Abstract
Background:
Neutrophil-to-lymphocyte ratio (NLR) is a biomarker of systemic inflammation that is associated with adverse oncologic and surgical outcomes. We investigated the use of NLR as a prognostic indicator of complications of head and neck cancer (HNC) surgeries.
Methods:
We conducted a retrospective study of 11,187 Veterans who underwent HNC surgery between 2000 and 2020. We calculated preoperative NLR values and fit logistic regression models adjusting for potential confounding factors, comparing high-NLR patients to low-NLR patients.
Results:
The cohort had a median age of 63 and was 98% men. High-NLR patients had increased odds of 30-day mortality (p<0.001), having 1+ perioperative complications (p<0.001), sepsis (p=0.03), failure to wean from mechanical ventilation (p=0.04), pneumonia (p<0.001), and pulmonary embolism (p=0.02) compared to low-NLR patients.
Conclusion:
NLR was a robust, independent predictor of 30-day mortality, having 1+ surgical complications, sepsis, failure to wean from mechanical ventilation, pneumonia, and pulmonary embolism.
Background
Head and neck cancer (HNC) was the 7th most common cancer worldwide in 2020, with 930,000 new cases and 460,000 deaths.1 Surgery retains an important role in HNC treatment. However, surgical complications are a significant cause of morbidity and mortality for HNC patients. Furthermore, surgical complications place patients at risk of missing time-sensitive adjuvant radiation therapy. Prognostic factors that identify patients at greatest risk of surgical complications are useful in treatment decision-making and tailoring therapy to minimize these complications.
Blood-based biomarkers have the potential to provide convenient and objective clinical risk stratification. The neutrophil-to-lymphocyte ratio (NLR) is an emerging measure that is correlated with systemic inflammation and poor oncologic prognosis.2 Systemic inflammation has been posited as a risk factor for adverse surgical outcomes as associated immunologic and coagulation disturbances may promote a variety of complications including infection, poor tissue repair and cardiovascular dysfunction.3 As the NLR is calculated from common components of standard preoperative evaluations, it may serve as a simple and useful index of systemic inflammatory response and resulting immunosuppression. Correspondingly, studies have shown that the NLR identifies patients with increased risk of perioperative complications after surgical treatment for solid tumors, such as esophageal, lung, gastric, bladder, and colorectal cancer.4–8
There has been little evaluation of NLR as a predictor of surgical complications from HNC surgery. Therefore, we used data from a high-quality database of surgical complications linked to laboratory and clinical data from a large cohort of Veterans to test our hypothesis that elevated NLR values would independently predict major perioperative events for patients undergoing surgeries for HNC.
Methods
Patient Selection
We performed a retrospective cohort study using data from the Veterans Affairs Surgical Quality Improvement Program (VASQIP), a high-quality data source for determining short-term surgical outcomes. VASQIP provides prospectively collected information on preoperative characteristics and 30-day postoperative outcomes for Veterans who undergo surgery. We identified 20,740 cases of head and neck cancer surgery based on Current Procedural Terminology (CPT) codes that were previously published, with some adjustments made by an expert head and neck cancer surgeon on our study team (SAR).9 We linked these cases to data from Veterans Health Affairs (VA) databases to obtain patient demographics, surgical information, comorbidities, and laboratory data. We excluded subsequent surgeries, cases with missing NLR data between 1 and 90 days prior to surgery, and surgery types with less than 50 cases (hypopharyngectomy). In total, we identified 11,187 cases of surgery for head and neck cancer with NLR data (Figure 1).
Figure 1.
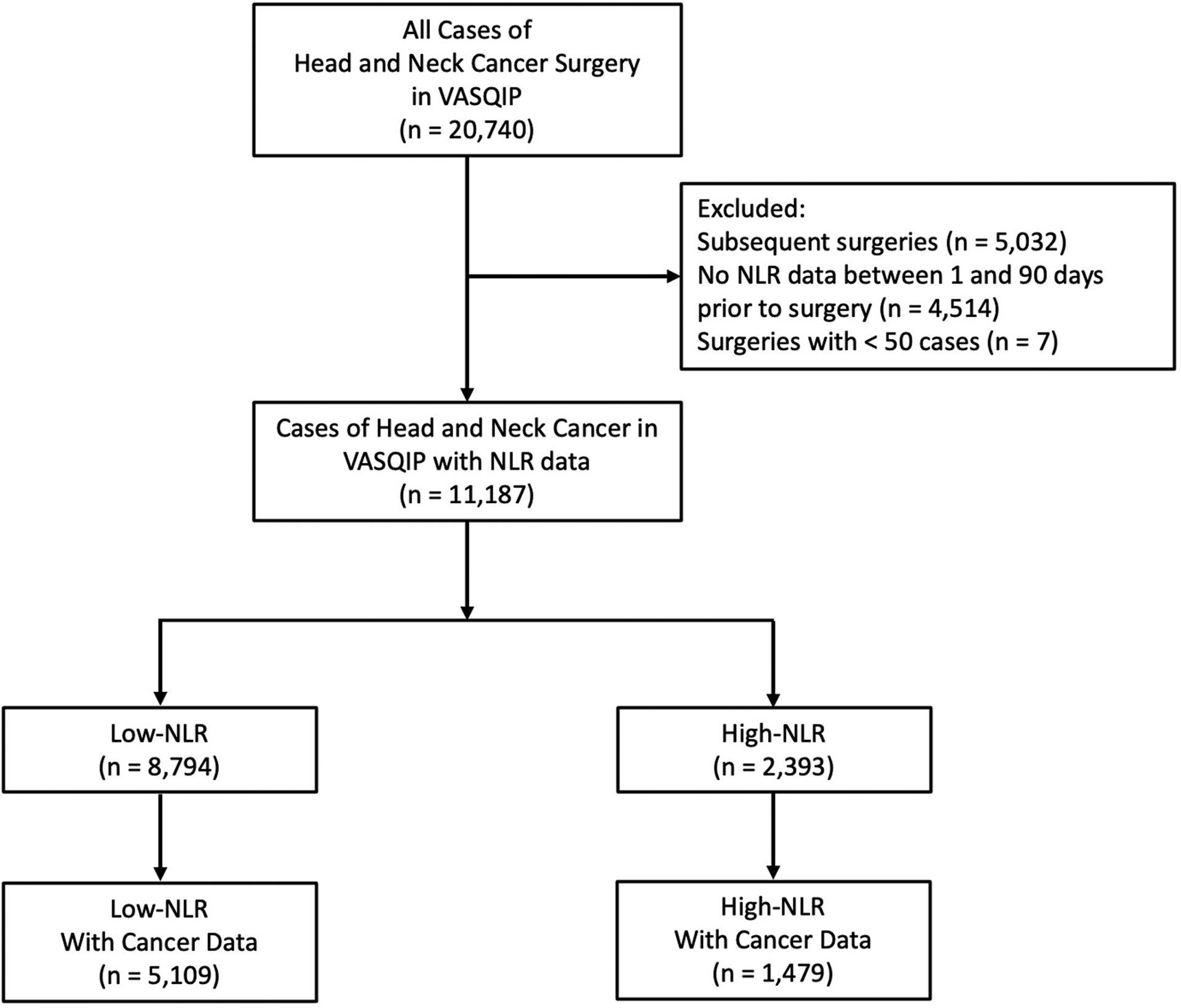
Consort diagram
Study Variables
Baseline sociodemographic variables (age, sex, smoking status, alcohol use), treatment data (year of surgery, surgical procedure, preoperative chemotherapy within 30 days), frailty or preoperative risk scores (risk analysis index [RAI]10, American Society of Anesthesiology [ASA] classification), and other widely available, potentially prognostic preoperative laboratory data (hematocrit, serum creatinine) were identified from VASQIP. VA databases were used to supplement VASQIP variables for race/ethnicity and body mass index (BMI). NLR values were calculated from neutrophil and lymphocyte counts obtained between 1 and 90 days prior to surgery date from laboratory data in VA databases. Charlson comorbidity scores were determined by comorbid illnesses identified by diagnostic codes in outpatient and inpatient VA claims databases within 12 months before surgery.11 We further analyzed a subset of cases, 6,588 patients, with linkable VA cancer registry data, including clinical stage (American Joint Committee on Cancer, 7th edition) and clinical T and N stage from VA databases.
Prospective 30-day postoperative outcomes were all identified from VASQIP. The following adverse events were analyzed: death within 30 days, a composite variable for one or more postoperative complications, return to the operating room within 30 days, cardiac arrest requiring CPR, myocardial infarction, coma lasting greater than 24 hours postoperative, cerebral vascular accident/stroke, bleeding requiring more than 4 units of packed red blood cells, deep vein thrombosis/thrombophlebitis, sepsis, failure to wean off ventilator greater than 48 hours postoperative, pneumonia, pulmonary embolism, reintubation for respiratory/cardiac failure, acute renal failure postoperative, progressive renal insufficiency, urinary tract infection, superficial surgical site infection, and deep wound surgical site infection. We considered death within 30 days the primary outcome as this was the most serious complication and of utmost interest.
Statistical Analysis
Using the Youden J-statistic, we dichotomized NLR against the primary outcome, death within 30 days, yielding a cutpoint of 4.99. The area under the receiver operating characteristic curve at this cutpoint was 0.61 (Figure 2). We then tested for differences in baseline and clinical characteristics between NLR groups with the t test for normal continuous variables and the chi-square test for categorical variables. Race/ethnicity, smoking status, alcohol use, ASA classification, BMI, preoperative hematocrit and serum creatinine, and chemotherapy had missing values (0.6%, 0.04%, 0.1%, 0.1%, 10.6%, 2.5%, 3.0%, 0.3%, respectively). Multiple imputation methods were used to estimate missing values for adjusted regression models.
Figure 2.
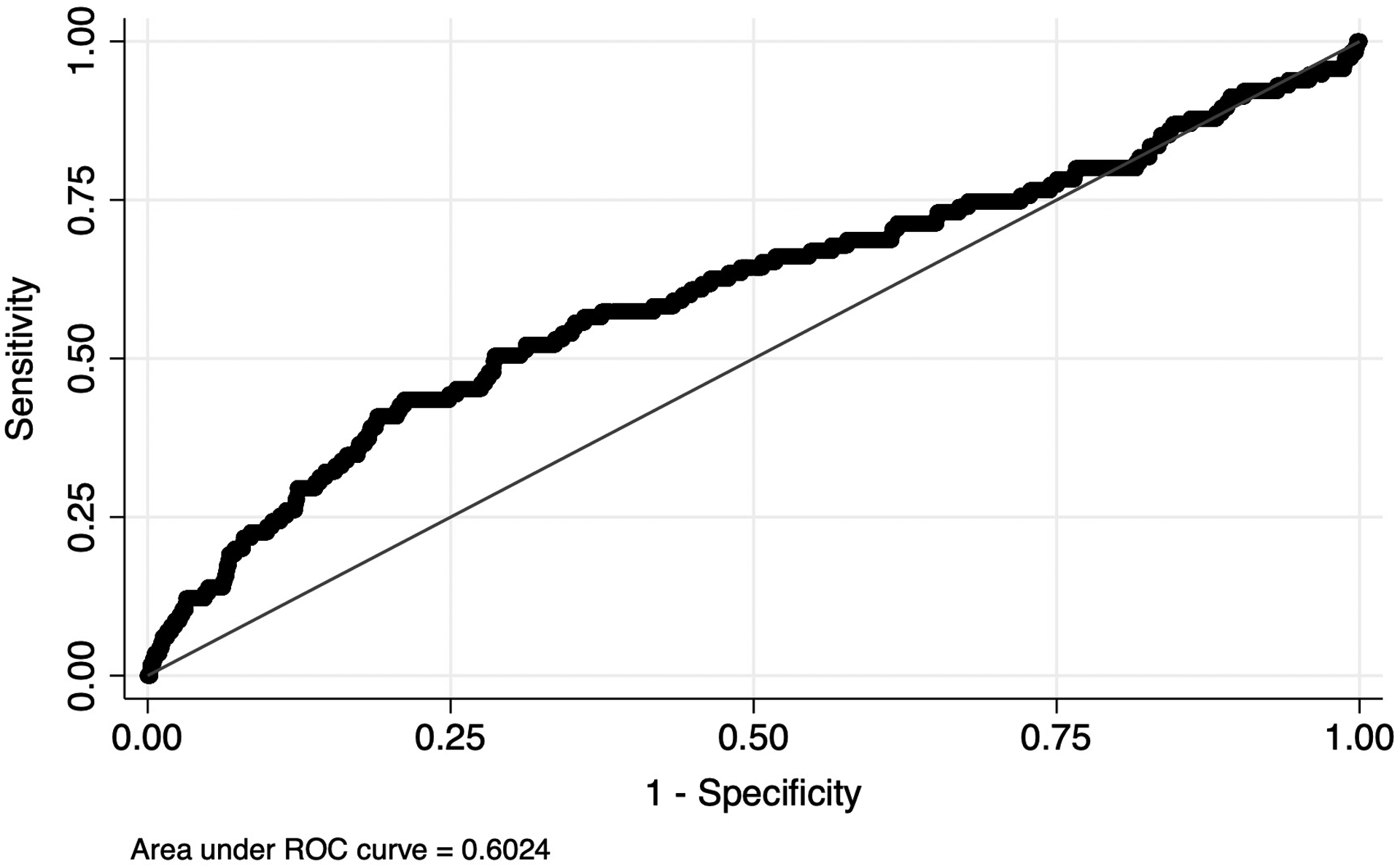
Receiver operating characteristic curve for NLR
We calculated the unadjusted prevalence of each outcome for the cohort and stratified by surgical procedure. Adjusted logistic regression models were then fit to test the association of NLR group with each outcome, adjusting for RAI, race/ethnicity, year of surgery, smoking status, alcohol use, Charlson comorbidity index, ASA classification, surgical procedure, pretreatment chemotherapy, and preoperative hematocrit. Variables included in the adjusted logistic regression models are listed in Table 1. Age, sex, heart failure, lung function, renal failure, cognitive decline, activities of daily living, independent living, and weight loss are all captured in RAI. We further performed subsequent adjusted analyses for death within 30 days and one or more postoperative complications outcomes stratified by surgical procedure. We then performed two sensitivity analyses, one analyzing a subset of the cohort with linked cancer registry data adjusting for tumor information and another excluding cases that received preoperative chemotherapy. All analyses were performed in STATA Version 13 (STATA Corp., College Station, TX). This study was approved by the James J Peters VA Medical Center Institutional Review Board.
Table 1.
Baseline cohort characteristics by NLR group
| Characteristic | Low NLR N=8,794 |
High NLR N=2,393 |
p-value |
|---|---|---|---|
| Age, mean (SD) | 63.2 (10.4) | 63.9 (9.5) | 0.003 |
| Men, No. (%) | 8,609 (97.9) | 2,376 (99.3) | <0.001 |
| Race/ethnicity, No. (%) | 0.005 | ||
| White | 6,068 (69.0) | 1,740 (72.7) | |
| Black | 982 (11.2) | 232 (9.7) | |
| Hispanic | 730 (8.3) | 181 (7.6) | |
| Other | 954 (10.9) | 232 (9.7) | |
| Missing | 60 (0.7) | 8 (0.3) | |
| Year of surgery, No. (%) | <0.001 | ||
| 2000–2005 | 3,199 (36.4) | 932 (39.0) | |
| 2006–2010 | 2,829 (32.2) | 747 (31.2) | |
| 2011–2015 | 2,537 (25.3) | 607 (25.4) | |
| 2016–2020 | 229 (2.6) | 107 (4.5) | |
| Current Smoker, No. (%) | 4,659 (53.0) | 1,285 (53.7) | 0.81 |
| Missing | 3 (0.03) | 1 (0.04) | |
| Alcohol Use*, No. (%) | 1,618 (18.4) | 416 (17.4) | 0.11 |
| Missing | 11 (0.13) | 0 (0.00) | |
| Charlson Comorbidity Index†, No. (%) | <0.001 | ||
| 0–1 | 6,298 (71.6) | 1,611 (67.3) | |
| 2–3 | 1,689 (19.2) | 543 (22.7) | |
| 4–5 | 532 (6.1) | 173 (7.2) | |
| 6+ | 275 (3.1) | 66 (2.8) | |
| ASA Classification, No. (%) | <0.001 | ||
| 1–2 | 1,584 (18.0) | 206 (8.6) | |
| 3 | 6,415 (73.0) | 1,816 (75.9) | |
| 4–5 | 782 (8.9) | 370 (15.5) | |
| Missing | 13 (0.2) | 1 (0.04) | |
| Risk Analysis Index, No. (%) | <0.001 | ||
| ≤26 | 6,573 (74.7) | 1,352 (56.5) | |
| 27–34 | 1,300 (14.8) | 444 (18.6) | |
| ≥35 | 921 (10.5) | 597 (25.0) | |
| Surgical Procedure, No. (%) | <0.001 | ||
| Laryngectomy | 1,274 (14.5) | 561 (23.4) | |
| Neck dissection | 2,372 (27.0) | 758 (31.7) | |
| Oral cavity | 2,577 (29.3) | 503 (21.0) | |
| Parotidectomy | 360 (4.1) | 61 (2.6) | |
| Cutaneous excision | 888 (10.1) | 169 (7.1) | |
| Thyroidectomy | 288 (3.3) | 44 (1.8) | |
| Sinus/Skull base | 285 (3.2) | 57 (2.4) | |
| Reconstruction | 496 (5.6) | 178 (7.4) | |
| Oropharyngectomy | 254 (2.9) | 62 (2.6) | |
| Chemotherapy‡, No. (%) | 109 (1.2) | 75 (3.1) | <0.001 |
| Missing | 26 (0.3) | 13 (0.5) | |
| Body Mass Index (BMI), mean (SD) | 26.3 (5.5) | 24.0 (5.3) | <0.001 |
| Missing, No. (%) | 913 (10.4) | 278 (11.6) | |
| Preoperative Hematocrit (%), mean (SD) | 41.1 (5.0) | 38.0 (5.7) | <0.001 |
| Missing, No. (%) | 245 (2.9) | 31 (1.3) | |
| Preoperative Serum Creatinine (mg/dl), mean (SD) | 1.0 (0.4) | 1.0 (0.6) | 0.35 |
| Missing, No. (%) | 283 (3.2) | 56 (2.3) |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; ASA, American Society of Anesthesiologists
>2 drinks/day in 2 weeks before admission
Diagnosed within 1 year prior to surgery
Chemotherapy for malignancy within 30 days prior to surgery
Results
Baseline Characteristics
In total, 11,187 cases of HNC surgery with NLR data were identified (Table 1). 8,794 (78.6%) had a preoperative NLR of less than 4.99 (low-NLR) and 2,393 (21.4%) had a NLR greater than or equal to 4.99 (high-NLR). Multiple differences were seen in baseline characteristics between low-NLR and high-NLR patients. High-NLR patients were more likely to be older (p=0.003), men (p<0.001), white (p=0.005), have an earlier year of surgery (p<0.001), a higher Charlson comorbidity index (p<0.001), a higher ASA classification (p<0.001), a higher RAI (p<0.001), receive neck dissection (p<0.001), and receive chemotherapy for malignancy within 30 days prior to surgery (p<0.001) than low-NLR patients. Low-NLR cases were more likely to have a higher BMI (p<0.001) and a higher preoperative hematocrit (p<0.001) than high-NLR cases.
Surgical Complications
For general postoperative outcomes, the mortality rate of all patients within 30 days was 1.0% (Table 2). 13.3% of the cohort had at least one postoperative complication and 13.2% returned to the operating room within 30 days. All other outcomes were infrequent, ranging from 0.1% (coma lasting greater than 24 hours, acute renal failure) to 2.9% (failure to wean off mechanical ventilation greater than 48 hours). In adjusted models, a high preoperative NLR significantly increased the odds ratio (OR) of six outcomes: death within 30 days (OR 2.12, 95% CI 1.42–3.16), having one or more perioperative complications (OR 1.39, 95% CI 1.22–1.58), sepsis (OR 1.51, 95% CI 1.05–2.17), failure to wean from mechanical ventilation for more than 48 hours (OR 1.30, 95% CI 1.01–1.68), pneumonia (OR 1.58, 95% CI 1.26–1.99), and pulmonary embolism (OR 2.51, 95% CI 1.17–5.37). Odds ratios for all other outcomes did not reach the level of significance.
Table 2.
Odds ratios for major postoperative outcomes by NLR group
| Outcome | Prevalence, % | Odds Ratio | 95% CI |
|---|---|---|---|
| General Postoperative Outcomes | |||
| Death within 30 days | 1.0 | 2.12* | 1.42–3.16 |
| 1 or more VASQIP complications | 13.3 | 1.39* | 1.22–1.58 |
| Return to OR within 30 days | 13.2 | 1.07 | 0.94–1.23 |
| Cardiac Postoperative Outcomes | |||
| Cardiac arrest requiring CPR | 0.5 | 1.25 | 0.68–2.30 |
| Myocardial infarction†‡ | 0.3 | 0.68 | 0.28–1.62 |
| Central Nervous System Postoperative Outcomes | |||
| Coma lasting >24 hours post-op‡ | 0.1 | 1.15 | 0.30–4.48 |
| Cerebral vascular accident/Stroke‡ | 0.3 | 1.29 | 0.61–2.73 |
| Other Surgical Postoperative Outcomes | |||
| Bleeding requiring >4 units pRBCs‡ | 0.2 | 1.72 | 0.75–3.94 |
| Deep vein thrombosis/Thrombophlebitis§ | 0.3 | 1.09 | 0.49–2.39 |
| Sepsis | 1.3 | 1.51* | 1.05–2.17 |
| Respiratory Postoperative Outcomes | |||
| Failure to wean off mechanical ventilation >48 hours | 2.9 | 1.30* | 1.01–1.68 |
| Pneumonia | 3.5 | 1.58* | 1.26–1.99 |
| Pulmonary embolism‡‖ | 0.3 | 2.51* | 1.17–5.37 |
| Reintubation for respiratory/cardiac failure | 2.1 | 1.25 | 0.92–1.70 |
| Urinary Tract Postoperative Outcomes | |||
| Acute renal failure post-op†‡ | 0.1 | 0.76 | 0.18–3.23 |
| Progressive renal insufficiency‡ | 0.2 | 1.20 | 0.50–2.88 |
| Urinary tract infection | 1.1 | 1.14 | 0.75–1.73 |
| Wound Postoperative Outcomes | |||
| Superficial surgical site infection | 2.6 | 1.13 | 0.85–1.49 |
| Deep wound surgical site infection | 1.2 | 1.42 | 0.96–2.09 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; CI, confidence interval; OR, operating room; pRBCs, packed red blood cells
Statistically significant
Race recategorized as White, Black, or Other
No chemotherapy in model
No ASA classification in model
No race in model
By Surgical Procedure
After stratifying cases by surgical procedure, oropharyngectomy had the highest prevalence of death within 30 days (1.6%), while cutaneous excision and thyroidectomy had the lowest (0.6% each) (Table 3). For one or more postoperative complications, laryngectomy had the highest prevalence (20.2%), while thyroidectomy had the smallest (8.1%).
Table 3.
Odds ratios for major postoperative outcomes by NLR group and stratified by surgical procedure
| Surgical Procedure | Death within 30 days | 1 or more VASQIP complications | ||||
|---|---|---|---|---|---|---|
| Prevalence, % | Odds Ratio | 95% CI | Prevalence, % | Odds Ratio | 95% CI | |
| All surgeries, N = 11,187 |
1.0 | 2.12* | 1.42–3.16 | 13.3 | 1.39* | 1.22–1.58 |
| Laryngectomy, N = 1,835 |
1.5 | 2.41* | 1.06–5.49 | 20.2 | 1.54* | 1.20–1.99 |
| Neck dissection,† N = 3,130 |
0.9 | 2.53* | 1.12–5.69 | 8.7 | 1.01 | 0.76–1.36 |
| Oral cavity, N = 3,080 |
1.0 | 1.34 | 0.58–3.12 | 14.1 | 1.66* | 1.29–2.14 |
| Parotidectomy, N = 421 |
0.7 | – | – | 12.8 | 2.55* | 1.23–5.29 |
| Cutaneous excision,ঠN = 1,057 |
0.6 | 1.13 | 0.11–11.89 | 9.5 | 0.74 | 0.40–1.38 |
| Thyroidectomy, N = 332 |
0.6 | – | – | 8.1 | 0.99 | 0.30–3.33 |
| Sinus/Skull base,§ N = 342 |
1.5 | 7.46 | 0.46–121.45 | 11.7 | 1.73 | 0.72–4.17 |
| Reconstruction,‖ N = 674 |
1.0 | 6.21 | 0.93–41.64 | 19.3 | 1.75* | 1.11–2.75 |
| Oropharyngectomy,¶ N = 316 |
1.6 | 0.79 | 0.05–13.29 | 17.7 | 1.52 | 0.75–3.15 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio
Stastically significant
No ASA in model for Death within 30 days
No alcohol use in model for Death within 30 days
No race in model for Death within 30 days
Race recategorized as White, Black, or Other for Death within 30 days
No chemo in model
Models would not converge
In age-adjusted analyses stratified by surgical procedure, high-NLR was significantly associated with an increased odds for death within 30 days in laryngectomy (OR 2.54, 95% CI 1.18–5.50), neck dissection (OR 3.52, 95% CI 1.66–7.49), parotidectomy (OR 14.29, 95% CI 1.24–164.58), and reconstruction (OR 7.11, 95% CI 1.35–37.39). High-NLR was significantly associated with one or more perioperative complications in laryngectomy (OR 1.67, 95% CI 1.32–2.12), oral cavity surgery (OR 1.97, 95% CI 1.55–2.51), parotidectomy (OR 2.69, 95% CI 1.37–5.29), and reconstructive surgery (OR 2.11, 95% CI 1.40–3.19) in similar age-adjusted models. In multivariate adjusted analyses stratified by surgical procedure, high-NLR was significantly associated with an increased odds for death within 30 days in laryngectomy (OR 2.41, 95% CI 1.06–5.49) and neck dissection (OR 2.53, 95% CI 1.12–5.69). For one or more perioperative complications, high-NLR was significantly associated with an increased odds in laryngectomy (OR 1.54, 95% CI 1.20–1.99), oral cavity surgery (OR 1.66, 95% CI 1.29–2.14), parotidectomy (OR 2.55, 95% CI 1.23–5.29), and reconstructive surgery (OR 1.75, 95% CI 1.11–2.75).
Sensitivity Analyses
In the subset of cases with cancer data, adjusted sensitivity analyses controlling for tumor characteristics in addition to previously included covariates showed that an elevated preoperative NLR similarly increases odds of death within 30 days (OR 2.15, 95% CI 1.27–3.64) and having one or more postoperative complications (OR 1.29, 95% CI 1.09–1.53) (Table 4). Tumor characteristics for this cohort are shown in Table 5. Likewise, excluding cases that received chemotherapy 30 days before surgery also demonstrated a significant association of a high NLR with death within 30 days (OR 2.09, 95% CI 1.39–3.13) and one or more postoperative complications (OR 1.38, 95% CI 1.21–1.57).
Table 4.
Odds ratios for major postoperative outcomes by NLR group after sensitivity analyses
| Outcome | Prevalence, % | Odds Ratio | 95% CI |
|---|---|---|---|
| With Cancer Data, N = 6,588 | |||
| Death within 30 days | 1.0 | 2.15* | 1.27–3.64 |
| 1 or more VASQIP complications | 14.5 | 1.29* | 1.09–1.53 |
| No Chemotherapy, N = 11,003 | |||
| Death within 30 days | 1.0 | 2.09* | 1.39–3.13 |
| 1 or more VASQIP complications | 13.2 | 1.38* | 1.21–1.57 |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; CI, confidence interval
Table 5.
Tumor characteristics by NLR group for the subset of cases with cancer data
| Tumor Characteristic | Low NLR N=5,109 |
High NLR N=1,479 |
p-value |
|---|---|---|---|
| Stage at Diagnosis, No. (%) | <0.001 | ||
| I | 1,181 (23.1) | 178 (12.0) | |
| II | 630 (12.3) | 175 (11.8) | |
| III | 735 (14.4) | 159 (10.8) | |
| IV | 1,995 (39.1) | 819 (55.4) | |
| Missing | 568 (11.1) | 148 (10.0) | |
| Clinical T Classification, No. (%) | <0.001 | ||
| 1 | 1,415 (27.7) | 260 (17.6) | |
| 2 | 1,280 (25.1) | 396 (26.8) | |
| 3 | 584 (11.5) | 258 (17.4) | |
| 4 | 618 (12.1) | 292 (19.7) | |
| Missing | 1,211 (23.7) | 273 (18.5) | |
| Clinical N Classification, No. (%) | <0.001 | ||
| 0 | 2,396 (46.9) | 534 (36.1) | |
| 1 | 627 (12.3) | 139 (9.4) | |
| 2 | 1,061 (20.8) | 515 (34.8) | |
| 3 | 95 (1.9) | 65 (4.4) | |
| Missing | 930 (18.2) | 226 (15.3) |
Abbreviations: NLR, neutrophil-to-lymphocyte ratio
Discussion
In a national cohort of Veterans undergoing surgery for HNC, we found that a high preoperative NLR was independently associated with increased odds of death within 30 days, one or more major postoperative complications, sepsis, prolonged mechanical ventilation, pneumonia, and pulmonary embolism. When stratified by surgical procedure, a high preoperative NLR was independently associated with death within 30 days in laryngectomy and neck dissections, while associated with one or more major complications in laryngectomy, oral cavity surgery, parotidectomy, and reconstructive surgery. These associations remained significant even after adjusting for tumor characteristics and excluding patients who received chemotherapy prior to surgery. This is the largest study to date that provides evidence that NLR may be a convenient biomarker of elevated HNC surgical risk, independent of conventional preoperative predictors.
We hypothesized that elevated NLR would predict adverse post-surgical events even after accounting for major comorbidities and standard surgical risk stratification criteria. Neutrophils drive inflammation through antigen presentation and secretion of cytokines, chemokines, prostaglandins, and leukotrienes and have the potential to directly inflict damage to tissue through the secretion of proteases and toxic oxygen metabolites.12 Lymphocytes, on the other hand, are part of the adaptive immune response and have been found to decrease in number in systemic inflammation, a well-studied indicator of poor prognosis.13 While measures of comorbidity or frailty may have subjective components or historical elements that may introduce error, NLR, as a biomarker, provides an objective measure of systemic inflammation and potentially impaired cell-mediated immunity.14 An elevated NLR therefore identifies dysregulated inflammation that may represent a category of post-surgical risk that is not well represented in other commonly used clinical information informing preoperative risk stratification.
NLR has been shown to be an independent predictor of poorer survival in head and neck cancer.15,16 We found that high preoperative NLR was also associated with major complications after head and neck cancer surgeries. Other surgical cohorts have found that NLR was predictive of adverse perioperative outcomes, particularly soft-tissue complications. The largest previous study was a prospective study of 369 patients undergoing head and neck cancer surgery that found that preoperative NLR was associated with a significantly increased risk of surgical site infection.17 In addition, a retrospective study of 146 patients with hypopharyngeal squamous cell carcinoma found that a greater percentage of high-NLR cases experienced postoperative wound complications after radical resection than low-NLR cases (p = 0.03).18 Finally, a retrospective study of 157 patients undergoing total laryngectomy found that an NLR greater than 2.5 was independently associated with a more than two-fold increase in the odds of pharyngocutaneous fistula.19 Our studies expands the list of complications found to be associated with high preoperative NLR to include death, sepsis, and adverse pulmonary outcomes.
NLR is a predictor of surgical complications in other oncologic procedures as well. A retrospective cohort study of 583 patients undergoing colorectal cancer surgery found that an elevated preoperative NLR was associated with a wide range major perioperative complications including infection-related and pulmonary adverse events.20 Two studies on gastrectomies for gastric cancer found that a preoperative NLR greater than 3–3.5 was associated with postoperative infectious complications and other high grade adverse events.6,21 Likewise, in studies of distal cholangiocarcinoma and rectal adenocarcinoma, a high preoperative NLR was an independent risk factor for postoperative complications.22,23 Similar to our findings, high preoperative NLR appears to be a predictor of a range of adverse events after oncologic surgery.
High NLR was independently associated with adverse pulmonary outcomes in our study. Pulmonary inflammatory conditions, such as COPD, have been associated with high NLR and it is possible that NLR provides an indirect measure of lung disease severity.24 A prospective observational study found that NLR was significantly elevated during COPD exacerbation compared to stable COPD and controls.25 Another study found that NLR was associated with disease severity and exacerbation in COPD patients with significant correlations with multiple adverse outcomes and measures of disease severity.14 Similar to our study, a prospective study of ICU patients found that an elevated NLR was an independent predictor of failure to wean from mechanical ventilation.26 Previous studies have found NLR to be associated with pneumonia as well.27 Last, elevated NLR has been found to be associated with thrombotic events, including pulmonary embolism, potentially predisposed by inflammatory conditions.28 This is consistent with our findings, as elevated NLR was predictive of pulmonary embolism following HNC surgery.
Our study used a NLR cutpoint of 4.99, which was determined using the pre-specified method of optimizing the Youden statistic for prediction of perioperative mortality. The normal NLR range in a healthy, adult, non-geriatric population has been reported to be 0.78–3.53.29 Therefore, we are confident in the specificity of our cutpoint to identify patients with abnormal NLR values. It also within the range of NLR value cutpoints from previous studies of poor outcomes for head and neck cancer patients, which ranged between 2.05 and 5 and was similar to other studies implementing formal cutpoint methods.15 Nonetheless, our cutpoint would benefit from validation in an external data source, which was not possible under the scope of our study.
The association between high NLR and adverse perioperative outcomes was strongest for laryngectomy, neck dissection, oral cavity surgery, parotidectomy, and reconstructive surgery. When stratifying by surgical procedures NLR was not independently associated with perioperative death (and in many cases major adverse events) for several surgery types. This is likely related to statistical power, as post-operative mortality was relatively rare overall. The probability of death within 30 days of the surgery was low (1%) for the overall cohort but was increased nearly five-fold in patients with high NLR who were older than age 65 with high ASA scores. Nonetheless, our pooled data provides validation of the utility of NLR as an independent prognostic marker for head and neck cancer surgeries.
The evidence of a strong association between NLR and post-surgical complications may assist patients and clinicians in deciding between surgical and non-surgical management of head and neck cancers. Oropharyngeal, laryngeal, and hypopharyngeal tumors can often be treated by either modality, making other factors, such as frailty, kidney function, and predictors of adverse events, important in decision-making.30 As surgical complications may delay the initiation of postoperative adjuvant therapy to beyond the window of optimal timing, after which the effectiveness of adjuvant therapy diminishes, patients identified as high risk for postoperative complications may consider non-surgical management.31–33 Preoperative NLR values may serve as a useful clinical decision-making tool in this regard.
The strengths of this study include that it is the largest, nationally representative study to evaluate NLR as a prognostic indicator of oncologic surgical complications. Our study utilized prospectively collected, high-quality 30-day outcomes, detailed clinical data, including body mass assessments, high quality vital status data, and extensive documentation of comorbidities, giving us a broader picture of each patient’s health. Some limitations include the lack of diagnostic codes and information on the hospital site, volume, and surgeon. Our study cohort consists of Veterans from the VA health system and was overwhelmingly male, limiting generalizability. We did not have quality of life data and therefore could not assess a full range of important patient-oriented outcomes. Nonetheless, this study utilized high-quality records on a wide range of surgical complications that were tested.
Conclusion
In a large cohort of 13,438 Veterans undergoing HNC surgery, NLR was a robust, independent predictor of post-operative 30-day mortality, having one or more surgical complications, failure to wean from mechanical ventilation, and pneumonia.
Footnotes
Conflicts of Interest: All authors: none
Data Availability Statement:
The data that support the findings of this study are available from the Veterans Health Administration. Restrictions apply to the availability of these data, which were used under license for this study. For questions about data access, please contact Dr. Keith Sigel or the VA Office of Research and Development.
DK had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. May 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. Jun 2014;106(6):dju124. doi: 10.1093/jnci/dju124 [DOI] [PubMed] [Google Scholar]
- 3.Becher RD, Hoth JJ, Miller PR, Meredith JW, Chang MC. Systemic inflammation worsens outcomes in emergency surgical patients. J Trauma Acute Care Surg. May 2012;72(5):1140–9. doi: 10.1097/TA.0b013e3182516a97 [DOI] [PubMed] [Google Scholar]
- 4.Xie X, Luo KJ, Hu Y, Wang JY, Chen J. Prognostic value of preoperative platelet-lymphocyte and neutrophil-lymphocyte ratio in patients undergoing surgery for esophageal squamous cell cancer. Dis Esophagus. Jan 2016;29(1):79–85. doi: 10.1111/dote.12296 [DOI] [PubMed] [Google Scholar]
- 5.Lan H, Zhou L, Chi D, et al. Preoperative platelet to lymphocyte and neutrophil to lymphocyte ratios are independent prognostic factors for patients undergoing lung cancer radical surgery: A single institutional cohort study. Oncotarget. May 23 2017;8(21):35301–35310. doi: 10.18632/oncotarget.13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohri Y, Tanaka K, Toiyama Y, et al. Impact of Preoperative Neutrophil to Lymphocyte Ratio and Postoperative Infectious Complications on Survival After Curative Gastrectomy for Gastric Cancer: A Single Institutional Cohort Study. Medicine (Baltimore). Mar 2016;95(11):e3125. doi: 10.1097/MD.0000000000003125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Kinoshita H, Yoshida K, et al. Perioperative change in neutrophil-lymphocyte ratio predicts the overall survival of patients with bladder cancer undergoing radical cystectomy. Jpn J Clin Oncol. Dec 2016;46(12):1162–1167. doi: 10.1093/jjco/hyw129 [DOI] [PubMed] [Google Scholar]
- 8.Li H, Zhao Y, Zheng F. Prognostic significance of elevated preoperative neutrophil-to-lymphocyte ratio for patients with colorectal cancer undergoing curative surgery: A meta-analysis. Medicine (Baltimore). Jan 2019;98(3):e14126. doi: 10.1097/MD.0000000000014126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascarella MA, Muthukrishnan N, Maleki F, et al. Above and Beyond Age: Prediction of Major Postoperative Adverse Events in Head and Neck Surgery. Ann Otol Rhinol Laryngol. Aug 20 2021:34894211041222. doi: 10.1177/00034894211041222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall DE, Arya S, Schmid KK, et al. Development and Initial Validation of the Risk Analysis Index for Measuring Frailty in Surgical Populations. JAMA Surg. Feb 1 2017;152(2):175–182. doi: 10.1001/jamasurg.2016.4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 12.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford). Sep 2010;49(9):1618–31. doi: 10.1093/rheumatology/keq045 [DOI] [PubMed] [Google Scholar]
- 13.Zahorec R Ratio of neutrophil to lymphocyte counts--rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 14.Treffers LW, Hiemstra IH, Kuijpers TW, van den Berg TK, Matlung HL. Neutrophils in cancer. Immunol Rev. Sep 2016;273(1):312–28. doi: 10.1111/imr.12444 [DOI] [PubMed] [Google Scholar]
- 15.Mascarella MA, Mannard E, Silva SD, Zeitouni A. Neutrophil-to-lymphocyte ratio in head and neck cancer prognosis: A systematic review and meta-analysis. Head & neck. May 2018;40(5):1091–1100. doi: 10.1002/hed.25075 [DOI] [PubMed] [Google Scholar]
- 16.Ferrandino RM, Roof S, Garneau J, et al. Neutrophil-to-lymphocyte ratio as a prognostic indicator for overall and cancer-specific survival in squamous cell carcinoma of the head and neck. Head & neck. Oct 2020;42(10):2830–2840. doi: 10.1002/hed.26329 [DOI] [PubMed] [Google Scholar]
- 17.Son HJ, Roh JL, Choi SH, Nam SY, Kim SY. Nutritional and hematologic markers as predictors of risk of surgical site infection in patients with head and neck cancer undergoing major oncologic surgery. Head & neck. Mar 2018;40(3):596–604. doi: 10.1002/hed.25031 [DOI] [PubMed] [Google Scholar]
- 18.Song Y, Liu H, Gao L, et al. Preoperative neutrophil-to-lymphocyte ratio as prognostic predictor for hypopharyngeal squamous cell carcinoma after radical resections. J Craniofac Surg. Mar 2015;26(2):e137–40. doi: 10.1097/SCS.0000000000001235 [DOI] [PubMed] [Google Scholar]
- 19.Aires FT, Dedivitis RA, Kulcsar MAV, Ramos DM, Cernea CR. Neutrophil-to-lymphocyte ratio as a prognostic factor for pharyngocutaneous fistula after total laryngectomy. Acta Otorhinolaryngol Ital. Feb 2018;38(1):31–37. doi: 10.14639/0392-100X-1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josse JM, Cleghorn MC, Ramji KM, et al. The neutrophil-to-lymphocyte ratio predicts major perioperative complications in patients undergoing colorectal surgery. Colorectal Dis. Jul 2016;18(7):O236–42. doi: 10.1111/codi.13373 [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol. May 2018;44(5):607–612. doi: 10.1016/j.ejso.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 22.Kumamoto Y, Kaizu T, Tajima H, et al. Neutrophil-to-lymphocyte ratio as a predictor of postoperative morbidity in patients with distal cholangiocarcinoma. Mol Clin Oncol. Oct 2018;9(4):362–368. doi: 10.3892/mco.2018.1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caputo D, Caricato M, Coppola A, La Vaccara V, Fiore M, Coppola R. Neutrophil to Lymphocyte Ratio (NLR) and Derived Neutrophil to Lymphocyte Ratio (d-NLR) Predict Non-Responders and Postoperative Complications in Patients Undergoing Radical Surgery After Neo-Adjuvant Radio-Chemotherapy for Rectal Adenocarcinoma. Cancer Invest. 2016;34(9):440–451. doi: 10.1080/07357907.2016.1229332 [DOI] [PubMed] [Google Scholar]
- 24.Furutate R, Ishii T, Motegi T, et al. The Neutrophil to Lymphocyte Ratio Is Related to Disease Severity and Exacerbation in Patients with Chronic Obstructive Pulmonary Disease. Intern Med. 2016;55(3):223–9. doi: 10.2169/internalmedicine.55.5772 [DOI] [PubMed] [Google Scholar]
- 25.Lee SJ, Lee HR, Lee TW, et al. Usefulness of neutrophil to lymphocyte ratio in patients with chronic obstructive pulmonary disease: a prospective observational study. Korean J Intern Med. Sep 2016;31(5):891–8. doi: 10.3904/kjim.2015.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo Z, Zheng Y, Yang L, et al. Neutrophil/lymphocyte ratio is helpful for predicting weaning failure: a prospective, observational cohort study. J Thorac Dis. Sep 2018;10(9):5232–5245. doi: 10.21037/jtd.2018.08.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Jager CP, Wever PC, Gemen EF, et al. The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. 2012;7(10):e46561. doi: 10.1371/journal.pone.0046561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soylu K, Gedikli O, Eksi A, et al. Neutrophil-to-lymphocyte ratio for the assessment of hospital mortality in patients with acute pulmonary embolism. Arch Med Sci. Feb 1 2016;12(1):95–100. doi: 10.5114/aoms.2016.57585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. Jan 3 2017;10(1):12. doi: 10.1186/s13104-016-2335-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forner D, Noel CW, Shuman AG, et al. Shared Decision-making in Head and Neck Surgery: A Review. JAMA Otolaryngol Head Neck Surg. Sep 1 2020;146(9):839–844. doi: 10.1001/jamaoto.2020.1601 [DOI] [PubMed] [Google Scholar]
- 31.Chen MM, Roman SA, Yarbrough WG, Burtness BA, Sosa JA, Judson BL. Trends and variations in the use of adjuvant therapy for patients with head and neck cancer. Cancer. Nov 1 2014;120(21):3353–60. doi: 10.1002/cncr.28870 [DOI] [PubMed] [Google Scholar]
- 32.Kiyota N, Tahara M, Fujii M. Adjuvant treatment for post-operative head and neck squamous cell carcinoma. Jpn J Clin Oncol. Jan 2015;45(1):2–6. doi: 10.1093/jjco/hyu195 [DOI] [PubMed] [Google Scholar]
- 33.Jensen AR, Nellemann HM, Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. Jul 2007;84(1):5–10. doi: 10.1016/j.radonc.2007.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the Veterans Health Administration. Restrictions apply to the availability of these data, which were used under license for this study. For questions about data access, please contact Dr. Keith Sigel or the VA Office of Research and Development.
DK had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.


