Abstract
Background
The triglyceride-glucose (TyG) index and the stress hyperglycaemia ratio (SHR) are both positively associated with cardiovascular (CV) risk in patients with coronary heart disease. However, the prognostic value of these two biomarkers has not been well elucidated in patients with chronic total occlusion (CTO). Therefore, this study aims to evaluate the association of the TyG index and the SHR with long-term prognosis in patients with CTO.
Methods
This prospective cohort study consecutively included 2740 angina patients with CTO from January 2017 to December 2018 at Fuwai Hospital. The outcomes are a composite of CV death and target vessel myocardial infarction (TVMI) and major CV cerebrovascular adverse events (MACCEs, including all-cause death, nonfatal MI, ischaemia-driven target vessel revascularization, and stroke). The association between biomarkers and prognosis was analysed by multivariable Cox proportional hazard models, and the predictive value was determined by a receiver-operating characteristic (ROC) curve.
Results
During the follow-up with a median time of 3 years, 179 (6.5%) cases of MACCEs and 47 (1.7%) cases of CV death or TVMI were recorded. Patients with a high TyG index (> 9.10) and a high SHR (> 0.87) showed a significantly increased risk of CV death/TVMI (TyG index: HR 4.23, 95% CI 1.58–11.37; SHR: HR 5.14, 95% CI 1.89–13.98) and MACCEs (TyG index: HR 2.47, 95% CI 1.54–3.97; SHR: HR 2.91, 95% CI 1.84–4.60) compared with those with a low Tyg index and a low SHR (TyG < 8.56, SHR < 0.76). The area under the curve (AUC) values were 0.623 (TyG index) and 0.589 (SHR) for CV death/TVMI and 0.659 (TyG index) and 0.624 (SHR) for MACCEs. Furthermore, patients with both a high TyG index and a high SHR showed the highest risk of clinical outcomes among patients with different levels of these two biomarkers, and the AUC for the TyG-SHR combination was larger than the TyG index alone in predicting MACCE risk.
Conclusions
The study revealed that a high TyG index and a high SHR were significantly correlated with poor prognosis in patients with CTO and suggested that these two biomarkers are reliable in predicting long-term prognosis in CTO patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-023-01883-8.
Keywords: Triglyceride-glucose index, Stress hyperglycaemia ratio, Chronic total occlusion, Coronary heart disease, Diabetes
Introduction
Chronic total occlusion (CTO) is a complex coronary lesion with a prevalence of 16–20% in patients with coronary heart disease (CHD) [1, 2]. In clinical practice, the CTO procedural success rate is not high (approximately 55–62% in Michigan in 2017), and patients with CTOs tend to have a high incidence of complications, long procedural duration, and high cost [2]. Regarding the prognosis of CTO patients, scholars reported a 10.4% 10 year cardiovascular (CV) mortality in those with successful procedures, indicating that a high CV risk existed in CTO patients [3]. Therefore, approaches identifying high-risk patients among those with CTO lesions are warranted.
The triglyceride-glucose (TyG) index was determined to be a reliable biomarker in evaluating insulin resistance [4], and the stress hyperglycaemia ratio (SHR) was suggested to precisely reflect background glucose metabolic status [5]. In previous studies, these two biomarkers have been revealed to be positively associated with the severity of coronary lesions and long-term CV risk in patients with CHD [6–8]. A high TyG index was suggested to correlate with poor prognosis in patients with CTOs in a previous retrospective study [9]. Although that was a well-conducted study, the sample size was relatively small (652 patients), which may have potentially biased the results [9]. Therefore, the prognostic value of the TyG index in CTO patients warrants investigation in a large population-based prospective cohort. Although glucose metabolism disorders were shown to be associated with a high incidence of adverse CV events in patients with CTO [10], the prognostic value of the SHR in patients with CTO lesions is still not clear.
In this large population-based prospective cohort study, we consecutively included 2740 patients with CTO lesions and conducted a follow-up with a median time of 3 years. The objective of this study was to evaluate the association of the TyG index and the SHR with long-term prognosis in CTO patients.
Methods
Study design and population
This was a single-centre prospective cohort study. From January 2017 to December 2018, a total of 3072 patients who underwent percutaneous coronary intervention (PCI) for CTO lesions were consecutively enrolled at FuWai Hospital, National Center for Cardiovascular Diseases. A CTO lesion was defined as a thrombolysis in myocardial infarction (TIMI) flow grade of 0 for a coronary artery with a duration of ≥ 3 months. The main exclusion criteria were incomplete data on the TyG index or SHR, severe liver and/or renal insufficiency, severe heart failure (HF), and loss to follow-up. Ultimately, a total of 2740 participants were analysed in the present study. The detailed flow chart is shown in Fig. 1.
Fig. 1.
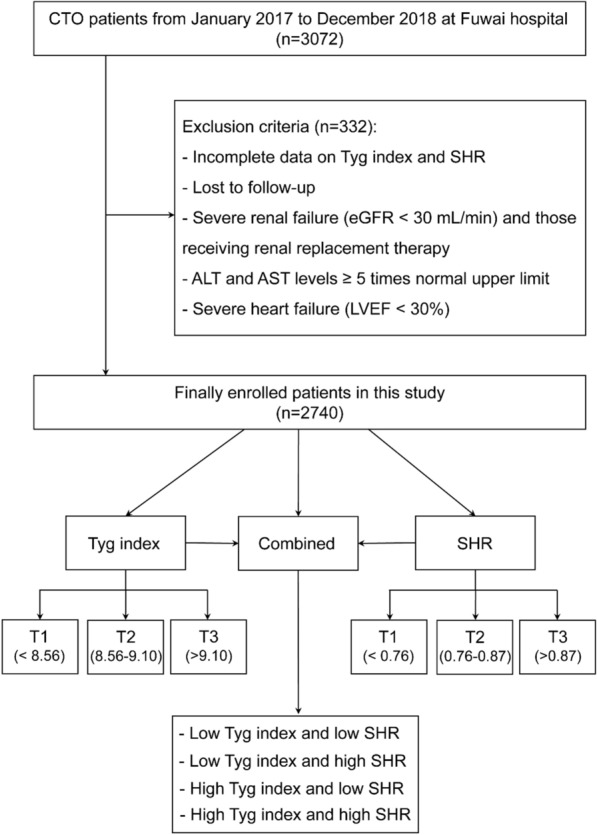
Flow chart of the enrolled patients. CTO chronic total occlusion, TyG riglyceride-glucose, SHR stress-hyperglycemia ratio, ALT alanine aminotransferase, AST aspartate aminotransferase, LVEF left ventricular ejection fraction
Participants enrolled in this study were divided into different groups according to the TyG index (T1: < 8.56; T2: 8.56–9.10; T3: > 9.10) and SHR (T1: < 0.76; T2: 0.76–0.87; T3, > 0.87). A high TyG index and SHR were defined as T3, and levels of T1 and T2 were considered to be ‘low’ groups. Participants were further divided into four groups according to the combination of the TyG index and the SHR: groups of low TyG and SHR, low TyG and high SHR, high TyG and low SHR, and high TyG and SHR.
This study was conducted in compliance with the Declaration of Helsinki and was approved by the Institutional Review Board of FuWai Hospital, National Center for Cardiovascular Diseases. All participants provided written informed consent before enrolment.
Study procedures and biochemical analysis
During hospitalization, all procedures and medical therapies were conducted according to the guideline’s recommendation and the cardiologist’s discretion. All patients received loading doses of aspirin (300 mg), clopidogrel (600 mg), or ticagrelor (180 mg) before PCI. The detailed PCI strategy was determined by experienced interventionists. All CTO lesions were first treated with the anterograde approach using dedicated coronary wires and devices; otherwise, the retrograde approach was adopted. The use of coronary wires and devices, as well as adjunctive examinations such as intravascular ultrasound and optical coherence tomography, were left to the discretion of interventionists. The procedural success of CTO-PCI was defined as residual stenosis < 30% with TIMI grade ≥ 2 antegrade flow. After patients were discharged, aspirin therapy was continued indefinitely (100 mg/day), and clopidogrel (75 mg/day) or ticagrelor (180 mg/day) was administered for ≥ 12 months.
Before coronary angiography, we obtained laboratory samples from each participant from the cubital vein after overnight fasting. All tests were conducted through the clinical chemistry department of our center. Concentrations of triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), fasting plasma glucose (FPG), and creatinine were analysed in an enzymatic assay by an automated biochemical analyser (Hitachi 7150, Tokyo, Japan). Glycosylated haemoglobin A1c (HbA1c) was measured using a Tosoh Automated Glycohemoglobin Analyser (HLC-723G8, Tokyo, Japan). Angiographic and procedural data were collected from catheter laboratory records by three experienced interventional cardiologists. Demographics, cardiovascular risk factors, clinical parameters, laboratory and imaging data, coronary angiographic and procedural details, and use of medications at discharge were prospectively collected with standardized questionnaires by independent research personnel. Notably, the TyG index was calculated using the following equation: Ln (fasting triglyceride (mmol/L) × FPG (mmol/L)/2) [11], while the SHR was calculated by the formula [FPG (mmol/L)]/[1.59 × HbA1c (%)− 2.59)] [12].
Patients who had a history of diabetes, received glucose-lowering therapy or had an FPG ≥ 7.0 mmol/L, HbA1c ≥ 6.5%, or 2 h plasma glucose ≥ 11.1 mmol/L in an oral glucose tolerance test (OGTT) were considered to have diabetes. Prediabetes was defined as FPG 5.6 ~ 6.9 mmol/L, HbA1c 5.7 ~ 6.4%, or 2 h plasma glucose 7.8 ~ 11.0 mmol/L in an OGTT according to the American Diabetes Association criteria [13]. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or the use of antihypertensive therapy [14]. Dyslipidaemia was defined as triglycerides ≥ 150 mg/dL, total cholesterol ≥ 200 mg/dL, LDL-C ≥ 130 mg/dL or HDL-C < 40 mg/dL, or the use of cholesterol-lowering therapy [15]. Heart failure [HF] was defined as having a history of HF or left ventricular ejection fraction [LVEF] < 50% [16]. Renal dysfunction was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 [17].
Follow‑up and endpoints
After discharge, participants were followed up at 6-month intervals until December 31, 2021. Data for endpoints were obtained from medical records, clinical visits, and/or telephone interviews by trained investigators who were blinded to the clinical data. The primary endpoint was the composite of CV death or target-vessel myocardial infarction (TVMI) and major adverse cardiovascular and cerebrovascular events (MACCEs), including all-cause death, nonfatal MI, ischaemia-driven target vessel revascularization, and stroke. The secondary endpoint was all-cause death, which was utilized in the survival analysis. Death was considered cardiac unless unequivocal noncardiovascular cause could be established. MI was defined as positive cardiac troponins with typical chest pain, typical electrocardiogram serial changes, identification of an intracoronary thrombus by angiography or autopsy, or imaging evidence of new loss of viable myocardium or a new regional wall-motion abnormality [18]. Ischaemia-driven lesions were defined as restenotic lesions ≥ 50% in angiography with ischaemic evidence or ≥ 70% in angiography irrespective of the ischaemic evidence. Target vessel revascularization was defined as any unplanned repeat percutaneous intervention or surgical bypass of the treated vessel with CTO lesions. Stroke was defined as a new focal neurological deficit lasting > 24 h confirmed by imaging evidence. All events were carefully verified and adjudicated by independent clinicians.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation if conformed to the normal distribution, otherwise shown as median (interquartile range), while categorical variables were expressed as frequencies (percentages). Differences between groups were compared using one-way ANOVA, Kruskal‒Wallis H test, Pearson's chi-square test, or Fisher’s exact test, when appropriate. The correlation between the TyG index, SHR, and other continuous variables was calculated using the Spearman rank correlation test. The cumulative incidence of clinical events was estimated using Kaplan‒Meier curves, and differences were assessed with the log-rank test. Single-variable and multivariable Cox regression analyses were conducted to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). In multivariable analysis, three Cox regression models were fitted. Model 1 was adjusted for age, sex, and body mass index (BMI); Model 2 was adjusted for the variables in Model 1 and smoking, diabetes, dyslipidaemia, hypertension, HF, prior MI, prior stroke, peripheral vascular disease, prior revascularization, and acute myocardial infarction; Model 3 was adjusted for the variables in Model 2 and multivessel disease, ostial lesion, bifurcation, number of lesions ≥ 2, number of stents ≥ 2, procedural success, eGFR, dual antiplatelet therapy, statin, and antidiabetic drug at discharge. The association of each co-variate with the risk of clinical outcomes with model 3 is presented in Additional file 1: Table S1, S2.
On a continuous scale, restricted cubic splines (RCSs) were used to examine the potential nonlinear relationships between the TyG index, SHR, and clinical outcomes. In addition, receiver operating characteristic (ROC) curve analysis was conducted, and areas under the curve (AUCs) were calculated to evaluate the predictive value of the TyG index, SHR, and their combination in predicting the prognosis of patients with CTO lesions. The cut-off values of the TyG index and SHR were identified by the Youden index using ROC curve analysis. The Youden Index equals the sum of sensitivity and specificity minus 1, and the optimal cut-off values refer to the TyG index and SHR that correspond to the maximum Youden Index. In addition to the primary endpoints, we conducted a survival analysis for the relationship between the TyG index, SHR, and life expectancy with the endpoint of all-cause death.
We further determined the association of the TyG index and SHR with the risk of clinical outcomes in patients with various glucose metabolism statuses (normal glucose tolerance [NGT], prediabetes, and T2DM) and procedural outcomes (success or failure). Categories were all based on the tertiles of the TyG index or SHR. Moreover, we examined the effect of the TyG index and SHR on the clinical outcomes through subgroup analysis based on important clinical variables, such as age, sex, BMI, smoking, dyslipidaemia, hypertension, HF with low LVEF (< 50%), renal dysfunction, and AMI. Sensitivity analysis was conducted by excluding patients who had clinical events within 90 days after the initial procedure. All statistical analyses were conducted with R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P value of < 0.05 was deemed statistically significant.
Results
Baseline characteristics according to categories of outcomes
Baseline characteristics and comparisons between patients with and without clinical outcomes are presented in Table 1. In the population enrolled in this study, the mean age was 58.5 ± 10.5 years old, and 82.9% of the patients were male. The average values of the TyG index and SHR were 8.8 ± 0.8 and 0.9 ± 0.2, respectively. Compared with survivors, those who developed clinical events during the follow-up tended to be older, have hypertension, HF (lower LVEF), renal dysfunction (lower eGFR), prior MI, and prior stroke, and hold higher TyG index and SHR values. Regarding the lesions and treatment characteristics, patients with clinical events were more likely to have multivessel coronary disease, receive more stent treatment and have a lower percentage of procedural success. In addition, the baseline characteristics grouped by the TyG index and SHR levels are shown in Additional file 1: Tables S3, S4, respectively.
Table 1.
Baseline patient characteristics grouped by outcomes
| All (n = 2740) | Survivors (n = 2571) | Patients with outcomes (n = 169) | P-value | |
|---|---|---|---|---|
| Age, years | 58.5 ± 10.5 | 58.3 ± 10.5 | 62.2 ± 10.6 | < 0.001 |
| Male, n (%) | 2272 (82.9) | 2139 (83.2) | 133 (78.7) | 0.132 |
| BMI, kg/m2 | 26.3 ± 3.3 | 26.3 ± 3.3 | 25.8 ± 3.5 | 0.042 |
| Smoking, n (%) | 880 (32.1) | 826 (32.1) | 54 (32) | 0.962 |
| Prediabetes, n (%) | 999 (36.5) | 943 (36.7) | 56 (33.1) | 0.354 |
| Diabetes, n (%) | 1280 (46.7) | 1193 (46.4) | 87 (51.5) | 0.200 |
| Hypertension, n (%) | 1777 (64.9) | 1643 (63.9) | 134 (79.3) | < 0.001 |
| Dyslipidemia, n (%) | 2182 (79.6) | 2046 (79.6) | 136 (80.5) | 0.780 |
| HF, n (%) | 211 (7.7) | 185 (7.2) | 26 (15.4) | < 0.001 |
| LVEF, (%) | 59.9 ± 8.7 | 60.1 ± 8.5 | 57.3 ± 10.8 | < 0.001 |
| Prior MI, n (%) | 901 (32.9) | 836 (32.5) | 65 (38.5) | 0.111 |
| Prior stroke, n (%) | 350 (12.8) | 320 (12.4) | 30 (17.8) | 0.045 |
| Peripheral vascular disease, n (%) | 188 (6.9) | 173 (6.7) | 15 (8.9) | 0.285 |
| Prior revascularization, n (%) | 870 (31.8) | 810 (31.5) | 60 (35.5) | 0.280 |
| PCI | 822 (30.0) | 765 (29.8) | 57 (33.7) | 0.275 |
| CABG | 86 (3.1) | 78 (3) | 8 (4.7) | 0.220 |
| Renal dysfunction, n (%) | 129 (4.7) | 108 (4.2) | 21 (12.4) | < 0.001 |
| Unstable angina, n (%) | 1162 (42.4) | 1092 (42.5) | 70 (41.4) | 0.788 |
| AMI, n (%) | 233 (8.5) | 220 (8.6) | 13 (7.7) | 0.696 |
| Silent ischeamia, n (%) | 541 (19.7) | 501 (19.5) | 40 (23.7) | 0.186 |
| Laboratory tests | ||||
| Hemoglobin, g/L | 4.9 (4.5, 5.2) | 4.9 (4.5, 5.2) | 4.7 (4.4, 5.3) | 0.269 |
| Platelet, × 109/L | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.5) | 0.902 |
| HbA1C, % | 6.5 ± 1.2 | 6.5 ± 1.2 | 6.6 ± 1.1 | 0.669 |
| FBG, mmol/L | 6.6 ± 2.4 | 6.6 ± 2.4 | 7.5 ± 3.0 | < 0.001 |
| TC, mmol/L | 3.8 (3.3, 4.6) | 3.8 (3.3, 4.6) | 3.9 (3.3, 4.6) | 0.75 |
| TG, mmol/L | 1.4 (1.0, 2.0) | 1.4 (1.0, 2.0) | 1.5 (1.2, 2.0) | 0.116 |
| LDL-C, mmol/L | 2.2 (1.8, 2.9) | 2.2 (1.8, 2.9) | 2.3 (1.8, 2.9) | 0.784 |
| HDL-C, mmol/L | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) | 1.0 (0.8, 1.2) | 0.308 |
| eGFR, mL/min/1.73 m2 | 98.8 ± 26.2 | 99.3 ± 25.9 | 91.3 ± 29.9 | < 0.001 |
| Hs-CRP, mmol/L | 2.7 ± 3.2 | 2.7 ± 3.2 | 3.5 ± 3.6 | 0.005 |
| TyG index | 8.8 ± 0.8 | 8.8 ± 0.8 | 9.0 ± 0.7 | < 0.001 |
| SHR | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.3 | < 0.001 |
| Angiographic characteristics, n (%) | ||||
| Multivessel disease | 2395 (87.4) | 2238 (87) | 157 (92.9) | 0.026 |
| Ostial lesion | 440 (16.1) | 413 (16.1) | 27 (16) | 0.976 |
| Bifurcation | 944 (34.5) | 893 (34.7) | 51 (30.2) | 0.227 |
| Intervention treatment, n (%) | ||||
| LM | 37 (1.4) | 37 (1.4) | 0 (0.0) | 0.167 |
| LAD | 940 (34.3) | 889 (34.6) | 51 (30.2) | 0.243 |
| LCX | 377 (13.8) | 358 (13.9) | 19 (11.2) | 0.327 |
| RCA | 1367 (49.9) | 1273 (49.5) | 94 (55.6) | 0.124 |
| Graft | 7 (0.3) | 6 (0.2) | 1 (0.6) | 0.360 |
| Number of lesions ≥ 2 | 732 (26.7) | 689 (26.8) | 43 (25.4) | 0.700 |
| Number of stents ≥ 2 | 1546 (56.4) | 1470 (57.2) | 76 (45) | 0.002 |
| Procedural success | 2233 (81.5) | 2105 (81.9) | 128 (75.7) | 0.047 |
| Medication at discharge, n (%) | ||||
| DAPT | 2716 (99.1) | 2548 (99.1) | 168 (99.4) | 1.000 |
| Aspirin | 2740 (100.0) | 2571 (100) | 169 (100) | 1.000 |
| Clopidogrel | 2539 (92.7) | 2379 (92.5) | 160 (94.7) | 0.301 |
| Ticagrelor | 599 (21.9) | 570 (22.2) | 29 (17.2) | 0.127 |
| β-blockers | 2509 (91.6) | 2358 (91.7) | 151 (89.3) | 0.284 |
| Statin | 2659 (97.0) | 2495 (97.0) | 164 (97.0) | 1.000 |
| Antidiabetic agents | 970 (35.4) | 905 (35.2) | 65 (38.5) | 0.390 |
Tyg triglyceride-glucose, CV cardiovascular, TVMI target vessel myocardial infarction, MACCEs, major adverse CV cerebral events, SHR stress-hyperglycemia ratio, BMI body mass index, T2DM type 2 diabetes mellitus, HF heart failure, LVEF left ventricular ejection faction, AMI acute myocardial infarction, PCI percutaneous coronary intervention, CABG coronary artery bypass grafting, PAD peripheral artery disease, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, HbA1c glycosylated hemoglobin A1c FBG fasting blood glucose, hs CRP high-sensitivity C-reactive protein, eGFR estimated glomerular filtration rate, LM left main, DAPT dual antiplatelet therapy
The TyG index and the long-term prognosis in CTO patients
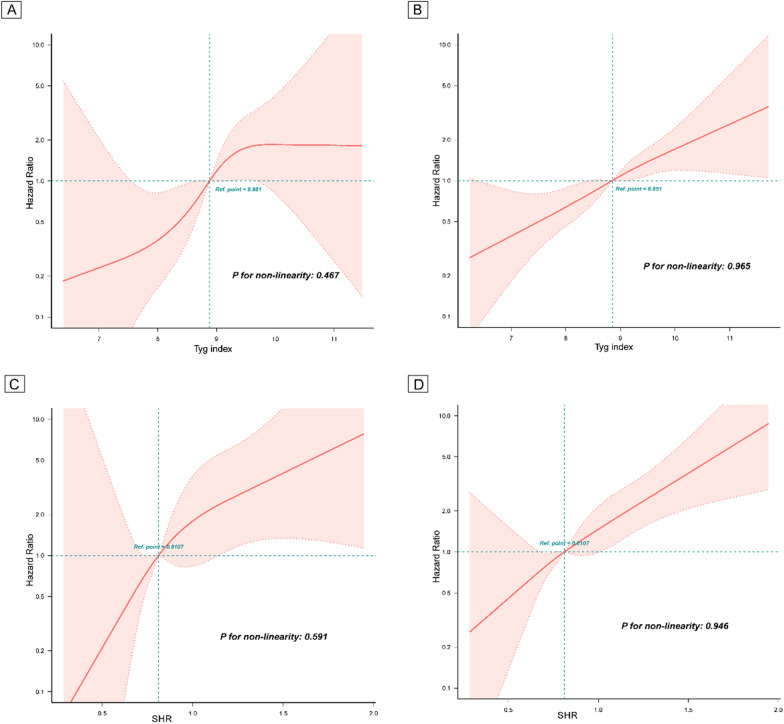
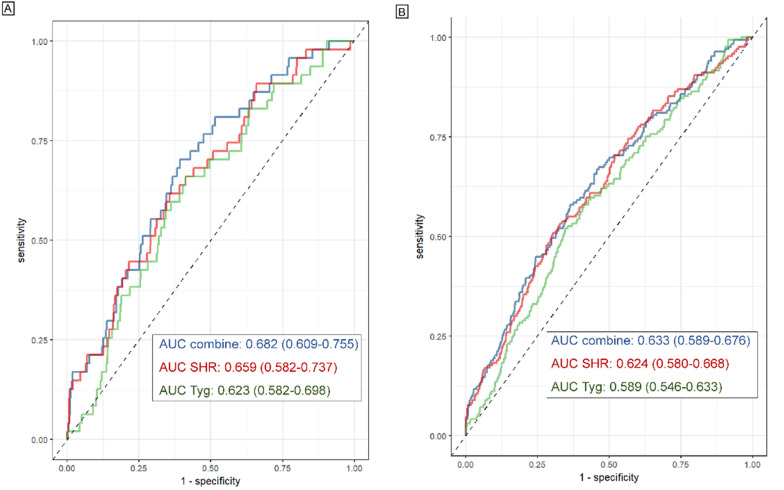
During the follow-up with a median time of 3 years, 179 (6.5%) cases of MACCEs, including 47 (1.7%) cases of CV death or TVMI, were recorded. The association of the TyG index with CV risk is shown in Table 2. Compared with patients with a low TyG index, those with a high TyG index showed a significantly higher risk of both CV death/TVMI (T3 vs. T1: HR 4.23, 95% CI 1.58–11.37) and MACCEs (T3 vs. T1: HR 2.47, 95% CI 1.54–3.97). KM plots for groups with different TyG index values revealed that the group with the highest TyG index values showed the highest risk of both CV death/TVMI and MACCEs (log-rank P < 0.001) (Additional file 1: Figure S1A, D). When analysed, the TyG index is a continuous variable, with the increase in per unit in the TyG index, the risk of CV death/TVMI and MACCEs elevated by 97% and 73%, respectively (Table 2). RCS curves showed that the TyG index was positively linearly correlated with the risk of CV death/TVMI and MACCEs (P for nonlinearity = 0.467 and 0.965) (Fig. 2A, B). The survival analysis also showed that a high TyG index was significantly associated with an increased risk of all-cause death (T3 vs. T1: HR 2.22, 95% CI 1.24–3.95) (Additional file 1: Table S5). For the predictive value of the TyG index for CV death/TVMI and MACCEs, the ROC curves showed that the AUCs of CV death/TVMI and MACCEs evaluated by the TyG index were 0.623 (95% CI: 0.582–0.698) and 0.589 (95% CI: 0.546–0.633), respectively (Table 3, Fig. 3). The optimal cut-off value of the TyG index was 8.98 for CV death/TVMI and 9.07 for MACCEs (Table 4, Fig. 3). The correlation between the TyG index and TC, LDL-C, and hs-CRP is presented in Additional file 1: Table S6, which shows that the TyG index was significantly positively correlated with TC (R2 0.64, 95% CI 0.56–0.73) and LDL-C levels (R2 0.25, 95% CI 0.18–0.33).
Table 2.
Cox regression models for the association of the TyG index and SHR with clinical outcomes
| Per one unit increasea | Groups | P for trend | |||
|---|---|---|---|---|---|
| T1 | T2 | T3 | |||
| TyG index | |||||
| CV death and TVMI | |||||
| No/Subject | 7/895 | 13/917 | 27/928 | ||
| Crude | 1.67 (1.15–2.43) | 1.00 | 1.78 (0.71–4.47) | 3.74 (1.62–8.56) | 0.001 |
| Model 1 | 1.94 (1.30–2.90) | 1.00 | 2.05 (0.81–5.16) | 4.76 (2.04–11.11) | < 0.001 |
| Model 2 | 2.15 (1.36–3.40) | 1.00 | 1.90 (0.75–4.83) | 5.07 (2.10–12.23) | < 0.001 |
| Model 3 | 1.97 (1.19–3.27) | 1.00 | 1.48 (0.53–4.09) | 4.23 (1.58–11.37) | 0.004 |
| MACCEs | |||||
| No/Subject | 40/895 | 56/917 | 83/928 | ||
| Crude | 1.50 (1.23–1.82) | 1.00 | 1.10 (0.72–1.68) | 2.04 (1.40–2.98) | < 0.001 |
| Model 1 | 1.62 (1.32–2.00) | 1.00 | 1.19 (0.77–1.82) | 2.35 (1.60–3.45) | < 0.001 |
| Model 2 | 1.67 (1.34–2.08) | 1.00 | 1.17 (0.76–1.79) | 2.46 (1.64–3.68) | < 0.001 |
| Model 3 | 1.73 (1.34–2.24) | 1.00 | 1.31 (0.81–2.14) | 2.47 (1.54–3.97) | < 0.001 |
| SHR | |||||
| CV death and TVMI | |||||
| No/Subject | 5/897 | 15/911 | 27/932 | ||
| Crude | 1.28 (1.15–1.41) | 1.00 | 2.94 (1.07–8.08) | 5.18 (1.99–13.45) | < 0.001 |
| Model 1 | 1.25 (1.14–1.38) | 1.00 | 3.09 (1.12–8.51) | 5.54 (2.13–14.40) | < 0.001 |
| Model 2 | 1.29 (1.15–1.44) | 1.00 | 2.88 (1.04–8.01) | 5.70 (2.16–15.04) | < 0.001 |
| Model 3 | 1.22 (1.14–1.32) | 1.00 | 2.53 (0.87–7.39) | 5.14 (1.89–13.98) | 0.001 |
| MACCEs | |||||
| No/Subject | 31/897 | 48/911 | 90/932 | ||
| Crude | 1.23 (1.16–1.30) | 1.00 | 1.51 (0.96–2.37) | 2.87 (1.91–4.32) | < 0.001 |
| Model 1 | 1.22 (1.15–1.29) | 1.00 | 1.56 (0.99–2.45) | 2.98 (1.98–4.48) | < 0.001 |
| Model 2 | 1.23 (1.16–1.32) | 1.00 | 1.55 (0.98–2.44) | 3.04 (2.01–4.61) | < 0.001 |
| Model 3 | 1.26 (1.11–1.44) | 1.00 | 1.58 (0.96–2.59) | 2.91 (1.84–4.60) | < 0.001 |
Model 1: adjusted for age, sex, and BMI
Model 2: adjusted for age, sex, BMI, smoking, T2DM, dyslipidemia, hypertension, prior HF, prior MI, prior stroke, peripheral vascular disease, prior revascularization, and AMI
Model 3: adjusted for age, sex, BMI, smoking, HF, T2DM, dyslipidemia, hypertension, prior MI, prior stroke, peripheral vascular disease, prior revascularization, AMI, multivessel disease, ostial lesion, bifurcation, number of lesions ≥ 2, number of stents ≥ 2, procedural success, eGFR, DAPT, statin, and anti-diabetic drug
TyG triglyceride-glucose, CV cardiovascular, eGFR estimated glomerular filtration rate, MI myocardial infarction, MACCEs major adverse cardiovascular cerebral events, SHR stress-hyperglycemia ratio, BMI body mass index, T2DM type 2 diabetes mellitus, HF heart failure, AMI acute myocardial infarction, DAPT dual anti-platelet therapy
aPer one unit refers to “1.00” in the Tyg index and “0.10” in SHR
Fig. 2.
RCS curves for the association of TyG index with the risk of CV death and TVMI A, the risk of MACCEs B, the association of SHR with the risk of CV death and TVMI C, and the risk of MACCEs D RCS Restricted cubic spline, TyG triglyceride-glucose, CV cardiovascular, TVMI target vessel myocardial infarction, MACCEs major adverse CV cerebral events, SHR stress-hyperglycemia ratio
Table 3.
Cox regression models for the association of the combination of Tyg index and SHR with clinical outcomes
| Groups | P for trend | ||||
|---|---|---|---|---|---|
| Low TyG and low SHR | Low TyG and high SHR | High TyG and low SHR | High TyG and high SHR | ||
| CV death and TVMI | |||||
| No/subject | 13/1353 | 7/460 | 7/455 | 20/472 | |
| Crude | 1.00 | 1.55 (0.62–3.89) | 1.60 (0.64–4.00) | 4.49 (2.23–9.02) | < 0.001 |
| Model 1 | 1.00 | 1.67 (0.67–4.19) | 2.01 (0.79–5.08) | 5.09 (2.51–10.29) | < 0.001 |
| Model 2 | 1.00 | 1.96 (0.77–5.00) | 2.35 (0.91–6.08) | 6.40 (2.92–14.05) | < 0.001 |
| Model 3 | 1.00 | 2.24 (0.82–6.13) | 2.42 (0.83–7.06) | 7.16 (2.90–17.69) | < 0.001 |
| MACCEs | |||||
| No/subject | 60/1353 | 26/460 | 19/455 | 64/472 | |
| Crude | 1.00 | 1.26 (0.79–1.99) | 1.02 (0.55–1.55) | 3.28 (2.30–4.66) | < 0.001 |
| Model 1 | 1.00 | 1.29 (0.81–2.05) | 1.05 (0.62–1.76) | 3.54 (2.48–5.06) | < 0.001 |
| Model 2 | 1.00 | 1.38 (0.87–2.2) | 1.16 (0.68–1.96) | 4.07 (2.75–6.02) | < 0.001 |
| Model 3 | 1.00 | 1.48 (0.88–2.47) | 1.25 (0.71–2.19) | 3.62 (2.32–5.64) | < 0.001 |
Model 1: adjusted for age, sex, and BMI
Model 2: adjusted for age, sex, BMI, smoking, T2DM, dyslipidemia, hypertension, prior HF, prior MI, prior stroke, peripheral vascular disease, prior revascularization, and AMI
Model 3: age, sex, BMI, smoking, HF, T2DM, dyslipidemia, hypertension, prior MI, prior stroke, peripheral vascular disease, prior revascularization, AMI, multivessel disease, ostial lesion, bifurcation, number of lesions ≥ 2, number of stents ≥ 2, procedural success, eGFR, DAPT, statin, and anti-diabetic drug
Low TyG index or SHR: T1-2; High Tyg index or SHR: T3
TyG triglyceride-glucose, CV cardiovascular, eGFR estimated glomerular filtration rate, MI myocardial infarction, MACCEs major adverse cardiovascular cerebral events, SHR stress-hyperglycemia ratio, BMI body mass index, T2DM type 2 diabetes mellitus, HF heart failure, AMI acute myocardial infarction, DAPT dual anti-platelet therapy
Fig. 3.
ROC curves for the use of TyG index, SHR, and their combination in predicting the prognosis of CTO patients A for the composite of CV death and TVMI; Fig. 3B: for MACCEs. Optimal cut-off: (1) the composite of CV death and TVMI: TyG, 8.98; SHR: 0.86; (2) MACCE: TyG, 9.07; SHR: 0.88 ROC receiver operating characteristic, AUC an area under the curve, TyG triglyceride-glucose, CV cardiovascular, TVMI target vessel myocardial infarction, MACCEs major adverse CV cerebral events, SHR stress-hyperglycemia ratio
Table 4.
Comparisons among the predictive value of the TyG index, SHR, and combined model for clinical outcomes
| AUC/△AUC | 95% CI | P value | |
|---|---|---|---|
| CV death and TVMI | |||
| AUC combined | 0.682 | 0.609–0.755 | – |
| AUC SHR | 0.659 | 0.582–0.737 | – |
| AUC TyG | 0.623 | 0.582–0.698 | – |
| AUC combined–AUC TyG | 0.058 | − 0.006–0.121 | 0.074 |
| AUC combined–AUC SHR | 0.022 | − 0.015–0.060 | 0.247 |
| AUC SHR– AUC TyG | 0.036 | − 0.057–0.128 | 0.452 |
| MACCEs | |||
| AUC combined | 0.633 | 0.589–0.676 | – |
| AUC SHR | 0.624 | 0.580–0.668 | – |
| AUC TyG | 0.589 | 0.546–0.633 | – |
| AUC combined–AUC TyG | 0.043 | 0.009–0.077 | 0.014 |
| AUC combined–AUC SHR | 0.009 | − 0.013–0.030 | 0.426 |
| AUC SHR– AUC TyG | 0.034 | − 0.084–0.016 | 0.179 |
Optimal cut-off: (1) the composite of CV death and TVMI: TyG, 8.98; SHR: 0.86; (2) MACCE: TyG, 9.07; SHR: 0.88
TyG triglyceride-glucose, CV cardiovascular, TVMI target vessel myocardial infarction, MACCEs major adverse CV cerebral events, SHR stress-hyperglycemia ratio
SHR and the long-term prognosis in CTO patients
As shown in Table 2, CTO patients with high SHR levels presented a significantly higher risk of CV death/TVMI (T3 vs. T1: HR 5.14, 95% CI 1.89–13.98) and MACCEs (T3 vs. T1: HR 2.91, 95% CI 1.84–4.60) than those with low SHR levels. KM plots also confirmed the highest risk of clinical outcomes in CTO patients with the highest SHR tertile (log-rank P < 0.001) (Additional file 1: Figure S1B, E). In addition, the risks of CV death/TVMI and MACCEs were shown to increase by 22% and 26%, respectively, per 0.1 unit increase in SHR (Table 2). RCS curves also showed a positive linear correlation between SHR and CV death/TVMI and MACCEs (P for nonlinearity = 0.591 and 0.946) (Fig. 2C, D). Compared with those with a low SHR, patients with a high SHR also showed an increased risk of all-cause death in the survival analysis (T3 vs. T1: HR 2.79, 95% CI 1.44–5.42) (Additional file 1: Table S5). The AUCs of CV death/TVMI and MACCEs evaluated by the SHR were 0.659 (95% CI 0.582–0.737) and 0.624 (95% CI 0.580–0.668), respectively, and the optimal cut-off value of the SHR was 0.86 for CV death/TVMI and 0.88 for MACCEs (Table 4, Fig. 3). Additional file 1: Table S6 shows that the SHR was positively correlated with TC (R2 0.30, 95% CI 0.04–0.56) and hs-CRP levels (R2 0.94, 95% CI 0.05–1.28) with statistical significance.
The association of the TyG index and SHR with long-term prognosis in CTO patients under different glucose metabolic statuses and procedural outcomes
When analysing patients with different glucose metabolic statuses (NG, prediabetes, and T2DM), we found that the positive association between a high TyG index and elevated long-term risk of clinical outcomes was particularly significant in patients with T2DM (Additional file 1: Table S7). For the SHR, it was shown that the association of a high SHR with increased CV risk was especially significant in patients with prediabetes and T2DM (Additional file 1: Table S7). We further analysed patients with different procedural outcomes (procedural success and failure) and determined that the positive association of both the TyG index and SHR with long-term CV risk was significant regardless of procedural outcomes (Additional file 1: Table S8).
The combination of the TyG index and SHR and the long-term prognosis in CTO patients
In Table 3, we show that patients with both a high TyG index and high SHR (Tertile 3) showed a significantly higher risk of CV death/TVMI (HR 7.16, 95% CI 2.90–17.69) and MACCEs (HR 3.62, 95% CI 2.32–5.64) when compared with those with low levels (Tertiles 1–2). KM plots showed that the group with both a high TyG index and SHR levels (Tertile 3) presented the highest risk of clinical outcomes during the follow-up (Additional file 1: Figure S1C, F). ROC curves of the TyG index-SHR combination showed that its AUCs of CV death/TVMI and MACCEs were 0.682 (95% CI 0.609–0.755) and 0.633 (95% CI 0.589–0.676), respectively (Fig. 3). Comparisons of predictive values of the TyG index, SHR, and TyG index-SHR combination for CV death/TVMI and MACCEs are shown in Table 4. The results showed that the predictive value of the TyG index-SHR combination was higher than that of the TyG index and SHR itself, although statistical significance was only present in the comparison between the TyG index-SHR combination and the TyG index for the risk of MACCEs (△AUC = 0.043, P value = 0.014) (Table 4). Survival analysis showed that patients with elevated levels of both TyG index and SHR exhibited the highest risk of mortality compared to the other groups with varying levels of TyG and SHR (Additional file 1: Table S9).
Subgroup and sensitivity analyses
Subgroup analyses of the association of the TyG index and SHR with clinical outcomes were performed according to age (≥ 60 years old), sex, BMI (≥ 30 kg/m2), smoking status, hyperlipidaemia, hypertension, HF with low LVEF (< 50%), renal dysfunction, and AMI, which showed consistent trends with the main results and no significant interaction in all subgroups (Additional file 1: Table S10–S13). In the sensitivity analysis, we excluded patients who presented clinical events within 90 days, and the association of the TyG index and SHR with clinical outcomes was still significant (Additional file 1: Table S14).
Discussion
The current large-population-based prospective cohort study included 2740 patients with CTO, conducted a clinical follow-up with a median time of 3 years, and investigated the association of the TyG index and SHR with the long-term prognosis. The major findings of this investigation included the following: 1) high Tyg index and SHR levels were related to an increase in the long-term risk of adverse events in CTO patients; 2) the association of the TyG index with high CV risk was especially significant in patients with T2DM, while the association of SHR with high CV risk was significant in patients with both prediabetes and T2DM; 3) the association of the TyG index and SHR with high CV risk was significant regardless of the procedural outcomes; and 4) patients with both high TyG index and SHR showed the worst prognosis among people with different levels of these two biomarkers, and the predictive value of the TyG index-SHR combination for the risk of MACCEs was more significant than the TyG index itself.
The TyG index has been widely demonstrated as a reliable marker to assess insulin resistance with high sensitivity and specificity, and it has been widely applied in clinical practice for its convenience, low cost, and wide-range usage [19]. The association between Tyg index levels and CVD was comprehensively discussed, and its predictive value for a high incidence of CHD has been extensively revealed in previous cohort studies and meta-analyses [20, 21]. Regarding the prognostic value of the TyG index in CHD patients, several cohort studies showed that patients with a high TyG index were independently associated with a high risk of repeat revascularization and hospital mortality [22, 23]. With respect to CTO, the prognostic role of the TyG index in patients with CTO lesions was previously investigated by several studies; however, the sample size of these studies was not satisfactory, and the evidence was not sufficient. In 2022, Li et al. conducted a rigorous retrospective cohort study and reported that a high TyG index was significantly related to an elevated CV risk [9]. Although that was a well-conducted study, there were only 652 CTO patients enrolled [9]. That small sample size might have potentially biased the results. Compared with the study conducted by Li et al. we not only validated the positive association of a high TyG index with poor prognosis in a much larger population (2740 patients) but also revealed the AUC and optimal cut-off of the TyG index in predicting CV adverse events in the current study. In addition, we conducted a subgroup analysis for this association in patients with differing glycaemic status and showed that the association of a high TyG index with poor prognosis was only significant among patients with T2DM, which was consistent with previous studies [9, 24]. Notably, we found that some results of this study differed from those of Li’s study. First, our subgroup analysis showed that the TyG index was significantly correlated with CV risk in patients with both successful and failed PCI procedures, while this correlation was only reported among patients who received successful CTO PCI in Li’s study [9]. Moreover, the RCS curves for the relationship between the TyG index and the risk of CV adverse events in our study showed a positive linear type, while Li et al. reported a “J”-shape relationship for the association between the TyG index and CV risk [9]. This kind of difference might be attributed to the discrepancy in sample size, and it is difficult to compare the accuracy of the conclusions from both studies due to the insufficiency of related research [9]. Therefore, further studies are needed to explore the association between the TyG index and CV risk in CTO patients in large, randomized controlled trials (RCTs).
Another focus of the current study is the association of the SHR with long-term prognosis in patients with CTO lesions. Ample literature suggests that the SHR could better reflect blood glucose status than FBG or HbA1C [5, 25]. Recently, scholars identified the SHR as an independent predictor of high CV risk in patients with CVD, especially for those with acute diseases such as acute coronary disease [26], AMI [27], critical HF [28], and MI with nonobstructive coronary arteries [29]. For chronic diseases, Xu et al. focused on patients with chronic coronary syndrome and reported a significant association of a high SHR with elevated in-hospital mortality, further indicating the potential prognostic value of the SHR in patients without stress conditions [30]. For patients with CTO lesions, there is no research elucidating the association of the SHR with long-term prognosis thus far. Herein, we focused on this point for the first time and revealed that a high SHR was associated with an increase in long-term CV risk in CTO patients undergoing PCI. Interestingly, the association with the SHR seems to be different in CHD patients with differing glycaemic status (NG, prediabetes, and T2DM). Zhang et al. conducted a retrospective cohort with 987 CHD patients and reported that a high SHR correlated with the improved risk of multivessel CHD in the prediabetes and DM groups, but this correlation was not significant in patients with NG [8]. Similar to Zhang’s study, we also determined that the predictive value of the SHR for poor prognosis in patients with CTO lesions was especially significant in those with prediabetes and T2DM. However, we reported different conclusions in a previous investigation, as we found that the SHR was significantly correlated with an increase in 2-year mortality in both diabetic and nondiabetic patients with AMI [31]. This paradox is potentially attributed to the different target diseases in the two studies (AMI and CTO). We speculated that the SHR in a patient with AMI might be mostly indicative of acute disease-related stress, and the stress extent, which was not affected greatly by basal glucose metabolism, had effects on patients with and without diabetes on an equal basis. The SHR in CTO patients was speculated to mostly reflect true glucose metabolism disorders and inflammation burden (as we showed that SHR was significantly correlated with levels of hs-CRP), which were more severe in patients with prediabetes and T2DM. Therefore, the relationship between the SHR and glycaemic status might be different in patients with different diseases. Nevertheless, this hypothesis has not yet been confirmed, and we anticipate that further studies could report more rigorous evidence.
This is the first study to combine the TyG index and the SHR and explore their combined predictive value for poor prognosis in patients with CHD. As the results showed in our study, patients with both a high TyG index and a high SHR presented a high risk of both CV death/TVMI and MACCEs, and the ROC curves showed that the AUC of the TyG index-SHR combination for predicting MACCE risk was larger than that of the TyG index itself. We speculated that this phenomenon was attributed to complementary effects between the TyG index and the SHR, as the TyG index mainly evaluated the extent of insulin resistance and the SHR could additionally reflect inflammation burden and glucose metabolism [32, 33]. However, since we only included patients with CTO lesions and no previous research combined the TyG index and the SHR, the predictive advantage of the TyG index-SHR combination cannot be confirmed in patients with CHD or other CVD diseases. This conclusion is expected to be validated among different populations.
HF and renal disease are recognized as significant factors contributing to the poor prognosis of patients with CTO. Previous studies have demonstrated that CTO patients with HF experience higher in-hospital mortality rates and a greater number of complications [34]. Similarly, renal disease, particularly contrast nephropathy, has been associated with increased mortality in CTO patients [35–37]. Consequently, identifying biomarkers with strong prognostic value in CTO patients with HF or renal disease is of great importance. In this study, we determined that a high TyG index and a high SHR were associated with an increased risk of adverse cardiac events in CTO patients with HF or renal disease, further strengthening the prognostic values of these two biomarkers. It is important to note that the specific type of nephropathy was not recorded in our study, thus limiting our ability to determine the extent to which the prognostic value of these biomarkers applies to CTO patients with contrast nephropathy. Future investigations should aim to address this knowledge gap.
There are several limitations to this study. First, the single-centre enrolment and the Chinese-only population limited the representativeness of this investigation. Second, the dynamic change in the TyG index and the SHR during follow-up was not presented in our study; thus, we are not able to evaluate the impact of changes in these two biomarkers on the prognosis. Third, clinical follow-up for angiographic results was not conducted in this study, which lacked the specific impact of the TyG index and the SHR on coronary lesions. Fourth, although we have adjusted potential confounders as covariates in multivariable regression models, the influence of uncollected confounders cannot be dismissed entirely. Fifth, it is worth noting that our study did not include a follow-up evaluation of the quality of life. Considering that one of the primary objectives of PCI in patients with CTO is to alleviate ischaemic symptoms, such as angina, it would be valuable to investigate the impact of a high TyG index and SHR on the quality of life in future studies. Finally, no randomization was included in our study; therefore, the conclusions of this study warrant validation in future RCTs.
Conclusion
This study, for the first time, confirmed the predictive value of the TyG index and the SHR for poor prognosis in patients with CTO lesions in a large population and verified conclusions in patients with differing glycaemic status and procedural outcomes. In addition, we combined the TyG index and the SHR and found that their combination had a more significant predictive value for CV risk. These findings indicated that the TyG index and the SHR held great prognostic value for patients with CTO lesions and thus supported the use of these two biomarkers as potential tools in identifying patients with high risk and guiding further treatment or follow-up strategies.
Supplementary Information
Additional file 1: Table S1. CV death and target vessel Mi risk according to baseline variables. Table S2. MACCE risk according to baseline variables. Table S3. Baseline patient characteristics grouped by TyG index levels. Table S4. Baseline patient characteristics grouped by SHR levels. Table S5. Survival analysis for the association of the TyG index and SHR with the risk of all-cause death. Table S6. Correlation analysis between the TyG index/SHR and TC, LDL-C, and hs-CRP. Table S7. Cox regression models for the association of TyG index and SHR with clinical outcomes in patients with different glucose statuses. Table S8. Cox regression models for the association of TyG index and SHR with clinical outcomes in patients with different procedural outcomes. Table S9. Survival analysis for the association of the combination of Tyg index and SHR with the risk of all-cause death. Table S10. Subgroup analyses of the associations between TyG index and the risk of the composite of CV death and target vessel MI. Table S11. Subgroup analyses of the associations between TyG index and the risk of MACCEs. Table S12. Subgroup analyses of the associations between SHR and the risk of the composite of CV death and target vessel MI. Table S13. Subgroup analyses of the associations between SHR and the risk of MACCEs. Table S14. Sensitivity analyses of the associations of TyG index and SHR with outcomes after excluding patients who presented clinical events within 90 days (n=2724). Figure S1. Kaplan–Meier curves for the cumulative incidence of clinical outcomes in patients grouped by the TyG index (Figure S1A: CV death and TVMI; Figure S1D: MACCEs), SHR (Figure S1B: CV death and TVMI; Figure S1E: MACCEs), and combination of two ratios (Figure S1C: CV death and TVMI; Figure S1F: MACCEs).
Acknowledgements
Not applicable.
Abbreviations
- AMI
Acute myocardial infarction
- AUC
Reas under the curve
- BMI
Body mass index
- CV
Cardiovascular
- CTO
Chronic total occlusion
- CIs
Confidence intervals
- CABG
Coronary artery bypass grafting
- DAPT
Dual antiplatelet therapy
- eGFR
Estimated glomerular filtration rate
- FBG
Fasting blood glucose
- HF
Heart failure
- HDL-C
High-density lipoprotein cholesterol
- HbA1c
Glycosylated hemoglobin A1c
- HRs
Hazard ratios
- hs CRP
High-sensitivity C-reactive protein
- LVEF
Left ventricular ejection faction
- LDL-C
Low-density lipoprotein cholesterol
- LM
Left main
- MACCEs
Major cardiovascular cerebrovascular adverse events
- PCI
Percutaneous coronary intervention
- PAD
Peripheral artery disease
- RCSs
Restricted cubic splines
- ROC
Receiver operating characteristic
- SHR
Stress hyperglycaemia ratio
- TyG
Triglyceride-glucose
- TVMI
Target vessel myocardial infarction
- T2DM
Type 2 diabetes mellitus
- TC
Total cholesterol
Author contributions
YS, KC, and MY performed study design, researched data, contributed to discussion, and wrote, reviewed, and edited the manuscript. YS and CS acquired the data, revised the manuscript’s intellectual content. DY and QD curated data and figures. YG and KD reviewed and edited the manuscript. All authors approved the final version of the manuscript. YG and KD are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study is supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-I2M-1-008).
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study process was in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Fuwai hospital. All subjects provided informed written consent for long-term follow-up before intervention.
Consent for publication
The manuscript was approved by all authors for publication.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yanjun Song, Kongyong Cui and Min Yang contributed equally to this article.
Kefei Dou and Ying Gao contributed equally as senior authors.
Contributor Information
Ying Gao, Email: cmuxyg@sina.com.
Kefei Dou, Email: drdoukefei@126.com.
References
- 1.Khatri JJ. Clinically meaningful definition of myocardial injury after chronic total occlusion intervention. JACC Cardiovasc Interv. 2019;12(19):1924–1926. doi: 10.1016/j.jcin.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Azzalini L, Karmpaliotis D, Santiago R, Mashayekhi K, Di Mario C, Rinfret S, Nicholson WJ, Carlino M, Yamane M, Tsuchikane E, et al. Contemporary issues in chronic total occlusion percutaneous coronary intervention. JACC Cardiovasc Interv. 2022;15(1):1–21. doi: 10.1016/j.jcin.2021.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Park TK, Lee SH, Choi KH, Lee JM, Yang JH, Song YB, Hahn JY, Choi JH, Gwon HC, Lee SH, et al. Late survival benefit of percutaneous coronary intervention compared with medical therapy in patients with coronary chronic total occlusion: A 10-year follow-up study. J Am Heart Assoc. 2021;10(6):e019022. doi: 10.1161/JAHA.120.019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sánchez-García A, Rodríguez-Gutiérrez R, Mancillas-Adame L, González-Nava V, Díaz González-Colmenero A, Solis RC, Álvarez-Villalobos NA, González-González JG. Diagnostic accuracy of the triglyceride and glucose index for insulin resistance: a systematic review. Int J Endocrinol. 2020;2020:4678526. doi: 10.1155/2020/4678526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O'Dea H, Stranks SN, Burt MG, Doogue MP. relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. 2015;100(12):4490–4497. doi: 10.1210/jc.2015-2660. [DOI] [PubMed] [Google Scholar]
- 6.Xiong S, Chen Q, Long Y, Su H, Luo Y, Liu H, Chen Y, Feng Q, Peng X, Jiang M, et al. Association of the triglyceride-glucose index with coronary artery disease complexity in patients with acute coronary syndrome. Cardiovasc Diabetol. 2023;22(1):56. doi: 10.1186/s12933-023-01780-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mone P, Lombardi A, Salemme L, Cioppa A, Popusoi G, Varzideh F, Pansini A, Jankauskas SS, Forzano I, Avvisato R, et al. Stress hyperglycemia drives the risk of hospitalization for chest pain in patients with ischemia and nonobstructive coronary arteries (INOCA) Diabetes Care. 2023;46(2):450–454. doi: 10.2337/dc22-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Song H, Bai J, Xiu J, Wu G, Zhang L, Wu Y, Qu Y. Association between the stress hyperglycemia ratio and severity of coronary artery disease under different glucose metabolic states. Cardiovasc Diabetol. 2023;22(1):29. doi: 10.1186/s12933-023-01759-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, He S, Wu Z, Li W, Jian W, Cheng Z, Wang C, Shi Y, Liu J. The predictive value of the triglyceride-glucose index for cardiovascular events in patients with coronary chronic total occlusion. Cardiovasc Diabetol. 2022;21(1):149. doi: 10.1186/s12933-022-01588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mashaly A, Rha SW, Choi BG, Baek MJ, Ryu YG, Choi SY, Byun JK, Li H, Shim MS, Jang WY, et al. Impact of diabetes mellitus on 5-year clinical outcomes in patients with chronic total occlusion lesions. Coron Artery Dis. 2018;29(2):119–126. doi: 10.1097/MCA.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, Jacques-Camarena O, Rodriguez-Moran M. The product of triglycerides and glucose, a simple measure of insulin sensitivity comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 12.Fu R, Cui K, Yang J, Xu H, Yin D, Song W, Wang H, Zhu C, Feng L, Wang Z, et al. Fasting stress hyperglycemia ratio and in-hospital mortality after acute myocardial infarction in patients with different glucose metabolism status: results from China acute myocardial infarction registry. Diabetes Res Clin Pract. 2023;196:110241. doi: 10.1016/j.diabres.2023.110241. [DOI] [PubMed] [Google Scholar]
- 13.Diabetes American 2 Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018;41(13):27. doi: 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 14.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39(33):3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 15.Rabar S, Harker M, O'Flynn N, Wierzbicki AS, Guideline Development G. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: summary of updated NICE guidance. BMJ. 2014;349:g4356. doi: 10.1136/bmj.g4356. [DOI] [PubMed] [Google Scholar]
- 16.Yang E, Spragg D, Schulman S, Gilotra NA, Kilic A, Salenger R, Whitman G, Metkus TS. Rate versus rhythm control in heart failure patients with post-operative atrial fibrillation after cardiac surgery. J Cardiac Fail. 2021;27(8):915–919. doi: 10.1016/j.cardfail.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang YS, Li YH, Lee IT. A synergistic effect of variability in estimated glomerular filtration rate with chronic kidney disease on all-cause mortality prediction in patients with type 2 diabetes: a retrospective cohort study. Cardiovasc Diabetol. 2021;20(1):209. doi: 10.1186/s12933-021-01399-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Writing Group on the Joint ESCAAHAWHFTFftUDoMI et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 19.Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. 2020;19(1):8. doi: 10.1186/s12933-019-0982-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao LC, Xu JN, Wang TT, Hua F, Li JJ. Triglyceride-glucose index as a marker in cardiovascular diseases: landscape and limitations. Cardiovasc Diabetol. 2022;21(1):68. doi: 10.1186/s12933-022-01511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20(1):76. doi: 10.1186/s12933-021-01268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo X, Shen R, Yan S, Su Y, Ma L. Triglyceride-glucose index for predicting repeat revascularization and in-stent restenosis in patients with chronic coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2023;22(1):43. doi: 10.1186/s12933-023-01779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Shi S, Chen W, Wang Y, Lin X, Zhao Y, Liao L, Guo Q, Zhang X, Li W, et al. Independent effects of the triglyceride-glucose index on all-cause mortality in critically ill patients with coronary heart disease: analysis of the MIMIC-III database. Cardiovasc Diabetol. 2023;22(1):10. doi: 10.1186/s12933-023-01737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin XL, Li QY, Zhao DH, Liu JH, Fan Q. A high triglyceride-glucose index associated with adverse cardiovascular events in patients with type 2 diabetes mellitus and chronic total occlusion after percutaneous coronary intervention. J Invest Med Off Publ Am Fed Clin Res. 2023 doi: 10.1177/10815589231152823. [DOI] [PubMed] [Google Scholar]
- 25.Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, Chirkov YY, Zeitz CJ, Horowitz JD. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49(3):304–310. doi: 10.1016/j.jacc.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 26.Yang J, Zheng Y, Li C, Gao J, Meng X, Zhang K, Wang W, Shao C, Tang YD. The impact of the stress hyperglycemia ratio on short-term and long-term poor prognosis in patients with acute coronary syndrome: insight from a large cohort study in Asia. Diabetes Care. 2022;45(4):947–956. doi: 10.2337/dc21-1526. [DOI] [PubMed] [Google Scholar]
- 27.Xu W, Yang YM, Zhu J, Wu S, Wang J, Zhang H, Shao XH. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: insights from a multi-center observational study. Cardiovasc Diabetol. 2022;21(1):48. doi: 10.1186/s12933-022-01479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y, Liu L, Huang H, Li N, He J, Yao H, Tang X, Chen X, Zhang S, Shi Q, et al. 'Stress hyperglycemia ratio and in-hospital prognosis in non-surgical patients with heart failure and type 2 diabetes. Cardiovasc Diabetol. 2022;21(1):290. doi: 10.1186/s12933-022-01728-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdu FA, Galip J, Qi P, Zhang W, Mohammed AQ, Liu L, Yin G, Mohammed AA, Mareai RM, Jiang R, et al. Association of stress hyperglycemia ratio and poor long-term prognosis in patients with myocardial infarction with non-obstructive coronary arteries. Cardiovasc Diabetol. 2023;22(1):11. doi: 10.1186/s12933-023-01742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu W, Song Q, Wang X, Zhao Z, Meng X, Xia C, Xie Y, Yang C, Guo Y, Zhang Y, et al. Association of stress hyperglycemia ratio and in-hospital mortality in patients with coronary artery disease: insights from a large cohort study. Cardiovasc Diabetol. 2022;21(1):217. doi: 10.1186/s12933-022-01645-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cui K, Fu R, Yang J, Xu H, Yin D, Song W, Wang H, Zhu C, Feng L, Wang Z, et al. Stress hyperglycemia ratio and long-term mortality after acute myocardial infarction in patients with and without diabetes: a prospective, nationwide, and multicentre registry. Diabetes Metab Res Rev. 2022;38(7):e3562. doi: 10.1002/dmrr.3562. [DOI] [PubMed] [Google Scholar]
- 32.Jeong S, Lee JH. The verification of the reliability of a triglyceride-glucose index and its availability as an advanced tool. Metab Off J Metab Soc. 2021;17(11):97. doi: 10.1007/s11306-021-01837-9. [DOI] [PubMed] [Google Scholar]
- 33.Esposito K, Marfella R, Giugliano D. Stress hyperglycemia, inflammation, and cardiovascular events. Diabetes Care. 2003;26(5):1650–1651. doi: 10.2337/diacare.26.5.1650-a. [DOI] [PubMed] [Google Scholar]
- 34.Tajstra M, Pyka Ł, Gorol J, Pres D, Gierlotka M, Gadula-Gacek E, Kurek A, Wasiak M, Hawranek M, Zembala MO, et al. Impact of chronic total occlusion of the coronary artery on long-term prognosis in patients with ischemic systolic heart failure: insights from the COMMIT-HF registry. JACC Cardiovasc Interv. 2016;9(17):1790–1797. doi: 10.1016/j.jcin.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Güzel T, Aktan A, Demir M, Özbek M, Aslan B. Relationship between contrast-induced nephropathy and long-term mortality after percutaneous coronary intervention in patients with chronic coronary total occlusion. Rev Assoc Med Bras. 2022;68(8):1078–1083. doi: 10.1590/1806-9282.20220283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aktürk E, Aşkın L, Taşolar H, Kurtoğlu E, Türkmen S, Tanrıverdi O, Uzel KE. Evaluation of contrast nephropathy in percutaneous treatment of chronic total occlusions. Int Med Appl Sci. 2019;11(2):95–100. doi: 10.1556/1646.11.2019.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee WC, Wu PJ, Fang CY, Chen HC, Wu CJ, Fang HY. Impact of chronic kidney disease on chronic total occlusion revascularization outcomes: a meta-analysis. J Clin Med. 2021 doi: 10.3390/jcm10030440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. CV death and target vessel Mi risk according to baseline variables. Table S2. MACCE risk according to baseline variables. Table S3. Baseline patient characteristics grouped by TyG index levels. Table S4. Baseline patient characteristics grouped by SHR levels. Table S5. Survival analysis for the association of the TyG index and SHR with the risk of all-cause death. Table S6. Correlation analysis between the TyG index/SHR and TC, LDL-C, and hs-CRP. Table S7. Cox regression models for the association of TyG index and SHR with clinical outcomes in patients with different glucose statuses. Table S8. Cox regression models for the association of TyG index and SHR with clinical outcomes in patients with different procedural outcomes. Table S9. Survival analysis for the association of the combination of Tyg index and SHR with the risk of all-cause death. Table S10. Subgroup analyses of the associations between TyG index and the risk of the composite of CV death and target vessel MI. Table S11. Subgroup analyses of the associations between TyG index and the risk of MACCEs. Table S12. Subgroup analyses of the associations between SHR and the risk of the composite of CV death and target vessel MI. Table S13. Subgroup analyses of the associations between SHR and the risk of MACCEs. Table S14. Sensitivity analyses of the associations of TyG index and SHR with outcomes after excluding patients who presented clinical events within 90 days (n=2724). Figure S1. Kaplan–Meier curves for the cumulative incidence of clinical outcomes in patients grouped by the TyG index (Figure S1A: CV death and TVMI; Figure S1D: MACCEs), SHR (Figure S1B: CV death and TVMI; Figure S1E: MACCEs), and combination of two ratios (Figure S1C: CV death and TVMI; Figure S1F: MACCEs).
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.