Abstract
This study examines the use of COVID-19 antiviral treatments in US nursing homes and the facility characteristics associated with use of oral antivirals and monoclonal antibodies.
Nursing home residents are at elevated risk for severe infection from SARS-CoV-2,1 making them a priority for antiviral treatment.2 Although oral antiviral use has been found to be greater in nursing homes than the community,3 overall use among nursing home residents has been low and may not be commensurate with residents’ elevated risk. Furthermore, multiple barriers to antiviral use exist in nursing homes, including treatment access, frequently changing authorizations and recommendations on the use of monoclonal antibodies (mAbs), patient and physician preferences, unfamiliarity with these medications, and treatment costs.4,5 We examined the use of COVID-19 antiviral treatments within US nursing homes and the facility characteristics associated with use.
Methods
The primary data source was the Centers for Disease Control and Prevention National Healthcare Safety Network (NHSN) Nursing Home COVID-19 database, which contains information regarding the number of residents treated with monoclonal antibodies (eg, bebtelovimab; complete list in the eAppendix in Supplement 1) and oral antivirals (nirmatrelvir-ritonavir and molnupiravir), as well as new resident COVID-19 weekly cases. All Medicare-certified nursing homes were required to submit information to NHSN. Facility characteristics were obtained from LTCFocus, the Centers for Medicare & Medicaid Services (CMS) Payroll-Based Journal, the CMS Nursing Home Compare database, and the CMS Physician Compare databases (eAppendix in Supplement 1).
We examined trends from May 31, 2021, through December 25, 2022, in the total number of oral antivirals and monoclonal antibodies (hereafter referred to as “treatments”) administered, confirmed new resident COVID-19 cases, treatment rates (ie, treatments divided by new cases), and the cumulative share of nursing homes that reported ever using treatments. Data from 106 facilities were omitted due to low data quality. Facility-level treatment rates were capped at 100%, and a 6-week moving average was used to estimate weekly rates. We used multivariable linear probability regression models (with county fixed effects) to estimate the relationship between facility characteristics and whether the nursing home reported ever (vs never) using treatments among facilities with at least 1 COVID-19 case, adjusted for variables in the Table (eAppendix in Supplement 1). Per Harvard policy, institutional review board approval and written informed consent were not required for publicly available data. Analyses were performed with Stata version 16. Statistical significance was determined by 95% CIs that did not include 0.
Table. Any Oral Antiviral or Monoclonal Antibody Use for COVID-19 by Nursing Home Characteristics, May 31, 2021-December 25, 2022a.
| Oral antiviral or monoclonal antibody use | Adjusted difference (95% CI) | ||
|---|---|---|---|
| None | Any | ||
| No. | 5696 | 8182 | NA |
| Facility characteristics | |||
| Bed size, mean (SD) | 106.6 (59.9) | 115.2 (67.9) | 5.68 (4.18 to 7.18) |
| Overall 5-star quality score, mean (SD) | 2.9 (1.4) | 3.3 (1.4) | 2.68 (1.66 to 3.70) |
| Profit status, No. (row %)b | |||
| Nonprofit | 920 (28.9) | 2266 (71.1) | [Reference] |
| Government owned | 284 (36.1) | 502 (63.9) | 1.11 (−2.92 to 5.14) |
| For profit | 4485 (45.3) | 5412 (54.7) | −2.42 (−4.71 to −0.14) |
| Part of a chain, No. (row %)b | |||
| No | 2053 (37.7) | 3396 (62.3) | [Reference] |
| Yes | 3305 (43.1) | 4360 (56.9) | −0.69 (−2.54 to 1.15) |
| Direct care hours per resident-day, mean (SD) | 3.6 (1.0) | 3.8 (0.9) | 1.68 (0.55 to 2.80) |
| No. of affiliated physicians per 100 beds, mean (SD)c | 2.7 (3.8) | 2.9 (3.4) | −0.96 (−2.14 to 0.23) |
| Any affiliated geriatrician, No. (row %)b,d | |||
| No | 3932 (41.1) | 5627 (58.9) | [Reference] |
| Yes | 916 (33.7) | 1805 (66.3) | 4.81 (2.50 to 7.11) |
| Staff COVID-19 vaccination rate, mean (SD) | 79.1 (13.6) | 82.6 (12.1) | 3.95 (2.59 to 5.32) |
| Resident characteristics | |||
| Percentage of residents who did not report race and ethnicity as White, mean (SD)e | 25.4 (23.3) | 17.1 (20.6) | −1.55 (−2.95 to −0.15) |
| Percentage of residents with Medicaid, mean (SD) | 63.7 (23.3) | 57.6 (22.7) | −1.89 (−3.03 to −0.76) |
| Resident acuity, mean (SD)f | 12.2 (1.9) | 12.2 (1.4) | 1.37 (0.33 to 2.40) |
| Resident age, mean (SD) | 76.2 (8.2) | 80.0 (6.2) | 6.32 (5.18 to 7.46) |
| Resident COVID-19 vaccination rate, mean (SD) | 85.4 (10.1) | 89.4 (7.7) | 3.92 (2.63 to 5.22) |
Abbreviation: NA, not applicable.
Sample restricted to 13 878 nursing homes with at least 1 resident COVID-19 case reported May 31, 2021, to December 25, 2022, in counties with more than 1 nursing home. Cell counts for categorical variables may not sum to column totals owing to missing information. Counts of missing observations are as follows: overall 5-star quality score, 123 (0.89%); profit status, 9 (0.1%); part of a chain, 764 (5.5%); direct care hours per resident-day, 346 (2.5%); number of affiliated physicians per 100 beds and any affiliated geriatrician, 1598 (11.5%); staff vaccination rate, 1 (0.01%); percentage of residents with non-White race and ethnicity, 1248 (9.0%); percentage of residents with Medicaid and resident acuity, 766 (5.5%); and resident age, 847 (6.1%). Adjusted differences in percentage points obtained from a multivariable linear regression estimating the probability of any oral antiviral or monoclonal antibody use as a function of all the characteristics contained in the table. Models also include county fixed effects. Positive estimates indicate increases in the likelihood of any treatment use; negative estimates indicate decreases in the likelihood of any use. Estimates for categorical characteristics are in comparison to the denoted reference group; estimates for continuous characteristics are per 1-SD change. SDs for continuous variables are as follows: bed size = 64.8; overall 5-star quality score = 1.4; direct care hours per resident-day = 0.9; affiliated physicians per bed = 3.6; staff COVID-19 vaccination rate = 12.8; percentage of residents with non-White race and ethnicity = 22.1; percentage of residents with Medicaid = 23.1; resident acuity = 1.6; resident age = 7.3; and resident COVID-19 vaccination rate = 9.0.
Categorical measure.
Facility affiliations are determined by the Centers for Medicare & Medicaid Services (CMS) using fee-for-service claims and are defined as a physician’s treating 3 unique patients and providing services on 3 unique dates at a facility during a 6-month period.
Geriatricians were identified as an affiliated physician with geriatric medicine listed as a specialty in the CMS Physician Compare database.
Race and ethnicity are self-reported in the Minimum Data Set by nursing home residents and reported in the LTCFocus data at the facility level. The non-White race and ethnicity categorization was used to minimize missing data owing to CMS censoring rules.
The acuity index indicates the level of care (eg, in activities of daily living and in specialized medical treatments) that is needed by residents of the facility. The index ranges from 0 to 24, with higher values indicating a greater need for care.
Results
During the study period, there were 763 340 resident cases of COVID-19 and 136 066 residents treated for COVID-19 among 15 092 nursing homes, equating to an overall oral antiviral or monoclonal antibody treatment rate of 17.8% (95% CI, 17.4%-18.3%) (Figure, A). The moving average treatment rate peaked at 32.7% (95% CI, 30.5%-34.8%) on November 28, 2021, coinciding with a decrease in new resident COVID-19 cases. During the last 6 weeks of the study, the mean treatment rate was 24.5% (95% CI, 23.2%-25.7%). Oral antiviral treatments replaced monoclonal antibodies as the dominant treatment in 2022; nirmatrelvir-ritonavir constituted 61.1% of all treatments in 2022, and molnupiravir constituted 18.2% (Figure, B). The share of facilities ever using a treatment increased steadily, but by the end of 2022, 41.0% of facilities still had not reported any use.
Figure. Oral Antiviral or Monoclonal Antibody Use for COVID-19 in US Nursing Homes.
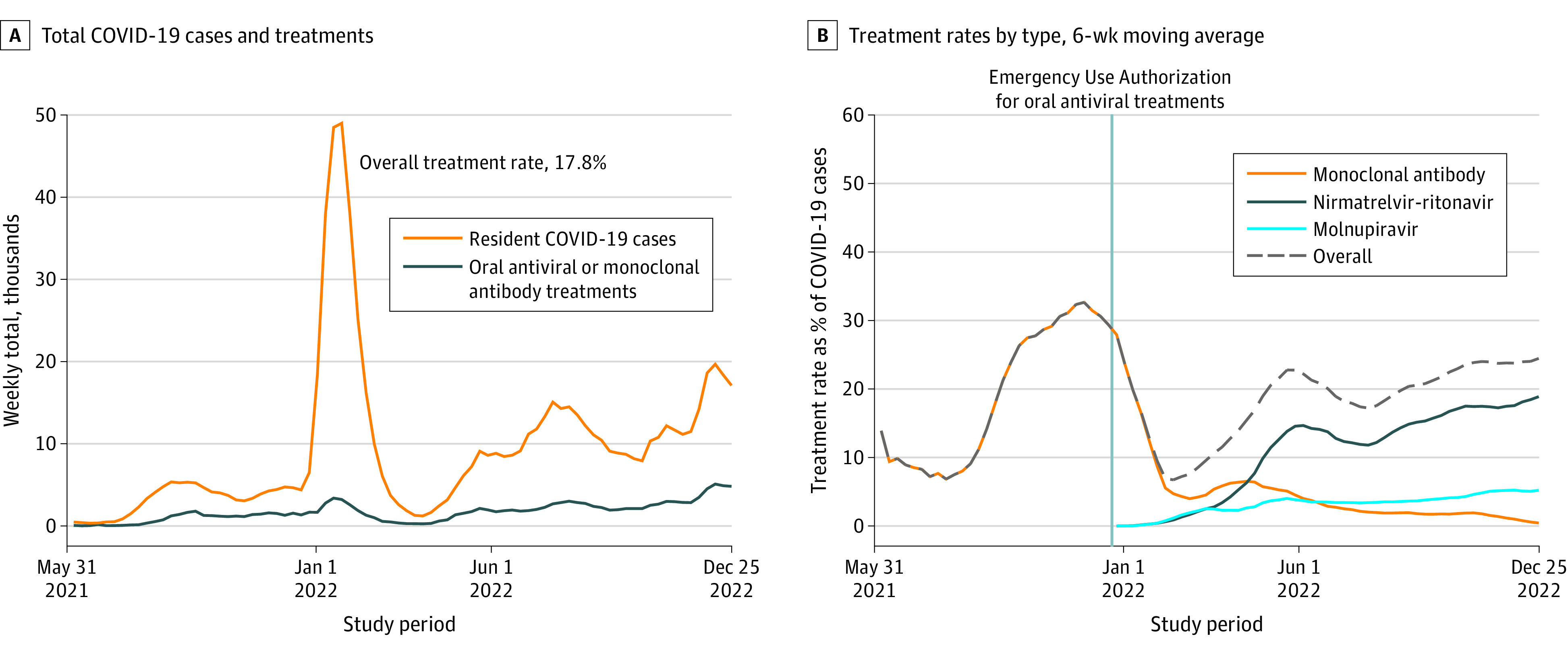
A, Data from the National Healthcare Safety Network (NHSN) database from 15 092 nursing homes. B, Moving 6-week average of Winsorized weekly treatment rates. Monoclonal antibodies include bamlanivimab, casirivimab plus imdevimab, bamlanivimab plus etesevimab, sotrovimab, and bebtelovimab. The NHSN data do not contain information on the use of remdesivir.
After adjustment, larger bed size, higher overall quality rating, greater direct care hours per resident-day, having an affiliated geriatrician, higher staff and resident vaccination rates, and greater mean resident age and acuity were positively associated with treatment use. Being for-profit and having higher shares of non-White race and Medicaid residents were significantly associated with lower probability of treatment use (Table).
Discussion
Despite their high risk, only 1 in 4 nursing home residents with COVID-19 had been treated with evidence-based antiviral treatments by the end of 2022.6 Overall treatment rates tracked closely with recent government estimates.3 Over 40% of nursing homes reported never administering any oral antiviral or monoclonal antibody treatment in the 19-month study window. Lower treatment rates in for-profit facilities and those with higher shares of Medicaid and non-White residents suggest that structural barriers may be contributing to underuse and disparities.
Study limitations include the use of facility-level data that prevent determining oral antiviral and monoclonal antibody treatment eligibility and receipt at the resident level, and an observational design that precludes causal interpretations.
Section Editors: Jody W. Zylke, MD, Deputy Editor; Kristin Walter, MD, Senior Editor.
eAppendix. Supplementary Details on Data and Methods
Data Sharing Statement
References
- 1.Centers for Medicare & Medicaid Services . COVID-19 nursing home data. Published 2023. Updated June 25, 2023. Accessed March 22, 2023. https://data.cms.gov/covid-19/covid-19-nursing-home-data
- 2.Department of Health and Human Services . Winter playbook for nursing homes and other long-term care facilities to manage COVID-19 and protect residents, staff, and visitors. Published 2022. Accessed May 17, 2023. https://www.whitehouse.gov/wp-content/uploads/2022/12/Winter-Playbook-for-Nursing-Homes-and-Other-Long-term-Care-Facilities-to-Manage-COVID-19-and-Protect-Residents-Staff-and-Visitors.pdf
- 3.Murphy SJ, Samson LW, Sommers BD. COVID-19 antivirals utilization: geographic and demographic patterns of treatment in 2022. Office of the Assistant Secretary for Planning and Evaluation. Published December 23, 2022. Accessed May 11, 2023. https://aspe.hhs.gov/reports/covid-19-antivirals-utilization
- 4.Tulledge-Scheitel S, Bell SJ, Larsen JJ, et al. A mobile unit overcomes the challenges to monoclonal antibody infusion for COVID-19 in skilled care facilities. J Am Geriatr Soc. 2021;69(4):868-873. doi: 10.1111/jgs.17090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kip KE, McCreary EK, Collins K, et al. Evolving real-world effectiveness of monoclonal antibodies for treatment of COVID-19: a cohort study. Ann Intern Med. 2023;176(4):496-504. doi: 10.7326/M22-1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewnard JA, McLaughlin JM, Malden D, et al. Effectiveness of nirmatrelvir-ritonavir in preventing hospital admissions and deaths in people with COVID-19: a cohort study in a large US health-care system. Lancet Infect Dis. 2023;23(7):806-815. doi: 10.1016/S1473-3099(23)00118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Supplementary Details on Data and Methods
Data Sharing Statement


