABY-025 was radiolabeled with gallium-68 with radiochemical purity of 43% (Figure S1) and the purity after NAP-5 purification was 99% (Figure S2). The radiochemical purity of [177Lu]Lu-PSMA-617 was over 99% after radiolabeling.
HER2 expression
The binding specificity assay (Figure 1A-D) demonstrated positive HER2 expression in PC-3 PIP and 22Rv1 cells. This was reflected by statistically significant reduction of [68Ga]Ga-ABY-025 uptake in both cell lines under excess amounts of unlabeled ABY-025 in comparison with the unsaturated cells. Although irradiated PC-3 PIP cells had slightly higher mean uptake of [68Ga]Ga-ABY-025 than untreated, there was no statistically significant increase in HER2 for irradiated cells in either cell line.
Figure 1.
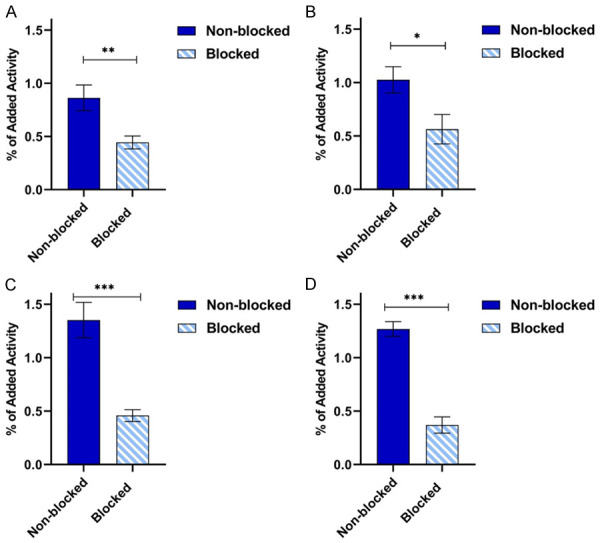
HER2 uptake in vitro. The in vitro binding assay of the HER2-targeting affibody [68Ga]Ga-ABY-025 per well in (A) non-treated PC-3 PIP cells, (B) X-ray irradiated PC-3 PIP cells, (C) non-treated 22Rv1 cells, and (D) X-ray irradiated 22Rv1 cells. The error bars represent the standard deviation. *Denote a P value <0.05, **denote a P value <0.01, and ***denote a P value <0.001.
Cell survival
The cell counting of control and treated groups (Table 1) showed a statistically significant reduction of cell count of the treated groups in comparison with control groups, this was evident for PC-3 PIP and 22Rv1 cells. However, there was no significant difference between the x-ray treated groups and the groups that additionally received Trastuzumab treatment.
Table 1.
Cell viability as measured using the WST-1 assay and cell survival as measured with the automated cell counter
| Group | Control PC-3 PIP | Control 22Rv1 | Only x-ray PC-3 PIP | x-ray + mAb PC-3 PIP | Only x-ray 22Rv1 | x-ray + mAb 22Rv1 |
|---|---|---|---|---|---|---|
| Cell viability | 100.00±8.86% | 100.00±13.0% | 102.04±0.78% | 98.52±13.4% | 16.62±2.40% | 17.87±2.88% |
| Cell survival | 100.00±10.86% | 100.00±7.86% | 66.39±5.75% | 73.09±6.49% | 45.93±4.72% | 50.93±4.63% |
Abbreviations: mAb, Trastuzumab antibody.
Cell viability
The microplate reader results of the WST-1 assay had some wells report a saturated value. One of the PC-3 PIP groups had 5 of these wells, so for analysis the 5 highest values (saturated or not) were removed from all PC-3 PIP groups. Results were calculated by setting the mean absorbance of the untreated cells as 100% viability and the mean absorbance of the control wells with only cell-free medium as 0%. See results in Table 1. Even when treating each well in the 96-well plate as an individual sample, the Independent-Samples Kruskal-Wallis Test did only show a statistically significant difference between the irradiated and unirradiated 22Rv1 cells but not between any PC-3 PIP groups.
In vivo therapy study
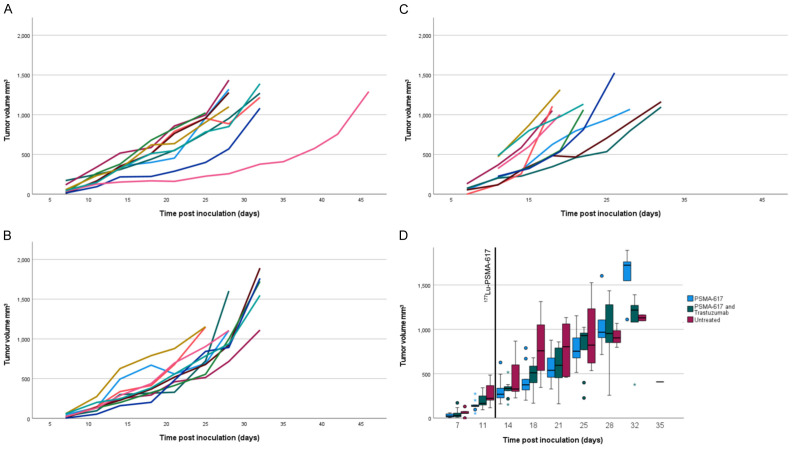
The tumor uptake of [177Lu]Lu-PSMA-617 at 24 h p.i. as measured by SPECT was not significantly different between groups, see Figure 2. The tumor sizes of all animals as measured can be seen in Figure 3. The only time point after therapy that the Independent-Samples Kruskal-Wallis Test with the Bonferroni correction showed significant differences in tumor size between groups was 5 days post injection. At that time the PSMA-only group had significantly smaller tumors than the untreated group (P=0.01). Animals were sacrificed when the tumor size reached 1000 mm3 and this is illustrated in the survival diagram in Figure 4. Mean survival post inoculation was 31.1 days for the PSMA-plus-Trastuzumab group, 29.4 days for the PSMA-only group and 24.7 days for the untreated group. The final day with any surviving animals was day 32 for PSMA-only and untreated groups, while one PSMA-plus-Trastuzumab animal survived until day 46. Comparing survival times between groups, the only significant difference using the Independent-Samples Kruskal-Wallis Test with the Bonferroni correction was a longer survival for the PSMA-plus-Trastuzumab group compared to the untreated group.
Figure 2.
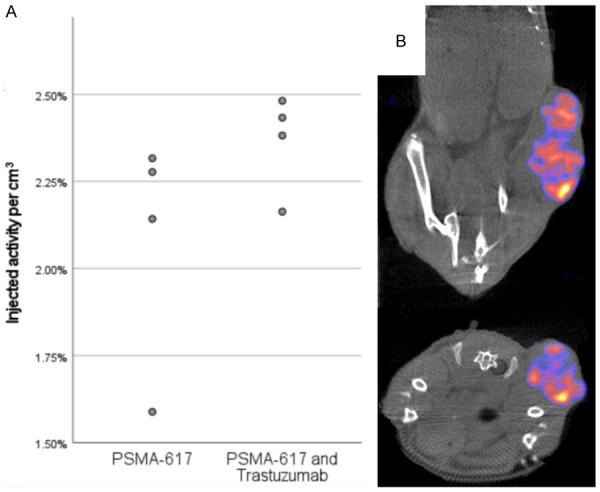
[177Lu]Lu-PSMA-617 uptake in tumor. Tumor uptake of [177Lu]Lu-PSMA-617 at 24 h p.i. in 4 animals per group as measured by SPECT (A) and coronal and transversal views of a SPECT/CT of a representative PSMA-617 and Trastuzumab animal (B). The SPECT data is filtered by a gaussian filter with 1 mm FWHM and windowed to best display the tumor uptake.
Figure 3.
Therapy effect on tumor size. Individual records of tumor size on animals in the PSMA-plus-Trastuzumab (A), PSMA-only (B) and untreated (C) groups. Boxplot of the mean of all animals per group up until day 35 (D). Note that half the untreated animals had measurements start later than the other half and that the number of animals at each point in the boxplot is different.
Figure 4.
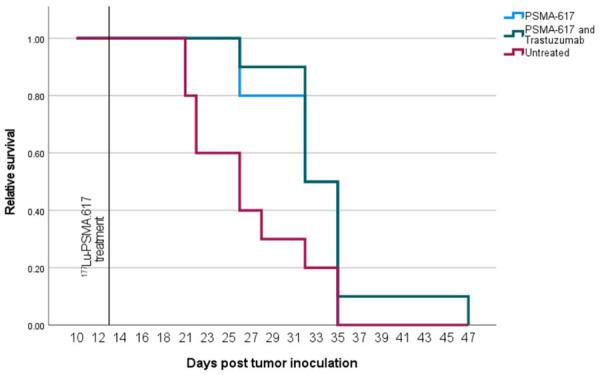
Therapy effect on animal sutvival. Relative survival of each group during the therapy study. Each group has n=10.
Histology, immunohistochemistry and autoradiography
While some additional necrotic areas could be observed in the tumors of [177Lu]Lu-PSMA-617 treated animals, it was not a dramatic difference to untreated controls. PSMA-expression was visible in all areas with tumor cells, although at different levels. The same can be said for HER2-expression, which was detected at different levels, co-localized with PSMA-expression. No significant difference in either HER2 or PSMA expression could be observed between the three groups. For the two tumors imaged by autoradiography, unfortunately both from the same PSMA-only group due to practical reasons, the radioactivity was at its highest concentration in areas that appeared necrotic and fibrous, with low PSMA-expression. Large areas of viable tumor cells, particularly along the edges of the tumors, had almost no detectable uptake of radioactivity. Representative examples of stained sections and autoradiography are shown in Figure 5A-F.
Figure 5.
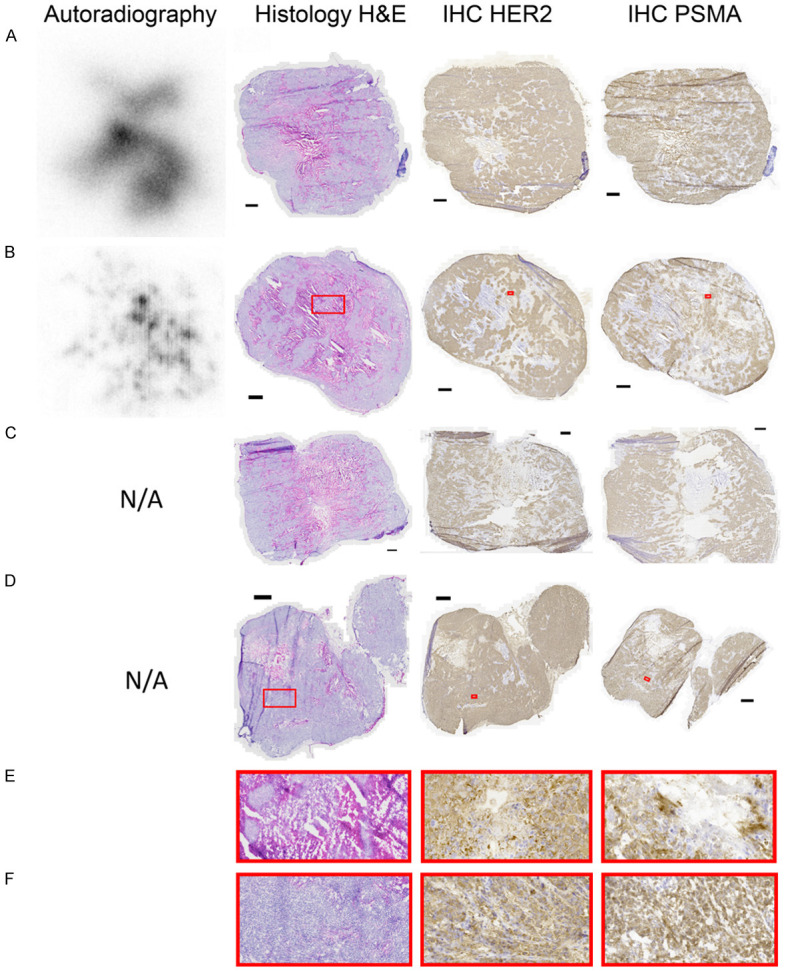
Tumor section images. Autoradiography (when available) and histology of the same tumor section, followed by immunohistochemistry for HER2 and PSMA of adjacent sections. Autoradiography images are individually scaled from 0% (white) to 100% (black) of radioactivity and each black scale bar represents 1 mm. (A) PSMA-only animal sacrificed 15 days p.i. (B) PSMA-only animal sacrificed 12 days p.i. (C) PSMA-and-Trastuzumab animal sacrificed 15 days p.i. (D) Untreated animal sacrificed 6 days p.i. (E) Magnification of treated areas annotated in (B). (F) Magnification of untreated areas annotated in (D).
Discussion
This study has shown that the co-treatment with anti-HER2 antibody Trastuzumab did not aid in therapy of prostate cancer either in vitro for 22Rv1 or PC-3 PIP cells in combination with external radiation or in vivo in combination with [177Lu]Lu-PSMA-617 therapy against PC-3 PIP tumors.
The in vitro HER2 expression study indicated positive expression in both PC-3 PIP and 22Rv1 cells. Our results demonstrated low uptake of the HER2-targeting affibody [68Ga]Ga-ABY-025 by both cell lines. This can be attributed to either low expression of HER2 or to the high concentration of radiolabeled affibody that was used in the assay, or to the contribution of both factors. Nevertheless, the significant reduction of uptake upon saturating HER2 confirms positive expression of HER2. We did not see any significant elevation of HER2 for irradiated cells, only a small and statistically insignificant elevation of uptake in PC-3 PIP cells post irradiation.
The in vitro cell survival results after Trastuzumab treatment differ from those in the Andersson et al. 2015 paper which used regular PC-3 cells but similar external irradiation at 6 Gy and Trastuzumab treatment as this study [7]. That paper found a 56% proliferation rate of PC-3 cells treated with both irradiation and Trastuzumab compared to controls. It also found a 50% higher HER2-expression per cell of the irradiated PC-3 cells compared to control 48 h post irradiation, which, although a slight difference in methodology, we did not. At the same time point post irradiation, we did not see significantly less viability or survival of the co-treated cells in either the automated cell-counter or using the WST-1 assay.
WST-1 is a common method to measure the viability of cells post irradiation, although it has been suggested that it works best for absorbed doses ≥10 Gy, higher than in this study [15]. The time of measurement will also affect the results, as this study did not find much effect on PC-3 PIP cells from only 6 Gy irradiation after 7 days using WST-1, whereas other studies found only about 20% viability of PC-3 cells directly post 6 Gy irradiation with the same assay [16]. ABY-025 binds to a different extracellular domain of HER2 than Trastuzumab and will not introduce a competitive interaction [17].
A rough estimate of the absorbed dose to tumors in the in vivo therapy study can be calculated from assuming all energy from emitted electrons, and only electrons, are deposited in tumors. With a constant 2.5% per gram uptake of 20 MBq 177Lu that gives 9.3 Gy absorbed dose to the tumor after 22 days, which is in the same range as the in vitro study but delivered with a much slower dose rate. The therapy effect might not appear to be very impressive, but it should be noted that the tumors were rather large, 300-400 mm3, at the initiation of therapy. The autoradiography results show that at least 12 and 15 days p.i. the highest activity concentrations are in low-PSMA areas of necrotic and fibrous tissue, possibly from therapy effects, but with new viable areas proliferating outside the irradiated zone. Autoradiography was only done for one group but there is no reason to believe that [177Lu]Lu-PSMA-617 would distribute differently in presence of Trastuzumab. There was no significant extended survival of animals treated with Trastuzumab in addition to [177Lu]Lu-PSMA-617 compared to radioligand therapy only and the immunohistochemistry did not reveal any elevated level of HER2 expression in irradiated tumors. This is in line with the in vitro results showing neither significant elevated HER2 expression post irradiation nor improved tumor cell therapy from Trastuzumab treatment. This means our in vivo results differ from Mitran et al. 2019 which had significantly longer survival when adding Trastuzumab treatment to [177Lu]Lu-PEG2-RM26 therapy of PC-3 tumors [8].
Since we have less effect of both in vitro and in vivo treatment with Trastuzumab than previous studies, it is necessary to delineate the differences that might have caused this. One difference common between in vitro and in vivo is the cell line. PC-3 PIP should be simply PC-3 transfected to express PSMA, but there is of course a possibility that this modification could have affected other characteristics of the cells. There are a few differences in the in vivo study, in contrast to Mitran et al. we employed a single injection of a PSMA-targeting therapy agent, and it is possible that there is a difference in biological response when using multiple injections of a GRPR-antagonist compared to a single-injection PSMA-ligand. Also, our tumors were around twice as large at the start of therapy than Mitran et al. It is possible that the addition of Trastuzumab would have improved survival more if the tumors had been treated with a higher absorbed dose rate, but 20 MBq of [177Lu]Lu-PSMA-617 per animal is already a higher dose per weight than what is administered to patients in the clinic [3] even if mice and humans are not directly comparable by weight.
In conclusion, our study confirmed that just as the positive results from in vitro treatment with Trastuzumab on PC-3 cells in Andersson et al. 2015 correctly predicted positive results in vivo on PC-3 tumors in Mitran et al. 2019, our negative results in vitro on PC-3 PIP cells predicted the negative results in vivo on PC-3 PIP tumors. These results give no support for co-treatment of radiosensitive prostate tumors with anti-HER2 immunotherapy, e.g. like PSMA-targeted radioligand therapy.
Acknowledgements
This study was funded by Berta Kamprad Stiftelse and the ALF Foundation of the Medical Faculty of Lund University. Lund University BioImaging Center (LBIC), Lund University, is gratefully acknowledged for providing experimental resources. We thank Crister Ceberg for use of the x-ray source for cell irradiation and Susanne Geres and Marcella Safi for aid in animal handling and measurements. Imagene-iT AB were pathology consultants. Anna Orlova, Uppsala University, contributed with valuable scientific discussions.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.James ND, Sydes MR, Mason MD, Clarke NW, Dearnaley DP, Spears MR, Millman R, Parker C, Ritchie AW, Russell JM. Docetaxel and/or zoledronic acid for hormone-naïve prostate cancer: first overall survival results from STAMPEDE ( NCT00268476) J. Clin. Oncol. 2015;33:5001. [Google Scholar]
- 2.Shore ND, Antonarakis ES, Cookson MS, Crawford ED, Morgans AK, Albala DM, Hafron J, Harris RG, Saltzstein D, Brown GA, Henderson J, Lowentritt B, Spier JM, Concepcion R. Optimizing the role of androgen deprivation therapy in advanced prostate cancer: challenges beyond the guidelines. Prostate. 2020;80:527–544. doi: 10.1002/pros.23967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sartor O, De Bono J, Chi KN, Fizazi K, Herrmann K, Rahbar K, Tagawa ST, Nordquist LT, Vaishampayan N, El-Haddad G, Park CH, Beer TM, Armour A, Pérez-Contreras WJ, DeSilvio M, Kpamegan E, Gericke G, Messmann RA, Morris MJ, Krause BJ VISION Investigators. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Osman I, Scher HI, Drobnjak M, Verbel D, Morris M, Agus D, Ross JS, Cordon-Cardo C. Her-2/neu (p185 neu) protein expression in the natural or treated history of prostate cancer. Clin Cancer Res. 2001;7:2643–2647. [PubMed] [Google Scholar]
- 5.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–285. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 6.Duru N, Fan M, Candas D, Menaa C, Liu HC, Nantajit D, Wen Y, Xiao K, Eldridge A, Chromy BA, Li S, Spitz DR, Lam KS, Wicha MS, Li JJ. HER2-associated radioresistance of breast cancer stem cells isolated from HER2-negative breast cancer cells. Clin Cancer Res. 2012;18:6634–6647. doi: 10.1158/1078-0432.CCR-12-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andersson J, Rosestedt M, Orlova A. Imaging of HER2 may improve the outcome of external irradiation therapy for prostate cancer patients. Oncol Lett. 2015;9:950–954. doi: 10.3892/ol.2014.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitran B, Rinne SS, Konijnenberg MW, Maina T, Nock BA, Altai M, Vorobyeva A, Larhed M, Tolmachev V, de Jong M, Rosenström U, Orlova A. Trastuzumab cotreatment improves survival of mice with PC-3 prostate cancer xenografts treated with the GRPR antagonist 177Lu-DOTAGA-PEG2-RM26. Int J Cancer. 2019;145:3347–3358. doi: 10.1002/ijc.32401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Altai M, Orlova A, Tolmachev V. Radiolabeled probes targeting tyrosine-kinase receptors for personalized medicine. Curr Pharm Des. 2014;20:2275–2292. doi: 10.2174/13816128113196660667. [DOI] [PubMed] [Google Scholar]
- 10.Ahlgren S, Orlova A, Wållberg H, Hansson M, Sandström M, Lewsley R, Wennborg A, Abrahmsén L, Tolmachev V, Feldwisch J. Targeting of HER2-expressing tumors using 111In-ABY-025, a second-generation affibody molecule with a fundamentally reengineered scaffold. J Nucl Med. 2010;51:1131–1138. doi: 10.2967/jnumed.109.073346. [DOI] [PubMed] [Google Scholar]
- 11.Sörensen J, Velikyan I, Sandberg D, Wennborg A, Feldwisch J, Tolmachev V, Orlova A, Sandström M, Lubberink M, Olofsson H, Carlsson J, Lindman H. Measuring HER2-receptor expression in metastatic breast cancer using [68Ga] ABY-025 affibody PET/CT. Theranostics. 2016;6:262–71. doi: 10.7150/thno.13502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velikyan I, Schweighöfer P, Feldwisch J, Seemann J, Frejd FY, Lindman H, Sörensen J. Diagnostic HER2-binding radiopharmaceutical, [68Ga]Ga-ABY-025, for routine clinical use in breast cancer patients. Am J Nucl Med Mol Imaging. 2019;9:12–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Current K, Meyer C, Magyar CE, Mona CE, Almajano J, Slavik R, Stuparu AD, Cheng C, Dawson DW, Radu CG, Czernin J, Lueckerath K. Investigating PSMA-targeted radioligand therapy efficacy as a function of cellular PSMA levels and intratumoral PSMA heterogeneity. Clin Cancer Res. 2020;26:2946–2955. doi: 10.1158/1078-0432.CCR-19-1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Örbom A, Ahlstedt J, Serén T, Auterinen I, Kotiluoto P, Hauge H, Östlund K, Olafsen T, Wu AM, Dahlbom M, Strand SE. Characterization of a double‐sided silicon strip detector autoradiography system. Med Phys. 2015;42:575–584. doi: 10.1118/1.4905049. [DOI] [PubMed] [Google Scholar]
- 15.Guertler A, Kraemer A, Roessler U, Hornhardt S, Kulka U, Moertl S, Friedl A, Illig T, Wichmann E, Gomolka M. The WST survival assay: an easy and reliable method to screen radiation-sensitive individuals. Radiat Prot Dosimetry. 2011;143:487–490. doi: 10.1093/rpd/ncq515. [DOI] [PubMed] [Google Scholar]
- 16.Elgqvist J, Timmermand OV, Larsson E, Strand SE. Radiosensitivity of prostate cancer cell lines for irradiation from beta particle-emitting radionuclide 177Lu compared to alpha particles and gamma rays. Anticancer Res. 2016;36:103–109. [PubMed] [Google Scholar]
- 17.Massicano AVF, Marquez-Nostra BV, Lapi SE. Targeting HER2 in nuclear medicine for imaging and therapy. Mol Imaging. 2018;17:1536012117745386. doi: 10.1177/1536012117745386. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.