Abstract
Purpose
Higher doses of cytarabine appear to improve long-term outcome in acute myeloid leukemia (AML), in particular for younger patients. To this end, the optimal dosage of single-agent cytarabine in consolidation therapy remains elusive. Here, we assessed the impact of different dosages of cytarabine consolidation after 7 + 3 induction on outcome in a large real-world data set from the German Study Alliance Leukemia-Acute Myeloid Leukemia (SAL-AML) registry.
Methods
Patients between 18 and 64 years of age, registered between April 2005 and September 2020, who attained complete remission after intensive induction and received at least one consolidation cycle with intermediate (IDAC) or high-dose cytarabine (HiDAC) were selected. To account for differences in patient and disease characteristics between both groups, the average treatment effect was estimated by propensity score weighting.
Results
Six-hundred-forty-two patients received HiDAC consolidation with median dosage of 17.6 (IQR (interquartile range), 16.5–18.0) g/m2 for a median number of 3 cycles (IQR, 2–3), whereas 178 patients received IDAC consolidation with 5.9 (IQR, 5.7–8.6) g/m2 for a median of 2 cycles (IQR, 1–3). Both groups differed significantly in some important characteristics (age, sex, cytogenetic risk group, ECOG performance status, disease status, HCT-CI, number of induction cycles). After propensity score weighting for differences in patient and disease characteristics, relapse-free survival after 2 years was comparable between HiDAC-treated (55.3%) and IDAC-treated (55.6%) patients (HR = 0.935, p = 0.69). Moreover, no significant differences in overall survival were observed after 2 years (84.7 vs. 80.6%, HR = 1.101, p = 0.65). Notably, more patients treated with IDAC received allogeneic hematopoietic cell transplantation in first remission (37.6 vs. 19.8%, p < 0.001). Censoring for allogeneic hematopoietic cell transplantation in first remission revealed no significant survival difference with regard to cytarabine dosage. Considering only of European LeukemiaNet (ELN) favorable-risk AML patients, there was no significant difference in outcome. Of note, significantly more patients treated with HiDAC suffered from ≥ 3 CTCAE infectious complications (56.7 [95%-CI 52.8–60.6%] vs. 44.1% [95%-CI 36.6–51.7%]; p = 0,004). The rate of other ≥ 3 CTCAE non-hematological toxicities and secondary malignancies was comparable in both treatment groups.
Conclusions
This retrospective analysis suggests no significant benefit of high-dose cytarabine compared to intermediate dosages in consolidation for AML patients under 65 years of age, independent of ELN risk group.
Trial registration
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-04356-9.
Keywords: Acute myeloid leukemia, Consolidation therapy, Cytarabine dosage, Real-world data
Background
Acute myeloid leukemia (AML) is an aggressive disease, which requires intensive treatment strategies to achieve curation. The mainstay of intensive anti-leukemic therapy comprises an induction with anthracyclines/mitoxantrone and cytarabine, commonly applied as the so-called 7 + 3 regimen with seven days cytarabine and three days of daunorubicin. Depending on genetic risk, induction therapy can vary by formulation of chemotherapy or addition of targeted therapies. However, AML is characterized by a high relapse rate, indicating insufficient clearance of leukemia-initiating cells (Dohner et al. 2017). Therefore, effective post-remission strategies are urgently needed to reduce risk of relapse. For genetically defined intermediate and adverse risk patients, according ELN 2017 classification, allogeneic hematopoietic cell transplantation is the most effective consolidation. However, patient-, donor- or transplantation-related issues limit its use. In these cases, as well as for genetically favorable AML patients with a lower relapse risk, consolidating chemotherapy should be applied. Of note, there is no clear benefit of intensified post-remission chemotherapy, including intermediate or high doses of cytarabine for elderly patients, in particular for adverse risk patients (Dohner et al. 2017; Itzykson et al. 2011). For younger patients, single-agent cytarabine at high doses as consolidating treatment proved to result in similar outcome compared to multiagent chemotherapeutic protocols (Dohner et al. 2017; Miyawaki et al. 2011; Schaich et al. 2013; Thomas et al. 2011). This entails the question to define the optimal dose of cytarabine after 7 + 3 induction therapy. Previous results from the Cancer and Leukemia Group B (CALBG) study group have demonstrated an advantage for high-dose cytarabine with six applications at 3000 mg/m2 compared to conventional cytarabine doses of 100 or 400 mg/m2 for patients below 60 years of age (Mayer et al. 1994). However, there is lacking evidence for increasing cytarabine doses above 2000 mg/m2 compared to intermediate doses of 1000 mg/m2 for consolidation treatment after 7 + 3 induction therapy. Numerous trials included comparisons of high-to-intermediate doses in consolidation, which do not show any advantage to raise the cytarabine dose above 1000 mg/m2 twice daily (Lowenberg 2013). However, these studies often contained different induction protocols, partly including higher doses of cytarabine already during induction treatment or multiagent protocols in consolidation (Miyawaki et al. 2011; Schaich et al. 2011). The different drug combinations during induction therapy and post-remission therapy might influence the therapeutic impact of the different cytarabine consolidation schedules in variable manners. Here, we retrospectively tested the significance of high-dose versus intermediate-dose cytarabine as monotherapy after uniform 7 + 3 induction treatment in patients under 65 years of age in a large real-world data set from the German Study Alliance Leukemia-Acute Myeloid Leukemia (SAL-AML) registry.
Methods
Patients between 18 and 64 years of age, registered between April 2005 and September 2020 with non-acute promyelocytic leukemia, who attained complete remission after intensive induction and received at least one consolidation cycle with intermediate (IDAC) or high-dose cytarabine (HiDAC), defined as 1–1.5 g/m2 and ≥ 2 g/m2, respectively, were selected from the SAL-AML registry (Fig. 1). Patients with initially palliative treatment but subsequent complete remission were excluded. Median follow-up time was 41.4 (IQR, 18.3–65.0) months. The study protocol has been approved by the ethics committees of all participating centers and the study is registered (NCT03188874).
Fig. 1.
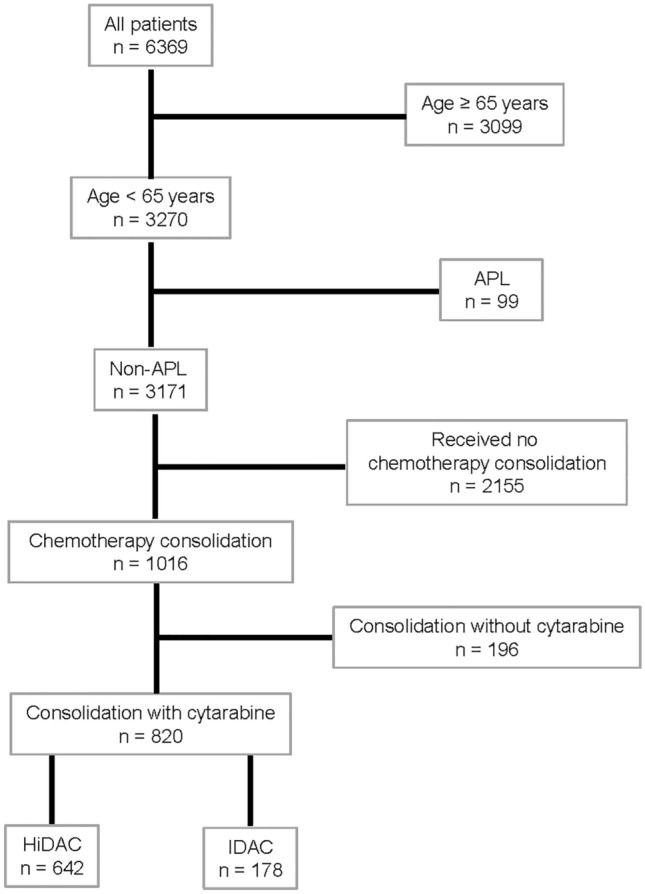
Patient selection for the present analysis. APL acute promyelocytic leukemia; HiDAC/IDAC high-dose/intermediate-dose cytarabine
Overall survival (OS) was defined as time from diagnosis to death from any cause. If no death was observed, OS time was censored on date of last follow-up. Relapse-free survival (RFS) was defined as time from date of first complete remission until relapse or death from any cause, whichever occurred first. If no relapse or death was observed, RFS time was censored on date of last follow-up. To assess the impact of allogeneic hematopoietic cell transplantation in sensitivity analyses, we also calculated OS and RFS with censoring on the date of allogeneic hematopoietic cell transplantation, if this occurred before the first event of interest. For estimation of adjusted survival of IDAC- and HiDAC-treated patients according to Kaplan–Meier propensity score weights for the average treatment effect were estimated, because both groups differed significantly in some important characteristics (age, sex, cytogenetic risk group, ECOG performance status, disease status, HCT-CI, number of induction cycles). A sufficient balance was reached with these propensity score weights. To assess the differential impact of IDAC versus HiDAC, multivariable Cox regression models with interactions of IDAC/HiDAC and the variable of interest were fitted. These models were adjusted for the parameters also used for estimation of the propensity score weights. Missing values in variables were imputed with simple imputation methods, if they were used for calculation of the propensity score or for adjustment of the multiple regression models. Missing values in categorical variables were imputed with the most frequent category of the observed values, missing values in continuous variables were imputed with the median of the observed values.
Results
Patient disposition
Eight-hundred-twenty patients from the database of the SAL-AML registry fulfilled the criteria and were included in the analyses (Fig. 1). 178 patients were treated with approximately 6 g/m2 cytarabine per cycle (median 5.9 (IQR, 5.7–8.6) g/m2), corresponding to 6 applications of 1 g/m2 cytarabine, compared to 642 patients who received approximately 18 g/m2 cytarabine per cycle (median 17.6 (IQR, 16.5–18.0) g/m2), which corresponds to 6 applications at 3 g/m2 (Table 1). Only 2.8% or 1.6% received additional agents during consolidation. Thus, the selected cohort was almost exclusively treated with single-agent cytarabine for consolidation. IDAC-treated patients were older (median (IQR) 58.5 (49–62) vs. 50 (41–56) years, p < 0.001) and had significantly more often secondary and therapy-related AML, as well as more adverse and less favorable genetic risk features according to the ELN 2017 classification (Table 1). Of note, 116 core binding factor AML (CBF AML) were treated with HiDAC, while only 20 received IDAC for consolidation. Furthermore, IDAC-treated patients had more comorbidities according to HCT-CI score (HCT-CI ≥ 2 43.8 vs. 22.3%, p < 0.001). Eighty, respectively, 90% of patients have been induced with the 7 + 3 regimen. Based on German recommendations, the number of induction cycles differed significantly with more patients within the HiDAC cohort receiving 2 cycles (76.8 vs. 61.2%, p < 0.001). Likewise, the median number of consolidation cycles was different with two (IQR, 1–3) in the IDAC group and three (IQR, 2–3) among HiDAC-treated patients. As a result of more unfavorable risk patients in elderly patients > 60 years, significantly more patients treated with IDAC received allogeneic hematopoietic cell transplantation in first remission (37.6 vs. 19.8%, p < 0.001). Whereas the rate of transplantation after relapse was higher among HiDAC-treated patients (30.8 vs. 20.2%, p = 0.007).
Table 1.
Patient and treatment characteristics of all patients
| IDAC (n = 178) |
HiDAC (n = 642) |
p value |
p value (after PS weighting) |
|
|---|---|---|---|---|
| Age at initial diagnosis (years, median (IQR)) | 58.5 years (IQR, 49–62) | 50.0 years (IQR, 41–56) | < 0.001 | 0.246 |
| Female sex, no./no. Available (%) | 88/178 (49.4%) | 314/642 (48.9%) | 0.968 | 0.867 |
| AML type, no./no. available (%) | ||||
| de novo AML | 147/178 (82.6%) | 594/640 (92.8%) | < 0.001 | 0.852 |
| sAML | 9/178 (5.1) | 20/640 (3.1) | ||
| tAML | 22/178 (12.4) | 26/640 (4.1) | ||
| ELN-Risk 2017 group, no./no. available (%) | ||||
| Favorable | 67/165 (40.6) | 336/600 (56) | < 0.001 | 0.915 |
| Intermediate | 74/165 (44.8) | 225/600 (37.5) | ||
| Adverse | 24/165 (14.5) | 39/600 (6.5) | ||
| Core binding factor AML | 20/168 (11.9) | 116/604 (19.2) | 0.037 | 0.585 |
| Complex karyotype, no./no. available (%) | 15/168 (8.9) | 29/609 (4.8) | 0.060 | 0.182 |
| FLT3-ITD | 38/161 (23.6) | 123/595 (20.7) | 0.486 | 0.927 |
| NPM1 mut | 68/168 (40.5) | 280/612 (45.8) | 0.258 | 0.663 |
| HCT-CI | ||||
| 0–1 | 100/178 (56.2) | 498/641 (77.7) | < 0.001 | 0.595 |
| 2–4 | 78/178 (43.8) | 143/641 (22.3) | ||
| Induction therapy | ||||
| 1 Cycle 7 + 3 | 67/178 (37.6) | 151/642 (23.5) | < 0.001 | < 0.001 |
| 2 Cycles 7 + 3 | 75/178 (42.1) | 428/642 (66.7) | ||
| 7 + 3/HAM | 8/178 (4.5) | 33/642 (5.1) | ||
| Others | 28/178 (15.7) | 30/642 (4.7) | ||
| Number of consolidation cycles (median (IQR)) | 2 (IQR,1–3) | 3 (IQR,2–3) | < 0.001 | < 0.001 |
| Cytarabine dose per chemo-consolidation cycle (median (IQR)) | 5891.85 mg/m2 per cycle | 17,580.38 mg/m2 per cycle | < 0.001 | < 0.001 |
| Additional substances | 5/178 (2.8) | 10/642 (1.6) | 0.432 | 0.107 |
| Allogeneic HCT in CR1 | 67/178 (37.6) | 127/642 (19.8) | < 0.001 | < 0.001 |
| Allogeneic HCT salvage | 36/178 (20.2) | 198/642 (30.8) | 0.007 | 0.017 |
HiDAC/IDAC high-dose/intermediate-dose cytarabine; IQR interquartile range; sAML secondary AML; tAML treatment-related AML; HCT-CI hematopoietic cell transplantation-comorbidity index; 7 + 3 induction treatment with standard-dose cytarabine for 7 d and daunorubicin for 3 d; HAM high-dose cytarabine plus mitoxantrone; HCT hematopoietic cell transplantation; CR1 first complete remission; PS propensity score
Effect of cytarabine dose on outcome
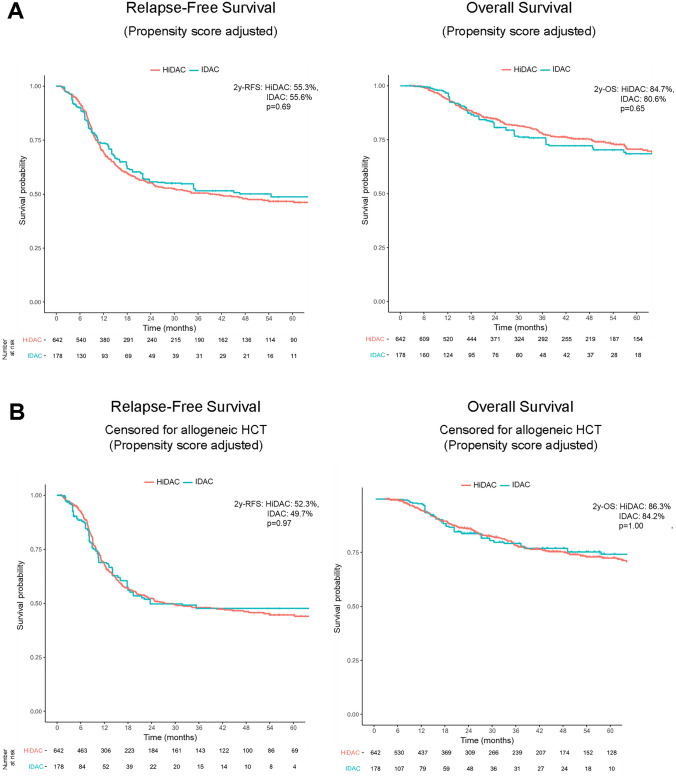
To explore the impact of cytarabine dose in post-remission therapy after intensive induction treatment on survival, propensity score weights were estimated for the average treatment effects, which allowed adjusting for imbalances in prognostic variables and estimating adjusted Kaplan–Meier curves. There was no difference in RFS with a 2-year survival probability of 55.3% in HiDAC- versus 55.6% in IDAC-treated patients (HR = 0.935, p = 0.69) (Fig. 2A). Also for OS, there were no significant differences with a 2-year OS probability of 84.7% in HiDAC vs. 80.6% in IDAC group (HR = 1.101, p = 0.65). To exclude the influence of allogeneic hematopoietic cell transplantation, we next assessed outcome with censoring on the date of allogeneic hematopoietic cell transplantation in first remission. In fact, there was no significant survival difference in dependence of cytarabine dosage, neither for RFS (2–year RFS HiDAC vs. IDAC, 52.3 vs. 49.7%, HR = 1.008, p = 0.97) nor OS (2–year OS HiDAC vs. IDAC, 86.3 vs. 84.1%, HR = 0.999, p = 1.00) (Fig. 2B).
Fig. 2.
Kaplan–Meier estimates of relapse-free survival (RFS, left) and overall survival (OS, right) for all patients after propensity score adjustment (A) and censored for allogeneic hematopoietic cell transplantation (HCT) in first complete remission (B)
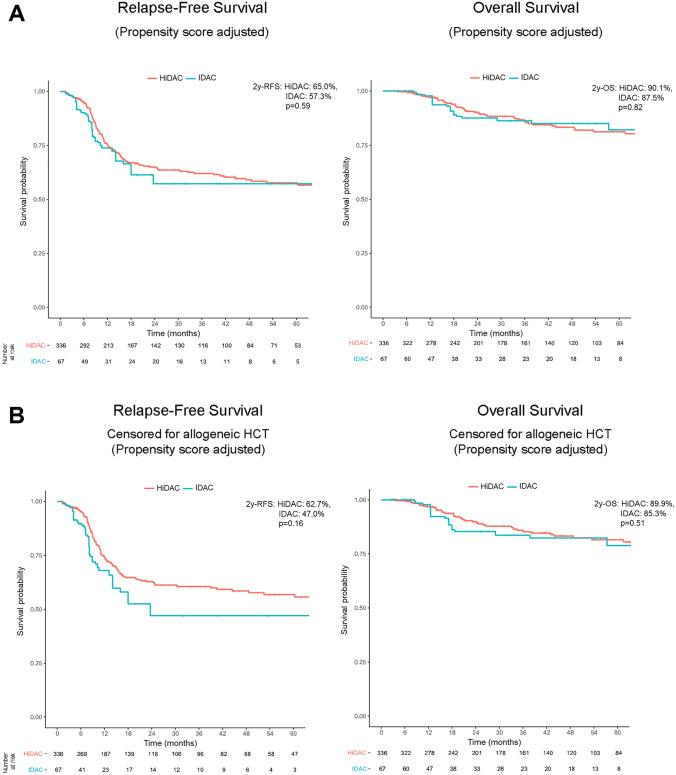
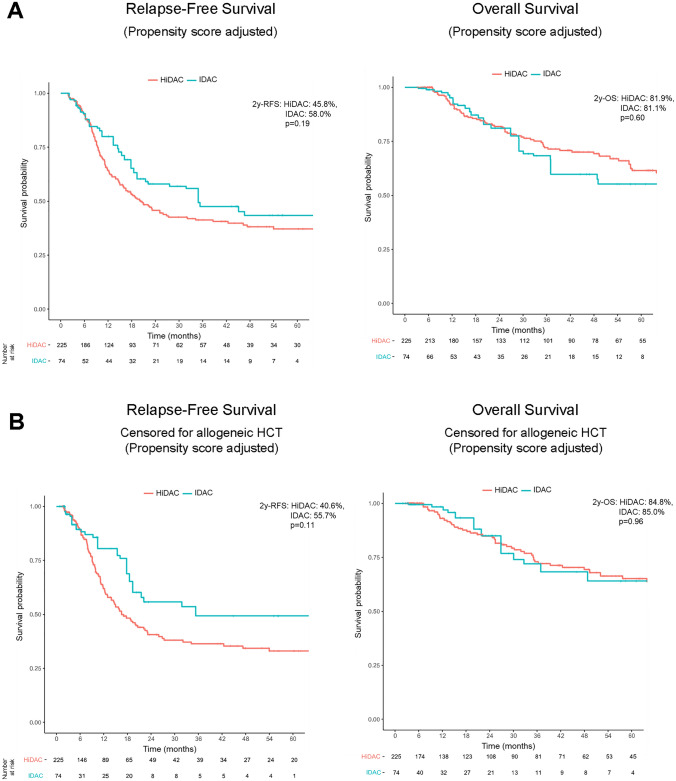
Looking at the subgroup of patients with favorable genetic features according to the ELN 2017 classification, which comprised 336 patients treated with HiDAC and 67 with IDAC, no significance difference for RFS (2-year RFS HiDAC vs. IDAC, 65.0 vs. 57.3%, HR = 1.151, p = 0.59) and OS was found (2-year OS HiDAC vs. IDAC, 90.1 vs. 87.5%, HR = 1.092, p = 0.82) (Fig. 3A). Though there was no significant difference in the rate of allogeneic hematopoietic cell transplantation in this subgroup, for better comparison, we also censored the ELN favorable cohort on the date of transplantation in first remission. A trend for superior RFS for HiDAC-treated patients appeared with a 2-year survival rate of 62.7% compared to 47.0% for patients consolidated with IDAC, which, however, did not reach statistical significance (HR = 1.453, p = 0.16) (Fig. 3B). For OS, there was no significant difference in dependence of cytarabine dose (2–year OS HiDAC vs. IDAC, 89.9 vs. 85.3%, HR = 1.326, p = 0.51) (Fig. 3B). Given previously reported evidence suggesting that HiDAC for consolidation is beneficial for core binding factor leukemia (Bloomfield et al. 1998; Miyawaki et al. 2011), we specifically assessed the subgroup of core binding factor AML. With only 20 patients in the IDAC and 116 patients in the HiDAC group, there was no significant difference for RFS and OS (2–year RFS HiDAC vs. IDAC, 59.5 vs. 38.2%, HR 1.742, p = 0.16; 2-year OS HiDAC vs. IDAC, 86.6 vs. 97%, HR 1.101, p = 0.86), though the small patient number prohibit any conclusion for this subgroup (data not shown). For AML patients at intermediate risk according to ELN 2017 classification, again, there was no significant difference in terms of RFS and OS in dependence of cytarabine dose (Fig. 4A). Given the significant differences in the number of allogeneic-transplanted patients between both cohorts, this subgroup was also censored on the date of allogeneic hematopoietic cell transplantation in first remission. There was a trend for inferior probability of RFS for ELN intermediate risk patients treated with HiDAC compared to IDAC, which, however, was not statistically significant (2-year RFS HiDAC vs. IDAC, 40.6 vs. 55.7%, HR = 0.626, p = 0.11) (Fig. 4B). Still, there was no difference in OS (2-year OS HiDAC vs. IDAC, 84.8 vs. 85.0%, HR = 0.979, p = 0.96) (Fig. 4B). For ELN adverse risk AML patients, there were no significant differences in outcome in dependence of cytarabine dose, though the numbers of patients were in both groups expectedly low (Fig. S1).
Fig. 3.
Kaplan–Meier estimates of relapse-free survival (RFS, left) and overall survival (OS, right) for ELN 2017 favorable-risk AML patients after propensity score adjustment (A) and censored for allogeneic hematopoietic cell transplantation (HCT) in first complete remission (B)
Fig. 4.
Kaplan–Meier estimates of relapse-free survival (RFS, left) and overall survival (OS, right) for ELN 2017 intermediate risk AML patients after propensity score adjustment (A) and censored for allogeneic hematopoietic cell transplantation (HCT) in first complete remission (B)
Finally, in multivariable analysis accounting for the influence of ELN risk, number of induction cycles, age, sex, performance and comorbidities, as well as AML type, the dose of cytarabine in post-remission therapy remained not prognostically significant for outcome (Table 2).
Table 2.
Hazard ratio for relapse-free and overall survival according to multivariable Cox regression models
| Relapse-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | p | Hazard ratio | 95% CI | p | |
| Cytarabine dose | ||||||
| HiDAC | Reference | Reference | ||||
| IDAC | 0.900 | 0.650–1.246 | 0.530 | 1.094 | 0.721–1.660 | 0.670 |
| ELN risk group | ||||||
| Intermediate | Reference | Reference | ||||
| Favorable | 0.704 | 0.514–0.965 | 0.029 | 0.435 | 0.273–0.694 | < 0.001 |
| Adverse | 1.147 | 0.745–1.767 | 0.540 | 1.771 | 0.988–3.174 | 0.055 |
| Number of 7 + 3 induction cycles | ||||||
| 1 cycle | 1.603 | 1.195–2.151 | 0.002 | 1.758 | 1.221–2.532 | 0.002 |
| 2 cycles | Reference | Reference | ||||
| Age (per 10 years) | 1.012 | 0.999–1.025 | 0.120 | 1.021 | 1.001—1.041 | 0.036 |
| Sex, Male | 1.151 | 0.860–1.539 | 0.350 | 1.163 | 0.788–1.718 | 0.450 |
| ECOG, > 1 | 1.071 | 0.704–1.629 | 0.750 | 1.653 | 1.001–2.728 | 0.050 |
| HCT-CI, > 1 | 1.220 | 0.887–1.677 | 0.220 | 1.425 | 0.968–2.099 | 0.072 |
| AML type | ||||||
| de novo | Reference | Reference | ||||
| sAML | 0.807 | 0.462–1.410 | 0.450 | 1.033 | 0.548–1.947 | 0.920 |
| tAML | 1.266 | 0.795–2.015 | 0.320 | 1.215 | 0.657–2.246 | 0.540 |
HiDAC/IDAC high-dose/intermediate-dose cytarabine; sAML secondary AML; tAML treatment-related AML; ECOG clinical performance status according to ECOG criteria; HCT-CI hematopoietic cell transplantation-comorbidity index; 7 + 3 induction treatment with standard-dose cytarabine for 7 d and daunorubicin for 3 d; CR1 first complete remission
Association of infectious complications with cytarabine dose
Significantly more patients treated with HiDAC suffered from ≥ 3 CTCAE infectious complications (56.7 [95%-CI 52.8–60.6%] vs. 44.1% [95%-CI 36.6–51.7%], p = 0.004) (Table 3), which was more striking in patients above 50 years of age (data not shown). The rate of other ≥ 3 CTCAE non-hematological toxicities and secondary malignancies was comparable in both treatment groups (Table 3).
Table 3.
Non-hematological grade 3 and 4 toxicities according to the Common Toxicity Criteria (CTC)
| IDAC (n = 178) (no./no. available (%)) |
HiDAC (n = 642) (no./no. available (%)) |
p | |
|---|---|---|---|
| Hemorrhage CTC ≥ 3° | 4/177 (2.3) | 21/642 (3.3) | 0.656 |
| Infections CTC ≥ 3° | 78/177 (44.1) | 364/642 (56.7) | 0.004 |
| ALAT/ASAT CTC ≥ 3°, | 6/177 (3.4) | 17/642 (2.6) | 0.786 |
| Bilirubin CTC ≥ 3° | 3/177 (1.7) | 10/642 (1.6) | 1.000 |
| Cardiac CTC ≥ 3° | 5/177 (2.8) | 19/642 (3) | 1.000 |
| Creatinine CTC ≥ 3° | 3/178 (1.7) | 3/642 (0.5) | 0.234 |
| Other AE CTC ≥ 3° | 33/177 (18.6) | 137/642 (21.3) | 0.498 |
| Secondary malignancies | 8/177 (5.0%) | 21/642 (3%) | 0.571 |
HiDAC/IDAC high-dose/intermediate-dose cytarabine; CTC common toxicity criteria; ALAT alanine aminotransferase; ASAT aspartate aminotransferase; AE adverse event
Discussion
Since the results from the Cancer and Leukemia Group B (CALBG) study group, which demonstrated an advantage for 3 g/m2 cytarabine compared to conventional cytarabine doses of 100 or 400 mg/m2 for patients below 60 years of age, the mainstay of conventional consolidation usually has comprised high-dose cytarabine (Mayer et al. 1994). Nevertheless, it is still controversial whether single doses as high as 3 g/m2 are necessary. Growing evidence suggests that 1–1.5 g/m2 may be similarly efficacious in preventing relapse while being less toxic. For remission induction therapy, Löwenberg et al. have clearly shown no advantage of increasing cytarabine above conventional doses while sparing excessive toxicities (Lowenberg et al. 2011). To contribute more information on the ongoing debate on the optimal cytarabine dose level and to add real-world evidence including patients outside clinical trials, we performed this large registry-based study. In this retrospective analysis, we did not detect any significant benefit on outcome after high-dose cytarabine compared to intermediate dosages. Alongside, we observed significantly more infectious complications among HiDAC-treated patients, while there were no other significant differences in tolerability, in particular no increase in early mortality. Our results are in line with findings of a recent retrospective study and a meta-analysis integrating ten randomized clinical trials comparing intermediate and higher cytarabine doses, amongst them eight studies in younger patients. Similar to our findings, high doses of cytarabine were not associated with significant differences in RFS or OS in younger AML patients (Magina et al. 2017; Tangchitpianvit et al. 2021). In addition, a combination of cytarabine with other classic cytotoxic agents did not lead to improved survival (Magina et al. 2017).
Implications that HiDAC for consolidation is beneficial for certain genetic subgroups, in particular core binding factor leukemia (Bloomfield et al. 1998; Kolla et al. 2021; Miyawaki et al. 2011) or RAS-mutated AML (Neubauer et al. 2008) compared to conventional cytarabine doses, did not withstand when comparing HiDAC with intermediate doses in different cytogenetic or molecular subgroups (Schaich et al. 2011). We did not observe any significant benefit for ELN favorable-risk AML patients, though there was a non-significant trend for superior RFS among HiDAC-treated patients, which did not result in any difference in OS. Again, this is exactly in line with meta-data from randomized trials (Magina et al. 2017), where prolonged RFS after consolidating HiDAC did not translate into an OS benefit, suggesting a good salvageability of favorable-risk patients in case of relapse. Of note, the risk classification in this meta-analysis was mainly based on cytogenetic criteria compared to the genetic definition by ELN2017 used in our study. Thus, we are able to show conclusive effects of cytarabine doses on survival, indicating that real-world data from a large cohort mirror the results of selected patients participating in randomized trials.
Being based on registry data, our study lacks information on minimal residual disease levels after induction therapy, which could have given valuable insights if intensified consolidation may be beneficial in dependence of residual disease burden. At least, there was no hint that the number of induction cycles among patients who received 7 + 3 induction therapy influenced the outcome in dependence of dose of cytarabine consolidation, as assessed by interaction analyses in multivariable Cox-Model testing (data not shown) and the obvious fact that the significantly higher number of induction cycles among HiDAC-treated patients did not result in survival benefit. The retrospective nature of this study, the risk of not accounting for unknown prognostic relevant factors, which have not been balanced for, as well as the long interval since 2005 might present limitations of this study.
Conclusions
In summary, this retrospective analysis shows no significant benefit of high-dose cytarabine compared to intermediate dosages in consolidation for AML patients under 65 years of age, independent of ELN risk group. Our results contribute to the growing body of evidence indicating similar efficacy of IDAC and HiDAC consolidation with slightly better tolerability. While evidence on the implications on CBF AML is limited, these real-word data underpin a recommendation for the use of IDAC rather than HiDAC in consolidation chemotherapy.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
MH, MK, CR designed the study. All authors collected clinical and/or genetic data. MH, MK, CR analyzed and interpreted the data; all authors had access to primary clinical trial data. MH, CR wrote the manuscript. All authors read the manuscript and gave their final approval for publication.
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Conflict of interest
AH: research support by Novartis, BMS, Pfizer, Incyte.
Ethical approval
The study protocol has been approved by the ethics committees of all participating centers and the study is registered (NCT03188874).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bloomfield CD, Lawrence D, Byrd JC, Carroll A, Pettenati MJ, Tantravahi R, Patil SR, Davey FR, Berg DT, Schiffer CA, et al. Frequency of prolonged remission duration after high-dose cytarabine intensification in acute myeloid leukemia varies by cytogenetic subtype. Cancer Res. 1998;58:4173–4179. [PubMed] [Google Scholar]
- Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzykson R, Gardin C, Pautas C, Thomas X, Turlure P, Raffoux E, Terre C, Fenaux P, Castaigne S, Dombret H, et al. Impact of post-remission therapy in patients aged 65–70 years with de novo acute myeloid leukemia: a comparison of two concomitant randomized ALFA trials with overlapping age inclusion criteria. Haematologica. 2011;96:837–844. doi: 10.3324/haematol.2010.036921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla BC, Halim NAA, Cao Q, Sachs Z, Warlick E, Weisdorf D, Ho AYL, Chuan WG, Lao Z, He F. High risk of relapse with intermediate dose cytarabine for consolidation in young favourable-risk acute myeloid leukaemia patients following induction with 7+3: a retrospective multicentre analysis and critical review of the literature. Br J Haematol. 2021;194:140–144. doi: 10.1111/bjh.17462. [DOI] [PubMed] [Google Scholar]
- Lowenberg B. Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood. 2013;121:26–28. doi: 10.1182/blood-2012-07-444851. [DOI] [PubMed] [Google Scholar]
- Lowenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, Ferrant A, Sonneveld P, Biemond BJ, Gratwohl A, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364:1027–1036. doi: 10.1056/NEJMoa1010222. [DOI] [PubMed] [Google Scholar]
- Magina KN, Pregartner G, Zebisch A, Wolfler A, Neumeister P, Greinix HT, Berghold A, Sill H. Cytarabine dose in the consolidation treatment of AML: a systematic review and meta-analysis. Blood. 2017;130:946–948. doi: 10.1182/blood-2017-04-777722. [DOI] [PubMed] [Google Scholar]
- Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, Omura GA, Moore JO, McIntyre OR, Frei E., 3rd Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- Miyawaki S, Ohtake S, Fujisawa S, Kiyoi H, Shinagawa K, Usui N, Sakura T, Miyamura K, Nakaseko C, Miyazaki Y, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood. 2011;117:2366–2372. doi: 10.1182/blood-2010-07-295279. [DOI] [PubMed] [Google Scholar]
- Neubauer A, Maharry K, Mrozek K, Thiede C, Marcucci G, Paschka P, Mayer RJ, Larson RA, Liu ET, Bloomfield CD. Patients with acute myeloid leukemia and RAS mutations benefit most from postremission high-dose cytarabine: a Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:4603–4609. doi: 10.1200/JCO.2007.14.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaich M, Rollig C, Soucek S, Kramer M, Thiede C, Mohr B, Oelschlaegel U, Schmitz N, Stuhlmann R, Wandt H, et al. Cytarabine dose of 36 g/m(2) compared with 12 g/m(2) within first consolidation in acute myeloid leukemia: results of patients enrolled onto the prospective randomized AML96 study. J Clin Oncol. 2011;29:2696–2702. doi: 10.1200/JCO.2010.33.7303. [DOI] [PubMed] [Google Scholar]
- Schaich M, Parmentier S, Kramer M, Illmer T, Stolzel F, Rollig C, Thiede C, Hanel M, Schafer-Eckart K, Aulitzky W, et al. High-dose cytarabine consolidation with or without additional amsacrine and mitoxantrone in acute myeloid leukemia: results of the prospective randomized AML2003 trial. J Clin Oncol. 2013;31:2094–2102. doi: 10.1200/JCO.2012.46.4743. [DOI] [PubMed] [Google Scholar]
- Tangchitpianvit K, Rattarittamrong E, Chai-Adisaksopha C, Piriyakhuntorn P, Rattanathammethee T, Hantrakool S, Tantiworawit A, Norasetthada L. Efficacy and safety of consolidation therapy with intermediate and high dose cytarabine in acute myeloid leukemia patients. Hematology. 2021;26:355–364. doi: 10.1080/16078454.2021.1912949. [DOI] [PubMed] [Google Scholar]
- Thomas X, Elhamri M, Raffoux E, Renneville A, Pautas C, de Botton S, de Revel T, Reman O, Terre C, Gardin C, et al. Comparison of high-dose cytarabine and timed-sequential chemotherapy as consolidation for younger adults with AML in first remission: the ALFA-9802 study. Blood. 2011;118:1754–1762. doi: 10.1182/blood-2011-04-349258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.