Abstract
Background
Avascular necrosis (AVN) of the femoral head can result from high-dose corticosteroid therapy. Given that severe COVID-19 pneumonia patients respond positively to corticosteroids, this study aimed to explore the incidence of femoral head AVN associated with corticosteroid therapy in 24 patients diagnosed with severe COVID-19 at a single center.
Material/Methods
The study included 24 patients who were diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection through real-time reverse transcription polymerase chain reaction test (rRT-PCR) and with COVID-19 pneumonia via high-resolution computed tomography (HRCT). Moderate cases received 2×4 mg Dexamethasone while severe cases were also administered with 3×40 mg Methylprednisolone. Diagnosis of femoral head AVN was confirmed with magnetic resonance imaging (MRI) and radiographs, which was subsequently treated by a total hip arthroplasty (THA) or a core decompression surgery (CDS) in line with the Ficat and Arlet classifications.
Results
Among the patients, 8 had a moderate infection course, while 16 were severe. The mean corticosteroid duration was 15±5 days for Dexamethasone and 30 days for Methylprednisolone. Severe patients presented with higher grade femoral head AVN and greater pain levels compared to moderate cases (p<0.05). Four patients developed bilateral AVN. The treatment resulted in 23 THAs and 5 CDSs.
Conclusions
The data from this study corroborate earlier studies and case reports, suggesting an increased occurrence of AVN of the femoral head during the COVID-19 pandemic due to the high-dose corticosteroid therapy employed for patients hospitalized with severe COVID-19 pneumonia.
Keywords: Arthroplasty, COVID-19, Osteonecrosis
Background
The current recommendations for the use of corticosteroids in the treatment of patients hospitalized with severe COVID-19 are dexamethasone in most patients that require conventional oxygen, and Petrov in all patients who require oxygen with a high-flow nasal cannula (HFNC), noninvasive ventilation (NIV), mechanical ventilation (MV), or extracorporeal membrane oxygenation (ECMO) [1]. Corticosteroid dosage for specific subgroups depending on the disease severity is yet to be discovered and optimized [2,3].
AVN of the femoral head is caused by traumatic and atraumatic reasons. The second most common etiology is corticosteroid-associated osteonecrosis [4]. The diagnosis is obtained by a clinical and imaging evaluation, and the timing of diagnosis can significantly affect outcomes.
The exact mechanism of osteonecrosis in association with corticosteroid use is unknown and under analysis. Sulewski et al described 10 cases of patients with AVN immediately after COVID-19 infection [5]. Li et al discovered glucocorticoid-induced osteonecrosis in up to 32% of the patients who were treated for severe infection [6].
Therefore, this study aimed to describe AVN of the femoral head associated with corticosteroid therapy in 24 patients diagnosed with severe COVID-19 at a single center.
Material and Methods
Ethics Statement
Ethics approval was obtained from the Institutional Review Board (IRB) at the University Hospital of Orthopedics “Prof. B. Boichev” EAD – Research and Ethics committee (№ 097/2023). This study was performed in accordance with the most recent version of the Helsinki Declaration. Written and verbal informed consent were taken. The participants’ full names were substituted with their initials to protect their rights and confidentiality. The authors declare no potential conflicts of interest.
Patients
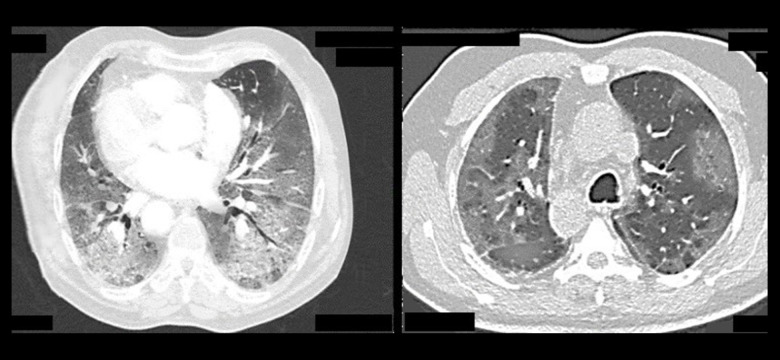
A retrospective study involving 24 patients was conducted. SARS-CoV-2 infection was diagnosed by an rRT −PCR test using 2 samples from the nose and throat. COVID-19 pneumonia was assessed by HRCT (Figure 1).
Figure 1.
Axial HRCT images showing severe changes in COVID-19 pneumonia.
The participants were divided into 2 groups – moderate (hospitalized, do not require oxygen supplementation) and severe COVID-19 (hospitalized, require oxygen in any form). Patients with moderate disease were treated with 2×4 mg dexamethasone. Severe cases received 3×40 mg methylprednisolone in addition to 2×4 mg dexamethasone. Dexamethasone was selected as a standard corticosteroid that was present in all COVID-19 protocols. The doses were based on expert opinion. Remdesivir was also administered, as well as the recommended anticoagulant therapy – prophylactic or therapeutic dose of heparin. The patients who required more than conventional oxygen (HFNC, NIV, MV, ECMO) were given methylprednisolone due to the unavailability of an immunomodulator at the institution (eg, Baricitinib, Tocilizumab) [1].
Orthopedic Assessment
The orthopedic clinical presentation consisted of mild groin pain that is increased in palpation, and limping. There was an increase in the pain with active movements, along with a reduction in hip range of motion. The Harris Hip Score (HHS) was used to assess the patients’ pain, function, and range of motion [7].
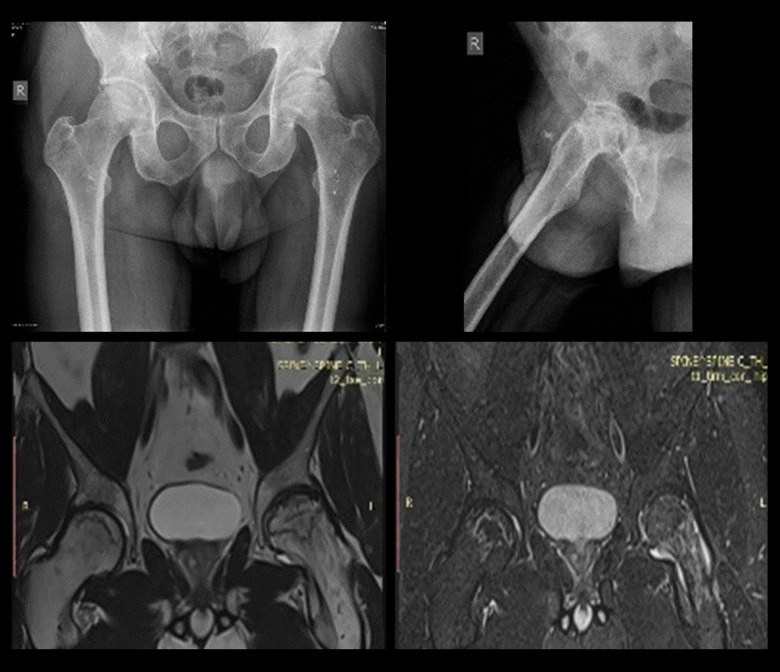
Plain radiographs and MRI were utilized and the patients were staged according to the Ficat and Arlet classification system (Figure 2) [8]. Focal sclerosis or cystic lesions that are seen on the radiographs as well as MRI data for edema confirmed Grade 2 AVN. Radiographic changes in femoral head sphericity and MRI data for bone necrosis confirmed Grade 3/4 AVN.
Figure 2.
Sample anteroposterior (AP) and lateral radiographs (top), and MRI images (bottom) of femoral head AVN.
Patients with Grades 3 and 4 received a THA and patients with a Grade 2 underwent a CDS. Grade 1 cases were not included in this study.
Statistics and Data
Demographic data and COVID-19-related patient information (severity of the infection, drug dosage, treatment duration, CRP, D-dimer) were recorded and analyzed (Table 1). The data were collected from the hospital’s registry to ensure accuracy and reliability. The timeframe was 25 months (from May 2020 until June 2022).
Table 1.
Summarized demographic and COVID-19-related patient data.
| Initials | Sex | Age | Therapy | COVID-19 | COVID-19 treatment duration | Days till AVN onset | D-dimer (μg/ml) | CRP (mg/L) | Orthopedic treatment |
|---|---|---|---|---|---|---|---|---|---|
| P.K.T. | M | 60 | 3×40 mg Methylprednisolone + 2×4 mg Dexamethasone | Severe | 7–30 days | 60 | 0.36 | 26 | THA |
| Y.G.A. | M | 59 | 2×40 mg Methylprednisolone | Severe | 7–30 days | 55 | 0.41 | 33 | THA |
| S.B.S. | M | 49 | 3×40 mg Methylprednisolone + 2×4 mg Dexamethasone | Severe | 7–30 days | 57 | 1.1 | 42 | THA + CDS |
| O.D.P. | F | 68 | 2×4 mg Dexamethasone | Moderate | 14 days | 66 | 27 | THA | |
| R.P.S. | M | 44 | 2×4 mg Dexamethasone | Moderate | 14 days | 46 | 25 | THA | |
| M.D.P. | M | 57 | 2×4 mg De×amathasone | Moderate | 21 days | 59 | 30 | THA | |
| A.H.D. | M | 68 | 2×40 mg Methylprednisolone + 2×4 mg Dexamethasone | Severe | 21 days | 65 | 0.29 | 25 | THA |
| W.G.P. | M | 49 | 2× 4 mg Dexamethasone | Moderate | 21 days | 58 | 24 | THA | |
| L.D.K. | M | 61 | 2×4 mg Dexamethasone | Severe | 21 days | 65 | 0.32 | 24 | THA |
| D.D.C. | M | 57 | 2×4 mg Dexamethasone | Moderate | 21 days | 59 | 33 | THA | |
| A.H.K. | F | 51 | 2×40 mg Methylprednisolone + 2×4 mg Dexamethasone | Severe | 14–30 days | 48 | 0.35 | 34 | THA |
| L.H.G. | F | 67 | 2×40 mg Methylprednisolone + 2×4 mg Dexamethasone | Severe | 14–30 days | 69 | 0.37 | 26 | THA |
| P.D.T. | M | 58 | 2×4 mg Dexamethasone | Severe | 21 days | 60 | 0.3 | 24 | CDS |
| M.G.H. | M | 39 | 3×40 mg Methylprednisolone + 2×4 mg Dexamethasone | Severe | 14–30 days | 47 | 0.4 | 30 | THA |
| L.K.Y. | F | 68 | 2×40 mg Methylprednisolone+ 2×4 mg Dexamethasone | Severe | 7–30 days | 65 | 0.44 | 31 | THA |
| P.D.M. | M | 49 | 3×40 mg Methylprednisolone +2×4 mg Dexamethasone | Severe | 14–30 days | 49 | 0.34 | 34 | THA + CDS |
| S.D.B. | M | 53 | 2×40 mg Methylprednisolone | Severe | 14–30 days | 60 | 0.41 | 36 | THA |
| G.I.B. | F | 61 | 2×4 mg Dexamethasone | Moderate | 21 days | 57 | 30 | THA | |
| S.I.L. | F | 43 | 2×40 mg Methylprednisolone | Severe | 7–30 days | 49 | 0.32 | 38 | THA |
| Y.Y.G. | M | 49 | 2×40 mg Methylprednisolone | Severe | 14–30 days | 51 | 0.44 | 36 | THA + CDS |
| P.G.H. | M | 57 | 2×40 mg Methylprednisolone | Severe | 14–30 days | 62 | 0.29 | 32 | THA |
| S.I.S. | M | 60 | 2×4 mg Dexamethasone | Moderate | 21 days | 55 | 35 | THA | |
| I.G.I. | F | 51 | 2×4 mg Dexamethasone | Moderate | 21 days | 50 | 24 | THA | |
| P.Y.P. | M | 57 | 2×40 mg Methylprednisolone | Severe | 14–30 days | 61 | 0.45 | 34 | THA + CDS |
The data are provided as the mean with standard deviation (SD) and p-values. The t test was used for analysis. Microsoft Excel 2019 and the Real Statistics Resource Pack for Excel 2019, Release 7.7.1 were used for processing. Results of p<0.05 were considered statistically significant.
Results
Participant Characteristics
We assessed a total of 119 patients for eligibility (Figure 3). There were 17 males and 7 females, with a male to female ratio of 2.4: 1. The mean age of the patients was 56±15 years, as the youngest one was 39 years old and the oldest one was 68 years old. The mean body mass index (BMI) was 34.3±2 kg/m2.
Figure 3.
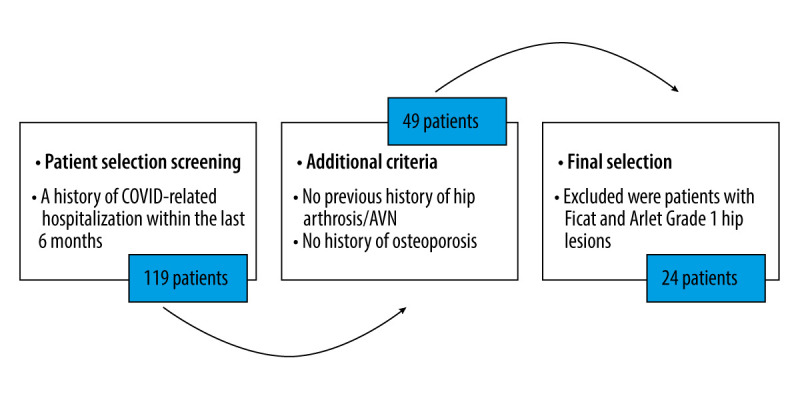
Patient eligibility assessment.
The course of infection was moderate in 8 patients and severe in 16. The moderate group consisted of 5 males and the mean age was 56±7 years. The severe group had 12 males and the mean age was 56 ±9 years.
Treatment and Clinical Outcomes
The mean corticosteroid duration was 15±5 days for dexamethasone, and 30 days for methylprednisolone. The mean time from the onset of infection to the onset of AVN symptoms was 57±12 days, and the mean time from first concern to diagnosis and orthopedic treatment was 87±45 days. At the time of surgical management, 100% of the participants had no symptoms of COVID-19 and all had a negative rRT-PCR test.
A bilateral involvement was discovered in 4 cases (17%), 24 patients with 28 hips underwent operative treatment, and 23 THAs (82%) and 5 CDSs (18%) were performed in total. The mean preoperative HHS was 59.2±11.4 compared to 86.8±7.2 postoperatively at 6 months follow-up (p<0.05). One case with CDS presented with worsened clinical and imaging signs of AVN at 7 months postoperatively, and was reoperated on with THA. No other complications were recorded.
Severe patients presented with a higher-grade femoral head AVN and greater pain levels than the moderate group (p<0.05) (Figure 4).
Figure 4.
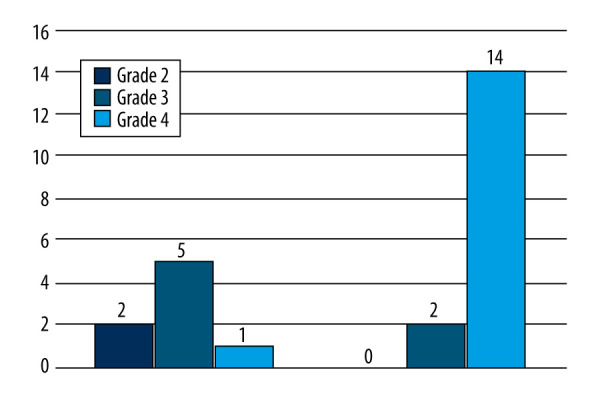
In the severe group, 88% presented with Grade 4 Ficat and Arlet femoral head lesions compared to 13% in the moderate group.
Laboratory Findings
The mean levels of D-dimer were 0.41 μg/ml and 31.6 mg/L for C-reactive protein (CRP) at the time of orthopedic treatment. The patients had elevated values of both D-dimer (normal values 0.23 μg/ml [230 ng/ml]) and CRP (normal values under 0.9 mg/L), corresponding to the severity index. These findings are in accordance with the systemic inflammation after COVID-19 and may be related to the AVN symptoms.
Vaccination Status
None of the patients in this study were vaccinated. Vaccination status is a part of the primary prophylaxis against the coronavirus infection and leads to fewer hospitalizations and lower incidence and mortality rates [9]. The absence of vaccination could be explained by the fact that the country has one of the lowest percentages in the European Unions – just above 30% [10].
Discussion
Twenty-four patients were treated with corticosteroid therapy for moderate to severe COVID-19 at a single institution. A combination of dexamethasone and methylprednisolone was given for a duration of 15±5 days and 30 days, respectively. Current National Institutes of Health (HIN) guidelines do not recommend the use of corticosteroids in patients who are hospitalized but do not require oxygen [1]. Dexamethasone is the preferred drug and it should be administered with a maximum daily dose of 6 mg with a duration of up to 10 days. The doses used in this study considerably exceeded the corticosteroid recommendations.
However, there is no consensus on steroid dosage and intake duration required to develop AVN. There are reports of a cumulative dose of 2000 mg prednisone (or its equivalent) [11]. Other studies have discovered a 700 mg dose is the minimum required to develop AVN [12]. McKee et al’s 15 patients who developed AVN had been treated with a mean steroid dose in prednisone equivalents of 850 mg (range, 290–3300 mg) [13]. Agarwala et al’s mean prednisolone equivalent steroid dose was 758 mg (range 400–1250 mg) [14]. This study has discovered a mean dose of methylprednisolone equivalent – 933 mg (range, 560–1680 mg) and a mean dose of dexamethasone of 207 mg (range, 112–240 mg).
Panin et al describe 3 patients with dexamethasone-related osteonecrosis in whom the first clinical signs of AVN appeared at days 80, 75, and 120 after the start of drug treatment against COVID-19 [15]. The mean duration from diagnosis of COVID-19 to onset of AVN symptoms in Agarwala et al’s paper was 58 days [13]. This study discovered that onset of AVN symptoms occurs after 57±12 days. According to some studies, the interval between corticosteroid intake and development of symptomatic AVN is usually 6 months to 1 year [16].
Four patients were discovered with bilateral involvement (17%). Li et al investigated 1406 patients with COVID-19; they detected bilateral AVN of the femoral head in one patient who was treated with 1960 mg methylprednisolone [6].
Several other studies described bone AVN after corticosteroid therapy for COVID-19 [17]. They are relatively small case series that involve up to 10 cases [5,14,15]. To the best of our knowledge, this study is the largest that describes AVN of the femoral head.
Limitations of the Study
The data from this study are from a single-center experience. The actual incidence may be higher. Another limitation is that the treatment protocols for COVID-19 have evolved rapidly since the pandemic. In the beginning very little was known on how to treat this infection; therefore, various corticosteroid dosing guidelines and regimes have been prescribed. The corticosteroid therapy for this group was partially based upon expert opinion, which is low-level evidence. This is a confounding factor.
Conclusions
The findings from this study support previous studies and case reports that cases of AVN of the femoral head increased during the COVID-19 pandemic due to the use of high-dose corticosteroid therapy in patients hospitalized with severe COVID-19 pneumonia.
Footnotes
Conflict of interest: None declared
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health; Accessed at https://www.covid19treatmentguidelines.nih.gov/ [PubMed] [Google Scholar]
- 2.Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID-19: Equity-related analyses and update on evidence. Cochrane Database Syst Rev. 2022;11(11):CD014963. doi: 10.1002/14651858.CD014963.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Z, Wang Y, Colunga-Lozano LE, et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: A systematic review and meta-analysis. CMAJ. 2020;192(27):E756–E767. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barney J, Piuzzi NS, Akhondi H. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Femoral head avascular necrosis. Accessed at: https://www.ncbi.nlm.nih.gov/books/NBK546658. [PubMed] [Google Scholar]
- 5.Sulewski A, Sieroń D, Szyluk K, et al. Avascular necrosis bone complication after active COVID-19 infection: Preliminary results. Medicina (Kaunas) 2021;57(12):1311. doi: 10.3390/medicina57121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Huang Z, Tan B, et al. General recommendation for assessment and management on the risk of glucocorticoid-induced osteonecrosis in patients with COVID-19. J Orthop Translat. 2021;31:1–9. doi: 10.1016/j.jot.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li F, Zhu L, Geng Y, Wang G. Effect of hip replacement surgery on clinical efficacy, VAS score and Harris hip score in patients with femoral head necrosis. Am J Transl Res. 2021;13(4):3851–55. [PMC free article] [PubMed] [Google Scholar]
- 8.Jawad MU, Haleem AA, Scully SP. In brief: Ficat classification: avascular necrosis of the femoral head. Clin Orthop Relat Res. 2012;470(9):2636–39. doi: 10.1007/s11999-012-2416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahmani K, Shavaleh R, Forouhi M, et al. The effectiveness of COVID-19 vaccines in reducing the incidence, hospitalization, and mortality from COVID-19: A systematic review and meta-analysis. Front Public Health. 2022;10:873596. doi: 10.3389/fpubh.2022.873596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coronavirus Resource Center. Bulgaria – COVID-19 Overview. Johns Hopkins; Accessed at: https://coronavirus.jhu.edu/region/bulgaria. [Google Scholar]
- 11.Jones JP. Osteonecrosis. In: Koopman WJ, editor. Arthritis and Allied Conditions: A Textbook of Rheumatology. 14th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 2143–64. [Google Scholar]
- 12.Anderton JM, Helm R. Multiple joint osteonecrosis following short-term steroid therapy. Case report. J Bone Joint Surg Am. 1982;64:139–41. [PubMed] [Google Scholar]
- 13.McKee MD, Waddell JP, Kudo PA, et al. Osteonecrosis of the femoral head in men following short-course corticosteroid therapy: A report of 15 cases. CMAJ. 2001;164:205–6. [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwala SR, Vijayvargiya M, Pandey P. Avascular necrosis as a part of ‘long COVID-19’. BMJ Case Rep. 2021;14(7):e242101. doi: 10.1136/bcr-2021-242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panin MA, Petrosyan AS, Hadjicharalambous K, Boiko AV. Avascular necrosis of the femoral head after COVID-19: A case series. Traumatology and Orthopedics of Russia. 2022;28(1):110–17. [Google Scholar]
- 16.Assouline-Dayan Y, Chang C, Greenspan A, et al. Pathogenesis and natural history of osteonecrosis. Semin Arthritis Rheum. 2002;32:94–124. [PubMed] [Google Scholar]
- 17.Wang H, Niu L. Can femoral head necrosis induced by steroid therapy in patients infected with coronaviruses be reversed? Bone Res. 2021;9(1):3. doi: 10.1038/s41413-020-00132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]