Abstract
Abdominal aortic aneurysms (AAAs) are prevelant with aging, and AAA rupture is associated with high mortality. There is currently no effective medical therapy for AAA rupture. Previous work demonstrated that the monocyte chemoattractant protein (MCP-1) / C-C chemokine receptor type 2 (CCR2) axis critically regulates AAA inflammation, matrix-metalloproteinase (MMP) production, and extracellular matrix (ECM) stability. Here we similarly observed that Ccr2−/− mice have significantly reduced AAA expansion and rupture. We therefore hypothesized that a dietary modulation of the CCR2 axis may therapeutically impact AAA risk of rupture. Since ketone bodies (KBs) can trigger repair mechanisms in response to inflammation, we specifically evaluated whether systemic ketosis in vivo can reduce CCR2 and AAA progression. Male Sprague-Dawley rats underwent surgical AAA formation using porcine pancreatic elastase (PPE), and received daily β-aminopropionitrile (BAPN) to promote AAA rupture. Animals with AAAs received either a standard diet (SD), ketogenic diet (KD), or exogenous KBs (EKB). Animals recieving KD and EKB reached a state of ketosis, and had significant reduction in AAA expansion and incidence of rupture. Ketosis also led to significantly reduced aortic CCR2 content, improved MMP balance, and reduced ECM degradation. In summary, this study demonstrates that ketosis plays a crucial role in AAA pathobiology, and provides the impetus for future clinical studies investigating the potential benefit of ketosis for prevention of AAA expansion and rupture.
Keywords: Abdominal Aortic Aneurysm, Aortic Rupture, Ketogenesis, C-C chemokine receptor 2, Aortic Inflammation, Matrix-Matalloproteinase
Introduction
Abdominal aortic aneurysm (AAA) formation, expansion, and rupture results from a complex series of biomolecular processes 1,2. AAAs are often asymptomatic during their formation and expansion stages, but lead to a high risk of morbidity and mortality when they spontaneously rupture 3,4. Unfortunately, there is currently no effective medical therapy to alleviate AAA expansion and the eventual risk of rupture. Invasive surgery is the only available management for AAAs that meet the traditional aortic diameter criteria or are rapidly expanding in diameter 5. Given the limited medical treatment options for individuals with small AAAs that do not yet meet criteria for surgical repair, expectant management is usually the only remaining option 6. Therefore, clinical stabilization of small AAAs remains a large unmet need, and a longer-term management strategy for individuals with AAA disease can be a tremendous value add 7.
Inflammation plays an essential role in AAA disease progression 8. From AAA formation due to wall microdissections, subsequent expansion, and eventual rupture, the release of inflammatory mediators within the aortic wall leads to a cascade of biomolecular signals that lead to the activation of matrix metalloproteinases (MMPs). Activated MMPs consequentially lead to extracellular matrix degradation 9–12, and leading to futher AAA expansion. The C-C chemokine receptor type 2 (CCR2) mediates trafficking of leukocytes to site of aortic tissue inflammation following initial and repeated injury 13. Our team previously demonstrated that CCR2 content in AAAs, as demonstrated by positron emission tomography (PET) / computed tomography (CT) imaging in a preclinical rodent model, highly correlates with the incidence of AAA expansion and rate of rupture 14,15. However, it remains unknown whether CCR2 content is essential for AAA rupture, and whether modulation of CCR2 can therefore help alleviate disease progression.
Various oral diets are known to impact immune function and inflammation 16. Ketogenesis in particular can dramatically impact anti-inflammatory signaling, and is reported to promote vascular tissue repair 16–18. As a natural physiologic process that leads to the production of ketone bodies (KBs) such as acetoacetate (AcAc), beta-hydroxybutyrate (βHB) and acetone, ketogenesis not only serves as alternative fuel source for organ systems, but it also activates signaling cascades that can impact various cell functions. Although high fat diets are linked to increased AAA expansion and aortic plaque formation 19,20, recent studies suggested that a high fat – low carbohydrate ketogenic diet, as well as exogenous ketone body supplementation, can reduce tissue inflammation and ameliorate the risk of vascular injury and atheroprogression 21,22. It is unknown whether these potential benefits are limited to atherosclerosis, or whether ketosis can also have a broader impact on degenerative aortopathies such as AAAs. Therefore, we hypothesized that nutritional ketosis, either in the form of a ketogenic diet or exogenous ketone body supplementation, can impact CCR2-mediated inflammation and improve MMP balance in aortic tissue to reduce the risk of AAA progression, aortic wall inflammation, extracellular matrix (ECM) content, and the incidence of AAA rupture.
Methods
Animals
Male Sprague-Dawley rats (200–300g) were obtained from Charles River Laboratories (Wilmington, MA; see supplemental methods). Male Ccr2−/− and Ccr2+/+ mice on a C57BL/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME; see supplemental methods). All animals were housed at 21°C in a 12/12 hour light/dark cycle and had access to food and water ad libitum. Anesthesia was administered with a mixture of ~ 1.5% isoflurane and oxygen for all procedures. The core body temperature was continously monitored and maintained with a heating pad at 37°C. Use of all animal experiments were performed in accordance with relevant guidelines and regulations, were approved by the Institutional Animal Care and Use Committee (IACUC) at Washington University School of Medicine in St. Louis, and reported in accordance with ARRIVE guidelines. At the conclusion of studies, live animals were sacrificed appropriately using anesthetic agents and cervical dislocation.
Induction of AAA
Rats were induced to develop infrarenal AAAs via an established model using porcine pancreatic elastase (PPE; 12 U/mL) as previously described 23. Ventral abdominal wall laparotomy is performed, and the infrarenal abdominal aorta was exposed. (Supplemental Fig. 1). A customized polyethylene catheter (Braintree Scientific, Braintree, MA) was introduced through an infrarenal aortotomy, and elastase was infused into the isolated aortic segment for 30 minutes. The exposed aortic segment was dilated to a maximal diameter, and constant pressure was maintained with the use of a syringe pump. Using a video micrometer, the baseline maximum aortic diameter was measured. After 14 days, all rat aortas were re- exposed via ventral abdominal laparotomy, maximal aortic diameters were measured, and aortic tissue was harvested for further analysis (Supplemental Fig. 1).
Promoting AAA Rupture
As previously described, β aminopropionitrile (BAPN) is reported to promote AAA tissue inflammation by day 6, and AAA rupture between days 7 and 14, but unlikely to cause rupture after day 14 if it has not already occurred 15. We therefore promoted AAA rupture with daily administration of BAPN on a specific cohort of rats (RAAA) starting 3 days before PPE exposure. Through drinking water, 300mg BAPN was administered daily (0.3% BAPN in 25mL water consumed per day by a 250g rat) 24. AAA growth was monitored for 1 week (6–7 days) or 2 weeks (14 days). At the 1 or 2-week timepoints, rats were sacrificed, AAA diameters were evaluated, and aortic tissue was harvested for further analysis. Rats that developed ruptured AAAs during the study period promptly underwent necropsy to confirm and analyze the pathology (Supplemental Fig. 1). Tissue harvested at week 1, prior to rupture, was mainly used to assess AAA tissue inflammation (Supplemental Fig. 2).
Animal Diets
We evaluated four different dietary interventions. First, control groups in the AAA (n = 5) and RAAA (n = 12) cohorts were fed with a standard chow diet (SD). Second, experimental groups in the AAA (n = 6) and RAAA (n = 8) cohorts were fed a very high fat diet with almost no carbohydrate, also known as a classic ketogenic diet one week prior to PPE exposure to induce a ‘priming’ keto-adapted status 25, before AAA induction (Supplemental Fig. 3). Ketogenic diet was then maintained prospectively in these groups following AAA formation. Third, experimental groups in the RAAA (n = 9) cohorts were separately started on ketogenic diets as a ‘treatment’ intervention 3 days after AAA induction. Lastly, experimental RAAA rats were administered a SD along with exogenous ketone body (EKB) supplementation starting 3 days after PPE exposure: RAAA + EKB (n = 10). As previously described 26, this EKB supplementation was performed with daily intragastric gavage of 1,3-Butanediol (BD; 5g per kg dose; Prod # B84785–100ML, St. Louis, MO) and animals achieved a ketosis state only for 8 hours per day (Supplemental Fig. 3).
Synthesis and Radiolabeling of DOTA-ECL1i
The ECL1i peptide (LGTFLKC) was synthesized from D-form amino acids by CPC Scientific (Sunnyvale, CA). DOTA-ECL1i was prepared following our previous report. Copper-64 (64Cu, t1/2 = 12.7 hour) was produced by the Washington University Cyclotron Facility. The DOTA-ECL1i conjugate was radiolabeled with 64CuCl2 (64Cu-DOTA-ECL1) as described, and the radiochemical purity was determined by radio- HPLC 15.
Animal PET/CT Imaging and Image Analysis
Dynamic PET scan and corresponding CT images were obtained using Inveon MM PET/CT (Siemens, Malvern, PA) at 45 to 60 minutes after a tail vein injection of 64Cu-DOTA-ECL1i (selective CCR2-targeting radiotracer; 11.1 MBq per rat) to minimize the effect of blood retention on AAA uptake. To localize tracer uptake, a CT contrast agent (1.0 mL, eXIA 160XL, Binitio, Canada) was administrated via tail vein after PET imaging. Contrast-enhanced CT (Bin of 2, 90 mm axial FOV, 60 kV, 500 μA, 500 ms exposure time, 10 ms settle time, no magnification, pixel size: 80–100 μm) was performed. The AAA uptake was calculated as standardized uptake value (SUV) in 3-dimensional regions of interest from PET images without correction for partial volume effect using Inveon Research Workplace software (Siemens). Dynamic (0–90 minutes) 18F-fluorodeoxyglucose (41.1 MBq per rat) PET was also performed in AAA rats at week 1 and 2 post-PPE exposure. Only a specific number of each group of rats received PET/CT imaging and analysis.
Ultrasound Aortic Assessments
Noninvasive ultrasound (GE, 12 MHz Zonare, Mountain View, CA), was used to evaluate serial aortic maximum diameter measurements. Relative to baseline aortic diameter prior to PPE exposure, the percentage increase in aortic diameter was evaluated at 1- and 2-weeks post-PPE exposure. As previously described, aortic aneurysms were defined as > 100% increase in the aortic maximum diameter relative to baseline diameter 23,27.
Blood βHB Assessments
Animal state of ketosis was evaluated via whole blood D-βHB (Keto-MoJo blood ketone meter; Keto-Mojo, Napa, CA, USA) concentrations 28. Tail vein puncture was used for blood sample, which was tested on day 0 pre-PPE exposure, and then 1- and 2-weeks following AAA induction. βHB values > 0.5 mmol/L were indicative of ketosis.
Animal Weight
Animal whole body weights were evaluated at day 0 pre-PPE exposure, and 1 and 2 weeks followed AAA induction. Body weight was evaluated by the difference between the values at the baseline (Day 0) and the values at week 1 and 2 respectively and then divided by the baseline to assess difference. All these absolute numbers were then multiplied by 100 to present it as the percentage of difference in weight throughout the time of the study.
Histology and Immunostaining
Aortic tissue was harvested from all animals. AAA tissue was fixed in Histochoice (VWR), and paraffin embedded. Paraffin blocks were sectioned at 5 μm, and deparaffinized. Processing for antigen retrieval was performed with Sodium Citrate solution, pH 6.0, for 10 min. Tissue sections were blocked with 10% serum, and sections were incubated with primary antibody anti-CD68, 1:100 [Bio-Rad, MCA341GA]. Sections were then incubated with anti-mouse secondary antibodies conjugated with HRP (Cell Signaling), DAB peroxidase substrate kit (Vector Laboratories), and counter stained with hematoxylin, imaged using an Olympus fluorescent microscope system. To evaluate AAA tissue morphology and pathology, tissue sections were also evaluated using Hematoxylin and Eosin (H&E) and Mason Trichrome (MT). AAA wall tissue-stained sections were then analyzed and quantified by Image J software via color deconvolution and shown as percentage of stained area for specific regions of interest (ROI).
ELISA and Cytokine Array Assessments
AAA tissue protein was extracted using RIPA buffer with proteinase inhibitor (Sigma #MCL1). Protein quantification was done by Bradford assay. For each AAA tissue samples 25ug of protein was analyzed for Tissue Inhibitor of Metalloproteinases 1 (TIMP1)-specific ELISA (RayBiotech, ELR-TIMP1-CL-2), MMP-9 (MyBioSource, MBS722532), CD68 (MyBioSource, MBS705029) and Cytokine multiplex assay (Millipore, RECYTMAG-65K) using manufacturer instructions.
MMP Activity Zymography
For each AAA tissue sample, 25ug of protein was loaded on wells of 10% Gelatin Zymogram electrophoresis gels. Gels were then incubated in Zymogram renature buffer for 30 min, followed by 36 hours of Zymogram development buffer at 37°C. Gels were then stained with Coomassie Brilliant Blue R-25 solution from BioRad for 30 min, followed by destaining buffer (20% Methanol, 20% Acetic acid, 60% DI water) until MMP bands were visualized. Gels were scanned on BioRad Chemi doc and analyzed using ImageJ software.
Immunoblotting
From each AAA sample 25μg of denatured protein was separated on 4–12% polyacrylamide by electrophoresis and then transferred to PVDF membrane. The membranes were then incubated with collagen type-1 (Millipore #ABT123, 1:2000), TGF-β1 (Sigma #AV44268, 1:1000), and α-SMA (Sigma #A2547, 1:1000). GAPDH (Sigma # G9545, .1mg/ml) and Caveolin-1 (Santa Cruz # sc-53564, 1:1000) were used as loading controls. Membranes were treated with HRP-conjugated secondary antibody at room temperature for 1 hour and evaluated with chemiluminescence. Blot band intensities were analyzed using ImageJ software.
Statistics
All data are presented as the mean ± SD. Most group comparisons were performed using unpaired t test. For comparisons that included one endpoint in more than one animal/diet groups, an ordinary one-way ANOVA with multiple comparison was performed. For comparisons that included more than one endpoint in more than one animal/diet group, we utilized a two-way ANOVA with multiple comparison. Data was considered statistically significant with p ≤ 0.05. Kaplan-Meier curve was generated to assess the survival of BAPN-exposed animals. GraphPad Prism 9 (La Jolla, CA) was used for all statistical analyses and graphical data representations. In certain circumstances outlier data points were excluded from the analysis if they met the pre-determinated criteria of the outlier was more than (1.5x Interquartile Range (IQR)) above the third quartile (QR) or below the first quartile (Q1). MT cross section staining’s were analyzed using ImageJ software by color deconvolution, adjust threshold and region of interest assessment of the AAA wall.
Results
CCR2 is Essential for AAA Formation and Rupture
To evaluate whether CCR2 plays an essential role in AAA pathology, we evaluated whether AAA progression and/or rupture were impacted in mice that underwent whole body gentic knockdown of Ccr2. Age-matched male wildtype (Ccr2+/+) and Ccr2−/− adult mice received angiotensin II osmotic pump administration to promote AAA formation14,29, and daily BAPN administration to promote AAA rupture 30 (Supplemental Fig. 4A). Over the subsequent 2 weeks, Ccr2−/− mice had significantly less AAA formation (p < 0.001; Supplemental Fig. 4B), significantly reduced incidence of AAA rupture (p = 0.003; Supplemental Fig. 4C), and 47% higher survival compared to Ccr2+/+ (Supplemental Fig. 4D). PET/CT with 64Cu-DOTA-ECL1i (selective CCR2-targeting PET radiotracer) demonstrated significantly reduced CCR2 content in the aorta of Ccr2−/− mice and no AAAs were observed (p < 0.001; Supplemental Fig. 4E & F). These data confirmed that CCR2 is essential for AAA formation and rupture, and led us to next evaluate whether an easy to implement dietary intervention can reduce CCR2 and ameliorate AAA pathology.
Ketosis Attenuates AAA Formation and Content of MMP9 in Aortic Tissue
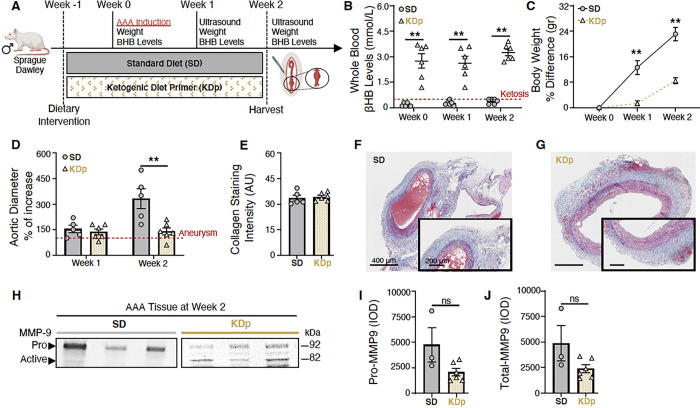
To evaluate the impact of a ketogenic diet on AAA pathology, we first compared to animals fed a standard diet (SD), to animals maintained on a ketogenic diet prior to AAA induction (KDp; Fig. 1A). KDp achieved a state of sustained ketosis from day 0–14 (Fig. 1B), and caused a moderate decrease in weight gain by week 1 and 2 (p < 0.001; Fig. 1C). By week 2 there was a substantial 42% decrease in AAA diameter in KDp animals (p = 0.008; Fig. 1D and Supplemental Fig. 5A). Aortic wall media demonstrated equivalent masson trichrome (MT)-stained collagen between animals maintained on SD and KDp (Fig. 1E–G). Zymography analysis of harvested aortic tissue at week 2 demonstrated a modest decrease in pro and total-MMP9 in KDp animals (Fig. 1H–J). These data suggested that diet-induced ketosis can inhibit AAA expansion, and that this may in part be due to a decrease in aortic wall total and pro-MMP9.
Figure 1. Ketosis attenuates AAA formation and MMP9.
(A) Animals underwent exposure to PPE for AAA creation. The experimental group was given a ketogenic diet that started one-week prior to PPE exposure (KDp; N=6) while the control group was fed a standard chow diet (SD; N=5). (B) Ketosis (βHB whole blood levels > 0.5 mM/L) was verified at week 0, 1 and 2 in SD (0.2±0.1, 0.3±0.1 and 0.4±0.1) and KDp animals (3±1, 3±1 and 3±0.5) respectively (p < 0.01). (C) Percent body weight difference in SD vs KDp animals at week 1 (13±5 vs 2±1.3) and at week 2 (23±5 vs 8±2) respectively (p < 0.001). (D) Percent aortic diameter in SD vs KDp animals at week 1 (154±48 vs 137±42; p = ns) and at week 2 (332±129 vs 140±152; p = 0.008) respectively (aneurysms were defined by a >100% increase in the aortic diameter compared with pretreatment measurements). (E) AAA collagen staining quantification for SD and KDp at week 2 (33±4 vs 34±2; p = ns) respectively. (F and G) Trichrome staining of abdominal aortas (cross-section of tissue slides) with 5x magnification for SD and KDp animals. Areas with blue staining signify areas with higher collagen deposition. (H) Zymogram demonstrating pro and active MMP9 levels were measured by integrated optical density (IOD).. (I) Pro MMP-9 levels for SD and KDp at week 2 (4.8±3×103 vs 2±0.9×103; p = ns) respectively. (J) Total MMP-9 levels for SD and KDp at week 2 (4.8±3×103 vs 2.4±0.9×103; p = ns) respectively. Data presented as mean ± standard deviation. ns > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001 using Student’s t test. No outliers were observed in the analyses, and all data was included in the figure.
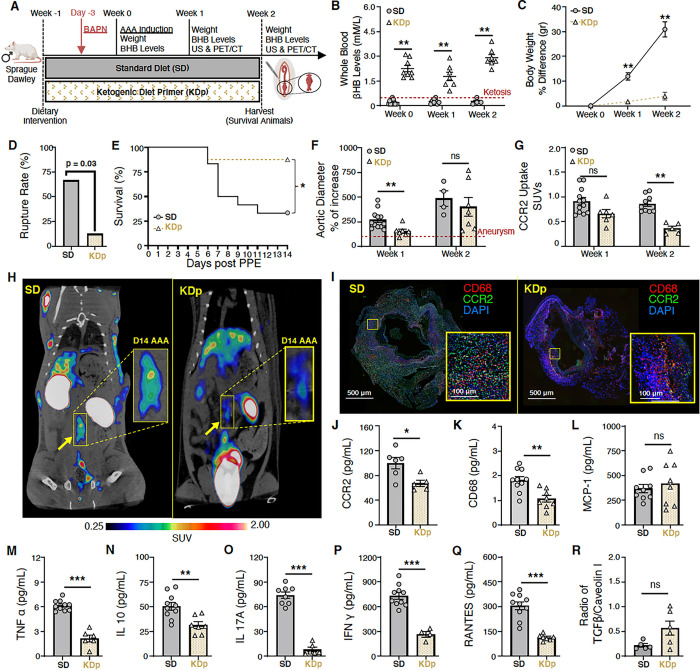
Sustained Ketosis Reduces AAA Expansion and CCR2 Content in Rupture-Prone Animals
To evaluate whether in a AAA rupture model dietary ketosis can impact CCR2 content and AAA pathology, we then evaluated a separate cohort of animals that received either SD or KDp after AAA induction with PPE, and daily BAPN administration to promote AAA rupture (Fig. 2A). By day 14, animals that survived (non-ruptured AAA; NRAAA) were evaluated via open surgical laparotomy. Animals that sustained AAA rupture (RAAA) were evaluated via necropsy. In vivo evaluations of aortic diameter were serially performed using ultrasound (US) and positron emission tomography (PET) / computed tomography (CT) with 64Cu-DOTA-ECL1i (selective CCR2-targeting PET radiotracer) were also performed (Fig. 2A). Animals fed KDp, and received daily BAPN, remained in a state of ketosis from days 0–14 (Fig. 2B & Supplemental Fig. 2B), and weight gain was similarly reduced at weeks 1 and 2 (p < 0.001 and p = 0.006, respectively; Fig. 2C & Supplemental Fig. 2C). Administration of BAPN did not significantly alter βHB levels between SD and KDp animals (Supplemental Fig. 6A). Interestingly, KDp animals also had significantly reduced AAA rupture (67% vs 12%; p = 0.03; Fig. 2D & E). Aneurysm diameter at week 1 was significantly decreased in KDp animals (p = 0.002 and p = 0.01; Fig. 2F & Supplemental Fig. 5B), and by week 2, AAA diameter was equivalent between groups. Interestingly, PET/CT demonstrated a significant reduction in CCR2 content in AAAs of KDp animals at week 1 (p = 0.05) and week 2 (p = < 0.0001; Fig. 2G & H). 18 F-fluorodeoxyglucose PET/CT performed in KDp and SD animals at week 1 revealed comparable AAA signal uptake 15,23,30, (p = ns; Supplemental Fig. 7A & B). These findings demonstrate that KDp animals developed smaller aneurysms with a combined 54% absolute risk reduction, and decreased CCR2 content in AAA tissue.
Figure 2. Sustained ketosis reduces AAA expansion and risk of rupture via CCR2 downregulation and Collagen 1 preservation.
(A) Animals underwent exposure to PPE to develop AAAs and were also treated with β-aminoprionitrile (BAPN) to promote AAA rupture. (B) Ketosis (βHB whole blood levels > 0.5 mM/L) in SD and KDp animals at week 0 (0.2±0.1 vs 2±0.5), week 1 (0.3±0.1 vs 1.8±0.7) and week 2 (0.2±0.1 vs 3±0.5; p < 0.01). (C) Percent body weight difference in SD and KDp animals at week 1 (12±5 vs 2±1.3; p < 0.001) and week 2 (31±6 vs 4±3; p = 0.006). (D) AAA rupture event rate with statistical analysis in SD and KDp animals (p = 0.03). (E) Kaplan-Meier curve demonstrating rate of survival following BAPN administration. 67% (8/12) of SD animals and 12% (1/8) KDp animals developed AAA rupture. (F) Percent aortic diameter in SD and KDp animals at week 1 (270±93 vs 154±53; p = 0.002) and week 2 (485±153 vs 401±246; p = ns). (G) Quantitative tracer uptake of CCR2 content in AAA tissue for SD and KDp animals at week 1 (0.9±0.2 vs 0.7±0.2; p = 0.05) and week 2 (0.9±0.2 vs 0.4±0.1; p < 0.001. (H) Representative PET/CT coronal images at day 14 post PPE exposure showed specific and intensive detection of AAA (yellow rectangle) in SD, compared with the low trace accumulations in the KDp group of animals. (I) Immunofluorescence staining of abdominal aortas (cross-sectional; 5x magnification and 10x magnification) marked with CCR2 (in green: CCR2+ cells) and CD68 marker (in red: CD68 + cells; macrophages) to visualize inflammatory cells infiltration within the AAA. (J) CCR2 content at week 1 in AAA tissue of SD and KDp animals (100±22 vs 67±12; p = 0.02). (K) Macrophage marker CD68 content at week 1 in AAA tissue of SD and KDp animals (1.8±0.4 vs 1±0.4; p = 0.002). (L) Chemokine MCP-1 content (3.7±1.2 ×102 vs 4.2±2.2 ×102; p = ns). Pro-inflammatory marker TNFα content (6.1±0.6 vs 2.1±1; p = 0.001), IL-10 content (5±1.3 ×101 vs 3.1±0.9 ×101 ; p = 0.03), (O) IL-17A content (7.4±1.1 ×101 vs 0.8±0.6 ×101 ; p < 0.001), (P) IFNg content (7.3±1.3 ×102 vs 2.6±0.7 ×102 ; p = 0.002), (Q) RANTES content (3±0.7 ×102 vs 1.1±0.2 ×102 ; p < 0.001) and (R) TGFβ (0.3±0.1 vs 0.5±0.1; p NS). Data presented as mean ± standard deviation. ns > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001 using Student’s t test.
Sustained Ketosis Inhibits Cytokine Profiles Downstream to CCR2 in AAA Tissue by Week 1
The risk of AAA progression correlates with CCR2-mediated pro-inflammatory signaling 15, 31–33. We therefore evaluated whether sustained ketosis impacts CCR2 in AAA tissue and downstream cytokine profiles during AAA formation. We observed that immunostaining of AAA tissue in KDp animals demonstrated a marked decrease in CCR2, and CD68 + macrophages (Fig. 2I & J; Supplemental Figs. 8 &9). Correspondingly, ELISA demonstrated that CCR2 and CD68 content was significantly decreased in AAAs of KDp animals at week 1 (p = 0.02 and p = 0.002, respectively; Fig. 2K & L). The CCR2 ligand, monocyte chemoattractant protein-1 (MCP-1) was unchanged between KDp and SD animals (Fig. 2M), but the pro-inflammatory cytokines TNFα, IL-10, IL-17A, and IFN γ were also decreased in AAA tissue of KDp animals (p = 0.001, p = 0.03, p < 0.001, and p = 0.002 respectively; Fig. 2N–Q). Similarly, RANTES (the ligand for C-C motif chemokine receptor 5; CCR5) was significantly reduced in KDp animals (p < 0.001; Fig. 2R). These results indicates that KDp notably decreases AAA macrophage infiltration, CCR2-mediated inflammation, and reduced AAA expansion and rupture.
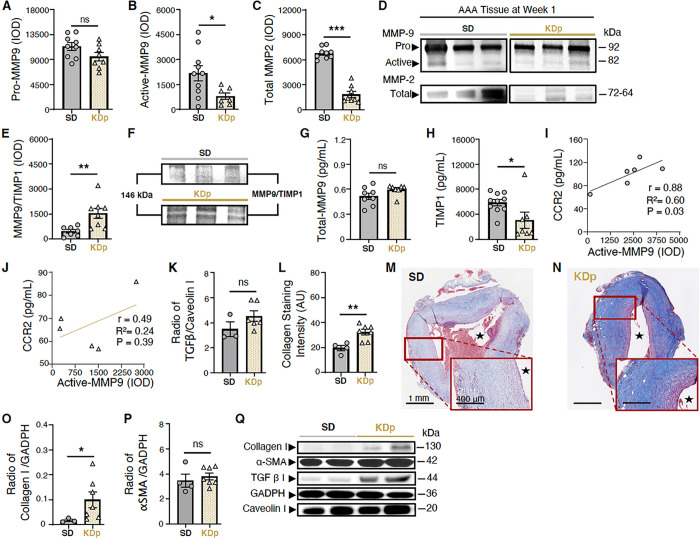
Ketosis Alters AAA Collagen Content and MMP Balance By Week 1
Previous work demonstrates that CCR2 is also alters MMP balance in favor of active enzymatic degridation of elastin, while decreasing transforming growth factor beta (TGFβ) signaling that contributes to collagen formation 34. Gelatin zymography of AAA tissue at week 1 demonstrated that although pro- MMP9 and total-MMP9 were equivalent between SD and KDp animals (Fig. 3A & D), active MMP9, known to promote AAA formation and rupture 35, was significantly reduced in KDp animals (p = 0.03; Fig. 3B & D). Similarly, total MMP 2, known to promote AAA expansion 36, was reduced in KDp animals (p < 0.001; Fig. 3C & D). Content of MMP9 and Tissue Inhibitor of Metalloproteinases 1 complex (MMP9/TIMP1; known to prevent MMP9 over-activation) was also significantly increased in KDp animals (p = 0.008; Fig. 3E & F). Correspondingly, AAA tissue in KDp animals demonstrated equivalent levels of total MMP-9 (Fig. 3G), and significantly reduced TIMP1 compared to SD animals (p = 0.03; Fig. 3H). Overall, these data demonstrate that sustained ketosis with KDp decreases active MMP9 while increasing MMP9/TIMP1 stabilizing complex in AAA tissue. Finally, we also observed a significant positive correlation between active MMP9 and CCR2 content in the AAA tissue in both SD and KDp animals (p = 0.03 and p = 0.39 respectively; Fig. 3I & 3J). These findings suggest that CCR2 content in AAA tissue may be responsible for activating MMPs, and therefore resulting in a higher incidence of AAA rupture.
Figure 3. Sustained ketosis downregulates CCR2 content and inhibit its downstream signals.
(A) Pro MMP9 levels at week 1 in AAA tissue of SD and KDp animals (11.3±2 ×103 vs 9.5±2.1 ×103; p=ns). (B) Active MMP9 levels in AAA tissue of SD and KDp animals (2.1±1.4 ×103 vs 0.8±0.5 ×103; p=0.03). One outlier data point in the KDp group was excluded based on pre-defined criteria prior to analysis (see methods). (C) Total MMP2 levels in AAA tissue of SD and KDp animals (7±0.7 ×103 vs 1.8±1 ×103; p<0.001). Pro and active MMP9 and total MMP2 levels were measured by integrated optical density (IOD). (D) Representative zymogram from AAA tissue homogenates at week 1 demonstrating pro and active MMP-9 and total MMP-2 levels in SD and KDp animals (E & F) Zymography demonstrating MMP9/TIMP1 complex levels in AAA tissue of SD and KDp animals (4.6±2.8 ×102 vs 15±8.7 ×102; p=0.008). One outlier data point in the KDp group was excluded based on pre-defined criteria prior to analysis. ELISA of AAA tissue homogenates in SD and KDp animals provided levels of (G) Total MMP9 (5±1.1 ×10−1 vs 6±0.6 ×10−1 ; p=ns), (H) TIMP 1 (5.9±1.6 ×103 vs 3.4±3 ×103 ; p=0.03). (I & J) Positive correlation analysis between CCR2 and Active MMP9 in SD and KDp respectively. (K) TGFβ−1 protein content expressed as a ratio to Caveolin 1 content in AAA tissue from SD and KDp animals at week 1 (2.2±0.8 ×10−1 vs 5.7±3.4 ×10−1; p = 0.2). (L) AAA collagen staining quantification for SD and KDp at week 2 (20±4 vs 32±6; p = 0.006). (M and N) Trichrome staining of abdominal aortas (cross-sectional) with 5x magnification and 10x magnification in SD and KDp animals to visualize collagen deposition in animal aortic tissue. (O) Collagen 1 protein content expressed as a ratio to GAPDH content in AAA tissue at week 2 for SD and KDp animals (1.4±0.8 ×10−2 vs 10±8.2 ×10−2, p = 0.03). One outlier data point in the SD group was excluded based on pre-defined criteria prior to analysis (see methods). (P) α-SMA protein content expressed as a ratio to GAPDH content in AAA tissue of SD and KDp animals (3.4±1 vs 3.8±0.7; p = ns). (Q) Representative Western blots of collagen 1, α-SMA, TGFβ−1, GAPDH and Caveolin 1 in AAA tissue. Data presented as mean ± standard deviation. ns > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001 using Student’s t test.
Comparted to week 1, TGFβ content in AAA tissue in KDp animals trended higher in week 2 (p = ns and p = 0.05, respectively; Fig. 3K & 3L). However, MT-staining demonstrated significantly higher collagen deposition in the AAA wall media by week 2 (p = 0.006; Fig. 3M–O). In particular, type 1 Collagen content was increased in KDp animals compared to SD at week 2 (p = 0.03), while α-smooth muscle actin (αSMA) content remained unchanged (Fig. 3P – Q).
Impact of Ketosis That is Initiated ‘Therapeutically’ After AAA Formation
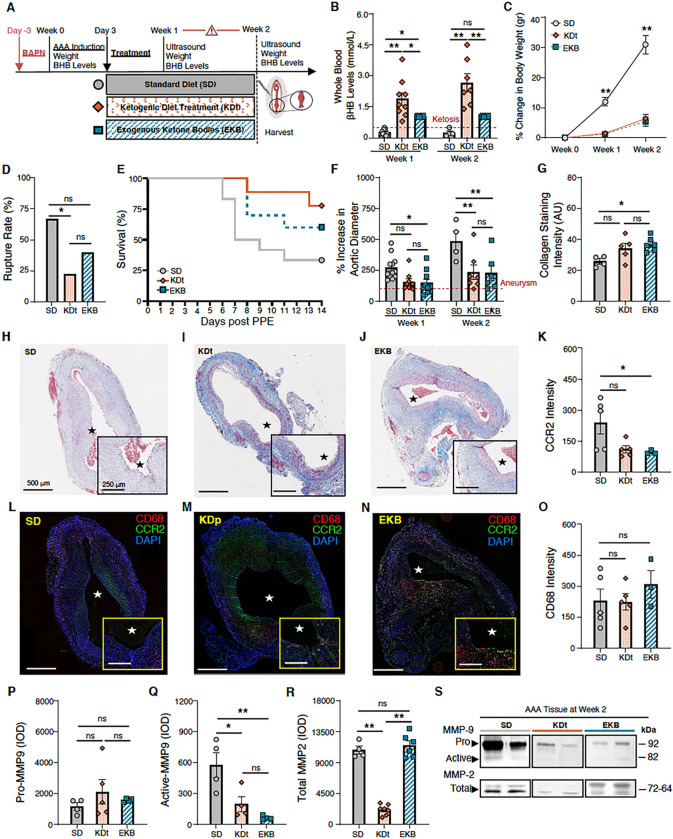
Animals treated with an abbreviated course of KD, therapeutically initiated 3 days post-AAA formation with PPE (KDt; Fig. 4A), also led to a state of ketosis (Fig. 4B). Animals treated with supplemental exogenous ketone bodies by oral daily gavage (EKB; Supplemental Fig. 2 & Fig. 4A) also led to ketosis, but only for 8-hour per day (Fig. 4B). Similar to KDp animals, KDt and EKB animals also had reduced weight gain at both week 1 and 2 when compared to SD animals (p < 0.001; Fig. 4C & Supplemental Fig. 2). Although, AAA rupture rate was reduced in KDt and EKB animals compared to SD animals (22% reduction with KDt, p = 0.03, and 40% reduction EKB, p = 0.12: Fig. 4D & E), the relative decrease in rupture was not as much as KDp animals (Fig. 4E & F). AAA absolute diameter and percentage of aortic diameter increase were also significantly reduced at both week 1 and 2 in EKB animals while only significantly reduced at week 2 in KDt animals (Fig. 4 & Supplemental Fig. 5C). These findings demonstrate that KDt and EKB therapeutic regimens lead to reduced AAA expansion and risk of rupture.
Figure 4. Impact of therapeutic ketosis on AAA risk of rupture.
(A) Animals underwent exposure to PPE to develop AAAs and were also treated with BAPN to promote AAA rupture. Following AAA induction, animals received a ketogenic ‘treatment’ via an oral diet (KDt) or exogenous supplement (EKB). (B) Ketosis (βHB whole blood levels > 0.5 mM/L) in SD, KDt, and EKB animals at week 1 (0.2±0.1, 1.8±0.9 and 1±0.02, respectively; p < 0.05) and at week 2 (0.2±0.1, 2.7±1.1, and 1±0.02 respectively; p < 0.01) analyzed using two-way ANOVA. (C) Percent body weight difference for SD, KDt, and EKB animals at week 1 (11±4, 2.5±1.4, and 2.4±1.3, respectively; p < 0.01) and at week 2 (31±6, 6±3.5, and 5±3.6, respectively; p < 0.01) analyzed using two-way ANOVA. (D) AAA rupture event rate in SD, KDt and EKB animals (p<0.05 between SD and KDt) using survival analysis. (E) Kaplan-Meier curve demonstrating survival following BAPN administration. 67% (8/12) of SD animals, 22% (2/9) of KDt animals (p=0.03), and 40% (4/10) of EKB animals (p = ns) developed AAA rupture by week 2. (F) Percent aortic diameter at week 1 in SD vs KDt animals (270±94 and 155±73; p = 0.06), and SD vs EKB animals (148±94; p = 0.04). At week 2, in SD vs KDt animals (485±153 and 234±151; p < 0.01), and SD vs EKB animals (227±147; p < 0.01 analyzed using two-way ANOVA. (G-J) Trichrome staining of AAA tissue at 5x and 10x magnifications to demonstrate collagen deposition in SD vs KDt animals (26±3 and 34±8; p = ns), and SD vs EKB animals (37±5; p = 0.02) analyzed using one-way ANOVA. (K) CCR2 ELISA content in AAA tissue of SD vs KDt animals (5.7±4 and 4.6±3; p = ns), and SD vs EKB animals (4.7±3; p = ns). (L-N) Immunofluorescent staining of AAA tissue at 5x and 10x magnifications to demonstrate CD68, CCR2, and DAPI positive cells. (O) Immunofluoresecent intensity of CD68 was analyzed using one-way ANOVA between SD, KDt, and EKB groups. (P) Pro MMP9 levels for SD vs KDt animals (1.2±0.5 ×103 and 2.1±1.7 ×103; p = ns), and SD vs EKB animals (1.6±0.12 ×103; p = ns). (Q) Active MMP9 levels for SD vs KDt (5.8±2.4 ×102 and 2±1.4 ×102; p = 0.02), and SD vs EKB animals (0.7±0.2 ×102; p = 0.005). (R) Total MMP-2 levels for SD vs KDt animals (10.8±1.1 ×103 and 2.1±0.7 ×103; p < 0.001), and SD vs EKB animals (11.5±1.7 ×103; p = ns). Pro and active MMP9 and total MMP2 levels were measured by integrated optical density (IOD) in AAA tissue, and analyzed using one-way ANOVA. (S) Representative separate zymogram gels of AAA tissue homogenates at week 2, demonstrating pro and active MMP-9 and total MMP-2 levels in animals fed SD, KDt, and EKB. Data presented as mean ± standard deviation. ns > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001 using one-way ANOVA, or two-way ANOVA with multiple comparison.
KDt and EKB animals also demonstrated increased AAA wall media Collagen content (p = 0.08 and p = 0.02, respectively; Fig. 4G–J), and reduced CCR2 immunostaining (p = 0.06 and p < 0.05, respectively; Fig. 4K–N). No difference was observed in CD68 immunostaining across groups (Fig. 4O). Equivalent levels of pro-MMP9 were observed among both treatment groups (Fig. 4P & S), but active MMP9 was significantly decreased in KDt and EKB animals (p = 0.02 and p = 0.001, respectively; Fig. 4Q & S). Total MMP2 was also notably attenuated in KDt animals (p < 0.001), but not in EKB animals (Fig. 4R & S). These data suggest that even an abbreviated therapeutic course of ketosis following AAA formation can help stabilize AAAs, preserve aortic wall collagen content, reduce CCR2 tissue content, and promote MMP balance.
Discussion
To our knowledge, our study is the first to demonstrate that Ccr2 is essential for the incidence of AAA rupture, and that diet-induced ketosis can also significantly decrease AAA progression and the risk of rupture. Using previously validated, pre-clinical murine and rat models for AAA 14,15, and different ketogenic supplementation strategies, we provide a robust and comprehensive assessment of the impact of dietary ketosis on AAA formation and the risk of rupture. We also specifically demonstrate that administration of either a ketogenic diet (KDp or KDt) or an oral ketone body supplementation (EKB) can reliably induce systemic ketosis, significantly reduced aortic wall CCR2 and pro-inflammatory cytokines, increase collagen content in the AAA media, and promote an MMP balance that minimizes elastin degredation (Fig. 5).
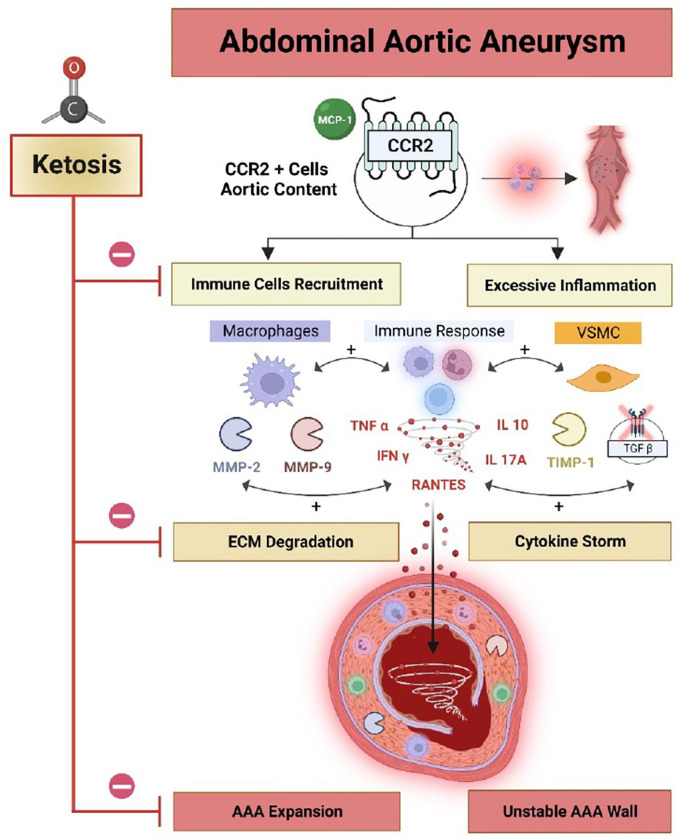
Figure 5. Ketosis impacts AAA expansion and risk of rupture.
AAA expansion and risk rupture is influenced by CCR2, which in turn recruits CD68+ pro-inflammatory macrophages, and also leads to cytokine release, and MMP activation. Vascular smooth muscle cells (VSMCs) production of TIMP1 can complex with MMP9 to help balance out the rate of MMP-medicated ECM degradation. Decreased TIMP1/MMP9 complex can lead to higher ECM degradation and AAA expansion. CCR2-mediated release of TNFa, RANTES, IL-10, IL-17A, and IFNg can further compound AAA tissue stability, and inhibition of TGFb can progress AAA instability, which further escalates the risk of rupture. Ketosis inhibits inflammation and ECM degradation thereby stabilizes AAA tissue and reduces the risk of rupture. Figure was made using BioRender.com.
Animals that received KDp demonstrated the most notable decrease in AAA expansion and risk of rupture. Animals that received KDt and EKB supplements also demonstrated differences in AAA progression, but not to the same extent. There was also mild to moderate variability in the KDt and EKB values of CCR2, CD68, and MMP content in AAA tissue. Administration of BAPN was reliable in inducing AAA rupture and did not appear to confound the impact of ketosis on AAA expansion and risk of rupture. Additionally, our complementary studies demonstrated that ketosis can impact pro-inflammatory CCR2-mediated signaling mechanisms that can lead to AAA progression. Therefore, this pre-clinical study demonstrates that a low-risk, and relatively easy dietary intervention, can potentially alter the course of AAA disease progression, and provides important insights that can be easily translated to human patients with AAAs who lack an effective medical management strategy.
Endogenous ketone body production mainly occurs in the liver, and results in a high glucagon/insulin ratio leading to an increased serum free fatty acids production in the circulation 37. This naturally occurs during periods of fasting, where βHB is released into the bloodstream as a byproduct of enzymatic degradation of ketone bodies within the mitochondrial matrix and is converted into ATP through oxidative phosphorylation 38 βHB rises to a few hundred micromolar (μM) concentrations within 12–16 hours of fasting, 1–2 mM after 2 days of fasting 39, and 6–8 mM with prolonged starvation 40. Ketogenic diets modify a host’s systemic energy metabolism to mimic the biochemical impact of starvation by significantly increasing serum βHB levels, lowering blood glucose, and increasing fatty acid concentrations 41. These regimens were originally introduced as a treatment for refractory epilepsy in children and have now become popular for weight loss programs, patients with diabetes, obesity, various types of cancer, and among high performance athletes 42–46. Standard ketogenic diets that are devoid of carbohydrates can lead to elevated βHB serum levels that are consistently > 2 mM 46. Recent studies demonstrate that βHB can serve as an important signaling mediator that can inhibit histone deacetylases 47, blunt tissue oxidative stress 48,49, active G-protein-coupled receptors 50,51, and regulate inflammatory mediators such as prostaglandin D2 52, interleukins 53, nuclear factor kappa B (NF-κB) 54, and NLRP3 inflammasome 55. Similarly, our study shows that animals with high serum βHB have blunted tissue inflammation and CCR2 content, which in part likely contributes to reduced pathological AAA expansion and risk of rupture.
Uniquely, our study administered three different ketosis regimens: two types of ketogenic diets (KDp and KDt), and an oral supplement regimen (EKB). KDp included a 1-week priming period prior to AAA formation, that imitates the phenomenon of keto-adaptation that occurs in humans who are maintained longer-term on a ketogenic diet 56. This regimen aided in determining whether a ketosis primer can have a ‘protective’ impact against AAA formation and expansion. On the other hand, KDt was designed to evaluate the potential ‘therapeutic’ impact of ketosis on expansion and rupture of AAA post-induction with PPE. This regimen would hypothetically be similar to how medical management would be prescribed in humans with small AAAs that do not yet meet the traditional size criteria for operative intervention. In the course of this study, we observed that animals tolerated both KDp and KDt, and that both were successful in inducing a sustained systemic state of ketosis. Interestingly, both regimens yielded significant reductions in AAA expansion and incidence of rupture relative to animals that received SD. However, the longer-term KDp regimen appeared to have a more protective impact, and a more impressive reduction of CCR2 content in AAA tissue. These findings suggest that the length of diet-induced ketosis may be an important variable in the extent of reduction of AAA tissue inflammation and risk of rupture.
With the recent advent of EKB supplements, oral regimens have been increasingly utilized to manipulate levels of circulating blood ketone body concentrations in humans for various health benefits 57. While most studies involving EKB supplementation have traditionally focused on its impact among high-performance athletes 58, these supplements are increasingly being studied as remedies for conditions such as epilepsy, heart failure, diabetes, and sepsis-related muscle atrophy 59. Our study evaluated the use of EKB to induce ketosis in animals with AAAs that are prone to rupture. Interestingly, we observed that EKB not only decreased AAA tissue inflammation (Supplemental Fig. 10), but also reduced AAA expansion and incidence of rupture (Fig. 4). The impact of EKB on CCR2 content and AAA rupture was variable from KDp and KDt, and we suspect this is because EKB only induced intermittent ketosis (limited to 8 hours per day). Nonetheless, these findings are the first to show that oral supplementation with ketone bodies can indeed serve as a minimally invasive method for the potential medical management of AAAs, and is a compelling topic for further exploration in future human clinical trials that completement prior efforts 60–62.
Our study results also suggest that ketosis has a multifaceted impact on aortic wall structure and function. Inflammation is the major molecular mediator of AAA disease progression (Fig. 5). Previous studies demonstrated that excessive aortic wall inflammation can inhibit reparative signaling, wall fibrosis, and collagen deposition, which can in turn accelerate AAA expansion and lead to a higher risk of rupture 63. Tissue macrophages are known to promote AAA disease, in particular subsets that highly express CCR2 12. We as well as others, also previously demonstrated that genetic or molecular targeting of CCR2 can reduce AAA progression 13–15. Here we provide further compleing evidence that CCR2 content indeed correlates with AAA disease progression, and that systemic ketosis in vivo can significantly reduce its both CCR2 content as well as downstream pro-inflammatory cytokines in AAA tissue.
Previous studies investigating the inflammasome in AAA tissue, demonstrated that TNFα and RANTES are both up-regulated in expanding AAA wall tissue 64,65. Inhibition of TNFα appears to decrease aortic wall MMP activity, reduce ECM disruption, and decrease aortic diameter in a murine pre-clinical AAA model 66. In another study, Empagliflozin, a sodium-glucose cotransporter 2 inhibitor that increases plasma ketone bodies 67,68, was found to reduce aortic aneurysm diameter and aortic wall RANTES in Apo E −/− mice 69. Similarly, in our study we observed that diet-induced ketosis can significantly decrease aortic wall pro-inflammatory cytokines TNFα and RANTES, as well as increase aortic wall Collagen content. Although the direct mechanism of action for this is yet to be fully elucidated, we suspect that the molecular interplay between macrophage and other pro-inflammatory cell types may be playing a critical role in the immune modulation of these processes and AAA progression 70,71.
A central pathological feature of AAA disease progression is excessive and aberrant extracellular matrix (ECM) remodeling. This results from increased MMP activity, which promotes rapid ECM breakdown and disruption of the integrity of the aortic wall 72,73. Previous work demonstrates that MMP2 plays a central role in the formation and early expansion of AAAs, while MMP9 is more related to late AAA expansion and risk of aneurysm rupture 30,74,75. Synergistic activation of both MMP2 and MMP9 provides an unfavorable environment that can accelerate AAA dilation and lead to a higher risk of aneurysm rupture 76. Previous studies also demonstrate that ketosis, high serum βHB, and signaling via NF-Kβ, play key roles in suppressing MMP-9 expression in colonic tissue 77. Our studies extend on this molecular mechanism of action, and demonstrate that ketosis and elevated serum βHB can also significantly attenuate both active MMP9, and total MMP2 in aortic tissue. In fact, a CCR2 antagonist has shown to downregulate MMP-9 expression in lung cancer cells, therefore mitigating cellular motility and metastatic invasion 78. These results may help explain why we observed a notable decrease in MMP-9 content in AAA tissue from animals with ketosis.
TIMPs are endogenous specific inhibitors of MMPs produced by vascular smooth muscle cells (VSMCs) as well as other cell types in AAA tissue 79, which inhibit zymogenesis of pro-MMPs and reduces overall MMP activation. Given their central role in maintaining the dynamic balance in ECM turnover in aortic wall tissue, the role of TIMPs in AAA progression continues to be an area of intense investigation 35. Our study demonstrates that while nutritional ketosis decreases the content of free TIMP1, it significantly increases the content of the stabilizing TIMP1/MMP9 complex in AAA tissue. This data suggests that complexed TIMP1 leads to a reduction in active MMP9 content, therefore decreasing AAA wall ECM degradation, further aneurysm expansion, and the overall risk of rupture (Fig. 5).
Our study also demonstrated a mild-moderate, but non-significant, increase in AAA tissue TGFβ content in animals treated with ketogenic diets (Fig. 5). TGFβ belongs to a superfamily of growth factors that regulate many cellular functions such as cell growth, adhesion, migration, differentiation, and apoptosis 80. TGFβ content appears to be significantly reduced in human AAA tissue 81. A recent study demonstrated that ketosis promoted TGFβ-induced myocardial fibrosis and Collagen 1 and 3 deposition in spontaneously hypertensive rats 82, suggesting that TGFβ up-regulation was deleterious in this setting. However, in aortic tissue, TGFβ appears to have a beneficial role. For example, administration of TGFβ neutralizing antibodies appeared to promote excessive monocyte-macrophage infiltration within murine and rat AAA tissue 34,83, while overexpression or administration of TGFβ1 significantly increased aortic wall collagen deposition 84, and collagen synthesis in normal arteries 85. This in part explains our observation that animals receiving a ketogenic diet had significantly increase aortic wall Collagen 1 deposition, which correlated with higher aortic tissue TGFβ content.
We acknowledge that there are some limitations in our study. First, all our data is derived from pre-clinical rodent models that are not necessarily representative of human AAA pathophysiology. However, the rat AAA rupture model was previously validated and shown to be the most reliable and consistent AAA rupture model currently available. Second, our studies did not systematically evaluate arterial blood pressure. This would have required sophisticated in dwelling sensors and the use of continuous telemetry. While such monitoring systems are feasible for shorter experimental protocols, our 2–3-week experimental protocol would have greatly complicated the experimental design and led to several confounding variables. We therefore elected to instead serially monitor AAA endpoints via ultrasound, which provided reliable and reproducible data. Third, our study used a single composition for the ketogenic diet intervention. We acknowledge that this is not fully representative of the wide variety of lipid and oil-based ketogenic diets consumed by humans, but this was selected to maintain consistency and adherence within all rodent study groups.
In conclusion, this study demonstrates that a ketogenic diet and EKB supplementation strategy that can significantly reduce AAA expansion and reduce the incidence of AAA rupture. Importantly, a ketogenic priming period appears to also be further protective, while EKB appears to be less effective than other dietary regimens. Ketogenic diets reduced CCR2 content, promoted MMP balance, and attenuated ECM degradation in AAA tissue. These findings provide the impetus for future pre-clinical and clinical studies geared to determine the role of ketosis as a medical management tool for human patients with AAAs that do not yet meet the criteria for surgical intervention.
Sources of Funding
This work was supported by grants from National Institute of Health, National Heart Lung and Blood Institute R01HL153436 (Mohamed A. Zayed), R01HL150891 (Mohamed A. Zayed), R01HL153262 (Mohamed A. Zayed).
Footnotes
Disclosures
The authors declare that they have no competing interests.
Contributor Information
Mohamed Zayed, Washington University in St. Louis School of Medicine.
Sergio Sastriques-Dunlop, Washington University in St. Louis School of Medicine.
Santiago Elizondo-Benedetto, Washington University in St. Louis School of Medicine.
Batool Arif, Washington University in St. Louis School of Medicine.
Rodrigo Meade, Washington University in St. Louis School of Medicine.
Mohamed Zaghloul, Washington University in St. Louis School of Medicine.
Hannah Luehmann, Washington University School of Medicine.
Gyu Heo, ashington University in St. Louis School of Medicine.
Sean English, ashington University in St. Louis School of Medicine.
Yongjian Liu, Wash U.
Data Availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1.Sakalihasan N, Limet R, Defawe OD. Abdominal Aortic Aneurysm. Lancet [Internet]. 2005;365:1577–89. Available from: www.thelancet.com [DOI] [PubMed] [Google Scholar]
- 2.Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, Van Herwaarden JA, Holt PJE, Van Keulen JW, Rantner B, et al. Management of Abdominal Aortic Aneurysms Clinical Practice Guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2011;41. [DOI] [PubMed] [Google Scholar]
- 3.Carino D, Sarac TP, Ziganshin BA, Elefteriades JA. Abdominal Aortic Aneurysm: Evolving Controversies and Uncertainties. Int J Angiol. 2018;27:58–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: Basic mechanisms and clinical implications. Curr Probl Surg. 2002;39:110–230. [DOI] [PubMed] [Google Scholar]
- 5.Golledge J, Moxon J V., Singh TP, Bown MJ, Mani K, Wanhainen A. Lack of an effective drug therapy for abdominal aortic aneurysm. J Intern Med. 2020;288:6–22. [DOI] [PubMed] [Google Scholar]
- 6.Brewster DC, Cronenwett JL, Hallett JW, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms: Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J. Vasc. Surg. 2003;37:1106–1117. [DOI] [PubMed] [Google Scholar]
- 7.Lindeman JH, Matsumura JS. Pharmacologic management of aneurysms. Circ. Res. 2019;124:631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carino D, Sarac TP, Ziganshin BA, Elefteriades JA. Abdominal Aortic Aneurysm: Evolving Controversies and Uncertainties. Int. J. Angiol. 2018;27:58–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Potteaux S, Tedgui A. Monocytes, Macrophages and Other Inflammatory Mediators of Abdominal Aortic Aneurysm. 2015. [DOI] [PubMed] [Google Scholar]
- 10.Li H, Bai S, Ao Q, Wang X, Tian X, Li X, Tong H, Hou W, Fan J. Modulation of Immune-Inflammatory Responses in Abdominal Aortic Aneurysm: Emerging Molecular Targets. 2018;Available from: 10.1155/2018/7213760 [DOI] [PMC free article] [PubMed]
- 11.Ijaz T, Tilton RG, Brasier AR. Cytokine amplification and macrophage effector functions in aortic inflammation and abdominal aortic aneurysm formation. J. Thorac. Dis. [Internet]. 2016. [cited 2022 Mar 21];8:E746–E754. Available from: https://jtd.amegroups.com/article/view/8271/html [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dale MA, Ruhlman MK, Baxter BT. Inflammatory cell phenotypes in AAAs; their role and potential as targets for therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Chen H, Liu L, Sun J, Shi MA, Sukhova GK, Shi G-P. Chemokine (C-C motif) receptor 2 mediates mast cell migration to abdominal aortic aneurysm lesions in mice. [cited 2022 Mar 21];Available from: https://academic.oup.com/cardiovascres/article/96/3/543/359557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA. Angiotensin II infusion promotes ascending aortic aneurysms: Attenuation by CCR2 deficiency in apoE−/− mice. Clin. Sci. 2010;118:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.English SJ, Sastriques SE, Detering L, Sultan D, Luehmann H, Arif B, Heo GS, Zhang X, Laforest R, Zheng J, et al. CCR2 positron emission tomography for the assessment of abdominal aortic aneurysm inflammation and rupture prediction. Circ. Cardiovasc. Imaging. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe M, Tozzi R, Risi R, Tuccinardi D, Mariani S, Basciani S, Spera G, Lubrano C, Gnessi L. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: A comprehensive review of the literature. Obes. Rev. 2020;21:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sayadi JJ, Sayadi L, Satteson E, Chopan M. Nerve injury and repair in a ketogenic milieu: A systematic review of traumatic injuries to the spinal cord and peripheral nervous tissue. PLoS One [Internet]. 2021;16:1–16. Available from: 10.1371/journal.pone.0244244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradshaw PC, Seeds WA, Miller AC, Mahajan VR, Curtis WM. COVID-19: Proposing a Ketone-Based Metabolic Therapy as a Treatment to Blunt the Cytokine Storm. Oxid. Med. Cell. Longev. 2020;2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto K, Kugo H, Tanaka H, Iwamoto K, Miyamoto C, Urano T, Unno N, Hayamizu K, Zaima N, Moriyama T. The effect of a high-fat diet on the development of abdominal aortic aneurysm in a vascular hypoperfusion-induced animal model. J. Vasc. Res. 2018;55:63–74. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Y, Qu H, Wang Y, Xiao W, Zhang Y, Shi D. Small rodent models of atherosclerosis. Biomed. Pharmacother. 2020;129. [DOI] [PubMed] [Google Scholar]
- 21.Castro R, Whalen CA, Gullette S, Mattie FJ, Florindo C, Heil SG, Huang NK, Neuberger T, Ross AC, Savastano S, et al. A Hypomethylating Ketogenic Diet in Apolipoprotein E-Deficient Mice: A Pilot Study on Vascular Effects and Specific Epigenetic Changes. 2021;Available from: 10.3390/nu13103576 [DOI] [PMC free article] [PubMed]
- 22.Zhang S jie, Li Z hua, Zhang Y dian, Chen J, Li Y, Wu F qing, Wang W, Cui ZJ, Chen GQ. Ketone Body 3-Hydroxybutyrate Ameliorates Atherosclerosis via Receptor Gpr109a-Mediated Calcium Influx. Adv. Sci. 2021;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.English SJ, Diaz JA, Shao X, Gordon D, Bevard M, Su G, Henke PK, Rogers VE, Upchurch GR, Piert M. Utility of 18 F-FDG and 11C-PBR28 microPET for the assessment of rat aortic aneurysm inflammation. EJNMMI Res. 2014;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu G, Su G, Davis JP, Schaheen B, Downs E, Roy RJ, Ailawadi G, Upchurch GR. A novel chronic advanced stage abdominal aortic aneurysm murine model. J. Vasc. Surg. 2017;66:232–242.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyde PN, Lustberg MB, Miller VJ, LaFountain RA, Volek JS. Pleiotropic effects of nutritional ketosis: Conceptual framework for keto-adaptation as a breast cancer therapy. Cancer Treat. Res. Commun. 2017;12:32–39. [Google Scholar]
- 26.Kesl SL, Poff AM, Ward NP, Fiorelli TN, Ari C, Van Putten AJ, Sherwood JW, Arnold P, D’Agostino DP. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats. Nutr. Metab. [Internet]. 2016;13:1–15. Available from: 10.1186/s12986-016-0069-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knipp BS, Ailawadi G, Sullivan V V, Roelofs KJ, Henke PK, Stanley JC, Upchurch GR. Ultrasound Measurement of Aortic Diameters in Rodent Models of Aneurysm Disease. 2003. [cited 2022 Mar 2];Available from: www.graphpad.com [DOI] [PubMed]
- 28.Deemer SE, Davis RAH, Roberts BM, Smith DL, Koutnik AP, Poff AM, D’Agostino DP, Plaisance EP. Exogenous Dietary Ketone Ester Decreases Body Weight and Adiposity in Mice Housed at Thermoneutrality. Obesity. 2020;28:1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fashandi AZ, Hawkins RB, Salmon MD, Spinosa MD, Montgomery WG, Cullen JM, Lu G, Su G, Ailawadi G, Upchurch GR. A novel reproducible model of aortic aneurysm rupture. Surg. (United States). 2018;163:397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.English SJ, Piert MR, Diaz JA, Gordon D, Ghosh A, D’Alecy LG, Whitesall SE, Sharma AK, DeRoo EP, Watt T, et al. Increased 18f-fdg uptake is predictive of rupture in a novel rat abdominal aortic aneurysm rupture model. Ann. Surg. 2015;261:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Middleton RK, Lloyd GM, Bown MJ, Cooper NJ, London NJ, Sayers RD. The pro-inflammatory and chemotactic cytokine microenvironment of the abdominal aortic aneurysm wall: A protein array study. J. Vasc. Surg. 2007;45:574–580. [DOI] [PubMed] [Google Scholar]
- 32.Wang S. Keisin, Green Linden A., Gutwein Ashley R., Drucker Natalie A., Motaganahalli Raghu L., Gupta Alok K., Fajardo Andres, and Murphy M Michael P.. Description of Human AAA by Cytokine and Immune Cell Aberrations Compared to Risk-Factor Matched Controls. Surgery. 2019;72:2964–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Puchenkova OA, Soldatov VO, Belykh AE, Bushueva OY, Piavchenko GA, Venediktov AA, Shakhpazyan NK, Deykin A V., Korokin M V., Pokrovskiy M V. Cytokines in Abdominal Aortic Aneurysm: Master Regulators With Clinical Application. Biomark. Insights. 2022;17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadière C, Rénia L, et al. TGF-β activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J. Clin. Invest. [Internet]. 2010;Available from: http://www.jci.orgvolume120number [DOI] [PMC free article] [PubMed]
- 35.Vandooren J, Van Den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013;48:222–272. [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Chang Q, Sun X, Qian X, Liu P, Pei H, Guo X, Liu W. Angiotensin II Induces an Increase in Matrix Metalloproteinase 2 Expression in Aortic Smooth Muscle Cells of Ascending Thoracic Aortic Aneurysms Through JNK, ERK1/2, and p38 MAPK Activation. J. Cardiovasc. Pharmacol. 2015;66:285–293. [DOI] [PubMed] [Google Scholar]
- 37.Cahill GF JR. Starvation in man. Clin. Endocrinol. Metab. 1976;5:397–415. [DOI] [PubMed] [Google Scholar]
- 38.Cotter DG, Schugar RC, Crawford PA. Ketone body metabolism and cardiovascular disease. Am J Physiol Hear. Circ Physiol [Internet]. 2013;304:1060–1076. Available from: www.ajpheart.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cahill GF, Herrera MG, Morgan AP, Soeldner JS, Steinke J, Levy PL, Reichard GA, Kipnis DM, Kline S, Labora-tories F. Hormone-Fuel Interrelationships during Fasting *. 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cahill GF. FUEL METABOLISM IN STARVATION. Annu. Rev. Nutr [Internet]. 2006;26:1–22. Available from: www.annualreviews.org [DOI] [PubMed] [Google Scholar]
- 41.Selvaraj S, Kelly DP, Margulies KB. Implications of Altered Ketone Metabolism and Therapeutic Ketosis in Heart Failure. 2020;141:1800–1812. Available from: http://ahajournals.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrero JR, Villarroya EC, Peñas JJG, Alcolea BG, Fernández BG, Macfarland LAP, Giner CP. Safety and effectiveness of the prolonged treatment of children with a ketogenic diet. Nutrients [Internet]. 2020;12. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moreno B, Crujeiras AB, Bellido D, Sajoux I, Felipe •, Casanueva F. Obesity treatment by very low-calorie-ketogenic diet at two years: reduction in visceral fat and on the burden of disease. Endocrine. 2020;54:681–690. [DOI] [PubMed] [Google Scholar]
- 44.Augustin K, Khabbush A, Williams S, Eaton S, Orford M, Cross H, Heales SJR, Walker MC, Williams RSB. Review Mechanisms of action for the medium-chain triglyceride ketogenic diet in neurological and metabolic disorders. 2018. [cited 2022 Feb 19];17. Available from: www.thelancet.com/neurology [DOI] [PubMed] [Google Scholar]
- 45.Ludwig DS, Willett WC, Volek JS, Neuhouser ML. Dietary fat: From foe to friend? [Internet]. Science (80-.). 2018;362:764–770. Available from: https://www.science.org [DOI] [PubMed] [Google Scholar]
- 46.Peterman MG. The ketogenic diet in epilepsy. J. Am. Med. Assoc. [Internet]. 1925;84:1979–1983. Available from: http://jama.jamanetwork.com/ [Google Scholar]
- 47.Gregoretti I V., Lee YM, Goodson H V. Molecular evolution of the histone deacetylase family: Functional implications of phylogenetic analysis. J. Mol. Biol. 2004;338:17–31. [DOI] [PubMed] [Google Scholar]
- 48.Nagao M, Toh R, Irino Y, Mori T, Nakajima H, Hara T, Honjo T, Satomi-Kobayashi S, Shinke T, Tanaka H, et al. b-Hydroxybutyrate elevation as a compensatory response against oxidative stress in cardiomyocytes. 2016;Available from: 10.1016/j.bbrc.2016.05.097 [DOI] [PubMed] [Google Scholar]
- 49.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, et al. Suppression of Oxidative Stress by β-Hydroxybutyrate, an Endogenous Histone Deacetylase Inhibitor NIH Public Access. Science (80-.). [Internet]. 2013. [cited 2022 Feb 23];339:211–214. Available from: http://www.sciencemag.org/cgi/content/full/science.1227166/DC1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. 2012. [cited 2022 Feb 23];Available from: [DOI] [PubMed] [Google Scholar]
- 51.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL. Short chain fatty acids and their receptors: New metabolic targets [Internet]. Transl. Res. 2013;161:131–140. Available from: 10.1016/j.trsl.2012.10.007 [DOI] [PubMed] [Google Scholar]
- 52.Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Müller-Fielitz H, Pokorná B, Vollbrandt T, Stölting I, Nadrowitz R, et al. ARTICLE The b-hydroxybutyrate receptor HCA 2 activates a neuroprotective subset of macrophages. 2014;Available from: www.nature.com/naturecommunications [DOI] [PubMed]
- 53.Fu S-P, Li S-N, Wang J-F, Li Y, Xie S-S, Xue W-J, Liu H-M, Huang B-X, Lv Q-K, Lei L-C, et al. BHBA Suppresses LPS-Induced Inflammation in BV-2 Cells by Inhibiting NF-í μí¼ B Activation. 2014;Available from: 10.1155/2014/983401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fu S-P, Wang J-F, Xue W-J, Liu H-M, Liu B, Zeng Y-L, Li S-N, Huang B-X, Lv Q-K, Wang W, et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherrier M, Li H. The impact of keto-adaptation on exercise performance and the role of metabolic-regulating cytokines. Am. J. Clin. Nutr. 2019;110:562–573. [DOI] [PubMed] [Google Scholar]
- 57.Newport MT, Vanitallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: Use of ketone ester in a case of Alzheimer’s disease. Alzheimer’s Dement. 2015;11:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dearlove DJ, Faull OK, Clarke K. Context is key: exogenous ketosis and athletic performance. Curr. Opin. Physiol. [Internet]. 2019. [cited 2022 Feb 21];10:81–89. Available from: 10.1016/j.cophys.2019.04.010 [DOI] [Google Scholar]
- 59.Soto-Mota A, Norwitz NG, Clarke K. Why a D-β-hydroxybutyrate monoester? 2020;Available from: 10.1042/BST20190240 [DOI] [PMC free article] [PubMed]
- 60.Koeslag J.H.. Post-exercise ketosis and the hormone response to exercise. Med Sci Sport. Exerc. 1982;14:327–334. [PubMed] [Google Scholar]
- 61.Tew GA, Batterham AM, Colling K, Gray J, Kerr K, Kothmann E, Nawaz S, Weston M, Yates D, Danjoux G. Randomized feasibility trial of high-intensity interval training before elective abdominal aortic aneurysm repair. Br. J. Surg. 2017;104:1791–1801. [DOI] [PubMed] [Google Scholar]
- 62.Perissiou M, Bailey TG, Windsor M, Greaves K, Nam MCY, Russell FD, O’Donnell J, Magee R, Jha P, Schulze K, et al. Aortic and Systemic Arterial Stiffness Responses to Acute Exercise in Patients With Small Abdominal Aortic Aneurysms. Eur. J. Vasc. Endovasc. Surg. [Internet]. 2019;58:708–718. Available from: 10.1016/j.ejvs.2019.02.021 [DOI] [PubMed] [Google Scholar]
- 63.Kaneko H, Anzai T, Takahashi T, Kohno T, Shimoda M, Sasaki A, Shimizu H, Nagai T, Maekawa Y, Yoshimura K, et al. Role of vascular endothelial growth factor-A in development of abdominal aortic aneurysm. Cardiovasc. Res. 2011;91:358–367. [DOI] [PubMed] [Google Scholar]
- 64.Kaneko H, Anzai T, Horiuchi K, Kohno T, Nagai T, Anzai A, Takahashi T, Sasaki A, Shimoda M, Maekawa Y, et al. Tumor necrosis factor-α converting enzyme is a key mediator of abdominal aortic aneurysm development. Atherosclerosis. 2011;218:470–478. [DOI] [PubMed] [Google Scholar]
- 65.Jones GT, Phillips LV, Williams MJA, van Rij AM, Kabir TD. Two C-C family chemokines, eotaxin and RANTES, are novel independent plasma biomarkers for abdominal aortic aneurysm. J. Am. Heart Assoc. 2016;5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Worth J, Persidsky Y, Baxter BT, Xiong W, Mactaggart J, Knispel R. Formation in a Murine Model Attenuates Aneurysm α Blocking TNF. J Immunol Ref. [Internet]. 2022;183:2741–2746. Available from: http://www.jimmunol.org/content/183/4/2741http://www.jimmunol.org/content/183/4/2741.full#ref-list-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrannini E, Muscelli E, Frascerra S, Baldi S, Mari A, Heise T, Broedl UC, Woerle H-J. Clinical medicine Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. 2014;124:499. Available from: http://www.jci.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ferrannini E, Baldi S, Frascerra S, Astiarraga B, Heise T, Bizzotto R, Mari A, Pieber TR, Muscelli E. Shift to Fatty Substrate Utilization in Response to Sodium-Glucose Cotransporter 2 Inhibition in Subjects Without Diabetes and Patients With Type 2 Diabetes. Diabetes [Internet]. 2016;65:1190–1195. Available from: 10.2337/db15-1356/-/DC1. [DOI] [PubMed] [Google Scholar]
- 69.Ortega R, Collado A, Selles F, Gonzalez-Navarro H, Sanz M-J, Real JT, Piqueras L. Arteriosclerosis, Thrombosis, and Vascular Biology SGLT-2 (Sodium-Glucose Cotransporter 2) Inhibition Reduces Ang II (Angiotensin II)-Induced Dissecting Abdominal Aortic Aneurysm in ApoE (Apolipoprotein E) Knockout Mice. Arter. Thromb Vasc Biol [Internet]. 2019. [cited 2022 Feb 18];39:1614–1628. Available from: 10.1161/ATVBAHA.119.312659. [DOI] [PubMed] [Google Scholar]
- 70.University S.Limiting AAA With Metformin (LIMIT) Trial (LIMIT) [Internet]. 2020;Available from: https://clinicaltrials.gov/ct2/show/NCT04500156.
- 71.Guangdong Provincial Peoplés Hospital. Low-dose Colchicine Inhibit Abdominal Aortic Aneurysm Growth Trial (COIN) [Internet]. 2022;Available from: https://clinicaltrials.gov/ct2/show/NCT05361772.
- 72.Zhang G, Miyake M, Lawton A, Goodison S, Rosser CJ. Matrix metalloproteinase-10 promotes tumor progression through regulation of angiogenic and apoptotic pathways in cervical tumors. BMC Cancer. 2014;14:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.García-Irigoyen O, Latasa MU, Carotti S, Uriarte I, Elizalde M, Urtasun R, Vespasiani-Gentilucci U, Morini S, Benito P, Ladero JM, et al. Matrix Metalloproteinase 10 Contributes To Hepatocarcinogenesis in a Novel Crosstalk With the Stromal Derived Factor 1/C-X-C Chemokine Receptor 4 Axis. Hepatology. 2015;62:166–178. [DOI] [PubMed] [Google Scholar]
- 74.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006;69:562–573. [DOI] [PubMed] [Google Scholar]
- 75.Koole D, Zandvoort HJA, Schoneveld A, Vink A, Vos JA, Van Den Hoogen LL, De Vries JPPM, Pasterkamp G, Moll FL, Van Herwaarden JA. Intraluminal abdominal aortic aneurysm thrombus is associated with disruption of wall integrity. J. Vasc. Surg. [Internet]. 2013;57:77–83. Available from: 10.1016/j.jvs.2012.07.003 [DOI] [PubMed] [Google Scholar]
- 76.Maleux G, Poorteman L, Laenen A, Saint-Lèbes B, Houthoofd S, Fourneau I, Rousseau H. Incidence, etiology, and management of type III endoleak after endovascular aortic repair. J. Vasc. Surg. [Internet]. 2017;66:1056–1064. Available from: 10.1016/j.jvs.2017.01.056 [DOI] [PubMed] [Google Scholar]
- 77.Zhang R, Wang Q, Chen J, Zhang N, Liu C, Wang T, Yang F, Siebert HC, Zheng X. Ketogenic diet elicits antitumor properties through inducing oxidative stress, inhibiting MMP-9 expression, and rebalancing M1/ M2 tumor-associated macrophage phenotype in a mouse model of colon cancer. J. Agric. Food Chem. 2020;68:11182–11196. [DOI] [PubMed] [Google Scholar]
- 78.An J, Xue Y, Long M, Zhang G, Zhang J, Su H. Targeting CCR2 with its antagonist suppresses viability, motility and invasion by downregulating MMP-9 expression in non-small cell lung cancer cells. Oncotarget. 2017;8:39230–39240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klaus V, Tanios-Schmies F, Reeps C, Trenner M, Matevossian E, Eckstein HH, Pelisek J. Association of Matrix Metalloproteinase Levels with Collagen Degradation in the Context of Abdominal Aortic Aneurysm. Eur. J. Vasc. Endovasc. Surg. [Internet]. 2017;53:549–558. Available from: 10.1016/j.ejvs.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 80.Schmierer B, Hill CS. TGFβ-SMAD signal transduction: Molecular specificity and functional flexibility. Nat. Rev. Mol. Cell Biol. 2007;8:970–982. [DOI] [PubMed] [Google Scholar]
- 81.Biros E, Walker PJ, Nataatmadja M, West M, Golledge J. Downregulation of transforming growth factor, beta receptor 2 and Notch signaling pathway in human abdominal aortic aneurysm. Atherosclerosis. 2012;221:383–386. [DOI] [PubMed] [Google Scholar]
- 82.You Y, Guo Y, Jia P, Zhuang B, Cheng Y, Deng H, Wang X, Zhang C, Luo S, Huang B. Ketogenic diet aggravates cardiac remodeling in adult spontaneously hypertensive rats. Nutr. Metab. [Internet]. 2020. [cited 2022 Jan 16];17. Available from: 10.1186/s12986-020-00510-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dai J, Michineau S, Franck G, Desgranges P, Becquemin J-P. Long Term Stabilization of Expanding Aortic Aneurysms by a Short Course of Cyclosporine A through Transforming Growth Factor-Beta Induction. PLoS One [Internet]. 2011;6:28903. Available from: www.plosone.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotra LA, Falangat V, Kehrl JH, et al. Transforming growth factor type,8: Rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nabel EG, Shumt L, Pompili VJ, Yang Z-Y, San H, Shu HB, Liptay S, Gold L, Gordonii D, Derynck R, et al. Direct transfer of transforming growth factor j81 gene into arteries stimulates fibrocellular hyperplasia (gene transfer/gene expression/extraceliular matrix/ceilular proliferation). 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.