Abstract
Despite revolutionizing cancer management, immunotherapies dysregulate the immune system leading to immune-mediated adverse events. These common and potentially dangerous toxicities are often treated with corticosteroids, which are among the most prescribed drugs in oncology for a wide range of cancer and non-cancer indications. While steroids exert several mechanisms to reduce immune activity, immunotherapies, such as immune checkpoint inhibitors (ICI), are designed to enhance the immune system’s inherent antitumor activity. Because ICI requires an intact and robust immune response, the immunosuppressive properties of steroids have led to a widespread concern they may interfere with antitumor responses. However, the existing data of the effect of systemic steroids on immunotherapy efficacy remain somewhat conflicted and unclear. To inform clinical decision making and improve outcomes, we review the impact of steroids on antitumor immunity, recent advances in the knowledge of their impact on ICI efficacy in unique populations and settings, associated precautions, and steroid-sparing treatment approaches.
Keywords: immunotherapy, immune checkpoint inhibitors, corticosteroids, oncology, immune-related adverse events
Introduction
The development of immunotherapy has transformed cancer treatment over the past decade. Instead of directly killing cancer cells, immune checkpoint inhibitors (ICI) activate stymied anti-tumor immune responses by targeting immune cells. This is primarily accomplished by pharmacologically removing inhibitory signals of T-cell activation and restoring cytotoxic immune effector function against cancer cells. ICIs include antibodies that block programmed death-1/ligand-1 (PD-1/PD-L1), cytotoxic T lymphocyte antigen-4 (CTLA-4), and lymphocyte activation gene-3 (LAG-3). ICIs are approved across nearly 20 different cancers and frequently lead to durable responses.
Despite improving anti-tumor outcomes for previously untreatable malignancies, the benefit of ICIs is constrained at times by severe adverse effects. Immune checkpoints, in the physiologic state, maintain immune tolerance; thus, their blockade results in autoreactive T cells (1). Thus, it is not surprising that immune checkpoint blockade triggers autoimmune manifestations, termed immune-related adverse events (irAEs). Clinically, irAEs most commonly impact organs that have epithelial interface with the external environment (e.g. skin, GI tract, and lung). However, irAEs can involve any tissue including heart, brain, and bone marrow. Events of any grade occur in up to 90% of patients treated with anti-CTLA-4 antibodies (2) and 70% treated with anti-PD-1/PD-L1 (3–5), with more severe events occurring in approximately 30% and 15%, respectively. While the overall patterns and incidence rates are well understood, the type and timing of irAEs appears highly idiosyncratic on an individual patient level.
While the general mechanisms of irAEs are intuitive (removal of self-tolerance mechanisms), the specific pathophysiology of these events remains a subject of intensive investigation. Several proposed mechanisms include stimulation of tissue-resident immune cells (6), immune activation against shared antigens between host and tumor tissue (7, 8), depletion of immune-tolerant cells (9), pre-existing subclinical autoimmunity (10), and environmental triggers such as viral infection (11), many of which are not mutually exclusive. Several studies have also suggested microbiome composition may also play a role in irAEs (12–14).
While irAEs are reversible when detected early and appropriately treated, they may cause severe morbidity, treatment discontinuation, or even death (15). Although treatment discontinuation may ease symptoms in some cases, the extended half-life of ICIs ensures that cessation alone is unlikely to forestall aberrant lymphocyte activation. As such, immune suppression with systemic corticosteroids (glucocorticoids) has become the first-line treatment for severe and protracted irAEs (16). Endocrine irAEs are a notable exception, commonly treated with hormone replacement. The treatment approach of irAEs primarily depends on organ-specific involvement and the Common Terminology Criteria for Adverse Events (CTCAE) (17) severity grade (18, 19). Generally, grade 2+ irAEs are treated with temporary or permanent ICI suspension and corticosteroids. Thus, steroids are the mainstay of treatment for moderate to severe irAEs, with other immune modulators (anti-TNF, rituximab, mycophenolate, etc.) reserved for second-line therapy (19, 20).
Although steroids can successfully treat irAEs, their associated immunosuppression and resulting effect on treatment efficacy must be carefully considered. Further, patients with cancer often receive steroids for a wide range of other cancer and non-cancer indications. However, the effect of systemic steroids on immunotherapy efficacy remains a complex topic which is still being explored. To better inform clinical decision-making, we review pharmacological and pathophysiological characteristics of steroids, recent data regarding their impact on immunotherapy effectiveness in unique settings, and potential steroid sparing approaches.
Steroids in Oncology
High-dose glucocorticoids (referred hereafter as “corticosteroids” or “steroids”) are commonly prescribed in oncology for cancer-related symptoms, treatment-related adverse events, anti-cancer effects (primarily in hematology), oncologic emergencies, and underlying comorbidities (e.g. autoimmune disease). Given their lympholytic and immunosuppressive properties, steroids are the primary treatment for autoimmune-mediated immunotherapy toxicities. However, due to concerns of inhibiting immune-mediated tumor regression, their ongoing use is also a common exclusion criterion for ICI clinical trials (21–23). Particularly in the palliative setting, moderate to high doses of steroids are used for cancer-related symptoms including fatigue, dyspnea, pain from bone metastases or cerebral edema from brain metastases. The widespread use of corticosteroids in patients with cancer coupled with the growing applications of immunotherapy highlight the importance of understanding their relationship.
Mechanism of Action of Steroids
Corticosteroids exert their anti-inflammatory effects by reducing gene expression of many pro-inflammatory genes including those encoding cytokines, prostaglandins, and eicosanoids. While a full review of their mechanism is beyond the scope of this review (and remains yet to be fully defined), steroids lead to immunosuppression by impairing IL-2 mediated effector T cell activation and increasing regulatory T cells (24–26). They also promote macrophage polarization and alter the microbiome. In cancer, steroids impact the release of tumor antigens, lymphocyte trafficking, and immune-mediated tumor killing (27). In many ways, therefore, ICIs and corticosteroids produce directly antagonistic effects (Figure 1).
Figure 1.
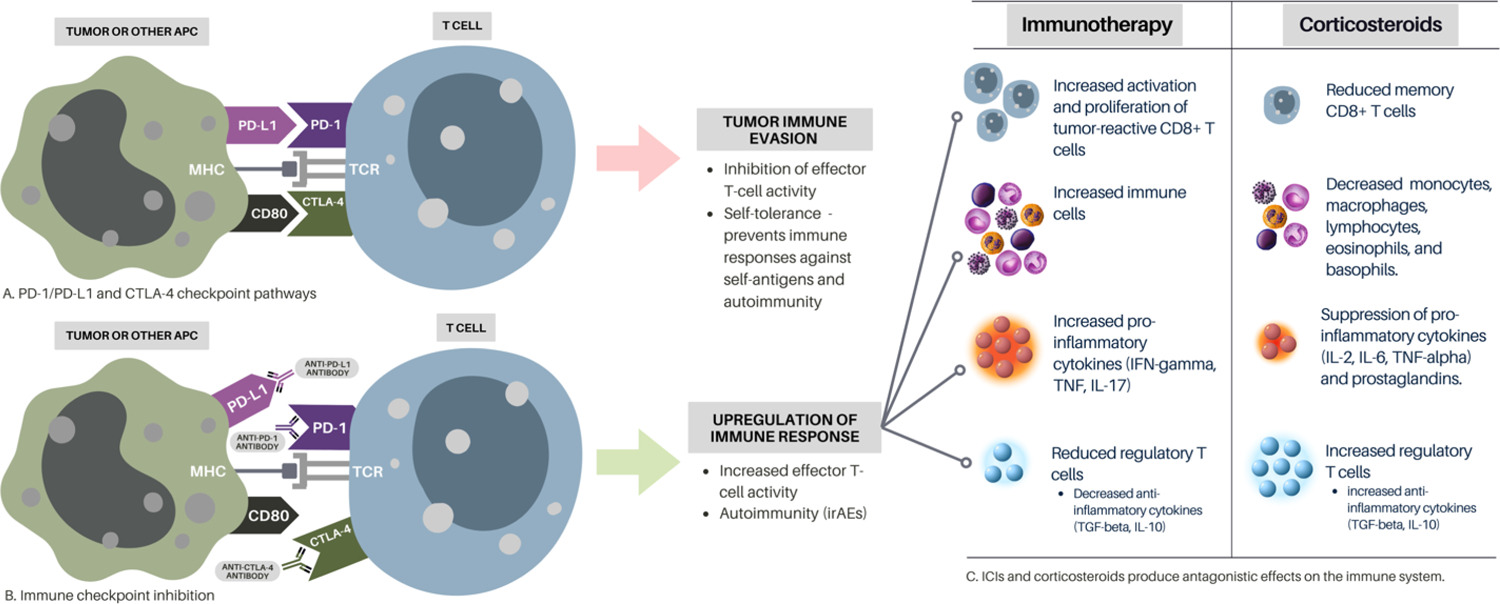
The Effects of Immunotherapy vs Corticosteroids on Immunity
(A) Tumor antigens are presented to T cells by antigen-presenting cells (APCs) via major histocompatibility complex (MHC) and T cell receptors. Several co-receptors act as negative modulators of the immune responses at different molecular checkpoints. Activated T cells upregulate PD-1, and inflammatory signals in the tumor microenvironment upregulate PD-L1, which inhibits effector T cell activity. CTLA-4 binds to CD80 leading to T cell inactivation. Tumors minimize antitumor immunity by exploiting PD-1/PD-L1 and CTLA-4 checkpoint pathways. (B) Monoclonal antibodies that block either CTLA-4 or PD1/PD-L1 pathways increase the activity of tumor-reactive T cells. While this immune checkpoint inhibition restores the antitumor immune response, it also leads to autoimmunity (immune-related adverse events). (C) ICIs and corticosteroids produce antagonistic effects on the immune system. This suggests that steroids may mechanistically undermine ICI efficacy by suppressing necessary anti-tumor immune functions. This figure contains elements from Canva Pro.
Several preclinical studies have provided insights into their concurrent use. One study, in an anti-PD-1 responsive murine model, showed that PD-1 blockade enhanced neoantigen-specific CD8+ T cell responses leading to tumor regression (28). With concurrent immunotherapy and steroid use, there was a reduction in low-affinity memory CD8+ T cells and blunted antitumor responses. Similarly, other preclinical models have shown reductions in both circulating CD4+ and CD8+ T cells and increased tumor growth in mice given steroids alone or in combination with anti-PD-1 therapy, thereby diminishing therapeutic efficacy (29). Interestingly, this effect was not seen in central nervous system tumors, a known immune-privileged site. Steroids may also impair immunotherapy functioning by enhancing the expression of PD-1 on T-cells (30). These studies raise concern that steroids may undermine ICI efficacy. To augment these preclinical studies, however, a wealth of clinical experience has provided further insights into factors that influence the complex interplay between steroids and ICIs.
Dosing and Timing of Steroid Administration
Two potentially important considerations surrounding steroids and ICI are their dosing and timing. One would intuitively presume that a short course of low-dose steroids administered months following a complete response might result in very different effects from higher doses given the same day of ICI initiation.
Baseline Steroid Use
Although most prospective clinical trials have excluded patients receiving doses of steroids above physiologic levels (e.g prednisone 7.5mg or equivalent) (22, 23, 31), several retrospective studies have consistently shown that baseline corticosteroid use at the time of ICI commencement appear to be associated with worse survival outcomes in several cancer types (27, 32–39).
Higher baseline steroid doses (>20mg of prednisone equivalents) were shown to be associated with worse outcomes than lower doses (10–20mg) in ICI-treated patients with NSCLC (40). However, this may be due to increased doses needed to mitigate severe cancer-related symptoms, reflecting independent adverse prognostic factors as a cause of worse outcomes, rather than increased steroid dose (41). However, higher doses of baseline steroids (>/= 50mg prednisone-equivalent) were independently associated with poor median overall survival of advanced melanoma on anti-PD-1 therapy when compared with lower baseline prednisone doses (0–49mg) after controlling for confounding variables, such as high ECOG scores and brain metastases (32). Hence, higher steroid doses at ICI initiation should be avoided when clinically appropriate and possible.
A further key question is the reason for steroid use. The Checkmate-204 study typifies this question; a phase II study of ipilimumab and nivolumab in patients with untreated brain metastases (42). Patients who were asymptomatic and did not require corticosteroids, had, unsurprisingly, dramatically better response rates and survival compared to patients who were symptomatic and/or receiving steroids. However, it is impossible to disentangle the impact of more severe/aggressive disease biology vs. the impact of steroids in this study.
Several other studies are worth highlighting, including a retrospective study of >2,000 patients on immunotherapy for advanced melanoma, NSCLC, and urothelial cancer (37). Baseline systematic corticosteroid use (defined as ≤ 14 days prior to, and up to 30 days after start of immunotherapy) was associated a 23%−47% increased risk of death compared with no use. Patients on baseline steroids were more likely to have advanced staging at diagnosis, distant metastases (including brain and liver), and poorer Eastern Cooperative Oncology Group (ECOG) performance scores. However, baseline steroids remained a significant factor even in multivariable analysis, suggesting a causal link. A subgroup of the Checkmate-204 study showed a that small cohort of melanoma patients on baseline dexamethasone for symptomatic brain metastases had poorer response rates (2 of 12; 17%) to combination nivolumab/ipilimumab than those with symptomatic brain metastases not on baseline dexamethasone (2 of 6; 33%) (38). Patients who were symptomatic and receiving baseline steroids had a higher disease burden than asymptomatic patients.
Several other retrospective studies demonstrated worse survival outcomes in ICI-treated patients with baseline steroid exposure (27, 35, 36, 40). While the pathologic characteristics were well balanced among those who did or did not receive steroids, those receiving steroids had poorer ECOG scores and increased history of brain metastases. Nonetheless, receiving >10mg/day of prednisone was independently associated with poorer clinical outcomes after adjusting for covariates, such as smoking history, performance status, and history of brain metastases. Steroid use within one month prior to ICI initiation was associated with worse survival outcomes than those no baseline steroid exposure and better outcomes than those who received steroids on the day of ICI initiation. A recent population-based study showed a similar association between shorter length of time between last steroid exposure and immunotherapy administration and significantly poorer outcomes in melanoma (34). Steroid exposure within 3 months prior to ICI initiation increased the risk of all-cause-mortality (ACM) by 51% for up to 6 months after ICI initiation. This risk was more than doubled with baseline steroid exposure within 1 month of ICI initiation.
A systematic review and meta-analysis of steroid use with survival in patients on ICIs showed that receiving baseline steroids (>10mg) within 24 hours of ICI initiation for cancer-related palliative indications was associated with poorer clinical outcomes when compared with patients receiving less than 10mg of prednisone or no steroids (43). However worse outcomes were not observed in patients receiving baseline steroids (>10mg) for non-palliative indications, such as autoimmune diseases and hypersensitivity reaction prophylaxis.
Taken together, these studies suggest that low-dose steroids (e.g. approximately 10mg/day of prednisone or equivalent) at baseline may not compromise anti-tumor efficacy, particularly when given for “inflammatory” reasons (e.g. pre-existing autoimmune disease). However, aggressive and severe disease requiring steroids for cancer-related reasons (e.g. cachexia, nausea, brain edema) is likely to result in poor anti-tumor outcomes which may be independent of steroid dose. While the existing literature does not dissect dosing at baseline, presumably increasing doses reduce the likelihood of benefit, though more studies are needed for confirmation.
Early Steroid Use
Steroid use shortly after starting therapy intuitively could also pose a heightened risk of adverse clinical outcomes compared with later use, potentially by forestalling a developing anti-tumor immune response. Steroids also increase neutrophil and decrease lymphocyte counts, a ratio which is associated with poor ICI responses (although it is not clear whether this causes the poor response or is correlated with other features of poor outcome – e.g. tumor-associated myeloid inflammation) (36). A retrospective study of patients receiving concurrent steroids during ICI treatment for several metastatic cancers found that those who initiated steroids greater than 2 months after starting ICI therapy had improved PFS (HR=0.30, p<0.001), OS (HR 0.34, p<0.0001), and ORR (39.8% versus 14.7%, p<0.001) than those who started them less than two months after starting ICI therapy (39). This finding persisted after adjusting for tumor type, ICI type, brain metastases, and irAEs. NSCLC patients with steroid exposures during the first cycle of nivolumab also had shorter overall survival rates (35). However, these patients also were more likely to receive fewer total cycles of nivolumab, and thus, their diminished therapeutic effect could be confounded by insufficient immunotherapy duration. Early high-dose steroid use was independently associated with worse PFS and OS in patients with advanced melanoma treated with anti-PD-1 monotherapy (44). Another cohort of patients treated with combination anti-PD-1 and ipilimumab that experienced hyperacute toxicities (within the first cycle) experienced seemingly worsening outcomes with increasing immunosuppression (no steroids > oral steroids > intravenous steroids > additional immunosuppression), although numbers were small (45). Early steroid use was also associated with increased hospitalization (34). Thus, as with baseline steroids use, early steroids should likely be avoided when possible.
Concurrent Steroid and Immunotherapy Use: Specific Steroid Indications
Oncologic Symptom Management
A systematic review and meta-analysis of steroid use with survival in patients on ICIs included 16 studies published from 2009 to 2019 (n=4045) and showed that patients taking steroids for any indication experienced a 34% increased risk of progression or death when compared with non-steroid users (21). However, those taking steroids for cancer-related symptoms experienced a more than 200% increased risk of death. These findings are consistent other studies showing patients receiving steroids for palliative indications experienced significantly worse outcomes than those who received the same or lower doses for cancer-unrelated symptoms or irAEs (43, 46). Patients receiving ≥10mg of steroids for cancer-unrelated indications experienced no significant differences in survival outcomes when compared with those receiving 0 to 10mg of steroids. Cancer patients receiving steroids are more likely to have high disease burden, distant disease spread, poor ECOG performance status scores, and other adverse prognostic factors. Hence, these studies finding poorer outcomes in steroid use for cancer-related symptoms may be simply attributed to poor prognostic factors rather than steroids undermining immunotherapy efficacy.
As the indications for combined chemo-immunotherapy regimens continue to expand for several cancers, it is also important to consider the clinical implications of steroid use as prophylactic antiemetics. A meta-analysis of studies of ICI + chemotherapy with or without dexamethasone pre-medications was performed with 12 trials enrolling 7155 patients (47). Notably, survival and outcomes were, perhaps counterintuitively, improved in treatment arms that included dexamethasone, although paradoxically toxicity was worse with dexamethasone as well. While this adds support to the safety of dexamethasone in this population, it is also very possible that more active (and potentially more toxic) chemotherapy regimens could have been used in dexamethasone-containing arms, thus confounding the results. National society guidelines recommend dexamethasone as first-line prophylaxis for nausea and vomiting in patients on combined ICI and chemotherapy regimens (48, 49). However, clinical trial data remain mixed (50–54). Given the paucity of data, some investigators are supporting steroid-minimizing approaches, such as 5-HT3 and NK1 antagonists and olanzapine, for antiemetic prophylaxis (53). However, it is clear that steroid-prophylaxis does not completely ablate responses to chemo-immunotherapy, as multiple studies have demonstrated improvement over chemotherapy alone, and available data, though incomplete, support its safety.
Immune-related Adverse Events
A fundamental question surrounding ICI activity is whether the benefit and toxicities of ICI are inextricably coupled. Most of the available evidence argues against such a relationship, particularly the fact that many durably-responding patients experience minimal to no toxicities. However, there is a broadly consistent association in numerous studies suggesting that patients with irAEs, particularly from anti-PD-(L)1, have better outcomes than patients who lack toxicities (46, 55–59). Thus, irAEs also may serve as a clinical biomarker for ICI response. Since steroids are the primary treatment for irAEs, clearly they do not preclude or ablate responses in all cases. However, there remains two possibilities: 1) steroids given for irAEs do not impact anti-tumor outcomes; or 2) steroids have a modest adverse impact which is masked by the favorable outcomes in this population (and may depend on timing, dose, and other factors). Additionally, successful treatment of irAEs with steroids may improve treatment efficacy by minimizing treatment disruption (46). Needless to say, disentangling the impact of irAEs and steroids is a major challenge.
In several studies, patients who did or did not receive steroids for irAEs had similar outcomes in melanoma (60) and NSCLC (61). Lower steroid doses for irAEs were associated with improved survival outcomes when compared with higher doses in one study (46). Steroids for irAEs did not seem to impact maintenance of disease control of ipilimumab for advanced melanoma (62, 63). However, one retrospective series that assessed patients with hypophysitis following ipilimumab suggested that patients who received physiologic doses of steroids had superior outcomes to those who received high-dose treatment (64). Although this was a modest-sized study, it has provoked unease among clinicians, given that it compared two cohorts of patients with the same toxicity and resulted in better outcomes in the low-dose steroid group. However, additional studies have not convincingly replicated this finding.
Taken together, the data suggests that irAEs are associated with a consistent albeit modest improvement in anti-tumor efficacy. Although there are hints that steroids may reduce this benefit, particularly if used early on treatment and in high doses, the weight of the evidence suggests that any detrimental effect is likely low. Thus, steroids should be used as per guidelines currently, although further research is needed into steroid-sparing approaches and optimal dosing/timing of tapers. It is also important to note that the current guidelines surrounding steroid treatment and dosing are not evidence-based, but rather reflect expert opinion.
CAR-T cells
Although not the focus of this review, assessing the impact of steroids on chimeric antigen receptor (CAR) T-cell therapies is instructive and may shed light on interactions between steroids and anti-tumor immunity in the context of aberrantly activated T cells (65). Like ICI, CAR-T cell therapy cause serious immune-mediated adverse effects, particularly cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Corticosteroids inhibit the proliferation of inflammatory cytokines from CAR-T cells and other immune cells (66). The antitumor implications of steroid use with concurrent CAR-T cell therapy remains a clinically relevant inquiry. A systematic review of glucocorticoid use and risk of impaired efficacy of Axicabtagene Ciloleucel, a CAR-T cell agent, showed that treatment outcomes were not significantly affected (65, 67). These results are consistent with other studies proposing that even high-dose steroids do not influence treatment outcomes of CAR-T cell treated B-cell acute lymphoblastic leukemia. However, other studies suggest survival outcomes may be affected by higher cumulative doses, prolonged duration, and early timing of steroid use (68, 69). High-grade ICANS are associated with shorter survival suggesting that worsened outcomes may be, in part, due to corticosteroid use (70). Interestingly, the early use of steroids for CAR-T cell-related toxicities may decrease the likelihood of more severe toxicities and allow for lower cumulative steroid use with no negative impact on antitumor responses (71). While the literature suggests concurrent short-duration, lower-dose steroid and CAR-T cell use does not cause negative antitumor implications, the underlying mechanisms of such interactions remain unanswered, and thus alternative steroid-sparing interventions for toxicities should be explored (72). Ultimately, the similarities between CAR-T and ICI therapies may provide cross-disciplinary lessons that will impact toxicity management in both fields.
Management Recommendations and Steroid-sparing Approaches
In addition to their immunosuppressive properties that may interfere with ICI efficacy, corticosteroids come with a significant side effect profile for which providers should monitor and mitigate. These include (1) neurological/psychiatric symptoms including insomnia, mood lability, psychosis, (2) musculoskeletal symptoms include proximal myopathy, arthralgias, avascular necrosis, and osteoporosis, (3) gastrointestinal symptoms such as dyspepsia and gastritis, (4) immunosuppression-related infections, (5) endocrine abnormalities such as hyperglycemia, Cushingoid habitus, and adrenal insufficiency, (6) cutaneous manifestations such as delayed wound healing, peripheral edema, and striae, and (7) ocular manifestations such as blurred vision and cataracts (73). Providers should counsel patients appropriately on the potential adverse effects. Given the substantial burden of potential side effects, steroid-sparing approaches should be considered when possible and appropriate independent of their antitumor implications. We will now explore management recommendations and potential steroid-sparing approaches in different settings.
Baseline and Early Steroids
The use and timing of steroid exposure preceding ICIs are important clinical considerations. Because baseline steroid is a common exclusion criterion in immunotherapy clinical trials, we propose that patients on baseline steroids be included in specific clinical trials (e.g. those with brain metastases, autoimmune disorders). Currently there are two ongoing randomized clinical trials among patients on nivolumab with autoimmune diseases, many of which are on steroids (NCT03656627, NCT03816345). Until we have more prospective data, providers should perform careful risk-benefit analyses for patients on steroids near the time of ICI initiation. If deemed clinically appropriate, steroids should be avoided or decreased prior to initiation. Otherwise, steroid-sparing approaches, delaying steroid exposure, or short delays of ICI induction to facilitate steroid taper may be considered when appropriate.
Cancer-related Symptoms
Receiving steroids for oncologic symptom management, such as cachexia or symptomatic brain metastases, or palliative indications may serve as a prognostic indicator rather than a driver of poor outcomes. Nonetheless, prescribing steroids in this population should be approached with caution. Less restraint seems to be necessitated when prescribing low-dose steroids for non-cancer-related symptoms while on concurrent immunotherapies. The ESMO Clinical Practice Guidelines provides practical symptom-specific management suggestions and highlights the widespread use of corticosteroids as well as potential steroid-sparing treatments (74). Radiation therapy is recommended for symptomatic cerebral metastases with the goal of minimizing the use of steroids (74). Corticorelin Acetate and Bevacizumab could potentially be alternatives to steroids in treating peritumoral cerebral edema (75) and melanoma brain metastases (76), respectively. While certain palliative indications necessitate steroid use, steroid sparing approaches should be utilized when clinically appropriate given the poor outcomes associated with concurrent steroid and immunotherapy use for cancer-related symptoms.
Steroid Alternatives for Immune-related Adverse Events
There is a known positive correlation between irAEs and immunotherapy efficacy, and steroids may slightly reduce this benefit. While treating irAEs with steroids does not appear to significantly hinder antitumor efficacy of immunotherapies, there are notable drawbacks. In addition to their inherent toxicities, up to 20% of irAEs fail to respond to steroids, and others may require prolonged courses (77). Further, they remain a “blunt instrument” to indiscriminately suppress immune function, rather than a rationally, mechanism-guided approach.
Monoclonal antibodies targeting specific cytokines, such as interleukin-6 (78, 79) and tumor necrosis factor alpha (80), and signaling kinases such as JAK and BTK are being studied as potential steroid-sparing approaches for irAEs. Preclinical and early data have suggested, at least for neutralizing antibodies to IL-6 and TNF-α, that they may enhance (or be neutral towards) anti-tumor efficacy while still mitigating toxicity (80). Hydroxychloroquine is an efficacious and well-tolerated steroid alternative for ICI-induced inflammatory arthritis and may allow continued therapy despite symptoms (81). Rescue agents for fulminant events may also play a role. Abatacept, a CTLA-4 fusion protein (which can be conceptually thought of as the “opposite” of ipilimumab) has demonstrated activity even in severe, critically ill patients with ICI-associated myocarditis (82, 83). IrAE phenotype and immunohistopathologic findings are also being utilized to develop more personalized steroid-sparing treatment options for immunotherapy toxicities, which may transcend our current, “one size fits all” paradigm (84, 85). It is important to consider these steroid-sparing agents may cause immunosuppression and may also interfere with antitumor immunity (particularly in the case of “rescue” agents like abatacept. Thus, these medications (at this juncture) should be reserved for severe, life-threatening toxicities, though further studies are needed to characterize their safety and efficacy.
Conclusions
The use of ICI continues to expand, and corticosteroids represent one of the most widely used medications for various cancer and non-cancer indications. Because immunotherapy requires an intact and robust immune response, the immunosuppressive properties of steroids have led to a widespread concern of their antitumor implications with concurrent use. Available data suggest that steroid timing is an important consideration as use preceding and soon after ICI initiation is associated with poorer clinical outcomes. It is important that providers are aware that specific indications of concurrent steroids influence immunotherapy efficacy, and thus the context matters. While steroid use for irAEs does not completely prevent or reverse antitumor response of ICIs, it remains to be seen whether there are modest impairments on antitumor immunity. Steroids for cancer-related symptoms or palliation often result in worse outcomes. However, it is important to consider that the poor prognostic factors of patients taking steroids for cancer-related symptoms make it difficult to delineate the true effect of steroids on treatment efficacy (Figure 2). Until more prospective data emerges, providers should perform careful risk-benefit analyses when prescribing steroids for patients on immunotherapy.
Figure 2.
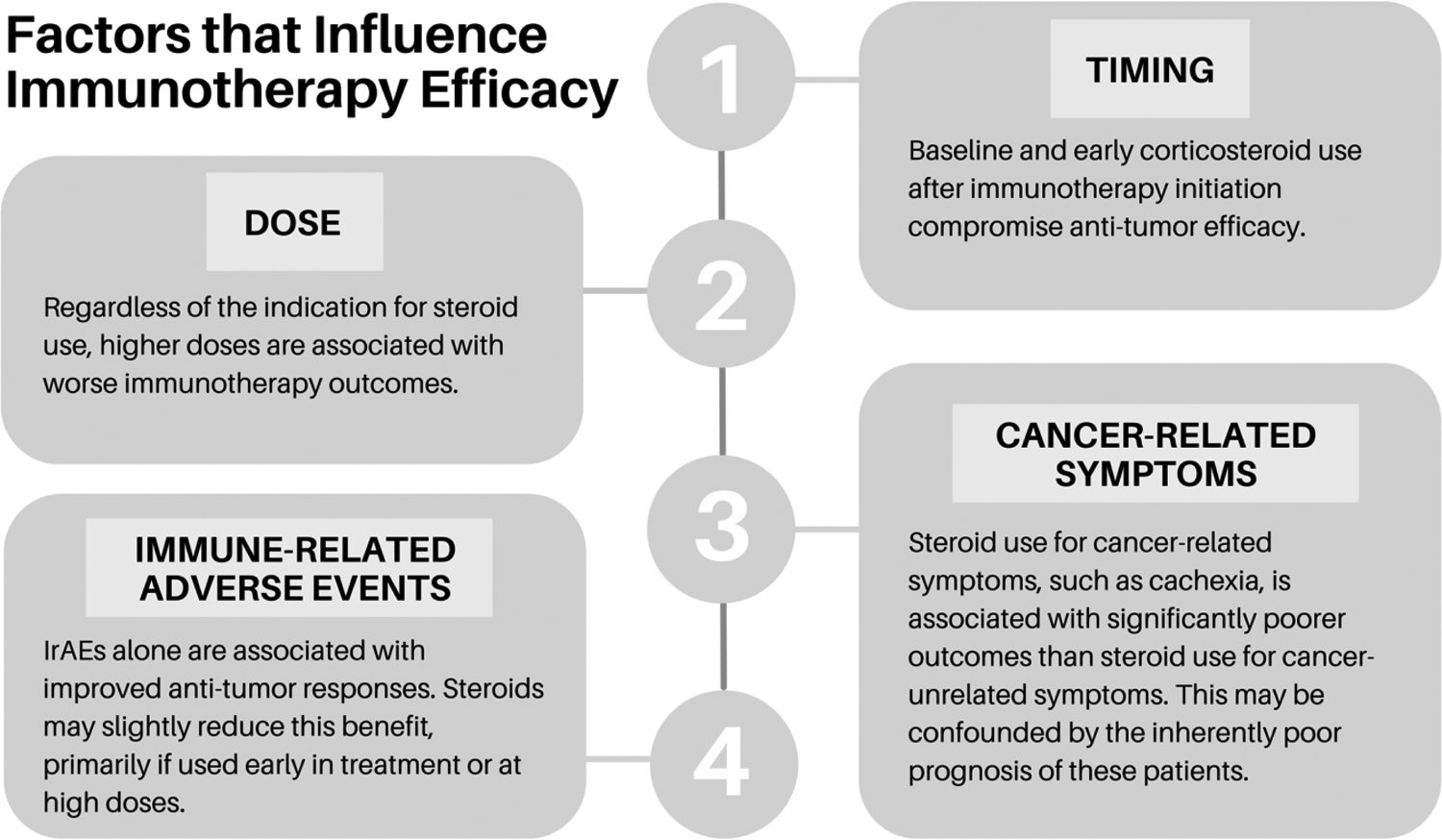
Factors that Influence Immunotherapy Efficacy
While timing and dose are important considerations when prescribing steroids for patients on immunotherapy, it is also important to consider the indication for steroid use.
Funding Sources:
RG receives funding from the SCRIPS Foundation. DBJ receives funding from the NCI R01CA227481, Susan and Luke Simons Directorship for Melanoma, the James C. Bradford Melanoma Fund, the Van Stephenson Melanoma Fund. JMB receives funding from NCI R01CA227481.
Conflicts of Interest:
DBJ has served on advisory boards or as a consultant for BMS, Catalyst Biopharma, Iovance, Jansen, Mallinckrodt, Merck, Mosaic ImmunoEngineering, Novartis, Oncosec, Pfizer, Targovax, and Teiko, has received research funding from BMS and Incyte, and has patents pending for use of MHC-II as a biomarker for immune checkpoint inhibitor response, and abatacept as treatment for immune-related adverse events. JB receives research support from Genentech/Roche, Bristol Myers Squibb, and Incyte Corporation, has received consulting/expert witness fees from Novartis, and is an inventor on patents regarding immunotherapy targets and biomarkers in cancer.
References
- 1.Weinmann SC, Pisetsky DS. Mechanisms of immune-related adverse events during the treatment of cancer with immune checkpoint inhibitors. Rheumatology (Oxford). 2019;58(Suppl 7):vii59–vii67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. New England Journal of Medicine. 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine. 2012;366(26):2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. New England Journal of Medicine. 2012;366(26):2455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luoma AM, Suo S, Williams HL, Sharova T, Sullivan K, Manos M, et al. Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell. 2020;182(3):655–71 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berner F, Bomze D, Diem S, Ali OH, Fassler M, Ring S, et al. Association of Checkpoint Inhibitor-Induced Toxic Effects With Shared Cancer and Tissue Antigens in Non-Small Cell Lung Cancer. JAMA Oncol. 2019;5(7):1043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. The New England journal of medicine. 2016;375(18):1749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Das R, Bar N, Ferreira M, Newman AM, Zhang L, Bailur JK, et al. Early B cell changes predict autoimmunity following combination immune checkpoint blockade. The Journal of clinical investigation. 2018;128(2):715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson DB, McDonnell WJ, Gonzalez-Ericsson PI, Al-Rohil RN, Mobley BC, Salem JE, et al. A case report of clonal EBV-like memory CD4(+) T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat Med. 2019;25(8):1243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nature communications. 2016;7:10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nature medicine. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simpson RC, Shanahan ER, Batten M, Reijers ILM, Read M, Silva IP, et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat Med. 2022. [DOI] [PubMed] [Google Scholar]
- 15.Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber JS, Antonia SJ, Topalian SL, Schadendorf D, Larkin JMG, Sznol M, et al. Safety profile of nivolumab (NIVO) in patients (pts) with advanced melanoma (MEL): A pooled analysis. Journal of Clinical Oncology. 2015;33(15_suppl):9018-. [Google Scholar]
- 17.Common terminology criteria for adverse events (CTCAE), version 5.0: US Department of Health and Human Resources; 2017 [Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdf.
- 18.Olsen TA, Zhuang TZ, Caulfield S, Martini DJ, Brown JT, Carthon BC, et al. Advances in Knowledge and Management of Immune-Related Adverse Events in Cancer Immunotherapy. Front Endocrinol (Lausanne). 2022;13:779915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol. 2021;39(36):4073–126. [DOI] [PubMed] [Google Scholar]
- 20.Henderson Berg MH, Del Rincon SV, Miller WH. Potential therapies for immune-related adverse events associated with immune checkpoint inhibition: from monoclonal antibodies to kinase inhibition. J Immunother Cancer. 2022;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrelli F, Signorelli D, Ghidini M, Ghidini A, Pizzutilo EG, Ruggieri L, et al. Association of Steroids use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers (Basel). 2020;12(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375(19):1823–33. [DOI] [PubMed] [Google Scholar]
- 23.Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med. 2017;376(25):2415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proceedings of the National Academy of Sciences. 2000;97(17):9573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, Oppenheim JJ, Winkler-Pickett RT, Ortaldo JR, Howard OZ. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3+ CD4+ CD25+ T regulatory cells in vivo and enhances their capacity to suppress EAE. European journal of immunology. 2006;36(8):2139–49. [DOI] [PubMed] [Google Scholar]
- 26.Libert C, Dejager L. How steroids steer T cells. Cell reports. 2014;7(4):938–9. [DOI] [PubMed] [Google Scholar]
- 27.Jove M, Vilariño N, Nadal E. Impact of baseline steroids on efficacy of programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) blockade in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(Suppl 4):S364–s8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tokunaga A, Sugiyama D, Maeda Y, Warner AB, Panageas KS, Ito S, et al. Selective inhibition of low-affinity memory CD8+ T cells by corticosteroids. Journal of Experimental Medicine. 2019;216(12):2701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maxwell R, Luksik AS, Garzon-Muvdi T, Hung AL, Kim ES, Wu A, et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. OncoImmunology. 2018;7(12):e1500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing K, Gu B, Zhang P, Wu X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: an insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunology. 2015;16(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(17):1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kartolo A, Deluce J, Holstead R, Hopman W, Lenehan J, Baetz T. Impact of Baseline Corticosteroids on Immunotherapy Efficacy in Patients With Advanced Melanoma. J Immunother. 2021;44(4):167–74. [DOI] [PubMed] [Google Scholar]
- 33.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(28):2872–8. [DOI] [PubMed] [Google Scholar]
- 34.Nikita N, Banks J, Keith SW, Song A, Johnson JM, Wilson M, et al. Is Timing of Steroid Exposure Prior to Immune Checkpoint Inhibitor Initiation Associated with Treatment Outcomes in Melanoma? A Population-Based Study. Cancers (Basel). 2022;14(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott SC, Pennell NA. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. Journal of Thoracic Oncology. 2018;13(11):1771–5. [DOI] [PubMed] [Google Scholar]
- 36.Fucà G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4(1):e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drakaki A, Dhillon P, Wakelee H, Chui S, Shim J, Kent M, et al. Association of baseline systemic corticosteroid use with overall survival and time to next treatment in patients receiving immune checkpoint inhibitor therapy in real-world US oncology practice for advanced non-small cell lung cancer, melanoma, or urothelial carcinoma. OncoImmunology. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tawbi HA, Forsyth PA, Hodi FS, Lao CD, Moschos SJ, Hamid O, et al. Safety and efficacy of the combination of nivolumab plus ipilimumab in patients with melanoma and asymptomatic or symptomatic brain metastases (CheckMate 204). Neuro Oncol. 2021;23(11):1961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maslov DV, Tawagi K, Kc M, Simenson V, Yuan H, Parent C, et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J Immunother Cancer. 2021;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–8. [DOI] [PubMed] [Google Scholar]
- 41.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune Checkpoint Inhibitor Outcomes for Patients With Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. Journal of Clinical Oncology. 2019;37(22):1927–34. [DOI] [PubMed] [Google Scholar]
- 42.Tawbi HA, Forsyth PA, Hodi FS, Algazi AP, Hamid O, Lao CD, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. The lancet oncology. 2021;22(12):1692–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricciuti B, Dahlberg SE, Adeni A, Sholl LM, Nishino M, Awad MM. Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol. 2019;37(22):1927–34. [DOI] [PubMed] [Google Scholar]
- 44.Bai X, Hu J, Betof Warner A, Quach HT, Cann CG, Zhang MZ, et al. Early Use of High-Dose Glucocorticoid for the Management of irAE Is Associated with Poorer Survival in Patients with Advanced Melanoma Treated with Anti-PD-1 Monotherapy. Clin Cancer Res. 2021;27(21):5993–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dearden H, Au L, Wang DY, Zimmer L, Eroglu Z, Smith JL, et al. Hyperacute toxicity with combination ipilimumab and anti-PD1 immunotherapy. Eur J Cancer. 2021;153:168–78. [DOI] [PubMed] [Google Scholar]
- 46.Riudavets M, Mosquera J, Garcia-Campelo R, Serra J, Anguera G, Gallardo P, et al. Immune-Related Adverse Events and Corticosteroid Use for Cancer-Related Symptoms Are Associated With Efficacy in Patients With Non-small Cell Lung Cancer Receiving Anti-PD-(L)1 Blockade Agents. Front Oncol. 2020;10:1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Y, He F, Liu S, Zhang Y, Li L, Wang B, et al. Effect of pretreatment with dexamethasone on the efficacy and immune-related adverse events of immunotherapy in first-line treatment for advanced non-small cell lung cancer: a network meta-analysis of randomized control trials. Am J Clin Exp Immunol. 2021;10(4):93–102. [PMC free article] [PubMed] [Google Scholar]
- 48.Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: ASCO guideline update. Journal of Clinical Oncology. 2020;38(24):2782–97. [DOI] [PubMed] [Google Scholar]
- 49.Berger MJ, Ettinger DS, Aston J, Barbour S, Bergsbaken J, Bierman PJ, et al. NCCN guidelines insights: antiemesis, version 2.2017. Journal of the National Comprehensive Cancer Network. 2017;15(7):883–93. [DOI] [PubMed] [Google Scholar]
- 50.O’Hara M, O’Reilly E, Varadhachary G, Wolff R, Wainberg Z, Ko A. An open-label, multicenter, phase 1b study evaluating the safety and efficacy of CD40 agonistic monoclonal antibody APX005M and chemotherapy, with or without nivolumab, for the treatment of metastatic pancreatic adenocarcinoma. Lancet Oncol. 2021;22:118–31. [DOI] [PubMed] [Google Scholar]
- 51.Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G Jr, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. The Lancet. 2019;394(10212):1915–28. [DOI] [PubMed] [Google Scholar]
- 52.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. New England Journal of Medicine. 2018;379(23):2220–9. [DOI] [PubMed] [Google Scholar]
- 53.Janowitz T, Kleeman S, Vonderheide RH. Reconsidering Dexamethasone for Antiemesis when Combining Chemotherapy and Immunotherapy. Oncologist. 2021;26(4):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. New England journal of medicine. 2018;378(22):2078–92. [DOI] [PubMed] [Google Scholar]
- 55.Rogado J, Sánchez-Torres JM, Romero-Laorden N, Ballesteros AI, Pacheco-Barcia V, Ramos-Leví A, et al. Immune-related adverse events predict the therapeutic efficacy of anti–PD-1 antibodies in cancer patients. European Journal of Cancer. 2019;109:21–7. [DOI] [PubMed] [Google Scholar]
- 56.Okada N, Kawazoe H, Takechi K, Matsudate Y, Utsunomiya R, Zamami Y, et al. Association Between Immune-Related Adverse Events and Clinical Efficacy in Patients with Melanoma Treated With Nivolumab: A Multicenter Retrospective Study. Clinical Therapeutics. 2019;41(1):59–67. [DOI] [PubMed] [Google Scholar]
- 57.Elias R, Yan F, Singla N, Levonyack N, Formella J, Christie A, et al. Immune-related adverse events are associated with improved outcomes in ICI-treated renal cell carcinoma patients. Journal of Clinical Oncology. 2019;37(7_suppl):645-. [Google Scholar]
- 58.Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S, Brave M, et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. Journal of Clinical Oncology. 2019;37(30):2730–7. [DOI] [PubMed] [Google Scholar]
- 59.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. Journal for ImmunoTherapy of Cancer. 2019;7(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leighl N, Gandhi L, Hellmann MD, Horn L, Ahn M-J, Garon EB, et al. Pembrolizumab for NSCLC: Immune-Mediated Adverse Events and Corticosteroid Use. Journal of Thoracic Oncology. 2015;10:S233. [Google Scholar]
- 62.Harmankaya K, Erasim C, Koelblinger C, Ibrahim R, Hoos A, Pehamberger H, et al. Continuous systemic corticosteroids do not affect the ongoing regression of metastatic melanoma for more than two years following ipilimumab therapy. Medical Oncology. 2011;28(4):1140–4. [DOI] [PubMed] [Google Scholar]
- 63.Amin A, DePril V, Hamid O, Wolchock J, Maio M, Neyns B, et al. Evaluation of the effect of systemic corticosteroids for the treatment of immune-related adverse events (irAEs) on the development or maintenance of ipilimumab clinical activity. Journal of Clinical Oncology. 2009;27(15_suppl):9037-. [Google Scholar]
- 64.Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–14. [DOI] [PubMed] [Google Scholar]
- 65.Sun Z, Xun R, Liu M, Wu X, Qu H. The Association Between Glucocorticoid Administration and the Risk of Impaired Efficacy of Axicabtagene Ciloleucel Treatment: A Systematic Review. Front Immunol. 2021;12:646450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paliogianni F, Ahuja SS, Balow JP, Balow JE, Boumpas DT. Novel mechanism for inhibition of human T cells by glucocorticoids. Glucocorticoids inhibit signal transduction through IL-2 receptor. J Immunol. 1993;151(8):4081–9. [PubMed] [Google Scholar]
- 67.Holtzman NG, Xie H, Bentzen S, Kesari V, Bukhari A, El Chaer F, et al. Immune effector cell-associated neurotoxicity syndrome after chimeric antigen receptor T-cell therapy for lymphoma: predictive biomarkers and clinical outcomes. Neuro Oncol. 2021;23(1):112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strati P, Ahmed S, Furqan F, Fayad LE, Lee HJ, Iyer SP, et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood. 2021;137(23):3272–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu S, Deng B, Yin Z, Pan J, Lin Y, Ling Z, et al. Corticosteroids do not influence the efficacy and kinetics of CAR-T cells for B-cell acute lymphoblastic leukemia. Blood Cancer Journal. 2020;10(2):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Strati P, Nastoupil LJ, Westin J, Fayad LE, Ahmed S, Fowler NH, et al. Clinical and radiologic correlates of neurotoxicity after axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(16):3943–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Topp M, Van Meerten T, Houot R, Minnema MC, Milpied N, Lugtenburg PJ, et al. Earlier Steroid Use with Axicabtagene Ciloleucel (Axi-Cel) in Patients with Relapsed/Refractory Large B Cell Lymphoma. Blood. 2019;134(Supplement_1):243-. [Google Scholar]
- 72.Strati P, Ahmed S, Kebriaei P, Nastoupil LJ, Claussen CM, Watson G, et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 2020;4(13):3123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arvold ND, Armstrong TS, Warren KE, Chang SM, DeAngelis LM, Blakeley J, et al. Corticosteroid use endpoints in neuro-oncology: Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2018;20(7):897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crawford GB, Dzierzanowski T, Hauser K, Larkin P, Luque-Blanco AI, Murphy I, et al. Care of the adult cancer patient at the end of life: ESMO Clinical Practice Guidelines. ESMO Open. 2021;6(4):100225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Recht L, Mechtler LL, Wong ET, O’Connor PC, Rodda BE. Steroid-Sparing Effect of Corticorelin Acetate in Peritumoral Cerebral Edema Is Associated With Improvement in Steroid-Induced Myopathy. Journal of Clinical Oncology. 2013;31(9):1182–7. [DOI] [PubMed] [Google Scholar]
- 76.Banks PD, Lasocki A, Lau PKH, Sandhu S, McArthur G, Shackleton M. Bevacizumab as a steroid-sparing agent during immunotherapy for melanoma brain metastases: A case series. Health Sci Rep. 2019;2(3):e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang LX, Quach HT, Moodabigil NV, Davis EJ, Sosman JA, Dusetzina SB, et al. Health care utilization and steroid-refractory toxicities from immune checkpoint inhibitors. Cancer. 2020;126(2):322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hailemichael Y, Johnson DH, Abdel-Wahab N, Foo WC, Bentebibel SE, Daher M, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;40(5):509–23.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dimitriou F, Hogan S, Menzies AM, Dummer R, Long GV. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer. 2021;157:214–24. [DOI] [PubMed] [Google Scholar]
- 80.Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer. 2019;7(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roberts J, Smylie M, Walker J, Basappa NS, Chu Q, Kolinsky M, et al. Hydroxychloroquine is a safe and effective steroid-sparing agent for immune checkpoint inhibitor-induced inflammatory arthritis. Clin Rheumatol. 2019;38(5):1513–9. [DOI] [PubMed] [Google Scholar]
- 82.Salem J-E, Allenbach Y, Vozy A, Brechot N, Johnson DB, Moslehi JJ, et al. Abatacept for Severe Immune Checkpoint Inhibitor–Associated Myocarditis. New England Journal of Medicine. 2019;380(24):2377–9. [DOI] [PubMed] [Google Scholar]
- 83.Wei SC, Meijers WC, Axelrod ML, Anang NAS, Screever EM, Wescott EC, et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov. 2021;11(3):614–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nature Reviews Clinical Oncology. 2020;17(8):504–15. [DOI] [PubMed] [Google Scholar]
- 85.Martins F, Sykiotis GP, Maillard M, Fraga M, Ribi C, Kuntzer T, et al. New therapeutic perspectives to manage refractory immune checkpoint-related toxicities. Lancet Oncol. 2019;20(1):e54–e64. [DOI] [PubMed] [Google Scholar]


