Abstract
Diabetic neuropathy (DN) is a condition in which nerve fibers are continually exposed to high glucose-induced free radicals. Recent discoveries demonstrated that melatonin is an indole hormone that contributes to neuroprotection through the modulation of autophagy. Herein, this study aims to examine the neuroprotective effects of melatonin on Schwann cells under high glucose conditions. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) assay was used to measure cell viability. The activation of autophagosomes was determined using acridine orange staining (AO). Western blot assay was used to measure the expression of proteins involved in autophagy and endoplasmic reticulum (ER) stress. Our results demonstrated that melatonin at 1 µM has the highest protective effects on high glucose-induced cell death. Melatonin concentrations of 5 and 10 µM were found to be the most effective in reducing autophagy induced by high glucose. Under high glucose conditions, the protein expressions of LC3, ATF4, ATF6, CHOP, PERK and eIF2-α were up-regulated in Schwann cells. However, melatonin attenuated these changes by downregulating LC3 and the ER stress markers ATF4, ATF6, CHOP, PERK and eIF2-α protein expressions in Schwann cells. In conclusion, melatonin alleviates high glucose-induced autophagy in Schwann cells through PERK-eIF2α-ATF4-CHOP signaling pathways.
Keywords: Melatonin, endoplasmic reticulum stress, autophagy, diabetic neuropathy, hyperglycemia, Schwann cells
Introduction
Diabetes mellitus (DM) affects 476.0 million people worldwide and causes 1.37 million deaths in 2017. These numbers are expected to increase to 570.9 million and 1.59 million by 2025, respectively [1]. DM is a metabolic disease caused by impaired insulin secretion and/or function and is characterised by chronic hyperglycemia state [2]. The complications associated with DM include damage to multiple organs such as the heart, kidneys, nerves, eyes and blood vessels. DN is one of the most common complications that occur when hyperglycemia causes loss of nerve fiber function due to oxidative stress. At least 50% of DM patients develop DN complications. Neuropathy refers to a group of clinical syndromes characterised by neuropathic pain, muscle weakness, cachexia, weight loss and autonomic dysfunction in the peripheral and autonomic nervous systems [4,5]. Schwann cells are the main glial cells of peripheral nerves. By synthesising the myelin sheath surrounding peripheral nerve fibers, they ensure their optimal function and structural integrity. One of the main pathogenic mechanisms involved in DN is hyperglycemia-induced Schwann cell death [6]. Melatonin, an indolamine hormone produced by the pineal gland, exhibits strong antioxidant properties [7]. Our previous study demonstrated that melatonin prevents oxidative stress-induced apoptosis and mitochondrial dysfunction in hyperglycaemia Schwann cells via upregulation of Bcl2, NF-κB, mTOR, Wnt signalling pathways, indicating that melatonin may serve as an anti-DN drug [8]. A number of studies have demonstrated that ER stress and autophagy pathways are involved in diabetic peripheral neuropathy [9,10]. However, the role of melatonin in high glucose-induced Schwann cells via endoplasmic reticulum (ER) stress and autophagy pathways remains unclear. Thus, this study aims to elucidate the involvement of ER stress and autophagy pathways in melatonin-treated hyperglycaemia Schwann cells.
Materials and methods
Cell culture
RT4-D6P2T rat Schwann cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Loughborough, UK) supplemented with 10% fetal bovine serum (FBS) (Gibco, Loughborough, UK) and incubated in a humidified atmosphere of 5% CO2 at 37°C. Subculture was performed after the cells reached 80% confluency. The stock solution of melatonin (215.275 µM) was dissolved in absolute ethanol and different concentrations were prepared in the culture medium. The stock solution of glucose (1 M) was dissolved in DMEM medium and different concentrations were prepared in the culture medium.
MTT assay
Schwann cells were seeded at a density of 10,000 cells/well into a 96-well plate (SPL life sciences, Pocheon-si, Korea) and allowed to adhere for 24 h. After 24 h, the supernatant was removed and the medium consisting of 100 mM glucose (Sigma Aldrich, Saint Louis, MO, USA) was added to each well, followed by adding various concentrations of melatonin (1, 5, 10 uM) (Sigma Aldrich, Saint Louis, MO, USA) and incubating for 24 h. The control group is the cells without being treated with glucose and melatonin. After 24 h, 20 μl of MTT solution (5 mg/ml) (Bio-basic, Toronto, ON, Canada) was added to each well and incubated for 4 h to measure cell viability. Next, medium containing MTT was removed and 100 μl of dimethyl sulfoxide (DMSO) crystals (Sigma Aldrich, Saint Louis, MO, USA) was added into each well to dissolve the formazan. Finally, the absorbance was measured at wavelength of 570 nm and reference wavelength of 630 nm using microplate reader Spectra Max3 Molecular Devices (San Jose, CA, USA).
AO fluorescence staining
Schwann cells were seeded into 24-well plate (SPL life sciences, Pocheon-si, Korea) and allowed to adhere for 24 h. An untreated group of wells was used as a control, while five other groups were treated with 100 mM glucose with or without melatonin (1, 5 and 10 μM) for 24 h. Autophagy was measured using AO staining. Under fluorescence microscope Nikon Eclipse Ti (Nikon, Tokyo, Japan) observation, AO-stained cells exhibit diffuse green fluorescence, whereas acidic organelles, such as acidic vesicular organelles (AVOs), exhibit bright red fluorescence, which indicates autophagy.
Western blot assay
Schwann cells were seeded into 60 mm tissue culture petri dishes (SPL life sciences, Pocheon-si, Korea) and allowed to adhere for 24 h. Untreated cells were used as a control, while the rest of the cells were treated with 100 mM glucose with or without melatonin (1, 5, 10 uM) for 24 h. Then, adherent and floating cells were collected. Cells were lysed with lysis buffer (10 mM Tris-HCl, pH 8; 0.32 mM sucrose; 5 mM EDTA; 2 mM DTT; 1 mM phenylmethyl sulfonyl fluoride; and 1% Triton X-100). After centrifugation, the supernatant was collected and measured for protein concentration using the Bradford assay. For electrophoresis, each sample was loaded with an equal amount of protein into the wells of 10% SDS-polyacrylamide gel. After electrophoresis, the proteins were electroblotted onto polyvinylidene difluoride (PVDF) membranes and incubated with diluted primary antibodies for 1 h at 25°C. After washing with PBS, the membranes were incubated with diluted horseradish peroxidase (HRP)-conjugated secondary antibodies for 30 min at 25°C. To detect and quantify the protein bands, the proteins were stained by chemiluminescence using ECL-Plus kit and quantified using Image Lab software (Bio-Rad Laboratories, California, USA).
Statistical analysis
The results were expressed as mean ± standard deviation (SD). Statistical analysis was performed using Student’s t-test. The statistical significance was set at P > 0.05, denoted by an *. The error bars denoted standard deviation.
Results
Effects of melatonin on cell viability in high glucose-treated Schwann cells
MTT assay was used to determine the cell viability of Schwann cells following the treatment with 100 mM glucose and different concentrations of melatonin (0, 1, 5, and 10 uM) for 24 h. A control group is without treatment with glucose and melatonin. Results showed that after treatment with 100 mM glucose, the cell viability of Schwann cells decreases to 59.6% as shown in Figure 1. However, after treatment with varying concentrations of melatonin, the cell viability was increased as compared to cells treated with glucose alone. At a concentration of 1 µM of melatonin, the increment in cell viability was the highest among the melatonin-treated groups, which is at 74.4%, showing that 1 µM of melatonin is the most effective concentration for counteracting the cytotoxicity of glucose on Schwann cells. However, as compared with 1 µM melatonin treatment, the viability of cells was slightly decreased after exposure to 5 and 10 µM melatonin Figure 1.
Figure 1.
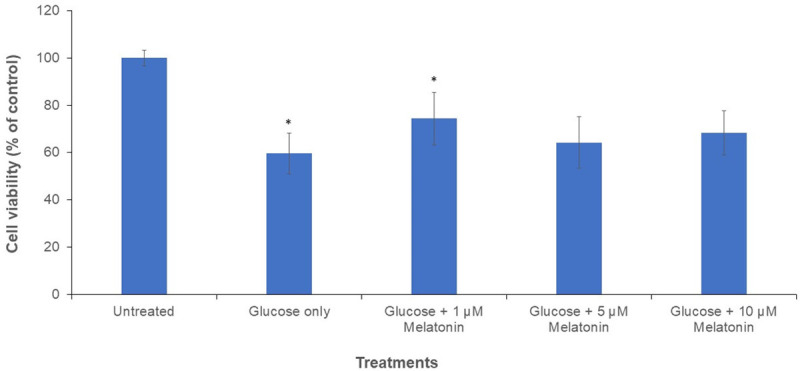
Graph of MTT cell viability of Schwann cells after 24 h treatment with 100 mM glucose with or without melatonin of varying concentrations (1, 5 and 10 µM). “*” indicates the statistically significant difference (P < 0.05) in melatonin-treated groups are compared with the group treated with 100 mM glucose alone.
Effects of melatonin on autophagy in high glucose-treated Schwann cells
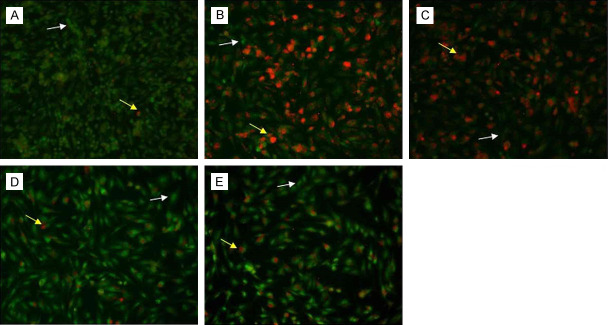
Cells treated with 100 mM glucose with or without melatonin (1, 5, and 10 µM) were visualized under inverted fluorescence microscope after staining with AO. The red colour in cells indicates the presence of autolysosomes Figure 2. Cells treated with glucose only depicted the highest percentage of autophagy activation (red fluorescence). Melatonin caused a concentration-dependent decrease in autophagy activation. The melatonin concentrations of 5 and 10 µM were the most effective in reducing autophagy, as confirmed by the statistically significant difference as shown in Figure 3.
Figure 2.
The images show autophagy activation after AO staining in 100 mM glucose-treated Schwann cells after 24 h with or without melatonin at varying concentrations (1, 5 and 10 µM). A. Control (Untreated); B. 100 mM Glucose; C. 1 µM melatonin + 100 mM glucose; D. 5 µM melatonin + 100 mM glucose; E. 10 µM melatonin + 100 mM glucose. The images shown are 40× magnification. Cells in green depict healthy non-autophagic cells (pointed by white arrows). Cells in red indicate autophagic activation (pointed by yellow arrows).
Figure 3.
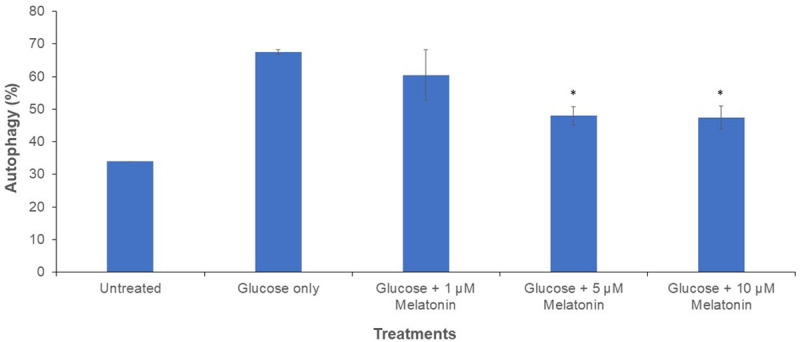
Graph of autophagy activation after AO staining in Schwann cells after 24 h treatment with 100 mM glucose with or without melatonin of varying concentrations (1, 5 and 10 µM). “*” indicates the statistically significant difference (P < 0.05) in melatonin-treated groups are compared with the group treated with 100 mM glucose alone.
Effect of melatonin on protein expressions in high glucose-treated Schwann cells
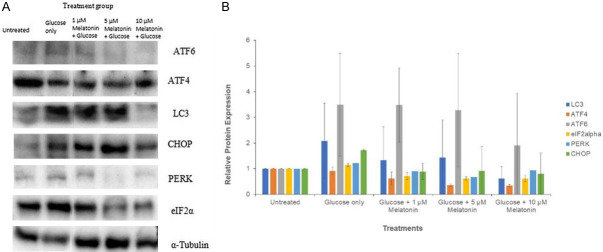
Western blot assay was performed to determine the relative expression of the autophagy marker LC3 and the ER stress pathway markers ATF6, PERK, ATF4, eIF2alpha and CHOP. Treatment of Schwann cells with the 100 µM glucose caused an increase in the relative expression of LC3, ATF6, PERK, eIF2alpha and CHOP, and this increase was statistically significant for the proteins PERK and eIF2alpha. As depicted by Figure 4, the results show a decrease in the expression of LC3, ATF4, ATF6, eIF2alpha, PERK and CHOP in cells treated with melatonin, as compared to those treated with glucose only. The relative expression of LC3 for the group of cells treated with glucose and 5 µM melatonin was significantly lower than that of the cells treated with glucose only. Similarly, eIF2alpha and ATF4 showed a significant decrease in their relative expression at 5 µM and 10 µM concentrations of melatonin in high glucose-treated cells. The reduction in the expression of ATF6, on the other hand, was statistically significant for the group of cells treated with glucose and 10 µM melatonin when compared to the treatment group with 100 mM glucose alone.
Figure 4.
Western blot analysis of protein expressions in Schwann cells after 24 h treatment with 100 mM glucose with or without melatonin of varying concentrations (1, 5 and 10 µM). A. Western blot analysis images showed the effect of melatonin on the expression of ATF6, ATF4, LC3, CHOP, PERK, and eIF2alpha, using alpha-tubulin as the housekeeping protein; B. Relative protein expression of ATF6, LC3, CHOP, PERK, and eIF2alpha. “*” indicates the statistically significant difference (P < 0.05) in melatonin-treated groups are compared with the group treated with 100 mM glucose alone.
Discussion
Autophagy belongs to type II programmed cell death and is different from type I programmed cell death, apoptosis. There is a wide debate over whether autophagy is harmful or beneficial, but research indicates that the outcome of autophagy varies depending on the cell line, cellular conditions, and the interplay between multiple pathways. It is possible that an excessive level of autophagy lead to the activation of autophagic cell death [11-14]. As seen in Figure 1, 100 mM high glucose-treated Schwann cells showed decreased cell viability, 59.6% of control, whereas the high glucose-treated cells co-treated with melatonin showed statistically significant increment in cell viability, 74.4% of control, counteracting hyperglycemia-induced cytotoxicity. This result supports our previous study in which melatonin ameliorated hyperglycemia-induced cytotoxicity in Schwann cells [8].
Interestingly, our fluorescence staining results demonstrated that excessive autophagy was present in the glucose-only treated group, which also correlated with the lowest cell viability as shown by the MTT assay. Autophagy, though an important homeostatic cellular process, can have deleterious effects on cells [15]. For the groups treated with 100 mM glucose and various concentration of melatonin (5 and 10 µM), the autophagy activation was significantly lower when compared to cells treated with 100 mM glucose only Figure 3. A previous study suggested that the autophagic cell death instead of apoptosis was observed in neurons, astrocytes, and oligodendrocytes at the lesion site in spinal cord injury [16]. Furthermore, in another study, the spinal cords of adult female C57BL/6J mice were hemitransected and electron microscopy revealed that the damaged cells had an increased number of autophagic vacuoles. It was found that autophagy, rather than apoptosis, was activated [17]. Based on the results of fluorescent staining, melatonin is capable of inhibiting autophagy activation, thereby protecting Schwann cells from autophagic cell death.
LC3 is the main autophagy marker. Our results showed that high glucose-treated Schwann cells have high levels of LC3 protein expression. Figure 4, correlated with the low cell viability as showed in Figure 1. However, after treatment with melatonin, LC3 protein expressions and cell death were decreased with increasing concentrations of melatonin treatment. Therefore, autophagic cell death induced by the hyperglycaemia condition was ameliorated via melatonin treatment. In other studies, the number of the LC3-positive cells significantly increased at the lesion site in adult female C57BL/6J mice spinal cord after hemisection, indicating the involvement of autophagic cell death [17]. Another study reported by Liu, et al. demonstrated that melatonin reduced oxidative stress and autophagic cell death in neural stem cells that had been treated with the Tri-ortho-cresyl phosphate, a cytotoxic organophophate [18].
Under hyperglycemia, Schwann cells exhibit elevated ER stress, which triggers autophagy to replenish the malfunctioning ER. However, the excessive activation of autophagy would be switched to autophagic cell death when the homeostasis of ER can no longer be maintained [19,20]. ATF6 is an ER transmembrane protein that is activated and stimulates CHOP activation, leading to an increase in autophagy-related gene expression. Similarly, activation eIF2alpha by PERK, causes it to stimulate ATF4 to directly induce the transcription of autophagy-related genes, as well as further stimulate the CHOP protein to increase the expression of autophagy related genes [21]. Li et al. reported that the nerve growth factor inhibited ER stress-mediated apoptosis in Schwann cells [22]. When Schwann cells were under hyperglycaemia condition, ER stress pathway was involved in the subsequent activation of cell death pathways [23,24]. Our results showed that there was a decrease in the expression of eIF2alpha, PERK, ATF6, ATF4 and CHOP proteins with increasing melatonin treatment concentrations in 100 mM glucose-treated Schwann cells, when compared to the cells treated with 100 mM glucose alone. The proteins expressions from the ER stress pathway correlated with the LC3 protein expression and cell viability, indicating the occurrence of ER stress-mediated autophagic cell death in Schwann cells under hyperglycaemia condition. In summary, treatment with melatonin in hyperglycaemia cells ameliorated ER-stress mediated autophagic cell death.
Acknowledgements
This work was supported by International Medical University; the grant number is BMS I-2020 (18).
Disclosure of conflict of interest
None.
References
- 1.Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan PF. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10:14790. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buschard K. The etiology and pathogenesis of type 1 diabetes - a personal, non-systematic review of possible causes, and interventions. Front Endocrinol (Lausanne) 2022;13:876470. doi: 10.3389/fendo.2022.876470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li S, Wang J, Zhang B, Li X, Liu Y. Diabetes mellitus and cause-specific mortality: a population-based study. Diabetes Metab J. 2019;43:319–341. doi: 10.4093/dmj.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feldman EL, Callaghan BC, Pop-Busui R, Zochodne DW, Wright DE, Bennett DL, Bril V, Russell JW, Viswanathan V. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5:41. doi: 10.1038/s41572-019-0092-1. [DOI] [PubMed] [Google Scholar]
- 5.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019;11:45–63. [PMC free article] [PubMed] [Google Scholar]
- 6.Liu YP, Shao SJ, Gao HD. Schwann cells apoptosis is induced by high glucose in diabetic peripheral neuropathy. Life Sci. 2020;248:117459. doi: 10.1016/j.lfs.2020.117459. [DOI] [PubMed] [Google Scholar]
- 7.Nuszkiewicz J, Woźniak A, Szewczyk-Golec K. Ionizing radiation as a source of oxidative stress-the protective role of melatonin and vitamin D. Int J Mol Sci. 2020;21:5804. doi: 10.3390/ijms21165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tiong YL, Ng KY, Koh RY, Ponnudurai G, Chye SM. Melatonin prevents oxidative stress-induced mitochondrial dysfunction and apoptosis in high glucose-treated Schwann cells via upregulation of Bcl2, NF-κB, mTOR, Wnt signalling pathways. Antioxidants. 2019;8:198. doi: 10.3390/antiox8070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du W, Wang N, Li F, Jia K, An J, Liu Y, Wang Y, Zhu L, Zhao S, Hao J. STAT3 phosphorylation mediates high glucose-impaired cell autophagy in an HDAC1-dependent and -independent manner in Schwann cells of diabetic peripheral neuropathy. FASEB J. 2019;33:8008–8021. doi: 10.1096/fj.201900127R. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa S, Urano F. The role of ER stress in diabetes: exploring pathological mechanisms using wolfram syndrome. Int J Mol Sci. 2022;24:230. doi: 10.3390/ijms24010230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019;26:605–616. doi: 10.1038/s41418-018-0252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung S, Jeong H, Yu SW. Autophagy as a decisive process for cell death. Exp Mol Med. 2020;52:921–930. doi: 10.1038/s12276-020-0455-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao Y, Wang C, Jiang D, An G, Jin F, Zhang J, Han G, Cui C, Jiang P. New insights into the interplay between autophagy and oxidative and endoplasmic reticulum stress in neuronal cell death and survival. Front Cell Dev Biol. 2022;10:994037. doi: 10.3389/fcell.2022.994037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erekat NS. Programmed cell death in diabetic nephropathy: a review of apoptosis, autophagy, and necroptosis. Med Sci Monit. 2022;28:e937766. doi: 10.12659/MSM.937766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ploumi C, Papandreou ME, Tavernarakis N. The complex interplay between autophagy and cell death pathways. Biochem J. 2022;479:75–90. doi: 10.1042/BCJ20210450. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Jones JW, Choi HMC, Sarkar C, Kane MA, Koh EY, Lipinski MM, Wu J. cPLA2 activation contributes to lysosomal defects leading to impairment of autophagy after spinal cord injury. Cell Death Dis. 2019;10:531. doi: 10.1038/s41419-019-1764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976) 2011;36:E1427–34. doi: 10.1097/BRS.0b013e3182028c3a. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Zhou W, Li Z, Ren J, Li X, Li S, Liu Q, Song F, Hao A, Wang F. Melatonin protects neural stem cells against Tri-Ortho-Cresyl phosphate-induced autophagy. Front Mol Neurosci. 2020;13:25. doi: 10.3389/fnmol.2020.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahangiri B, Saei AK, Obi PO, Asghari N, Lorzadeh S, Hekmatirad S, Rahmati M, Velayatipour F, Asghari MH, Saleem A, Moosavi MA. Exosomes, autophagy and ER stress pathways in human diseases: cross-regulation and therapeutic approaches. Biochim Biophys Acta Mol Basis Dis. 2022;1868:166484. doi: 10.1016/j.bbadis.2022.166484. [DOI] [PubMed] [Google Scholar]
- 20.Song S, Tan J, Miao Y, Li M, Zhang Q. Crosstalk of autophagy and apoptosis: involvement of the dual role of autophagy under ER stress. J Cell Physiol. 2017;232:2977–2984. doi: 10.1002/jcp.25785. [DOI] [PubMed] [Google Scholar]
- 21.Cai Y, Arikkath J, Yang L, Guo ML, Periyasamy P, Buch S. Interplay of endoplasmic reticulum stress and autophagy in neurodegenerative disorders. Autophagy. 2016;12:225–244. doi: 10.1080/15548627.2015.1121360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Wu Y, Zou S, Wang X, Li Y, Xu K, Gong F, Liu Y, Wang J, Liao Y, Li X, Xiao J. NGF attenuates high glucose-induced ER stress, preventing Schwann cell apoptosis by activating the PI3K/Akt/GSK3β and ERK1/2 pathways. Neurochem Res. 2017;42:3005–3018. doi: 10.1007/s11064-017-2333-6. [DOI] [PubMed] [Google Scholar]
- 23.Padilla A, Descorbeth M, Almeyda AL, Payne K, De Leon M. Hyperglycemia magnifies Schwann cell dysfunction and cell death triggered by PA-induced lipotoxicity. Brain Res. 2011;1370:64–79. doi: 10.1016/j.brainres.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Guan R, Pan L. Mechanism of Schwann cells in diabetic peripheral neuropathy: a review. Medicine (Baltimore) 2023;102:e32653. doi: 10.1097/MD.0000000000032653. [DOI] [PMC free article] [PubMed] [Google Scholar]