Abstract
Desert organisms have evolved physiological, biochemical, and genomic mechanisms to survive the extreme aridity of desert environments. Studying desert-adapted species provides a unique opportunity to investigate the survival strategies employed by organisms in some of the harshest habitats on Earth. Two of the primary challenges faced in desert environments are maintaining water balance and thermoregulation. We collected data in a simulated desert environment and a captive colony of cactus mice (Peromyscus eremicus) and used lab-based experiments with real time physiological measurements to characterize the response to water-deprivation. Mice without access to water had significantly lower energy expenditures and in turn, reduced water loss compared to mice with access to water after the first 24 hours of the experiment. Additionally, we observed significant weight loss likely related to dehydration-associated anorexia a response to limit fluid loss by reducing waste and the solute load as well as allowing water reabsorption from the kidneys and gastrointestinal tract. Finally, we observed body temperature correlated with sex, with males without access to water maintaining body temperature when compared to hydrated males while body temperature decreased for females without access to water compared to hydrated, suggesting daily torpor in females.
Keywords: energy expenditure, Peromyscus, physiology, respirometry, total water loss, dehydration
Introduction
Water is arguably the single most important factor for life on Earth and in organisms, water is stored in intracellular and extracellular spaces (Fitzsimons 1963). Dehydration occurs where there is a decrease in extracellular fluid volume caused when the loss is outpaced by fluid intake and metabolic water production, leading to a negative fluid balance and increased serum osmolality (Thomas et al. 2008). Regardless of the habitat, animals must regulate body fluids to protect against or cope with dehydration (Takei 2015). Mammals have developed many different mechanisms for body fluid regulation (Christian and Matson 1978; Frank 1988; Jirimutu et al. 2012; Marra et al. 2014; Yang et al. 2016; Kordonowy et al. 2017) and these mechanisms could aid in survival given the most well-supported climate change scenarios predict increased aridity (Mirzabaev et al., 2019).
Climate change is rapidly reshaping habitats globally and is predicted to continue (Hughes 2000; Parmesan and Yohe 2003; Parmesan 2006), modifying selective pressures for many populations (Hochachka and Somero 2002; Pörtner 2002; Pörtner and Farrell 2008). Understanding environmental tolerance and the capacity for adaptation in one species can provide insight into the potential for similar species to respond to increasingly extreme climatic patterns which are likely to affect many habitats. In recent years, many habitats have recorded some of the hottest temperatures to date (IPCC 2019; Stillman 2019), resulting in environmental thresholds that may exceed organismal tolerance. Furthermore, climate change is increasing global desertification rates, increasing water stress among wildlife (Loarie et al. 2009; Mirzabaev et al. 2019). To maintain viable populations, organisms must survive and successfully reproduce under climate warming and aridification by either using existing phenotypes and phenotypic plasticity, rapid evolution, or geographic range and phenological shifts (Hofmann and Todgham 2010; Hoffmann and Sgrò 2011; Brown et al. 2016). Despite climate change altering habitats and impacting populations, habitat distributions for rodents have remained remarkably stable over the last century of climate change, largely due to behavioral changes (Pardi et al. 2020; Riddell et al. 2021).
Mice of the genus Peromyscus have the widest distributions of any North American mammal and have unparalleled habitat diversity (Bedford and Hoekstra 2015). Several members of the genus, including the cactus mouse (Peromyscus eremicus) are native inhabitants of the arid deserts in southwest North America (Murie 1961; Pavlik 2008). Past studies have shown that cactus mice are extremely efficient at water retention (Kordonowy et al. 2017), have limited tissue damage when dehydrated (MacManes 2017), the slowest metabolism amongst the Peromyscus species (Mueller and Diamond 2001), have a suite of genomic adaptations (MacManes 2017; Tigano et al. 2020; Colella et al. 2021a), but lack the kidney modification present in kangaroo rats (Dewey et al. 1966; MacManes 2017). Furthermore, animals of this genus can be held in captivity (Crossland et al., 2014), have extensive genomic resources (Colella et al., 2021a; Tigano et al., 2020), and a wealth of samples collected historically and contemporaneously in natural history museums (Pergams and Lawler, 2009). Together, these features make the cactus mouse ideal for investigating water economy.
Desert habitats are characterized by such an extreme lack of precipitation which exerts a controlling effect on biological processes (Rocha et al., 2021). Daily temperatures in the Sonoran Desert can fluctuate by as much as 30–50 °C (Reid 1987; Sheppard et al. 2002). During the summer months, temperatures can reach upwards of 50 °C during the day, while at night they may drop to as low as 15 °C (Coppernoll-Houston and Potter 2018). In the winter, daytime temperatures are typically between 20 – 30°C, while nighttime temperatures can drop to near freezing (Boyd Deep Canyon Desert Research Center). Daily rainfall in the Sonoran Desert is relatively rare, with most areas receiving less than a centimeter of rain per year (Boyd Deep Canyon Desert Research Center). Organisms that are adapted to live in desert habitats must manage their water budgets over long dry and hot periods of time.
Here we expand on the long history of studies of organismal water management in desert taxa (Albright et al., 2017; Blumstein et al., 2022; Bradford, 1974; Cortés et al., 2000; Frank, 1988; Hayes et al., 1998; Kordonowy et al., 2017; MacMillen, 1983; Schmidt-Nielsen and Schmidt-Nielsen, 1952; Schmidt-Nielsen and Schmidt-Nielsen, 1952) to assess the physiological response to water deprivation in a hot and dry environment. To accomplish this, we compared multiple physiological responses, rate of water loss, energy expenditure, respiratory quotient, a suite of electrolytes, body weight, and body temperature, for mice with and without access to water for 72 hours to understand how animals survive the extreme head and aridity of deserts and further characterize P. eremicus’ response to water deprivation.
Methods
Animal Care and Experimental Model
All animals used in this study were captive born, sexually mature, non-reproductive healthy adult male and female P. eremicus. Individuals were descended from wild caught animals from a dry-desert population in Arizona and maintained at the University of South Carolina Peromyscus Genetic Stock Center (Columbia, South Carolina, USA). Animal care procedures were approved by the University of New Hampshire Institutional Animal Care and Use Committee under protocol number 210602 and followed guidelines established by the American Society of Mammologists (Sikes and the Animal Care and Use Committee of the American Society of Mammalogists, 2016). Mice were housed in a large walk-in environmental chamber designed to simulate the temperature, humidity, and photoperiod of their native desert environment (Kordonowy et al. 2017; Colella et al. 2021b; Blumstein et al. 2022). The daytime (light) phase lasted for 12 hours (08:00 to 20:00) at a room temperature of 32°C and 10% RH followed by a one-hour transition period to the nighttime (dark) phase which lasted for 9 hours (21:00 to 06:00) at a room temperature of 24°C and 25% RH. To compete the cycle a two-hour transition period occurs to return the room to light phase conditions (Kordonowy et al. 2017; Colella et al. 2021b; Blumstein et al. 2022). Mice were provided a standard diet and fed ad libitum (LabDiet® 5015*, 26.101% fat, 19.752% protein, 54.148% carbohydrates, energy 15.02 kJ/g, food quotient [FQ] 0.89).
Prior to experimental conditions mice were weighed (rounded to the nearest tenth of a gram) on a digital scale. A temperature-sensing passive integrated transponder (PIT) tag (BioThermo13, accuracy ±0.02°C, BioMark®, Boise, ID, USA) was implanted subdermally between the shoulders of each rodent using a tag injector (Biomark® MK10). Animals were then allowed to recover individually in an experimental chamber for one week of observation before the experiments were started. Body temperature was recorded at noon and midnight via a Biomark® HPR Plus reader and weight was measured every noon over the duration of the experiment. A randomly selected set of animals were assigned to the two water treatment groups (n=9 of each treatment, female mice with water, female mice without water, male mice with water, and male mice without water, total n=36). At the start of the experiment (day 0, time 0, 10:00), water was removed from three of the chambers corresponding to those animals in the dehydration group. No mortality occurred during these experiments. Three days later, at the conclusion of the experiment, mice were euthanized via isoflurane overdose and decapitation, and we collected 120 μl of trunk blood for serum electrolyte measurement using an Abaxis i-STAT® Alinity machine. Using i-STAT CHEM8+ cartridges (Abbott Park, IL, USA, Abbott Point of Care Inc), we measured the concentration of sodium (Na, mmol/L), potassium (K , mmol/L), blood urea nitrogen (BUN, mmol/L), hematocrit (Hct, % PCV), ionized calcium (iCa, mmol/L), glucose (Glu, mmol/L), osmolality (mmol/L), hemoglobin (Hb, g/dl), chlorine (Cl, mEq/L), total CO2 (TCO2, mmol/L), and Anion gap (AnGap, mEq/L). Using Na, Glu, and BUN, we calculated serum osmolality. The experimental setup was repeated six times, three male batches and three female batches.
Metabolic phenotyping
During the experiment mice were exposed to either water deprivation or normal conditions for three continuous days while being housed in transparent 9.5L respirometry chambers with dried cellulose-based bedding. Air was continuously pulled from the chambers using a pull flow-through respirometry system from Sable Systems International (SSI) starting with SS-4 Sub-Sampler Pumps, one for each chamber, at a rate of 1600 ml min−1 (96 l h−1). The SSI MUXSCAN was used to multiplexed air streams, measuring each chamber 120s approximately twice every hour. Finally, the Field Metabolic System (FMS, zeroed and spanned between each 72-hour experiment using dry gas with known concentrations of CO2 and O2) sub-sampled the airstream at 250 ml min−1 and measured water vapor, CO2, and O2 with no scrubbing.
Calculations and Statistical Analysis
We analyzed our data using methods fully described in Colella et al. (2021b) and Blumstein et al. (2022). Rates of CO2 production, O2 consumption, and water loss were calculated using equations 10.6, 10.5, and 10.9, respectively, from Lighton (2018). Respiratory quotient (RQ, the ratio of VCO2 to VO2) and Energy expenditure (EE) kJ hr−1 was calculated as in Lighton (2018, eq. 9.15). All downstream statistical analyses were conducted in R v 4.0.3 (R Core Team 2020). The R package mgcv::gamm was used and included the fixed effects; access to water and sex, and interacting nonlinear smoothing regression terms with pairwise fixed effect combinations as interactions; time in days and diurnal cycle (Lin and Zhang 1999; Wood 2017) and visualized using gratia (Simpson 2023). Experimental batches and the mice nested within the experimental batch were used as random effects to ensure we were not violating the assumption of independence. This allows us to explain the average differences between groups of mice instead of explaining differences between individual mice. To test for statistical significant (p < 0.05) differences in electrolytes after the treatments were applied and for each time point weight and body temperature were collected we used a student’s two-tailed t-test (stats::t.test) between the sexes for each experimental group.
Results
Rate of Water Loss
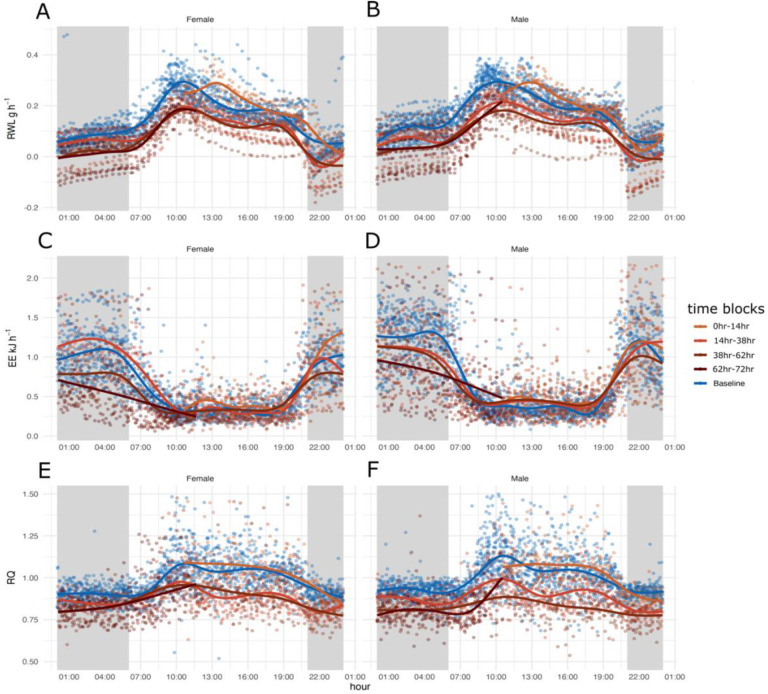
Both experimental groups, water access and water deprivation, had diurnal patterning of rate of water loss (RWL) with the highest occurring during the light phase and lowest during the dark phase (Figure 1A and 1 B). Each day of the experiment had similar patterns regardless of the treatment however, RWL was higher in males without access to water and for females lower or similar to the groups with water ad lib during day one of the experiment. For days two and three both males and females without access to water had lower RWL (Figure 1A and 1B).
Figure 1.
72 hours of respirometry data collection spit by sex for 18 adult males and 18 adult females plotted in a 24-hour window to display circadian patterns for each group: Baseline measurements of mice with water access (blue) and measurements of mice without access to water for one, two, three, and four days (four shades of brown). Shaded sections in gray indicate the dark phase when animals are active, and unshaded blocks indicate light phase when animals are inactive. A and B) 24-hour rate of water loss (RWL, H2O g hr−1) C and D) energy expenditure (EE kJ hr−1), and E and F) respiratory quotient (RQ), for females (A, C, E) and males (B, D, F).
Generalized additive modeling (GAM) analysis explained 77.2% of the deviance in RWL (Supplemental Figure 1, Supplemental Table 1). Significant predictors of RWL included sex (p < 2−16) and water access (p < 2−16) but not sex by water access (p = 0.13). All treatment combination splines were significant (Supplemental Table 1. For both males and females without access to water, the curves for time in days by 24-hour cycle were very complex, oscillating with the light dark cycle and decreasing over time (Figure 1A and 1B). The curves for time in days for males and females with access to water oscillated with the light dark cycle as well (Figure 1A and 1B). Generally, mice without water had higher RWL during the first 24 hours and lower RWL for the remainder of the experiment based on GAM analysis and visualization (Supplemental Figure 1, Supplemental Table 1, Figure 1A and 1B). When comparing the four curves (males without water, males with water, females without water, females with water), RWL was similar to mice with access to water converged during the light to dark transition phases, with the exception of the first transition (Supplemental Figure 1).
Energy Expenditure
Males and females in both experimental groups, water access and water deprivation, show diurnal pattering, with the highest EE occurring during the dark (active) phase and the lowest EE occurring during the light (inactive) phase (Figure 1C and 1D). Each day of the experiment for males and females with and without water has a similar pattern, elevated during the dark phase, and reduced during the light phase, regardless of the treatment.
During the first 24 hours, EE was highest for females without access compared to males without access to water and all mice with access to water. EE decreased over the subsequent 48 hours for mice without access to water with females without access to water having the lowest EE compared to males without access to water and all mice with access to water during the dark phase of days two and three of the experiment (Supplemental Figure 2, Supplemental Table 2, Figure 1C and 1D). Mice were manually weighted at 12:00 every day, resulting in a transient increase of EE at that time (Figure 1C and 1D). The GAM analysis explained 61.4% of the deviance in EE with significant predictors being sex (p < 2−16), water access (p < 2−16), and sex * water access (p = 0.0464). All treatment combination splines were significant (Supplemental Table 2).
Respiratory Quotient
RQ had diurnal patterning for both experimental groups and for both sexes (Figure 1E and 1F). RQ was highest during the light phases (Figure 1E and 1F) and lowest and comparable to the FQ during the dark phases (Figure 1E and 1F) 43.3% of the deviance was explained in the GAM analysis with significant predictors being sex (p < 2−16), water access (p = 4.14−08), and the interaction between sex and access to water (p = 1.41−07). All treatment combination splines were significant (Supplemental table 3) and complex, oscillating with the light dark cycle (Figure 1E and 1F, Supplemental Figure 3). Males and females without water access had higher RQ compared to mice with water access during the first 24-hours based on GAM analysis and visualization (Supplemental Figure 3, Supplemental Table 3). Interestingly, males without access to water had the lowest RQ of any group over the course of the entire experiment during the second dark phase and for the remainder of the experiment (Supplemental Figure 3).
Electrolytes, Weight, and Body Temperature
Several electrolytes were significantly different when comparing males with and without access to water and females with and without access to water (male and female Na p = 0.0016 and p = 0.0026 respectively, BUN p = 0.001/0.003, Hct p = 0.002/0.001, osmolality p = 8.2−05/0.0001, Cl p = 0.02/0.007, Hb p = 0.017/0.009, and TCO2 female p = 0.017) (Figure 2). No electrolytes were significantly different when comparing males to females for either water treatment (Figure 2).
Figure 2.
Violin plots showing the distribution of serum electrolyte measurements (Na = sodium (mmol/L), K = potassium (mmol/L), BUN = blood urea nitrogen (mmol/L), Hct = hematocrit (% PCV), iCa = ionized calcium (mmol/L), Glu = glucose (mmol/L), osmolality (mmol/L), Hb = hemoglobin (g/dl), Cl = chlorine (mEq/L), TCO2 = total CO2 (mmol/L), and AnGap = Anion gap (mEq/L), for female and male Peromyscus eremicus with (blue) or without (brown) access to water for 72 hours. Observations (n=9 of each treatment, total n=36) are represented by black dots. P-values from pairwise t-tests are reported above the brackets.
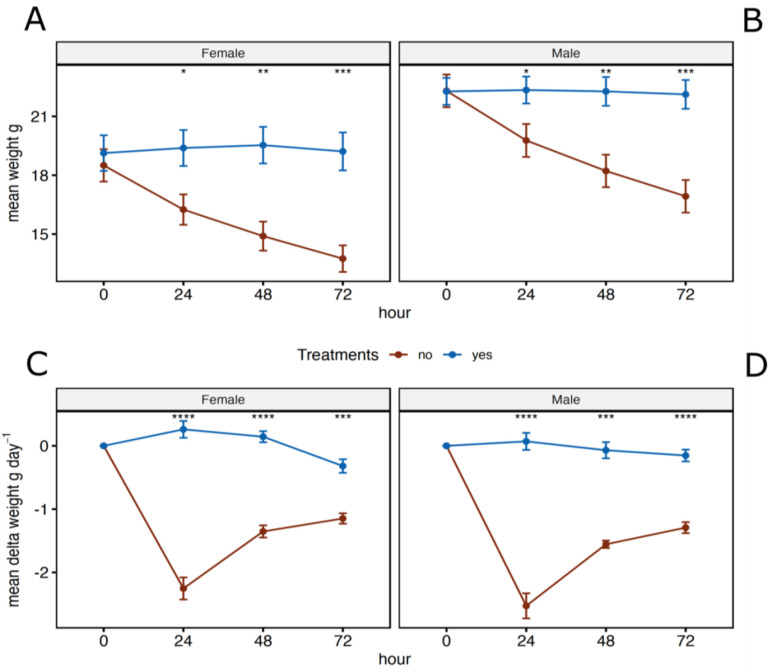
While the weights of males and females where insignificant at the beginning of the experiment, both sexes lost weight over the course of the water deprivation experiment with the most weight loss occurring in the first 24 hours without water (Figure 3). When comparing males without access to water to males with water access and females without access to water to females with water access, mice without water weighted significantly less then mice with water at 24 hours (p = 0.024, 0.019), 48 hours (p = 0.004, 0.002) and 72 hours (p = 0. 001, 0.005) (Figure 3A and 3B). Only animals held without water lost weight (Figure 3C and 3D), and analysis of these changes were significantly different at all timepoints after water had been removed (24 hours, p = 4.1−05, 4.1−05), (48 hours, p = 0.001, 4.1−05), and (72 hours, p = 4.1−05, 0.001).
Figure 3.
Mean weights (A and B) and mean delta weights (C and D) for female and male Peromyscus eremicus with (blue) or without (brown) access to water every 24 hours over the course of the 72-hour experiment. Error bars represent the standard error and * across the top denote statical significance from t-tests between the two treatments, with and without water, at each timepoint (* p <= 0.05, **: p <= 0.01, ***: p <= 0.001, ****: p <= 0.0001).
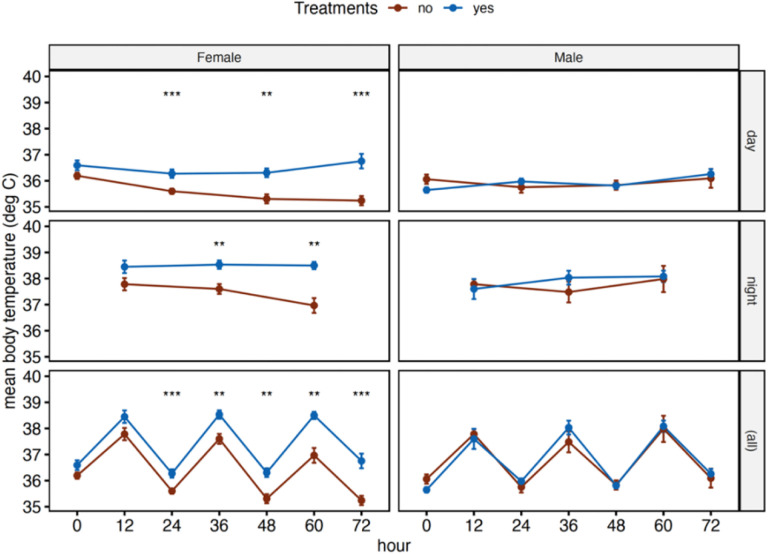
Body temperature showed diurnal pattering with the highest body temperature during the dark (active) phase and the lowest during the light phase (Figure 4. For females, body temperature followed a similar pattern as described above and were significantly lower for mice without access to water at 24 hours (p = 0. 001), 36 hours (p = 0.005), 48 hours (p = 0.001), 60 hours (p = 0.002), and 72 hours (p = 0.0003) while males were not significantly different at any of the time points (Figure 4).
Figure 4.
Mean body temperatures for female and male Peromyscus eremicus with (blue) or without (brown) access to water every 12 hours over the course of the 72-hour experiment. The top row of graphs are measurements taken only during the light phase, middle row are measurements taken only during the dark phase, and bottom row represents all the measurements. Error bars represent the standard error and * across the top denote statical significance from t-tests between the two treatments, with and without water, at each timepoint (* p <= 0.05, **: p <= 0.01, ***: p <= 0.001, ****: p <= 0.0001).
Discussion
Physiological mechanisms can act as a buffer, expanding organismal tolerance to new or extreme environments (Bijlsma and Loeschcke, 2005; Gabriel, 2005; Lau et al., 2017; Lui et al., 2015; Wilson and Franklin, 2002), however, biochemical and physical constraints eventually limit physiological capacity (Campbell-Staton et al., 2021; Velotta and Cheviron, 2018; Velotta et al., 2018), and ultimately determine a population’s persistence (Parmesan, 2006; Parmesan and Yohe, 2003; Van der Putten et al., 2010). In xeric habitats, further increased ambient temperature is compounded by reduced water availability, potentially affecting an animal’s ability to maintain homeostasis of body temperature and fluids (Reece et al., 2015). Given the current rapid pace of climate change (IPCC 2019), it is vital that we understand how species are responding to changes in their environment. Increased drought and changes in precipitation patterns are having several impacts on the availability of water, both in terms of availability, quantity, and quality (IPCC, 2019; Mirzabaev et al., 2019).
Organisms maintain water homeostasis in several ways, including seeking out sources of free-flowing water, preformed dietary water (Frank 1988; Orr et al. 2015) and/or water produced by metabolism. However, if adequate water is not acquired, dehydration can negatively affect an animal’s ability to regulate its body temperature, impair its’ cardiovascular function, and decrease perfusion to organ systems. Specifically, dehydration results in a decrease in blood volume and increase in osmolality, primarily driven by the increase in serum sodium levels (Leib et al., 2016; Thornton, 2010). As a result, several neurohormonal systems are activated to maintain blood pressure to perfuse tissues appropriately (Kaufmann et al., 2020). Water is recovered in the gastrointestinal tract (Thiagarajah and Verkman, 2018) and in the kidneys it is reabsorbed from the tubule system back into the blood stream (Fuller et al., 2020; Kortenoeven and Fenton, 2014). In severe cases, dehydration can lead to organ failure and death.
We explored the relationships and tradeoffs between thermoregulation, osmoregulation, and energy expenditure, of desert adapted mice without access to drinking water for three days while housed in an environmental chamber that simulated the desert environment. There are multiple avenues of water loss, including loss via urine and feces, as well as via cutaneous evaporation and respiration, and the measurements presented here represent their sum. We observed that when water was removed, energy expenditure and evaporative water loss are reduced in both sexes (presumably to conserve body water) at the expense of homeothermy, resulting in lower core body temperature in females but not in males. Though it may save water and/or energy, these physiological shifts could ultimately increase the risk of mortality and decrease fitness if water continues to be unavailable for extended periods of time.
Weight loss and water deprivation
Our study targeted responses to water deprivation, investigating how physiological variables changed in response to dehydration throughout the course of the experiment. We found that in response to water deprivation, cactus mouse phenotypic responses changed rapidly. During the first 24-hours of the water-deprivation experiment both males and females increased EE, resulting in an increase in RWL, and a significant decrease in body weight. The reasons behind this dramatic shift are unclear but may be a result of 1) a behavioral response related to searching for drinking water and or 2) suppression of food intake as suggested by pilot studies.
The relationship between eating and drinking has been extensively studied (Fitzsimons and Le Magnen, 1969; Kissileff, 1969; Smith, 2000; Watts, 1998; Zorrilla et al., 2005) and it has been documented that dehydration-anorexia that is an adaptive response to limit fluid loss (Watts and Boyle, 2010), as typically the processing of food requires the use of water. Previous studies have demonstrated that dehydrated animals with ad lib food match the same attributes of food restricted animals, such as expression of hypothalamic neuropeptide genes, leptin and insulin levels, and corticosterone concentrations (Watts et al., 1999). Furthermore, the reduction of food intake results in a series of adaptive responses that target GI function, allowing for the absorption of the osmotically sequestered water that is normally in the GI (Kutscher, 1966; Lepkovsky et al., 1957; Schoorlemmer and Evered, 1993). Finally, reduced food intake also reduces the solute load (Rowland, 2007) and the need for removal of waste products via urinary water loss (Schoorlemmer and Evered, 1993). In the study discussed herein, several tissues, including the GI tract, were removed at the conclusion of the experiment for future RNAseq analysis, and the GI tract was empty of food and feces (unpublished observations), suggesting that the intake of solid food had been decreased dramatically. As mentioned above, we saw a significant decrease in weight during the first 24 hours of the experiment, however, the RWL during the first 24 hours is not enough to account for the weight loss, suggesting weight loss through other means such as dehydration-anorexia (Armstrong et al., 1980; Hamilton and Flaherty, 1973).
Previous studies have found that access to water (Hochman and Kotler 2006; Shrader et al. 2008; Levy et al. 2016) and specific dietary composition (Blumstein et al., 2022; Frank, 1988; Manlick et al., 2021; Orr et al., 2015; Schmidt-Nielsen, 1975; Schmidt-Nielsen and Adolph, 1964; Wolf and del Rio, 2003) strongly effects populations living in arid environments. These external factors influence species distributions (McKee et al. 2015), modifying foraging decisions (Gedir et al. 2016, 2020), and altering behavior and reproduction (Douglas 2001; McKinney et al. 2001; Cain et al. 2008). In the wild, cactus mice have been documented shifting diet seasonally, consuming arthropods during the winter (Hope and Parmenter, 2007), and transitioning to the consumption of cactus seeds and/or fruits during the summer (Hope and Parmenter, 2007; Orr et al., 2015). In addition to preformed water, the composition of diet is also very important for P. eremicus as described in Blumstein et al. (2022). Specifically, mice fed a diet low in fat with ad lib water lost significantly more water and had electrolyte levels suggesting dehydration compared to mice fed a diet higher in fat, suggesting a limited capacity to tolerate water deprivation if optimal foods become less abundant (Blumstein et al. 2022). Furthermore, the temperatures required to balance evaporative water loss with metabolic water production on dry seed are much lower than what occurs during the summers in desert regions (MacMillen and Hinds 1983; Walsberg 2000), suggesting that P. eremicus may not be able to survive on a only a dry diet, unlike the Heteromyids, which survive on dry diets alone (Frank, 1988; Schmidt-nielsen et al., 1948).
Consistent with predictions of altered physiology and behavior mediated by water restriction, we recorded a decrease in EE, RWL, body weight, and body temperature and a shift in serum electrolytes in water deprived P. eremicus during all three 24-hour time blocks. While males and females without access to water had different magnitudes of change in EE and RWL throughout the duration of the study, both metabolic rates and the rate at which water is lost decreased, similar to what has been recorded in other desert organisms (Schmidt-Nielsen et al. 1967; Taylor 1969; Finch and King 1982). EE and RWL are inherently related in animals as lower EE leads to lower water loss by decreasing the amount of dry air passing through the respiratory track (McFarlane and Howard 1972). Furthermore, catabolism of different diets vary in the amount of available energy (Sánchez-Peña et al., 2017), water potential, as well as their obligatory water loss (Schmidt-Nielsen, 1975). At lower humidity, oxidation of carbohydrates produces a net metabolic water gain while lipids and proteins result in water loss, mainly through urination which is required to remove products of their metabolism like urea.
Sexual Dimorphism
Interestingly, males and females responded differently to lack of water, with body temperature being the most notable difference. Females decreased their body temperature while males maintained their body temperature when compared to their hydrated counterparts. Whether this sexually dimorphic response is a strategy or consequence is an open-ended question that cannot be answered using the data presented here, this response may be the product of high costs of reproduction in females, but not males. Indeed, similar patterns of sexual dimorphism in response to resource availability has been observed in other rodent species (Cranford 1977; Randolph et al. 1977; Murray and Smith 2012). Previous studies hypothesized that sexual dimorphism differences can be explained by differences in body size, metabolism, respiratory rate, or activity (Cryan and Wolf, 2003). While we do not have direct measurements of respiratory rate or activity, the production of CO2 follows the patterns of EE, providing indirect yet strong evidence that respiratory and metabolic rates (EE) as well as activity are all sexually dimorphic, consistent with observations in humans (Glucksmann 1974; Mittendorfer 2005; Pomatto et al. 2018) and has also been observed in P. eremicus by McNab and Morrison (1963) and Colella et al. (2021b).
Male reproduction is mainly limited by access to females (Bateman 1948), therefore, torpor or estivation by males could reduce male reproductive success. Furthermore, sperm quantity and quality is dependent on body temperature (Moore, 1926; Pérez-Crespo et al., 2008) and while typically resolved by externalizing the testes to the scrotum during excessive heat, a decrease in body temperature, as is seen in females (discussed below), could reduce sperm viability. Maintaining consistent body temperatures also allows for regular biological reactions, such as enzymatic processes and protein folding which have evolved to function best at a single temperature and can influence a series of functions not directly related to reproduction, such as growth rate, metabolic biorhythms, and environmental sensing (Glucksmann 1974; Hochachka and Somero 2002; McPherson and Chenoweth 2012; Calisi et al. 2018). Our data supports this as body temperature was unchanged for dehydrated males compared to their hydrated counterparts for the entire experiment, suggesting a maintenance of reproductive investment at the cost of long-term survival.
In contrast, female reproduction is primarily limited by their access to resources (Bateman 1948), in this case water. During the course of our study, female body temperature, EE, and RWL all decreased, suggesting torpor and or estivation, consistent with MacMillen (1983). Specifically, homeostatic responses such as adaptive heterothermy, a process which reduces evaporation by storing body heat, reduces the air to body temperature gradient thus decreasing inward heat flow, minimizes water loss from evaporative cooling (Schmidt-Nielsen et al. 1956; Schoen 1972; Taylor 1972; Cain et al. 2008), and in small endotherms with high surface area to volume ratios heterothermy can lead to substantial energy and water savings (Walsberg 2000; Speakman and Król 2010; Turbill and Stojanovski 2018). For females, reproductive demands are especially high, particularly during pregnancy and in lactating females (not measured in this study, Sorensen et al. 2005; Murray and Smith 2012), and minimizing energy costs or allocating pulses of resources to reproductive energy could increase reproductive success (Smith et al. 2014; Flores-Manzanero et al. 2019). While homeostatic responses are quite common among endotherms (Boyles et al. 2011, 2013; Canale et al. 2012; McGuire et al. 2014; Dammhahn et al. 2017) and are essential for short term survival, they incur energetic, resource, and or fitness costs when the disturbance lasts longer than the homeostatic tolerance (Wingfield et al. 1992; Boonstra 2004; Canale et al. 2012; McGuire et al. 2014; Dammhahn et al. 2017).
Electrolytes
In order to gain a deeper understanding of how water deprivation affects the physiological functioning of endotherms in desert environments, we collected serum electrolyte data from both males and females with and without access to water at the end of the experimental period. Electrolytes are essential for all physiological functions, including regulating fluid balance, transmitting nerve impulses, and maintaining the acid-base balance (Hasona and Elasbali, 2016). Additionally, electrolyte levels can provide insight into an individual’s overall metabolic state, renal function, and can be indicative of dehydration, kidney disease, or heart failure (Kutscher 1968; Cheuvront et al. 2010).
The kidney typically ensures that fluid and electrolyte balance remain within a narrow range, and this is conducive to efficient biochemical and physiological processes. Altered electrolytes, such as K, iCa, and Na, are associated with dehydration (Abubakar and Sule, 2010; Cheuvront et al., 2010; Kutscher, 1966), and may result in fatigue, cognitive disfunction, and changes in osmotic pressure which may affect blood pressure. More severe electrolyte abnormalities may cause cardiac arrhythmias, and lead to death (Abubakar and Sule, 2010). We uncovered significant differences in electrolyte values between water treatments (Na, BUN, Hct, osmolality, Hb, Cl, and total CO2), suggestive of dehydration, but synthetic markers of renal function were unchanged. Together, supporting findings from (Kordonowy et al., 2017), this suggests that end-organ perfusion is maintained despite dehydration.
Despite being statistically insignificant, glucose trended downwards for males and females without water when compared to their hydrated counterparts. During fasting, blood glucose levels decrease due to a lack of glucose absorbed from the GI tract (Jensen et al., 2013). Previous studies have shown that the duration of fasting significantly affects blood glucose levels up to 72 hours, but after 72 hours there is no further decrease (Jensen et al., 2013). In humans, glucose concentrations are maintained regardless of the duration of starvation (Watford, 2015). Initially, carbohydrates are depleted during the first 24 hours, however, during prolonged starvation gluconeogenesis provides glucose by breaking down skeletal muscle proteins (Watford, 2015). While the current study does not measure food intake, glucose is being maintained, suggesting they could be reducing in food intake consistent with other studies of dehydration-anorexia in rodents and or shift toward increased glycogenolysis and lipolysis to maintain glucose concentrations (Salter and Watts, 2003; Schoorlemmer and Evered, 2002; Watts and Boyle, 2010), meaning the liver is possibly serving as a buffer for blood glucose concentration.
Conclusion
The extreme aridity of desert environments plays a role in shaping biological processes however, the physiological mechanisms that allow animals to maintain salt and water homeostasis are still not well understood. Rapid climate change can challenge this tolerance. The cactus mouse (Peromyscus eremicus) is native to the arid deserts in southwest North America. Past studies have shown that cactus mice are highly adapted to desert conditions, with efficient water retention and dehydration tolerance. Therefore, cactus mice represent an interesting experimental model to examine physiological adaptations and thresholds.
In this study, we explore the physiological mechanisms that enable cactus mice to survive in desert habitats. By integrating laboratory-based experiments with long-term physiological data collected from a captive colony of cactus mice in a simulated desert environment, we investigate their response to water deprivation. Our findings reveal that mice without access to water exhibit significantly lower energy expenditures, leading to reduced water loss compared to mice with access to water. Moreover, significant weight loss was observed during the first 24 hours, likely attributed to dehydration anorexia—an adaptive response aimed at limiting fluid loss by reducing waste and the solute load, while facilitating water reabsorption from the kidneys and gastrointestinal tract. Furthermore, our observations indicate a relationship between body temperature and sex. Males without access to water maintained their body temperature compared to hydrated males, while females without access to water experienced decreased body temperature, suggesting the occurrence of daily torpor in females as an adaptive response, likely related to reproductive investment.
By examining the physiological responses of water deprived P. eremicus, we gain valuable insights into how adaptations developed over long evolutionary timescales. Given the current global climate change and the escalating desertification trends, it becomes imperative to investigate the plasticity and mechanisms of response in desert-adapted species. Such investigations hold the potential to enhance our understanding of organismal responses to the increasingly unpredictable climatic conditions.
Supplementary Material
Acknowledgments
We would like to thank members of the MacManes lab including Ella Caughran, Molly Kephart, Sean Pierre-Louis, Sarah Nicholls for helpful comments and support on previous versions of the manuscript. The Animal Resources Office and veterinary care staff at the University of New Hampshire for colony maintenance and care. This work was supported by the National Institute of Health National Institute of General Medical Sciences (R35 GM128843 to M.D.M.).
Footnotes
Competing Interests
No competing interests declared.
Data Availability
Macro processing files, processed respirometry data, and cage sampling scheme files are available on Zenodo: https://zenodo.org/record/8091766. All R scripts used in this project are available through GitHub at: https://github.com/DaniBlumstein/dehy_phys.
Refrences
- Albright T. P. et al. 2017. Mapping evaporative water loss in desert passerines reveals an expanding threat of lethal dehydration. Proceedings of the National Academy of Sciences 114:2283–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A. J. 1948. Intra-sexual selection in Drosophila. Heredity 2:349–368. [DOI] [PubMed] [Google Scholar]
- Bedford N. L., and Hoekstra H. E.. 2015. Peromyscus mice as a model for studying natural variation. eLife 4:e06813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein D. M., Colella J. P., Linder E., and MacManes M. D.. 2022. High total water loss driven by low-fat diet in desert-adapted mice. bioRxiv. [Google Scholar]
- Boonstra R. 2004. Coping with changing northern environments: the role of the stress axis in birds and mammals. Integrative and Comparative Biology 44:95–108. [DOI] [PubMed] [Google Scholar]
- Boyles J. G., Smit B., and McKechnie A. E.. 2011. A new comparative metric for estimating heterothermy in endotherms. Physiological and Biochemical Zoology 84:115–123. [DOI] [PubMed] [Google Scholar]
- Boyles J. G., Thompson A. B., McKechnie A. E., Malan E., Humphries M. M., and Careau V.. 2013. A global heterothermic continuum in mammals. Pp. 1029–1039 in Global ecology and biogeography. Wiley Online Library. [Google Scholar]
- Bradford D. F. 1974. Water Stress of Free-Living Peromyscus Truei. Ecology 55:1407–1414. [Google Scholar]
- Brown C. J. et al. 2016. Ecological and methodological drivers of species’ distribution and phenology responses to climate change. Global change biology 22:1548–1560. [DOI] [PubMed] [Google Scholar]
- CAIN J. W. III, Krausman P. R., Morgart J. R., Jansen B. D., and Pepper M. P.. 2008. Responses of desert bighorn sheep to removal of water sources. Wildlife Monographs 171:1–32. [Google Scholar]
- Calisi R. M., Austin S. H., Lang A. S., and MacManes M. D.. 2018. Sex-biased transcriptomic response of the reproductive axis to stress. Hormones and behavior 100:56–68. [DOI] [PubMed] [Google Scholar]
- Canale C. I., Levesque D. L., and Lovegrove B. G.. 2012. Tropical heterothermy: does the exception prove the rule or force a re-definition? Living in a seasonal world: thermoregulatory and metabolic adaptations:29–40. [Google Scholar]
- Cheuvront S. N., Kenefick R. W., Montain S. J., and Sawka M. N.. 2010. Mechanisms of aerobic performance impairment with heat stress and dehydration. Journal of Applied Physiology 109:1989–1995. [DOI] [PubMed] [Google Scholar]
- Christian D.P. and Matson J.O. 1978. Comparative water balance in two species of Liomys (Rodentia: Heteromyidae). Comparative Biochemistry and Physiology A. [Google Scholar]
- Colella J. P. et al. 2021a. Limited Evidence for Parallel Evolution Among Desert-Adapted Peromyscus Deer Mice. Journal of Heredity 112:286–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colella J. P., Blumstein D. M., and MacManes M. D.. 2021b. Disentangling environmental drivers of circadian metabolism in desert-adapted mice. Journal of Experimental Biology 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés A., Rosenmann M., and Bozinovic F.. 2000. Water economy in rodents: evaporative water loss and metabolic water production. Revista chilena de historia natural 73. [Google Scholar]
- Cranford J. A. 1977. Home Range and Habitat Utilization by Neotoma fuscipes as Determined by Radiotelemetry. Journal of Mammalogy 58:165–172. [Google Scholar]
- Dammhahn M., Landry-Cuerrier M., Réale D., Garant D., and Humphries M. M.. 2017. Individual variation in energy-saving heterothermy affects survival and reproductive success. Functional Ecology 31:866–875. [Google Scholar]
- Dewey G. C., Elias H., and Appel K.-R.. 1966. Stereology of the renal corpuscles of desert and swamp deermice. Nephron 3:352–365. [DOI] [PubMed] [Google Scholar]
- Douglas C. L. 2001. Weather, disease, and bighorn lamb survival during 23 years in Canyonlands National Park. Wildlife Society Bulletin:297–305. [Google Scholar]
- Finch V. A., and King J. M.. 1982. Energy-conserving mechanisms as adaptation to undernutrition and water deprivation in the African zebu. Use of tritiated water in studies of production and adaptation in ruminants. [Google Scholar]
- Fitzsimons J. T. 1963. The effects of slow infusions of hypertonic solutions on drinking and drinking thresholds in rats. The Journal of Physiology 167:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Manzanero A., Luna-Bárcenas M. A., Dyer R. J., and Vázquez-Domínguez E.. 2019. Functional connectivity and home range inferred at a microgeographic landscape genetics scale in a desert-dwelling rodent. Ecology and Evolution 9:437–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C. L. 1988. Diet Selection by a Heteromyid Rodent: Role of Net Metabolic Water Production. Ecology 69:1943–1951. [Google Scholar]
- Gedir J. V., Cain J. W. III, Krausman P. R., Allen J. D., Duff G. C., and Morgart J. R.. 2016. Potential foraging decisions by a desert ungulate to balance water and nutrient intake in a water-stressed environment. PloS one 11:e0148795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedir J. V., Cain J. W. III, Swetnam T. L., Krausman P. R., and Morgart J. R.. 2020. Extreme drought and adaptive resource selection by a desert mammal. Ecosphere 11:e03175. [Google Scholar]
- Glucksmann A. 1974. Sexual dimorphism in mammals. Biological Reviews 49:423–475. [DOI] [PubMed] [Google Scholar]
- Hayes J. P., Bible C. A., and Boone J. D.. 1998. Repeatability of Mammalian Physiology: Evaporative Water Loss and Oxygen Consumption of Dipodomys merriami. Journal of Mammalogy 79:475–485. [Google Scholar]
- Hochachka P. W., and Somero G. N.. 2002. Biochemical adaptation: mechanism and process in physiological evolution. Oxford university press. [Google Scholar]
- Hochman V., and Kotler B. P.. 2006. Effects of food quality, diet preference and water on patch use by Nubian ibex. Oikos 112:547–554. [Google Scholar]
- Hoffmann A. A., and Sgrò C. M.. 2011. Climate change and evolutionary adaptation. Nature 470:479–485. [DOI] [PubMed] [Google Scholar]
- Hofmann G. E., and Todgham A. E.. 2010. Living in the now: physiological mechanisms to tolerate a rapidly changing environment. Annu Rev Physiol 72:127–45. [DOI] [PubMed] [Google Scholar]
- Hughes L. 2000. Biological consequences of global warming: is the signal already apparent? Trends in ecology & evolution 15:56–61. [DOI] [PubMed] [Google Scholar]
- IPCC. 2019. Global Warming of 1.5 C An IPCC Special Report on the Impacts of Global Warming of 1.5 C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change. Sustainable Development, and Efforts to Eradicate Poverty. https://www.ipcc.ch/sr15/. Accessed 1.
- Jirimutu et al. 2012. Genome sequences of wild and domestic bactrian camels. Nature Communications 3:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordonowy L. et al. 2017. Physiological and biochemical changes associated with acute experimental dehydration in the desert adapted mouse, Peromyscus eremicus. Physiological Reports 5:e13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutscher C. 1968. Plasma volume change during water-deprivation in gerbils, hamsters, guinea pigs and rats. Comparative Biochemistry and Physiology 25:929–936. [DOI] [PubMed] [Google Scholar]
- Levy O., Dayan T., Porter W. P., and Kronfeld-Schor N.. 2016. Foraging activity pattern is shaped by water loss rates in a diurnal desert rodent. The American Naturalist 188:205–218. [DOI] [PubMed] [Google Scholar]
- Lighton J. R. B. 2018. Measuring Metabolic Rates: A Manual for Scientists. 2nd edition. Oxford University Press. [Google Scholar]
- Lin X., and Zhang D.. 1999. Inference in Generalized Additive Mixed Models by Using Smoothing Splines. Journal of the Royal Statistical Society. Series B (Statistical Methodology) 61:381–400. [Google Scholar]
- Loarie S. R., Duffy P. B., Hamilton H., Asner G. P., Field C. B., and Ackerly D. D.. 2009. The velocity of climate change. Nature 462:1052–1055. [DOI] [PubMed] [Google Scholar]
- Shrader, A. M., Kotler B. P., Brown J. S., and IH Kerley G.. 2008. Providing water for goats in arid landscapes: effects on feeding effort with regard to time period, herd size and secondary compounds. Oikos 117:466–472. [Google Scholar]
- MacManes M. D. 2017. Severe acute dehydration in a desert rodent elicits a transcriptional response that effectively prevents kidney injury. Renal Physiol:11. [DOI] [PubMed] [Google Scholar]
- MacMillen R. E. 1983. Water regulation in Peromyscus. Journal of Mammalogy 64:38–47. [Google Scholar]
- MacMillen R. E., and Hinds D. S.. 1983. Water Regulatory Efficiency in Heteromyid Rodents: A Model and Its Application. Ecology 64:152–164. [Google Scholar]
- Marra N. J., Romero A., and DeWoody J. A.. 2014. Natural selection and the genetic basis of osmoregulation in heteromyid rodents as revealed by RNA-seq. Molecular Ecology 23:2699–2711. [DOI] [PubMed] [Google Scholar]
- McFarlane W. V., and Howard B.. 1972. Comparative water and energy economy of wild and domestic animals. Pp. 261–296 in Symposia of the Zoological Society of London. [Google Scholar]
- McGuire L. P., Jonasson K. A., and Guglielmo C. G.. 2014. Bats on a Budget: Torpor-Assisted Migration Saves Time and Energy. PLoS ONE 9:e115724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee C. J. et al. 2015. Spatial distributions and resource selection by mule deer in an arid environment: Responses to provision of water. Journal of Arid Environments 122:76–84. [Google Scholar]
- McKinney T., Smith T. W., and Hanna J. D.. 2001. Precipitation and desert bighorn sheep in the Mazatzal Mountains, Arizona. The Southwestern Naturalist:345–353. [Google Scholar]
- McNab B. K., and Morrison P.. 1963. Body Temperature and Metabolism in Subspecies of Peromyscus from Arid and Mesic Environments. Ecological Monographs 33:63–82. [Google Scholar]
- McPherson F. J., and Chenoweth P. J.. 2012. Mammalian sexual dimorphism. Animal reproduction science 131:109–122. [DOI] [PubMed] [Google Scholar]
- Mirzabaev A. et al. 2019. Desertification. In: Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. <https://www.ipcc.ch/srccl/chapter/chapter-3/> (24 May 2021). [Google Scholar]
- Mittendorfer B. 2005. Sexual dimorphism in human lipid metabolism. The Journal of nutrition 135:681–686. [DOI] [PubMed] [Google Scholar]
- Moore C. R. 1926. The Biology of the Mammalian Testis and Scrotum. The Quarterly Review of Biology 1:4–50. [Google Scholar]
- Moritz C., Patton J. L., Conroy C. J., Parra J. L., White G. C., and Beissinger S. R.. 2008. Impact of a Century of Climate Change on Small-Mammal Communities in Yosemite National Park, USA. Science 322:261–264. [DOI] [PubMed] [Google Scholar]
- Mueller P., and Diamond J.. 2001. Metabolic rate and environmental productivity: Well-provisioned animals evolved to run and idle fast. Proceedings of the National Academy of Sciences 98:12550–12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murie M. 1961. Metabolic Characteristics of Mountain, Desert and Coastal Populations of Perormyscus. Ecology 42:723–740. [Google Scholar]
- Murray I. W., and Smith F. A.. 2012. Estimating the influence of the thermal environment on activity patterns of the desert woodrat (Neotoma lepida) using temperature chronologies. Canadian Journal of Zoology 90:1171–1180. [Google Scholar]
- Orr T. J., Newsome S. D., and Wolf B. O.. 2015. Cacti supply limited nutrients to a desert rodent community. Oecologia 178:1045–1062. [DOI] [PubMed] [Google Scholar]
- Pardi M. I., Terry R. C., Rickart E. A., and Rowe R. J.. 2020. Testing climate tracking of montane rodent distributions over the past century within the Great Basin ecoregion. Global Ecology and Conservation 24:e01238. [Google Scholar]
- Parmesan C. 2006. Ecological and Evolutionary Responses to Recent Climate Change. Annual Review of Ecology, Evolution, and Systematics 37:637–669. [Google Scholar]
- Parmesan C., and Yohe G.. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42. [DOI] [PubMed] [Google Scholar]
- Pavlik B. M. 2008. The California deserts: an ecological rediscovery. Univ of California Press. [Google Scholar]
- Pomatto L. C., Tower J., and Davies K. J.. 2018. Sexual dimorphism and aging differentially regulate adaptive homeostasis. The Journals of Gerontology: Series A 73:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pörtner H. O., and Farrell A. P.. 2008. Physiology and climate change. Science 322:690–692. [DOI] [PubMed] [Google Scholar]
- Pörtner H.-O. 2002. Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 132:739–761. [DOI] [PubMed] [Google Scholar]
- R Core Team. 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. [Google Scholar]
- Randolph P. A., Randolph J. C., Mattingly K., and Foster M. M.. 1977. Energy costs of reproduction in the cotton rat, Sigmodon hispidus. Ecology 58:31–45. [Google Scholar]
- Riddell E. A. et al. 2021. Exposure to climate change drives stability or collapse of desert mammal and bird communities. Science 371:633–636. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K., Crawford E. C. Jr, Newsome A. E., Rawson K. S., and Hammel H. T.. 1967. Metabolic rate of camels: effect of body temperature and dehydration. American Journal of Physiology-Legacy Content 212:341–346. [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielsen K., Schmidt-Nielsen B., Jarnum S. A., and Houpt T. R.. 1956. Body temperature of the camel and its relation to water economy. American Journal of Physiology-Legacy Content 188:103–112. [DOI] [PubMed] [Google Scholar]
- Schoen A. 1972. Studies on the environmental physiology of a semi-desert antelope, the dikdik. East African Agricultural and Forestry Journal 37:325–330. [Google Scholar]
- Sikes R. S., and Gannon W. L.. 2011. Guidelines of the American Society of Mammalogists for the use of wild mammals in research. Journal of Mammalogy 92:235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson G. 2023. gratia: Graceful ggplot-Based Graphics and Other Functions for GAMs Fitted using mgcv. <https://gavinsimpson.github.io/gratia/>.
- Smith F. A., Murray I. W., Harding L. E., Lease H. M., and Martin J.. 2014. Life in an extreme environment: a historical perspective on the influence of temperature on the ecology and evolution of woodrats. Journal of Mammalogy 95:1128–1143. [Google Scholar]
- Sorensen J. S., McLister J. D., and Dearing M. D.. 2005. Plant secondary metabolites compromise the energy budgets of specialist and generalist mammalian herbivores. Ecology 86:125–139. [Google Scholar]
- Speakman J. R., and Król E.. 2010. Maximal heat dissipation capacity and hyperthermia risk: neglected key factors in the ecology of endotherms. Journal of Animal Ecology 79:726–746. [DOI] [PubMed] [Google Scholar]
- Stillman J. H. 2019. Heat Waves, the New Normal: Summertime Temperature Extremes Will Impact Animals, Ecosystems, and Human Communities. Physiology 34:86–100. [DOI] [PubMed] [Google Scholar]
- Takei Y. 2015. From Aquatic to Terrestrial Life: Evolution of the Mechanisms for Water Acquisition. Zoological Science 32:1–7. [DOI] [PubMed] [Google Scholar]
- Taylor C. R. 1969. The eland and the oryx. Scientific American 220:88–97. [DOI] [PubMed] [Google Scholar]
- Taylor C. R. 1972. The desert gazelle: a paradox resolved.
- Thomas D. R. et al. 2008. Understanding Clinical Dehydration and Its Treatment. Journal of the American Medical Directors Association 9:292–301. [DOI] [PubMed] [Google Scholar]
- Tigano A., Colella J. P., and MacManes M. D.. 2020. Comparative and population genomics approaches reveal the basis of adaptation to deserts in a small rodent. Molecular Ecology 29:1300–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbill C., and Stojanovski L.. 2018. Torpor reduces predation risk by compensating for the energetic cost of antipredator foraging behaviours. Proceedings of the Royal Society B: Biological Sciences 285:20182370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsberg G. E. 2000. Small Mammals in Hot Deserts: Some Generalizations Revisited. BioScience 50:109. [Google Scholar]
- Westerterp K. R. 1993. Food quotient, respiratory quotient, and energy balance. The American Journal of Clinical Nutrition 57:759S–765S. [DOI] [PubMed] [Google Scholar]
- Wingfield J. C., Vleck C. M., and Moore M. C.. 1992. Seasonal changes of the adrenocortical response to stress in birds of the Sonoran Desert. Journal of Experimental Zoology 264:419–428. [DOI] [PubMed] [Google Scholar]
- Wood S. 2017. Generalized Additive Models: An Introduction with R, Second Edition. [Google Scholar]
- Yang J. et al. 2016. Whole-Genome Sequencing of Native Sheep Provides Insights into Rapid Adaptations to Extreme Environments. Molecular Biology and Evolution 33:2576–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Macro processing files, processed respirometry data, and cage sampling scheme files are available on Zenodo: https://zenodo.org/record/8091766. All R scripts used in this project are available through GitHub at: https://github.com/DaniBlumstein/dehy_phys.