Abstract
Purpose
Endometrial cancer is the most common gynecological malignancy. The helicase RIG-I, a part of the innate immune system, and EFTUD2, a splicing factor which can upregulate RIG-I expression, are shown to influence tumor growth and disease progression in several malignancies. For endometrial cancer, an immunogenic cancer, data about RIG-I and EFTUD2 are still missing. The aim of this study was to examine the expression of RIG-I and EFTUD2 in endometrial cancer.
Methods
225 specimen of endometrial cancer were immunohistochemically stained for RIG-I and EFTUD2. The results were correlated to clinicopathological data, overall survival (OS) and progression-free survival (PFS).
Results
High RIG-I expression correlated with advanced tumor stages (FIGO: p = 0.027; pT: p = 0.010) and worse survival rates (OS: p = 0.009; PFS: p = 0.022). High EFTUD2 expression correlated to worse survival rates (OS: p = 0.026; PFS: p < 0.001) and was determined to be an independent marker for progression-free survival.
Conclusion
Our data suggest that the expression of RIG-I and EFTUD2 correlates with survival data, which makes both a possible therapeutic target in the future.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-04271-z.
Keywords: RIG-I, DDX58, EFTUD 2, Endometrial cancer, Survival, Innate immune system
Introduction
Endometrial carcinoma (EC) is the most common gynecologic malignancy and the sixth most common cancer among women worldwide (Bray et al. 2018) with an increasing incidence (Society. 2014). In 2018, an incidence of 382.069 cases and a mortality of almost 90.000 worldwide was estimated by The International Agency for Research and Cancer (Bray et al. 2018). Several risk factors are known, including obesity, diabetes mellitus, early menarche, nullipara or therapy with tamoxifen (Braun et al. 2016). It was thought that all those factors could contribute to an imbalance in the estrogen level which favors cell transformation. Previously, Bokhman differentiated between EC Type I (estrogen-dependent) and Type II (estrogen-independent) (Bokhman 1983). Today, EC is classified according to the ProMisE algorithm. This classification system includes MMR deficiency, POLE mutation, p53 wildtype and p53 aberrancy (Kommoss et al. 2018). The therapy for EC is multimodal and contains surgery, radiotherapy and chemotherapy (Concin et al. 2021). In Germany, a relative 10-year survival rate of 74% is estimated (RKI 2021). Nevertheless, about 20% are diagnosed in advanced stages (FIGO III-IV) and up to 12% suffer from recurrence (Huijgens and Mertens 2013, Hong et al. 2022). In these situations, the prognosis is worse with an estimated 5-year survival rate of just about 17% due to limited therapeutic options (Legge et al. 2020, Siegel et al. 2021). Therefore, new therapeutic strategies are needed and there is a rising interest in immunecheckpoint inhibitors. The PD-1-inhibitor Pembrolizumab showed promising results in clinical trials and in combination with the tyrosine kinase inhibitor lenvatinib even significantly better survival rates (Ott et al. 2017; Marabelle et al. 2020; Makker et al. 2022). Recent studies identified immune-related genes and proteins as well as the microenvironment of the tumor as relevant prognostic factors in EC (Chen et al. 2020; Ding et al. 2020). As many cancer cells develop strategies to escape the immune response, new insights into the understanding of this process could contribute to the development of new therapeutic options.
A major initiator of the innate immune response is the retinoic acid-inducible gene I, RIG-I, a cytosolic helicase. RIG-I is encoded by the gen DDX58 and together with MDA-5 and LGP-2, it belongs to the family of RIG-I-like receptors (Onoguchi et al. 2011) and detects viral single and double stranded RNA particles. By binding to viral RNA, a signaling process is initiated. This leads to an activation of the transcription factor NF-kB and finally to an upregulation of proinflammatory genes, including type I and III interferons and IFN stimulated genes (Quicke et al. 2017). In malignancies activation of innate immune signaling has been widely reported in absence of infections and plays and active role in the regulation of broader immune response. In ovarian cancer, RIG-I activation leads to an upregulation of MHC class I and to a secretion of proinflammatory mediators leading to the hypothesis that this could increase antitumor activity within microenvironment (Kübler et al. 2010). In HCC, a downregulation of RIG-I leads to enhanced levels of IFN alpha and was associated to poorer prognosis (Hou et al. 2014). It seems to be clear, that RIG-I has multiple roles in cancer tissue (Xu et al. 2018), including induction of intrinsic and extrinsic apoptosis (Elion et al. 2018; Castiello et al. 2019, Rameshbabu et al. 2021). RIG-I can be induced by many upstream factors, among them an induction by the Elongation factor Tu GTP-binding domain containing 2 (EFTUD2) is notable (Breiman et al. 2005; Stok et al. 2020).
EFTUD2 is a component of the spliceosome and an important protein regulating alternative splicing. EFTUD2 mutations are reported to cause mandibulofacial dysostosis with microcephaly (Lines et al. 2012), in which context it was originally detected. In the past years, it has been demonstrated that EFTUD2 is involved in various biological processes as it modulates cell differentiation, influences the innate immune response (Asghar and Meyer 2012; Zhu et al. 2015a, b) and the spliceosome activation (Guo et al. 2014).
To the best of our knowledge, this is the first study examining the protein expression of RIG-I and EFTUD2 on EC by immunohistochemistry.
Materials and methods
Patients and specimen
Native tissue samples were systematically accumulated between 1990 and 2001 from patients who underwent surgery at the Department of Gynecology and Obstetrics, Ludwig-Maximilians-University Munich, Germany. The surgically removed material (hysterectomy specimens and if indicated pelvic/paraaortic lymph nodes (for details about indication of lymphonodectomie see: (AGO-S3-Leitlinie: Diagnostik, T. u.N.d.P.m.E. 2018)) was immediately fixed in formaldehyde and embedded in paraffin for diagnosis and for further experiments.
In this study, we included a total number of 225 patients with endometrioid EC.
The average age at first diagnosis was 65 years, with the youngest patient being 35 and the oldest 85 years old. The average time of follow-up was about 11 years (131 months). In this context, we distinguished between overall survival (OS = time between diagnosis and all-cause death of the patient or last follow-up) and progression-free survival (PFS = time from diagnosis to any kind of progression, also including all-cause death).
Staging was performed based on the criteria formulated by the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) based on its version of 2020 (AGO-S3-Leitlinie), when all samples were re-staged by a pathologist according to the current staging system (Supplement 1). Grading types were assigned according to the WHO criteria by a gynecological pathologist, who also verified the histological subtype.
The majority of patients was diagnosed at an early stage of the disease (> 80% in stage I and II) and more than half of the tumor specimens presented a low-grade carcinoma (56% G1). Details concerning the study cohort are provided in (Table 1).
Table 1.
Clinic pathological variables of the patients included in this study
| Item | Patient no.(n = 225) | % |
|---|---|---|
|
Age at diagnosis (years) ≤ 65 > 65 |
109 116 |
48.4 51.6 |
|
Tumor size (pT) pT1 pT2 pT3 pT4 Not available |
175 16 30 3 1 |
77.8 6.4 13.3 1.3 0.4 |
|
FIGO staging I II III IV Not available |
167 15 36 6 1 |
74.2 6.7 16.0 2.7 0.4 |
|
Grade G1 G2 G3 Not available |
127 77 20 1 |
56.4 34.2 8.9 0.4 |
|
Nodal status (pN) pN0 pN1 pNX |
142 21 62 |
63.1 9.3 27.6 |
|
Metastases (pM) pM0 pM1 pMX |
109 5 111 |
48.4 2.2 49.3 |
|
Survival Alive Dead Not available |
93 132 0 |
41.3 58.7 0.0 |
| Progression | ||
|
None At least one Not available |
180 45 0 |
80.0 20.0 0.0 |
pT: Tumor size (pT1: The cancer is found only in the uterus; pT2: The tumor has spread from the uterus to the cervical stroma but not to other parts of the body; pT3: The cancer has spread beyond the uterus, but it is still only in the pelvic area; pT4: The cancer has spread to the mucosa of the rectum or bladder). FIGO: classification of the International Federation of Gynecology and Obstetrics (FIGO I: The cancer is found only in the uterus; FIGO II: The tumor has spread from the uterus to the cervical stroma but not to other parts of the body; FIGO III: The cancer has spread beyond the uterus, but it is still only in the pelvic area; FIGO IV: The cancer has spread to the mucosa of the rectum or bladder or the cancer has spread to lymph nodes in the groin area, and/or it has spread to distant organs, such as the bones or lungs). pN: status regarding lymph node. pN0: histopathological lymphnodes without cancer infiltration. pN1: histopathological lymphnodes with cancer infiltration. pNX: no information about lymph nodes in the histopathological examination, due to missing lymph node sampling. pM: status regarding distant metastasis. pM0: histopathological examination without sign for distant metastasis. pM1: histopathological examination with sign for distant metastasis. pMX: no information about distant metastasis in the histopathological examination
The patient and tumor characteristics as well as the follow-up data were provided by the Munich cancer registry. The data are completely anonymized.
Methods
Immunohistochemistry of RIG-I and EFTUD2
The EC samples were fixed in neutral-buffered formalin and underwent paraffin embedding following a standard protocol. To perform the following examinations at a more standardized level and for a better comparison, tissue microarrays (TMAs) were established. For this purpose, the tumor was marked in the paraffin block and three columnar biopsies were taken from each of it. Those tumor columns were cast again with paraffin into blocks, now containing tissue samples of several patients at ones, which made the staining process more effective, and the scoring less error-prone due to randomized selection of visual fields within the tumor. The blocks were cut into 3 µm thick slices using a microtome (Hn40 Schlitten-Mikrotom, then Reichert-Jung, today part of Leitz, Wetzlar, Germany) and mounted on microscope slides. After deparaffinisation in Roticlear® (Carl Roth, Arlesheim, Switzerland) and rinsing in ethanol, the tissue sections were blocked with 3% H2O2 in methanol at room temperature for 20 min to inactivate the endogenous peroxidase and subsequently rehydrated in a descending ethanol gradient. Afterwards, the slides were cooked in a pressure cooker using either an EDTA-based buffer containing surfactant (for RIG-I-staining) or a trisodium citrate buffer solution with pH = 6 (for EFTUD2 staining) aiming for epitope retrieval. Blocking solution was applied for blocking of the non-specific binding of the primary antibody. Tissue sections were incubated with the primary antibody. Subsequently, substrate and chromogen were added to the slides. For detailed staining process, see Table 2. After counterstaining with Mayer’s acidic haematoxylin (Waldeck, Münster, Germany), the slides passed through an ascending alcohol series and were finally cover slipped. Appropriate negative and positive controls were included in the staining (Supplement 2).
Table 2.
Overview of antibodies and chemicals used in the staining process
| RIG-I | EFTUD2 |
|---|---|
| Blocking solutiona: 20 min | Blocking solutionb: 5 min |
| Primary antibodyc | Primary antibodyd |
| 1:100 in ADe | 1:300 in PBSf |
| Incubation 1 h; 21 °C | Incubation 16,5 h; 4 °C |
| ImmPRESS polymer reagentg: 30 min | PostBlockh: 20 min |
| HRP Polymeri: 30 min | |
| Chromogen: DABj, 3 min | Chromogen: DABj, 2 min |
aNormal Horse Serum (NHS), 2.5%, ImmPRESS™ Anti-Mouse IgG Polymer Kit, VECTOR laboratories, Newark, United States; catalogue number: MP-7402
bZytoChem Plus HRP Polymer Kit (Mouse/Rabbit) 3 × 100, Zytomed Systems GmbH, Berlin, Germany; catalogue number POLHRP-100
cRIG-I-antibody: monoclonal IgG (mouse); LSBio, Seattle, United States; catalogue number: LS-C331000
dEFTUD2-antibody: polyclonal IgG (rabbit); Abcam, Cambridge, United Kingdom; catalogue number: ab72456
eAntibody Diluent, Agilent Technologies, Santa Clara, United States; catalogue number: S3022
fDulbecco ‘s Phosphate Buffered Saline, SigmaAlderich, Saint Louis, Missouri, United States
gAnti-Mouse IgG Polymer Kit, VECTOR laboratories, Newark, United States; catalogue number: MP-7402
hZytoChem Plus HRP Polymer Kit (Mouse/Rabbit); Zytomed Systems GmbH, Berlin, Germany
iVectastain Elite ABC Kit, diluted NORMAL serum; Vector laboratories, Newark, United States
jLiquid DAB + Substrate Chromogen System, REF K3468; Abcam, Cambridge, United Kingdom
To evaluate the staining, the immunoreactive score (IRS) was used (Remmele et al. 1986). By this semi-quantitative score, the intensity of the staining (0 = not stained; 1 = low intensity; 2 = moderate intensity; 3 = high intensity) and the percentage of stained cells (0 = 0%; 1 = 1–10%; 2 = 11–50%; 3 = 51–80%; 4 > 80%) was multiplied, resulting in a number between 0 (weak expression) and 12 (strong expression). Since we evaluated three different tumor areas from each patient, this also resulted in three IRS values, whereas we calculated the average.
Statistical analysis
The statistical analysis was carried out by Microsoft Excel and SPSS Statistics 26 (SPSS Inc., Chicago, Illinois). Comparisons of independent groups were conducted using non-parametric tests for two (Wilcoxon–Mann–Whitney U test) or multiple groups (Kruskal–Wallis test). All histopathological variables as provided in the table above and the expression of RIG-I/EFTUD2 were tested for correlations using Spearman’s Rho. Survival times were compared by Kaplan–Meier analysis, and differences in OS and PFS of patients were tested for significance by the log-rank test. A multivariate Cox regression model was established for the analysis of survival to compare the risk of death in patients depending on the expression. In addition to the expression levels, the effects of further independent factors such as age at diagnosis, tumor grade, tumor size, lymph node status and FIGO classification were accounted for in this model. The one in ten rule (Vittinghoff and McCulloch 2007) was respected and only variables with a complete dataset were included. P values were two sided and had to be lower than p < 0.05 for being considered to be statistically significant.
Ethics approval
The study was approved by the local ethics committee of the Ludwig-Maximilians-University of Munich (reference number 19-249, 2019) and was performed in accordance to the Declaration of Helsinki.
Results
pT, FIGO and grade correlate to RIG-I expression
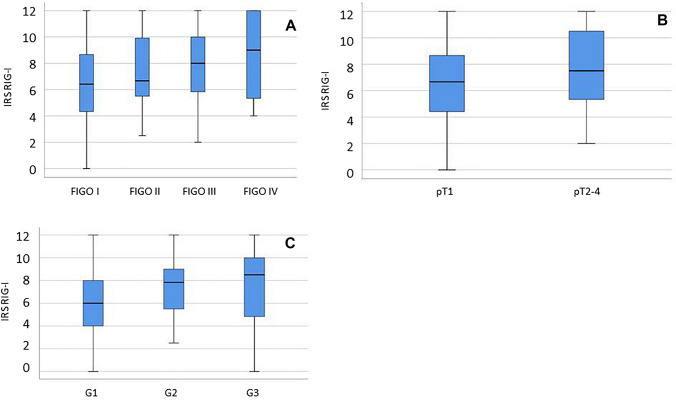
99% of all EC specimen expressed RIG-I in the cytoplasm. The median IRS was 7.0 (5.4%). RIG-I was found to be expressed in the nucleus only very weakly for which reason the following results refer to cytoplasmic RIG-I expression. A low cytoplasmic expression (IRS ≤ 4) was detected in 21.2% compared to a high expression (IRS > 4) in 78.8% of all cases. In the further course, we correlated clinical-pathological data with the IRS of RIG-I (Table 3). RIG-I expression was significantly increased in higher FIGO stages (p = 0.027, Fig. 1A; Supplementary 3). Furthermore, RIG-I levels correlated with the size of the primary tumor (pT stage): patients whose cancer was diagnosed early as a pT1 tumor showed significantly lower RIG-I expression (p = 0.010, Fig. 1B; Supplementary 3). The higher the tumor grade the patients presented, the higher the IRS we determined on the corresponding histological specimens (p = 0.007; Fig. 1C; Supplementary 3). There was no significant correlation with RIG-I in terms of lymph node (pN) and distant metastases (pM).
Table 3.
Staining results and correlation analysis
| RIG-I | EFTUD2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Median IRS (± SD) |
% | p (NPAR) | ρ | Median IRS (± SD) |
% | p (NPAR) | ρ | |
| FIGO1 | ||||||||
|
FIGO I FIGO II FIGO III FIGO IV |
6.4 (± 2.6) 6.7 (± 2.9) 8.0 (± 2.7) 9.0 (± 3.4) |
/* 6.7 11.1 0 |
0.027 | 0.201 |
6.0 (± 3.1) 4.3 (± 2.7) 5.8 (± 3.4) 6.3 (± 3.2) |
7.5 6.7 /* 0.0 |
0.815 | − 0.044 |
| pT2 | ||||||||
|
T1 T2/3/4 |
6.7 (± 2.6) 7.5 (± 3.0) |
2.3 2.0 |
0.010 | 0.174 |
6.00 (± 3.1) 5.7 (± 3.1) |
7.7 8.2 |
0.928 | 0.006 |
| Grade1 | ||||||||
|
G1 G2 G3 |
6.0 (± 2.6) 7.8 (± 2.4) 8.5 (± 3.7) |
10.3 /* 0 |
0.007 | 0.210 |
5.7 (± 3.1) 6.0 (± 3.2) 6.8 (± 3.5) |
3.2 10.7 /* |
0.303 | 0.096 |
| pN2 | ||||||||
|
N − N + |
6.7 (± 2.7) 8.0 (± 2.9) |
1.4 19.0 |
0.753 | 0.110 |
6.0 (± 3.1) 5.7 (± 3.5) |
8.0 4.8 |
0.657 | − 0.035 |
| pM2 | ||||||||
|
M − M + |
7.0 (± 2.5) 5.3 (± 4.5) |
6.5 20.0 |
0.539 | 0.025 |
5.5 (± 3.1) 6.7 (± 4.3) |
/* 20.0 |
0.464 | 0.102 |
SD standard deviation, % percentage of the subgroup with median IR, NPAR non-parametric test, p p value, ρ correlation coefficient. 1correlation tested by Kruskall–Wallis test (p; NPAR) and Spearman-correlation (ρ).2correlation tested by Mann–Whitney test (p; NPAR) and Spearman-correlation (ρ).
FIGO: classification of the International Federation of Gynecology and Obstetrics (FIGO I: The cancer is found only in the uterus; FIGO II: The tumor has spread from the uterus to the cervical stroma but not to other parts of the body; FIGO III: The cancer has spread beyond the uterus, but it is still only in the pelvic area; FIGO IV: The cancer has spread to the mucosa of the rectum or bladder or the cancer has spread to lymph nodes in the groin area, and/or it has spread to distant organs, such as the bones or lungs). pT: Tumor size (pT1: The cancer is found only in the uterus; pT2: The tumor has spread from the uterus to the cervical stroma but not to other parts of the body; pT3: The cancer has spread beyond the uterus, but it is still only in the pelvic area; pT4: The cancer has spread to the mucosa of the rectum or bladder). pN: status regarding lymph node. pN0: histopathological lymphnodes without cancer infiltration. pN1: histopathological lymphnodes with cancer infiltration. pM: status regarding distant metastasis. pM0: histopathological examination without sign for distant metastasis. pM1: histopathological examination with sign for distant metastasis. *Values resulted from calculations, no sample with this value exists. Significant results are shown in bold
Fig. 1.
RIG-I expression and clinicopathological variables. RIG-I expression is significant lower in FIGO I (tumor is found only in the uterus) compared to FIGO IV (tumor has spread to the mucosa of rectum/bladder or to lymph nodes in the groin area or to distant organs) as shown in the boxplot (A). Regarding pT (tumor size) stages, RIG-I expression is lower in pT1 (tumor is only found in the uterus) compared to higher pT stages (B). Low graded endometrial cancers have significant less RIG-I expression than high graded samples, see in boxplot (C)
EFTUD2 does not correlate to clinicopathological variables
Due to technical issues (specimen floating off the slides during the staining process), we could only use 219 samples in our analysis of EFTUD2 expression. Out of these 219 samples, none or very low EFTUD2 expression (IRS ≤ 1) was displayed in 5.5% of the specimens, whereas an IRS ≥ 9 was shown in 51 (23.3%) cases. The median IRS of EFTUD2 staining was 6.00. 55.3% of all samples showed an EFTUD2 expression ≤ 6, whereas 44.7% scored higher than the median IRS (example see Fig. 2). Examining the EFTUD2 expression with regards to tumor size, pN-/pM-status, grade and FIGO classification, we could not find statistically significant differences and there was no significant correlation between IRS and any of the clinicopathological parameters mentioned above (Table 3).
Fig. 2.

Exemplary image of endometrial cancer tissue with high (IRS = 8) EFTUD2 expression (B). Magnification: big pictures *25, small pictures *100
High RIG-I level is correlated with poor survival in EC
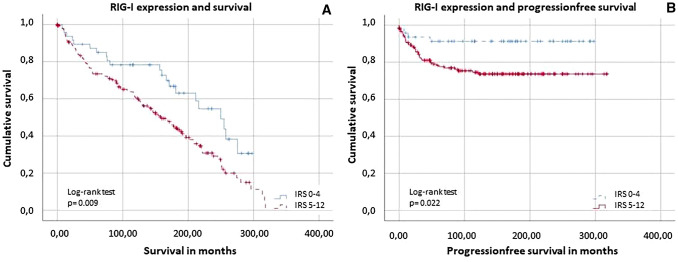
As shown in the data above, RIG-I expression correlates at a significant level with various clinicopathological data. Additionally, our data strongly suggest that RIG-I levels in endometrioid adenocarcinoma are associated with both PFS and OS. With a cut-off at IRS = 4, univariate time-to-event analyses using the Kaplan–Meier estimator showed a significantly reduced OS in patients with high RIG-I expression (p = 0.009, Fig. 3A). Patients with low RIG-I levels survived 207 months on average, whereas patients with a high IRS score only survived 159 months after diagnosis (95% confidence interval of the median: 197–303 vs. 129–189 months; for distribution of the groups see Supplement 4). Furthermore, high RIG-I levels were also associated with poor PFS in endometrioid adenocarcinoma patients (p = 0.022, Fig. 3B). In the group of patients with low expression (IRS ≤ 4), 8.5% of the study participants experienced progression within the observation period. This contrasts with 23.4% of patients suffering progression who presented with high RIG-I levels (IRS > 4). To assess whether RIG-I expression was an independent predictor for survival, we performed a multivariate Cox regression analysis. Neither OS nor PFS showed independent prognostic significance (Tables 4, 5). For correlation in between the histopathological variables and the categorization of the variables, see Supplement 5 and 6.
Fig. 3.
Survival rates depending of RIG-I expression. Patients with low RIG-I expression (IRS ≤ 4) have significant better overall survival- (p = 0.009; A) and progression-free survival- (p = 0.022; B) rates (tested by log-rank test). For distribution of the patient group, see Supplement 4
Table 4.
Multivariate COX regression of RIG-I regarding overall survival
| Significance | HR | L95% | U95% | |
|---|---|---|---|---|
| RIG-I expression | 0.733 | 1.092 | 0.658 | 1.813 |
| Age at diagnosis | 0.000 | 1.062 | 1.039 | 1.086 |
| Grade | 0.137 | 1.315 | 0.917 | 1.887 |
| pT | 0.056 | 4.009 | 0.966 | 17.403 |
| pN | 0.000 | 2.462 | 1.683 | 3.601 |
| FIGO | 0.324 | 0.488 | 0.117 | 2.030 |
HR hazard ratio, L95% Lower 95% CI of Exp(B), U95% upper 95% CI of Exp(B), pT tumor size, pN status regarding lymph node. FIGO: classification of the International Federation of Gynecology and Obstetrics. For categorization of variables, see Supplement 7. Significant results are shown in bold
Table 5.
Multivariate COX regression of RIG-I regarding progression-free survival
| Significance | HR | L95% | U95% | |
|---|---|---|---|---|
| RIG-I expression | 0.173 | 2.100 | 0.722 | 6.104 |
| Age at diagnosis | 0.901 | 0.998 | 0.967 | 1.030 |
| Grade | 0.040 | 1.926 | 1.032 | 3.596 |
| pT | 0.291 | 1.970 | 0.560 | 6.935 |
| pN | 0.019 | 2.184 | 1.137 | 4.195 |
| FIGO | 0.459 | 1.634 | 0.446 | 5.987 |
HR hazard ratio, L95% Lower 95% CI of Exp(B), U95% upper 95% CI of Exp(B), pT tumor size, pN status regarding lymph node. FIGO: classification of the International Federation of Gynecology and Obstetrics. For categorization of variables, see Supplement 7. Significant results are shown in bold
High EFTUD2 level is correlated with poor survival in endometrial cancer
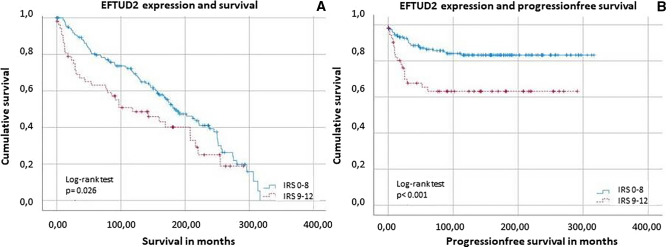
Although EFTUD2 expression was not associated with tumor stage, its expression correlated with OS and PFS. As shown in Fig. 4, patients with low EFTUD2 expression (IRS ≤ 8) had significantly (p = 0.026/p < 0.001) better OS and PFS than patients with higher EFTUD2 expression. The analysis also showed that the median survival time of patients with high EFTUD2 (IRS > 8) expression was only 118 moths (95% confidence interval of the median: 150–218 months) and an event of progression occurred in 33.96% of cases. In comparison, patients with lower (IRS ≤ 8) EFTUD2 expression showed a median survival time of 184 moths (95% confidence interval: 39–198 months) and only progressed in 15.06% of cases.
Fig. 4.
Kaplan–Meier analysis for overall survival A and progression-free survival B with regards to EFTUD2 expression with significant better survival rates when EFTUD2 expression was IRS 0–8 (tested by log-rank test). For distribution of the patient group, see Supplement 5
EFTUD2 expression turned out to be an independent marker for OS and PFS in the multivariate Cox regression (Tables 6, 7). For the distribution of the groups, see Supplement 7.
Table 6.
Multivariate COX regression of EFTUD2 regarding overall survival
| Significance | HR | L95% | U95% | |
|---|---|---|---|---|
| EFTUD2 | 0.064 | 1.474 | 0.978 | 2.221 |
| Age at diagnosis | 0.000 | 1.059 | 1.037 | 1.081 |
| Grade | 0.215 | 1.263 | 0.873 | 1.827 |
| pT | 0.051 | 4.234 | 0.996 | 18.004 |
| pN | 0.000 | 2.669 | 1.811 | 3.934 |
| FIGO | 0.311 | 0.479 | 0.115 | 1.989 |
Significant results are shown in bold
HR hazard ratio, L95% lower 95% CI of Exp(B), U95% upper 95% CI of Exp(B), pT tumor size, pN status regarding lymph node. FIGO: classification of the International Federation of Gynecology and Obstetrics. For categorization of variables, see Supplement 7
Table 7.
Multivariate COX regression of EFTUD2 regarding progression-free survival
| Significance | HR | L95% | U95% | |
|---|---|---|---|---|
| EFTUD2 | 0.005 | 2.497 | 1.326 | 4.701 |
| Age at diagnosis | 0.783 | 0.996 | 0.965 | 1.027 |
| Grade | 0.152 | 1.603 | 0.841 | 3.055 |
| pT | 0.353 | 1.824 | 0.512 | 6.494 |
| pN | 0.017 | 2.236 | 1.152 | 4.338 |
| FIGO | 0.258 | 2.109 | 0.578 | 7.686 |
Significant results are shown in bold
HR hazard ratio, L95% Lower 95% CI of Exp(B), U95% upper 95% CI of Exp(B), pT Tumor size, pN status regarding lymph node. FIGO: classification of the International Federation of Gynecology and Obstetrics. For categorization of variables, see Supplement 7
Discussion
RIG-I is expressed in most human cells and plays an important role in the innate immune system (Horvath et al. 2020). As already revealed in functional experiments, its expression can be upregulated by the splicing factor EFTUD2 (Zhu et al. 2015a, b). Although EC is known as an “immunogenic” cancer, this is—to the best of our knowledge—the first study examining RIG-I and EFTUD2 in EC. We detected that a high expression of RIG-I correlated with high FIGO and pT stages as well as higher grade. Additionally, high expression of RIG-I and EFTUD2 was associated with poor PFS.
RIG-I is a nucleoid acid receptor in the cytoplasm (Wicherska-Pawlowska et al. 2021). In addition, recent studies showed that RIG-I can be located in the nucleus in some cells (Liu et al. 2018). The ligands of RIG-I (various RNA molecules) are typically (but not exclusively) parts of the viral RNA (Gack et al. 2007). After ligand biding, RIG-I interacts with the MAVS (Breiman et al. 2005; Matsumiya and Stafforini 2010). This process results in the activation of NFĸB and IRF-3 and leads to the production of Type I IFN, chemokines and other cytokines (Seth et al. 2005). By these substances, effector T cells and NK cells are stimulated leading to a tumor-suppressive effect (Elion and Cook 2018). RIG-I can also activate intrinsic and extrinsic apoptosis pathways (Rameshbabu et al. 2021). Taking these effects and pathways together, RIG-I as part of the innate immune system has become more and more important over the last years.
In several tumors, RIG-I activation follows the described mechanisms and leads to a tumor-suppressive milieu. Well-studied examples are pancreatic cancer and melanoma (Poeck et al. 2008; Ellermeier et al. 2013). RIG-I stimulation by endogenous RNAs enhanced therapy resistance by a STAT-1-dependent pathway and NOTCH signaling (Boelens et al. 2014). In breast cancer, a high RIG-I expression was also described to be associated with poor outcome: RIG-I stimulation by endogenous RNA lead to increased tumor growth and metastasis in vitro (Nabet et al. 2017).
This trend also appeared in our results as well as in ovarian cancer. In a retrospective study with 141 cases of ovarian cancer, RIG-I was overexpressed (compared to healthy ovarian tissue) and a high expression correlated with higher tumor grade as well as to poorer outcome (Wolf et al. 2020). Additionally, correlations with interferon beta, PDL-1 and FOXP3 levels were detected in ovarian cancer (Wolf et al. 2020). The immune-suppressive system of PD-L1 was found to be associated with poor survival rates in ovarian cancer as well as in EC (Wieser et al. 2018; Zhang et al. 2021). The authors suggest that in ovarian cancer the antiviral and tumor-suppressive effects of RIG-I seem to be neutralized by an immune-escape phenomenon in the tumor microenvironment (Wolf et al. 2020). Regarding the tumor microenvironment in EC, it is known that regulatory T cells and their marker FOXP3 are correlated with worse survival rates (de Jong et al. 2009; Xi et al. 2019). By interaction with the tumor microenvironment RIG-I seems to lose its tumor-suppressive effect in ovarian cancer (Wolf et al. 2020). Discovery of mechanism how this protective function has been aborted could help to develop strategies for more effective use of immunotherapies in both EC and OC.
Still, it is important to keep in mind, that our results refer to the expression of RIG-I and not to its activation, similar to the study in ovarian cancer (Wolf et al. 2020). Nevertheless, RIG-I remains a potential target in further therapies and agonists as well as antagonists are in development (Rawling et al. 2020; Onomoto et al. 2021).
As an upregulation of RIG-I by EFTUD2 was described (Zhu et al. 2015a, b), we also investigated the expression of this splicing factor. EFTUD2 seems to be involved in viral infections and miscarriages (Zhu et al. 2015a, b; Löb et al. 2020). Only limited data are available examining the role of EFTUD2 in cancer and, to the best of our knowledge, this is the first study examining EFTUD2 in EC. We observed a significant correlation between EFTUD2 and survival rates in EC: a high expression was associated with low PFS and OS and turned out to be an independent marker for PFS. EFTUD2 is known to be a modulator of the innate immune system: in colorectal cancer, EFTUD2 seems to promote tumor growth, especially in a colitis-associated environment and by modulation of macrophages (Lv et al. 2019). Lv et al. demonstrated in a mouse model of colorectal cancer that a knockdown of EFTUD2 resulted in a reduced secretion of proinflammatory cytokines and tumorigenic factors (Lv et al. 2019). This reduction of inflammation and tumor development was related to an impaired activation of TLR-4-NF-κB signaling cascade in macrophages due to an altered EFTUD2 expression (Lv et al. 2019). In hepatic cellular cancer, EFTUD2 was upregulated compared to healthy liver tissue (Lv et al. 2021). In analogy to our results in EC, a negative correlation between survival rates and EFTUD2 expression was shown. Also, a reduced tumor growth in case of EFTUD2 knockdown was detected, suggesting it as an independent prognostic factor for patients with hepatic cellular cancer (Tu et al. 2020; Lv et al. 2021).
Furthermore, another limitation of this study needs to be discussed: although the data regarding FIGO/pT/grade and survival rates are consistent and clear, RIG-I expression did not turn out to be an independent marker for survival. This assumes that underlying mechanisms which are not fully understood play an important role. Further studies are, therefore, necessary to understand these processes.
In summary, our data show that a high RIG-I expression is correlated with advanced tumor stages and thus subsequently to worse survival rates. EFTUD2 turned out to be an independent marker for progression-free survival. Therefore, RIG-I and EFTUD2 seem to be negative predictors in patients with EC. To examine the underlying mechanisms, further studies are necessary.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work is part of the doctoral thesis of Sophie Mitter and Lena Marie Müller
Abbreviations
- EC
Endometrial cancer
- EFTUD2
Elongation factor Tu GTP-binding domain containing 2
- OS
Overall survival
- PFS
Progression-free survival
- RIG-I
Retinoic acid-inducible gene I
Author contributions
SM and LM: performed the staining and the experiments, analyzed the data and wrote the paper. TK: design of experiments and assistance in writing the paper and approvement of the manuscript. ES: supervision. UJ and SB: design of the experiment and supervision. All authors interpreted the data and read the paper and approved the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported by Foundation in favor of the medical school of the LMU-Cluster 1 (Stiftungen zugunsten der Medizinischen Fakultät-Cluster 1).
Data availability
All data generated or analyzed during this study are included in this published article. For any questions, please contact S. Beyer.
Declarations
Conflict of interest
F. Trillsch declares Research support, advisory board, honoraria and travel expenses from Astra-Zeneca, Clovis, Eisai, Medac, MSD, PharmaMar, Roche, Tesaro/GSK. A. Burges declares advisory board and honoraria from AstraZeneca, Roche, Tesaro. S. Mahner has received Research support, advisory board, honoraria and travel expenses from AstraZeneca, Clovis, Eisai, GlaxoSmithKline, Medac, MSD, Novartis, Olympus, PharmaMar, Roche, Sensor Kinesis, Teva, Tesaro. T. Kolben has a relative employed at Roche and holds stock of Roche. All other authors declare that they have no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the ethics committee of the Ludwig-Maximilians-University Munich (reference number: 19–249, 2019). Patient data were anonymized.
Consent to participate
Not applicable as all data are anonymized.
Consent to publication
Not applicable as all data are anonymized.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AGO-S3-Leitlinie: Diagnostik, Version 1.0 – April 2018. Available from: https://www.awmf.org/uploads/tx_szleitlinien/032-034OLl_S3_Endometriumkarzinom-Diagnostik-Therpie-Nachsorge_2018-04.pdf. Accessed 15 Jul 2022
- Asghar U, Meyer T (2012) Are there opportunities for chemotherapy in the treatment of hepatocellular cancer? J Hepatol 56(3):686–695 [DOI] [PubMed] [Google Scholar]
- Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y, Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, Ter Brugge PJ, Jonkers J, Slingerland J, Minn AJ (2014) Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell 159(3):499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15(1):10–17 [DOI] [PubMed] [Google Scholar]
- Braun MM, Overbeek-Wager EA, Grumbo RJ (2016) Diagnosis and management of endometrial cancer. Am Fam Physician 93(6):468–474 [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424 [DOI] [PubMed] [Google Scholar]
- Breiman A, Grandvaux N, Lin R, Ottone C, Akira S, Yoneyama M, Fujita T, Hiscott J, Meurs EF (2005) Inhibition of RIG-I-dependent signaling to the interferon pathway during hepatitis C virus expression and restoration of signaling by IKKepsilon. J Virol 79(7):3969–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiello L, Zevini A, Vulpis E, Muscolini M, Ferrari M, Palermo E, Peruzzi G, Krapp C, Jakobsen M, Olagnier D, Zingoni A, Santoni A, Hiscott J (2019) An optimized retinoic acid-inducible gene I agonist M8 induces immunogenic cell death markers in human cancer cells and dendritic cell activation. Cancer Immunol Immunother 68(9):1479–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Yang Y, Zhang Y, Jiang S, Li X, Wan J (2020) Identification of prognostic immune-related genes in the tumor microenvironment of endometrial cancer. Aging (Albany NY) 12(4):3371–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza MR, Sessa C (2016) ESMO-Esgo-Estro consensus conference on endometrial cancer: diagnosis treatment and follow-up. Int J Gynecol Cancer 26(1):2–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concin N, Matias-Guiu X, Vergote I, Cibula D, Mirza MR, Marnitz S, Ledermann J, Bosse T, Chargari C, Fagotti A, Fotopoulou C, Gonzalez Martin A, Lax S, Lorusso D, Marth C, Morice P, Nout RA, O’Donnell D, Querleu D, Raspollini MR, Sehouli J, Sturdza A, Taylor A, Westermann A, Wimberger P, Colombo N, Planchamp F, Creutzberg CL (2021) ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 31(1):12–39 [DOI] [PubMed] [Google Scholar]
- de Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, Nijman HW (2009) Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol 114(1):105–110 [DOI] [PubMed] [Google Scholar]
- Ding H, Fan GL, Yi YX, Zhang W, Xiong XX, Mahgoub OK (2020) Prognostic implications of immune-related genes’ (IRGs) signature models in cervical cancer and endometrial cancer. Front Genet 11:725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion DL, Cook RS (2018) Harnessing RIG-I and intrinsic immunity in the tumor microenvironment for therapeutic cancer treatment. Oncotarget 9(48):29007–29017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion DL, Jacobson ME, Hicks DJ, Rahman B, Sanchez V, Gonzales-Ericsson PI, Fedorova O, Pyle AM, Wilson JT, Cook RS (2018) Therapeutically active RIG-I agonist induces immunogenic tumor cell killing in breast cancers. Cancer Res 78(21):6183–6195 [DOI] [PubMed] [Google Scholar]
- Ellermeier J, Wei J, Duewell P, Hoves S, Stieg MR, Adunka T, Noerenberg D, Anders HJ, Mayr D, Poeck H, Hartmann G, Endres S, Schnurr M (2013) Therapeutic efficacy of bifunctional siRNA combining TGF-β1 silencing with RIG-I activation in pancreatic cancer. Cancer Res 73(6):1709–1720 [DOI] [PubMed] [Google Scholar]
- Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU (2007) TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 446(7138):916–920 [DOI] [PubMed] [Google Scholar]
- Guo R, Zheng L, Park JW, Lv R, Chen H, Jiao F, Xu W, Mu S, Wen H, Qiu J, Wang Z, Yang P, Wu F, Hui J, Fu X, Shi X, Shi YG, Xing Y, Lan F, Shi Y (2014) BS69/ZMYND11 reads and connects histone H3.3 lysine 36 trimethylation-decorated chromatin to regulated pre-mRNA processing. Mol Cell 56(2):298–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Cho HW, Ouh YT, Lee JK, Chun Y, Gim JA (2022) Genomic landscape of advanced endometrial cancer analyzed by targeted next-generation sequencing and the cancer genome atlas (TCGA) dataset. J Gynecol Oncol. 10.3802/jgo.2022.33.e29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath L, Thienpont B, Zhao L, Wolf D, Pircher A (2020) Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC) - novel approaches and future outlook. Mol Cancer 19(1):141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Zhou Y, Zheng Y, Fan J, Zhou W, Ng IO, Sun H, Qin L, Qiu S, Lee JM, Lo CM, Man K, Yang Y, Yang Y, Yang Y, Zhang Q, Zhu X, Li N, Wang Z, Ding G, Zhuang SM, Zheng L, Luo X, Xie Y, Liang A, Wang Z, Zhang M, Xia Q, Liang T, Yu Y, Cao X (2014) Hepatic RIG-I predicts survival and interferon-α therapeutic response in hepatocellular carcinoma. Cancer Cell 25(1):49–63 [DOI] [PubMed] [Google Scholar]
- Huijgens AN, Mertens HJ (2013) Factors predicting recurrent endometrial cancer. Facts Views vis Obgyn 5(3):179–186 [PMC free article] [PubMed] [Google Scholar]
- Kommoss S, McConechy MK, Kommoss F, Leung S, Bunz A, Magrill J, Britton H, Kommoss F, Grevenkamp F, Karnezis A, Yang W, Lum A, Krämer B, Taran F, Staebler A, Lax S, Brucker SY, Huntsman DG, Gilks CB, McAlpine JN, Talhouk A (2018) Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann Oncol 29(5):1180–1188 [DOI] [PubMed] [Google Scholar]
- Kübler K, Gehrke N, Riemann S, Böhnert V, Zillinger T, Hartmann E, Pölcher M, Rudlowski C, Kuhn W, Hartmann G, Barchet W (2010) Targeted activation of RNA helicase retinoic acid-inducible gene-I induces proimmunogenic apoptosis of human ovarian cancer cells. Cancer Res 70(13):5293–5304 [DOI] [PubMed] [Google Scholar]
- Legge F, Restaino S, Leone L, Carone V, Ronsini C, Di Fiore GLM, Pasciuto T, Pelligra S, Ciccarone F, Scambia G, Fanfani F (2020) Clinical outcome of recurrent endometrial cancer: analysis of post-relapse survival by pattern of recurrence and secondary treatment. Int J Gynecol Cancer 30(2):193–200 [DOI] [PubMed] [Google Scholar]
- Lines MA, Huang L, Schwartzentruber J, Douglas SL, Lynch DC, Beaulieu C, Guion-Almeida ML, Zechi-Ceide RM, Gener B, Gillessen-Kaesbach G, Nava C, Baujat G, Horn D, Kini U, Caliebe A, Alanay Y, Utine GE, Lev D, Kohlhase J, Grix AW, Lohmann DR, Hehr U, Böhm D, Majewski J, Bulman DE, Wieczorek D, Boycott KM (2012) Haploinsufficiency of a spliceosomal GTPase encoded by EFTUD2 causes mandibulofacial dysostosis with microcephaly. Am J Hum Genet 90(2):369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Lu Y, Thulasi Raman SN, Xu F, Wu Q, Li Z, Brownlie R, Liu Q, Zhou Y (2018) Nuclear-resident RIG-I senses viral replication inducing antiviral immunity. Nat Commun 9(1):3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löb S, Vattai A, Kuhn C, Schmoeckel E, Mahner S, Wöckel A, Kolben T, Szekeres-Bartho J, Jeschke U, Vilsmaier T (2020) Spliceosome protein EFTUD2 is upregulated in the trophoblast of spontaneous miscarriage and hydatidiform mole. J Reprod Immunol 140:103149 [DOI] [PubMed] [Google Scholar]
- Lv C, Li XJ, Hao LX, Zhang S, Song Z, Ji XD, Gong B (2021) Over-activation of EFTUD2 correlates with tumor propagation and poor survival outcomes in hepatocellular carcinoma. Clin Transl Oncol 24:93–103 [DOI] [PubMed] [Google Scholar]
- Lv Z, Wang Z, Luo L, Chen Y, Han G, Wang R, Xiao H, Li X, Hou C, Feng J, Shen B, Wang Y, Peng H, Guo R, Li Y, Chen G (2019) Spliceosome protein Eftud2 promotes colitis-associated tumorigenesis by modulating inflammatory response of macrophage. Mucosal Immunol 12(5):1164–1173 [DOI] [PubMed] [Google Scholar]
- Makker V, Colombo N, CasadoHerráez A, Santin AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay S, Ray-Coquard I, Shapira-Frommer R, Ushijima K, Sakata J, Yonemori K, Kim YM, Guerra EM, Sanli UA, McCormack MM, Smith AD, Keefe S, Bird S, Dutta L, Orlowski RJ, Lorusso D (2022) Lenvatinib plus pembrolizumab for advanced endometrial cancer. N Engl J Med 386(5):437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marabelle A, Le DT, Ascierto PA, Giacomo AMD, Jesus-Acosta AD, Delord J-P, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang Y-J, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr (2020) Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 38(1):1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumiya T, Stafforini DM (2010) Function and regulation of retinoic acid-inducible gene-I. Crit Rev Immunol 30(6):489–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabet BY, Qiu Y, Shabason JE, Wu TJ, Yoon T, Kim BC, Benci JL, DeMichele AM, Tchou J, Marcotrigiano J, Minn AJ (2017) Exosome RNA unshielding couples stromal activation to pattern recognition receptor signaling in cancer. Cell 170(2):352-366.e313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoguchi K, Yoneyama M, Fujita T (2011) Retinoic acid-inducible gene-I-like receptors. J Interferon Cytokine Res 31(1):27–31 [DOI] [PubMed] [Google Scholar]
- Onomoto K, Onoguchi K, Yoneyama M (2021) Regulation of RIG-I-like receptor-mediated signaling: interaction between host and viral factors. Cell Mol Immunol 18(3):539–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott PA, Bang YJ, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, Puzanov I, Mehnert JM, Aung KL, Lopez J, Carrigan M, Saraf S, Chen M, Soria JC (2017) Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol 35(22):2535–2541 [DOI] [PubMed] [Google Scholar]
- Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, Kirschnek S, Gaffal E, Landsberg J, Hellmuth J, Schmidt A, Anz D, Bscheider M, Schwerd T, Berking C, Bourquin C, Kalinke U, Kremmer E, Kato H, Akira S, Meyers R, Häcker G, Neuenhahn M, Busch D, Ruland J, Rothenfusser S, Prinz M, Hornung V, Endres S, Tüting T, Hartmann G (2008) 5’-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med 14(11):1256–1263 [DOI] [PubMed] [Google Scholar]
- Quicke KM, Diamond MS, Suthar MS (2017) Negative regulators of the RIG-I-like receptor signaling pathway. Eur J Immunol 47(4):615–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameshbabu S, Labadie BW, Argulian A, Patnaik A (2021) Targeting innate immunity in cancer therapy. Vaccines (basel) 9(2):138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawling DC, Jagdmann GE, Potapova O, Pyle AM (2020) Small-molecule antagonists of the RIG-I innate immune receptor. ACS Chem Biol 15(2):311–317 [DOI] [PubMed] [Google Scholar]
- Remmele W, Hildebrand U, Hienz HA, Klein PJ, Vierbuchen M, Behnken LJ, Heicke B, Scheidt E (1986) Comparative histological, histochemical, immunohistochemical and biochemical studies on oestrogen receptors, lectin receptors, and Barr bodies in human breast cancer. Virchows Arch A Pathol Anat Histopathol 409(2):127–147 [DOI] [PubMed] [Google Scholar]
- RKI (2021) “Robert-Koch-Institut. Zentrum für Krebsregisterdaten. Cancer Ger.”.
- Seth RB, Sun L, Ea CK, Chen ZJ (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122(5):669–682 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistic. CA: A Cancer J Clin 71(1):7–33 [DOI] [PubMed] [Google Scholar]
- Society AC (2014) "Cancer Facts and Figures " http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. Accessed 27 Oct 2014.
- Stok JE, Vega Quiroz ME, van der Veen AG (2020) Self RNA sensing by RIG-I-like receptors in viral infection and sterile inflammation. J Immunol 205(4):883–891 [DOI] [PubMed] [Google Scholar]
- Tu M, He L, You Y, Li J, Yao N, Qu C, Huang W, Xu L, Luo R, Hong J (2020) EFTUD2 maintains the survival of tumor cells and promotes hepatocellular carcinoma progression via the activation of STAT3. Cell Death Dis 11(10):830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittinghoff E, McCulloch CE (2007) Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 165(6):710–718 [DOI] [PubMed] [Google Scholar]
- Wicherska-Pawłowska K, Wróbel T, Rybka J (2021) Toll-like receptors (TLRs), NOD-like receptors (NLRs), and RIG-I-like receptors (RLRs) in innate immunity. TLRs, NLRs, and RLRs ligands as immunotherapeutic agents for hematopoietic diseases. Int J Mol Sci 22(24):13397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser V, Gaugg I, Fleischer M, Shivalingaiah G, Wenzel S, Sprung S, Lax SF, Zeimet AG, Fiegl H, Marth C (2018) BRCA1/2 and TP53 mutation status associates with PD-1 and PD-L1 expression in ovarian cancer. Oncotarget 9(25):17501–17511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D, Fiegl H, Zeimet AG, Wieser V, Marth C, Sprung S, Sopper S, Hartmann G, Reimer D, Boesch M (2020) High RIG-I expression in ovarian cancer associates with an immune-escape signature and poor clinical outcome. Int J Cancer 146(7):2007–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Z, Jing L, Le-Ni K, Zhu L, Ze-Wen D, Hui Y, Ming-Rong X, Guang-Dong L (2019) Evaluation of PTEN and CD4+FOXP3+ T cell expressions as diagnostic and predictive factors in endometrial cancer: a case control study. Medicine (baltimore) 98(30):e16345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XX, Wan H, Nie L, Shao T, Xiang LX, Shao JZ (2018) RIG-I: a multifunctional protein beyond a pattern recognition receptor. Protein Cell 9(3):246–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Liu Q, Zhu Y, Zhang S, Peng Q, Strickland AL, Zheng W, Zhou F (2021) PD-L1 expression in endometrial serous carcinoma and its prognostic significance. Cancer Manag Res 13:9157–9165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xiao F, Hong J, Wang K, Liu X, Cai D, Fusco DN, Zhao L, Jeong SW, Brisac C, Chusri P, Schaefer EA, Zhao H, Peng LF, Lin W, Chung RT (2015a) EFTUD2 is a novel innate immune regulator restricting hepatitis c virus infection through the RIG-I/MDA5 pathway. J Virol 89(13):6608–6618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Xiao F, Hong J, Wang K, Liu X, Cai D, Fusco DN, Zhao L, Jeong SW, Brisac C, Chusri P, Schaefer EA, Zhao H, Peng LF, Lin W, Chung RT, Ou J-HJ (2015b) EFTUD2 is a novel innate immune regulator restricting hepatitis C virus infection through the RIG-I/MDA5 pathway. J Virol 89(13):6608–6618 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article. For any questions, please contact S. Beyer.