Abstract
Background and Objective: Lysine is an essential amino acid involved in several biochemical pathways. It has been shown to enhance blood supply and target growth factors, leading to improved wound healing. The present study aimed to evaluate the efficacy and tolerability of a 15% lysine cream in treating diabetic foot ulcers, as measured by the Bates-Jensen Wound Assessment Tool (BWAT). Materials and Method: A randomized, open-label, interventional study was conducted on 40 volunteers with diabetic ulcers. The treatment group (n=20) received well-known treatment along with lysine cream (15%) twice daily, while the control group (n=20) received standard therapy alone. Wound healing was evaluated using the BWAT. The student t-test and one-way ANOVA were used to compare the clinical assessment parameters to the baseline. Results: Both groups showed a significant decrease in ulcer size, depth, edges, undermining, necrotic tissue type, necrotic tissue amount, exudate type, and exudate amount over six weeks, with no significant difference between the groups after the first week. The lysine-treated group showed a significant improvement in wound healing compared to the control group (P<0.05). Conclusion: The present study demonstrates that a 15% lysine cream can significantly improve wound healing in diabetic foot ulcer patients, as measured by the BWAT, compared to standard treatment alone. Further research is needed to confirm these findings and to explore the underlying mechanisms of lysine’s therapeutic effects.
Keywords: Diabetic foot ulcer, lysine cream, Bates-Jensen wound assessment
Introduction
Diabetes mellitus represents a growing public health concern, affecting 536.6 million cases worldwide in 2021, and is expected to rise to 783.2 million by 2045 [1]. One of the most severe complications of diabetes is diabetic foot ulceration (DFU), which increases the risk of morbidity, and mortality and lowers the quality of life [1]. The estimated lifetime risk of developing a foot ulcer is about 19-34% of persons with diabetes mellitus have a history of foot ulceration [2].
Wound healing combines cellular and molecular mechanisms to restore the original architecture of injured tissues. Several epigenetic factors have been found to influence how the healing process in various tissues is modulated [3]. The skin restoration process is arguably the most well-understood one. It consists of four stages: the immediate early response, brought on by mechanical stimuli and biochemicals released at the site of injury; inflammation; proliferation and migration of wound-edge epithelium; and healing closure. Epigenetic processes control each of these processes [4,5]. The restoration procedures are no longer offered in a select few exceptional cases, such as patients with ulceration and other co-morbidities like peripheral vascular disease, due to the absence of pedal pulsations [4].
Although many treatments are available for DFU, the healing process is often slow and challenging. Treatment of diabetic ulcers begins with surgical debridement, which converts the chronic wound to an acute injury, after which collagenase can be used for adequate debridement [6]. Local wound care is initiated and continued until the wound is clean and ready for reconstruction; it is a secondary intention allowed to heal an ideal wound dressing that keeps the wound moist and clean, preventing pressure and mechanical trauma, reducing edema, stimulating repair, and being affordable [7].
Newer methods, such as growth factors, are promising for treating chronic diabetic ulcers [8]. Growth factors attract cells into the wound, stimulate their proliferation, and profoundly influence extracellular matrix deposition. The U.S. Food and Drug Administration only authorized growth factor for treating diabetic foot ulcers is recombinant human platelet-derived growth factor-BB (rhPDGF-BB) [9].
Recent reports also suggest the beneficial role of nutrition plays in wound healing [10]. Amino acids aid in the recovery of wounds related to multiple co-morbidities. Human tripeptide GHK-Cu has effectively promoted wound healing, drawing in immune cells, possessing antioxidant and anti-inflammatory properties, producing collagen and glycosaminoglycan in skin fibroblasts, and promoting blood vessel growth [11].
Lysine is an essential amino acid that plays a crucial role in wound healing, with studies showing its ability to stimulate angiogenesis and promote cell proliferation. Recently, there have been reports of lysine’s potential therapeutic effect in treating diabetic foot ulcers [12,13]. Histopathological analysis of wounds treated with lysine showed that the dermo-epidermal layer thickened and cell proliferation increased but was controlled [14]. In a clean-cut wound model, the molecule offers qualitatively superior, noticeably quicker healing with less scarring and distortion. It is believed that lysine stimulates angiogenesis by acting as a cell surface bridge to connect growth factors to their receptors [15]. Therefore, this study aims to compare topical lysine’s effectiveness to standard moist dressing with saline in treating diabetic foot ulcers. The results of this study could provide valuable insights into the potential of lysine as a treatment option for DFU.
Materials and methods
Study design
Between April and November 2009, a randomized, open-label, comparative, prospective, and parallel-group study was carried out at the Diabetology departments of Chennai, Tamil Nadu’s Madras Medical College and Government General Hospital.
Diabetic patients with grade II foot ulcers, visited the outpatient department, between April and November of 2009 were enrolled in the study. Each participant underwent treatment for eight weeks, including eight visits. Each participant underwent the examination for eight weeks, including eight separate visits. After receiving approval from the institution’s ethical review committee, the study was carried out.
Inclusion criteria
(1) Age: 30-70 years. (2) Both genders. (3) Fasting blood sugar of 130 mg/dl or postprandial blood sugar of greater than 130 and greater than 180 mg/dl. (4) Patients with type-2 diabetes who have a grade 2 diabetic ulcer that has lasted more than eight weeks.
Exclusion criteria
(1) Grade-I, III, IV, V diabetic ulcer. (2) Patients who have been diagnosed with vascular occlusion. (3) Mothers who are pregnant or breastfeeding. (4) Patients who refuse to provide informed consent. (5) Pathogenic bacteria were found in a wound swab.
Study design, study participants, and sample size
Forty volunteers were screened and divided into two groups: Group A (case) and Group B (control). Each group of 20 participants received drug treatment and management for eight weeks.
Case
Routine ulcer care (n=20) standard therapy with lysine 15% cream. Dressing with non-adherent absorbent gauze.
Control
Routine ulcer care (n=20). Standard treatment (2% Mupirocin). Using absorbent non-adherent gauze for dressing.
Evaluation and assessment tool
The improvement in the patient’s wound condition was assessed by Bates-Jensen wound assessment tool (BWAT): The BWAT is a standardized, methodical, visual wound evaluation technique that has proven effective for all chronic wounds. The instrument assesses the extent of the wound, its depth, margins, undermining, the type and quantity of necrotic tissue, the amount of granulation and epithelialization tissue, the type and quantity of exudate, and the color of the skin around the wound, edema, and induration. These are rated using a modified Likert scale, with 1 denoting the most conducive to health features and 5 representing the opposite. A 13 out of 65 scores indicates regeneration.
Size of the ulcer assessed as per BWAT
1. 4sq.cms in length and width. 2. 4q.cm in length and width. 3. Dimensions: length 16.1-36q.cms width 16.1-36q.cms. 4. Dimensions: length 36.1-80q.cm width 36.1-80q.cm. 5. Length >80q.cm width.
Depth of the ulcer assessed as per BWAT
1. Erythema of intact skin that is not blanchable. 2. Substantial epidermal thickness erosion, including the epidermis and dermis. 3. Comprehensive epidermis loss and subcutaneous tissue damage or necrosis may extend to but not via underlying fascia, mixed partial and complete thickness and tissue layer covered by granulation tissue. 4. Skin loss with significant destruction, tissue necrosis or muscle injury, necrosis of bone or supporting structures.
Visits to receive study drug
Tubes were only accessible for one week. They were asked to return the empty tube at the end of the first week (to assess compliance) and receive a new line for the second week. The exact process was used throughout the entire six-week period. The patient was instructed to notify the investigator immediately if any adverse effects occurred. But during the study period, there were no adverse events reported.
Wound assessment of the scales was done at baseline and each follow-up visit.
Photographs were taken for the patients in both the groups at the baseline (visit 1), interim visit (visit 5) and at the end of the visit (visit 9).
Fasting blood sugar values were taken at the baseline (visit 1), interim visit (visit 5) and at the end of the visit (visit 9).
Swab collection
A cotton-tipped Tran swab should be lightly rubbed across the wound surface while moving the wipe over the entire area in a zigzag manner Swabs should be wetted with 0.9 percent sodium chloride or transport medium before cotton swab the wound. On the same day, the swab should be sent to the laboratory. The request form should include all essential details (Patient name, age, gender, address, wound location, ID number).
Ethical consideration
The study was conducted after obtaining approvals from the institutional ethical committee. At Madras Medical College and Government General Hospital in Chennai, patients with diabetic foot ulcers who were already receiving standard diabetic ulcer treatment were informed in the local language about the study’s objectives, methods, and possible adverse effects. Participants in the study provided informed consent in an acceptable form and in the local language. The illiterate patients’ left thumb impressions were taken before a neutral witness.
Statistical analysis
All eligible patients enrolled in the study were considered for statistical analysis. The clinical assessment parameters will be analysed using the Student’s t-test and one-way ANOVA compared to their initial values. p-value of 0.05 were regarded as significant. Scoring was per formed on both scales. The rate of recovery was also evaluated.
Results
The study participated in the outpatient clinic, Diabetology department, Madras Medical College, and Government General Hospital in Chennai. Out of 76 people screened, 40 patients with grade II diabetic ulcers were chosen for the study.
Group A (n=20) received 15% lysine cream in addition to the recommended therapy. The study drug was administered for eight weeks, and the changes were tracked for eight weeks. Group B (n=20): only standard therapy was used for treatment. Factors like age distribution, body mass index, and sex distribution were taken into account to determine the statistical significance of demographic characteristics. The mean age distribution of the study groups was found to be 54.75 and 53.80 after the age distribution of the study groups was analyzed. There was no statistical difference between the study groups (P=0.720).
There were nine female patients and eleven male patients in group A. For group B, 10 male and 10 female patients were chosen. No statistically significant difference was found by statistical analysis (P=0.752). This shows that the gender distribution in both groups was equal. For the study groups, the mean BMI was 26.26 for group A and 26.40 for group B. There are no statistically significant differences between the study groups, according to the data analysis (P=0.833).
Assessment of wound healing by measuring the size and depth of the ulcer using BWAT
The mean ± SD values indicate the ulcer’s size and depth from the first to the ninth week. After initial treatment with lysine and standard therapy, there was no significant reduction in ulcer size in the test group during the first week of assessment (P=0.650); however, significant changes occurred in subsequent visits 6, 7, and 8, and ulcer depth was observed in all participants. The data presented in Table 1 revealed this statistically significant distinction between the groups. Data significance was greater at the most recent visit (P<0.001) than in previous visits and the control group. Results indicate that twice-daily topical application of lysine for eight weeks in the test group reduced ulcer size and depth as measured by BWAT.
Table 1.
Size of the ulcer
| Sl. No. | Size of the Ulcer | Mean ± Standard deviation | Student Independent t-Test p-value | |
|---|---|---|---|---|
|
| ||||
| Study Groups | Study Groups | |||
| A | B | |||
| 1. | Visit-2 | 2.85±1.04 | 2.7±1.031 | 0.650 |
| 2. | Visit-3 | 2.85±1.04 | 2.7±1.031 | 0.650 |
| 3. | Visit-4 | 2.55±0.826 | 2.7±1.031 | 0.614 |
| 4. | Visit-5 | 2.3±0.657 | 2.55±0.945 | 0.337 |
| 5. | Visit-6 | 2.1±0.526 | 2.45±0.83 | 0.310 |
| 6. | Visit-7 | 1.85±0.813 | 2.15±0.813 | 0.250 |
| 7. | Visit-8 | 1.5±0.513 | 1.95±0.826 | 0.045 |
| 8. | Visit-9 | 1.00±0.00 | 1.9±0.788 | <0.001 |
| One way ANOVA | P<0.001 | P=0.007 | - | |
Each row and column represents the Mean ± SD of the size of the ulcer. The differences in Mean ± SD values showed a significant difference between the study groups (P<0.001). P<0.05 was considered statistically substantial as derived from the descriptive method followed by a two-tailed Student’s t-test. SD, Standard deviation.
The ulcer depths measured at each visit from the first to the eighth week in the control and test groups are shown in Figure 1 as Mean ± SD values. The depth of the ulcer was significantly reduced in the test groups compared to the control group, which only received standard care (P 0.001). The lysine-treated groups had significantly quicker ulcer healing after five weeks of treatment than the lysine-untreated groups. In both groups, the mean value of the ulcer’s size significantly decreased (P=0.01), as did the mean value of the ulcer’s edges during treatment (P=0.01), and there was a statistical difference between the groups (P=0.001) (Table 2).
Figure 1.
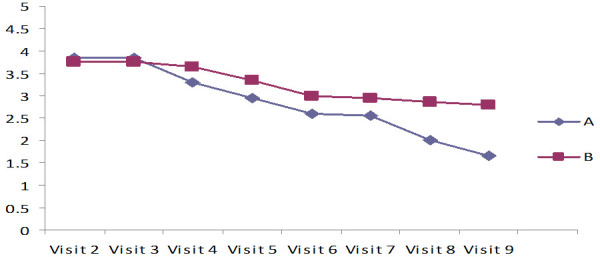
Measurement of depth of the foot ulcer assessed by Bates-Jensen wound assessment tool (BWAT).
Table 2.
Edges of the ulcer
| Sl. No. | Edges of the ulcer | Mean ± Standard deviation | Student Independent t-Test p-value | |
|---|---|---|---|---|
|
| ||||
| Study Groups | Study Groups | |||
| A | B | |||
| 1. | Visit-2 | 3.4±0.598 | 3.3±0.657 | 0.618 |
| 2. | Visit-3 | 3.4±0.598 | 3.3±0.657 | 0.618 |
| 3. | Visit-4 | 3.15±0.489 | 3.3±0.657 | 0.418 |
| 4. | Visit-5 | 2.7±0.571 | 3.2±0.616 | 0.011 |
| 5. | Visit-6 | 2.4±0.542 | 3.45±0.606 | 0.093 |
| 6. | Visit-7 | 2.3±0.47 | 2.6±0.598 | 0.086 |
| 7. | Visit-8 | 1.8±0.696 | 2.5±0.688 | 0.003 |
| 8. | Visit-9 | 1.4±0.598 | 2.5±0.688 | <0.001 |
| One way ANOVA | P<0.001 | P<0.001 | - | |
Each row and column represents the Mean ± SD of the Edges of the ulcer. The differences in Mean ± SD are significant. P<0.05 was considered statistically substantial as derived from the descriptive method followed by a two-tailed Student’s t-test.
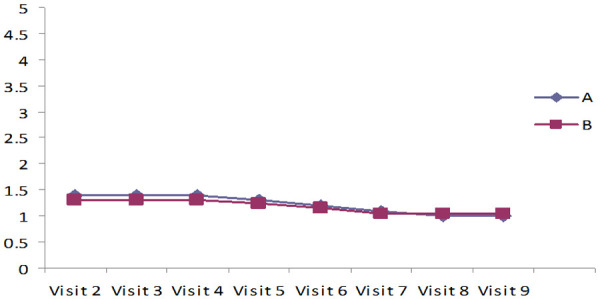
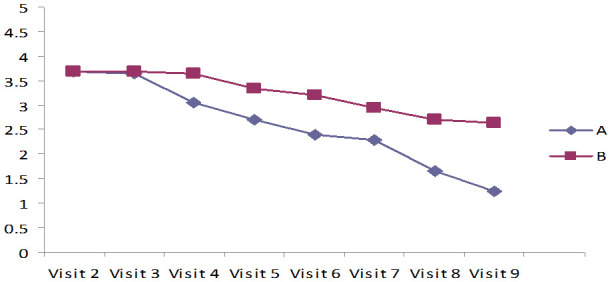
Assessment of wound healing by measuring undermining, necrotic tissue type, exudate type of the ulcer, and skin color surrounding the wound as BWAT
The results in Tables 3, 4 and 5 and Figures 2, 3 indicated Mean ± SD values of undermining, Necrotic tissues type, Exudate type of the ulcer surrounding the wound, and skin color of the ulcer of both the groups revealed that they improved the wound healing and significantly reduced the undermining of the ulcer, Exudate type, and amount of the ulcer, surrounding the wound and skin color of the ulcer in both the groups, which was statistically significant (P<0.001). The lysine-treated group showed little higher significance and faster wound healing compared to the untreated lysine group (P<0.001).
Table 3.
Necrotic tissue type
| Sl. No. | Necrotic tissue type | Mean ± SD | Student Independent t-Test p-value | |
|---|---|---|---|---|
|
| ||||
| Study Groups | Study Groups | |||
| A | B | |||
| 1. | Visit-2 | 2.95±0.394 | 3.00±0.459 | 0.714 |
| 2. | Visit-3 | 2.95±0.394 | 3.00±0.459 | 0.714 |
| 3. | Visit-4 | 2.50±0.513 | 2.90±0.553 | 0.023 |
| 4. | Visit-5 | 2.40±0.524 | 2.80±0.562 | 0.034 |
| 5. | Visit-6 | 2.15±0.587 | 2.70±0.571 | 0.005 |
| 6. | Visit-7 | 1.75±0.444 | 2.35±0.671 | 0.002 |
| 7. | Visit-8 | 1.25±0.444 | 2.20±0.696 | <0.001 |
| 8. | Visit-9 | 1.05±0.224 | 2.00±0.795 | <0.001 |
| One way ANOVA | P<0.001 | P<0.001 | - | |
Each row and column represent the Mean ± SD of Necrotic tissue type. The differences in Mean ± SD are significant. P<0.05 was considered statistically substantial as derived from the descriptive method followed by a two-tailed Student’s t-test.
Table 4.
Exudate type
| Sl. No. | Exudate type | Mean ± Standard deviation | Student Independent t-Test p-value | |
|---|---|---|---|---|
|
| ||||
| Study Groups | Study Groups | |||
| A | B | |||
| 1. | Visit-2 | 4.15±0.745 | 4.00±0.725 | 0.523 |
| 2. | Visit-3 | 4.01±0.718 | 4.00±0.725 | 0.664 |
| 3. | Visit-4 | 3.50±0.607 | 3.90±0.718 | 0.065 |
| 4. | Visit-5 | 3.05±0.759 | 3.50±0.827 | 0.081 |
| 5. | Visit-6 | 2.85±0.709 | 3.20±0.764 | 0.062 |
| 6. | Visit-7 | 2.45±0.605 | 3.00±0.725 | 0.013 |
| 7. | Visit-8 | 1.75±0.639 | 2.65±0.933 | 0.001 |
| 8. | Visit-9 | 1.15±0.366 | 2.15±0.933 | <0.001 |
| One way ANOVA | P<0.001 | P<0.001 | - | |
Each row and column represents the Mean ± SD of the Exudate type. The differences in Mean ± SD show -significant. P<0.05 was considered statistically significant as derived from the descriptive method followed by a two-tailed Student’s t-test.
Table 5.
Skin color surrounding wound
| Sl. No. | Skin color surrounding wound | Mean ± Standard deviation | Student Independent t-Test p-value | |
|---|---|---|---|---|
|
| ||||
| Study Groups | Study Groups | |||
| A | B | |||
| 1. | Visit-2 | 4.2±0.696 | 4.05±0.759 | 0.519 |
| 2. | Visit-3 | 4.2±0.696 | 4.05±0.759 | 0.519 |
| 3. | Visit-4 | 3.85±0.685 | 4±0.759 | 0.438 |
| 4. | Visit-5 | 3.65±0.671 | 4±0.758 | 0.085 |
| 5. | Visit-6 | 3.1±0.718 | 3.6±0.598 | 0.022 |
| 6. | Visit-7 | 2.7±0.733 | 3.3±0.733 | 0.014 |
| 7. | Visit-8 | 1.95±0.887 | 3.2±0.768 | P<0.001 |
| 8. | Visit-9 | 1.65±0.671 | 3.2±0.768 | P<0.001 |
| One way ANOVA | P<0.001 | P<0.001 | - | |
Each row and column represent the Mean ± SD of the Skin color surrounding wound. The differences in Mean ± SD show -significant. P<0.05 was considered statistically significant as derived from the descriptive method followed by a two-tailed Student’s t-test.
Figure 2.
Measurement of Undermining of the Ulcer assessed using Bates-Jensen wound assessment tool (BWAT).
Figure 3.
Measurement of Necrotic tissue amount assessed using Bates-Jensen wound assessment tool (BWAT).
Assessment of wound healing by measuring peripheral edema, granulation tissue, epithelialization, and Bates-Jensen wound assessment scale - score by BWAT
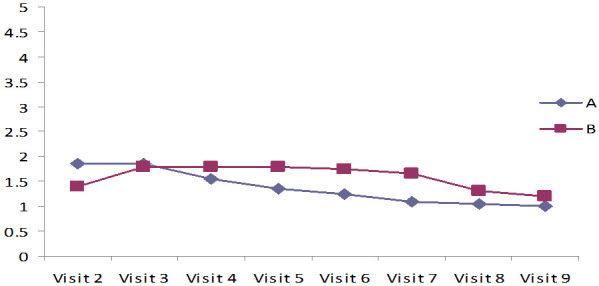
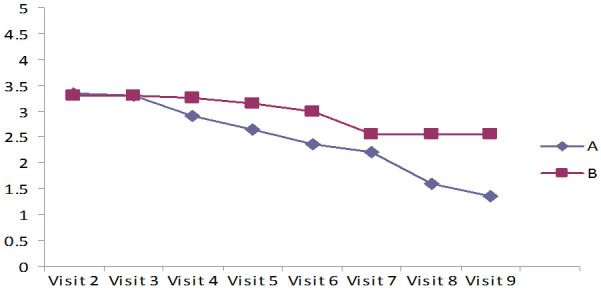
The results in Tables 6, 7 and Figures 4, 5 indicate Mean ± SD values of significant reduction of peripheral edema, peripheral tissue induration, granulation tissue, and epithelization of the wound among both the groups (P<0.001). Group A (case) was shown a faster-wound healing rate and significant wound healing properties when compared to the control group (P<0.001). In addition, the Bates Jensen scale score of 13 out of 65 points indicates regeneration. In group A, the score was 25.3 at the study’s end, showing significant ulcer healing. In group B, the score was 10.74, which indicates poor healing (Table 8). Compared to the patients treated with standard care with 2% mupirocin, the test groups receiving the standard care with 2% lysine cream showed significantly less ulcer depth and size (Figures 6 and 7).
Table 6.
Peripheral tissue edema
| Sl. No. | Peripheral tissue edema | Mean ± Standard deviation | Student Independent t-Test p-value | |
|---|---|---|---|---|
|
| ||||
| Study Groups | Study Groups | |||
| A | B | |||
| 1. | Visit-2 | 1.8±0.733 | 1.7±0.733 | 1.000 |
| 2. | Visit-3 | 1.7±0.733 | 1.7±0.733 | 1.000 |
| 3. | Visit-4 | 1.4±0.503 | 1.7±0.733 | 0.139 |
| 4. | Visit-5 | 1.2±0.41 | 1.6±0.754 | 0.046 |
| 5. | Visit-6 | 1.1±0.32 | 1.55±0.543 | 0.032 |
| 6. | Visit-7 | 1±0 | 1.25±0.444 | 0.021 |
| 7. | Visit-8 | 1±0 | 1.2±0.41 | 0.042 |
| 8. | Visit-9 | 1±0 | 1.2±0.41 | 0.042 |
| One way ANOVA | P<0.001 | P=0.006 | - | |
Each row and column represents Mean ± SD of Peripheral Tissue Edema. The differences in Mean ± SD are significant. P<0.05 was considered statistically significant as derived from the descriptive method followed by a two-tailed Student’s t-test.
Table 7.
Epithelialisation
| Sl. No. | Epithelialization | Mean ± Standard deviation | Student Independent t-Test p-value | |
|---|---|---|---|---|
|
| ||||
| Study Groups | Study Groups | |||
| A | B | |||
| 1. | Visit-2 | 4.8±0.444 | 4.75±0.444 | 1.000 |
| 2. | Visit-3 | 4.75±0.444 | 4.75±0.444 | 1.000 |
| 3. | Visit-4 | 4.15±0.671 | 4.65±0.489 | 0.010 |
| 4. | Visit-5 | 4.00±0.543 | 4.55±0.492 | 0.010 |
| 5. | Visit-6 | 3.60±0.503 | 4.40±0.503 | <0.001 |
| 6. | Visit-7 | 3.20±0.768 | 4.20±0.696 | <0.001 |
| 7. | Visit-8 | 2.60±0.821 | 3.85±0.671 | <0.001 |
| 8. | Visit-9 | 2.15±0.875 | 3.85±0.671 | <0.001 |
| One way ANOVA | P<0.001 | P<0.001 | - | |
Each row and column represents the Mean ± SD of Epithelialisation. The differences in Mean ± SD are significant. P<0.05 was considered statistically significant as derived from the descriptive method followed by a two-tailed Student’s t-test.
Figure 4.
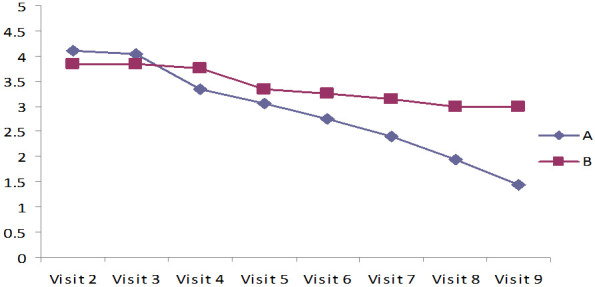
Measurement of Exudate Amount assessed using Bates-Jensen wound assessment tool (BWAT).
Figure 5.
Measurement of Peripheral tissue induration using Bates-Jensen wound assessment tool (BWAT).
Table 8.
Bates-Jensen wound assessment scale - score
| Sl. No. | Visit | Study Groups | Study Groups |
|---|---|---|---|
| A | B | ||
| 1. | Visit 2 | 42.4 | 40.79 |
| 2. | Visit 9 | 17.1 | 30.05 |
| Score* | 25.3 | 10.74 |
Scale Score = Previous stage - Current Stage.
Figure 6.
Granulation tissue. Measurement of Granulation tissue using Bates-Jensen wound assessment tool (BWAT).
Figure 7.
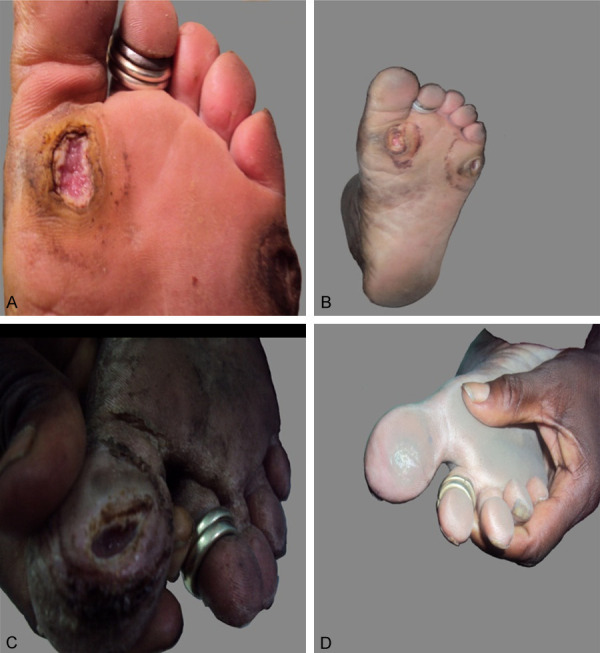
At visit (A), a patient with a grade II diabetic foot ulcer treated with 2% mupirocin cream and standard care showed a significant decrease in the depth and size of the ulcer at the 8th visit (B). The case group (C) is treated with 2% lysine cream in addition to standard care, the size and depth of the ulcer are significantly reduced when compared to the control group at the eighth visit (D).
Discussion
In diabetic patients, foot ulceration is a common occurrence, and the financial costs of treating these ulcers are substantial. Over 85% of diabetic patients’ lower extremity amputations begin with non-healing foot ulcers. Since the beginning, numerous treatment modalities have been investigated for treating diabetic ulcers; however, none have proven to be the most effective ulcer healer. So, the quest for a miracle cure for ulcers continues [16].
Ulcer-based studies have shown a decrease in the growth factor in the chronic wound setup. Hence, it has been hypothesized that exogenous supplementation of damages with growth factors will promote wound healing. Amino acids like glycine, proline, arginine, and lysine act as nonspecific binding molecules between the various growth factors and their receptors [8,10]. Thus, it has been theorized that it increases the available concentration of growth factors, leading to accelerated wound healing [17].
To date, studies have been conducted with only multicomponent amino acid preparations like tripeptide GHK and tetrapeptide AcSDKT as a topical supplementation therapy to evaluate this theory. In this study, we have assessed the efficacy and tolerability of a cream containing lysine alone for treating chronic diabetic ulcers as a supplement to the standard therapy [18,19].
The study showed a significant change in Bates Jensen scale 0 parameters, including ulcer size, depth, margins, necrotic tissue type, necrotic tissue amount, exudate type, exudate amount, skin color surrounding the lesion, granulation tissue, and epithelialization, were found by statistical analysis. Size (P=0.01), depth (P=0.001), necrotic tissue type (P=0.001), necrotic tissue amount (P=0.01), exudate type (P=0.001), exudate amount (P=0.01), skin color surrounding the wound (P=0.01), granulation tissue (P=0.01), and epithelialization (P=0.001) are the p values for these parameters. This is a sign that group A has significantly improved.
BWAT tool for assessment of wound severity and improvements over time, have been used in clinical research and in clinical practice [20-22]. Regeneration is indicated by a BWAT scale score of 13 out of 65. After the study, group A score was 25.3; this value denotes a significant degree of healing. Group B received a score of 10.74, which denotes subpar healing. Lamin gel (GHK-Cu) was the subject of a multicentric, randomized, placebo-controlled clinical study by Mulder et al. to treat diabetic ulcers [23]. Compared to placebo and standard therapy, the rate of healing was quicker (P<0.01). The closure rate was three times faster than the present study’s double healing rates. However, Mulder et al., noted the prevalence of ulcer infections, which our study did not find clinically evident [24]. The rate of healing was faster than the standard treatment and placebo (P<0.01). The closure rate was three times faster compared to the present study’s double healing rates. But, Mulder records the incidence of ulcer infections, which was not clinically evident in our study [23].
The present study provides promising evidence that a 15% lysine cream can improve wound healing in diabetic foot ulcer patients, although there are some opportunities for future research to enhance the validity and generalizability of the findings. While the study had a small sample size and an open-label design, it represents an important initial step in demonstrating the potential therapeutic effects of lysine cream. Future studies could build on these findings by conducting larger randomized controlled trials with longer follow-up periods and more detailed measurements of wound healing. Additionally, the study highlights the potential benefits of using lysine cream in conjunction with standard treatment. However, further research is needed to determine the cost-effectiveness of this approach and explore the use of lysine cream in combination with other treatments to enhance wound healing. Overall, this study offers promising insights into the use of lysine cream as a potential treatment option for diabetic foot ulcers, and opens up exciting possibilities for further research in this area.
Conclusion
The study evaluated the efficacy of a cream containing 15% lysine alone for treating chronic diabetic foot ulcers as a supplement to standard therapy. The results showed a significant improvement in wound healing parameters with the use of lysine cream, indicating its potential as a treatment option for diabetic foot ulcers. However, further research is needed to determine its cost-effectiveness and to explore its use in combination with other treatments to enhance wound healing.
Disclosure of conflict of interest
None.
References
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 3.Lewis CJ, Stevenson A, Fear MW, Wood FM. A review of epigenetic regulation in wound healing: implications for the future of wound care. Wound Repair Regen. 2020;28:710–718. doi: 10.1111/wrr.12838. [DOI] [PubMed] [Google Scholar]
- 4.Rafehi H, El-Osta A, Karagiannis TC. Epigenetic mechanisms in the pathogenesis of diabetic foot ulcers. J Diabetes Complications. 2012;26:554–561. doi: 10.1016/j.jdiacomp.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Kalal BS, Modi PK, Najar MA, Behera SK, Upadhya D, Prasad TSK, Pai VR. Hyperphosphorylation of HDAC2 promotes drug resistance in a novel dual drug resistant mouse melanoma cell line model: an in vitro study. Am J Cancer Res. 2021;11:5881–5901. [PMC free article] [PubMed] [Google Scholar]
- 6.Manna B, Nahirniak P, Morrison CA. StatPearls. Treasure Island (FL): StatPearls Publishing; 2022. Wound debridement. [PubMed] [Google Scholar]
- 7.Chhabra S, Chhabra N, Kaur A, Gupta N. Wound healing concepts in clinical practice of OMFS. J Maxillofac Oral Surg. 2017;16:403–423. doi: 10.1007/s12663-016-0880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Declair V. The importance of growth factors in wound healing. Ostomy Wound Manage. 1999;45:64–68. 70–72, 74 passim. [PubMed] [Google Scholar]
- 9.Al-Zube L, Breitbart EA, O’Connor JP, Parsons JR, Bradica G, Hart CE, Lin SS. Recombinant human platelet-derived growth factor BB (rhPDGF-BB) and beta-tricalcium phosphate/collagen matrix enhance fracture healing in a diabetic rat model. J Orthop Res. 2009;27:1074–1081. doi: 10.1002/jor.20842. [DOI] [PubMed] [Google Scholar]
- 10.Bechara N, Gunton JE, Flood V, Hng TM, McGloin C. Associations between nutrients and foot ulceration in diabetes: a systematic review. Nutrients. 2021;13:2576. doi: 10.3390/nu13082576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickart L, Vasquez-Soltero JM, Margolina A. The human tripeptide GHK-Cu in prevention of oxidative stress and degenerative conditions of aging: implications for cognitive health. Oxid Med Cell Longev. 2012;2012:324832. doi: 10.1155/2012/324832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grace A, Murphy R, Dillon A, Smith D, Cryan SA, Heise A, Fitzgerald-Hughes D. Modified poly(L-lysine)-based structures as novel antimicrobials for diabetic foot infections, an in-vitro study. HRB Open Res. 2022;5:4. doi: 10.12688/hrbopenres.13380.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng H, Li B, Shen Q, Zhang C, Kuang L, Chen R, Wang S, Ma Z, Li G. Mechanisms of diabetic foot ulceration: a review. J Diabetes. 2023;15:299–312. doi: 10.1111/1753-0407.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shashikumara S, Purushotham K, Darshan CL, Kalal BS. Characterization of antidepressant activity of Saraca asoca flower (Roxb.) Wilde in mice subjected to acute restraint stress. Am J Transl Res. 2022;14:5014–5023. [PMC free article] [PubMed] [Google Scholar]
- 15.Vani J, Kumara S, Prathima C. A randomized, open-label, comparative study of lysine cream 15% with standard therapy in the management of non-diabetic foot ulcer assessing by Bates-Jensen wound assessment tool. Natl J Physiol Pharm Pharmacol. 2019;9:907–909. [Google Scholar]
- 16.Everett E, Mathioudakis N. Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 2018;1411:153–165. doi: 10.1111/nyas.13569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry-Kilgour C, Cabral J, Wise L. Advancements in the delivery of growth factors and cytokines for the treatment of cutaneous wound indications. Adv Wound Care (New Rochelle) 2021;10:596–622. doi: 10.1089/wound.2020.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickart L, Margolina A. Regenerative and protective actions of the GHK-Cu peptide in the light of the new gene data. Int J Mol Sci. 2018;19:1987. doi: 10.3390/ijms19071987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shetty R, Rai M, Chandrashekar R, Kalal BS. Diabetogenic effect of gluten in Wistar albino rats: a preliminary preclinical screening. Med Pharm Rep. 2020;93:47–52. doi: 10.15386/mpr-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Chao N, Yin D. A net meta-analysis of the effectiveness of different types of dressings in the treatment of diabetic foot. Comput Math Methods Med. 2022;2022:4915402. doi: 10.1155/2022/4915402. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Brown S, Nixon J, Ransom M, Gilberts R, Dewhirst N, McGinnis E, Longo R, Game F, Bojke C, Chadwick P, Chandrasekar A, Chetter I, Collier H, Fernandez C, Homer-Vanniasinkam S, Jude E, Leigh R, Lomas R, Vowden P, Wason J, Sharples L, Russell D. Multiple interventions for diabetic foot ulcer treatment trial (MIDFUT): study protocol for a randomised controlled trial. BMJ Open. 2020;10:e035947. doi: 10.1136/bmjopen-2019-035947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gould LJ, Serena TE, Sinha S. Development of the BWAT-CUA scale to assess wounds in patients with calciphylaxis. Diagnostics (Basel) 2021;11:730. doi: 10.3390/diagnostics11040730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulder GD, Patt LM, Sanders L, Rosenstock J, Altman MI, Hanley ME, Duncan GW. Enhanced healing of ulcers in patients with diabetes by topical treatment with glycyl-l-histidyl-l-lysine copper. Wound Repair Regen. 1994;2:259–269. doi: 10.1046/j.1524-475X.1994.20406.x. [DOI] [PubMed] [Google Scholar]
- 24.Mulder G, Tenenhaus M, D’Souza GF. Reduction of diabetic foot ulcer healing times through use of advanced treatment modalities. Int J Low Extrem Wounds. 2014;13:335–346. doi: 10.1177/1534734614557925. [DOI] [PubMed] [Google Scholar]


