Abstract
Purpose
Atrial fibrillation (AF) and diabetes mellitus (DM) are common pathogenic diseases. Diabetes is an independent risk factor for AF, and coexisting AF is a risk factor for the diabetic pa-tient’s progression. The purpose of this study was to see if two-dimensional-speckle tracking echocardiography (2D-STE) might provide valuable criteria for determining the risk of AF in diabetic patients.
Patients and Methods
This retrospective study compared 30 adult diabetic patients with documented paroxysmal atrial fibrillation (PAF) with 30 age- and sex-matched diabetic patients without PAF. Inclusion criteria were: age ≥18 years, sinus rhythm, diabetes mellitus type 2, and the ability to sign the informed consent. Exclusion criteria included: moderate or severe valvular disease, previous myocardial infarction, left ventricular ejection fraction (LVEF) <50%, congenital heart disease, a history of cardiac surgery, paced atrial or ventricular rhythm, inadequate echocardiography imaging. The medical history, clinical, biochemical data and the results of the transthoracic cardiac ultrasound examination were registered during their evaluation at the outpatients cardiology clinics.
Results
The mean age of the patients was 62.5±1.7 years, 60% were men. Diabetic patients who experienced PAF episodes demonstrated significantly impaired left atrial (LA) deformation patterns, with decreased LA strains and increased LA stiffness (p < 0.05).
Conclusion
The present study demonstrates that LA strains and LA stiffness are significantly associated with the occurrence of PAF in diabetic patients. As 2D-STE of the LA is more sensitive than routine echocardiographic examination, it should be performed in patients suspected of being suffering from PAF.
Keywords: diabetes mellitus, paroxysmal atrial fibrillation, 2D-speckle tracking echography, left atrial strains, left atrial stiffness
Introduction
With a current incidence of 2–4%, atrial fibrillation (AF) is the most prevalent persistent cardiac arrhythmia in people around the world.1 It is expected that one in every three Europeans over the age of 55 will have this disorder.2 AF patients have a 5-fold risk of having a stroke, a higher prevalence of heart failure, and a higher death rate.3
AF is frequent in patients with type 2 diabetes, with a prevalence rate estimated to be at least twice compared to nondiabetic individuals.4 Diabetes mellitus (DM) has been linked to an increased risk of cardiovascular disease and, when this condition is present, it doubles the chance of a fatal event.5 Diabetes, independent of hypertension or diastolic function, is also associated with an increase in the size of the left atrium (LA); it also induces sympathetic and parasympathetic denervation of the latter.1
AF prevalence is approximately three times greater in diabetic patients with hypertension, than in AF patients with either diabetes or hypertension.6
There is disagreement over whether type 2 diabetes is an independent risk factor for AF.4,7,8 Several studies, however, have found a link between type 2 diabetes and atrial fibrillation.5,9 Furthermore, diabetes is one of the risk factors included in the CHA2DS2-VASc-score risk stratification scheme.10
LA size is recognized to be a useful predictor of paroxysmal atrial fibrillation (PAF) in the general population as well as in patients with a variety of pathologies.11,12 Atrial dilatation does not discriminate between valvular and non-valvular AF since it also occurs in left ventricular diastolic failure and arterial hypertension. Nevertheless, PAF has been found in individuals with normal LA dimensions.13–15 Recent studies have shown that LA function parameters surpass LA size measurements as a predictor of PAF, particularly when investigating deformation anomalies using two-dimensional speckle-tracking echocardiography (2D-STE).14–16
The purpose of this study was to see if LA 2D-STE might provide valuable parameters that are significantly linked with the occurrence of PAF in diabetic patients.
Materials and Methods
Patients’ Selection
From December 2021 to December 2022, 600 Caucasian diabetic patients were referred from the diabetologist for cardiological evaluation at the outpatient’s cardiology clinics of Timisoara Institute of Cardiovascular Diseases. Among them, 150 (40%) were referred for the evaluation of palpitations, with suspected PAF. The diabetic patients in whom PAF was documented were enrolled in the study group. Inclusion criteria in the study group were: age ≥18 years, sinus rhythm, diabetes mellitus type 2, documented PAF, and the ability to sign the informed consent. Exclusion criteria included: moderate or severe valvular disease, previous myocardial infarction, left ventricular ejection fraction (LVEF) <50%, congenital heart disease, a history of cardiac surgery, paced atrial or ventricular rhythm, inadequate echocardiography imaging. The control group consisted of age- and sex-matched diabetic adults without palpitations and without documented PAF, and also without any of the exclusion criteria.
The medical history, clinical, biochemical data and the results of the 12-lead resting electrocardiogram, the 24 hours Holter ECG monitoring were performed during their evaluation at the outpatient’s cardiology clinics, before the transthoracic cardiac ultrasound examination.
Definition of Covariates
PAF was defined as an AF identified by a 12-lead standard resting electrocardiogram (ECG) or a 24-hour Holter ECG monitoring, with a minimal length of 30 seconds and a spontaneous or therapeutic termination within 7 days.17 It was documented by reviewing medical records when at least one episode was reported at any point in the patient’s history, during the hospitalization, or at the 1-month post-hospitalization assessment.
Diabetes mellitus and systemic arterial hypertension were defined according to the current European Society of Cardiology Guidelines.18,19
Heart failure with preserved ejection fraction (HFpEF) was identified in patients with signs or symptoms of heart failure, left ventricular (LV) ejection fraction ≥50% and NT-proBNP levels ≥125 pg/mL at sinus rhythm.20
Echocardiography
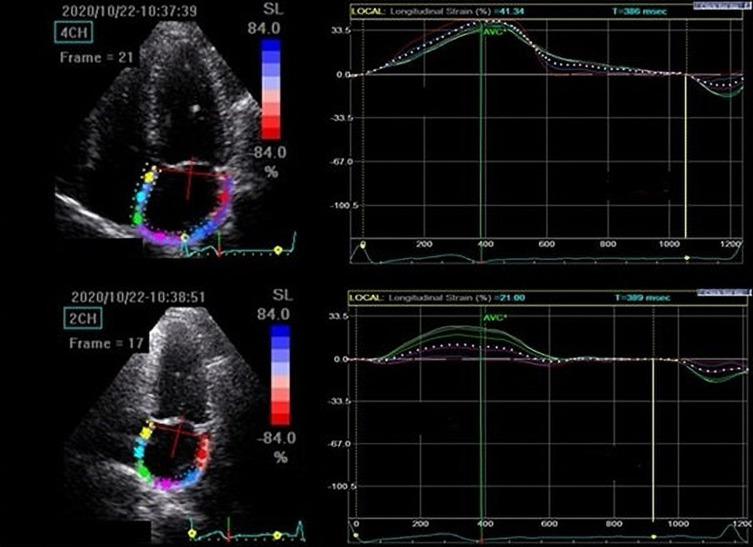
Standard 2-dimensional transthoracic echocardiography (TTE) was performed in all patients according to the American Society of Echocardiography and the European Association Updated Guidelines of Cardiovascular Imaging.21 A Vivid 5S (General Electrics) echocardiograph with a 3.4 MHz transducer was used for standard TTE. The echographer modified machine parameters to improve endocardial and myocardial gray scale definition. The images were taken during an episode of end-expiratory breath-hold. We saved four cardiac cycle loops. The offline analysis was carried out with the help of the Echo PAC 201 software package from GE Healthcare. The LA di-ameter (LAD) was measured in the parasternal long-axis view at the end of systole. During end-ventricular systole, the maximal LA volume was determined using the bi-plane disk approach in apical 4 and apical 2-chamber views, as shown in Figure 1. We assessed the left ventricular end-systolic and end-diastolic volumes, as well as the left ventricular ejection fraction (LVEF), in all subjects. Mitral flow velocity (E) was measured using pulsed-wave Doppler from the apical 4 chamber view, with the sample volume positioned in diastole between the tips of the mitral leaflets. The peak flow velocities were averaged across five consecutive cardiac cycles.
Figure 1.
Two-dimensional speckle tracking ultrasound of the left atrium.
The mitral annulus’ early diastolic velocity (E’) was measured using TDI. The E/E’ ratio was used to calculate ventricular filling pressures.21
Using the offline 2D-STI approach, the maximal LA peak systolic longitudinal strain (LAS) was determined. The LA endocardium was automatically tracked during peak systole, which corresponded to the closing of the aortic valve. If the tracking of the LA endocardium was not acceptable, manual corrections were undertaken. In both 4 and 2 chamber perspectives, LAS was measured by splitting the LA wall into 6 regions. LAS was determined for all segments from the annulus to the LA’s roof and then averaged. The peak LA-pool strain was assessed immediately before the mitral valve opened, and the peak LA-pump strain was measured right at the onset of the P wave. LA stiffness was calculated by dividing the E/A ratio by the peak LA-pool strain value. The peak LA-pool strain was measured just before the mitral valve opening, and the peak LA-pump strain just before the P wave. LA stiffness was estimated as the E/A value divided by the peak LA-pool strain.22,23
The echocardiographic measurements, including the LA assessment, were done by the same investigator with substantial echocardiography experience that was blinded to all other information and that performed all the echocardiographic evaluations, according to the common approach for the patients with PAF in the echocardiography laboratory.
Ethics
All study participants gave written informed consent. The study was conducted in accordance with the requirements of the Helsinki Declaration and was authorized by the Ethics Committee of the “Victor Babeș” University of Medicine and Pharmacy Timișoara.
Statistical Analysis
MedCalc statistical software for Windows, version 20.218, was used for statistical analysis (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2023). Chi square tests for categorical data and Student’s t tests for continuous variables were applied to compare differences between study groups. Covariates considered for potential impact with PAF included obesity, smoking status, coronary artery disease, arterial hypertension, heart failure with preserved ejection fraction, dyslipidemia, glycated hemoglobin value, NT-proBNP levels, the CHA2DS2-VASc score, the conventional and two-dimensional echocardiography measurements of the left ventricle and left atrium, the duration of diabetes and the medication of the patients. For assessing the involvement of each variable in the studied outcomes, univariate and multivariate logistic regression models were established. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated using logistic regression models. Multivariate regression analysis with backward stepwise method was used for all parameters that were associated with the occurrence of PAF at univariate analysis. Receiver–operating characteristic (ROC) curves were utilized to determine the sensitivity and specificity of the analyzed parameters. The discrimination ability of the analyzed parameters was estimated by the area under the ROC curve (AUC). A p-value <0.05 was used as the statistical significance level.
Results
Characteristics of the Enrolled Subjects
PAF was documented in 35 subjects (5% of all examined patients with DM and 23% of those referred with the suspicion of PAF). Eight patients had to be excluded from the research, 5 from the study group and 3 from the control group, due to inadequate cardiac ultrasound images. Finally, the study and the control group consisted of 30 patients, with a mean age of 62.5±1.7 years, and 60% were males.
Table 1 shows the general clinical features of the recruited diabetic individuals. The diabetic patients with PAF received more frequent oral anticoagulation and anti-arrhythmic medication.
Table 1.
Baseline Clinical Characteristics of Diabetic Patients with and without Atrial Fibrillation
| Diabetes with PAF | Diabetes without PAF | P value | |
|---|---|---|---|
| n = 30 | n = 30 | ||
| BMI (Kg/m2) | 33.6 ± 5.2 | 31.8 ± 1.6 | 0.07 |
| Obesity n (%) | 7 (23) | 5 (18) | 0.63 |
| CAD | 20 (68) | 17 (57) | 0.38 |
| HTN | 25 (82) | 23 (76) | 0.57 |
| HFpEF | 13 (43) | 12 (40) | 0.81 |
| History of stroke/TIA | 1 (3) | 0 (0) | 0.27 |
| Dyslipidemia | 17 (56) | 16 (53) | 0.81 |
| Smoking (current, %) | 2 (9) | 3 (10) | 0.89 |
| Systolic BP (mmHg) | 143±15 | 141±16 | 0.61 |
| Diastolic BP (mmHg) | 85.4±11 | 82.7 ±9 | 0.30 |
| Heart rate (beats/min) | 85.1±13 | 79.6±13 | 0.20 |
| ABI | 1.09±0.19 | 1.13±0.17 | 0.39 |
| Total cholesterol (mg/dL) | 174±40 | 183±62 | 0.50 |
| HDL (mg/dL) | 40.5 ± 10.2 | 44.0±14.7 | 0.28 |
| LDL (mg/dL) | 129.4±31 | 130.5±30 | 0.88 |
| Triglyceride (mgl/dL) | 172.14±57 | 158.7±69 | 0.41 |
| FPG (mg/dL) | 153.6±50 | 161.1 ±61 | 0.50 |
| HbA1c (%) | 7.1±0.9 | 6.9±0.7 | 0.34 |
| ASAT (U/L) | 24±9 | 23±5 | 0.59 |
| ALAT (U/L) | 37±7 | 36±5 | 0.52 |
| NT-proBNP (pg/mL) | 412.2±248 | 344.0±266 | 0.24 |
| eGFR | 62.5±6 | 63.7±9 | 0.54 |
| CHA2DS2-VASc score (mean ± 1 SD) | 4.6±1.5 | 4.2±1.2 | 0.23 |
| 0–1 | 5 (17) | 9 (31) | |
| ≥2 | 25 (83) | 21 (69) | 0.20 |
| LVEF | 48.5±6.2 | 47.7±6.1 | 0.64 |
| Duration of diabetes (years) | 6.5±4 | 5.3±2 | 0.14 |
| Diabetes treatment | |||
| ● Oral antidiabetic drugs | 21 (70%) | 19 (62%) | 0.51 |
| ● Insulin | 8 (25%) | 10 (30%) | 0.66 |
| ● SGLT2i | 5 (18) | 6 (21) | 0.77 |
| Statins | 15 (60) | 17 (57) | 0.81 |
| Beta blockers | 24 (82) | 24 (80) | 0.84 |
| ACE-i/ARB | 48 (80) | 23 (75) | 0.64 |
| CCB | 10 (33) | 12 (40) | 0.57 |
| Diuretics | 16 (52) | 14 (48) | 0.75 |
| ASA | 5 (17) | 16 (52) | <0.0001 |
| Clopidogrel | 15 (51) | 14 (48) | 0.81 |
| Acenocumarol | 30 (100) | 0 (0) | <0.0001 |
| Amiodarone | 10 (32) | 0 (0) | <0.001 |
Notes: The data is given as a mean, standard deviation, or number (percentage).
Abbreviations: ABI, ankle brachial index; ACEI, angiotensin converting enzyme inhibitors; AF, atrial fibrillation; ALAT, alanine amino transferase; ARB, angiotensin-1 receptor blockers; ASA, Aspirin; ASAT, aspartate amino-transferase; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blocker; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled), vascular disease, age 65 to 74, sex category (female); FPG, fasting plasma glucose; eGFR, estimated glomerular filtration rate; HbA1c, glycosylated hemoglobin; HDL, high density lipoprotein; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HTN, hypertension; LDL, low density lipoprotein; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro- brain type natriuretic peptides; U, units; SGLT2i, sodium-glucose cotransporter-2 inhibitors; TIA, transient ischemic attack.
Echocardiography Results
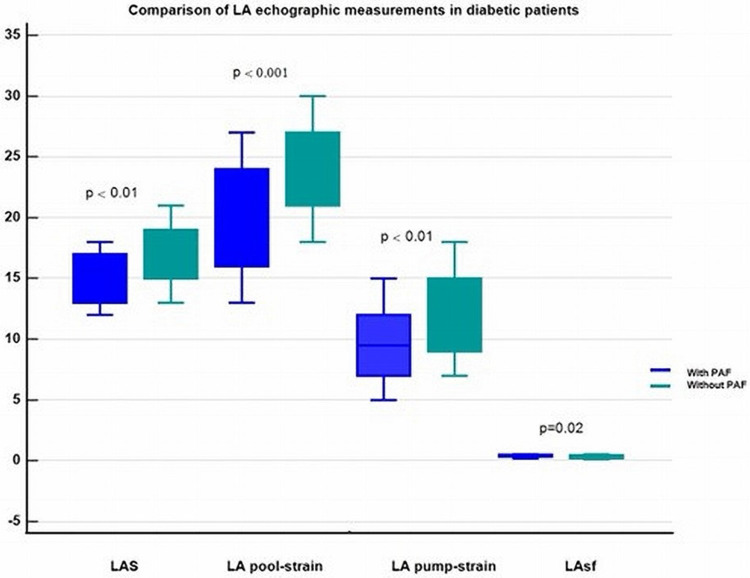
The standard echography evaluations of the PAF- and non-PAF patients revealed no disparities in the echocardiographic assessment. However, the LA strain parameters differed significantly between the two groups. Diabetic patients who experienced PAF episodes demonstrated significantly impaired LA deformation patterns (lower strains and increased stiffness), as shown in Table 2 and Figure 2.
Table 2.
Echocardiography Parameters in Diabetic Patients
| Diabetes with PAF | Diabetes without PAF | P value | |
|---|---|---|---|
| n = 30 | n = 30 | ||
| LVEDV (mL) | 97.8±21.0 | 102.5±17.9 | 0.34 |
| LVESD (mL) | 46.1 ± 10.2 | 49.3±12.6 | 0.28 |
| LVMI (g/m2) | 143 ± 41 | 139 ± 35 | 0.68 |
| LVEF (%) | 57 ± 2 | 60 ± 14 | 0.25 |
| Mitral E/A-ratio | 1.3 ± 0.4 | 1.2 ± 0.2 | 0.22 |
| Septal E/E´ average ratio | 13.9 ± 4.2 | 13.1 ± 3.6 | 0.43 |
| TAPSE (cm) | 2.5 ± 0.43 | 2.4 ± 0.49 | 0.40 |
| GLS (%) | −14.4 ± 3.1 | −15.2 ± 3.2 | 0.32 |
| LAVI (mL/m2) | 42.1 ± 9.3 | 43.5 ± 10.3 | 0.58 |
| LAEF (%) | 47.7 ± 4.7 | 49.7 ± 5.9 | 0.15 |
| LA global strain (%) | 14.9 ± 1.9 | 16.8 ± 2.5 | <0.01 |
| LA-pool strain (%) | 20.0 ± 4.3 | 23.8 ± 3.6 | <0.001 |
| LA- pump strain (%) | 9.6 ± 3.0 | 12.3 ± 3.4 | <0.01 |
| LAsf (%) | 0.42±0.09 | 0.35±0.11 | 0.02 |
Abbreviations: LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volumes; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; E, peak transmitral early diastolic inflow; A, peak transmitral late diastolic inflow; TAPSE, tricuspid annular plane systolic excursion, GLS, global longitudinal strain, LA, left atrium; LAVI, indexed left atrial volume; LAEF, left atrial total emptying fraction; LAsf, left atrial stiffness.
Figure 2.
Comparison of left atrial echocardiographic measurements in diabetic patients with and without paroxysmal atrial fibrillation.
Abbreviations: LA, left atrium; PAF, paroxysmal atrial fibrillation; LAS, left atrial global strain; LAsf, left atrial stiffness.
Correlations Between Echocardiography Data and PAF
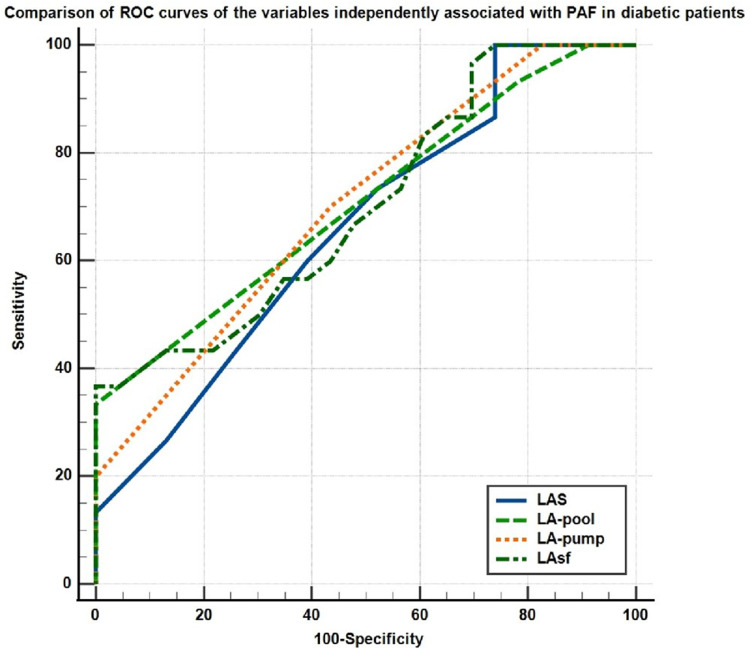
Both univariate and multivariate logistic regression analysis identified LA global strain, LA-pool strain, LA-pump strain and LA stiffness as independently associated with PAF in diabetic patients (Table 3). The comparison of the ROC curves for these parameters is shown in Figure 3 (LA global strain, AUC = 0.661, 95% CI 0.51–0.78: LA-pool strain, AUC = 0.669, 95% CI 0.56–0.8; LA-pump strain, AUC = 0.700, 95% CI 0.56–0.82; LA stiffness, AUC = 0.703, 95% CI 0.56–0.82).
Table 3.
Predictors of Paroxysmal Atrial Fibrillation in Diabetic Patients
| Parameter | Univariate Analysis OR (95% CI) | P value | Multivariate Analysis OR (95% CI) | P value |
|---|---|---|---|---|
| LAS | 0.73 (0.56–0.96) | 0.02 | 0.58 (0.37–0.92) | 0.02 |
| LA-pool strain | 0.78 (0.64–0.94) | <0.01 | 0.74 (0.58–1.95) | 0.018 |
| LA- pump strain | 0.78 (0.64–0.94) | <0.01 | 0.68 (0.50–0.94) | 0.019 |
| LAsf | 0.73 (0.56–0.96) | 0.01 | 5.2 (4.05–7.17) | 0.007 |
Abbreviations: OR, Odds ratio; CI, confidence interval; LAS, left atrial global strain; LAsf, left atrial stiffness.
Figure 3.
Comparison of the ROC curves of LA strain parameters associated with PAF in diabetic patients.
Abbreviations: ROC, receiver operating curve; LA, left atrial; LAS, left atrial global strain; PAF, paroxysmal atrial fibrillation.
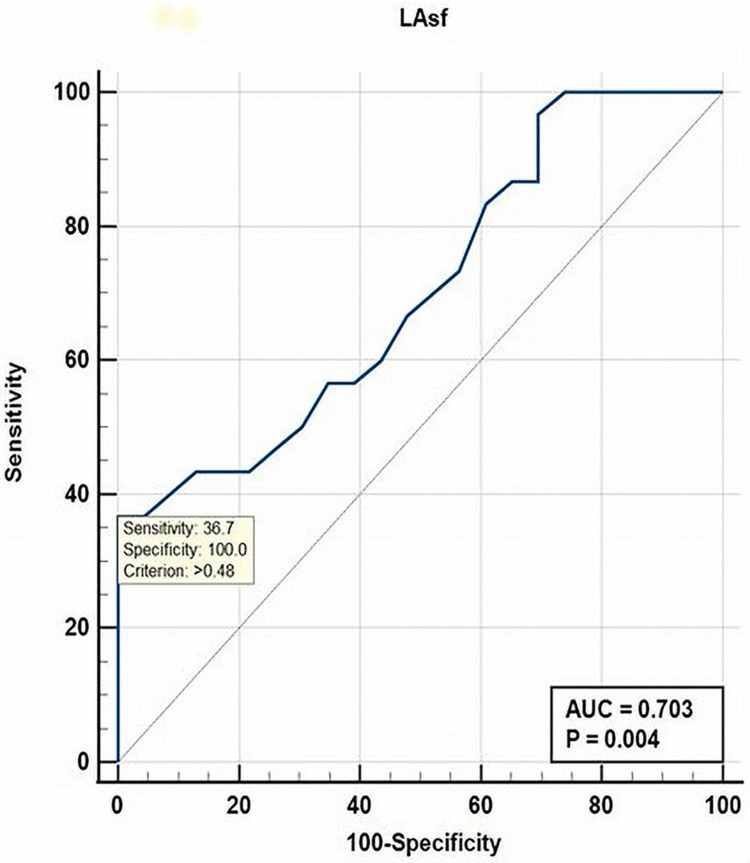
The parameter that showed a strong positive correlation with PAF was LA stiffness (p = 0.005). LAsf was also a strong independent parameter associated with the occurrence of PAF in diabetic patients, with a cut-off value of >0.48% (AUC = 0.704, sensitivity 36.7%, specificity 100%, P = 0.004), as shown in Figure 4.
Figure 4.
ROC curve analysis for LAsf as independent predictor of PAF in diabetic patients.
Abbreviations: ROC, receiver operating curve; AUC, area under the curve; LAsf, left atrial stiffness; PAF, paroxysmal atrial fibrillation.
Discussion
The hypothesis of this study was to verify whether left atrial 2D-speckle tracking echocardiography might predict the occurrence of paroxysmal atrial fibrillation in diabetic patients. This hypothesis was confirmed by our research that found that LA global strain, LA-pool strain, LA-pump strain and LA stiffness are independently associated with PAF in diabetic patients. A strong independent parameter associated with PAF in DM patients was LA stiffness, with a cut-off value >0.48% (p = 0.004). These results have important practical value, as they allow the early identification of diabetic patients that are at high risk for AF.
Holter-monitoring is commonly used for identifying PAF. Its efficiency is limited, as this arrhythmia is sporadic and frequently not recognized by the patients. Long-term monitoring by insertable loop recorders represents a more efficient method, but it is invasive and expensive and cannot be achieved in all patients.
Ischemic strokes caused by AF are typically severe, recurring, and followed by fatal outcomes or permanent disabilities. Anticoagulation therapy is advised as soon as AF is discovered, regardless of the length of the arrhythmia.17 If the heart rate in patients with AF is not well controlled, tachy-cardiomyopathy and heart failure may develop, both with high morbidity and mortality rates.24
In cardiology, atrial fibrillation (AF) and diabetes mellitus (DM) are frequently associated. About 15% of persons with diabetes mellitus develop AF, and about 30% of AF episodes occur in diabetic patients.25
Several studies found that diabetes, particularly type 2 diabetes, is an independent risk factor for the occurrence of AF and that coexisting AF is a comorbidity and a risk factor for the diabetic patient’s unfavorable outcome.5,9 Diabetes can operate as a metabolic determinant at the cell level, alone or in conjunction with other risk factors/comorbidities (hypertension, heart failure, coronary artery disease) that are also implicated in atrial remodeling and generation of atrial tachyarrhythmias. It is recognized that cardiovascular problems are the most serious diabetes-linked complications and the leading cause of death in DM. There are evidences indicating a variety of alterations in diabetic hearts, including interstitial fibrosis of the myocardium, hypertrophy and apoptosis of the cardiomyocytes.26,27 It has also been proven that functional remodeling occurs prior to structural remodeling, resulting in heart chamber dilatation. The final pathogenic and the underlying mechanism for the occurrence and maintenance of atrial fibrillation is LA remodeling that implies three interdependent forms in diabetes (mechanical, electrical, and neurogenic autonomous).28
Our study compared clinical, laboratory, and echocardiographic data in diabetic adults with and without PAF. While clinical, biochemical and conventional echocardiographic parameters such as LA and LV diameters, volumes, and ejection fractions did not differ significantly between the groups, the 2D-STI indicated more frequently aberrant LA deformation patterns in those with PAF. We found a significant association between reduced LA strains and increased LA stiffness and the presence of PAF in diabetic patients. In a previous study, the pattern of myocardial deformation was significantly linked to the degree of myocardial fibrosis as evaluated by cardiac magnetic resonance imaging or on clinicopathologic specimens.29
There were several hypotheses that showed mechanisms of type 2 diabetes mellitus influences on LA structure and function.30,31 First, sustained hyperglycemia induces interstitial fibrosis not only in the LV but also in LA. Second, hyperglycemia is related to enhanced pro-fibrotic signaling molecules that provoke collagen synthesis by cardiac fibroblasts implying that these factors can promote atrial fibrosis in DM.32,33 In addition, LA stiffness, which is related to LA reservoir function and LV filling pressure, increases with LA remodeling and is recognized as a further indicator of LA performance.34 Khurram et al reported that LA stiffness is a strong independent predictor AF recurrence after ablation.35 Additionally, several studies revealed that LA stiffness is associated with poor clinical outcomes in patients with heart failure and reduced ejection fraction.36,37
Limitations of the Study
There are various limitations to our research. This is a retrospective observational study conducted at a single cardiology center with a small number of patients and a single echocardiographer that performed all blinded echocardiographic measurements. We did not determine GAL-3 or other markers of fibrosis in the heart. Moreover, due to the short duration of PAF, the lack of long-term ECG monitoring by insertable loop recorders, as well as the possibility of asymptomatic PAF episodes, many occurrences may have gone unnoticed. Our results need to be validated by larger studies with a prospective design, in order to assess the AF risk in diabetic adults.
Conclusion
The present study demonstrates that LA strains and LA stiffness are significantly and independently associated with the occurrence of PAF in diabetic patients. As 2D-STE of the LA is more sensitive than routine echocardiographic examination, it should be performed in patients suspected of being suffering from PAF.
Acknowledgments
We would like to offer our grateful appreciation to those who participated in the peer review process and provided their valuable suggestions and opinions.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Staerk L, Wang B, Preis SR, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the Framingham Heart Study. BMJ. 2018;1453:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 4.Movahed MR, Hashemzadeh M, Jamal MM, et al. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315–318. doi: 10.1016/j.ijcard.2005.02.050 [DOI] [PubMed] [Google Scholar]
- 5.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostgren CJ, Merlo J, Rastam LLindblad L, Lindblad U. Atrial fibrillation and its association with type 2 diabetes and hypertension in a Swedish community. Diabetes Obes Metab. 2004;6(5):367–374. doi: 10.1111/j.1462-8902.2004.00358.x [DOI] [PubMed] [Google Scholar]
- 7.Wilhelmsen L, Rosengren A, Lappas G. Hospitalizations for atrial fibrillation in the general male population: morbidity and risk factors. J Intern Med. 2001;250(5):382–389. doi: 10.1046/j.1365-2796.2001.00902.x [DOI] [PubMed] [Google Scholar]
- 8.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. Framingham Heart Study. JAMA. 1994;271:840–844. doi: 10.1001/jama.1994.03510350050036 [DOI] [PubMed] [Google Scholar]
- 9.Aune D, Feng T, Schlesinger S, et al. Diabetes mellitus, blood glucose and the risk of atrial fibrillation: a systematic review and meta-analysis of cohort studies. J Diabetes Complications. 2018;32(5):501–511. doi: 10.1016/j.jdiacomp.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 10.Lane DA, Lip GY. Use of the CHA(2)DS(2)-VASc and HAS-BLED scores to aid decision making for thromboprophylaxis in nonvalvular atrial fibrillation. Circulation. 2012;126:860–865. doi: 10.1161/CIRCULATIONAHA.111.060061 [DOI] [PubMed] [Google Scholar]
- 11.Kamel H, Bartz TM, Longstreth WT, et al. Cardiac mechanics and incident ischemic stroke: the cardiovascular health study. Sci Rep. 2021;11:17358. doi: 10.1038/s41598-021-96702-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fatema K, Barnes ME, Bailey KR, et al. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009;10:282–286. doi: 10.1093/ejechocard/jen235 [DOI] [PubMed] [Google Scholar]
- 13.Pagola J, González-Alujas T, Flores A, et al. Left atria strain is a surrogate marker for detection of atrial fibrillation in cryptogenic strokes. Stroke. 2014;45:e164–e166. doi: 10.1161/STROKEAHA.114.005540 [DOI] [PubMed] [Google Scholar]
- 14.Olsen FJ, Jørgensen PG, Møgelvang R, et al. Predicting paroxysmal atrial fibrillation in cerebrovascular ischemia using tissue Doppler imaging and speckle tracking echocardiography. J Stroke Cerebrovasc Dis. 2016;25:350–359. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 15.Ble M, Benito B, Cuadrado-Godia E, et al. Left atrium assessment by speckle tracking echocardiography in cryptogenic stroke: seeking silent atrial fibrillation. J Clin Med. 2021;10:3501–3511. doi: 10.3390/jcm10163501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnăutu SF, Morariu VI, Arnăutu DA, et al. Left atrial strain helps identifying the cardioembolic risk in transient ischemic attacks patients with silent paroxysmal atrial fibrillation. Ther Clin Risk Manag. 2022;Volume 18:213–222. doi: 10.2147/TCRM.S359490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hindricks G, Potpara T, Dagres G, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery. Eur Heart J. 2020;2020:1–125. [Google Scholar]
- 18.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – a new world- wide definition. A consensus statement from the international diabetes federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x [DOI] [PubMed] [Google Scholar]
- 19.Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;2018:1–98. [DOI] [PubMed] [Google Scholar]
- 20.McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging European. Eur Heart J Cardiovasc Imaging. 2015;16:233–271. doi: 10.1093/ehjci/jev014 [DOI] [PubMed] [Google Scholar]
- 22.Fabiani I, Pugliese NR, Santini V, et al. Speckle-tracking imaging, principles and clinical applications: a review for clinical cardiologists. In: Lakshmanadoss U, editor. Echocardiography in Heart Failure and Cardiac Electrophysiology [Internet]. London: IntechOpen; 2016. Available from: https://www.intechopen.com/chapters/51931. Accessed July 7, 2023. [Google Scholar]
- 23.Cottrell C, Kirkpatrick JN. Echocardiographic strain imaging and its use in the clinical setting. Expert Rev Cardiovasc Ther. 2010;8(1):93–102. doi: 10.1586/erc.09.165 [DOI] [PubMed] [Google Scholar]
- 24.Friberg J, Buch P, Scharling H, et al. Rising rates of hospital admissions for atrial fibrillation. Epidemiology. 2003;14(6):666–672. doi: 10.1097/01.ede.0000091649.26364.c0 [DOI] [PubMed] [Google Scholar]
- 25.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation, developed in collaboration with EACTS, ESC, EHRA of the ESC. Europ Heart J. 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Wang J, Zhang B, et al. Diabetes mellitus and cause-specific mortality: a population-based study. Diabetes Metab J. 2019;43(3):319–341. doi: 10.4093/dmj.2018.0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan Y, Zhang Z, Zheng C, et al. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. 2020;17(9):585–607. doi: 10.1038/s41569-020-0339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gherasim L, Gherasim M. Association of atrial fibrillation with diabetes mellitus, high risk comorbidities. Mædica. 2022;17:143–152. doi: 10.26574/maedica.2022.17.1.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69:1691–1705. doi: 10.1136/gutjnl-2020-320622 [DOI] [PubMed] [Google Scholar]
- 30.Kassan M, Choi SK, Galán M, et al. Enhanced NF-kB activity impairs vascular function through PARP- 1-, SP- 1-, and COX-2-dependent mechanisms in type 2 diabetes. Diabetes. 2013;62:2078–2087. doi: 10.2337/db12-1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohne LJ, Johnson D, Rose RA, et al. The association between diabetes mellitus and atrial fibrillation: clinical and mechanistic insights. Front Physiol. 2019;10:135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosmala W, Sanders P, Marwick TH. Subclinical myocardial impairment in metabolic diseases. JACC Cardiovasc Imaging. 2017;10(6):692–703. doi: 10.1016/j.jcmg.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 33.Sonaglioni A, Barlocci E, Adda G, et al. The impact of short-term hyperglycemia and obesity on biventricular and biatrial myocardial function assessed by speckle tracking echocardiography in a population of women with gestational diabetes mellitus. Nutr Metab Cardiovasc Dis. 2022;32(2):456–468. doi: 10.1016/j.numecd.2021.10.011 [DOI] [PubMed] [Google Scholar]
- 34.Cameli M, Mandoli GE, Loiacono F, et al. Left atrial strain: a new parameter for assessment of left ventricular filling pressure. Heart Fail Rev. 2016;21(1):65–76. doi: 10.1007/s10741-015-9520-9 [DOI] [PubMed] [Google Scholar]
- 35.Khurram IM, Maqbool F, Berger RD, et al. Association between left atrial stiffness index and atrial fibrillation recurrence in patients undergoing left atrial ablation. Circ Arrhythm Electrophysiol. 2016;9(3):e003163. doi: 10.1161/CIRCEP.115.003163 [DOI] [PubMed] [Google Scholar]
- 36.Bytyçi I, D’Agostino A, Bajraktari G, et al. Left atrial stiffness predicts cardiac events in patients with heart failure and reduced ejection fraction: the impact of diabetes. Circ Arrhythm Electrophysiol. 2016;9:e003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bytyçi I, Dini FL, Bajraktari A, et al. Speckle tracking-derived left atrial stiffness predicts clinical outcome in heart failure patients with reduced to mid-range ejection fraction. J Clin Med. 2020;9:1244–1252. doi: 10.3390/jcm9051244 [DOI] [PMC free article] [PubMed] [Google Scholar]