Abstract
Objective
To investigate clinicians’ perspectives on the current use of wearable technology for detecting COPD exacerbations, and to identify potential facilitators and barriers to its adoption in clinical settings.
Methods
A mixed-method survey was conducted through an online survey platform involving clinicians working with COPD patients. The questionnaires were developed by an expert panel specialising in respiratory medicine at UCL. The questionnaire evaluated clinicians’ perspectives on several aspects: the current extent of wearable technology utilisation, the perceived feasibility, and utility of these devices, as well as the potential facilitators and barriers that hinder its wider implementation.
Results
Data from 118 clinicians were included in the analysis. Approximately 80% of clinicians did not currently use information from wearable devices in routine clinical care. A majority of clinicians did not have confidence in the effectiveness of wearables and their consequent impact on health outcomes. However, clinicians highlighted the potential value of wearables in helping deliver personalised care and more rapid assistance. Ease of use, technical support and accessibility of data were considered facilitating factors for wearable utilisation. Costs and lack of technical knowledge were the most frequently reported barriers to wearable utilisation.
Conclusion
Clinicians’ perspectives of the use of wearable technology to detect and monitor COPD exacerbations are variable. While accessibility and technical support facilitate wearable implementation, cost, technical issues, and knowledge act as barriers. Our findings highlight the facilitators and barriers to using wearables in patients with COPD and emphasise the need to assess patients’ perspectives on wearable acceptability.
Keywords: wearable technology, COPD exacerbations, clinicians’ perspectives
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is currently the third leading cause of death worldwide.1 The latest GOLD report defines COPD as
A heterogeneous lung condition characterised by chronic respiratory symptoms (dyspnea, cough, sputum production, exacerbations) due to abnormalities of the airways (bronchitis, bronchiolitis) and/ or alveoli (emphysema) that cause persistent, often progressive airflow obstruction.2
Some COPD patients are susceptible to acute exacerbations, defined by the latest Global Initiative for Chronic Obstructive Lung Disease (GOLD) as
An exacerbation of chronic obstructive pulmonary disease (ECOPD) is defined as an event characterised by increased dyspnea and/or cough and sputum that worsens in < 14 days which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and systemic inflammation caused by infection, pollution, or other insult to the airways.2
Exacerbations have been linked to a significantly increased risk of developing complications, a decrease in health-related quality of life (HRQoL) and an increase in mortality.3–5 More than 20% of patients hospitalised with a severe exacerbation die within one year of discharge.4 One of the most important unmet clinical needs in COPD is the early detection, management, and monitoring of exacerbations. Early detection of an exacerbation, along with timely self-management by patients, has the potential to prevent hospitalisation and positively impact morbidity and mortality rates.6
In the latest GOLD report, the past exacerbation history remains the best predictor for future exacerbation risk.2,7 The introduction of advanced technologies, such as wearable devices, which could assess and measure variables associated with a COPD exacerbation, has the potential to enhance the clinical care of COPD patients.6,8 Through real-time monitoring of physiological variables, wearable technology could become routine part of future care. Several studies have found wearable technologies to be feasible and reliable for continuous measurement of vital signs (heart rate, respiratory rate, skin temperature) and physical activity, which thus include physiological parameters associated with monitoring and detecting exacerbations.9–12 However, studies have also reported a number of challenges, including low compliance and high study drop-out rates.13–15 Studies also found that missing data and technical issues were a concern and reported that users who had previously experienced exacerbations showed less adherence to continually wearing the devices.16,17
There has been growing interest in developing and implementing telehealth initiatives globally, supported by several studies and a Cochrane systematic review highlighting the potential benefits of telehealth.2,18 The specific role of wearables in detecting COPD exacerbations has been investigated in a few studies, but the results have been varied.17,19,20 This indicates that further research, particularly in understanding clinicians’ perspectives on the value of wearable technologies in COPD, is necessary. This study is set to assess clinicians’ perspectives of the current practice and potential of wearable technology in the detection of COPD exacerbations. We aim to better understand the facilitators and barriers that may limit the adaptation of wearable technology for patients with COPD.
Methods
Study Design and Population
A mixed-methods survey was conducted through an online survey platform (SurveyMonkey) to understand how wearable technology (“wearables”) could be used in the COPD care pathway. The perspectives of clinicians were collected, analysed, and evaluated. Surveyed individuals included qualified clinicians working with COPD patients. Conditional branching was used to distinguish clinicians with existing experience of wearable technology from clinicians who did not currently use wearable devices.
Questionnaire Design and Data Collection
The technology acceptance model (TAM) was used to understand the level of acceptance that clinicians have for the introduction of wearable devices into clinical settings for COPD.21 The TAM model states that to predict the level of acceptance of any given technology, it is important to understand the behavioural intention of the user/s, with behavioural intentions being influenced by the perceived usefulness of the technology in question, and the perceived ease of use of technology. As such, this study focused not only on existing user experiences of wearable technology but on the perceived views of clinicians, to understand whether this population group would be open to implementing the use of wearable devices in their respective clinical settings. For this study, wearables were defined as electronic devices worn as accessories, embedded within clothing, kept close to the body or attached to the skin via adhesive patches, collecting and transmitting data to the user.22
We conducted a literature review to identify the wearables currently in use, under development, or being clinically trialed.23 These include smartwatches, wristband trackers, smart rings, skin-based wearables, wireless body sensors, headbands, armbands, chest bands, waistbands/straps, smart vests/shirts, smart socks, pedometers, wearable stethoscopes, and smart mats. Furthermore, our review recognised potential physiological variables that could be used for monitoring ECOPD, such as heart rate, respiratory rate, oxygen saturation, temperature, physical activity, breathing effort, cough, sleep pattern, air quality, breathing sounds, quality of life, usage data from apps recording digital medications, and fluid retention.24
A panel at University College London (UCL), specialising in respiratory medicine, developed a questionnaire aimed at gathering insights into clinicians’ perspectives on the use of wearable technology in COPD clinical settings. The questionnaire evaluated clinicians’ perspectives on several aspects: the current extent of wearable technology utilisation, the perceived feasibility, and utility of these devices, as well as the potential facilitators and barriers that hinder its wider implementation, from both clinicians’ and patients’ perspectives. Responses were predominantly close-ended, either based on a checkbox or multiple-choice format or rated on a Likert Scale, with a 5-point range. The questionnaire also collected standard demographic data, such as age, job role, and country of clinical practice. Upon finalisation, the survey was disseminated via email and various social media platforms.
The survey was emailed to hospital respiratory teams, clinical academics, GP practices, and community respiratory teams across different countries. It was also shared on social media, including LinkedIn, Twitter and Facebook. Responses were collected and analysed after a 7-week period of distribution between June 2022 and July 2022.
Data was downloaded from surveymonkey.com® to Microsoft Excel to check for missing data. This was followed up by a transfer of the complete data from Microsoft Excel to SPSS 29 (IBM SPSS Statistics, New York, USA),25 with categorical variables reported using frequencies and percentages. Thematic analysis was conducted using NVivo 12, a qualitative data analysis software by QSR International. Each theme is represented by quotes to further understand clinicians’ perspectives regarding wearable technology.
This study took the form of a service evaluation survey, which was anonymised and based on informed consent with voluntary participation. As such, according to the UCL Research Ethics Committees’ definition of research, the Research Governance Framework (2005) and the Health Research Authority description, this study did not require ethical approval.26 To maintain an ethical study, informed consent was obtained from clinicians, with a consent statement being outlined clearly at the beginning of the questionnaire. Clinicians were informed that the data collected was intended for publication without identifiable information. The study followed the UKRI ethics and governance policy on informed consent.27
Results
General Characteristics Data
A total of 118 clinicians participated in the survey. 88 responded to the whole questionnaire. 80% (n=94/118) did not currently use wearables in COPD care. Most clinicians were from the UK and were predominately from a younger demographic (<44 years). The clinicians came from varied medical professions including physicians (30%), nurses (21%), and physiotherapists (21%). Table 1 provides details on the demographic characteristics of the responding clinicians.
Table 1.
General Characteristics Data
| Do You Currently Provide Clinical Care to COPD Patients Using Wearables? | ||||
|---|---|---|---|---|
| Yes | No | Total | ||
| Continent | Europe | 21 | 70 | 91 (77%) |
| Asia | 0 | 14 | 14 (12%) | |
| Middle East | 1 | 7 | 8 (7%) | |
| United States | 1 | 3 | 4 (3%) | |
| Australia | 1 | 0 | 1 (1%) | |
| Total | 24 (20%) | 94 (80%) | 118 (100%) | |
| Profession | ||||
| Physicians | 7 | 28 | 35 (30%) | |
| Nurse | 1 | 24 | 25 (21%) | |
| Occupational Therapist | 1 | 1 (0.8%) | ||
| Physiotherapist | 3 | 22 | 25 (21%) | |
| Dietitian | 1 | 1 (0.8%) | ||
| Clinical Research | 9 | 5 | 14 (12%) | |
| Respiratory Therapist | 4 | 13 | 17 (14%) | |
| Total | 24 (20%) | 94 (80%) | 118 (100%) | |
| Age | 18–24 | 1 | 5 | 6 (5%) |
| 25–34 | 14 | 21 | 35 (30%) | |
| 35–44 | 4 | 36 | 40 (34%) | |
| 45–54 | 3 | 25 | 28 (24%) | |
| 55–64 | 2 | 6 | 8 (7%) | |
| 65+ | 0 | 1 | 1 (1%) | |
| Total | 24 (20%) | 94 (80%) | 118 (100%) | |
Note: Data Reported as Frequency/ (Percentage %).
Wearable devices and variables
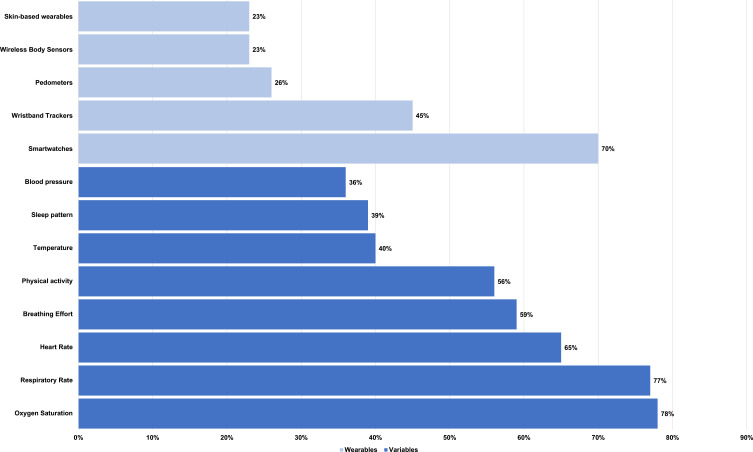
With regards to clinicians’ usage of wearable devices with patients, for the minority who did use them, (25%, n=6/24) used smartwatches, (21%, n=5/24) used wristband trackers, and (4%, n=1/24) used smart rings and waistbands. These clinicians mostly used the technology to monitor physical activity (42%, n=10/24), respiratory rate (33%, n=8/24), and heart rate (29%, n=7/24).
Among those who were not using wearable devices, clinicians reported that they would most likely use smartwatches (70%, n=66/94), wristband trackers (45%, n=42/94), and pedometers (26%, n=24/94). Devices such as smart mats (1%, n=1/94), and smart socks (2%, n=2/94) were the least likely to be used. These clinicians would most frequently seek to use wearables to measure oxygen saturation (78%, n=73/94), respiratory rate (77%, n=72/94), and heart rate (65%, n=61/94) (Figure 1).
Figure 1.
Wearables and variables that clinicians found desirable to monitor COPD patients.
Clinicians’ confidence in wearable technology to monitor and detect exacerbation
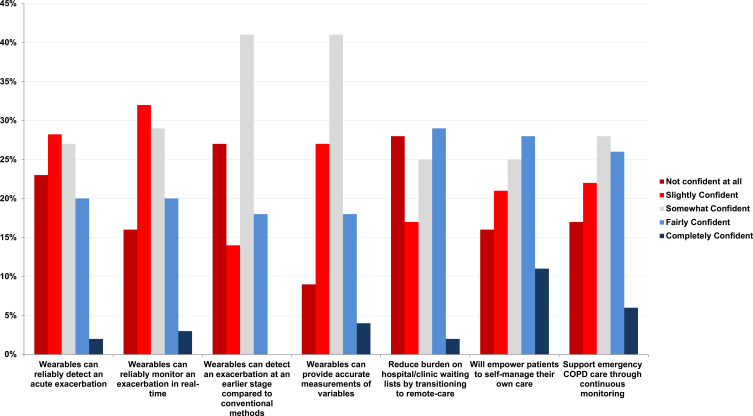
To examine clinicians’ confidence in the reliability of wearable devices for monitoring exacerbation, we assessed their level of confidence. The response rate was (80%, n=94/118). More than half of the clinicians (61%, n=57/94) reported being either “slightly confident” or “somewhat confident”. A minority of clinicians reported being “fairly confident” (20%, n=19/94) or “completely confident” (3%, n=3/94). On the other hand, a smaller number of clinicians (16%, n=15/94) reported having no confidence at all in wearables’ ability to reliably monitor an acute exacerbation (Figure 2).
Figure 2.
Clinicians’ confidence in wearable technology - Please select how you feel about the effectiveness of wearables in the following scenarios.
When asked to rate their confidence in the statement “wearable devices reliably detect an acute exacerbation”, the response rate was (80%, n=94/118). Slightly more than half of clinicians (54%, n=51/94) were either “slightly confident” or “somewhat confident”. A minority of clinicians were “fairly confident” (20%, n=19/94); and an even smaller number were “completely confident” (2%, n=2/94). However, a considerable proportion of clinicians (23%, n=22/94) were “not confident at all” (Figure 2).
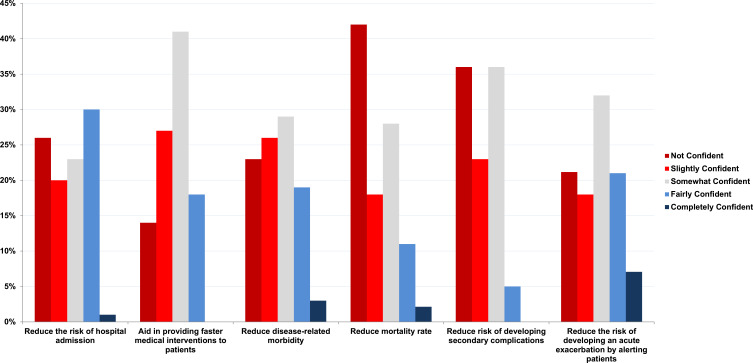
When asked about the impact of wearable devices on health outcomes, the response rate was (80%, n=94/118). The most frequent response was “not confident” in reducing mortality (41%, n=39/94). However, some clinicians were “somewhat confident” (28%, n=26/94), or “slightly confident” (18%, n=17/94). The responses were variable in relation to the utility of wearable devices in reducing the risk of hospitalisation, preventing secondary complications, and reducing the risk of exacerbations (Figure 3).
Figure 3.
Clinicians’ confidence on patient health outcomes - Please select how you feel implementing wearables will affect patient health? (Please select all that apply).
Financial Factors Associated with Wearable Device Implementation
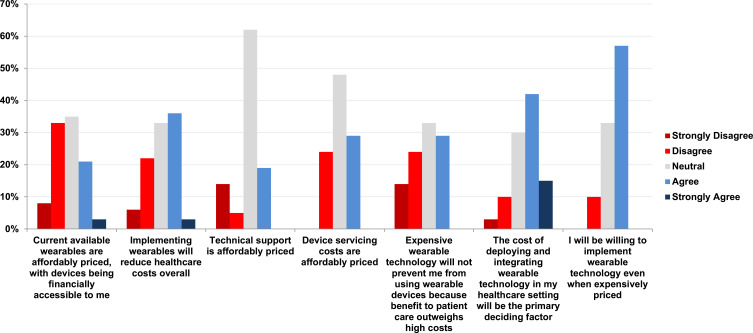
Clinicians were asked if the cost of deploying wearable devices will be the primary factor in deciding whether to use or not use wearable technology. The response rate was (75%, n=89/118). More than half of clinicians (64%, n=57/89) did have some level of agreement with financial factors being the primary deciding factor to use or not use wearable devices. Of this majority, (15%, n=13/89) selected “strongly agree”, and (42%, n=37/89) chose “Agree” (Figure 4).
Figure 4.
Financial costs, how strongly do you agree/disagree with the following statements?.
Advantages of wearable devices
Clinicians were asked if wearable devices could encourage patients to seek medical help in a shorter time. Response rate was (75%, n=88/118). Among those clinicians who responded to this question (74%, n=65/88) had some level of agreement with this. Furthermore, more than half of clinicians (72%, n=63) perceived wearables would make monitoring acute exacerbations more convenient. Clinicians were then asked to provide perspectives on wearable devices facilitating remote care, who would otherwise be challenging to reach face-to-face. The majority (68%, n=60) had some level of agreement.
Facilitating Factors Enhancing Rollout of Wearable Devices
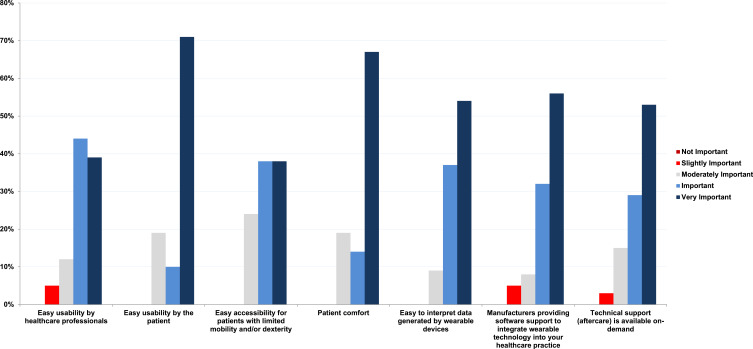
The majority of clinicians highlighted the following facilitating factors as “important” or “very important” when considering the use of wearables in COPD patients. The most important factor was the support from device manufacturers to integrate wearable technology into healthcare settings (Figure 5). Other key factors included the ease of use for patients and clinicians, the accessibility of the devices for patients with limited mobility and dexterity, patient comfort, the ease of data interpretation, and the availability of on-demand technical support (Figure 5).
Figure 5.
Clinicians’ perspectives – how important are the following facilitating factors when deciding to use wearable technology with COPD patients?.
Barriers Which Restrict Rollout of Wearable Devices
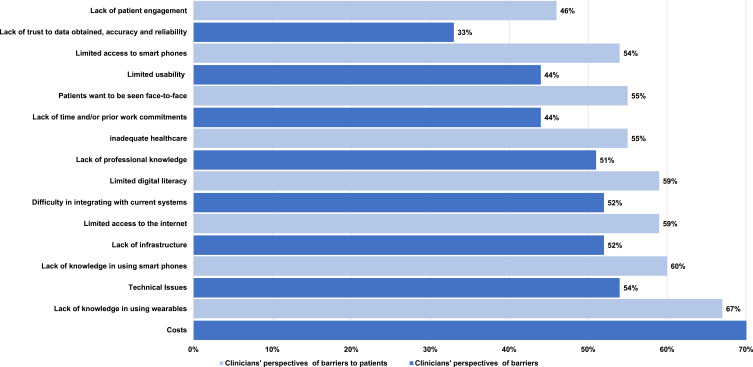
The most commonly reported barriers to using wearable devices were the “cost of implementation of wearable technology” (73%, n=62/85) followed by “technical issues” (54%, n=46/85), and “lack of professional knowledge” (50%, n=43/85). The least common perceived barrier was clinicians “not having an interest in using wearables” (12%, n=10/85) (Figure 6).
Figure 6.
Clinician’s perspectives of barriers that preventing use of wearables.
Clinicians were then asked to consider barriers to implementing wearable devices from the patients’ perspective. The most common barrier identified was a “lack of knowledge in using wearables” (67%, n=57/85), followed by “lack of knowledge in using smartphones” (60%, n=51/85), and “limited access to the internet” (59%, n=50/85). The least common perceived barrier was a “language barrier” (36%, n=31/85) (Figure 6).
When discussing the future of wearable devices in a medical setting, it is important to ask clinicians about their intentions to promote such technology. The most common response was “undecided” (38%, n=32/85). The next most common response was “likely” (35%, n=30/85), with only a small number of clinicians indicating they were “unlikely” (9%, n=8/85) or “very unlikely” (6%, n=5/85) to promote this technology.
Finally, clinicians were asked for any further suggestions on how wearable devices could be better utilised in the care of COPD patients. The response rate was (28%, n=24), and the question was open-ended. Thematic analysis was conducted, and each theme is represented by the quotes below to further understand how wearable devices could enhance the care of COPD patients. The clinicians identified a number of barriers and challenges to the implementation of wearables.
Lack of Robust Evidence and Misconceptions About Effectiveness
I have yet to see any good quality PROSPECTIVE data that has shown any usefulness of any wearable devices in COPD care. One device (cost and implementation) will probably be more expensive than a pulmonary rehabilitation session. If you could show some/ANY good quality data, my responses would have been a lot more positive. (ID 68, Physician)
Staff Shortages and Additional Workload
Hospital chest doctors are so busy with in-patient medical and out-patient referrals that I can’t see them having time for this and it may be best with the community respiratory nurses although they are well over-stretched in our area and repeatedly leaving and going on to different posts in their community contracts. (ID 13, Physician)
Inappropriate Technology Infrastructure
Needs a lot of support/resources we don’t have. If it’s a choice between a team member answering phones/ seeing patients or setting up/supporting wearables, wearables will lose on utilitarian basis/ stewardship of resources. (ID 117, Physician)
Reduce Sense of Responsibility
In the past we have trialed remote monitoring of obs, using a tele-health system, however found patients to be too reliant on HCP to tell them they were unwell. Essentially, it made them less likely to self-manage, & understand their own changing symptoms. (ID 61, Physiotherapist)
Lack of Awareness and Knowledge
I do not have enough knowledge of wearables and have never seen any apart from overnight oximetry. Keen to find out if they would be suitable for some of our COPD patients. (ID 33, Nurse)
I am not aware of any patients in my area being offered wearables to use. I am not aware of any schemes that are intending to do this in the near future in my area. (ID 64, Physiotherapist)
Frail Patients
Older patients who cannot use the necessary technology in addition to multiple issues related to cognition, vision, cost, & interpreting the information correctly. (ID 61, Physiotherapist)
I feel they would be useful in group settings but the wider issues of this is the patient types that we have for eg language barriers, dyslexia, visual impairments. It would not be something that would suit all. (ID 73, Respiratory Therapist)
Implementation
Wearables have a potential role but need to be supported with adequate nursing resource to handle the data and patient concerns. My impression is that most of the pilot initiatives to date have involved spending capital on buying in technology, but not investing in the revenue costs for staffing to respond to the increased patient support then required. (ID 77, Physician)
Discussion
In this international study, clinicians’ perspectives were variable towards the feasibility and utility of wearable devices in COPD care to detect and monitor exacerbations. Clinicians recognise the potential advantages wearables could have for the detection and/or monitoring of exacerbations, as well as the convenience of being able to monitor the condition remotely. Although 80% (n=94/118) did not currently use wearables in COPD care, clinicians were in strong agreement that wearable devices might reduce the time it takes for patients to seek medical care. However, a significant proportion of clinicians felt only slightly or somewhat confident in the reliability of wearable devices to detect and monitor exacerbations.
Our findings likely reflect the existing, conflicting data. A 12-month RCT trial of home telemonitoring found that patients who were monitored in home-based settings had a reduction in hospital visits compared to patients who did not have access to home telemonitoring services.28 Another study of patients with COPD demonstrated a reduced risk of exacerbation in the home monitoring group.29 However, data from previous studies which assessed the reliability of wearable devices in hospital settings and free-living conditions, reported inaccuracy and wide limits of agreement existed when clinical parameters (variables) were measured in clinicians. A systematic review examining fourteen studies, which collectively assessed seven key clinical parameters, ie, heart rate, skin temperature, blood pressure, fall risk, sleep duration, SpO2 and respiratory rate, found many of these variables to be inaccurately measured using wearables when compared to gold standard methods of measurement. Interestingly, many studies examining wearable device use to monitor clinical variables do not validate results against gold-standard assessment methods. Furthermore, many devices used in these studies are not medical grade but are, in fact, consumer-grade devices which have not been validated for use in clinical settings.
Previous research studies which focused on healthcare settings identified several obstacles limiting the deployment and implementation of specific wearable technologies.30,31 One major barrier was clinicians’ scepticism towards the accuracy, reliability and utility of wearable technology.15 This distrust in technology limits the acceptability of wearable devices, preventing adoption into clinical settings for use in COPD.32
Clinicians’ perspectives in our study regarding the potential benefits of implementing wearable devices to improve patient outcomes like the ability of wearable devices to reduce morbidity and mortality. Most clinicians had a low level of confidence in wearable devices decreasing morbidity or mortality in COPD. Previous studies showed no significant reduction in mortality between telemonitoring and control groups.33
Wearable devices using accelerometers or pedometers have the ability to monitor the physical activity of COPD patients remotely.22,34,35 In a 15 month study, Wan et al29 found that people with chronic obstructive pulmonary disease (COPD) who used pedometers had a significantly reduced acute COPD exacerbation witth (rate ratio = 0.51, 95% confidence interval = 0.31–0.85). Furthermore, several previous studies examining the use of wearable devices have demonstrated that activity trackers can improve physical activity and health outcomes.34–36
Clinicians were more willing to accept the implementation of wearable devices into their clinical practice if provided with hardware and software support to integrate technology into their current systems. Overall, clinicians were keen to highlight the importance of the seamless integration of wearable technology into existing IT systems.
While monitoring vital signs is a routine procedure in hospital settings, many barriers limit the deployment of wearable devices in free-living conditions. The most commonly perceived barrier by clinicians limiting the deployment of wearables devices was cost (68%). However, it should be noted that the economic burden associated with exacerbations is considerable, accounting for 50–70% of total COPD costs.37 In the US alone, there is a large economic burden associated with exacerbations, with costs estimated to be in the region of $50 billion USD in 2010, with 60% of expenditure ($30 billion) associated with direct costs of healthcare.37 In the UK, the indirect cost of COPD is estimated to be £800 million GBP annually, and COPD exacerbation is one of the most expensive inpatient conditions treated by the NHS.38
However, the widespread commercialisation of wearable technology by competing manufacturers has gradually reduced purchase costs to more affordable levels.36,39–41 Studies have shown that implementing some wearable devices into patient care can reduce healthcare costs, with cost savings per patient over a one-year period averaging $6621.39,41 Costs of exacerbations are not restricted solely to direct healthcare burden, but also have far-reaching effects with a significant socioeconomic burden. Therefore, governments looking to reduce the burden of healthcare expenditure may find it lucrative to promote the use of wearable devices in national healthcare settings where there is robust evidence of efficacy.42
Currently, there are many challenges and barriers which hinder the deployment of wearable technology into healthcare settings. One of the key issues is that the role of wearable technology in exacerbation detection is not clear.2,10,17,19,20,28,29,43,44 As such, there is concern around how efficacious these devices are in a real-world setting. Recommendations would be to ensure that a regulatory framework is set up in which all devices considered for use in patients are validated clinically. These devices would need to undergo controlled trials to ensure reliability, accuracy and safety of device measurements and be registered as a medical grade device, going through the same certification process as other devices prescribed in healthcare, to ensure the safety of patients and efficacy of the interventions.
The strength of this survey is that our survey is the first to assess COPD clinicians’ perspectives on the current use of wearable technology for detecting COPD exacerbations. This study also provides knowledge of the various wearable technologies currently available for use in COPD patients. Our findings provide a baseline understanding of clinicians’ current perspectives on wearable and may encourage further research to investigate the potential of wearables in COPD patients. Nonetheless, our study has several limitations of this study include the sample size of 118 clinicians, which may not be representative of wider clinicians working with COPD patients. Another limitation was the limited geographical reach and the lack of patients’ perspectives about the acceptability of wearables in COPD. In addition, the data on clinicians’ perspectives were collected prior to the release of the latest GOLD report that introduced the new ECOPD definition/classification. Therefore, the perspectives captured might be different now. Finally, the study on clinicians’ perspectives was conducted at a time when there was a lack of literature supporting the use of wearables.
Conclusion
Clinicians’ perspectives of the value of wearable technology in COPD patients are variable. While cost, technical issues, and lack of knowledge in using wearables are barriers to adoption, facilitators like accessibility, simple data interpretation and technical support can promote their use. Wearable technology has the potential to improve health outcomes, but several challenges need to be addressed to increase the evidence based to support wider wearable utilisation.
Disclosure
Dr Swapna Mandal reports grants from Imperial College, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.World Health Organisation. Chronic Obstructive Pulmonary Disease (COPD). World Health Organisation; 2022. [Google Scholar]
- 2.GOLD. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: Global Initiative for Chronic Obstructive Lung Disease. Global Initiative for Chronic Obstructive Lung Disease; 2023. [Google Scholar]
- 3.Hurst JR, Anzueto A, Vestbo J. Susceptibility to exacerbation in COPD. Lancet Respir Med. 2017;5(9):e29. [DOI] [PubMed] [Google Scholar]
- 4.Hurst JR, Skolnik N, Hansen GJ, et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med. 2020;73:1–6. doi: 10.1016/j.ejim.2019.12.014 [DOI] [PubMed] [Google Scholar]
- 5.Rabe KF, Hurst JR, Suissa S. Cardiovascular disease and COPD: dangerous liaisons? Eur Respir Rev. 2018;27(149):180057. doi: 10.1183/16000617.0057-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dixon LC, Ward DJ, Smith J, Holmes S, Mahadeva R. New and emerging technologies for the diagnosis and monitoring of chronic obstructive pulmonary disease: a horizon scanning review. Chron Respir Dis. 2016;13(4):321–336. doi: 10.1177/1479972316636994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurst JR, Donaldson GC, Quint JK, Goldring JJ, Patel AR, Wedzicha JA. Domiciliary pulse-oximetry at exacerbation of chronic obstructive pulmonary disease: prospective pilot study. BMC Pulm Med. 2010;10(1):1–11. doi: 10.1186/1471-2466-10-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendoza L, Horta P, Espinoza J, et al. Pedometers to enhance physical activity in COPD: a randomised controlled trial. Eur Respir J. 2015;45(2):347–354. doi: 10.1183/09031936.00084514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Rajeh AM, Aldabayan YS, Aldhahir A, et al. Once daily versus overnight and symptom versus physiological monitoring to detect exacerbations of chronic obstructive pulmonary disease: pilot randomized controlled trial. JMIR mHealth and uHealth. 2020;8(11):e17597. doi: 10.2196/17597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah SA, Velardo C, Farmer A, Tarassenko L. Exacerbations in chronic obstructive pulmonary disease: identification and prediction using a digital health system. J Med Internet Res. 2017;19(3):e7207. doi: 10.2196/jmir.7207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pépin J-L, Degano B, Tamisier R, Viglino D. Remote monitoring for prediction and management of Acute Exacerbations in Chronic Obstructive Pulmonary Disease (AECOPD). Life. 2022;12(4):499. doi: 10.3390/life12040499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bentley CL, Powell L, Potter S, et al. The use of a smartphone app and an activity tracker to promote physical activity in the management of chronic obstructive pulmonary disease: randomized controlled feasibility study. JMIR Mhealth Uhealth. 2020;8(6):e16203. doi: 10.2196/16203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz J, Brooks D, Marques A. Home telemonitoring in COPD: a systematic review of methodologies and patients’ adherence. Int J Med Inform. 2014;83(4):249–263. doi: 10.1016/j.ijmedinf.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 14.Hoaas H, Andreassen HK, Lien LA, Hjalmarsen A, Zanaboni P. Adherence and factors affecting satisfaction in long-term telerehabilitation for patients with chronic obstructive pulmonary disease: a mixed methods study. BMC Med Inform Decis Mak. 2016;16(1):1–14. doi: 10.1186/s12911-016-0264-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright SP, Hall Brown TS, Collier SR, Sandberg K. How consumer physical activity monitors could transform human physiology research. Am J Physiol Regul Integr Comp Physiol. 2017;312(3):R358–R67. doi: 10.1152/ajpregu.00349.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeffs E, Vollam S, Young JD, Horsington L, Lynch B, Watkinson PJ. Wearable monitors for patients following discharge from an intensive care unit: practical lessons learnt from an observational study. J Adv Nurs. 2016;72(8):1851–1862. doi: 10.1111/jan.12959 [DOI] [PubMed] [Google Scholar]
- 17.Hawthorne G, Greening N, Esliger D, et al. Usability of wearable multiparameter technology to continuously monitor free-living vital signs in people living with chronic obstructive pulmonary disease: prospective observational study. JMIR Human Factors. 2022;9(1):e30091. doi: 10.2196/30091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janjua S, Pike KC, Carr R, Coles A, Fortescue R, Batavia M. Interventions to improve adherence to pharmacological therapy for chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2021;9(9):Cd013381. doi: 10.1002/14651858.CD013381.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu CT, Li GH, Huang CT, et al. Acute exacerbation of a chronic obstructive pulmonary disease prediction system using wearable device data, machine learning, and deep learning: development and cohort study. JMIR Mhealth Uhealth. 2021;9(5):e22591. doi: 10.2196/22591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valeiro B, Rodríguez E, Pérez P, et al. Promotion of physical activity after hospitalization for COPD exacerbation: a randomized control trial. Respirology. 2023;28(4):357–365. doi: 10.1111/resp.14394 [DOI] [PubMed] [Google Scholar]
- 21.Davis FD. Perceived usefulness, perceived ease of use, and user acceptance of information technology. MIS Quart. 1989;13:319–340. doi: 10.2307/249008 [DOI] [Google Scholar]
- 22.Guk K, Han G, Lim J, et al. Evolution of wearable devices with real-time disease monitoring for personalized healthcare. Nanomaterials. 2019;9(6):813. doi: 10.3390/nano9060813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranjan Y, Althobiani M, Jacob J, et al. Remote assessment of lung disease and impact on physical and mental health (RALPMH): protocol for a prospective observational study. JMIR Res Protoc. 2021;10(10):e28873. doi: 10.2196/28873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mönninghoff A, Kramer JN, Hess AJ, et al. Long-term effectiveness of mHealth physical activity interventions: systematic review and meta-analysis of randomized controlled trials. J Med Internet Res. 2021;23(4):e26699. doi: 10.2196/26699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corp I. IBM SPSS Statistics for Windows, Version 29.0. Armonk, NY: IBM Corp; 2021. [Google Scholar]
- 26.Department of Health. Research governance framework for health and social care. Health Soc Care Community. 2005;10(1):1–54. [DOI] [PubMed] [Google Scholar]
- 27.UKRI. Framework for Research Ethics- ESRC. United Kingdom Research and Innovation; 2022. [Google Scholar]
- 28.Shany T, Hession M, Pryce D, et al. A small-scale randomised controlled trial of home telemonitoring in patients with severe chronic obstructive pulmonary disease. J Telemed Telecare. 2017;23(7):650–656. doi: 10.1177/1357633X16659410 [DOI] [PubMed] [Google Scholar]
- 29.Wan ES, Kantorowski A, Polak M, et al. Long-term effects of web-based pedometer-mediated intervention on COPD exacerbations. Respir Med. 2020;162:105878. doi: 10.1016/j.rmed.2020.105878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange J, Schneider A, Jordi C, Lau M, Disher T. Formative study on the wearability and usability of a large-volume patch injector. Med Devices Evid. 2021;Volume 14:363–377. doi: 10.2147/MDER.S337670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Surantha N, Atmaja P, Wicaksono M, Wicaksono M. A review of wearable internet-of-things device for healthcare. Procedia Comput Sci. 2021;179:936–943. doi: 10.1016/j.procs.2021.01.083 [DOI] [Google Scholar]
- 32.Ferguson T, Olds T, Curtis R, et al. Effectiveness of wearable activity trackers to increase physical activity and improve health: a systematic review of systematic reviews and meta-analyses. Lancet Digit Health. 2022;4(8):e615–e26. doi: 10.1016/S2589-7500(22)00111-X [DOI] [PubMed] [Google Scholar]
- 33.Sul A-R, Lyu D-H, Park D-A. Effectiveness of telemonitoring versus usual care for chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Telemed Telecare. 2020;26(4):189–199. doi: 10.1177/1357633X18811757 [DOI] [PubMed] [Google Scholar]
- 34.Bayerle P, Kerling A, Kück M, et al. Effectiveness of wearable devices as a support strategy for maintaining physical activity after a structured exercise intervention for employees with metabolic syndrome: a randomized controlled trial. BMC Sports Sci Med Rehabil. 2022;14(1):24. doi: 10.1186/s13102-022-00409-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li B, Dong Q, Downen RS, et al. A wearable IoT aldehyde sensor for pediatric asthma research and management. Sens Actuators B Chem. 2019;287:584–594. doi: 10.1016/j.snb.2019.02.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panel E. Implantable & wearable medical devices for chronic obstructive pulmonary disease; 2019.
- 37.Dixit D, Bridgeman MB, Madduri RP, Kumar ST, Cawley MJ. Pharmacological management and prevention of exacerbations of chronic obstructive pulmonary disease in hospitalized patients. J Clin Pharm Ther. 2016;41(11):703. doi: 10.12659/MSM.890210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakerly ND, Davies C, Dyer M, Dhillon P. Cost analysis of an integrated care model in the management of acute exacerbations of chronic obstructive pulmonary disease. Chron Respir Dis. 2009;6(4):201–208. doi: 10.1177/1479972309104279 [DOI] [PubMed] [Google Scholar]
- 39.Nherera L, Larson B, Cooley A, Reinhard P. An economic analysis of a wearable patient sensor for preventing hospital-acquired pressure injuries among the acutely ill patients. Int J Health Econ Manag. 2021;21(4):457–471. doi: 10.1007/s10754-021-09304-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Degroote L, Hamerlinck G, Poels K, et al. Low-cost consumer-based trackers to measure physical activity and sleep duration among adults in free-living conditions: validation study. JMIR mHealth and uHealth. 2020;8(5):e16674. doi: 10.2196/16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dinesen B, Haesum LK, Soerensen N, et al. Using preventive home monitoring to reduce hospital admission rates and reduce costs: a case study of telehealth among chronic obstructive pulmonary disease patients. J Telemed Telecare. 2012;18(4):221–225. doi: 10.1258/jtt.2012.110704 [DOI] [PubMed] [Google Scholar]
- 42.Care DoHaS. Whole System Demonstrator Programme: Headline Findings. Department of Health London; 2011. [Google Scholar]
- 43.Demeyer H, Louvaris Z, Frei A, et al. Physical activity is increased by a 12-week semiautomated telecoaching programme in patients with COPD: a multicentre randomised controlled trial. Thorax. 2017;72(5):415. doi: 10.1136/thoraxjnl-2016-209026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tabak M, Brusse-Keizer M, van der Valk P, Hermens H, Vollenbroek-Hutten M. A telehealth program for self-management of COPD exacerbations and promotion of an active lifestyle: a pilot randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2014;935–944. doi: 10.2147/COPD.S60179 [DOI] [PMC free article] [PubMed] [Google Scholar]