Abstract
Some clinical algorithms incorporate a person's race, ethnicity, or both as an input variable or predictor in determining diagnoses, prognoses, treatment plans, or risk assessments. Inappropriate use of race and ethnicity in clinical algorithms at the point of care may exacerbate health disparities and promote harmful practices of race-based medicine. This article describes a comprehensive search of online resources, the scientific literature, and the FDA Drug Label Information that uncovered 39 race-based risk calculators, six laboratory test results with race-based reference ranges, one race-based therapy recommendation, and 15 medications with race-based recommendations. These clinical algorithms based on race are freely accessible through an online database. This resource aims to raise awareness about the use of race-based clinical algorithms and track the progress made toward eradicating the inappropriate use of race. The database will be actively updated to include clinical algorithms based on race that were previously omitted, along with additional characteristics of these algorithms.
Keywords: clinical algorithms, race, ethnicity, database
INTRODUCTION
Clinical algorithms are tools that support medical decision-making in a variety of medical conditions and procedures. Examples include diagnostic calculators that predict that a specific disease or condition is present, prognostic algorithms that assess the risk of developing a specific disease or a clinical outcome in the future (1), treatment guidelines for managing chronic conditions, interpretation recommendations for laboratory test results and directions for medication use (2, 3). Such algorithms enable standardization of care, increased efficiency, and improved clinical decision-making quality (2).
Some clinical algorithms incorporate the individual's race, ethnicity, or both as an input variable or predictor. In medicine, race and ethnicity are used to describe certain population characteristics that may have implications for health outcomes and health care. While these terms are often used interchangeably, they each have distinct meanings and refer to distinct aspects of human identity and ancestry (4). Race is a categorization system that classifies individuals based on visible physical traits such as skin color, facial features, hair texture, and eye shape. Influenced by historical and political factors, race has been used to classify people into broad groups, such as white, Black, or Asian, among others. In contrast, ethnicity is a categorization system that groups individuals based on customs, language, religion, traditions, and other aspects of shared cultural heritage passed down through generations. Shaped by ancestry and geographical location, ethnicity reflects an individual's sense of cultural identity, such as Hispanic, Chinese, or Navajo, among many others. Despite the distinction between race and ethnicity, we will frequently use the term race to refer to both race and ethnicity for the sake of brevity.
Race is widely used in medicine in studying genetic variations, disease prevalence, treatment responses, and health disparities. However, using race as a proxy for biological or genetic differences can oversimplify complex health issues and contribute to disparities in health. Frequent causes of health disparities include, among others, biological, environmental, socioeconomic, healthcare access, discrimination, and cultural factors. Although clinical algorithms incorporating race are intended to improve healthcare outcomes, they can inadvertently exacerbate racial health disparities in several ways. They can perpetuate racial prejudices and stereotypes because of historical and societal biases, and the inclusion of race as an input variable can inadvertently reinforce these biases. Medical students are taught to associate race with diseases such as sickle cell anemia, sarcoidosis, or cystic fibrosis, which reinforces their implicit understanding of race as a biological characteristic (5). Using race as a proxy for genetic variation oversimplifies the complex interactions between genes, environment, and disease (6), and there is more intra-racial genetic variation within racial groups than between them (7). By presuming biological and genetic differences between racial groups, race-based clinical algorithms may result in differential treatment or incorrect diagnoses in minority populations (8). In addition, the use of race in clinical algorithms raises ethical concerns regarding discrimination, equity, and equality of opportunity (9). It may violate the principles of justice and equality if individuals are treated differently based on their race instead of their individual health requirements.
Race is now widely accepted as a social construct and is a poor proxy for biological differences among individuals. As a result of growing recognition that race-based diagnosis and treatment reflect flawed social, biological, and genetic assumptions, the use of race in clinical decision-making is coming under increasing scrutiny. Several high-profile articles in the past few years have identified and deemed problematic examples of clinical algorithms that include race in a wide range of clinical specialties, such as nephrology (10), urology (11), obstetrics (12), and cardiology (5, 13, 14). Further, leading organizations such as the American Society of Nephrology and the American College of Obstetricians and Gynecologists have recommended race-free risk assessments.
However, because race is not a biological feature, it is not clear that its inclusion as a predictor to inform clinical decision-making will automatically perpetuate long-standing disparities in health care (15). Till recently, it was assumed that clinical prediction algorithms should include all observed patient variables with predictive power to produce more accurate predictions. And a recent study demonstrated that dropping race may propagate systemic inequities and discrimination (9). Thus, eliminating race is more nuanced than removing it from all clinical algorithms.
Our goal in this study was to create an up-to-date database of race-based clinical algorithms that is a resource for raising awareness of the use of race-based clinical algorithms and tracking the progress made toward eliminating the inappropriate use of race. We conducted a systematic search and analysis of dedicated online resources and published literature for clinical algorithms and identified and classified those that use race.
METHODS
Clinical algorithms include risk calculators for diagnostic and prognostic settings, flowcharts, lookup tables, nomograms, and guidelines. A risk calculator uses a formula or a statistical model to assess the presence of a condition or predict the occurrence of a future condition, such as determining current osteoporosis status and predicting future fracture risk associated with osteoporosis. A flowchart is a branching decision tree, such as a diagnostic flowchart, for determining the etiology of chest pain. A lookup table enables quick reference of data, such as a table containing energy and nutritional content of various foodstuffs. A nomogram is a graphical tool used for a specific calculation, such as a nomogram of height and weight measurements that can be used to find the surface area of a person. This study focused on identifying and cataloging clinical algorithms such as race-based risk calculators, laboratory test results with race-based reference ranges, therapy recommendations based on race, and medications with race-based guidelines.
Data sources and search strategy
We identified online resources with clinical calculators using Google search (query: (medical OR clinical) AND calculator). Websites were excluded if there was no contact information, no references (i.e., PubMed), contained only hyperlinks to external websites, described smartphone apps for calculators, and did not implement the calculator for online data entry and result output. From the included online resources, we created a list of race-based risk calculators; we included a calculator if it had at least one PubMed reference, and race was an input variable.
We also identified peer-reviewed articles with race-based clinical calculators using PubMed search (query: (medical OR clinical) AND calculator AND race AND bias). We retained only those articles that contained a table of race-based clinical calculators. From the included articles, we extracted a list of race-based clinical calculators. The list of race-based clinical calculators obtained from online resources was merged with the list obtained from peer-reviewed articles, and duplicates were removed to create a final list.
We identified medications with potential race-based guidelines by searching Micromedex, which is one of the largest web-based pharmacological knowledge bases that provide detailed information on drugs and their clinical significance. We generated independent lists for each of the following keywords: ancestry, descent, ethnicity, heritage, and peoples. We removed duplicates and excluded medications with no U.S. FDA Drug Label Information, medications with no race-based guidelines in the Drug Label Information, and combination medications if a member of the combination was included independently.
Data extraction
We independently reviewed the original publication or source for each included calculator and extracted the pertinent information. The information included the name of the calculator, its purpose, a description of its use of race, the potential harm caused by the inclusion of race, the calculator's input variables, a reference, and a description of any modifications (especially in terms of handling race) made after its introduction. In addition, we categorized each calculator based on its intended use and identified its clinical specialty.
We independently reviewed the relevant FDA Drug Product Labeling for each medication and extracted pertinent information. The information included the name of the drug, a description of the drug, a description of the use of race, a rationale for the use of race, a reference to the FDA Drug Label Information, and the section(s) in Drug Label Information that contained the race-based information. In addition, we categorized each medication according to the racial context of its use and identified its clinical specialty.
Online database
After identification, data extraction, and validation, we created an online database that provides free access to the results. The authors will regularly update this open-access database as new race-based calculators and medications are identified. Additionally, we added a submission feature to the database, allowing the community to submit new calculators and medications that our search missed. Before being added to the database, all community submissions will be verified and cross-checked.
RESULTS
We report separately clinical calculators with race-based guidelines (including risk calculators, laboratory tests, and therapy recommendations) and medications with race-based guidelines.
Clinical calculators
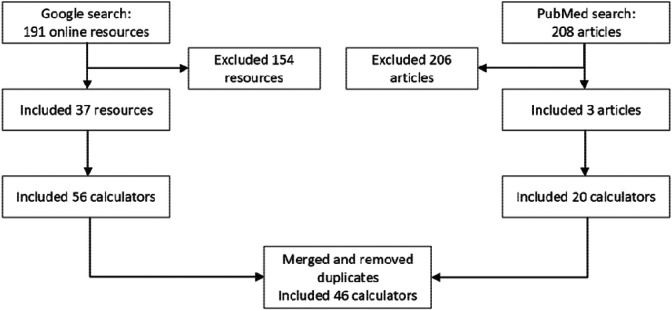
We identified 191 online resources with clinical calculators, of which 37 met the inclusion criteria and 208 articles, of which three met the inclusion criteria (Figure 1). After merging calculators identified from the online resources and the three articles, the final list contained 46 calculators. Detailed information for each calculator is provided in Supplementary Information. We located an online implementation for every calculator except for one. Though it does not use race as a variable, we included pulse oximetry since it has been shown to be less accurate in darker-skinned individuals.
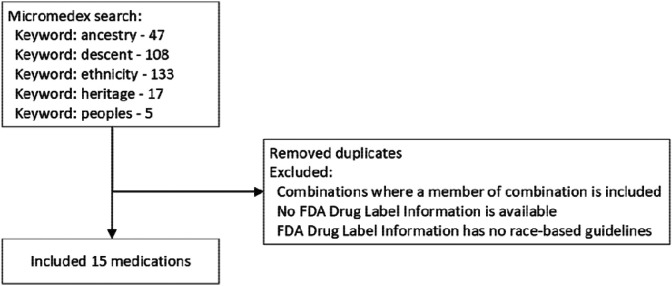
Figure 1.
Selection of risk calculators, laboratory tests, and therapy recommendations with race-based guidelines.
Among the online resources, the most comprehensive are MDCalc, UpToDate Medical Calculators, and MDApp. MDCalc is widely used globally, and in the U.S., over 65 percent of physicians use it every month (16). UpToDate is a widely used database of point-of-care information that also contains a comprehensive calculator resource (17). MDApp is a U.K.-based company that implements clinical calculators. The MD Anderson Cancer Center has created several cancer-related calculators to predict response to treatment outcomes, survival, and clinical outcomes.
The clinical calculators were categorized into risk calculators, laboratory tests, and therapy recommendations (see Table 1). The calculators were categorized into ten specialties, including cardiac surgery (1), cardiology (5), endocrinology (3), infectious diseases (4), nephrology (3), obstetrics (13), oncology (10), pulmonology (3), surgery (2), and urology (2). The rationale for the use of race mostly comes from epidemiological data that recorded race, and a statistical analysis found a difference based on race. Four of the 46 calculators have been respecified to exclude race as a variable (Anemia in pregnancy, MDRD GFR Equation, Vaginal Birth After Cesarean (VBAC), and UTICalc), and efforts are underway to eliminate the use of race in two other calculators (Kidney Donor Risk Index (KDRI) and Spirometry Reference Value Calculator).
Table 1.
Summary of risk calculators, laboratory tests, and therapy recommendations with race-based guidelines.
| Category | Description | Number of calculators |
|---|---|---|
| risk calculator | differential risk of a clinical event is predicted based on race | 39 |
| laboratory test | differential abnormal laboratory test values are defined based on race | 6 |
| therapy recommendation | differential therapy is recommended based on race | 1 |
Race and ethnicity are treated as separate variables in only two calculators (Predict COVID-19 Test Result and Predict Hospitalization Risk for COVID-19 Positive); in the majority of the calculators, race and ethnicity are treated as a single variable or only race are included as a variable. A total of 49 distinct race/ethnicity categories were identified in the calculators. Common categories included white, Black, other, Asian, Caucasian, East Asian, mixed, and South Asian.
Medications
From Micromedex, we identified 47 (keyword: ancestry), 108 (keyword: descent), 133 (keyword: ethnicity), 17 (keyword: heritage), and 5 (keyword: peoples) medications with potential race-based guidelines. After applying the exclusion criteria and removing duplicates, the final list contained 15 medications for which we verified that the FDA Drug Product Labeling included race-based treatment guidelines (see Figure 2). Detailed information for each medication is provided in Supplementary Information. They were categorized into medications with race-based indication, race-based dose adjustment, race-based monitoring, and race-based pharmacogenetic screening (see Table 2). The rationale for the use of race mostly comes from studies that recorded race and found a difference in genetics or pharmacokinetics based on race.
Figure 2.
Selection of medications with race-based guidelines.
Table 2.
Summary of medications with race-based guidelines.
| Category | Description | Number of medications |
|---|---|---|
| race-based indication | medication is recommended for a specific race or races | 2 (isosorbide dinitrate and hydralazine, isoniazid) |
| race-based dose adjustment | differential dosing is recommended based on race | 6 (rosuvastatin, eltrombopag, tacrolimus, warfarin, omeprazole, simeprevir) |
| race-based monitoring | differential frequency of monitoring is recommended based on race | 2 (simvastatin, alvimopan) |
| race-based pharmacogenetic screening | pharmacogenetic screening is recommended for a specific race or races | 5 (pegloticase, rasburicase, carbamazepine, oxcarbazepine, allopurinol) |
DISCUSSION
Clinical algorithms are embedded in electronic health records, guidelines, and decision support tools, and it is feared that the use of algorithms that include race as a predictor may lead to disparities in health care, especially in racial minority populations. As our understanding of race in medicine evolves, efforts are being made to investigate the role of race in clinical tools. Recently, the Agency for Healthcare Research and Quality conducted a stakeholder review of 18 algorithms based on race in health care (13). With the beginning of this new era, it is crucial for the medical community to have an up-to-date catalog of clinical algorithms that use race in order to anticipate and measure progress in the antiracist reformulation of these tools. We conducted an exhaustive search of online resources, the scientific literature, and the FDA Drug Label Information and identified 39 race-based risk calculators, six laboratory test results with race-based reference ranges, one race-based therapy recommendation, and 15 medications with race-based recommendations. Information about these 61 entities is accessible in an online database at http://www.race-based-clinical-algorithms.org/.
A key issue is the lack of standardization in the racial categorization systems currently in use. Typically, an optimal categorization system has consistent definitions for universally applicable categories, and the categories are mutually exclusive. A good system is also complete and capable of absorbing entities that have not yet been identified without requiring system revisions. All of these features are lacking in racial categorization systems. There is no single global racial categorization system in use. The Office of Management and Budget in the U.S. defines five racial categories: white, Black or African American, American Indian or Alaskan Native, Asian, and Native Hawaiian or Other Pacific Islander, as well as two ethnic categories: Hispanic and not Hispanic. In the U.K., the Office of National Statistics defines five high-level ethnic groups: "Asian, Asian British, Asian Welsh," "Black, Black British, Black Welsh, Caribbean or African," "Mixed or Multiple," "White," and "Other ethnic group." These systems make racial categories difficult to define in practice, and there are no well-defined rules governing what constitutes a race or which race a person belongs to. For example, Caucasians are often called whites or Europeans, even though many Caucasians are neither. Blacks are often called Africans, even though many Blacks are not African. Because racial categories are not mutually exclusive, individuals can belong to multiple races at the same time. Finally, the addition of new racial categories leads to rearrangements of the system. For example, in 1977, the U.S. Census Bureau established four racial categories, including white, Black, American Indian or Alaskan Native, and Asian or Pacific Islander. Two decades later, the Bureau split the Asian or Pacific Islander category into two, namely Asian and Native Hawaiian and Pacific Islander categories. Further, racial categories are used inconsistently across different research papers and data sets. A recent review showed that the U.S. racial categories of white, Black, and Asian mapped to 66, 62, and 49 different racial or ethnic categories, respectively (18). This lack of standardization leads to ambiguities in operationalizing race-based clinical algorithms for clinical use. For example, guidelines are silent on how race adjustment should be applied to a patient with a white mother and a Black father (14).
Five medications have race-based pharmacogenetic screening recommendations (see Table 2), including testing for glucose-6-phosphate dehydrogenase (G6PD) deficiency in pegloticase and rasburicase, testing for the HLA-B*1502 variant in carbamazepine and oxcarbazepine, and testing for the HLA-B*5801 variant in allopurinol. Race-based pharmacogenetic screening focuses on determining who should undergo specific pharmacogenetic testing so that testing is pursued only for those who are most likely to require it. However, the use of broad racial categories complicates the application of these guidelines in practice. For instance, while the HLA-B*1502 variant is present in over 10% of individuals from Indonesia, Hong Kong, and Vietnam, it is present in less than 1.5% of individuals from Japan and Korea. Therefore, the broad racial category of Asians is inadequate for identifying which patients are at the greatest risk and would most likely benefit from genetic testing (19). Hence, pharmacogenetic screening recommendations based on race may result in considerable disparities in health care with the potential for adverse clinical outcomes.
Concerns regarding the inappropriate use of race in clinical algorithms have prompted calls for the elimination of race adjustments in clinical algorithms both in the academic literature and by organizations such as the Coalition to End Racism in Clinical Algorithms (20) and the Kaiser Family Foundation (21). These efforts have resulted in the recent removal of race from four calculators. In 2021, the American College of Obstetricians and Gynecologists eliminated race-based cutoffs for hematocrit levels for screening for iron deficiency anemia in pregnancy (22), data from the Cesarean Registry of the Maternal-Fetal Medicine Units Network was reanalyzed to develop a new Vaginal Birth After Cesarean (VBAC) calculator without race and ethnicity (23), and a Task Force established by the National Kidney Foundation and the American Society of Nephrology recommended the use of an updated eGFR equation without race (24). In 2022, the original UTICalc calculator was replaced by a race-free calculator with comparable predictive performance (25). In addition, investigators have recommended eliminating the use of race in the Kidney Donor Risk Index (KDRI) (26) and Spirometry Reference Value Calculator (27) based on evidence that these race-based calculators lead to disparities in health care for racial and ethnic minorities.
However, it is critical to thoroughly investigate the impact of race on clinical decision-making and health disparities. While replacing race in clinical decision-making with a person's unique genetic makeup, environmental factors, and other relevant factors is obviously preferable, including race in understanding health and healthcare disparities may still be prudent (28). Race is often correlated with health disparities due to various factors such as socioeconomic status, access to healthcare, environmental conditions, and historical systemic racism. Removing race from algorithms without addressing these underlying disparities may overlook important risk factors and perpetuate health inequities. And completely ignoring race could lead to an incomplete understanding of health outcomes for marginalized communities. Some diseases and genetic variations are known to have higher prevalence rates among specific racial or ethnic groups. For instance, sickle cell anemia is more common in individuals of African or Mediterranean descent, and genetic mutations causing G6PD deficiency are more common in persons of African, Asian, and Mediterranean descent. In such instances, using race for screening may be acceptable in the interim until a better biological substitute is developed. With rapid progress in precision medicine, it is preferable to avoid race and to use suitable blood and genetic tests. And as genetic tests, including multigene panels and whole genome sequencing, become more affordable, race-based pharmacogenomic will become obsolete. The predictive models underlying clinical algorithms are frequently derived from datasets that were assembled from processes of clinical care. These datasets may contain racial biases due to historical disparities in healthcare access and diagnosis. Simply removing race without addressing the underlying biases might perpetuate or amplify existing inaccuracies, leading to misdiagnoses and inappropriate treatment decisions.
There are limitations to our approach. We concentrated on a narrow subset of the extensive clinical usage of race. Beyond race-based risk calculators, race-based reference ranges for laboratory test results, and race-based guidelines for medications, race is used widely to distinguish among variations in physiological processes, genetics, behavior, and cultural characteristics (29). In the future, we intend to add usages of race outside of calculators and medication guidelines. We did not include online implementations of calculators that have been modified to exclude racial information because, in most cases, the original implementations were not available. The addition of race-based implementations of these algorithms will be useful for historical reasons as well as to evaluate if the revised formulations are indeed less biased. We did not include information on the potential harm or equity concerns related to the use of race. This is because it is contentious if the inclusion of race in these algorithms indeed propagates disparities when used clinically. Some authors have pointed out that under certain frameworks of social utility and fairness, all individuals are served better when clinical decisions are guided by all predictors, including race. Further, the particular use of an algorithm may inform if race should be included or not. For example, a recent simulation study showed that removing race from diagnostic algorithms could make healthcare inequities worse, while including race in prognostic algorithms that help decide how to allocate resources can worsen inequities.
The database will be actively updated to include additional race-based clinical algorithms and clinical uses of race. In the future expansion of the database, we want to offer additional details of algorithms, such as the actual equations and statistical models. In addition, we intend to include descriptions of the datasets from which the algorithms were derived and, if possible, provide access to the datasets themselves.
ACKNOWLEDGEMENTS
This work was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1 TR001857. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. 2015;162(1):55–63. [DOI] [PubMed] [Google Scholar]
- 2.Dziadzko MA, Gajic O, Pickering BW, Herasevich V. Clinical calculators in hospital medicine: availability, classification, and needs. Comput Methods Programs Biomed. 2016;133:1–6. [DOI] [PubMed] [Google Scholar]
- 3.Green TA, Whitt S, Belden JL, Erdelez S, Shyu CR. Medical calculators: prevalence, and barriers to use. Comput Methods Programs Biomed. 2019;179:105002. [DOI] [PubMed] [Google Scholar]
- 4.Lu C, Ahmed R, Lamri A, Anand SS. Use of race, ethnicity, and ancestry data in health research. PLOS Glob Public Health. 2022;2(9):e0001060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerdeña JP, Plaisime MV, Tsai J. From race-based to race-conscious medicine: how antiracist uprisings call us to act. Lancet. 2020;396(10257):1125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sirugo G, Tishkoff SA, Williams SM. The quagmire of race, genetic ancestry, and health disparities. J Clin Invest. 2021;131(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rebbeck TR, Mahal B, Maxwell KN, Garraway IP, Yamoah K. The distinct impacts of race and genetic ancestry on health. Nat Med. 2022;28(5):890–3. [DOI] [PubMed] [Google Scholar]
- 8.Eneanya ND, Boulware LE, Tsai J, Bruce MA, Ford CL, Harris C, et al. Health inequities and the inappropriate use of race in nephrology. Nat Rev Nephrol. 2022;18(2):84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu A. Use of race in clinical algorithms. Sci Adv. 2023;9(21):eadd2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt IM, Waikar SS. Separate and unequal: race-based algorithms and implications for nephrology. J Am Soc Nephrol. 2021;32(3):529–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kessler L, Watts K, Abraham N. Should we correct the use of race in urological risk calculators? J Urol. 2023;209(1):17–20. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien J, Clare CA. Race-based versus race-conscious medicine in obstetrics and gynecology. Clin Obstet Gynecol. 2023;66(1):95–106. [DOI] [PubMed] [Google Scholar]
- 13.Jain A, Brooks JR, Alford CC, Chang CS, Mueller NM, Umscheid CA, et al. Awareness of racial and ethnic bias and potential solutions to address bias with use of health care algorithms. JAMA Health Forum. 2023;4(6):e231197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyas DA, Eisenstein LG, Jones DS. Hidden in plain sight - reconsidering the use of race correction in clinical algorithms. N Engl J Med. 2020;383(9):874–82. [DOI] [PubMed] [Google Scholar]
- 15.Manski CF. Patient-centered appraisal of race-free clinical risk assessment. Health Econ. 2022. [DOI] [PubMed] [Google Scholar]
- 16.Elovic A, Pourmand A. MDCalc medical calculator app review. J Digit Imaging. 2019;32(5):682–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKinney A. UpToDate: evidence-based medicine database. Journal of electronic resources in medical libraries. 2012;9(1):56–64. [Google Scholar]
- 18.Zhang F, Finkelstein J. Inconsistency in race and ethnic classification in pharmacogenetics studies and its potential clinical implications. Pharmgenomics Pers Med. 2019;12:107–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman CW, Brett AS. Race and pharmacogenomics-personalized medicine or misguided practice? Jama. 2021;325(7):625–6. [DOI] [PubMed] [Google Scholar]
- 20.Khazanchi R, Soled DR, Yearby R. Racism-conscious praxis: a framework to materialize anti-oppression in medicine, public health, and health policy. Am J Bioeth. 2023;23(4):31–4. [DOI] [PubMed] [Google Scholar]
- 21.Schor EL. The Henry J. Kaiser Family Foundation. Acad Med. 1990;65(1):26–7. [PubMed] [Google Scholar]
- 22.Anemia in Pregnancy: ACOG Practice Bulletin, Number 233. Obstet Gynecol. 2021;138(2):e55–e64. [DOI] [PubMed] [Google Scholar]
- 23.Grobman WA, Sandoval G, Rice MM, Bailit JL, Chauhan SP, Costantine MM, et al. Prediction of vaginal birth after cesarean delivery in term gestations: a calculator without race and ethnicity. Am J Obstet Gynecol. 2021;225(6):664.e1–.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, et al. A unifying approach for GFR estimation: recommendations of the NFK-ASN task force on reassessing the inclusion of race in diagnosing kidney disease. J Am Soc Nephrol. 2021;32(12):2994–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaikh N, Lee MC, Stokes LR, Miller E, Kurs-Lasky M, Conway I, et al. Reassessment of the role of race in calculating the risk for urinary tract infection: a systematic review and meta-analysis. JAMA Pediatr. 2022;176(6):569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doshi MD, Schaubel DE, Xu Y, Rao PS, Sung RS. Clinical utility in adopting race-free kidney donor risk index. Transplant Direct. 2022;8(7):e1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhakta NR, Bime C, Kaminsky DA, McCormack MC, Thakur N, Stanojevic S, et al. Race and ethnicity in pulmonary function test interpretation: an official American Thoracic Society statement. Am J Respir Crit Care Med. 2023;207(8):978–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choy KW. From race-based to race-conscious medicine: proceed with care. Intern Med J. 2021;51(2):309. [DOI] [PubMed] [Google Scholar]
- 29.Cerdeña JP, Asabor EN, Plaisime MV, Hardeman RR. Race-based medicine in the point-of-care clinical resource UpToDate: a systematic content analysis. EClinicalMedicine. 2022;52:101581. [DOI] [PMC free article] [PubMed] [Google Scholar]