Abstract
The essential oil isolated by hydrodistillation of the oleogum resin of Araucaria heterophylla has been analyzed by GC–MS. Twenty-four components accounting to 99.89% of the total detected constituents of this essential oil were identified. The major ones were: caryophyllene oxide (14.8%), ( +)-sabinene (12.07%), D-limonene (11.22%), caryophyllene (10.36%), α-copaene (8.00%), β-pinene (6.44%), trans-verbenol (5.88%) and α-pinene oxide (5.18%). The in vitro inhibitory activities of this oil against aldose reductase, BuCHE, COX-2 and SARS-CoV-2 Mpro enzymes were evaluated. This revealed promising inhibitory activity of the essential oil against both aldose reductase and BuCHE enzymes. The molecular docking study of the major components of the Araucaria heterophylla essential oil was carried out to correlate their binding modes and affinities for aldose reductase and BuCHE enzymes with the in vitro results. In conclusion, the in vitro inhibitory activity of the essential oil attributed to the synergistic effect between its components and the in silico study suggested that compounds containing epoxide and hydroxyl groups may be responsible for this activity. This study is preliminary screening for the oil to be used as antidiabetic cataract and Alzheimer’s disease therapeutics and further investigations may be required.
Subject terms: Drug discovery, Plant sciences
Introduction
The genus Araucaria belongs to the family Araucariaceae which is famous for evergreen coniferous ornamental trees. It includes approximately 19 species with limited existence in the southern hemisphere. Several classes of phytoconstituents have been reported in different Araucaria species, mainly flavonoids, sesqui- and di-terpenes, and phenylpropanoids compounds. Since ancient times, Araucaria species were well known for their medicinal properties as anti-inflammatory, antipyretic, anti-ulcerative, antiviral, antimicrobial, neuro-protective, anti-coagulant, and anti-depressant1–5. Araucaria heterophylla, one of Araucaria species known as ‘Norfolk Island Pine’, was famous for columnar tree used as Christmas tree. Its aerial parts were used in folk medicine for a toothache. It was reported that the chloroform extract of its oleogum resin is rich in labdane diterpenes which exhibited significant antioxidant, antiulcer and anticancer activities. The essential oil (EO) was reported as one of the main constituents of Araucaria species. The major compound of the EO derived from the leaves of Australian A. heterophylla was α-pinene. Whereas the major compounds of the EO derived from the leaves of Indian A. heterophylla were found to be 13-epidolabradiene, beyerene, rimuene, and dolabradiene. While the most abundant compounds identified in the oleogum resin of the same plant were α-copaene, germacrene D, γ-gurjunene, and δ-cadinene4,6–8. Up to date, there is only one study of the composition of EO of the oleogum resin of A. heterophylla collected from Egyptian culture4.
Aldose reductase, a NADPH-dependent oxidoreductase enzyme, is included in reduction of glucose into sorbitol. Large quantities of sorbitol are produced because of the increased flux during hyperglycemia associated with diabetes. As a result of sorbitol deposition and redox imbalance after depletion of NADPH, osmotic stress is produced and causes organ injury and cell damage, which lead to neuropathy, cataract formation and other diabetic complications. Thus, targeting this enzyme is significant in the improvement of diabetes complications9–11.
Butyryl cholinesterase (BuChE) and acetyl cholinesterase (AChE) are hydrolysis enzymes for ACh and BuCh neurotransmitters, which have an important role in cognition and memory. These neurotransmitters are observed to be declined in Alzheimer’s disease (AD) patients. Thus, the inhibition of BuChE and AChE enzymes has been a potential treatment for AD12,13. Butyrylcholinesterase, which plays a recognized role in the Ach regulation, increases progressively in brain of AD patients while AChE concentration remains unchanged or decreases. Thus, (BuChE)-inhibition can better increase acetylcholine (ACh) levels in treating AD14,15.
Cyclooxygenase (COX-2), one of the cyclooxygenase enzymes (COX-1 & COX-2), is an inducible enzyme responsible for producing prostaglandins (PGs) from membrane and releasing arachidonic acid in inflammatory and tumorigenic settings. The dramatic increase of COX-2 expression in inflamed tissue leads to increased prostaglandins synthesis and subsequent development of the cardinal signs of acute inflammation. So, blocking COX-2 enzyme can relieve such signs16,17.
SARS-CoV-2 main protease (Mpro) or (3-chymotrypsin-like protease 3CLpro) is an enzyme responsible for the proteolysis and release of essential functioning peptides, playing a great role in replication, and thus the life cycle of the coronaviruses. It is considered one of decisive factors in the infectious route of the virus in addition to papain-like protease (PLpro), and RNA-dependent RNA polymerase (RdRp) of SARS-CoV-2 enzymes, which have been reported as important targets for therapeutic strategies18.
Nowadays, the use of plant-derived natural products with therapeutic significance attracts great attention due to their availability, affordability and relative safety. This motivates further search into traditional medicine to explore highly effective and safer natural inhibitors for some important enzymes involved in certain disorders and diseases.
In the framework of continuing research on Araucaria heterophylla tree, this study is concerned with analyzing the essential oil isolated from the oleogum resin exuded from its trunk. Moreover, discovering potential anti-diabetic cataract, anti-Alzheimer’s disease, anti-inflammatory and anti-COVID-19 leads based on the evaluation of the ability of the major components of this essential oil to inhibit certain enzymes as aldose reductase, BUCHE, COX-2 and SARS-CoV-2 Mpro using in vitro and in silico studies.
To the best of our knowledge, this study is the second one to study the composition of EO of the oleogum resin of A. heterophylla collected from Egyptian culture. Also, it is the first one to report the inhibitory activity of this essential oil against aldose reductase and BUCHE enzymes.
Results and discussion
Identification of the essential oil constituents
The analysis of the essential oil of Araucaria heterophylla and the identification of its components were carried out depending on their retention indices19, their mass spectral fragmentation patterns20,21 and/or stored data on the mass spectral database NIST/ ChemStation data system.
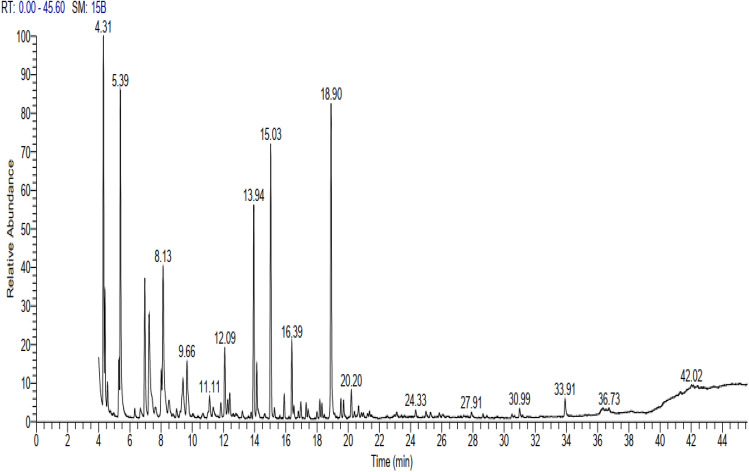
The analysis of the essential oil resulted in the identification of 24 major components representing 99.89% of the total oil composition (Table 1, Fig. 1). The non-oxygenated fraction of this oil was (59.23%). Monoterpenes hydrocarbons (32.93%) were the most abundant non-oxygenated components and ( +)-sabinene (12.07%) was the main constituent followed by D-limonene (11.22%), β-pinene (6.44%) in addition to other minors. Caryophyllene (10.36%) was the major sesquiterpene present followed by α-copaene (8.00%). The oxygenated fraction represented about 40.66% in a descending order by oxides (epoxides) and dioxides (22.27%), alcohols (15.50%) and ketones (2.89%). Caryophyllene oxide (14.82%) was the dominant oxide component followed by α-pinene oxide (5.18%). Trans-verbenol (5.88%) was the major alcohol, while levoverbenone was the major ketone present.
Table 1.
Chemical compositions of the essential oil of Araucaria heterophylla oleogum resin.
| No | Compound name | Rt | % Area | RI | M+ peak | M.F | Base peak |
|---|---|---|---|---|---|---|---|
| 1 | ( +)- Sabinene | 4.30 | 12.07 | 980 | 136.2 | C10H16 | 93.2 |
| 2 | β-pinene | 4.40 | 6.44 | 985 | 136.2 | C10H16 | 93.2 |
| 3 | β-Myrcene | 4.56 | 0.88 | 997 | 136.3 | C10H16 | 93.2 |
| 4 | O-Cymene | 5.29 | 1.70 | 1011 | 134.2 | C10H14 | 119.2 |
| 5 | D-limonene | 5.39 | 11.22 | 1023 | 136.2 | C10H16 | 68 |
| 6 | α-Pinene oxide | 6.95 | 5.18 | 1143 | 152.23 | C10H16O | 67.1 |
| 7 | Cis-verbenol | 7.23 | 4.39 | 1146 | 152.2 | C10H16O | 109.2 |
| 8 | Trans-pinocarveol | 8.00 | 1.23 | 1148 | 152.2 | C10H16O | 92.2 |
| 9 | Trans-Verbenol | 8.13 | 5.88 | 1151 | 152.2 | C10H16O | 109.2 |
| 10 | (−)-Myrtenol | 9.41 | 2.01 | 1188 | 152.23 | C10H16O | 79.2 |
| 11 | Levoverbenone | 9.66 | 2.89 | 1210 | 150.2 | C10H14O | 107.2 |
| 12 | Limonene dioxide | 11.11 | 1.52 | 1144 | 168.2 | C10H16O2 | 67.1 |
| 13 | 5-Tridecene, (E) | 11.83 | 0.62 | 1288 | 182.3 | C13H24 | 55.2 |
| 14 | Patchoulane | 12.40 | 0.89 | 1189 | 206.36 | C15H26 | 91.1 |
| 15 | α-Copaene | 13.94 | 8.00 | 1350 | 204.35 | C15H24 | 105.1 |
| 16 | β-bourbonene | 14.14 | 2.06 | 1380 | 204.35 | C15H24 | 81.2 |
| 17 | Caryophyllene | 15.03 | 10.36 | 1400 | 204.3 | C15H24 | 93.1 |
| 18 | Humulene | 15.90 | 0.91 | 1442 | 204.2 | C15H24 | 93.2 |
| 19 | γ-Muurolene | 16.39 | 3.49 | 1480 | 204.2 | C15H24 | 161.2 |
| 20 | γ-Cadinene | 17.30 | 0.59 | 1510 | 204.35 | C15H24 | 161.2 |
| 21 | Caryophyllene oxide | 18.18 | 14.82 | 1540 | 220.35 | C15H24O | 79.2 |
| 22 | Germacra-4(15),5,10(14)-triene-1-α-ol | 18.31 | 1.29 | 1577 | 220.35 | C15H24O | 131.2 |
| 23 | Humulene oxide II | 19.53 | 0.75 | 1600 | 220.35 | C15H24O | 67.1 |
| 24 | ( +)-Agathadiol | 33.90 | 0.70 | 1634 | 306.48 | C20H34O2 | 81.1 |
| Total % | 99.89% |
| Non oxygenated compounds | 59.23% | Oxygenated compounds | 40.66% | ||||
|---|---|---|---|---|---|---|---|
| Monoterpenes | 32.93% | Monoterpenoids | 23.1% | ||||
| Sesquiterpenes | 26.3% | Sesquiterpenoids | 16.86% | ||||
| Diterpenes | – | Diterpenoids | 0.70% |
| Oxygenated compounds | 40.66% | ||||||
|---|---|---|---|---|---|---|---|
| Epoxide and dioxide | 22.27% | Alcohols | 15.50% | Ketone | 2.89% |
Significant values are in bold.
Rt (min) retention time, RI retention index, M.F. molecular formula.
Figure 1.
GC–MS chromatogram of essential oil of Araucaria heterophylla oleogum resin.
By comparing the composition of EO in our study with previous reported literature4, differences in some components and in some percentage of others was found. The EO of the previous study showed that the most abundant component was α-pinene (44.88%) followed by germacrene-D (10.25%), α-copaene (4.72%) and sabinene (4.44%). Also, it showed low percentage of oxygenated components (5.66%) compared to the EO of our study (40.66%). In addition to caryophyllene oxide that exhibited very low percentage (0.33%). In contrast, it was the most abundant component in our study (14.82%).
These variations in the composition of the essential oil could be explained by the impact of extraction technique including solvent, temperature and time of extraction. Also, these variations could be ascribed to the environmental, climatic, genetic factors, age of the plant, soil type or time of harvesting. Thus, it could exert significant effects on the chemical profile of the essential oils derived4,22,23.
It was worth mentioning that our plant material was collected from the coastal city, Alexandria while in the previously reported literature, it was collected from Mansoura city in Delta region4.
Enzyme inhibitory activity of the essential oil
Aldose reductase enzyme inhibition
Up to our knowledge there was no study that evaluated the inhibitory activity of any Araucaria species extract or their essential oils against aldose reductase enzyme, thus our study was the first one to report the activity of essential oil of Araucaria heterophylla oleogum resin against aldose reductase enzyme. It was found that the essential oil exhibited high significant inhibitory activity against aldose reductase enzyme with potent IC50 (0.133 ± 0.006 µg/mL) compared to the control epalrestat (0.165 ± 0.008 µg/mL) (Table 2).
Table 2.
Aldose reductase, BUCHE, COX-II and SARS-CoV-2 Mpro inhibitory activities of the essential oil of Araucaria heterophylla oleogum resin compared to standards.
| Sample | Aldose reductase IC50 µg/mL | Sample | BUCHE IC50 µg/mL |
|---|---|---|---|
| EO | 0.133 ± 0.006 | EO | 0.154 ± 0.009 |
| Epalrestat (standard) | 0.165 ± 0.008 | Rivastigmine (standard) | 0.078 ± 0.005 |
| Sample | COX-II IC50 µg/mL | Sample | SARS-CoV-2 Mpro IC50 µg/mL |
|---|---|---|---|
| EO | 10.3 ± 0.29 | EO | 32.1 ± 1.44 |
| Celecoxib (standard) | 2.6 ± 0.09 | Tipranavir (standard) | 4.7 ± 0.21 |
All data are presented as mean value ± SD for three independent experiments.
BUCHE enzyme inhibition
It was reported that some essential oils of genus Araucaria had inhibitory activities against BUCHE enzyme as the EO of Araucaria brasiliensis, so this encouraged us to evaluate the activity of Araucaria heterophylla essential oil as inhibitor of BUCHE enzyme5. The tested essential oil showed comparable inhibitory activity against BUCHE enzyme with an IC50 of 0.154 ± 0.009 µg/mL compared to the control rivastigmine with an IC50 of 0.078 ± 0.005 µg/mL (Table 2).
COX-II enzyme inhibition
In vitro and in silico COX enzyme inhibitory activity was previously reported for A. heterophylla, A. bidwillii and A. cunninghamii leaves extracts, so they could be a possible source of natural anti-inflammatory drugs24. The essential oil of A. heterophylla resin in our study revealed moderate inhibitory activity against COX-II enzyme with an IC50 of 10.3 ± 0.29 µg/mL compared to the control celecoxib with an IC50 of 2.6 ± 0.09 µg/mL (Table 2).
SARS-CoV-2 Mpro enzyme inhibition
Previous reported molecular docking study of compounds identified in A. heterophylla besides A. bidwillii and A. cunninghamii leaves extracts revealed possible effect of these compounds against SARS-CoV-2, so they probably have anti-COVID effects24. Our essential oil exhibited low inhibitory activity against SARS-CoV-2 Mpro enzyme with an IC50 of 32.1 ± 1.44 µg/mL compared to the control tipranavir with an IC50 of 4.7 ± 0.21 µg/mL (Table 2).
Molecular docking simulation
As the EO of A. heterophylla oleogum resin showed the highest inhibitory activity against aldose reductase and BUCHE enzymes compared to the other two enzymes under investigation, thus molecular docking study was carried out to demonstrate the major 8 components of the EO binding modes and their affinities for these two enzymes, thus exploring the components of the EO responsible for the inhibitory activities against these enzymes and their mode of action.
Molecular docking simulation against aldose reductase active site
Docking results of the tested essential oil components to aldose reductase active site, using epalrestat as reference inhibitor, showed the binding interaction types and binding scores of essential oil components (Tables 2 and 3 and Fig. 2). The reference epalrestat (IC50 = 0.165 µg/mL, binding score = − 11.0 kcal/mol) bound to the active site through its amide and carboxylate moieties by two hydrogen bonds with Lys21 and another H-bond with Trp20, it also bound to active site through arene-cation interaction with Arg268 by its phenyl group. In addition, it formed strong hydrophobic interactions with Lys262, Pro215 and Asp216 amino acid residues.
Table 3.
The aldose reductase inhibition docking scoresa and type of binding interactions of the major components of the essential oil & the standard (Epalrestat).
| Comp | Binding energy (Kcal/mol)a (docking score) | Type of binding interactions |
|---|---|---|
| Epalrestat | − 11.0 | Two H-bonds with Lys21 |
| H-bond with Trp20 | ||
| Arene-cation interaction with Arg268 Strong hydrophobic interaction with Lys262, Pro215 and Asp216 | ||
| Ah major components | ||
| Caryophyllene | − 8.0 | Strong hydrophobic interaction with Arg268, Pro215 and Leu228 |
| Caryophyllene oxide | − 9.0 | H-bond with Lys262 |
| Strong hydrophobic interaction with Arg268 and Pro215 | ||
| ( +)- Sabinene | − 7.1 | Strong hydrophobic interaction with Arg268 and Lys262 |
| D-limonene | − 6.7 | Strong hydrophobic interaction with Arg268 and Lys262 |
| Alpha-Copaene | − 8.5 | Strong hydrophobic interaction with Arg268 and Lys262 |
| Beta-pinene | − 6.8 | Strong hydrophobic interaction with Arg268 and Lys262 |
| Trans-verbenol | − 7.9 | Two H-bonds with Arg268 and Ser263 |
| Strong hydrophobic interaction with Pro215 and Lys262 | ||
| Alpha-pinene oxide | − 7.7 | Two H-bonds with Arg268 and Ser263 |
| Strong hydrophobic interaction with Pro215, Asp216 and Lys262 | ||
Significant values are in bold.
Epalrestat was used as reference aldose reductase inhibitor compound.
Docking was carried out following the reported procedures26 against the aldose reductase enzyme pocket (PDB code ID: 3RX2).
aMore negative score refers to better capability of a molecule to dock with the target and make more desirable interactions.
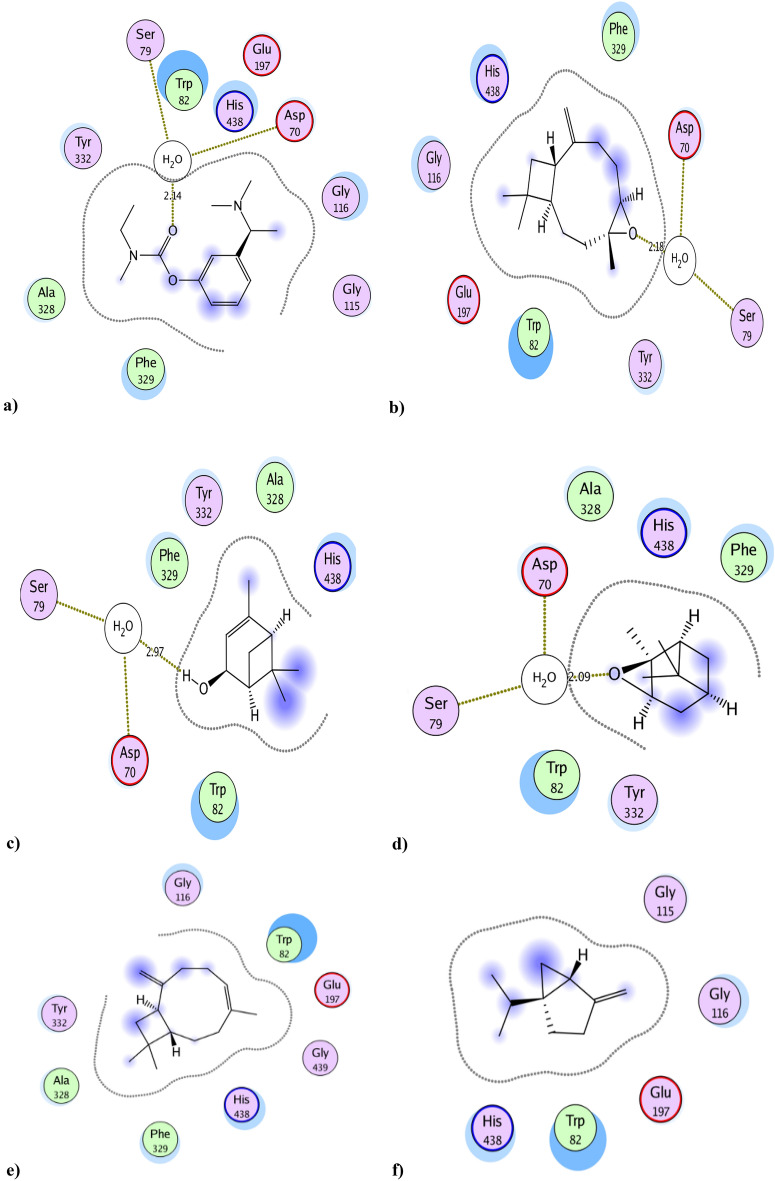
Figure 2.
Docking results of the major compounds of the essential oil & the standard epalrestat in the active site of aldose reductase enzyme (3RX2). 2D interactions of (a) Standard epalrestat. (b) Caryophyllene oxide. (c) Trans-verbenol. (d) Alpha-pinene oxide. (e) Caryophyllene. (f) ( +)- Sabinene. (g) D-limonene. (h) Alpha-Copaene. (i) Beta-pinene.
Compounds caryophyllene oxide, trans-verbenol and alpha-pinene oxide proved to have the best docking results (binding score values of − 9.0, − 7.9 and − 7.7 kcal/mol, respectively). They shared epalrestat in binding with Arg268, Lys262 and Pro215 by various binding interactions. Caryophyllene oxide bound to active site by its epoxide moiety with Lys262 and other hydrophobic interactions with Arg268 and Pro215. Trans-verbenol and alpha-pinene oxide showed better binding interactions by binding with active site Arg268 and Ser263 amino acid residues with two hydrogen bonds through their hydroxyl and epoxide moieties, respectively. They formed also strong hydrophobic interactions with Pro215 and Lys262 (Tables 2 and 3 and Fig. 2).
Other essential oil major components showed weaker binding interactions with aldose reductase active site. This may be due to their non-polar structure. They only bound to target by hydrophobic bonds. So, it could be predicted that the activity of Araucaria heterophylla essential oil against aldose reductase enzyme (IC50 = 0.133 µg/mL), that was more potent than the reference epalrestat itself (IC50 = 0.165 µg/mL) may be attributed to trans-verbenol and alpha-pinene oxide components and in a less extent to caryophyllene oxide.
The other predicted interactions of the tested components with aldose reductase active site were shown in (Table 3, Fig. 2, Tables S1 and S2) and the 3D interactions of the best binding compounds were visualized in (Fig. S1).
Molecular docking simulation against BuChE active site
Docking results of the tested compounds to BuChE active site, using rivastigmine as standard inhibitor, showed the types of binding interactions and docking scores of essential oil components. Rivastigmine (IC50 = 0.078 µg/mL, binding score = − 13.7 kcal/mol) bound to BuChE active site through its carbamate moiety by forming extensive interactions with water molecule strongly anchored to the target by hydrogen bonding to Asp70 and Ser79, the main catalytic amino acids in BuChE enzyme, it bound also to BuChE through strong hydrophobic interaction with Trp82, His438, Gly116 and Phe329 (Tables 2 and 4 and Fig. 3).
Table 4.
The BuChE inhibition docking scoresa and type of binding interactions of the major compounds of the essential oil & the standard (Rivastigmine).
| Comp | Binding energy (Kcal/mol)a (docking score) | Type of binding interactions |
|---|---|---|
| Rivastigmine | − 13.7 | Interaction with a water molecule of the binding site that form hydrogen bond with Asp70 and Ser79 |
| Strong hydrophobic interaction with Trp82, His438, Gly116 and Phe329 | ||
| Ah major components | ||
| Caryophyllene | − 8.9 | Strong hydrophobic interaction with Trp82 and His438 |
| Caryophyllene oxide | − 12.0 | Interaction with a water molecule of the binding site that form hydrogen bond with Asp70 and Ser79 |
| Strong hydrophobic interaction with Trp82, His438 and Phe329 | ||
| ( +)- Sabinene | − 6.6 | Strong hydrophobic interaction with Trp82 and His438 |
| D-limonene | − 6.7 | Strong hydrophobic interaction with Trp82 and His438 |
| Alpha-Copaene | − 8.6 | Strong hydrophobic interaction with Trp82 and His438 |
| Beta-pinene | − 7.0 | Strong hydrophobic interaction with Trp82 and His438 |
| Trans-verbenol | − 7.8 | Interaction with a water molecule of the binding site that form hydrogen bond with Asp70 and Ser79 |
| Strong hydrophobic interaction with Trp82 and His438 | ||
| Alpha-pinene oxide | − 9.5 | Interaction with a water molecule of the binding site that form hydrogen bond with Asp70 and Ser79 |
| Strong hydrophobic interaction with Trp82, His438 and Phe329 | ||
Significant values are in bold.
Rivastigmine was used as reference butyryl choline esterase inhibitor compound.
Docking was carried out following the reported procedures27 against the BuChE enzyme pocket (PDB code ID: 4BDS).
aMore negative score refers to better capability of a molecule to dock with the target and make more desirable interactions.
Figure 3.
Docking results of the major compounds of the essential oil & the standard rivastigmine in the active site of BuChE enzyme (4BDS). 2D interactions of (a) Standard rivastigmine. (b) Caryophyllene oxide. (c) Trans-verbenol. (d) Alpha-pinene oxide. (e) Caryophyllene. (f) ( +)- Sabinene. (g) D-limonene. (h) Alpha-Copaene. (i) Beta-pinene.
Compounds caryophyllene oxide, trans-verbenol and alpha-pinene oxide showed the best docking results (binding score = − 12.0, − 7.8 and − 9.5 kcal/mol, respectively). They shared rivastigmine in binding with Asp70 and Ser79 by the same binding manner and also in the strong hydrophobic interaction with almost the same amino acid residues. Caryophyllene oxide and alpha-pinene oxide formed interactions with water molecule anchored to the target by hydrogen bonding to Asp70 and Ser79 through their epoxide moiety, while trans-verbenol formed this interaction through its hydroxyl group (Table 2 and 4 and Fig. 3). To the best of our knowledge, it was reported that caryophyllene oxide exhibited strong cholinesterase inhibitory activities25.
Other essential oil major components showed poor interactions with BuChE active site. This may be due to their lipophilic character and absence of any hydrogen bonding functional groups in their structures. They only bound to target by hydrophobic interactions. So, it could be predicted that the BuChE inhibition of essential oil (IC50 = 0.154 µg/mL), nearly similar to rivastigmine (IC50 = 0.078 µg/mL), may be attributed to caryophyllene oxide, trans-verbenol and alpha-pinene oxide components.
The other predicted binding interactions of the tested components with BuChE active site were shown in (Table 4, Fig. 3, Table S3 and S4) and the 3D interactions of the best binding compounds were visualized in (Fig. S2).
From in vitro and in silico study, our findings shed light toward the potential use of the EO from A. heterophylla as a green source of aldose reductase and BUCHE inhibitor.
Conclusion
This study is the first one that reports the in vitro aldose reductase, BUCHE, COX-2 and SARS-CoV-2 Mpro inhibitory activities of Araucaria heterophylla essential oil. Our findings attracted the attention to the possibilities of using the EO of A. heterophylla as a new medicinal natural inhibitor against aldose reductase and BUCHE enzymes as it exhibited promising significant inhibitory activity compared to the standards. Thus, this EO may be suggested for treatment of diabetic cataract as well as Alzheimer’s disease.
Material and methods
Plant material
The oleogum resin of cultivated Araucaria heterophylla (Salisb.) were collected from the Gardens of Al Montazah, Alexandria, Egypt, in November 2021. The plant identity was confirmed by Associate Prof. Dr Mahmoud Makram Qassem, Department of Vegetables & Floriculture, Faculty of Agriculture, Mansoura University, Egypt. Voucher specimens were coded as Ah-1–2021 and kept in Pharmacognosy Department, Faculty of Pharmacy, Mansoura University, Egypt.
Essential oil isolation
Araucaria heterophylla oleogum resin, weighted 250 g, were subjected after collection, to hydro distillation for 6 h. using a Clevenger-type apparatus based on using water for the extraction of essential oils that were characterized by their hydrophobic nature. The hydrated sample was heated to vaporize volatile constituents, then two layers (aqueous and oil-rich) were produced and oil could be further separated via separating funnels. The hydrodistillation method achieved three main physicochemical processes: hydrodiffusion, hydrolysis and heat decomposition28. This extraction was repeated three times to afford 5 ml of essential oil. The oil was dried over anhydrous sodium sulfate then stored at + 4 °C in the dark until tested.
For experimental research and field studies on plants
All procedures were conducted in accordance to the relevant institutional, national, and international guidelines and legislation.
Analysis of essential oil by gas chromatography-mass spectrometry (GC/MS)
GC/MS analysis was carried out using an Agilent 19091S-433 system with a mass selective detector. HP-5 MS capillary column (30 m × 0.25 mm, film thickness: 0.25 μm); injection mode: splitless; split-flow: 10 ml/min; splitless time: 0.80 min; injector and detector temperature: 250 °C; oven temperature was programmed as follows: 60 °C for 2 min and then 5 °C/min programmed at 240 °C; the carrier gas was helium with a constant flow of 1 mL/min; injection volume: 1 µL of diluted essential oil (1% w/v in CH2Cl2).
Enzyme inhibitory activity of the essential oil
Aldose reductase enzyme inhibition
For measuring the aldose reductase activity: (Catalog # K369-100) colorimetric kit was used. Also, epalrestat was included as a positive control (155 S. Milpitas Blvd., Milpitas, CA 95035 USA, Email: tech@biovision.com).
The aldose reductase inhibitory activity of the essential oil sample was done according to previous literature10. The principle of the assay depends on the ability of aldose reductase enzyme to catalyze the oxidation of NADPH to NADP. The absorption of NADPH at 340 nm was measured in a mixture containing NADPH, the enzyme and its substrate in addition to test samples.
BUCHE enzyme inhibition
For measuring butyryl choline esterase activity: (Catalog Number EIABCHEF (192 tests) fluorescent kit was used. Also, rivastigmine was included as a positive control (Life Technologies Corporation | Carlsbad, CA 92,008 USA | Toll Free in USA 1 800 955 6288).
The cholinesterase inhibitory activity of the test sample was determined according to the previously published procedure29 using Ellman’s microplate spectrophotometric method. The substrate butyryl thiocholine chloride is hydrolyzed by BuChE to give butyrate and thiocholine. In neutral and alkaline media, thiocholine reacts with 5,5-Dithiobis [2-nitrobenzoic acid] (DTNB) to give yellow-colored 2-nitro-5-thiobenzoate, which can be detected spectrophotometrically at 412 nm.
COX-II enzyme inhibition
For measuring COX-2 activity: (Catalog # K547-100) fluorometric kit was used. Also, celecoxib was included as a positive control (155 S. Milpitas Blvd., Milpitas, CA 95035 USA, Email: tech@biovision.com).
The Cox-II inhibitory activity of tested sample was carried out according to previous literature30. The assay is based on the fluorometric detection of prostaglandin G2, the intermediate product generated from arachidonic acid by the action of COX enzyme at 535/587 nm.
SARS-CoV-2 Mpro enzyme inhibition
For measuring main protease (SARS-CoV-2): (Catalog #79,955–1) fluorometric kit was used. Also, tipranavir was included as a positive control (6042 Cornerstone Court West, Ste. BSan Diego CA 92,121, Email: info@bpsbioscience.com).
The inhibitory activity against the SARS-CoV-2 main protease (Mpro or 3CLpro) assay was carried out based on the FRET-based activity assay31. The principle of the assay depends on the C-terminal of the peptide substrate being linked to a fluorophor (Edans) and the N-terminal has a fluorescence quencher (Dabcyl) that quenches the fluorescence signal of Edans. Thus, the peptide substrate exhibits low fluorescence because the fluorescence intensity of Edans in the C-terminal is quenched by the Dabcyl in the N-terminal of the substrate.
Statistical analysis of the data was performed using GraphPad Instat version 8 software package ((GraphPad Software Inc. V8, San Diego, CA, USA). Graphs were sketched using GraphPad Prism. Statistical tests used were one-way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons test for statistical comparison between parametric data.
The results were expressed as percentage inhibition, which was calculated using the formula:
Inhibitory activity (%) = (1 − A test/A control) × 100.
Where, A test is the reading* in the presence of test substance and A control is the reading* of control. *Absorbance or inflouresence.
Molecular docking studies
Molecular docking was used as a tool that predicting the binding mode of the essential oil major components with the targeted enzymes. The molecular docking studies in both two- & three-dimensional visualizations were used to predict the binding affinity in Kcal/mol between the essential oil major components and the enzymes active sites, then comparing their results with a reference. Therefore, molecular docking studies helped to pick up the essential oil components that are responsible for the activity. These studies were done only on two enzymes, aldose reductase and BUCHE because of their promising in vitro results.
Ligand and protein preparation
The essential oil major components structures were adjusted in their least energetic and neutral conformers using the builder of Molecular Operating Environment (MOE) version 2009.10 (Chemical Computing Group Inc. software. https://www.chemcomp.com). They were prepared for docking into the aldose reductase and BuChE active sites. Energy was minimized via MMFF94 technique with root mean square gradient of 0.01 kcal/mol Å. The tested compounds were docked into the active site of crystal structure of human aldose reductase complexed with sulindac sulfone (ID: 3RX2)26 and human butyrylcholinesterase in complex with tacrine (BuChE) (ID: 4BDS)27. Targets were prepared and active site was detected following the reported procedures32,33.
Supplementary Information
Acknowledgements
Dr. Esam Rashwan (Head of the confirmatory diagnostic unit VACSERA-EGYPT) is acknowledged for carrying out the biological assays.
Author contributions
A.F.S.: Suggested the research point, operated the hydrodistillation of plant materials, revised of the manuscript and took over the publishing process. G.A.: Participated in the identification of the oil components, followed up the biological part, writing the manuscript and took over the publishing process. M.A.S.: did the molecular docking study of the major components of the Araucaria heterophylla essential oil to explain their binding modes and affinities for aldose reductase and BuCHE enzymes.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
The datasets used during the current study available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-38143-4.
References
- 1.Santi-Gadelha T, et al. Purification and biological effects of Araucaria angustifolia (Araucariaceae) seed lectin. Biochem. Biophys. Res. Commun. 2006;350:1050–1055. doi: 10.1016/j.bbrc.2006.09.149. [DOI] [PubMed] [Google Scholar]
- 2.Aslam MS, Choudhary BA, Uzair M, Ijaz AS. Phytochemical and ethno-pharmacological review of the genus Araucaria—Review. Trop. J. Pharm. Res. 2013;12(4):651–659. [Google Scholar]
- 3.Branco C, et al. Chemical constituents and biological activities of Araucaria angustifolia (Bertol.) O. Kuntze: A review. Org. Inorg. Chem. 2016;2:1–8. [Google Scholar]
- 4.Elshamy AI, et al. Essential oil and its nanoemulsion of Araucaria heterophylla resin: Chemical characterization, anti-inflammatory, and antipyretic activities”. Ind. Crops Prod. 2020;148:112272. doi: 10.1016/j.indcrop.2020.112272. [DOI] [Google Scholar]
- 5.Jaramillo D, et al. Chemical characterization and biological activity of the essential oil from Araucaria brasiliensis collected in Ecuador. Molecules. 2022;27:3793. doi: 10.3390/molecules27123793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brophy JJ, Goldsack RJ, Wu MZ, Fookes CJ, Forster PI. The steam volatile oil of Wollemia nobilis and its comparison with other members of the Araucariaceae (Agathis and Araucaria) Biochem. Syst. Ecol. 2000;28:563–578. doi: 10.1016/S0305-1978(99)00090-3. [DOI] [PubMed] [Google Scholar]
- 7.Verma RS, et al. Chemical composition and antibacterial activity of foliage and resin essential oils of Araucaria cunninghamii Aiton ex D. Don and Araucaria heterophylla (Salisb.) Franco from India. Ind. Crops Prod. 2014;61:410–416. doi: 10.1016/j.indcrop.2014.07.040. [DOI] [Google Scholar]
- 8.Elkady WM, Ayoub IM. Chemical profiling and antiproliferative effect of essential oils of two Araucaria species cultivated in Egypt. Ind. Crops Prod. 2018;118:188–195. doi: 10.1016/j.indcrop.2018.03.051. [DOI] [Google Scholar]
- 9.Kim TH, et al. Aldose reductase inhibitory activity of compounds from Zea mays L. Biomed. Res. Int. 2013;8:727143. doi: 10.1155/2013/727143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel Motaal A, et al. Flavonol glycosides: in vitro inhibition of DPPIV, aldose reductase and combating oxidative stress are potential mechanisms for mediating the antidiabetic activity of Cleome droserifolia. Molecules. 2020;25:5864. doi: 10.3390/molecules25245864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed M, et al. Investigation of antidiabetic properties of shikonin by targeting aldose reductase enzyme: In silico and in vitro studies. Biomed. Pharmacother. 2022;150:112985. doi: 10.1016/j.biopha.2022.112985. [DOI] [PubMed] [Google Scholar]
- 12.Cavdar H, et al. Inhibition of acetylcholinesterase and butyrylcholinesterase with uracil derivatives: Kinetic and computational studies. J. Enzyme Inhib. Med. Chem. 2019;34(1):429–437. doi: 10.1080/14756366.2018.1543288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korolev IO. Alzheimer’s disease: A clinical and basic science review. Am. Med. Stud. Res. J. 2014;4(1):24–33. [Google Scholar]
- 14.Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002;14(S1):77–91. doi: 10.1017/S1041610203008676. [DOI] [PubMed] [Google Scholar]
- 15.Kamal AM, et al. Inhibition of butyrylcholinesterase with fluorobenzylcymserine, an experimental alzheimer's drug candidate: Validation of enzoinformatics results by classical and innovative enzyme kinetic analyses. CNS Neurol. Disord. Drug Targets. 2017;16(7):820–827. doi: 10.2174/1871527316666170207160606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greenhough A, et al. The COX-2/PGE 2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumor microenvironment. Carcinogenesis. 2009;30(3):377–386. doi: 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 17.Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arter. Thromb. Vasc. Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdel Wahab G, Aboelmaaty WS, Lahloub MF, Sallam A. In vitro and in silico studies of SARS-CoV-2 main protease Mpro inhibitors isolated from Helichrysum bracteatum. RSC Adv. 2022;12:18412–18424. doi: 10.1039/D2RA01213H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van den Dool H, Kratz PD. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 20.Halim A, Mashaly M, Sandra P. Constituents of the essential oil of Mentha microphylla C. Koch. Egypt. J. Pharm. Sci. 1990;31(1–4):437–441. [Google Scholar]
- 21.Adams RP. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. Allured Publishing Corporation Carol Stream IL; 2007. [Google Scholar]
- 22.Kamal GM, Anwar F, Hussain AI, Sarri N, Ashraf MY. Yield and chemical composition of Citrus essential oils as affected by drying pretreatment of peels. Int. Food Res. J. 2011;18(4):1275–1282. [Google Scholar]
- 23.Mukarram M, et al. Lemongrass essential oil components with antimicrobial and anticancer activities. Antioxidants. 2022;11:20. doi: 10.3390/antiox11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El-Hawary SS, et al. Metabolomic profiling of three Araucaria species, and their possible potential role against COVID-19. J. Biomol. Struct. Dyn. 2022;40(14):6426–6438. doi: 10.1080/07391102.2021.1885494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karakaya S, et al. A caryophyllene oxide and other potential anticholinesterase and anticancer agent in Salvia verticillata subsp. amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae) J. Essent. Oil Res. 2020;32(6):512–525. doi: 10.1080/10412905.2020.1813212. [DOI] [Google Scholar]
- 26.Zheng X, et al. The molecular basis for inhibition of sulindac and its metabolites towards human aldose reductase. FEBS Lett. 2012;586(1):55–59. doi: 10.1016/j.febslet.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Nachon F, et al. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer's drugs targeting acetyl-and butyryl-cholinesterase. Biochem. J. 2013;453(3):393–399. doi: 10.1042/BJ20130013. [DOI] [PubMed] [Google Scholar]
- 28.Fagbemi KO, Aina DA, Olajuyigbe OO. Soxhlet extraction versus hydrodistillation using the Clevenger apparatus: A comparative study on the extraction of a volatile compound from Tamarindus indica seeds. Sci. World J. 2021;8:5961586. doi: 10.1155/2021/5961586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obregon AD, et al. Effects per se of organic solvents in the cerebral acetylcholinesterase of rats. Neurochem. Res. 2005;30(3):379–384. doi: 10.1007/s11064-005-2612-5. [DOI] [PubMed] [Google Scholar]
- 30.Çavuşoğlu BK, et al. Design, synthesis, biological evaluation, and docking studies of some novel chalcones as selective COX-2 inhibitors. Arch. Pharm. 2021;354:2000273. doi: 10.1002/ardp.202000273. [DOI] [PubMed] [Google Scholar]
- 31.Morse JS, Lalonde T, Xu S, Liu WR. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-nCoV. ChemBioChem. 2020;21(5):730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali MY, et al. Inhibition of aldose reductase by ginsenoside derivatives via a specific structure activity relationship with kinetics mechanism and molecular docking study. Molecules. 2022;27(7):2134. doi: 10.3390/molecules27072134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waly OM, et al. Synthesis, biological evaluation and molecular modeling simulations of new heterocyclic hybrids as multi-targeted anti-Alzheimer's agents. Eur. J. Med. Chem. 2022;231:114152. doi: 10.1016/j.ejmech.2022.114152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study available from the corresponding author upon request.