Abstract
Purpose
To construct and validate a combined nomogram model based on magnetic resonance imaging (MRI) radiomics and Albumin-Bilirubin (ALBI) score to predict therapeutic response in unresectable hepatocellular carcinoma (HCC) patients treated with hepatic arterial infusion chemotherapy (HAIC).
Methods
The retrospective study was conducted on 112 unresectable HCC patients who underwent pretherapeutic MRI examinations. Patients were randomly divided into training (n = 79) and validation cohorts (n = 33). A total of 396 radiomics features were extracted from the volume of interest of the primary lesion by the Artificial Kit software. The least absolute shrinkage and selection operator (LASSO) regression was applied to identify optimal radiomic features. After feature selection, three models, including the clinical, radiomics, and combined models, were developed to predict the non-response of unresectable HCC to HAIC treatment. The performance of these models was evaluated by the receiver operating characteristic curve. According to the most efficient model, a nomogram was established, and the performance of which was also assessed by calibration curve and decision curve analysis. Kaplan–Meier curve and log-rank test were performed to evaluate the Progression-free survival (PFS).
Results
Using the LASSO regression, we ultimately selected three radiomics features from T2-weighted images to construct the radiomics score (Radscore). Only the ALBI score was an independent factor associated with non-response in the clinical model (P = 0.033). The combined model, which included the ALBI score and Radscore, achieved better performance in the prediction of non-response, with an AUC of 0.79 (95% CI 0.68–0.90) and 0.75 (95% CI 0.58–0.92) in the training and validation cohorts, respectively. The nomogram based on the combined model also had good discrimination and calibration (P = 0.519 for the training cohort and P = 0.389 for the validation cohort). The Kaplan–Meier analysis also demonstrate that the high-score patients had significantly shorter PFS than the low-score patients (P = 0.031) in the combined model, with median PFS 6.0 vs 9.0 months.
Conclusion
The nomogram based on the combined model consisting of MRI radiomics and ALBI score could be used as a biomarker to predict the therapeutic response of unresectable HCC after HAIC.
Keywords: Hepatocellular carcinoma, Radiomics, Hepatic arterial infusion chemotherapy, Albumin-Bilirubin score, Nomogram, Therapeutic response
Introduction
Hepatocellular carcinoma (HCC), the fourth leading cause of cancer death, is a common malignant tumor with more than 840,000 new cases worldwide each year (Bray et al. 2018). A comparative study of over 8,000 HCC cases showed that fewer than 10% of patients met the criteria of hepatectomy (Roayaie et al. 2015). For unresectable HCC, transcatheter arterial chemoembolization (TACE) is a well-established treatment. It is generally believed to be an effective treatment for preventing tumor growth and improving prognosis in unresectable HCC patients (2018; Shim et al. 2012). However, in the last few years, hepatic arterial infusion chemotherapy (HAIC) has been demonstrated to have fewer side effects and thus was considered to be superior to TACE in the treatment of unresectable HCC (He et al. 2017; Kudo et al. 2014). In addition, some studies have shown that HAIC might improve the median overall survival (OS) and disease-free survival (DFS) compared to sorafenib. Therefore, HAIC has been widely recommended as the first-line therapy for unresectable HCC patients in western countries (Choi et al. 2018; Lyu et al. 2018). Additionally, in several retrospective studies, the HAIC responders had significantly higher survival rates than HAIC non-responders (Miyaki et al. 2012; Nagano et al. 2011). Therefore, it is necessary to evaluate response to HAIC treatment as early as possible. According to Response Evaluation Criteria in Solid Tumors (RECIST), the conventional methods of assessing treatment response are imaging studies, including contrast-enhanced CT and MRI (Eisenhauer et al. 2009). These imaging studies are performed 1 month after each serial HAIC treatment. This means that the evaluation of therapeutic response can not be confirmed until at least the first course is completed. Therefore, to improve the prognosis and avoid hepatic function impairment in patients with HCC, predicting therapeutic response before deciding on HAIC protocol is of vital importance.
Radiomics has emerged as a new field in tumor treatment research in recent years (Gillies et al. 2016). It can transform massive image features into high-dimensional data that can be combined with clinical data, genetic information, and so on, to develop and validate various models using machine learning algorithms or artificial intelligence to help in medical decision-making (Aerts et al. 2014; Lambin et al. 2012; Mao et al. 2019a, b). In studies of HCC, radiomics has been used to forecast patients' prognoses and help make treatment choices (Li et al. 2016; Liu et al. 2021b; Simpson et al. 2015). In addition, the Albumin-Bilirubin (ALBI) score has been validated as an effective and objective marker of liver reserve function and a prognostic indicator after interventional therapy in middle and advanced-stage HCC patients (Chen et al. 2022; Ni et al. 2020). Thus, we hypothesized that an MRI-based radiomics score and ALBI score might be used to predict therapeutic response prior to HAIC treatment.
To our knowledge, the value of an MRI-based radiomics combined with ALBI scores in predicting the HCC’s therapeutic response after HAIC has not yet been investigated. Thus, the purpose of the current study was to construct and validate a novel nomogram based on a pretherapeutic MRI-based radiomics score combined with an ALBI score to predict the therapeutic response of unresectable HCC after HAIC.
Materials and methods
Patients
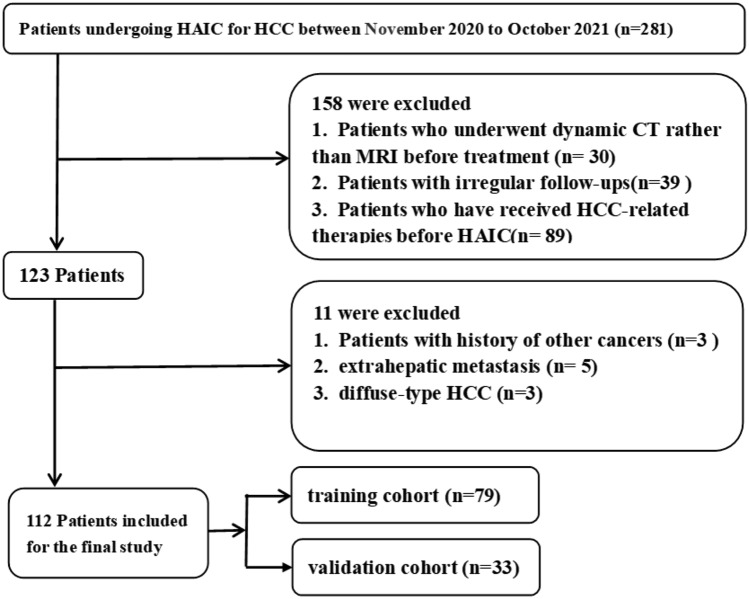
This single-center retrospective study was conducted in accordance with the Declaration of Helsinki and was approved by the research ethics committee of the Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, and the requirement for written informed consent was waived. According to the American Association for the Study of Liver Disease (AASLD) practice guidelines (Heimbach et al. 2018), 112 HCC patients were included in our study. The study population was screened from 281 HCC patients who received HAIC treatment in our hospital between November 2020 and October 2021, and their characteristics are shown in Table 1. The inclusion criteria were as follows: (1) diagnosed with unresectable HCC by our hospital's multidisciplinary team; (2) HCC cases with Barcelona Clinic Liver Cancer (BCLC) stage B or C; (3) received HAIC at our hospital; (4) performance status (PS) score < 2; and (5) Child–Pugh stage: A or B (7–8 points). Exclusion criteria were as follows: (1) HCC diagnosed with CT rather than MRI; (2) lost to follow-up or irregular follow-ups, not enough clinical data for assessing therapeutic response; (3) received HCC-related therapies before HAIC; (4) history of other cancers; (5) extrahepatic metastasis; and (6) diffuse-type HCC.
Table 1.
Patient characteristics in the training and validation cohorts
| Characteristics | Training cohort (n = 79) | Validation cohort (n = 33) | ||||
|---|---|---|---|---|---|---|
| PR (n = 27) | PD + SD (n = 52) | p | PR (n = 11) | PD + SD (n = 22) | p | |
| Gender | 0.355 | 0.632 | ||||
| Male | 23 (85.2%) | 49 (94.2%) | 8 (72.7%) | 19 (86.4%) | ||
| Female | 4 (14.8%) | 3 (5.8%) | 3 (27.3%) | 3 (13.6%) | ||
| Age (y) | 51.7 ± 13 | 50.8 ± 12.9 | 0.761 | 54.7 ± 8.2 | 53.9 ± 10.4 | 0.810 |
| Maximum tumor size (mm) | 1.000 | 0.701 | ||||
| > 100 | 18 (66.7%) | 34 (65.4%) | 8 (72.7%) | 13 (59.1%) | ||
| ≤ 100 | 9 (33.3%) | 18 (34.6%) | 3 (27.3%) | 9 (40.9%) | ||
| BCLC stage | 0.456 | 1.000 | ||||
| B | 1 (3.7%) | 6 (11.5%) | 1 (9.1%) | 3 (13.6%) | ||
| C | 26 (96.3%) | 46 (88.5%) | 10 (90.9%) | 19 (86.4%) | ||
| Tumor number (N) | 0.318 | 1.000 | ||||
| 1 | 11 (40.7%) | 14 (26.9%) | 4 (36.4%) | 9 (40.9%) | ||
| > 1 | 16 (59.3%) | 38 (73.1%) | 7 (63.6%) | 13 (59.1%) | ||
| PVTT type | 0.456 | 1.000 | ||||
| None | 1 (3.7%) | 6 (11.5%) | 2 (18.2%) | 3 (13.6%) | ||
| I | 6 (22.2%) | 14 (26.9%) | 3 (27.3%) | 1 (4.5%) | ||
| II | 11 (40.7%) | 16 (30.8%) | 5 (45.5%) | 11 (50.0%) | ||
| III | 8 (29.6%) | 15 (28.8%) | 1 (9.1%) | 6 (27.3%) | ||
| IV | 1 (3.7%) | 1 (1.9%) | 0 (0.0%) | 1 (4.5%) | ||
| Cause of disease | 0.348 | 1.000 | ||||
| HBV | 27 (100%) | 48 (92.3%) | 10 (90.9%) | 20 (90.9%) | ||
| Othersa | 0 (0%) | 4 (7.7%) | 1 (9.1%) | 2 (9.1%) | ||
| Child–Pugh class | 1.000 | 0.150 | ||||
| A | 23 (85.2%) | 45 (86.5%) | 11 (100.0%) | 16 (72.7%) | ||
| B | 4 (14.8%) | 7 (13.5%) | 0 (0.0%) | 6 (27.3%) | ||
| DCP (mAU/ml) | 1.000 | 0.678 | ||||
| ≤ 1000 | 4 (14.8%) | 7 (13.5%) | 4 (36.4%) | 5 (22.7%) | ||
| > 1000 | 23 (85.2%) | 45 (86.5%) | 7 (63.6%) | 17 (77.3%) | ||
| ALT (U/L) | 0.151 | 1.000 | ||||
| ≤ 40 | 8 (29.6%) | 7 (13.5%) | 2 (18.2%) | 5 (22.7%) | ||
| > 40 | 19 (70.4%) | 45 (86.5%) | 9 (81.8%) | 17 (77.3%) | ||
| AST (U/L) | 1.000 | 1.000 | ||||
| ≤ 40 | 2 (7.4%) | 4 (7.7%) | 1 (9.1%) | 2 (9.1%) | ||
| > 40 | 25 (92.6%) | 48 (92.3%) | 10 (90.9%) | 20 (90.9%) | ||
| ALB (g/L) | 0.245 | 0.314 | ||||
| ≤ 35 | 3 (11.1%) | 13 (25.0%) | 1 (9.1%) | 7 (31.8%) | ||
| > 35 | 24 (88.9%) | 39 (75.0%) | 10 (90.9%) | 15 (68.2%) | ||
| TBIL (μmol/L) | 0.915 | 0.900 | ||||
| ≤ 20 | 12 (44.4%) | 21 (40.4%) | 7 (63.6%) | 12 (54.5%) | ||
| > 20 | 15 (55.6%) | 31 (59.6%) | 4 (36.4%) | 10 (45.5%) | ||
| PT (s) | 0.591 | 0.105 | ||||
| ≤ 13 | 10 (37.0%) | 24 (46.2%) | 9 (81.8%) | 10 (45.5%) | ||
| > 13 | 17 (63.0%) | 28 (53.8%) | 2 (18.2%) | 12 (54.5%) | ||
| AFP (ng/ml) | 0.667 | 0.850 | ||||
| ≤ 20 | 2 (7.4%) | 7 (13.5%) | 2 (18.2%) | 2 (9.1%) | ||
| 20–200 | 3 (11.1%) | 9 (17.3%) | 2 (18.2%) | 3 (13.6%) | ||
| ≥ 200 | 22 (81.5%) | 36 (69.2%) | 7 (63.6%) | 17 (77.3%) | ||
| ALBI | − 2.5 ± 0.5 | − 2.3 ± 0.4 | 0.025 | − 2.5 ± 0.4 | − 2.3 ± 0.4 | 0.082 |
DCP des-γ-carboxy prothrombin, ALT alanine aminotransferase, AST aspartate aminotransferase, ALB albumin, TBIL total bilirubin, PT prothrombin time, AFP Alpha fetoprotein level, PVTT portal vein tumor thrombus, ALBI Albumin-Bilirubin score
aIncluding 4 case of HCV and 3 cases with unknown causes
Finally, the patients were randomly divided into training and validation cohorts at a ratio of 7:3. A flow chart of patient selection is presented in Fig. 1.
Fig. 1.
Flow chart for screening HCC patients treated with HAIC in our hospital
HAIC therapy procedure
All routine laboratory blood data were obtained within 3 days before HAIC. A femoral arterial puncture was performed in every cycle of treatment. A microcatheter was inserted into the feeding arteries of the HCC. The FOLFOX4 regimen was used via the hepatic artery: leucovorin 400 mg/m2, oxaliplatin 85 mg/m2, and fluorouracil 400 mg/m2 on the first day, and fluorouracil 2400 mg/m2 for 46 h. Repeat HAIC treatments were performed after every 3 weeks. Then, the therapeutic effect was assessed by imaging studies after every therapy cycle.
HAIC treatment could be combined with targeted therapy and immunotherapy.
Targeted therapies included Lenvatinib or Bevacizumab, and immunotherapies included Nivolumab or Atezolizumab.
Assessment of response to HAIC and follow-up
The response to the course of HAIC treatment was evaluated by the RECIST criteria. The best imaging response (Miyaki et al. 2015) was assessed by experienced radiologists, who were uninformed about the treatment, using either a contrast-enhanced CT or MRI. All best imaging responses were assessed at least 3 weeks after the first HAIC treatment. Objective response rate (ORR) is defined as the rate of complete response (CR) plus partial response (PR). Disease control rate (DCR) is defined as the CR rate plus PR rate plus stable disease (SD) rate. PFS is defined from the day of initial HAIC treatment to the date of disease progression, death or the end day of the follow-up. In this study, we compared the HAIC treatment response between responders (CR + PR) and non-responders (PD + SD).
The follow-up ended in August 2022. Liver MR or CT imaging was performed every 3–4 weeks. Laboratory data, including the serum alpha-fetoprotein (AFP) level and liver function test results, were collected during each HAIC treatment cycle. The next treatment was determined by our hospital's multidisciplinary team (MDT). The ALBI score was calculated by the formula: ALBI score = (lg bilirubin [μmol/L] × 0.66) − (albumin [g/L] × 0.085).
MR imaging acquisitions
The preoperative MRI examination was performed with 3.0 T MRI scanner (Achieva; Philips Medical Systems, Best, the Netherlands) with 16 channels phased array coil serving as the receiver coil. The MRI protocol included: (1) a respiration-triggered turbo spin-echo (TSE) T2-w fat-suppressed axial imaging; TR = 3000 ms, TE = 70 ms, slice thickness = 5 mm, intersection gap = 1.1 mm, matrix = 320 × 280, and field of view (FOV) = 36 × 36 cm; (2) plain and contrast-enhanced T1-w imaging; T1-w 3D sequence (T1 high-resolution isotropic volume examination, THRIVE) was performed following the intravenous injection of Magnevist (Bayer Healthcare, Germany, 0.1 mmol/kg); TR = 4.1 ms, TE = 1.4 ms, slice thickness = 1 mm, matrix = 252 × 198, and FOV = 36 × 36 cm; 15 s acquisition time for one phase. In this study, only T2-w images were selected for radiomics analysis.
Texture features extraction
Axial T2-w images (T2WI) were transferred into an open-source ITK-SNAP software (version 3.6.0, www. itksnap.org) for manual segmentation; the region of interest (ROI) on the MR images cannot be obtained automatically. As a result, an experienced radiologist (with more than 10 years of experience in abdominal radiology), blinded to the patients’ clinical status, reviewed the aforementioned MR images and manually traced the border of the lesions slice by slice to acquire a 2D ROI. Subsequently, the three-dimensional volume of interest (3D VOI) was constructed automatically based on the 2D ROIs of the intrahepatic lesion. Subsequently, the radiomics features from each VOI were extracted using in-house software (Artificial Intelligence Kit, A.K, GE Healthcare). A total of 396 radiomics features were extracted from MRI images, including (1) histogram features, such as entropy, energy, kurtosis, uniformity, and skewness; (2) form factor features, including compactness, surface area, sphericity, maximum 3D diameter, surface volume ratio, volume CC, spherical disproportion, and volume MM; (3) texture features, such as gray-level size zone matrix (GLZSM), gray-level co-occurrence matrix (GLCM), gray-level run-length matrix (GLRLM), and Haralick parameters.
Feature selection and construction of RadScore
For the training cohort, mRMR (Max-Relevance and Min-Redundancy) was performed to eliminate the redundant and irrelevant features, and finally, 30 features were retained. Then LASSO (least absolute shrinkage and selection operator) regression was used to choose the optimized subset of features to construct the radiomics model (Gui and Li 2005; Huang et al. 2016). The Radscore was calculated by weighting the respective coefficients of linear combinations of the optimal radiological features selected from the training cohort to predict the non-response (PD + SD) with HAIC treatment for each patient. The area under the receiver–operator characteristic (ROC) curve (AUC) was used to assess the performance of the Radscore in both the training and validation cohort.
Prognostic model construction
We used univariate analysis and multivariate regression to select optimal clinical factors. Then these significant factors were used to construct the clinical model by logistic regression. Lastly, these clinical independent risk factors and Radscore were utilized to build a combined model in the training cohort. The discriminative power of the model was evaluated by the area under the ROC curve with 95% confidence intervals. In addition, the accuracy, sensitivity, specificity, positive-predictive value (PPV), and negative-predictive value (NPV) for the clinical model, radiomics score, and combined model, were calculated in both cohorts. Then, a nomogram was established based on the most efficient model. Furthermore, the prediction accuracy of this nomogram model was explored using the calibration curve. And the decision curve analysis (DCA) was applied to assess the actual usefulness of the nomogram.
Figure 2 shows the radiomics workflow of this study.
Fig. 2.
Workflow of the study: A Tumor segmentation on MR images. Radiomic feature extraction from VOI. B The LASSO regression was applied to identify optimal radiomic features and construct the Radscore. C The nomogram model was established based on the most efficient model. ROC and calibration curves were constructed to assess the model performance
Statistical analysis
The R3.5.1 software was used for statistical analyses. P < 0.05 was regarded as statistically significant. The Mann–Whitney U-test or independent-sample t test were used to compare the continuous variables. The most discriminating radiomics features were selected by LASSO regression. A radiomics signature was constructed via tenfold cross-validation based on the minimum criteria. The significant clinical factors were used to construct the clinical model via multivariate logistic regression analysis. The “rms” package program was applied to construct a nomogram and execute calibration curves. The calibration curve, ROC curve, and DCA were employed to assess the efficiency of the nomogram. Potential correlation of the combined model with PFS was evaluated using Kaplan–Meier analysis and log-rank test.
Results
A total of 112 cases with confirmed unresectable HCC [median age, 53 years; interquartile range (IQR), 43–60 years; 13 women and 99 men] were included in our study, including 79 cases [median age, 52 years; interquartile range (IQR), 41–60 years; 7 women and 72 men] in the training and 33 cases [median age, 55 years; interquartile range (IQR), 47–60 years; 6 women and 27 men] in the validation cohort. The number of patients who presented with CR, PR, SD, and PD was 0 (0%), 38 (33.9%), 35 (31.2%), and 39 (34.8%), respectively. The ORR was 33.9%, and DCR was 65.2%. A total of 21 (18.7%) patients died before the end day of the follow-up. The differences in clinical characteristics between the PR and PD + SD groups in both cohorts are shown in Table 1. Of note, the number for targeted therapy and immunotherapy combined with HAIC was shown in Table 2. The proportion of combination therapies after HAIC in two cohorts showed no significant difference (P = 0.158). Hence, the influencing factor of treatment on therapeutic response was excluded, which make the statistical analysis in this study more reliable.
Table 2.
Combined treatment with HAIC
| Training cohort (n = 79) | Validation cohort (n = 33) | p value | |
|---|---|---|---|
| HAIC alone | 11 | 9 | 0.158 |
| HAIC + targeted therapy | 21 | 10 | |
| HAIC + targeted therapy + immunotherapy | 47 | 14 |
Feature selection and rad-score establishment
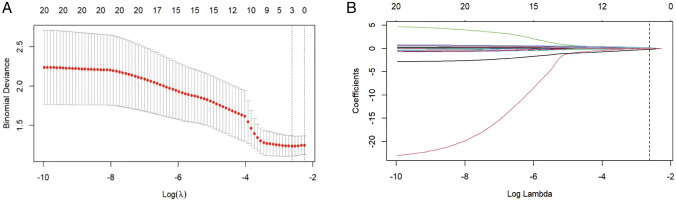
For the 396 MRI radiomics features, the LASSO regression selected three significant radiomics features, namely, wavelet_LLH_glcm_Correlation,wavelet_HHL_glcm_Correlation, and log_sigma_2_0_mm_3D_glszm_ZoneEntropywere to construct radiomics model from the training cohort (Fig. 3). These features were used to calculate the Radscore using the following formula: Radscore = 0.637 × (Intercept) + 0.045 × wavelet_LLH_glcm_Correlation − 0.072 × wavelet_HHL_glcm_Correlation − 0.144 × log_sigma_2_0_mm_3D_glszm_ZoneEntropy.
Fig. 3.
LASSO algorithm was used to choose optimal radiomic features to construct Radscore model: A mean square error path using tenfold cross-validation; B LASSO coefficient profiles of the radiomics features
The radiomics model based on the Radscore yielded an AUC of 0.70 (95% CI 0.58–0.82) in the training cohort and 0.69 (95% CI 0.51–0.88) in the validation cohort in predicting the SD + PD responses.
Independent indicators of therapeutic response
Univariate analysis and multivariate logistic regression analysis showed that among all clinical features, only the ALBI score was an independent factor for therapeutic response (P = 0.033). The hazard ratio (HR) for the ALBI score was 3.69 (95% CI 1.10–12.30). Then, the clinical model was constructed based on the ALBI score to predict SD + PD responses, with an AUC of 0.68 (95% CI 0.55–0.81) in the training cohort and 0.71 (95% CI 0.51–0.91) in the validation cohort.
After combining the Radscore with the above clinic feature, the ALBI score and the Radscore were independent indicators of therapeutic response (P = 0.024 and 0.004). The HR for the ALBI score and the Radscore were 2.14 (95% CI 1.10–4.15) and 124.45 (95% CI 5.86–1347), respectively (Table 3).
Table 3.
Logistic analysis of Radscore and clinical features for evaluation of HAIC response in training cohort
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| P | HR (95% CI) | P | HR (95% CI) | |
| Gender (male/female) | 0.194 | 2.840 (0.586–13.746) | ||
| Age (y) (< 60/ ≥ 60) | 0.758 | 0.994 (0.958–1.031) | ||
| Maximum tumor size (> 100 mm/ ≤ 100 mm) | 0.909 | 0.944 (0.353–2.524) | ||
| BCLC stage (B/C) | 0.270 | 0.294 (0.033–2.584) | ||
| Tumor number (N) (1/ > 1) | 0.213 | 1.866 (0.698–4.983) | ||
| PVTT (none/I/II/III/IV) | 0.270 | 0.294 (0.033–2.584) | ||
| HBV/others | 0.989 | 0.775 (0.480–1.252) | ||
| Child–Pugh class (A/ B) | 0.869 | 0.894 (0.237–3.372) | ||
| DCP (≤ 1000/> 1000) | 0.264 | 0.647 (0.301–1.388) | ||
| ALT (≤ 40/> 40 U/L) | 0.088 | 2.706 (0.859–8.526) | ||
| AST (≤ 40/> 40 U/L) | 0.963 | 0.960 (0.164–5.607) | ||
| ALB (≤ 35 /> 35 g/L) | 0.155 | 2.666 (0.688–10.332)) | ||
| TBIL (≤ 20 /> 20 μmol/L) | 0.728 | 1.180 (0.461–3.022) | ||
| PT (≤ 13/> 13 s) | 0.438 | 0.686 (0.264–1.779) | ||
| AFP (≤ 20/20–200/ ≥ 200) | 0.264 | 0.647 (0.301–1.388) | ||
| ALBI score | 0.033 | 3.686 (1.104–12.300) | 0.024 | 2.140 (1.10–4.15) |
| Radscore | 0.005 | 130.95 (4.25–3644.14) | 0.004 | 124.45 (5.86–1347) |
The nomogram establishment
In the training cohort, the AUC of the combined model, the Radscore model, and the clinical model were 0.79, 0.70, and 0.68, respectively, while in the validation cohort, the AUC of these three models were 0.75, 0.69, and 0.71, respectively (Fig. 4). There were significant differences between the ROC of the combined model and the clinical model (Z = − 1.935, P = 0.046), but no significant differences were found between the ROC of the combined model and the Radscore model (Z = − 1.894, P = 0.058) using DeLong’s test. Furthermore, the combined model had the greatest accuracy (accuracy: 81.0%; specificity: 77.7%; sensitivity: 82.6%; NPV: 70.0%; PPV:87.7%) in predicting SD + PD responses (Table 4). Therefore, the nomogram was established based on the combined model (Fig. 5A).
Fig. 4.
Receiver operating characteristic (ROC) curve for predicting the SD + PD response in the training cohort (A) and validation cohort (B)
Table 4.
Accuracy and predictive value of three models
| Accuracy | 95% CI | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|
| Training cohort | ||||||
| Clinical model | 0.696 | 0.582–0.794 | 0.730 | 0.629 | 0.791 | 0.548 |
| Radiomic model | 0.746 | 0.636–0.837 | 0.942 | 0.370 | 0.742 | 0.769 |
| Combined model | 0.810 | 0.706–0.889 | 0.826 | 0.777 | 0.877 | 0.700 |
| Validation cohort | ||||||
| Clinical model | 0.636 | 0.451–0.795 | 0.590 | 0.727 | 0.812 | 0.470 |
| Radiomic model | 0.636 | 0.451–0.795 | 0.727 | 0.454 | 0.727 | 0.454 |
| Combined model | 0.696 | 0.512–0.844 | 0.727 | 0.636 | 0.800 | 0.538 |
CI, confifidence interval; NPV, negative-predictive value; PPV, positive-predictive value
Fig. 5.
A The nomogram model based on Radscore and ALBI score for predicting SD + PD response of HCC to HAIC. B calibration curves of the nomogram model in the training cohort. C Calibration curves of the nomogram model in the validation cohort. The X-axis represents the predicted probability, and the Y-axis is the actual probability for the SD + PD response of HCC. Calibration curves indicate that there was a good agreement between the predicted probability and the actual state of treatment response
The calibration curve of the nomogram’s probability in predicting SD + PD responses had a good agreement between the observed and predicted results in the training cohort and validation cohorts (Fig. 5B,C). The Hosmer–Lemeshow test showed no significant difference between ideal curves and calibration curves in the training (P = 0.519) and validation cohorts (P = 0.389), which represented a good fitting of the model.
The clinical utility of these three models was evaluated via DCA (Fig. 6). The nomogram showed a larger net benefit than did the radiomics and clinical models, which demonstrated the nomogram represented the best clinical utility for the prediction of therapeutic response of HCC after HAIC.
Fig. 6.
DCA curve for the nomogram model in the validation cohort. Compared to other models, the nomogram model demonstrated the highest area under the curve, and is the optimal decision that achieved the maximal net benefit in predicting SD + PD response of HCC after HAIC
Kaplan–Meier analysis for PFS
The Kaplan–Meier analysis was performed to assess the risk factor to PFS based on the aforementioned combined model in whole cohort. Using a cutoff value of 0.587 by ROC curve, patients were stratified into the low-score and high-score groups (low-score < 0.587 predicted HAIC-response, whereas high-score > 0.587 predicted HAIC-nonresponse). The high-score patients had significantly shorter PFS than the low-score patients (P = 0.031), with median PFS 6.0 vs 9.0 months (Fig. 7).
Fig. 7.
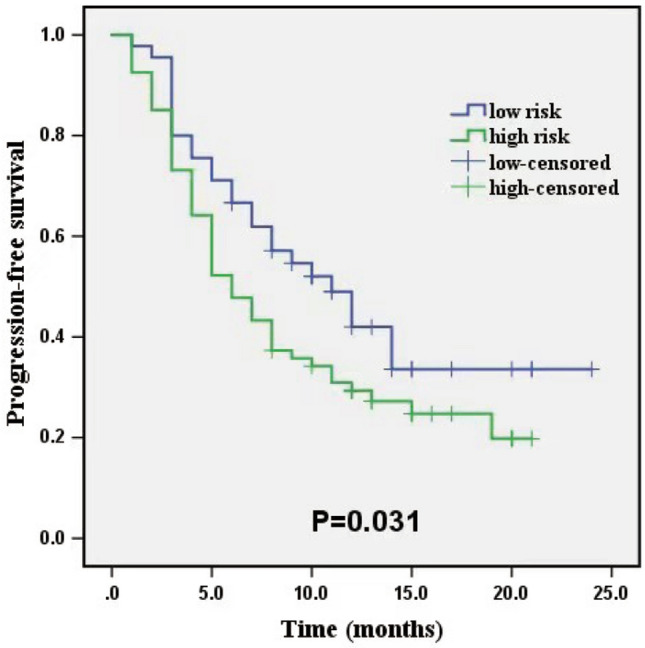
Kaplan–Meier curve of the low-risk and high-risk to nonresponse stratified by the combined model in the whole cohort
Discussion
The current study evaluated the potential value of MRI-based radiomics combined with ALBI score in the prediction of the therapeutic response to HAIC in patients with unresectable HCC. We proved that intratumoral radiomics from pretherapeutic MRI imaging could improve the performance of clinical features in the prediction of therapeutic response. The combined model achieved an optimal predictive efficiency in patients with unresectable HCC. A nomogram combining radiomics features and ALBI score could efficiently separate those with SD + PD response from those with PR response in both training and validation cohorts. Meanwhile, the survival analysis also demonstrate that the patients with high-risk to non-response had significantly shorter PFS than the low-risk patients in the combined model. To the best of our knowledge, among all the related radiomics studies for HCC, our study was the first to establish a nomogram from the combined model to predict therapeutic response to HAIC in patients with HCC.
HAIC has been regarded as an effective treatment for advanced HCC in Asia. In several studies, HAIC responders have been proven to have longer survival rates than HAIC non-responders; thus, performing HAIC before administering molecular targeted therapy is recommended. Clinical features and tumor markers were used to predict the therapeutic response to HAIC in most of the previous studies; one of which reported that the early change of tumor markers and the imaging assessment 2 weeks after HAIC treatment were useful in predicting therapeutic response and prognosis (Miyaki et al. 2015). Yamamoto et al. (2020) suggested that the combination of optimal cutoff values for the AFP ratio and the DCP ratio after the initiation of HAIC enabled the prediction of non-responders and improved prognosis in patients with advanced HCC. Compared with these biomarkers, radiomics may serve as a relatively inexpensive and non-invasive biomarker as it has the potential to provide a more specific tumor characterization and guide clinicians in individualized treatment planning before therapy.
In our study, a nomogram combined with MRI radiomics and ALBI score showed remarkable differences between PR and SD + PD groups. Furthermore, radiomics score was identified by the univariate and multivariate logistic regression models as an independent predictor of non-response. In a previous study, VWF:Ag/ADAMTS13:AC ratio was used as a biomarker of treatment response in HCC patients before the initiation of HAIC treatment, and ROC curves were used to evaluate predictive efficiency, yielding an AUC of 0.715 (Takaya et al. 2020). However, our study showed that the radiomics-based nomogram in the training group had a higher AUC (AUC of 0.79). Therefore, we believe that, compared with clinical features, radiomics score may be a more powerful predictor. The nomogram based on a combined model in our study can serve as an adaptive strategy to improve the prognosis of HCC patients.
Currently, clinical MR scans are routinely acquired for diagnosis and staging in HCC. These MRI images contain important information about tumor heterogeneity, including biological characteristics determined by underlying micro-level components of the tumor tissue (Gillies et al. 2016; Rogers et al. 2020). Based on previous research, the calculation of radiomics features from T2WI imaging may be more accurate than from T1WI and enhanced T1WI, and can be further utilized as a reliable indicator for objective and high throughput image analysis (Liu et al. 2021a, b). Therefore, our study analyzed radiomics features from T2W images to predict the therapeutic response after HAIC.
In previous studies of HCC, radiomics obtained from pretreatment CT was thought to be a good predictor of the HCC treatment response after TACE, which could prevent unnecessary treatment (Park et al. 2017). Another study suggested that MRI-based radiomics may predict the prognosis of HCC treated with TACE combined with microwave ablation (Liu et al. 2021b). CT imaging radiomics was also useful for stratifying patients with HCC to determine appropriate treatment options between liver resection and TACE (Li et al. 2016). All relevant studies showed that radiomics has additional value to clinical features in predicting therapeutic response and prognosis of HCC. The results of our study indicated that the Radscore was an independent risk factor obviously related to the non-response of HCC to HAIC. Moreover, the HR of the Radscore was considerably higher than that of clinical features, which suggests that Radscore may be more helpful than the clinical characteristics in predicting the therapeutic response of unresectable HCC after HAIC.
In recent years, The ALBI score, a scoring system based solely on serum levels of albumin and total bilirubin, has been proposed as a simple and objective model to assess the liver reserve function in patients with HCC (Johnson et al. 2015). The elevation of serum bilirubin and reduction of albumin level usually indicates liver injury. Meanwhile, the reduction of albumin level is also associated with worse nutritional status of the body, leading to a decrease in immune function and, subsequently, tumor recurrence. HCC patients with higher ALBI scores have poorer liver reserve function, lower grade tumor biological behavior, worse systemic condition, and thus a worse prognosis. Our study has found that the higher ALBI score was an independent risk factor for non-response after the HAIC. The calibration curve of our nomogram based on the combined model (ALBI score and Radscore) verified that the predictive probability of the model fitted well with the actual therapeutic response, proving that the model has high accuracy. Therefore, the nomogram in this study can guide the prediction of the treatment response in HCC patients after HAIC.
This study has several limitations. First, selection bias might exist because of the retrospective design in our study. Second, although our cohort remains one of the largest in terms of radiomics features and interventional treatment of HCC, the patients were enrolled from a single institution with limited sample size, so the conclusions need to be verified by further prospective studies. Third, our study conducted image segmentation manually; an automated method for image segmentation may provide greater stability (de Hoop et al. 2009). Fourth, feature extraction was based on a single T2WI image for analysis in this study, but in practice, it is convenient for the clinician to extract radiomics features for analysis.
Conclusion
In conclusion, the nomogram based on the combined model that incorporated MRI-based radiomics features and ALBI score exhibited favorable performance in predicting the response of unresectable HCC to HAIC. The pretherapeutic MRI-based radiomics score could be used as a non-invasive biomarker to help clinicians make reasonable clinical decisions, thereby avoiding the overtreatment of patients with HCC.
Acknowledgements
We thank Yigang Pei for assistance in the study.
Author contributions
JL: conceptualization, methodology; HL: data analysis; YZ: writing original draft; SL:visualization, investigation; LJ: supervision; FH: validation; XX:writing—reviewing.
Funding
This work has been supported by Scientific Research Project of Hunan Provincial Health Commission (Grant Number: 202109011033, Changsha, China), the Natural Science Foundation of Hunan Province (Grant Number: 2019JJ80099, Changsha, China), Hunan Cancer Hospital Climb Plan (Grant Number: ZX2020002, Changsha, China).
Data availability
The data used to support the fndings of this study are available from the corresponding author upon request.
Declarations
Conflict of interest
There was no any interest conflict in this paper for all authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yang Zhao and Fang Huang contributed equally to this work.
References
- Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 68(6):394–424 [DOI] [PubMed] [Google Scholar]
- Chen HY, Kee KM, Lu SN et al (2022) Incorporating albumin-bilirubin grade and up-to-seven criteria to predict outcomes of patients with intermediate stage hepatocellular carcinoma after transarterial (chemo)embolization. J Formosan Med Assoc Taiwan Yi Zhi 121(4):778–786 [DOI] [PubMed] [Google Scholar]
- Choi JH, Chung WJ, Bae SH et al (2018) Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother Pharmacol 82(3):469–478 [DOI] [PubMed] [Google Scholar]
- de Hoop B, Gietema H, van Ginneken B et al (2009) A comparison of six software packages for evaluation of solid lung nodules using semi-automated volumetry: what is the minimum increase in size to detect growth in repeated CT examinations. Eur Radiol 19(4):800–808 [DOI] [PubMed] [Google Scholar]
- Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 11). Eur J Cancer (oxford, England: 1990) 45(2):228–247 [DOI] [PubMed] [Google Scholar]
- European Association For The Study Of The Liver (2018) EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol 69(1):182–236 [DOI] [PubMed] [Google Scholar]
- Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278(2):563–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui J, Li H (2005) Penalized Cox regression analysis in the high-dimensional and low-sample size settings, with applications to microarray gene expression data. Bioinformatics (oxford, England) 21(13):3001–3008 [DOI] [PubMed] [Google Scholar]
- He MK, Le Y, Li QJ et al (2017) Hepatic artery infusion chemotherapy using mFOLFOX versus transarterial chemoembolization for massive unresectable hepatocellular carcinoma: a prospective non-randomized study. Chin J Cancer 36(1):83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach JK, Kulik LM, Finn RS et al (2018) AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (baltimore, MD) 67(1):358–380 [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu Z, He L et al (2016) Radiomics signature: a potential biomarker for the prediction of disease-free survival in early-stage (I or II) non-small cell lung cancer. Radiology 281(3):947–957 [DOI] [PubMed] [Google Scholar]
- Johnson PJ, Berhane S, Kagebayashi C et al (2015) Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol 33(6):550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M, Matsui O, Izumi N et al (2014) JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the liver cancer study group of Japan. Liver Cancer 3(3–4):458–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer (oxford, England: 1990) 48(4):441–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Fu S, Zhu Y et al (2016) Computed tomography texture analysis to facilitate therapeutic decision making in hepatocellular carcinoma. Oncotarget 7(11):13248–13259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhang X, Zheng T et al (2021a) Optimisation and evaluation of the random forest model in the efficacy prediction of chemoradiotherapy for advanced cervical cancer based on radiomics signature from high-resolution T2 weighted images. Arch Gynecol Obstet 303(3):811–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Pei Y, Zhang Y et al (2021b) Predicting the prognosis of hepatocellular carcinoma with the treatment of transcatheter arterial chemoembolization combined with microwave ablation using pretreatment MR imaging texture features. Abdom Radiol (new York) 46(8):3748–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu N, Kong Y, Mu L et al (2018) Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 69(1):60–69 [DOI] [PubMed] [Google Scholar]
- Mao N, Wang Q, Liu M et al (2019a) Computerized image analysis to differentiate benign and malignant breast tumors on magnetic resonance diffusion weighted image: a preliminary study. J Comput Assist Tomogr 43(1):93–97 [DOI] [PubMed] [Google Scholar]
- Mao N, Yin P, Wang Q et al (2019b) Added value of radiomics on mammography for breast cancer diagnosis: a feasibility study. JACR 16:485–491 [DOI] [PubMed] [Google Scholar]
- Miyaki D, Aikata H, Honda Y et al (2012) Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma according to Child-Pugh classification. J Gastroenterol Hepatol 27(12):1850–1857 [DOI] [PubMed] [Google Scholar]
- Miyaki D, Kawaoka T, Aikata H et al (2015) Evaluation of early response to hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma using the combination of response evaluation criteria in solid tumors and tumor markers. J Gastroenterol Hepatol 30(4):726–732 [DOI] [PubMed] [Google Scholar]
- Nagano H, Wada H, Kobayashi S et al (2011) Long-term outcome of combined interferon-α and 5-fluorouracil treatment for advanced hepatocellular carcinoma with major portal vein thrombosis. Oncology 80:63–69 [DOI] [PubMed] [Google Scholar]
- Ni JY, Fang ZT, Sun HL et al (2020) A nomogram to predict survival of patients with intermediate-stage hepatocellular carcinoma after transarterial chemoembolization combined with microwave ablation. Eur Radiol 30(4):2377–2390 [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JH, Choi SY et al (2017) Prediction of therapeutic response of hepatocellular carcinoma to transcatheter arterial chemoembolization based on pretherapeutic dynamic CT and textural findings. Am J Roentgenol 209(4):W211–W220 [DOI] [PubMed] [Google Scholar]
- Roayaie S, Jibara G, Tabrizian P et al (2015) The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology (baltimore, MD) 62(2):440–451 [DOI] [PubMed] [Google Scholar]
- Rogers W, Thulasi Seetha S, Refaee TAG et al (2020) Radiomics: from qualitative to quantitative imaging. Br J Radiol 93(1108):20190948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JH, Lee HC, Kim SO et al (2012) Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology 262(2):708–718 [DOI] [PubMed] [Google Scholar]
- Simpson AL, Adams LB, Allen PJ et al (2015) Texture analysis of preoperative CT images for prediction of postoperative hepatic insufficiency: a preliminary study. J Am Coll Surg 220(3):339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya H, Namisaki T, Moriya K et al (2020) Association between ADAMTS13 activity-VWF antigen imbalance and the therapeutic effect of HAIC in patients with hepatocellular carcinoma. World J Gastroenterol 26(45):7232–7241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Onishi H, Takaki A et al (2020) The early decline of α-fetoprotein and Des-γ-carboxy prothrombin predicts the response of hepatic arterial infusion chemotherapy in hepatocellular carcinoma patients. Gastrointest Tumors 7(3):83–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the fndings of this study are available from the corresponding author upon request.