Abstract
Background:
Global coverage with the third dose of diphtheria–tetanus–pertussis vaccine among children under 1 year of age stagnated at ~ 83–84% during 2008–13.
Sources of data:
Annual World Health Organization and UNICEF-derived national vaccination coverage estimates.
Areas of agreement:
Incomplete vaccination is associated with poor socioeconomic status, lower education, non-use of maternal-child health services, living in conflict-affected areas, missed immunization opportunities and cancelled vaccination sessions.
Areas of controversy:
Vaccination platforms must expand to include older ages including the second year of life. Immunization programmes, including eradication and elimination initiatives such as those for polio and measles, must integrate within the broader health system.
Growing points:
The Global Vaccine Action Plan (GVAP) 2011–20 is a framework for strengthening immunization systems, emphasizing country ownership, shared responsibility, equity, integration, sustainability and innovation.
Areas timely for developing research:
Immunization programmes should identify, monitor and evaluate gaps and interventions within the GVAP framework.
Keywords: immunization, vaccination, routine immunization, Expanded Programme on Immunization, vaccination coverage, global health
Introduction
Building on the momentum generated by smallpox eradication efforts, the World Health Organization (WHO) launched the Expanded Programme on Immunization (EPI) in 1974.1 At that time, <5% of the world’s children were vaccinated against six vaccine-preventable diseases: diphtheria, pertussis, tetanus, measles, poliomyelitis and tuberculosis. The goal of the EPI was to protect every child against these high-incidence diseases, for which affordable vaccines were available. Although some infrastructure was in place as a result of smallpox eradication efforts, many countries needed to develop and establish routine immunization systems to effectively implement the EPI.
A successful routine immunization system requires the synchronization of multiple programme components to provide a child the opportunity to be successfully vaccinated. Vaccines must be procured, and successfully delivered to the service delivery level, while constantly maintained through a functioning cold chain. Health workers must be trained in vaccine management, handling and administration; data recording and reporting and appropriate interaction with caregivers of young children. Creating community demand for immunization is critical to ensuring that caregivers value vaccination, and know when and where to bring their children to be vaccinated. The overall coordination, management and implementation of these activities require political support, sustained financing, supervision and the appropriate monitoring and use of high-quality data.
Monitoring immunization system performance
As routine immunization systems became established globally in the 1970s and 1980s, standardized indicators of immunization programme performance were established and monitored. Multiple indicators are necessary to monitor the various components of the programme and to assess overall programme performance (Table 1).2 Globally, vaccination coverage, which is calculated as the percentage of persons in the target age group who received a particular vaccine dose by a specified age, is the indicator most closely followed. Within an individual immunization programme, coverage with different vaccines and with different doses of the same vaccine may vary, and coverage with each vaccine dose reflects different attributes of the programme. For example, coverage with the first diphtheria–tetanus–pertussis (DTP) vaccine dose (DTP1) is an indicator of access to health care services; whereas coverage with the third DTP dose (DTP3) reflects the ability of a family both to access and utilize immunization services on multiple visits. Therefore, DTP3 coverage is widely accepted as the standardized indicator of immunization programme performance. The first dose of measles-containing vaccine (MCV1) is closely followed because it historically has been the last childhood vaccine offered, although new vaccines have been introduced into immunization programmes, more vaccines are being recommended during the second year of life and later. The percentage of fully vaccinated children (A child vaccinated with all recommended vaccines during the first year of life.), another indicator of immunization programme performance, is also closely followed.
Table 1.
Indicators that can be used to monitor immunization programme performance2
| Programme component | Indicators |
|---|---|
| Programme outputs | Fully vaccinated children—proportion of children aged 12–23 months who have received the following:
|
| Equity | Percentage of districts with ≥80% DTP3 coverage in infants* |
| Equity | Percentage of districts with ≥90% MCV1 coverage in infants* |
| Service delivery | Percentage of planned outreach sessions that were conducted on schedule |
| Percentage of planned fixed site sessions that were conducted on schedule | |
| Access to services | Up-to-date BCG, DTP1/OPV1 and HepB (if in country’s schedule) by age 2 months |
| Tracking activities | ‘Dropout’: difference in percentage receiving DTP1/OPV1 and DTP3/OPV3 or measles vaccine |
| Use of all opportunities | Percentage of children receiving all vaccines for which they are eligible at each visit |
| Safety | Proportion of districts that have been supplied with adequate (equal or more) number of auto-disable syringes for all routine immunizations during the year* |
| Logistics and cold chain | Proportion of districts that had no interruption in vaccine supply* |
| Percentage of facilities storing vaccines at recommended temperatures | |
| Transport | Kilometres/vehicle or motorbike/month (high km = high utilization) |
| Percentage of use for service delivery and service delivery support (higher = more effective) | |
| Policy of PPM and percentage of PPM activities conducted | |
| Full cost per km (low cost = more efficient use of vehicles/motorbikes) | |
| Surveillance/monitoring | Proportion of district disease surveillance reports received at national level* |
| Proportion of district coverage reports received at national level* | |
| Management and supervision | Country has 5-year immunization plan |
| Proportion of districts having microplans (annual work plans) that include immunization activities* | |
| Proportion of districts that did at least one supervisory visit to all health facilities in last year* | |
| Provider knowledge | Proportion of providers who know and follow recommended guidelines, including those for simultaneous administration, contraindications and safe injection procedures |
BCG, bacilli Calmette–Guérin; DTP, diphtheria and tetanus toxoids and whole cell pertussis vaccine; HepB, hepatitis B vaccine; Hib, Haemophilus influenzae type b vaccine; HPV, human papillomavirus vaccine; IPV, inactivated poliovirus vaccine; MCV, measles-containing vaccine; OPV, oral poliovirus vaccine; PPM, planned preventive maintenance.
Reported annually to WHO-UNICEF via WHO-UNICEF Joint Reporting Form.
Pentavalent vaccine = DTP + HepB + Hib.
To be valid, a dose must have a minimum age at first dose, 6 wk; minimum interval, 4 wk.
Vaccination coverage can be estimated using a number of different methods. The administrative method estimates coverage as the aggregated number of doses of a specific vaccine dose administered to children in the target age group through routine immunization services, divided by the estimated target population. Administrative coverage estimates are convenient and timely, but may overestimate or underestimate coverage if there are inaccuracies in the numerator (i.e. number of doses administered) or the denominator (i.e. census data). Immunization coverage surveys estimate vaccination coverage by visiting a representative sample of households to identify children in the target age group, and transcribing dates of vaccination from children’s vaccination cards or asking caregivers to report whether the child received a particular vaccine dose. Coverage surveys are not dependent on knowing target population size, nor are they subject to some limitations of administrative data sources (e.g. dependency on denominator data); however, they are costly and do not provide timely information to guide programmes. In addition, coverage survey results for multi-dose antigens are increasingly subject to bias as prevalence of vaccination cards in the home declines and reliance on maternal recall for more vaccines and multiple doses increases.3
WHO and UNICEF derive national vaccination coverage estimates through an annual country-by-country review of all available data, including administrative and survey-based coverage; as new data are incorporated, revisions of past coverage estimates and updates are published on their websites.4 These estimates are used to monitor international goals for vaccination coverage and for assessments of global vaccination coverage.
Global vaccination coverage: 1974–2013
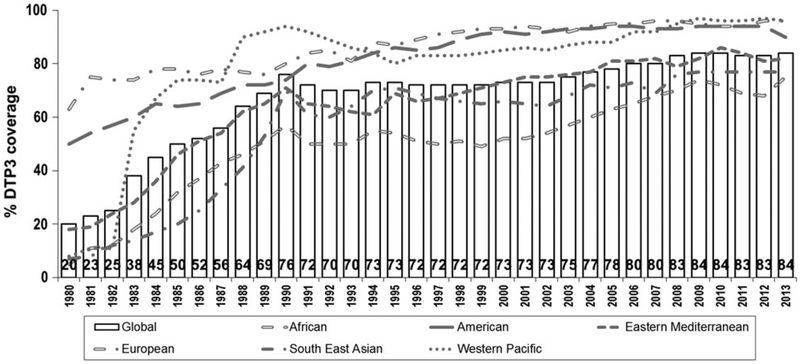
After the EPI was established in 1974, coverage began to increase, although DTP3 coverage among children <1 year of age had only reached 20% by 1980.5 Global DTP3 coverage did improve to 84% by 2013 but at a very non-linear rate (Fig. 1).5 During the 1980s, as infrastructure improved for EPI globally, and as countries were able to capitalize on the momentum generated by the successes of smallpox eradication and by the adoption of the Universal Childhood Immunization initiative, DTP3 coverage rapidly increased to 76% by 1990.6 During the 1990s, however, global immunization coverage stagnated, and by 2000, DTP3 coverage worldwide was 73%.5 Many factors likely contributed to this lack of progress in vaccination coverage, including reduced funding of immunization programmes, decreased prioritization of immunizations within national health programmes and increased decentralization leading to diminished influence and authority of Ministries of Health towards national immunization programmes.7 Since 2000, there has been renewed global emphasis on immunization, with funding support from sources such as Gavi, the Vaccine Alliance, a global public and private sector alliance dedicated to creating equal access to new and underutilized vaccines for children in the world’s poorest countries, and the Bill and Melinda Gates Foundation and by the development of the Global Immunization and Vaccine Strategy (GIVS) for 2006–15, which outlined strategies to build on past achievements to immunize more people against more diseases, introduce new vaccines and technologies and integrate other health interventions at immunization contacts. DTP3 coverage improved during the first decade of the millennium to peak at 84%. However, from 2008 to 2013, DTP3 coverage has stagnated at ~83–84%, leaving 22.6 million children or nearly one-fifth of the world’s children vulnerable to vaccine-preventable diseases in 2013.
Fig. 1.
Global DTP3 coverage, 1980–2013.5 Source: WHO/UNICEF coverage estimates 2013 revision. July 2014. Immunization Vaccines and Biologicals (IVB), World Health Organization. DTP3 = third dose of diphtheria–tetanus–pertussis containing vaccine.
Current global vaccination coverage estimates
Although an estimated 84% of children <1 year of age—111.8 million—received three doses of DTP vaccine in 2013 globally, substantial geographic variability in coverage8 can obscure important areas of low vaccination coverage, particularly in countries with high disease burden. Among the six geographic regions of WHO, DTP3 coverage was as high as 90, 96 and 96% in the American, European and Western Pacific Regions, respectively, but was only 82, 77 and 75% in the Eastern Mediterranean, South East Asian and African Regions, respectively.8 Among the 21.8 million children globally who did not receive 3 DTP doses during the first year of life, 14.8 million (68%) lived in 10 countries (India, Nigeria, Pakistan, Ethiopia, Democratic Republic of Congo, Indonesia, Vietnam, Mexico, South Africa and Kenya); more significantly, 10.9 million (50%) of these children lived in just 3 countries: India, Nigeria and Pakistan.8 Although 129 (66%) countries achieved ≥90% national DTP3 coverage in 2012, only 56 (29%) achieved ≥80% DTP3 coverage in every district,8 highlighting the need to reduce coverage disparities within countries. This disconnect between the national immunization coverage and subnational coverage highlights the current challenge faced by countries, which must address inequity between subnational levels to improve national coverage and to improve herd immunity.
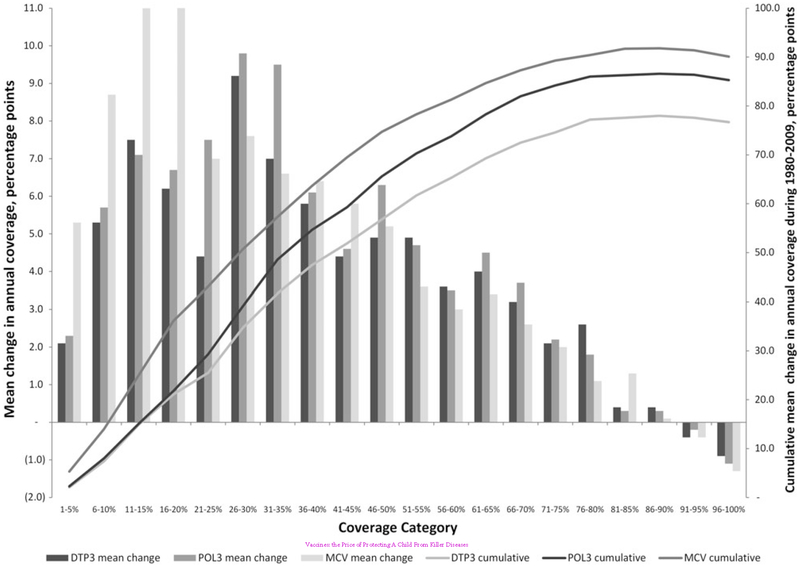
The rates of change in global vaccination coverage over time are consistent with patterns observed at regional and national levels. Historically, the rate of coverage increase at national and regional levels tends to be non-linear (Fig. 2).9 Marginal increases at the lowest coverage levels occur when infrastructure for immunization systems, such as supply chains and human resources, tends to be poor. As infrastructure improves, more rapid increases in coverage tend to be observed, but the rate of improvement eventually slows especially when coverage exceeds 80%. This slow rate of improvement may represent programmes experiencing diminishing returns and reaching a saturation point in the effectiveness of existing strategies to reach all children and complete their vaccination series. Although this analysis reflects trends at national levels, and is seen for all routinely recommended vaccines, a similar saturation point may have been reached at the global level.
Fig. 2.
Mean and cumulative absolute annual rate of change in global vaccination coverage, by coverage category, 1980–2009.10 MCV, measles-containing vaccine; POL3, third dose of polio vaccine; DTP3, third dose of diphtheria–tetanus–pertussis containing vaccine. Source: Wallace AS, Ryman TK, Dietz V. Overview of global, regional and national routine vaccination coverage trends and growth patterns from 1980 to 2009: implications for vaccine-preventable disease eradication and elimination initiatives. J Infect Dis 2014;210(Suppl. 1):S514–22. 180 × 127 mm (300 × 300 DPI).
Factors affecting changes in coverage
Many factors contributed to improvements in vaccination coverage during the first decade of the millennium.10 In addition to the GIVS, the increase and improvement in national multi-year immunization plans have led to improved national planning. Improvements in national budget lines for immunization, from domestic sources, as well as external sources such as Gavi have led to improved financing for immunization services and operations. In areas with poor infrastructure and coverage, periodic intensification of routine immunization (PIRI) activities, which use mass vaccination campaign-like features to deliver routine immunizations, has demonstrated efficacy in reaching hard-to-reach populations and in raising vaccination coverage rapidly.11,12 However, unless health care infrastructure is improved, or campaigns are repeated, gains in coverage levels may not be sustained. Little information is available on whether PIRIs strengthen or hamper the development of routine services. To be successful, PIRIs require a well-coordinated and planned effort with the identification of specific goals, intensive social promotion and strong management.13
Another element in the improvement in global coverage during the early 2000s was the improved implementation and management of immunization programmes, particularly at local levels. In 2002, global immunization partners including WHO and UNICEF developed the Reaching Every District (RED) strategy to strengthen immunization systems through a more localized approach, particularly in areas with low coverage. The strategy includes five components meant to be implemented as a package to strengthen immunization systems at the district level. The components include re-establishment of regular outreach services, use of supportive supervision for on-site training, community links with service delivery, monitoring and use of data for action and better planning and management of human and financial resources. Soon after the development of the RED strategy, GIVS endorsed RED as an operational framework to be implemented at local levels. Although the impact of the RED strategy is difficult to evaluate, evidence suggests that implementation has led to improvement in the delivery of routine immunization services.10,14–18
Current challenges
Immunization programmes have become increasingly more complex and expensive since establishment of the EPI in 1974. Initially, EPI’s focus was on six vaccine-preventable diseases; however, WHO now recommends that all countries provide vaccinations against six additional pathogens: hepatitis B virus, Haemophilus influenzae type b, pneumococcus, rota-virus, rubella virus and human papillomavirus.19 The growth of EPI has led to a 27-fold increase in the price of purchasing a full vaccination course for a child in a Gavi-eligible country from $1.37 for the traditional EPI vaccines to $38.80 for all recommended vaccines in 2011.20 This price does not even include other programmatic costs, or costs associated with vaccine wastage.
With the rapid rise in cost to fully vaccinate a child, the need for more precise monitoring and improved data quality has emerged, leading to demand for larger scale coverage surveys with increased precision and cost. At the outset, EPI programmes focused on vaccinating children <1 year old. With recommendations for booster doses of some vaccines and the use of newer vaccines such as human papillomavirus vaccine (HPV), immunization programmes have had to expand their target populations to include children in older age groups. For example, the second dose of measles vaccine is typically recommended during the second year of life, and HPV is recommended for school-aged children. This has led to the implementation of additional strategies to access children in older age groups including the use of school entry requirements as well as school-based platforms to administer vaccines. But administering vaccines to children beyond the first year of life has also led to challenges in monitoring coverage. The low prevalence of retained vaccination cards in some countries makes it difficult to estimate immunization coverage with surveys; these obstacles will be further exacerbated when cards need to be retained for much longer to estimate coverage in older age groups. In addition, the multiplicity of vaccines and vaccine doses makes care-giver recall bias an increasing problem in obtaining accurate information.
The increase in the number of recommended vaccines has led to increased challenges for the entire routine immunization system, especially related to supply chain, logistics and cold chain requirements, as well as caregiver concern about multiple injections. The use of some multivalent and combined vaccines (e.g. pentavalent vaccine consisting of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenza type B vaccines) has somewhat alleviated this concern, but as new vaccines are developed and introduced, there will continue to be an increase in the number of recommended injections.
Because immunization programmes generally achieve the highest coverage among preventive health interventions, increased efforts have been made to integrate immunization service delivery with other health interventions such as bed net distribution, deworming and vitamin A supplementation. Integration of multiple health interventions can theoretically lead to improved coverage with all the interventions;21 however, delivery of additional public health measures along with vaccines does not always translate into higher coverage for vaccines or the other intervention. Experience has shown that there can be problems with logistics, and the added interventions can place an increased burden on resource-limited health systems. There is also a need for better communication with caregivers.22–28
Substantial efforts have been made to identify reasons for a child not starting or completing the vaccination series. Reviews of the published literature, the grey literature and of large national level coverage surveys (Demographic Health Surveys or Multiple Indicators Cluster Surveys) identified some family and health service characteristics associated with non-vaccination and under-vaccination.2,29–31 Family characteristics commonly reported as being associated with not vaccinating or under-vaccinating a child include poor socioeconomic status, poor education (especially of the mother) and lack of use of maternal-child health services. Living in or recently having migrated from conflict-affected areas has also been reported as a risk factor for under-vaccination in many settings, and will likely continue to contribute to suboptimal vaccination coverage in the coming years. Health service barriers commonly reported as being associated with not vaccinating or under-vaccinating a child include missed opportunities and cancellation of planned sessions. Missed opportunities, defined as any contact with a health service that did not result in an eligible child or woman receiving a needed vaccine, may result from vaccine stock-outs, concern about wastage, concern about multiple injections or health care workers’ misunderstandings about vaccine contraindications. Reasons for cancellation of planned sessions may include transport failure, cold chain failure, vaccine stock-outs and staff absences.
Moving forward
Multiple interventions and strategies within a given programme may be necessary to improve the routine immunization system. Within countries, increased resources are needed for the subnational areas (e.g. provinces, states, districts) with the largest number of unimmunized children and the ones with lowest coverage. However, such prioritization is highly dependent on the use of high quality data, which are often limited in developing countries. In such settings, improving data quality will be a necessary requirement to improve vaccination coverage.
In 2012, the World Health Assembly approved the Global Vaccine Action Plan (GVAP) 2011–20, the next global framework for strengthening routine childhood immunization programmes. The guiding principles of the GVAP are country ownership, shared responsibility, equity, integration, sustainability and innovation. At a global level, coordinated strategies to improve routine immunization systems and vaccination coverage will need to be linked to the GVAP framework. The GVAP highlights the key issues that will need to be addressed for routine immunization systems to improve in the coming years.
Country ownership is currently a large barrier in several countries, particularly many of the priority countries with the largest number of unimmunized children. In these settings, poor national planning combined with weak capacity for developing evidence-based national policies has led to poor management of national immunization programmes.
Shared responsibility refers to individuals and communities needing to understand the value of vaccines and demand immunization as both their right and responsibility. As the number of recommended vaccines has increased, caregiver concern about multiple injections has increased. In addition, there are groups that oppose immunization for a variety of philosophical and political reasons. The number of ‘vaccine-hesitant’ individuals—those who may refuse some vaccines, but agree to others, delay vaccines or reluctantly accept vaccines32—has increased in both developed and developing countries. Improving communications and stimulating demand will become increasingly important activities to overcome vaccine hesitancy and improve vaccination coverage.
In the coming years, improving equity will become a key issue for improving routine vaccination coverage, and hard-to-reach and marginalized populations will become areas of focus for immunization services. Better tools for identifying, mapping and tracking these populations will be critical for immunization programmes to improve. Humanitarian emergencies, including those from natural disasters and civil strife, have become formidable in recent years, including violence directed at vaccinators in some areas,33 and will continue to be a major barrier to improving vaccination coverage. Current strategies such as RED, which aim to improve programme performance at local levels will need to further refine and identify inequities within individual districts, and hence evolve into a ‘Reaching Every Community’ strategy. In addition, equity among age groups will become an essential issue for improving coverage with many vaccines. Over the last 40 years, the EPI has successfully established the first year of life as a platform for vaccination, but for improvements in vaccination coverage and routine immunization systems to occur, the platform needs to be expanded to older age groups. HPV vaccination already requires a vaccination platform in pre-adolescent years while malaria and dengue vaccines that may become prequalified in the near future will likely require vaccination in the second year of life. The efforts and resources needed to expand the age platform should benefit the entire EPI programme by also creating opportunity to catch up children with vaccines provided in the first year of life such as MCV1 and DTP3 and by providing opportunities for the second dose of MCV and booster doses of DTP.
Further integration of immunization programmes will need to continue to evolve to maximize the impact of services in resource-limited settings. Within immunization programmes, eradication and elimination initiatives, such as those for polio and measles, need to be integrated within routine immunization systems. The Polio Eradication and Endgame Strategic Plan 2013–18 places renewed emphasis on strengthening routine immunization. As the Global Polio Eradication Initiative transitions human resources to routine immunization activities, routine immunization programmes worldwide will be introducing inactivated polio vaccine to facilitate polio eradication.34 And within the overall health system, immunization programmes need to work with other health sectors to improve integration of surveillance and monitoring systems, health worker capacity and logistics. Most integration studies to date have focused on trying to improve coverage with other interventions while they are integrated with the more established infrastructure of immunization programmes. Future work and research is needed to find opportunities to also improve vaccination coverage through integration with other programmes such as antenatal programmes. More importantly, integration efforts will need to focus more broadly towards health system strengthening rather than on specific health interventions. A weak public health system has contributed to the rapid dissemination of ebola virus in Liberia, Sierra Leone and Guinea. However, when the Ebola virus was imported into Nigeria via Lagos, a densely populated urban centre, a swift public health response based on the infrastructure of Nigeria’s polio eradication programme led to rapid control and end of the outbreak.35,36
An essential component of improving vaccination coverage is ensuring that success is sustainable especially in terms of access to financing and quality supply. As the costs to fully vaccinate a child continue to increase at a substantial rate, the need for national programmes to increase their own financial commitments will be necessary. There will still be a need for external sources such as Gavi to provide financial assistance to lower income countries. However, middle income countries that are not eligible for Gavi support face enormous challenges, and will require more innovative financing mechanisms.
Finally, innovation is required to help improve immunization programmes and, ultimately, vaccination coverage. Research and development at the global level are needed to develop new vaccines and to improve vaccine delivery systems, with such strategies as microneedle patches, more combination vaccines and reduced dependence on needles and cold chain. At more operational levels, innovative means to implement and manage immunization programmes, through improved use of information systems and mobile technologies, will be necessary to improve efficiencies.
Conclusion
Although global vaccination coverage has substantially improved from <5% of children vaccinated in 1974 to 84% DTP3 coverage in 2013, vast challenges remain to further improve global vaccination coverage. Despite the increasing complexities and cost of modern day immunization systems, opportunities exist to strengthen routine immunization systems with increased funding opportunities and global partnerships. For global vaccination coverage to improve sustainably, investments and efforts will be required in multiple areas, and will need to be tailored to the specific needs of individual programmes.
Acknowledgements
The authors would like to acknowledge Jacqueline Gindler for her help in reviewing and editing this manuscript.
Footnotes
Conflict of Interest statement
The authors have no potential conflicts of interest.
References
- 1.Chan M Beyond expectations: 40 years of EPI. Lancet 2014;383:1697–8. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell V, Dietz V, Okwo-Bele JM, et al. Immunization in developing countries. In: Plotkin S, Orenstein W, Offit W (eds). Vaccine, 6th edn. Amsterdam: Elsevier Saunders, 2013, 1369–94. [Google Scholar]
- 3.Cutts FT, Izurieta HS, Rhoda DA. Measuring coverage in MNCH: design, implementation, and interpretation challenges associated with tracking vaccination coverage using household surveys. PLoS Med 2013;10:e1001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton A, Monasch R, Lautenbach B, et al. WHO and UNICEF estimates of national infant immunization coverage: methods and processes. Bull World Health Organ 2009;87:535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO/UNICEF. WHO and UNICEF estimates of coverage: 2013 revision. http://www.who.int/immunization/monitoring_surveillance/data/en/ (15 January 2015, date last accessed).
- 6.Okwo-Bele JM, Cherian T. The expanded programme on immunization: a lasting legacy of smallpox eradication. Vaccine 2011;29(Suppl. 4):D74–9. [DOI] [PubMed] [Google Scholar]
- 7.Cutts FT. Advances and challenges for the expanded programme on immunization. Br Med Bull 1998;54:445–61. [DOI] [PubMed] [Google Scholar]
- 8.Harris JB, Gacic-Dobo M, Eggers R, et al. Global routine vaccination coverage, 2013. MMWR Morb Mortal Wkly Rep 2014;63:1055–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Wallace AS, Ryman TK, Dietz V. Overview of global, regional, and national routine vaccination coverage trends and growth patterns from 1980 to 2009: implications for vaccine-preventable disease eradication and elimination initiatives. J Infect Dis 2014;210(Suppl. 1):S514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duclos P, Okwo-Bele JM, Gacic-Dobo M, et al. Global immunization: status, progress, challenges and future. BMC Int Health Hum Rights 2009;9(Suppl. 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliphant NP, Mason JB, Doherty T, et al. The contribution of child health days to improving coverage of periodic interventions in six African countries. Food Nutr Bull 2010;31:S248–63. [DOI] [PubMed] [Google Scholar]
- 12.Ryman TK, Trakroo A, Ekka JB, et al. Contribution of immunization weeks toward improving coverage, access to services, and completion of recommended childhood vaccinations in Assam, India. Vaccine 2012;30:2551–5. [DOI] [PubMed] [Google Scholar]
- 13.Dietz V, Cutts F. The use of mass campaigns in the expanded program on immunization: a review of reported advantages and disadvantages. Int J Health Serv 1997;27:767–90. [DOI] [PubMed] [Google Scholar]
- 14.Enkhtuya B, Badamusuren T, Dondog N, et al. Reaching every district—development and testing of a health micro-planning strategy for reaching difficult to reach populations in Mongolia. Rural Remote Health 2009;9:1045. [PubMed] [Google Scholar]
- 15.Ryman T, Macauley R, Nshimirimana D, et al. Reaching every district (RED) approach to strengthen routine immunization services: evaluation in the African region, 2005. J Public Health (Oxf) 2010;32:18–25. [DOI] [PubMed] [Google Scholar]
- 16.Ryman TK, Elsayed EA, Mustafa AA, et al. Implementation of the reaching every district (RED) approach: experience from North Sudan. East Mediterr Health J 2011;17:804–12. [DOI] [PubMed] [Google Scholar]
- 17.Ryman TK, Trakroo A, Wallace A, et al. Implementation and evaluation of the Reaching Every District (RED) strategy in Assam, India, 2005–2008. Vaccine 2011;29: 2555–60. [DOI] [PubMed] [Google Scholar]
- 18.Vandelaer J, Bilous J, Nshimirimana D. Reaching Every District (RED) approach: a way to improve immunization performance. Bull World Health Organ 2008;86:A–B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. Immunization, Vaccines and Biologicals: WHO recommendations for routine immunization—summary tables volume 2014. http://www.who.int/immunization/policy/immunization_tables/en/ (15 January 2015, date last accessed).
- 20.Campaign MSFA. Vaccines: the Price of Protecting A Child From Killer Diseases. Geneva, Switzerland: Médecins Sans Frontières, 2012,1–4. [Google Scholar]
- 21.Anand A, Luman ET, O’Connor PM. Building on success—potential to improve coverage of multiple health interventions through integrated delivery with routine childhood vaccination. J Infect Dis 2012;205 (Suppl. 1):S28–39. [DOI] [PubMed] [Google Scholar]
- 22.Goodson JL, Finkbeiner T, Davis NL, et al. Evaluation of using routine infant immunization visits to identify and follow-up HIV-exposed infants and their mothers in Tanzania. J Acquir Immune Defic Syndr 2013;63:e9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryman TK, Briere EC, Cartwright E, et al. Integration of routine vaccination and hygiene interventions: a comparison of 2 strategies in Kenya. J Infect Dis 2012;205 (Suppl. 1):S65–76. [DOI] [PubMed] [Google Scholar]
- 24.Ryman TK, Wallace A, Mihigo R, et al. Community and health worker perceptions and preferences regarding integration of other health services with routine vaccinations: four case studies. J Infect Dis 2012;205(Suppl. 1): S49–55. [DOI] [PubMed] [Google Scholar]
- 25.Wallace A, Dietz V, Cairns KL. Integration of immunization services with other health interventions in the developing world: what works and why? Systematic literature review. Trop Med Int Health 2009;14:11–9. [DOI] [PubMed] [Google Scholar]
- 26.Wallace A, Kimambo S, Dafrossa L, et al. Qualitative assessment of the integration of HIV services with infant routine immunization visits in Tanzania. J Acquir Immune Defic Syndr 2014;66:e8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wallace AS, Ryman TK, Dietz V. Experiences integrating delivery of maternal and child health services with childhood immunization programs: systematic review update. J Infect Dis 2012;205(Suppl. 1):S6–19. [DOI] [PubMed] [Google Scholar]
- 28.Partapuri T, Steinglass R, Sequeira J. Integrated delivery of health services during outreach visits: a literature review of program experience through a routine immunization lens. J Infect Dis 2012;205(Suppl. 1):S20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rainey JJ, Watkins M, Ryman TK, et al. Reasons related to non-vaccination and under-vaccination of children in low and middle income countries: findings from a systematic review of the published literature, 1999–2009. Vaccine 2011;29:8215–21. [DOI] [PubMed] [Google Scholar]
- 30.Bosch-Capblanch X, Banerjee K, Burton A. Unvaccinated children in years of increasing coverage: how many and who are they? Evidence from 96 low- and middle-income countries. Trop Med Int Health 2012;17: 697–710. [DOI] [PubMed] [Google Scholar]
- 31.Favin M, Steinglass R, Fields R, et al. Why children are not vaccinated: a review of the grey literature. Int Health 2012;4:229–38. [DOI] [PubMed] [Google Scholar]
- 32.Larson HJ, Jarrett C, Eckersberger E, et al. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine 2014;32:2150–9. [DOI] [PubMed] [Google Scholar]
- 33.Wassilak SG, Oberste MS, Tangermann RH, et al. Progress toward global interruption of wild poliovirus transmission, 2010–2013, and tackling the challenges to complete eradication. J Infect Dis 2014;210(Suppl. 1): S5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdelwahab J, Dietz V, Eggers R, et al. Strengthening the partnership between routine immunization and the Global Polio Eradication Initiative to achieve eradication and assure sustainability. J Infect Dis 2014;210(Suppl. 1): S498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shuaib F, Gunnala R, Musa EO, et al. Ebola virus disease outbreak—Nigeria, July-September 2014. MMWR Morb Mortal Wkly Rep 2014;63:867–72. [PMC free article] [PubMed] [Google Scholar]
- 36.Team WER. Ebola virus disease in West Africa—the first 9 months of the epidemic and forward projections. N Engl J Med 2014;371:1481–95. [DOI] [PMC free article] [PubMed] [Google Scholar]