Abstract
Two event-related potentials (ERPs) elicited following errors, the error-related negativity (ERN) and error positivity (Pe), have been proposed to reflect cognitive control, though the specific processes remain debated. Few studies have examined the ERN and Pe’s relations with individual differences in cognitive control/executive functioning using well-validated tests administered separately from the inhibition tasks used to elicit the ERN/Pe. Additionally, neurocognitive tests of executive functions tend to strongly predict ADHD symptoms, but the extent to which task-based and EEG-based estimates of executive functioning/cognitive control account for the same variance in ADHD symptoms remains unclear. The current study addressed these limitations by examining relations between the ERN/Pe and three core executive functions (working memory, inhibitory control, set shifting) in a clinically-evaluated sample of 53 children ages 8–12 (Mage=10.36, SD=1.42; 77.4% White/Non-Hispanic; 16 girls) with and without ADHD. Results demonstrated that neither the ERN nor Pe were related to overall cognitive control/executive functioning, or to working memory or set shifting specifically (all 95%CIs include 0.0). In contrast, a larger Pe was associated with better-developed inhibitory control (β=−.35, 95%CI excludes 0.0), but does not capture aspects of inhibitory control that are important for predicting ADHD symptoms. Neither the ERN nor Pe predicted ADHD symptoms (95%CIs include 0.0). Results were generally robust to control for age, sex, SES, ADHD symptom cluster, and anxiety, and emphasize the need for caution when interpreting the ERN/Pe as indices of broad-based cognitive control/executive functioning, as well as using the ERN/Pe to examine cognitive processes contributing to ADHD symptomatology.
Keywords: Error-related negativity, error positivity, executive function, cognitive control, ADHD
Event-related potentials (ERPs) are a useful tool for assessing cognitive processes because they are a direct measure of brain activity and have demonstrated clinical utility in predicting both risk for and developmental trajectories of psychopathology (Hajcak et al., 2019). Two ERPs that are elicited following errors on speeded decision-making tasks, the error-related negativity (ERN) and error positivity (Pe), have been proposed to reflect cognitive control mechanisms given that errors represent a breakdown in performance that is detected and subsequently acted upon using performance monitoring processes (Cavanagh & Shackman, 2015; Falkenstein, 2004; Gehring et al., 2012; Overbeek et al., 2005). However, despite the common reification of the ERN and Pe as indices of cognitive control, relatively few studies have examined their relations with individual differences in cognitive control/executive functioning (Meyer & Hajcak, 2019). As such, it is also unclear if and how the ERN and Pe may overlap with the executive function/cognitive control processes that are associated with both the etiology and behavioral symptom expression of attention-deficit/hyperactivity disorder (ADHD), a common neurodevelopmental disorder (e.g., Kofler et al., 2019). The present study examined the extent to which the ERN and Pe are related to overall and specific components of cognitive control/executive functioning (working memory, inhibitory control, set shifting) to inform characterization of the ERN and Pe as proxies of cognitive control, as well as their interrelations with ADHD symptoms in a relatively small but carefully phenotyped and clinically evaluated sample of children with and without ADHD.
Executive Functions and Cognitive Control
Executive functions refer to a set of interrelated, higher-order processes that regulate and enable cognition, goal-directed behaviors, and problem solving (Miyake et al., 2000). Cognitive control, a closely related construct, refers to mental processes that enable the continuous adjustment of behaviors in accordance with actively changing goals and task demands to optimize performance (Botvinick & Cohen, 2014; Nigg, 2017)i. Executive function deficits are theorized to play an etiological role in several forms of psychopathology, and are considered to play a prominent/primary role in the development and behavioral expression of ADHD (e.g., Rapport et al., 2013). Miyake et al. (2000) proposed three primary executive function domains: working memory, inhibitory control, and set shifting. Briefly, working memory involves the active, top-down manipulation of information held in short-term memory through interrelated functions of updating, dual-processing, and temporal/serial reordering (Fosco et al., 2020). Inhibitory control refers to the processes that support the ability to deliberately withhold or stop a dominant and/or on-going response (Alderson et al., 2007). Finally, set shifting involves cognitive flexibility in shifting back and forth between mental sets or tasks (Irwin et al., 2019; Miyake et al., 2000).
ERN, Pe, and Executive Function
The ERN is characterized by a negative deflection about 50 to 100 ms after an individual makes an error, and is most readily observed at frontocentral scalp locations (Falkenstein et al., 1991; Gehring et al., 1993, 2012). Evidence suggests that the ERN is generated in the anterior cingulate cortex (ACC; Mathewson et al., 2005; van Veen & Carter, 2002), a brain region implicated in conflict and outcome monitoring (Botvinick et al., 2004). The Pe typically follows the ERN and is marked by a slow positive wave occurring centroparietally on the scalp between about 200 and 400 ms after an individual makes an error (Falkenstein et al., 1991; Overbeek et al., 2005).
Support for the ERN and Pe as proxies of executive functioning/cognitive control includes evidence that the ERN is associated with accuracy-speed tradeoffs (Gehring et al., 1993) and that a larger ERN is related to greater post-error slowing in adults during response inhibition tasks (Hirsh & Inzlicht, 2010; for review, see Cavanagh & Shackman, 2015). However, while behavioral data in some ERN studies found that fewer errors on inhibition tasks were associated with a larger ERN in children and adolescents (Lo et al., 2017; Mehra & Meyer, 2022; Overbye et al., 2019), others did not find this relation (Gorday & Meyer, 2018; Grammer et al., 2018; Kim et al., 2016). Similarly, an increased Pe in adults has been associated with fewer errors (Falkenstein et al., 2000; Mathewson et al., 2005) and increased post-error slowing (Chang et al., 2014; Hajcak et al., 2003) during response inhibition tasks. However, studies relating the Pe and inhibitory control in children and adolescents are mixed (Grammer et al., 2018; Kim et al., 2016; Overbye et al., 2019), and to our knowledge no studies have examined these relations specifically in school-aged children – a critical omission given that (a) the ERN and Pe follow different developmental trajectories, with the Pe maturing earlier and the ERN continuing to increase into early adulthood (Boen et al., 2022; Downes et al., 2017); and (b) school-aged children’s executive functioning/cognitive control abilities also follow different developmental trajectories (Best et al., 2009; Best & Miller, 2010), with working memory and inhibitory control reflecting unique executive functions in early childhood (Lerner & Lonigan, 2014) and set shifting becoming a distinct ability in later school-aged children (Karr et al., 2018).
Although the extant literature is informative for understanding the relations between the ERN/Pe and inhibitory control, it is unclear if such findings extend to executive functioning/cognitive control overall, or to executive functions beyond inhibitory control (i.e., working memory, set shifting). In addition, the evidence is based primarily on performance parameters measured during the same inhibitory control tasks from which the ERPs are derived (Meyer & Hajcak, 2019), which questions the extent to which these findings may be artifacts of mono-task bias (i.e., attributable to illusory associations when multiple metrics from the same task/measure are compared). Given evidence supporting the reliability and validity of these ERPs as individual difference markers (Riesel et al., 2013; Weinberg et al., 2012), there is a need for studies using (a) construct valid executive function/cognitive control batteries completed separately from the task used to capture ERN/Pe parameters, and (b) measures of working memory and set shifting in addition to inhibitory control – yet few studies have used this approach (Meyer & Hajcak, 2019).
A partial exception to this critique comes from a recent adult study that suggests associations between the ERN/Pe and global estimates of executive functioning/cognitive control (Larson & Clayson, 2011). Similarly, a recent study with young children found that an increased ERN was associated with greater cognitive control (Meyer & Klein, 2018). However, the conclusions that can be drawn from these studies may be limited because they relied primarily on tests that were developed to assess gross neuropsychological functioning and questionnaire-based assessments of executive function/cognitive control processes that show poor correspondence with specific tests of these cognitive processes (for reviews, see Snyder et al., 2015 and Soto et al., 2020). However, two studies conducted with college students addressed these limitations with construct valid working memory tests, and found that a larger ERN and Pe were associated with better working memory (Coleman et al., 2018; Miller et al., 2012).
ADHD, Executive Functioning, and Error-related ERPs
Given that (a) the ERN and Pe have been reified as indices of executive functioning/cognitive control, and (b) many if not most children with ADHD have deficits in executive functioning (e.g., Kofler et al., 2019), it is unsurprising that multiple studies have examined the ERN and Pe in individuals with ADHD. However, as with the ERN/Pe-executive function findings in the developmental/cognitive literatures, results from these ADHD studies are mixed (for reviews, see Meyer & Hajcak, 2019; Shiels & Hawk, 2010). A meta-analysis of seven studies found that individuals with ADHD exhibit a blunted (i.e., significantly reduced) ERN compared to controls (d = 0.50), but that a blunted Pe may be detectable only during certain types of inhibition tasks such as the go/no-go task used in the current study (d = 0.68; Geburek et al., 2013). In contrast, a more recent meta-analysis found the Pe to be blunted in ADHD groups compared to non-ADHD groups (d = −0.39), while no significant group differences were found in the ERN (d = 0.21; Kaiser et al., 2020). Similarly, Meyer & Hajcak (2019) conducted a box score review and found that 14 studies showed a blunted ERN in ADHD, 11 showed no differences between ADHD and control groups, and still 1 showed an increased ERN in ADHD. Indeed, in a review of the use of EEG in ADHD research, McLoughlin and colleagues (2022) conclude that while research on group differences in the ERN remain inconclusive, a reduced Pe in ADHD groups is more consistently, albeit not always, found.
Few studies have taken a dimensional approach to examining the relation between ADHD and the ERN and Pe. While some have found greater ADHD symptoms to be associated with a smaller ERN amplitude in adolescents and adults (Marquardt et al., 2018; Rommel et al., 2019), others have not found this relation (Herrmann et al., 2009; Wiersema et al., 2009). Similarly, an association between greater ADHD symptoms and a reduced Pe has been found in some studies (Herrmann et al., 2009; Wiersema et al., 2009), but not others (Rommel et al., 2019). The mixed results at both the categorical and dimensional level have been hypothesized to be attributable to varying participant characteristics and methodological approaches across studies (Kaiser et al., 2020; McLoughlin et al., 2022).
Current Study
Taken together, the current literature provides some support for associations between the ERN/Pe and neurocognitive test-based estimates of executive functioning/cognitive control, as well as evidence linking test-based and potentially EEG-based executive function/cognitive control tests with ADHD symptoms. However, no study to date has examined whether the test- and EEG-based indices predict the same variance in ADHD symptoms. The current study addresses this limitation and is the first to be conducted specifically with school-aged children, use well-validated assessments of all three core executive functions (working memory, inhibitory control, and set shifting) that were administered separately from the tasks used to evoke the ERN and Pe, and consider the extent to which the ERN/Pe are capturing the aspects of executive functioning/cognitive control that are predictive of ADHD behavioral symptoms. We hypothesized that an increased ERN and Pe would be associated with better-developed executive functioning/cognitive control overall, and with better-developed working memory and inhibitory control specifically; no specific hypotheses regarding set shifting were offered due to the paucity of prior research. Additionally, we hypothesized that blunted ERN and Pe metrics would be associated with greater ADHD symptoms, and would capture the same variance in the executive function/cognitive control tests associated with ADHD symptoms.
Method
Participants
The sample included 53 children between the ages of 8 and 12 years (M = 10.36, SD = 1.42; 16 girls) from the Southeastern U.S. who were (a) recruited through community resources from 2018–2020 for a clinical research study of neurocognitive mechanisms underlying pediatric attention and behavior problems; and (b) completed EEG testing during evaluation. Recruitment to this study was closed in March 2020 due to the COVID-19 pandemic. The sample size reflects consecutive cases whose evaluations were completed prior to the COVID-19 shutdown. The Florida State University IRB approved the study prior to and throughout data collection, and parents and children gave informed consent/assent. Sample ethnicity consisted of 41 White Not Hispanic or Latino (77.4%), 6 Black or African American (11.3%), 2 Hispanic or Latino (3.8%), and 4 multiracial (7.5%) children. All children spoke English.
All children and caregivers completed a comprehensive evaluation that included semi-structured clinical interviewing and multiple norm-referenced parent and teacher questionnaires. A detailed account of the comprehensive psychoeducational evaluation can be found in the larger study’s preregistration: [LINK]. The final sample comprised 53 children, including 14 children with ADHD only; 31 children with ADHD and common comorbidities (18 anxiety, 1 depression, 6 oppositional-defiant disorder, 6 autism spectrum disorder); and 8 with common clinical diagnoses but not ADHD (5 anxiety, 2 depression, 1 autism spectrum disorder). Further, eight children with ADHD and two children without ADHD met diagnostic criteria for a learning disorder. Psychostimulants (Nprescribed= 14; 26.4%) were withheld ≥24 hours for neurocognitive testing. Psychoeducational evaluations were provided to caregivers. Children were excluded from the larger study if they presented with gross neurological, sensory, or motor impairment; non-stimulant medications that could not be withheld for testing; or history of seizure disorder, psychosis, or intellectual disability.
Procedure
Children completed the executive function tasks and EEG recording as part of a larger battery of neurocognitive testing that involved two sessions of approximately three hours each. All tasks were counterbalanced to minimize order effects. EEG data collection was conducted at the end of the second testing session following an extended break. Children received breaks after each task and preset longer breaks every 2–3 tasks to minimize fatigue. Task performance was monitored at all times by the examiner, who was stationed just outside of the testing room (out of the child’s view) to provide a structured setting while minimizing performance improvements associated with examiner demand characteristics (Gomez & Sanson, 1994).
Measures
Executive Function Test Battery and Dimension Reduction
Descriptions, psychometric properties, and scoring information for the working memory, inhibitory control, and set shifting tasks are depicted in Table 1. Task impurity was controlled by computing Bartlett maximum likelihood component scores based on intercorrelations among all 7 executive function tests (Distefano et al., 2009), which parsed the 3 working memory, 2 inhibitory control, and 2 set-shifting tasks into 3 component scores (35.31% of variance explained; Supplementary Table 1). A three-component solution based on the larger sample (Soto et al., 2022) was specified a priori to derive separate estimates of working memory, inhibitory control, and set shifting based on theory and previous empirical work (e.g., Miyake et al. 2000). These principal components analysis-derived component scores provide estimates of reliable, construct-level variance attributable to domain-general working memory, inhibitory control, and set-shifting. This formative method for estimating executive functioning was selected because (a) such methods have been shown to provide higher construct stability relative to confirmatory/reflective approaches (Willoughby et al., 2016); and (b) estimating executive functioning at the construct-level rather than measure-level was expected to maximize associations with the study’s outcomes via the removal of task-specific and error variance. These component scores were used for all analyses. Higher scores reflect better working memory and set shifting, but worse inhibitory control.
Table 1.
Executive function test psychometric properties and scoring
| Executive Function Test | Description/Key Task Parameters | Psychometric Support | Indicators and Scoring | Trials |
|---|---|---|---|---|
|
| ||||
| Inhibitory Control | ||||
| Stop-Signal | -Go-stimuli were uppercase letters X and O. -Stop-stimulus was a 1000 Hz auditory tone presented randomly on 25% of trials. -Stop-signal delay (SSD; the latency between go- and stop-stimuli presentation) was initially set to 250 ms and dynamically adjusted ±50 ms contingent on the child’s performance. |
Internal consistency: α = .83–.89 3-week test-retest: r=.72 (Soreni et al., 2009) Internal consistency current study: α = .67 |
Speed of the inhibitory stop process (iSSRT) estimated separately for each of the task blocks (Verbuggen et al., 2013 integration method) Higher scores indicate worse inhibition |
4 blocks 32 trials/block |
| Go/No-Go | -Children presented with a randomized series of vertical (go stimuli) and horizontal (no-go stimuli) rectangles. - A ratio of 80:20 go:no-go stimuli was selected to maximize prepotency (Kane & Engle, 2003; Unsworth & Engle, 2007). |
Internal consistency: α=.95 (Kofler et al., 2019) Internal consistency current sample: α=.94 |
Commission errors (i.e., incorrectly responding to no-go trials) Lower scores indicate better inhibition |
4 blocks 25 trials/block |
| Set Shifting | ||||
| Global-Local | -Navon (1977) figures were used, which consist of a larger, “global” shape made up of smaller, “local” shapes. Figures were presented one at a time in one of four quadrants. -To minimize memory demands, on-screen cues (“big shape,” “small shapes”) were positioned next to each quadrant. -Children were required to shift their response between global and local features depending on which quadrant the figures appeared (top quadrants: global; bottom quadrants: local). |
Internal consistency: α=.86–.90) (Kofler et al., 2019) Increases in shift costs and accuracy costs relative to control conditions: ω2 = .12, .14 (Irwin et al., 2019) Internal consistency current sample: α=.82–.87 for shift and no-shift trials, respectively |
Speed shift costs (Speed shift cost = RTshift − RTnon-shift for correct trials) (Miyake et al., 2000; Irwin et al., 2019) Lower speed shift costs indicate better set-shifting |
4 blocks 30 trials/block |
| Number-Color | -Children clicked either the larger or smaller single-digit number depending on the font color, which was presented semi-randomly to require shifting every other trial. -To minimize memory demands, on-screen instructions (blue bigger, yellow smaller) remained visible throughout the task. |
Internal consistency: α = .87–.95 (Kofler et al., 2019) Internal consistency current study: α=.92–.95 for shift and no-shift trials, respectively |
Speed shift costs (Speed shift cost = RTshift – RTnon-shift for correct trials) (Miyake et al., 2000; Irwin et al., 2019) Lower speed shift costs reflect better set-shifting |
4 blocks 30 trials/block |
| Working Memory | ||||
| Rapport Phonological and Visuospatial Working Memory Tasks | -Phonological: A series of jumbled numbers and a capital letter presented. Children verbally recalled numbers in order from smallest to largest, and to say the letter last (e.g., 4H62 is correctly recalled as 246H). -Visuospatial: A series of 2.5 cm diameter dots (3, 4, 5, or 6) were presented sequentially in one of the nine squares arranged in three offset vertical columns. All but one dot presented within the squares was black—the exception being a red dot. Children reordered the dot locations (black dots in serial order, red dot last). |
Internal consistency: α = .82–.97 (Kofler et al., 2019) 1- to 3-week test-retest reliability: r=.76–.90 (Sarver et al., 2015) Convergent validity: r = .61–.69 (Wells et al., 2018) Internal consistency current sample: α = .84–.85 |
Partial-credit unit scoring (i.e., stimuli correct per trial) (Conway et al., 2005) computed separately for the phonological and visuospatial working memory tests Higher scores reflect better working memory |
24 total trials per task 6 trials/set size (3–6 stimuli/trial) |
| Letter Updating | -Letters presented on the screen one at a time, and children kept track of the last three letters presented. -Children were instructed to rehearse out loud the last three letters by mentally adding the most recent letter and dropping the fourth letter back (Miyake et al. 2000). |
Internal consistency: α=.75 (Kofler et al., 2019) Large magnitude ADHD/Non-ADHD between group differences (Fosco et al., 2020; Kofler et al., 2019). Internal consistency current sample: α=.82 |
Mean stimuli correct per trial (correct serial order) Higher scores reflect better working memory |
4 blocks 3 trials/block (4–8 stimuli/trial) |
EEG Task
Children completed an age-appropriate go/no-go task that was administered while EEG was being recorded. Alien (go stimuli) and astronaut (no-go stimuli) images appeared on the screen one at a time for 200 ms (inter-trial interval of 1000–2000 ms). Children were instructed to “shoot” the aliens as soon as alien images appeared by clicking the mouse and “save” the astronauts by withholding from clicking when astronaut images appeared. After five practice trials, children completed three blocks of 100 trials, with a ratio of 75:25 go:no-go stimuli. Behavioral data were recorded, including task accuracy and reaction times for go and no-go trials.
EEG Data Acquisition and Processing
EEG data was recorded at 9 electrode sites (Fz, FCz, Cz, Pz, Oz, F3, F4, CMS, and DRL) using an elastic cap, along with two electrodes on the left and right mastoids, using a BioSemi ActiveTwo system (BioSemi, Amsterdam, Netherlands) during the EEG go/no-go task described above. The electrooculogram (EOG) data were collected using four electrodes: two each 1 cm outside the outer edge of the right and left eyes to record horizontal eye movements and two each 1 cm above and below the right eye to capture vertical eye movements and blinks. The EEG signal was preamplified at the electrode and amplified with a gain of one. Online, all active electrodes were referenced to a common mode sense (CMS) active electrode producing a monopolar (non-differential) channel. EEG were recorded with a low-pass fifth order sinc filter with a half-power cutoff of 204.8 Hz and digitized at a 2-bit resolution with a sampling rate of 1024 Hz.
Offline, EEG data were referenced to the mean of the left and right mastoids and band-pass filtered between 0.1 and 30 Hz. Ocular artifacts (i.e., eye blinks and eye movement) were corrected following the steps outlined in Gratton et al. (1983). Specific intervals containing artifacts were eliminated from individual channels in each trial. Artifacts were detected and rejected using a semi-automatic approach if the interval included a voltage step of more than 50.0 μV between sample points, a voltage difference of 300.0 μV within a trial, or a maximum voltage difference of less than 0.50 μV within 100-ms intervals. All EEG processing was conducted by trained researchers masked to child diagnostic status and without access to the executive function/cognitive control data.
The EEG was segmented to −500 to 1000 ms before and after response onset for each trial and the response-locked ERPs (ERN and Pe) were averaged separately for correct and error trial types. Response-locked ERPs were baseline corrected using the pre-response interval between −500 and −300 ms. The ERN and Pe were extracted from electrodes where error-related brain activity was maximal (i.e., FCz, Pz) based on the current study data. The ERN was defined as the average voltage between 0 and 100 ms following the response for each child and calculated separately for error and correct trials. This method for extracting the ERN was selected over peak detection due to increased split-half reliability (Spearman-Brown adjusted) in the current sample (error trials: r = .61 vs. .49; correct trials: r = .76 vs. .66)ii. The Pe was defined as the mean voltage between 200 and 500 ms following the response and calculated separately for error and correct trials. The Pe’s split-half reliability (Spearman Brown adjusted) in the current sample was similar to the ERN (error: r = .67; correct: r = .68).
Validity Check.
A minimum of six commission errors during the EEG go/no-go task was required for ERP extraction, as recommended (Meyer et al., 2014), and all participants in the current sample met this threshold (M = 29.77, SD = 9.02; range = 10–50). Inspection of task performance data showed a mean reaction time of 439.12 ms (SD=74.57) on correct go-trials. Within-subjects ANOVAs with trial type (error, correct) were conducted to confirm that error-related ERPs were elicited/captured by the task. For the ERN, neural activity was more negative during error trials (M = −9.56, SD = 8.12) compared to correct trials (M = 1.21, SD = 5.67; η2p = 0.63; p < .001) between 0 and 100 ms after response as expected (Figure 1, left). For the Pe, neural activity was more positive during error trials (M = 14.42, SD = 9.73) compared to correct trials (M = 3.62, SD = 5.69; η2p = 0.57; p < .001) between 200 and 500 ms after response as expected (Figure 1, right). In other words, error trials elicited significant and large/very large magnitude ERN and Pe.
Figure 1.

ERN and Pe Grand Average Waveforms
Note. Grand average correct, error, and difference waveforms at FCz and Pz. The error-related negativity (ERN) was extracted from FCz (0 to 100 ms) and the error positivity (Pe) was extracted from Pz (200 to 500 ms). The difference wave represents activity during error trials minus activity during correct trials.
ERN and Pe Dependent Variables.
Following Meyer et al. (2017), the ERN and Pe were computed as unstandardized residualized difference scores (average voltage for correct trials regressed out of average voltage for error trials) to reflect the difference wave between correct and erroneous responsesiii, separately for the ERN (R2 = .09, p = .03) and Pe (R2 = .11, p = .02). More negative values reflect a larger ERN and more positive values reflect a larger Pe.
ADHD Symptoms
The ADHD Rating Scale (ADHD-RS-5; DuPaul et al., 2016) was used to assess the frequency and severity of DSM ADHD symptoms in children and adolescents (18 items; 4-point Likert scale). The ADHD-RS-5 comprises two symptom subscales: Inattention (9 items) and Hyperactivity-Impulsivity (9 items). Psychometric support for the ADHD-RS-5 includes high internal consistency (α=0.94) and test-retest reliability (r=0.79 to 0.85; DuPaul et al., 2016). Teacher-reported ADHD symptoms were selected a priori given evidence that teacher-reported ADHD symptoms are more strongly associated with executive function task performance (Cho et al., 2011; Wang et al., 2015) and more predictive of ADHD diagnosis (Tripp et al., 2006). Higher raw scores reflect greater quantity/severity of ADHD symptoms.
Anxiety Symptoms
The Multidimensional Anxiety Scale for Children 2nd Edition Self-Report (MASC-2; March, 2012) was added for sensitivity analyses given evidence linking anxiety with increased error-related neural activity (Meyer, 2016). The MASC-2 Total Score measures the overall extent to which the child is experiencing anxiety symptoms. Child self-reported anxiety was used because self-reports appear to be more sensitive to early symptom emergence than parent reports (Cole et al., 2002); the presence/absence of anxiety diagnoses based on differential diagnoses considering all informant data was also probed as described below. The MASC-2 Total Score has demonstrated high internal consistency (α=.92) and 1- to 4-week test-retest reliability (r=.89; March, 2012). Higher scores reflect greater quantity/severity of anxiety symptoms.
Global Intellectual Functioning (IQ) and Socioeconomic Status (SES)
All children were administered the Short Form of the WISC-V (Wechsler, 2014). Hollingshead (1975) SES was estimated based on caregiver(s)’ education and occupation.
Data Analysis Overview
We used a series of bias-corrected, bootstrapped conditional effects modeling using the R package medmod as implemented in jamovi v.1.6.23 (the jamovi project, 2021) separately for the ERN and Pe to examine the relations between each executive function (working memory, inhibitory control, and set shifting), the ERN/Pe, and ADHD symptoms. As such, these analyses were used to determine whether each executive function predicted ADHD symptoms and the extent to which these hypothesized relations were conveyed via associations between executive function and the ERN/Pe. Based on prior research indicating directional, if not causal, effects of executive functioning on ADHD behavioral symptom frequency/severity (e.g., Kofler et al., 2010; Rapport et al., 2009), the executive functions were modeled as predictors of ADHD symptoms. Each executive function was also modeled as a predictor of the ERN and Pe to determine the extent to which variability in the ERN and Pe reflected executive function abilities.
Finally, the ERN and Pe were modeled as predictors of ADHD symptoms as we were interested in the extent to which the hypothesized aspects of executive function shared between the ERN/Pe and executive function tests predict ADHD symptom expression. Our a priori plan called for modeling ADHD symptoms as a whole to conserve power; sensitivity analyses were added to probe the extent to which the findings were driven by one or both ADHD symptom domains (inattention, hyperactivity/ impulsivity). Age was controlled in all models. As described below, additional sensitivity analyses indicated that the primary model findings were generally robust to control for sex, SES, anxiety, separating overall ADHD symptoms into separate inattentive and hyperactive/impulsive symptom clusters, and modeling overall executive functioning/cognitive control instead of the 3 primary executive functions separately.
Notably, the current study is cross-sectional, inhibiting our ability to test competing models regarding the direction of effects (i.e., reversing arrows does not distinguish plausible models; Thoemmes, 2015). Effects are statistically significant if their 95% CI does not contain 0.0. Effect ratios (ER) for significant indirect effects indicate the proportion of the total effect (c pathway) that is conveyed via the indirect pathway (ab; i.e., ER=ab/c), and reflect the extent to which the ERN/Pe and the executive function performance metrics are capturing the same components of executive function/cognitive control that predict ADHD behavioral symptoms.
Results
Power Analysis
Power analysis for the conditional effects models was conducted using Monte Carlo simulation (Schoemann et al., 2017). Standardized path coefficients were imputed iteratively to delineate the proposed path model. Results indicated that for α=.05, our N of 53 is expected to detect significant effects at power=.80, assuming medium/large associations between executive functions and the ERN/Pe, medium associations between the ERN/Pe and ADHD symptoms, and conservatively assuming partial mediation (i.e., small/medium associations between executive functions and ADHD symptoms remaining after accounting for the ERN and Pe). Effects of this magnitude were considered reasonable given previous evidence of (a) medium to large relations between executive functions and the ERN and Pe (η2=.19-.25, r=.27-.72; Coleman et al., 2018; Miller et al., 2012; Grammer et al., 2018; Mathewson et al., 2005; Larson & Clayson, 2011); (b) medium relations between the ERN and Pe and ADHD symptoms (d=0.21–0.68; Geburek et al., 2013; Kaiser et al., 2020); and (c) medium to large magnitude relations between executive functions and ADHD symptoms (d=0.60–1.40; Kasper et al., 2012; Kofler et al., 2019; Wright et al., 2014; Willcutt et al., 2005). Thus, we concluded that the study was likely to be sufficiently powered to detect clinically meaningful effects.
Preliminary Analyses
Each of the independent and dependent variables were screened for univariate outliers, defined as values three standard deviations above or below the mean. Outliers were corrected to the next most extreme value in the sample (1.18% of data points affected). Missing data were imputed using expectation maximization based on all available data and were determined to be missing completely at random (Little’s MCAR test: χ2 = 34.08, p > .99). This affected 2.25% of data points. Sample demographics are shown in Table 2. The zero-order correlation matrix is shown in Table 3.
Table 2.
Sample and demographic characteristics
| Variable | M | SD | Min | Max |
|---|---|---|---|---|
|
| ||||
| N (Boys/Girls) | 53 (37/16) | |||
| Age | 10.36 | 1.42 | 8.22 | 12.84 |
| SES | 49.28 | 9.46 | 31 | 66 |
| FSIQ | 101.70 | 14.37 | 73 | 128 |
| Ethnicity (B, BR, H, W) | (6, 4, 2, 41) | |||
| Teacher Total ADHD Symptoms (raw score) | 28.42 | 12.96 | 2 | 51 |
| Executive Functioning (z-scores) | ||||
| Working Memory Component Score | −.12 | 1.03 | −3.27 | 1.65 |
| Inhibitory Control Component Score | .05 | .77 | −1.37 | 3.14 |
| Set Shifting Component Score | −.15 | .70 | −1.45 | 2.03 |
| Executive Function Component Score | −.14 | 1.01 | −3.27 | 1.74 |
| EEG Error-Related ERPs | ||||
| ERN (residualized score) | 0.00 | 7.73 | −13.32 | 16.08 |
| ERN (error trials)* | −9.56 | 8.12 | −24.97 | 3.48 |
| ERN (correct trials)* | 1.21 | 5.67 | −8.36 | 17.49 |
| Pe (residualized score) | 0.11 | 9.23 | −17.56 | 27.07 |
| Pe (error trials)* | 14.42 | 9.73 | −4.58 | 41.10 |
| Pe (correct trials)* | 3.62 | 5.69 | −11.85 | 16.74 |
Note: B = Black and/or African American; BR = Biracial; H = Hispanic and/or Latino; SES = Hollingshead SES total score; FSIQ = Short-Form IQ; W = White/Non-Hispanic
amplitudes are in microvolts (μV)
Table 3.
Zero-order and partial correlations
| ERN | Pe | WM | IC | SS | General EF | ADHD Symptoms | Sex | Age | SES | SFIQ | EEG GNG Commission Errors | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| ERN | -- | .36 ** | −.08 | −.14 | −.06 | −.03 | −.01 | .06 | -- | −.08 | .07 | −.22 |
| Pe | .36** | -- | .08 | −.30* | −.05 | .18 | −.02 | −.17 | -- | −.01 | .20 | −.11 |
| WM | −.04 | .06 | -- | .20 | .31 * | .93 ** | −.22 | .00 | -- | .16 | .44 ** | −.19 |
| IC | −.14 | −.28 | −.06 | -- | .00 | −.17 | .25 | −.01 | -- | −.07 | −.11 | .25 |
| SS | −.07 | −.05 | .13 | .07 | -- | .35 * | −.07 | .19 | -- | .01 | .39 ** | −.03 |
| General EF | .00 | .13 | .96** | −.35* | .13 | -- | −.31* | .02 | -- | .18 | .50 ** | −.29* |
| ADHD Symptoms | −.02 | −.02 | −.36** | .33* | −.01 | −.43** | -- | −.22 | -- | −.05 | −.11 | .26 |
| Sex | .06 | −.17 | −.01 | .00 | .19 | .00 | .20 | -- | -- | .20 | −.07 | .07 |
| Age | .04 | −.01 | .60** | −.35** | −.18 | .66** | −.32* | −.02 | -- | -- | -- | -- |
| SES | −.08 | −.01 | .09 | −.04 | .02 | .09 | −.03 | .20 | −.06 | -- | .37 ** | .21 |
| SFIQ | −.07 | .20 | .31* | −.08 | .40** | .33* | −.08 | −.07 | −.07 | .37** | -- | −.07 |
| EEG GNG Commission Errors | −.22 | −.11 | −.11 | .21 | −.04 | −.17 | .22 | .07 | .08 | .21 | −.07 | -- |
Note. ERN: error-related negativity (residualized score), General EF: general executive function component score, GNG: Go/no-go, IC: inhibitory control factor score, Pe: error positivity (residualized score), SES: Hollingshead socioeconomic status Total Score, SFIQ: WISC-V short-form IQ score, SS: set shifting factor score, ADHD Symptoms: teacher-rated total ADHD symptoms, WM: working memory factor score. = p <.05
= p<.01.
Zero-order correlations are shown in the bottom triangle; partial correlations controlling for age are shown in italics in the upper triangle.
Conditional Effects Models
Bias-corrected conditional effects models with 5,000 bootstrap resamples were conducted, separately for the ERN (Figure 2) and Pe (Figure 3); results are organized by pathway. Results of both models indicated that better working memory (path c; β = −.34) and inhibitory control (path c; β = .31) each uniquely predicted lower levels of ADHD symptoms. In contrast, set shifting did not predict ADHD symptoms (path c; β = .02, ns).
Figure 2.

Path diagram depicting primary ERN model results. Significant pathways are indicated by 95% CIs that exclude zero, and are shown in black/bold font. Nonsignificant pathways are shown in grey font.
Note. ERN: error-related negativity (residualized score); ADHD Symptoms: teacher-rated total ADHD symptoms. Age was included as a covariate but is not depicted for clarity.
Figure 3.
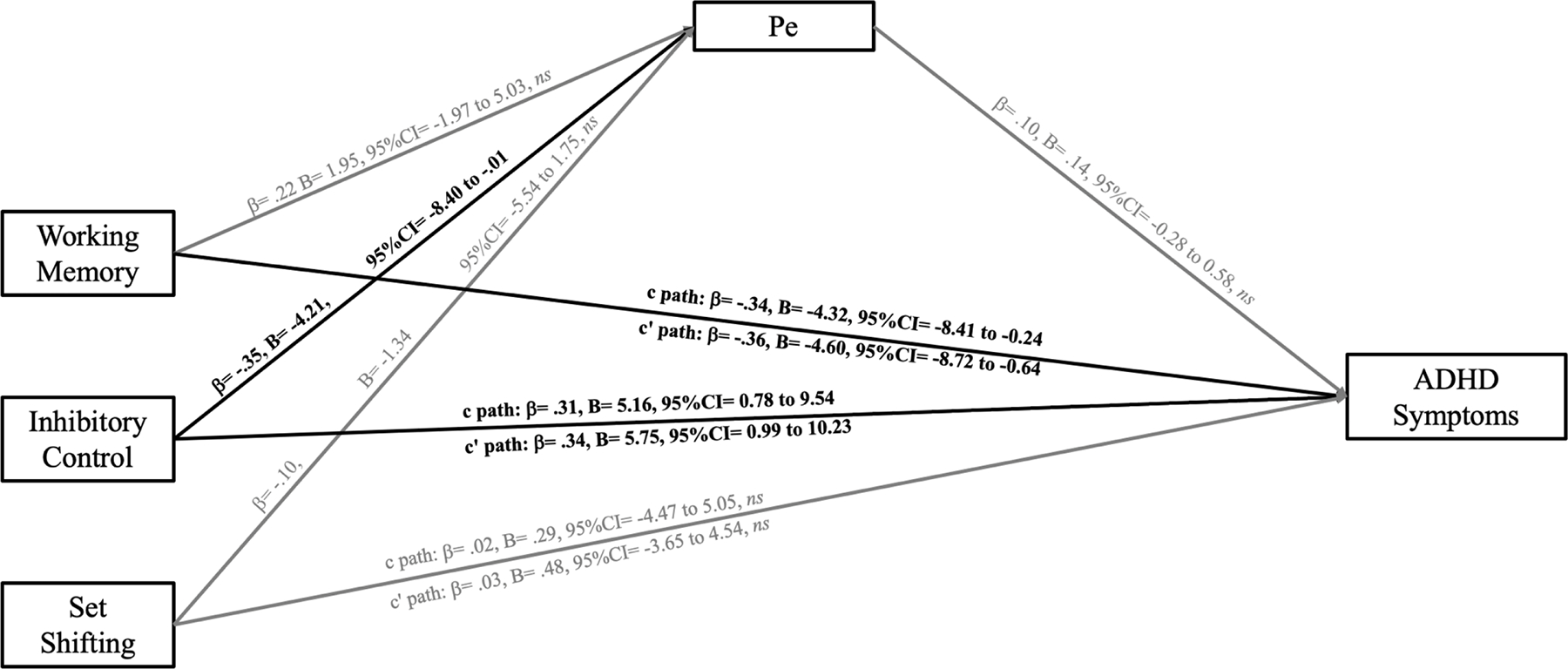
Path diagram depicting primary Pe model results. Significant pathways are indicated by 95% CIs that exclude zero, and are shown in black/bold font. Nonsignificant pathways are shown in grey font.
Note. Pe: error positivity (residualized score); ADHD Symptoms: teacher-rated total ADHD symptoms. Age was included as a covariate but is not depicted for clarity.
In the ERN model, working memory (path a; β = −.04, ns), inhibitory control (path a; β = −.14, ns), and set shifting (path a; β = −.05, ns) all failed to predict the ERN. Additionally, the ERN did not predict ADHD symptoms (path b; β = .01, ns). Further, there were no significant indirect effects of the three executive functions on ADHD symptoms through the ERN pathway (path ab; all 95% CIs include 0.0). Overall, these findings indicate that the ERN is not capturing meaningful variance in children’s executive functioning/cognitive control either overall or with regard to the aspects of working memory and inhibitory control that predict ADHD behavioral symptom expression.
In contrast, in the Pe model, better-developed inhibitory control was associated with a greater Pe (path a; β = −.35), whereas working memory (path a; β = .22, ns) and set shifting (path a; β = −.10, ns) failed to predict the Pe. Similar to the ERN model, the Pe was not significantly related to ADHD symptoms (path b; β = .10, ns) and there was no evidence to suggest conditional effects of the executive functions on ADHD symptoms through the Pe (path ab; all 95% CIs include 0.0). In other words, the Pe is capturing meaningful variance associated with inhibitory control, but similar to the ERN is not capturing the aspects of working memory and inhibitory control that are predictive of ADHD behavioral symptom expression.
Taken together, these results indicate that better working memory and inhibitory control, but not set shifting, predict fewer ADHD symptoms, whereas variability in the ERN and Pe is not capturing the aspects of working memory and inhibitory control that underlie these relations. Interestingly, better-developed inhibitory control predicted an increased Pe as hypothesized, whereas none of the three executive functions predicted the ERN. In contrast, neither the ERN nor Pe were predictive of ADHD symptoms.
Sensitivity Analyses
Sensitivity analyses were conducted to probe the extent to which the pattern of results reported above was impacted by our a priori decisions to (a) model ADHD symptoms as a whole; (b) exclude sex and SES as covariates to conserve power; (c) model the three EFs separately rather than as a single indicator reflecting overall executive functioning/cognitive control; and (d) include children with and without anxiety disorders. First, the overall ADHD symptoms variable was replaced with separate indicators for inattention and hyperactivity/ impulsivity symptoms. Better-developed working memory (β = −.34) and inhibitory control (β = .28) continued to predicted inattention symptoms, but were not significantly associated with hyperactivity/impulsivity (all 95% CIs included 0.0). Consistent with the primary models, the ERN and Pe were not significant predictors of either ADHD symptom cluster (all 95% CIs included 0.0; Supplementary Tables 2–5).
Next, we repeated the primary models again controlling for sex and SES. Results were highly consistent with those reported above, with inhibitory control significantly predicting the Pe (β = −.36); working memory (β = −.38) and inhibitory control (β = .32) predicting ADHD symptoms; and no other significant relations detected (95%CIs include 0.0; Supplementary Tables 6 & 7). Third, we probed the extent to which the ERN and/or Pe might show stronger associations with executive functioning/cognitive control in general rather than the specific executive functions. This involved creating a single executive function component score based on the same 7 executive function tests described above (21.29% of variance explained; Supplementary Table 1). We then repeated the primary models above with the overall executive functioning/cognitive control estimate instead of the three separate executive function predictors. Results were generally consistent with the primary model in that better executive functioning predicted fewer ADHD symptoms (β = −.40), whereas the ERN and Pe did not (both 95%CIs include 0.0). In contrast, overall executive functioning/cognitive control did not significantly predict the ERN (β = −.04, ns) or the Pe (β = .24, ns). There were also no indirect effects of executive functioning/cognitive control on ADHD symptoms via the ERN or Pe pathways (95% CIs included 0.0; Supplementary Tables 8 & 9).
Finally, given converging evidence linking anxiety disorders with increased error-related neural activity (Meyer, 2016), we explored the extent to which the results may have been impacted by our a priori decision to include children with anxiety disorders (n=23 of 53). This involved re-running the primary models twice, once with anxiety disorder status (No/Yes)iv and once with child-reported anxiety symptoms (MASC-2) as additional covariates. Results were generally consistent with the primary models, with better inhibitory control predicting a larger Pe (β=−.35 for both models) and no other significant relations between the ERPs and executive functions or ADHD symptoms, with one minor exception: the relations between working memory (β=−.34 in the primary model vs. −.27) and inhibitory control (β=.31 vs. .21) no longer significantly predicted ADHD symptoms as the relations were slightly attenuated when controlling for anxiety disorder status (95%CIs include 0.0). However, working memory (β=−.33) and inhibitory control (β=−.28) continued to predict ADHD symptoms when covarying self-reported anxiety symptoms.
Discussion
Although the ERN and Pe are frequently interpreted as indices of cognitive control (Gehring et al., 2012), few studies have tested this interpretation empirically outside of data from the same inhibitory control tasks used to derive the ERPs (Meyer & Hajcak, 2019) or examined whether test-based and EEG-based estimates predict the same variance in behavioral outcomes of executive function/cognitive control difficulties (i.e., ADHD symptoms). The current study addressed these limitations in a clinically evaluated and carefully phenotyped school-aged sample by examining the ERN/Pe’s shared and unique relations with ADHD symptoms and a well-validated battery of all three core executive functions. Contrary to our hypotheses, the ERN was not associated with general or specific executive functioning/cognitive control abilities, whereas a larger Pe was only related to better inhibitory control. These findings add to a largely mixed literature on error-related neural activity and cognitive control/executive function. Our results are inconsistent with studies finding relations between a larger ERN and better working memory and inhibitory control (Coleman et al., 2018; Miller et al., 2012; Overbye et al., 2019), as well as better overall cognitive control/executive function (Larson & Clayson, 2011; Meyer & Klein, 2018). In contrast, the significant relation between a larger Pe and better inhibitory control is consistent with most prior work finding that the Pe is related to performance metrics from inhibition tasks in adults (Chang et al., 2014; Hajcak et al., 2003; Mathewson et al., 2005) and children (Kim et al., 2016; Overbye et al., 2019; c.f. Grammer et al., 2018). Interestingly, the Pe was not related to cognitive control/executive function overall, and did not capture meaningful variance in inhibitory control that was associated with ADHD behavioral symptoms, potentially suggesting the need for increased specificity when interpreting this metric as discussed below.
The characteristics of the current sample may be an important factor contributing to our findings that the ERN was not associated with executive function/cognitive control, while the Pe was related to inhibitory control only. Prior work suggesting that an increased ERN is related to better working memory ability was conducted with undergraduate students (Coleman et al., 2018; Miller et al., 2012), while the current study examined a clinically evaluated, school-aged sample. Studies examining the Pe and inhibitory control have also largely focused on healthy adult populations, with the few pediatric studies focusing on children younger than our age group (Grammer et al., 2018; Kim et al., 2016) or a wider age range spanning school-age and adolescence (Overbye et al., 2019). This distinction is important given evidence for differences in the developmental trajectories of the ERN, Pe, and specific executive functions. For example, the ERN continues to increase into early adulthood and the Pe matures earlier in development (Downes et al., 2017). Similarly, working memory and inhibitory control reflect distinct abilities around the age of school entry, while set shifting only emerges as a distinct executive function in older school-aged children (Karr et al., 2018; Lerner & Lonigan, 2014). Thus, it is possible that the ERN and Pe are reflective of different cognitive functions in different age groups.
Alternatively, the linkage between inhibitory control and the Pe but not ERN may be attributable to the increased use of top-down/effortful control processes for the former relative to the latter. Inhibitory control abilities are characterized by the active and goal-directed withholding or stopping of a response that relies on deliberate and conscious control processes (Alderson et al., 2007; Mirabella, 2021). However, prior work suggests that an ERN is detectable regardless of whether an individual recognizes that they made an error, whereas the Pe is most pronounced following trials where an individual has conscious awareness of the error (Endrass et al., 2007; Nieuwenhuis et al., 2001; O’Connell et al., 2007). Thus, it seems reasonable to hypothesize that the Pe may reflect effortful inhibitory control, whereas the ERN may be a more automatic monitoring process. In other words, the Pe may represent top-down regulation of ongoing behavioral processes, while the ERN may be reflective of more automatic bottom-up processes that would be less affected by effortful cognitive control/executive function processes (Wells et al., 2019). Indeed, Boen and colleagues (2022) suggested such a distinction as a potential mechanism underlying the differing developmental trajectories of the ERN and Pe.
Interestingly, we found that the aspects of inhibitory control captured by the Pe did not overlap with those aspects that are predictive of ADHD behavioral symptoms, despite better inhibitory control abilities predicting fewer ADHD symptoms. These findings contribute to a fairly large body of mixed evidence in which about half of the studies find that a greater ERN and/or Pe is related to fewer ADHD symptoms and the other half find no such relations (Herrmann et al., 2009; Marquardt et al., 2018; Rommel et al., 2019; Wiersema et al., 2009). Similar results are seen in ADHD/Control between-group studies in the ERN (for review see Meyer & Hajcak, 2019), while a reduced Pe in ADHD groups appears to be a more consistent finding (Kaiser et al., 2020; McLoughlin et al., 2022). Given this pattern, it seems likely that these mixed results, at least for the ERN, reflect study-level sampling error around a true effect that is small or null. Alternatively, we speculated that the mixed findings may be related to most studies examining ADHD symptoms overall as opposed to probing across the ADHD inattentive and hyperactivity/impulsivity domains. Our sensitivity analyses suggest that this explanation is unlikely given that the ERN and Pe were also unrelated to both ADHD symptom domains, but future studies with larger samples would be helpful to confirm/refute this interpretation.
Limitations and Future Directions
The current study’s sample size was relatively small due to discontinued recruitment as a result of the COVID-19 pandemic. Although our sample size was similar to most prior work on this topic (e.g., Grammer et al., 2018; Marquardt et al., 2018, Miller et al, 2012; cf. Meyer & Klein, 2018; Rommel et al., 2019) and our power analyses indicated that the sample size was sufficient to detect meaningful effects, it is possible that the sample was not large enough to detect small magnitude relations with the ERN and Pe in the current study. However, we found significant associations between better inhibitory control and an increased Pe, which is consistent with prior work (Kaiser et al., 2020; McLoughlin et al., 2022), and robust relations between working memory/inhibitory control and ADHD symptoms. To the extent that the true relation between the ERN and ADHD symptoms is close to zero as suggested by prior literature (Kaiser et al., 2020; Meyer & Hajcak, 2019), it seems likely that much larger samples would be needed to reliably detect this effect if it is present. Interestingly, while go/no-go tasks seem to elicit greater group differences in the Pe than flanker tasks (Geburek et al., 2013; Lutz et al., 2021), the opposite may be true of the ERN (McLoughlin et al., 2022). Thus, it is possible that our use of a go/no-go task during EEG recording may have optimized the potential to observe relations between the Pe, but not the ERN, and ADHD symptoms. In contrast, the lack of ERP relations with overall and specific executive functions in 7 of 8 cases (with the only significant relation being the Pe and inhibitory control) – as well as the non-significant associations between the ERPs and commission errors on the EEG go/no-go task – suggests challenges for attributing the same construct to these performance-based and electrophysiological measures. That is, if the current findings are replicated in larger and more diverse samples, a parsimonious conclusion would be that both the performance-based tests and error-related ERP metrics are reliable assessment methods, but are primarily measuring different constructs – or at best that terms like ‘cognitive control/executive function’ are too broad to accurately capture the cognitive processes reflected by the ERPs. This latter hypothesis is consistent with our finding that the ERN/Pe were not related to ADHD symptoms, and the Pe is not capturing the aspects of inhibitory control important for understanding children’s inattentive or hyperactive/impulsive behavior.
Importantly, the current study recruited a clinically heterogeneous sample. Although more representative of pediatric populations seeking assessment for behavior and emotional concerns that have been linked with executive function/cognitive control (e.g., Kofler et al., 2019; Snyder, 2013), it is possible that the diverse clinical symptom presentation complicated our ability to detect relations between the ERN/Pe and executive function/cognitive control. In particular, an increased ERN is frequently reported in children with anxiety disorders, and has been proposed to be a biomarker of anxiety (Meyer, 2016). In contrast, the relation between the Pe and anxiety is more variable (Hajcak et al., 2004; Ladouceur et al., 2006; McDermott et al., 2009). Thus, we conducted sensitivity analyses to examine the extent that our results were confounded by the inclusion of children with anxiety disorders in the sample. We found that the ERP primary findings held with anxiety disorder status and child self-report of anxiety symptoms covaried, suggesting increased confidence in the findings. A more nuanced examination of this topic in future research is warranted given (a) the empirical and theorized relations between anxiety and both error-related neural activity and executive functions (Derakshan et al., 2009; Eysenck et al., 2007; Meyer, 2016; Wong et al., 2013); (b) the multicomponent nature of inhibitory control (e.g., Alderson et al., 2008); and (c) the current finding that the Pe did not capture the variance in inhibitory control that is important for predicting ADHD symptoms. A speculative hypothesis is that the Pe may instead be capturing aspects of inhibitory control that are linked with anxiety symptoms and/or other behavioral expressions rather than ADHD symptoms (e.g., Lutz et al., 2021), particularly given evidence that anxiety may serve as a protective factor for children with ADHD in at least some domains (Chan et al., 2022; Klymkiw et al., 2020; Maric et al., 2018).
Conclusion
Taken together, the current study found that the Pe appears to be capturing meaningful variance associated with inhibitory control abilities, whereas neither the Pe nor the ERN were associated with overall cognitive control/executive functioning in school-aged children. These findings call into question the use of ERN and/or Pe as broad indices of cognitive control and, if replicated, suggest the need for more specificity in the construct reification of these reliable indices. Based on results from the current study, we hypothesize that the Pe may be reflective of the top-down, effortful components of inhibitory control (Boen et al., 2022.; Nieuwenhuis et al., 2003; O’Connell et al., 2007) that are distinct from the aspects of inhibitory control implicated in the behavioral symptomatology of ADHD. In contrast, the ERN appears to reflect more passive monitoring processes that do not overlap meaningfully with the active, top-down processes that characterize cognitive control/executive function (Boen et al., 2022; Nieuwenhuis et al., 2001; O’Connell et al., 2007). Further research is needed to replicate these findings and identify additional cognitive and non-cognitive processes that are reflected in the ERN and Pe, toward a more refined understanding of the processes that these reliable ERP metrics are, and are not, capturing, and their utility in study of neurocognitive processes in ADHD.
Supplementary Material
Acknowledgements:
This work was supported by NIH grant R01 MH115048 (PI: Kofler). The sponsor had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Nigg (2017) concluded that cognitive control overlaps significantly with specific facets of executive function, particularly the core ‘lower-level’ executive functions that appear first developmentally (working memory and response inhibition, followed by set shifting; Diamond, 2013; Karr et al., 2018; Nigg, 2017). The subtle distinction between the two terms is rooted in their development within different disciplines (Nigg, 2017). Given that cognitive control and executive functioning are closely related constructs from separate disciplines, we use the terms interchangeably in the present study. Relatedly, our use of the term ‘executive functions’ is consistent with Nigg (2017)’s conceptualization of ‘lower-level executive functions’, which reflect the ‘core’ executive functions in the Miyake et al. (2000) model (Kofler et al., 2019).
Sensitivity analyses were conducted using Peak scores for the ERN and produced no deviations from the primary study analyses.
Sensitivity analyses were conducted using differences scores for the ERN and Pe (average voltage for error trials minus average voltage for correct trials) and produced no deviations from the primary study analyses.
Due to prior research demonstrating that generalized anxiety, social anxiety, and obsessive-compulsive disorders are most reliably associated with error-related neural activity (Meyer et al. 2016), the models were also run only covarying the presence of these three disorders. The results were unchanged from those reported when anxiety disorder status was included as a covariate.
Conflict of Interest:
The principal investigator (Michael Kofler) holds a patent for a neurocognitive intervention that targets central executive working memory and is licensed to Sky Therapeutics, where Michael Kofler serves as Chief Science Officer and consultant. This intervention was not used in the current study, and there are no other current licensing, financial, or other conflicts to report.
References
- Alderson RM, Rapport MD, & Kofler MJ (2007). Attention-deficit/hyperactivity disorder and behavioral inhibition: A meta-analytic review of the stop-signal paradigm. Journal of Abnormal Child Psychology, 35(5), 745–758. [DOI] [PubMed] [Google Scholar]
- Alderson RM, Rapport MD, Sarver DE, & Kofler MJ (2008). ADHD and behavioral inhibition: A re-examination of the stop-signal task. Journal of Abnormal Child Psychology, 36(7), 989–998. [DOI] [PubMed] [Google Scholar]
- Baddeley A (2007). Working memory, thought, and action. Oxford University Press. [Google Scholar]
- Best JR, & Miller PH (2010). A developmental perspective on executive function. Child Development, 81(6), 1641–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Miller PH, & Jones LL (2009). Executive functions after age 5: Changes and correlates. Developmental Review, 29(3), 180–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boen R, Quintana DS, Ladouceur CD, & Tamnes CK (2022). Age-related differences in the error-related negativity and error positivity in children and adolescents are moderated by sample and methodological characteristics: A meta-analysis. Psychophysiology, 59(6), e14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, & Cohen JD (2014). The computational and neural basis of cognitive control: Charted territory and new frontiers. Cognitive Science, 38(6), 1249–1285. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, & Carter CS (2004). Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences, 8(12), 539–546. [DOI] [PubMed] [Google Scholar]
- Cavanagh JF, & Shackman AJ (2015). Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. Journal of Physiology, Paris, 109(1–3), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ESM, Groves NB, Marsh CL, Miller CE, Richmond KP, & Kofler MJ (2022). Are there resilient children with ADHD? Journal of Attention Disorders, 26(5), 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A, Chen C-C, Li H-H, & Li C-SR (2014). Event-related potentials for post-error and post-conflict slowing. PLOS ONE, 9(6), e99909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S-C, Kim H-W, Kim B-N, Shin M-S, Yoo HJ, Kim J-W, Bhang S-Y, & Cho IH (2011). Are teacher ratings and parent ratings differently associated with children’s intelligence and cognitive performance? Psychiatry Investigation, 8(1), 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DA, Tram JM, Martin JM, Hoffman KB, Ruiz MD, Jacquez FM, & Maschman TL (2002). Individual differences in the emergence of depressive symptoms in children and adolescents: A longitudinal investigation of parent and child reports. Journal of Abnormal Psychology, 111(1), 156–165. [PubMed] [Google Scholar]
- Coleman JR, Watson JM, & Strayer DL (2018). Working memory capacity and task goals modulate error-related ERPs. Psychophysiology, 55(3), e12805. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, & Engle RW (2005). Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review, 12(5), 769–786. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, & Eysenck MW (2009). Anxiety, inhibition, efficiency, and effectiveness. Experimental Psychology, 56(1), 48–55. [DOI] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64(1), 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distefano C, Zhu M, & Mîndrilă D (2009). Understanding and using factor scores: Considerations for the applied researcher. Practical Assessment, Research, and Application, 14(20), 1–11. [Google Scholar]
- Downes M, Bathelt J, & De Haan M (2017). Event-related potential measures of executive functioning from preschool to adolescence. Developmental Medicine and Child Neurology, 59(6), 581–590. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, & Reid R (2016). ADHD Rating Scale-5 for children and adolescents: Checklists, norms, and clinical interpretation. Guilford Press. [Google Scholar]
- Endrass T, Reuter B, & Kathmann N (2007). ERP correlates of conscious error recognition: Aware and unaware errors in an antisaccade task. The European Journal of Neuroscience, 26(6), 1714–1720. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, & Calvo MG (2007). Anxiety and cognitive performance: Attentional control theory. Emotion, 7(2), 336–353. [DOI] [PubMed] [Google Scholar]
- Falkenstein M (2004). Errors, conflicts, and the brain: A review of the contributions to the error conference, Dortmund 2003. Journal of Psychophysiology, 18(4), 153–163. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, & Blanke L (1991). Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology, 78(6), 447–455. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, & Hohnsbein J (2000). ERP components on reaction errors and their functional significance: A tutorial. Biological Psychology, 51(2–3), 87–107. [DOI] [PubMed] [Google Scholar]
- Fosco WD, Kofler MJ, Groves NB, Chan ESM, & Raiker JS (2020). Which “working” components of working memory aren’t working in youth with ADHD? Journal of Abnormal Child Psychology, 48(5), 647–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geburek AJ, Rist F, Gediga G, Stroux D, & Pedersen A (2013). Electrophysiological indices of error monitoring in juvenile and adult attention deficit hyperactivity disorder (ADHD)—A meta-analytic appraisal. International Journal of Psychophysiology, 87(3), 349–362. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, & Donchin E (1993). A neural system for error detection and compensation. Psychological Science, 4(6), 385–390. [Google Scholar]
- Gehring WJ, Liu Y, Orr JM, & Carp J (2012). The error-related negativity (ERN/Ne). In Luck SJ & Kappenman ES (Eds.), The Oxford handbook of event-related potential components (pp. 231–291). Oxford University Press. [Google Scholar]
- Gomez R, & Sanson AV (1994). Effects of experimenter and mother presence on attentional performance and activity of hyperactive boys. Journal of Abnormal Child Psychology, 22(5), 517–529. [DOI] [PubMed] [Google Scholar]
- Gorday JY, & Meyer A (2018). Linking puberty and error-monitoring: Relationships between self-reported pubertal stages, pubertal hormones, and the error-related negativity in a large sample of children and adolescents. Developmental Psychobiology, 60(4), 483–490. [DOI] [PubMed] [Google Scholar]
- Grammer JK, Gehring WJ, & Morrison FJ (2018). Associations between developmental changes in error-related brain activity and executive functions in early childhood. Psychophysiology, 55(3), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MGH, & Donchin E (1983). A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology, 55(4), 468–484. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Klawohn J, & Meyer A (2019). The utility of event-related potentials in clinical psychology. Annual Review of Clinical Psychology, 15, 71–95. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, & Simons RF (2003). To err is autonomic: Error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology, 40(6), 895–903. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, & Simons RF (2004). Error-related psychophysiology and negative affect. Brain and Cognition, 56(2), 189–197. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Saathoff C, Schreppel TJ, Ehlis A-C, Scheuerpflug P, Pauli P, & Fallgatter AJ (2009). The effect of ADHD symptoms on performance monitoring in a non-clinical population. Psychiatry Research, 169(2), 144–148. [DOI] [PubMed] [Google Scholar]
- Hirsh JB, & Inzlicht M (2010). Error-related negativity predicts academic performance. Psychophysiology, 47(1), 192–196. [DOI] [PubMed] [Google Scholar]
- Irwin LN, Kofler MJ, Soto EF, & Groves NB (2019). Do children with attention-deficit/hyperactivity disorder (ADHD) have set shifting deficits? Neuropsychology, 33(4), 470–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A, Aggensteiner P-M, Baumeister S, Holz NE, Banaschewski T, & Brandeis D (2020). Earlier versus later cognitive event-related potentials (ERPs) in attention-deficit/hyperactivity disorder (ADHD): A meta-analysis. Neuroscience and Biobehavioral Reviews, 112, 117–134. [DOI] [PubMed] [Google Scholar]
- Kane MJ, & Engle RW (2003). Working-memory capacity and the control of attention: The contributions of goal neglect, response competition, and task set to Stroop interference. Journal of Experimental Psychology: General, 132(1), 47–70. [DOI] [PubMed] [Google Scholar]
- Karr JE, Areshenkoff CN, Rast P, Hofer SM, Iverson GL, & Garcia-Barrera MA (2018). The unity and diversity of executive functions: A systematic review and re-analysis of latent variable studies. Psychological Bulletin, 144(11), 1147–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper LJ, Alderson RM, & Hudec KL (2012). Moderators of working memory deficits in children with attention-deficit/hyperactivity disorder (ADHD): A meta-analytic review. Clinical Psychology Review, 32(7), 605–617. [DOI] [PubMed] [Google Scholar]
- Kim MH, Grammer JK, Marulis LM, Carrasco M, Morrison FJ, & Gehring WJ (2016). Early math and reading achievement are associated with the error positivity. Developmental Cognitive Neuroscience, 22, 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymkiw DF, Milligan K, Lackner C, Phillips M, Schmidt LA, & Segalowitz SJ (2020). Does anxiety enhance or hinder attentional and impulse control in youth with ADHD? An ERP analysis. Journal of Attention Disorders, 24(12), 1746–1756. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Irwin LN, Soto EF, Groves NB, Harmon SL, & Sarver DE (2019). Executive functioning heterogeneity in pediatric ADHD. Journal of Abnormal Child Psychology, 47(2), 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, Sarver DE, & Raiker JS (2010). ADHD and working memory: The impact of central executive deficits and exceeding storage/rehearsal capacity on observed inattentive behavior. Journal of Abnormal Child Psychology, 38(2), 149–161. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, & Ryan ND (2006). Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry, 47(10), 1073–1082. [DOI] [PubMed] [Google Scholar]
- Larson MJ, & Clayson PE (2011). The relationship between cognitive performance and electrophysiological indices of performance monitoring. Cognitive, Affective & Behavioral Neuroscience, 11(2), 159–171. [DOI] [PubMed] [Google Scholar]
- Lerner MD, & Lonigan CJ (2014). Executive function among preschool children: Unitary versus distinct abilities. Journal of Psychopathology and Behavioral Assessment, 36(4), 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo SL, Schroder HS, Fisher ME, Durbin CE, Fitzgerald KD, Danovitch JH, & Moser JS (2017). Associations between disorder-specific symptoms of anxiety and error-monitoring brain activity in young children. Journal of Abnormal Child Psychology, 45(7), 1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz MC, Kok R, Verveer I, Malbec M, Koot S, van Lier PAC, & Franken IHA (2021). Diminished error-related negativity and error positivity in children and adults with externalizing problems and disorders: A meta-analysis on error processing. Journal of Psychiatry & Neuroscience: JPN, 46(6), E615–E627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric M, Bexkens A, & Bögels SM (2018). Is clinical anxiety a risk or a protective factor for executive functioning in youth with ADHD? A Meta-regression analysis. Clinical Child and Family Psychology Review, 21(3), 340–353. [DOI] [PubMed] [Google Scholar]
- Marquardt L, Eichele H, Lundervold AJ, Haavik J, & Eichele T (2018). Event-related-potential (ERP) correlates of performance monitoring in adults with attention-deficit hyperactivity disorder (ADHD). Frontiers in Psychology, 9(485), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KJ, Dywan J, & Segalowitz SJ (2005). Brain bases of error-related ERPs as influenced by age and task. Biological Psychology, 70(2), 88–104. [DOI] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, & Fox NA (2009). A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biological Psychiatry, 65(5), 445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin G, Gyurkovics M, & Aydin Ü (2022). What has been learned from using EEG methods in research of ADHD? Current Topics in Behavioral Neurosciences, 57, 415–444. [DOI] [PubMed] [Google Scholar]
- Mehra LM, Hajcak G, & Meyer A (2022). The relationship between stressful life events and the error-related negativity in children and adolescents. Developmental Cognitive Neuroscience, 55, 101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A (2016). Developing psychiatric biomarkers: A review focusing on the error-related negativity as a biomarker for anxiety. Current Treatment Options in Psychiatry, 3, 356–364. [Google Scholar]
- Meyer A, Bress JN, & Proudfit GH (2014). Psychometric properties of the error-related negativity in children and adolescents. Psychophysiology, 51(7), 602–610. [DOI] [PubMed] [Google Scholar]
- Meyer A, & Hajcak G (2019). A review examining the relationship between individual differences in the error-related negativity and cognitive control. International Journal of Psychophysiology, 144, 7–13. [DOI] [PubMed] [Google Scholar]
- Meyer A, & Klein DN (2018). Examining the relationships between error-related brain activity (the ERN) and anxiety disorders versus externalizing disorders in young children: Focusing on cognitive control, fear, and shyness. Comprehensive Psychiatry, 87, 112–119. [DOI] [PubMed] [Google Scholar]
- Meyer A, Lerner MD, De Los Reyes A, Laird RD, & Hajcak G (2017). Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology, 54(1), 114–122. [DOI] [PubMed] [Google Scholar]
- Miller AE, Watson JM, & Strayer DL (2012). Individual differences in working memory capacity predict action monitoring and the error-related negativity. Journal of Experimental Psychology. Learning, Memory, and Cognition, 38(3), 757–763. [DOI] [PubMed] [Google Scholar]
- Mirabella G (2021). Inhibitory control and impulsive responses in neurodevelopmental disorders. Developmental Medicine & Child Neurology, 63(5), 520–526. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, & Wager TD (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. [DOI] [PubMed] [Google Scholar]
- Navon D (1977). Forest before trees: The precedence of global features in visual perception. Cognitive Psychology, 9(3), 353–383. [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, & Kok A (2001). Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology, 38(5), 752–760. [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, & Ridderinkhof KR (2003). Electrophysiological correlates of anterior cingulate function in a go/no-go task: Effects of response conflict and trial type frequency. Cognitive, Affective, & Behavioral Neuroscience, 3(1), 17–26. [DOI] [PubMed] [Google Scholar]
- Nigg JT (2017). Annual research review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58(4), 361–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell R, Dockree P, Bellgrove M, Kelly S, Hester R, Garavan H, Robertson I, & Foxe J (2007). The role of cingulate cortex in the detection of errors with and without awareness: A high-density electrical mapping study. The European Journal of Neuroscience, 25, 2571–2579. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, & Ridderinkhof KR (2005). Dissociable components of error processing: On the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology, 19(4), 319–329. [Google Scholar]
- Overbye K, Walhovd KB, Paus T, Fjell AM, Huster RJ, & Tamnes CK (2019). Error processing in the adolescent brain: Age-related differences in electrophysiology, behavioral adaptation, and brain morphology. Developmental Cognitive Neuroscience, 38, 100665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapport MD, Bolden J, Kofler MJ, Sarver DE, Raiker JS, & Alderson RM (2009). Hyperactivity in boys with attention-deficit/hyperactivity disorder (ADHD): A ubiquitous core symptom or manifestation of working memory deficits? Journal of Abnormal Child Psychology, 37(4), 521–534. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Orban SA, Kofler MJ, & Friedman LM (2013). Do programs designed to train working memory, other executive functions, and attention benefit children with ADHD? A meta-analytic review of cognitive, academic, and behavioral outcomes. Clinical Psychology Review, 33(8), 1237–1252. [DOI] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A, & Hajcak G (2013). The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biological Psychology, 93(3), 377–385. [DOI] [PubMed] [Google Scholar]
- Rommel A-S, James S-N, McLoughlin G, Michelini G, Banaschewski T, Brandeis D, Asherson P, & Kuntsi J (2019). Impairments in error processing and their association with ADHD symptoms in individuals born preterm. PLOS ONE, 14(4), e0214864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver DE, Rapport MD, Kofler MJ, Raiker JS, & Friedman LM (2015). Hyperactivity in attention-deficit/hyperactivity disorder (ADHD): Impairing deficit or compensatory behavior? Journal of Abnormal Child Psychology, 43(7), 1219–1232. [DOI] [PubMed] [Google Scholar]
- Schoemann AM, Boulton AJ, & Short SD (2017). Determining power and sample size for simple and complex mediation models. Social Psychological and Personality Science, 8(4), 379–386. [Google Scholar]
- Shiels K, & Hawk LW (2010). Self-regulation in ADHD: The role of error prrocessing. Clinical Psychology Review, 30(8), 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, & Hankin BL (2015). Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology, 6(328), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreni N, Crosbie J, Ickowicz A, & Schachar R (2009). Stop signal and Conners’ continuous performance tasks: Test--retest reliability of two inhibition measures in ADHD children. Journal of Attention Disorders, 13(2), 137–143. [DOI] [PubMed] [Google Scholar]
- Soto EF, Kofler MJ, Singh LJ, Wells EL, Irwin LN, Groves NB, & Miller CE (2020). Executive functioning rating scales: Ecologically valid or construct invalid? Neuropsychology, 34(6), 605–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoemmes F (2015). Reversing arrows in mediation models does not distinguish plausible models. Basic and Applied Social Psychology, 37(4), 226–234. [Google Scholar]
- Tripp G, Schaughency EA, & Clarke B (2006). Parent and teacher rating scales in the evaluation of attention-deficit hyperactivity disorder: Contribution to diagnosis and differential diagnosis in clinically referred children. Journal of Developmental & Behavioral Pediatrics, 27(3), 209–218. [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Engle RW (2007). The nature of individual differences in working memory capacity: Active maintenance in primary memory and controlled search from secondary memory. Psychological Review, 114(1), 104–132. [DOI] [PubMed] [Google Scholar]
- van Veen V, & Carter CS (2002). The anterior cingulate as a conflict monitor: FMRI and ERP studies. Physiology & Behavior, 77(4–5), 477–482. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Chambers CD, & Logan GD (2013). Fictitious inhibitory differences: How skewness and slowing distort the estimation of stopping latencies. Psychological Science, 24(3), 352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-J, Chen C-K, & Huang Y-S (2015). Neurocognitive performance and behavioral symptoms in patients with attention-deficit/hyperactivity disorder during twenty-four months of treatment with methylphenidate. Journal of Child and Adolescent Psychopharmacology, 25(3), 246–253. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, & Hajcak G (2012). Integrating multiple perspectives on error-related brain activity: The ERN as a neural indicator of trait defensive reactivity. Motivation and Emotion, 36(1), 84–100. [Google Scholar]
- Wells EL, Day TN, Harmon SL, Groves NB, & Kofler MJ (2019). Are emotion recognition abilities intact in pediatric ADHD? Emotion, 19(7), 1192–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells EL, Kofler MJ, Soto EF, Schaefer HS, & Sarver DE (2018). Assessing working memory in children with ADHD: Minor administration and scoring changes may improve digit span backward’s construct validity. Research in Developmental Disabilities, 72, 166–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersema JR, van der Meere JJ, & Roeyers H (2009). ERP correlates of error monitoring in adult ADHD. Journal of Neural Transmission, 116(3), 371–379. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Pennington BF, Olson RK, Chhabildas N, & Hulslander J (2005). Neuropsychological analyses of comorbidity between reading disability and attention deficit hyperactivity disorder: In search of the common deficit. Developmental Neuropsychology, 27(1), 35–78. [DOI] [PubMed] [Google Scholar]
- Willoughby MT, Blair CB, & Family Life Project Investigators. (2016). Measuring executive function in early childhood: A case for formative measurement. Psychological Assessment, 28(3), 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong IY, Mahar D, Titchener K, & Freeman J (2013). The impact of anxiety on processing efficiency: Implications for the attentional control theory. The Open Behavioral Science Journal, 6(1), 7–15. [Google Scholar]
- Wright L, Lipszyc J, Dupuis A, Thayapararajah SW, & Schachar R (2014). Response inhibition and psychopathology: A meta-analysis of go/no-go task performance. Journal of Abnormal Psychology, 123(2), 429–439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


