Abstract
Background
This is an update of the original review that was published in The Cochrane Database of Systematic Reviews, 2009, Issue 2. Gestational trophoblastic neoplasia (GTN) are malignant disorders of the placenta that include invasive hydatidiform mole, choriocarcinoma, placental‐site trophoblastic tumour (PSTT) and epithelioid trophoblastic tumour (ETT). Choriocarcinoma and invasive hydatidiform mole respond well to chemotherapy: low‐risk tumours are treated with single‐agent chemotherapy (e.g. methotrexate or actinomycin D), whereas high‐risk tumours are treated with combination chemotherapy (e.g. EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine)). Various drug combinations may be used for high‐risk tumours; however, the comparative efficacy and safety of these regimens is not clear.
Objectives
To determine the efficacy and safety of combination chemotherapy in treating high‐risk GTN.
Search methods
For the original review, we searched the Cochrane Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL; Issue 2, 2008), MEDLINE, EMBASE and CBM in May 2008. For the updated review, we searched Cochrane Group Specialised Register, CENTRAL, MEDLINE and EMBASE to September 2012 and on 1 June 2015. In addition, we searched online clinical trial registries for ongoing trials.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs comparing first‐line combination chemotherapy interventions in women with high‐risk GTN.
Data collection and analysis
Two review authors independently collected data using a data extraction form. Meta‐analysis could not be performed as we included only one study.
Main results
We included one RCT of 42 women with high‐risk GTN who were randomised to MAC (methotrexate, actinomycin D and chlorambucil) or the modified CHAMOCA regimen (cyclophosphamide, hydroxyurea, actinomycin D, methotrexate, doxorubicin, melphalan and vincristine). There were no statistically significant differences in efficacy of the two regimens; however women in the MAC group experienced statistically significantly less toxicity overall and less haematological toxicity than women in the CHAMOCA group. During the study period, six women in the CHAMOCA group died compared with one in the MAC group. This study was stopped early due to unacceptable levels of toxicity in the CHAMOCA group. We identified no RCTs comparing EMA/CO with MAC or other chemotherapy regimens.
Authors' conclusions
CHAMOCA is not recommended for GTN treatment as it is more toxic and not more effective than MAC. EMA/CO is currently the most widely used first‐line combination chemotherapy for high‐risk GTN, although this regimen has not been rigorously compared to other combinations such as MAC or FAV in RCTs. Other regimens may be associated with less acute toxicity than EMA/CO; however, proper evaluation of these combinations in high‐quality RCTs that include long‐term surveillance for secondary cancers is required. We acknowledge that, given the low incidence of GTN, RCTs in this field are difficult to conduct, hence multicentre collaboration is necessary.
Plain language summary
Combinations of anti‐cancer drugs to treat high‐risk cancers arising from the placenta, known as high‐risk gestational trophoblastic neoplasia (GTN)
GTN is a cancer that most often arises after a molar pregnancy but can arise after any type of pregnancy. Molar pregnancies occur due to abnormal growth of placental tissue that is usually benign and treated by evacuation of the womb (D&C). However, in less than 10% of molar pregnancies in the UK, the growth remains after D&C and transforms into a cancer (GTN) that needs treatment with anti‐cancer drugs (chemotherapy). GTN can be low‐risk or high‐risk. Anti‐cancer drugs are very effective, especially in low‐risk GTN, which is usually cured with single‐drug treatment. However, high‐risk GTN needs to be treated with combinations of anti‐cancer drugs for the best effect. These drugs can produce toxic side effects that are more likely to occur when used in combination with each other. The most commonly administered drug combination is abbreviated as EMA/CO, which stands for Etoposide, Methotrexate, Actinomycin D, Cyclophosphamide and Oncovin® (vincristine), but several other combinations are also in use.
We undertook this review to try to determine which combination/s of drugs are the most effective for the first‐line drug treatment of high‐risk GTN, and with the least side effects. We found only one small, older study that compared a drug combination abbreviated as CHAMOCA with one called MAC. The CHAMOCA regimen, which is no longer recommended for GTN treatment, was found to be extremely toxic to the blood and bone marrow, with no greater effect against the cancer than the MAC regimen. Based on the available evidence, it is currently not possible to determine whether EMA/CO is the most effective and least toxic drug combination as no high‐quality studies have been conducted comparing this combination with other combinations. GTN is a rare cancer and so studies in this field are difficult to conduct, therefore researchers need to collaborate in order to produce the necessary high‐quality evidence.
Background
Description of the condition
This is an update of the original review that was published in The Cochrane Database of Systematic Reviews, 2009, Issue 2. Gestational trophoblastic neoplasia (GTN) together with hydatidiform moles (HM) are collectively known as gestational trophoblastic disease (GTD), a spectrum of tumours that arise from placental tissue. GTN represents the malignant forms of the disease that includes invasive mole, choriocarcinoma, placental‐site trophoblastic tumour (PSTT) and epithelioid trophoblastic tumour (ETT) (Lurain 2011). GTNs most frequently occur following a molar pregnancy (Seckl 2009a); however, they may occur following any antecedent pregnancy including a term pregnancy, miscarriage or an ectopic pregnancy.
HMs are benign tumours that are described as partial or complete based on the morphological, karyotypic and clinical features (Szulman 1978). Complete moles (CMs) are paternally derived and have a diploid karyotype (usually 46 XX) whereas partial moles (PMs) usually have a triploid karyotype (Fisher 2009). CMs are associated with total hydatidiform enlargement of the villi, whereas the hydatidiform changes of the villi in PMs tend to be localised (Driscoll 1981). The p57 gene is not present in CMs as p57 is paternally imprinted but maternally expressed, therefore p57 immunostaining can distinguish CMs from PMs or hydropic abortions (Popiolek 2006). HMs usually resolve spontaneously following one or more uterine evacuations; however, in approximately 6% to 20% of CMs and 0.5% to 1% of PMs the disease persists and transforms into GTN (Seckl 2009a). Overall, Seckl 2009a estimates that 8% of HMs in the UK will transform and need chemotherapy.
The incidence of GTD varies geographically with some countries showing an increase in GTD rates over time, while others showing a decline (Lee 2009). Europe, Japan and North America report GTD rates of ≤ 1/1000 pregnancies, whereas India, Turkey and Indonesia report the highest rates (Lee 2009). A study carried out to determine the incidence rate of GTD in south‐east Anatolia (Turkey) from July 1998 to October 2003 reported that out of 6016 deliveries, 73 cases of GTD were identified, giving an incidence of 12/1000 deliveries. Of these GTD cases, two (2.7%) had invasive mole and five (6.9%) had choriocarcinoma (Harma 2005), which is equivalent to an incidence of GTN in this region of 1/1000 deliveries.
Although the aetiology of GTN is not well understood, the occurrence of this tumour has been associated with several factors including the extremes of reproductive age (younger than 20 years and older than 40 years), previous HM and particular ABO blood groups (Bagshawe 1986; Hayashi 1982). In addition, ethnicity, poor nutrition, viral infections and environmental factors may play a role (Lee 2009).
The three types of GTN have distinct morphological features. Invasive moles and choriocarcinomas arise from fetal tissue within the maternal host and are composed of both syncytiotrophoblastic and cytotrophoblastic cells (WHO 1983). Invasive moles have the same histological features as CMs but are characterised by myometrial invasion without involvement of intervening endometrial stroma (Lurain 1982). Choriocarcinomas have a unique histology distinct from that of moles: microscopically, the neoplasm is composed of a disordered array of syncytiotrophoblastic and cytotrophoblastic elements, frequent mitoses and multinucleated giant cells (Mazur 1987). Vascular invasion occurs early, with resultant metastases to the lungs, vagina, brain, kidneys, liver and gastrointestinal tract (Decherney 2003). PSTTs are derived from intermediate trophoblast cells of the placenta, which are identified by the secretion of placental lactogen and small amounts of β‐human chorionic gonadotrophin (β‐hCG) (Kurman 1984).
GTNs are characterised by aggressive biological cell behaviour and a propensity for widespread metastases, especially choriocarcinomas (Song 2004). All GTNs secrete hCG, which is a unique and characteristic tumour marker that can be used in the monitoring of treatment and in follow‐up (Pastorfide 1974).
Description of the intervention
GTNs are the only disseminated solid tumours that are highly curable by chemotherapy (Schorge 2003), except for PSTTs and ETTs, which are rare and relatively resistant to chemotherapy (Lurain 2011). The majority of invasive moles and choriocarcinomas will be cured with first‐line chemotherapy; however, some will persist or metastasise, thereby needing secondary treatments, adjuvant treatments (such as surgery or radiotherapy) or a combination. Bagshawe 1976 introduced a scoring system to identify those tumours that were at a higher risk of metastases or persistence, and the World health Organization (WHO) subsequently adapted this system (WHO 1983) to classify tumours as low‐risk (score ≤ 4), medium risk (score 5 to 7) and high‐risk (score ≥ 8) based on various prognostic factors including: age, antecedent pregnancy, interval since antecedent pregnancy, hCG level, ABO blood group, largest tumour site(s) of metastases, site of metastases and previous chemotherapy. More recently, the International Federation of Obstetrics and Gynecology (FIGO) has modified the WHO system to include low‐risk (score ≤ 6) and high‐risk (score ≥ 7) only (FIGO 2009; Table 1). The scoring system of FIGO differs from the WHO system in that the ABO blood group risk factor is eliminated and the risk factor for liver metastasis is upgraded from two, to highest group, four. FIGO recommends denoting the anatomical stage (Table 2) and the risk score for individual patients (FIGO 2009).
1. Modified WHO Prognostic Scoring System for GTN as adapted by FIGO.
| Scores | 0 | 1 | 2 | 4 |
| Age (years) | < 40 | ≥40 | – | – |
| Antecedent pregnancy | mole | abortion | term | – |
| Interval months from index pregnancy | < 4 | 4 to 6 | 7 to 12 | > 12 |
| Pre‐treatment serum hCG (IU/L) | < 103 | 103 to 104 | 104 to 105 | > 105 |
| Largest tumour size (including uterus) (cm) | < 3 | 3 to 4 | ≥ 5 | – |
| Site of metastases | lung | spleen, kidney | gastrointestinal | liver, brain |
| Number of metastases | – | 1 to 4 | 5 to 8 | > 8 |
| Previous failed chemotherapy | – | – | single drug | ≥ 2 drugs |
| To stage and allot a risk factor score, a patient's diagnosis is allocated to a stage as represented by a Roman numeral I, II, III, and IV. This is then separated by a colon from the sum of all the actual risk factor scores expressed in Arabic numerals, i.e. stage II:4, stage IV:9. This stage and score will be allotted for each patient (FIGO 2009). A score ≤ 6 indicates low‐risk; > 6 indicates high‐risk. | ||||
2. FIGO anatomical staging *.
| Stage I | Disease confined to the uterus |
| Stage II | GTN extends outside of the uterus, but is limited to the genital structures (adnexa, vagina, broad ligament) |
| Stage III | GTN extends to the lungs with or without known genital tract involvement |
| Stage IV | All other metastatic sites |
*From FIGO 2009.
Low‐risk GTN is usually treated with single‐agent chemotherapy and cure rates approximate 100% (Seckl 2009b). Both methotrexate (with folinic acid) and low‐dose actinomycin D are effective (Alazzam 2012a). For high‐risk GTN, appropriate and timely treatment can prevent life‐threatening complications (Decherney 2003). This treatment involves multi‐agent or combination chemotherapy with or without adjuvant radiotherapy and surgery, and cure rates with current regimens are estimated to be around 80% to 90% (Goldstein 2012).
The following regimens have been described:
EMA/CO: etoposide, methotrexate, actinomycin D (EMA) plus cyclophosphamide and vincristine (CO) (Newlands 1986; Bower 1997; Newlands 1998; Tang 2003; Seckl 2009b; Goldstein 2012);
MAC: methotrexate, actinomycin D, cyclophosphamide or chlorambucil (CB1348) (Hammond 1973; Curry 1989);
EMA/EP: etoposide, methotrexate, actinomycin D (EMA) plus etoposide and cisplatin (EP) (Ghaemmaghami 2004; Cyriac 2011b);
CHAMOCA or CHAMOMA: cyclophosphamide, hydroxyurea, actinomycin D, methotrexate, doxorubicin, melphalan and vincristine (Bagshawe 1977; Curry 1989);
EMA: etoposide, methotrexate and actinomycin D (Matsui 2000; Dobson 2000);
FA (5‐fluorouracil (5‐FU) and actinomycin D) or FAV (5‐FU, actinomycin D and vincristine) (Zhao 2009; Feng 2011); and
MEF (methotrexate, etoposide, 5‐FU) (Wang 2006).
How the intervention might work
Most high‐risk GTNs will respond to various chemotherapy regimens. The MAC regimen was commonly used in the 1970s and 1980s until etoposide was found to have high activity against GTNs (Goldstein 2012). CHAMOCA was compared with MAC but was found to be associated with significantly more haematological toxicity (Curry 1989) and subsequently abandoned. One UK study reported using EMA/CO (Table 3) in 272 women with high‐risk GTN (primary and secondary), inducing remission rates of 86% of women (Bower 1997; Newlands 1998).Thereafter EMA/CO became the preferred first‐line combination regimen in North America (Goldstein 2012), Europe (Seckl 2009b) and elsewhere. However, 5‐FU combinations are reported to be preferred in parts of China (Feng 2011). Currently, EMA/EP is recommended for salvage therapy of EMA/CO‐resistant tumours (Goldstein 2012; Seckl 2009b); however, it has also been used for first‐line therapy (Ghaemmaghami 2004; Cyriac 2011b).
3. EMA‐CO chemotherapy regimen *.
| Schedule | |
| Day 1 (EMA) | |
| Etoposide | 100 mg/m2 by intravenous infusion for 30 min |
| Actinomycin D | 0·5 mg intravenous bolus |
| Methotrexate | 300 mg/m2 by intravenous infusion for 12 h |
| Day 2 | |
| Etoposide | 100 mg/m2 by intravenous infusion for 30 min |
| Actinomycin D | 0·5 mg intravenous bolus |
| Folinic acid rescue† | 15 mg intramuscularly or orally every 12 h for 4 doses |
| Day 8 (CO) | |
| Vincristine | 1 mg/m2 intravenous bolus (maximum 2 mg) |
| Cyclophosphamide | 600 mg/m2 intravenous infusion for 30 min |
| EMA alternates with CO every week. †Rescue begun 24 h after start of methotrexate infusion. The problem of myelosuppression is usually overcome by administering G‐CSF (e.g. filgrastim) and increasing the frequency (4 doses daily) and duration (up to 4 days) of folinic acid rescue. | |
*From Seckl 2010.
Combination chemotherapy regimens are frequently associated with side effects that may result in significant morbidity and treatment delays or changes. Some agents, such as etoposide, have been associated with an increased risk of secondary tumours, including acute myeloid leukaemia (AML), colon cancer, breast cancer and melanoma (Schorge 2003). The main side effects of EMA/CO are haematological toxicity, gastrointestinal toxicity and reversible alopecia (Zhao 2000; Seckl 2010). Although the most favoured initial chemotherapy regimen in high‐risk GTN patients is EMA/CO, the relative side effects of this regimen compared to other chemotherapy regimens are unclear.
Why it is important to do this review
We originally conducted this review in 2006 with the aim of comparing all RCTs of combination chemotherapy used as treatment for high‐risk GTN, to determine their relative efficacies and safety. However, we were only able to include one study (CHAMOCA versus MAC; Curry 1989) in the original review. Furthermore we identified no RCTs comparing the more frequently used combinations (e.g. EMA/CO and MAC). Combination chemotherapy regimens may be associated with side effects that result in significant morbidity and treatment delays or changes, therefore comparing these regimens with respect to efficacy and toxicity is important. We continue to update this review periodically, in order to identify new studies that may be helpful in providing treatment guidelines for these rare tumours. Chemotherapy for low‐risk GTN and salvage chemotherapy for recurrent and resistant GTN are the subject of separate reviews (Alazzam 2012a; Alazzam 2012b).
Objectives
To determine the efficacy and safety of combination chemotherapy in the treatment of high‐risk GTN.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised controlled trials (RCTs) and quasi‐RCTs comparing first‐line combination chemotherapy regimens for the treatment of high‐risk GTN.
Types of participants
Inclusion criteria
Women diagnosed with high‐risk GTN who had a pathological diagnosis and a high‐risk prognostic score as determined by any prognostic scoring system, such as FIGO (Kohorn 2001), WHO (WHO 1983) or Bagshawe (Bagshawe 1976).
Exclusion criteria
Women with PSTT.
Women receiving secondary or salvage chemotherapy for recurrent or resistant GTN.
Types of interventions
Combination chemotherapy regimens compared with other combination chemotherapy (e.g. EMA/CO versus MAC).
Types of outcome measures
Primary outcomes
Death from any cause (overall survival).
Death from cancer (cancer‐specific survival).
Progression or recurrence of the cancer (progression‐free survival).
Secondary outcomes
Level of β‐hCG.
Adverse events, according to International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines (ICH 1997), Common Terminology Criteria for Adverse Events (CTCAE) guidelines (CTCAE 2010) or as defined by the investigators.
Secondary cancers.
Quality of life (QoL).
Search methods for identification of studies
Electronic searches
For the original review, we searched the following databases: Cochrane Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 2, 2008); MEDLINE (from 1966 to 15 June 2008); EMBASE (from 1980 to 15 June 2008); Chinese Biomedical database (CBM; from 1978 to 15 June 2008 (Appendix 1)) and China National Knowledge Infrastructure (CNKI; from 1997 to 15 June 2008). For the updated review, we searched Cochrane Group Specialised Register, CENTRAL (Appendix 2), MEDLINE (Appendix 3) and EMBASE (Appendix 4) (to 12 September 2012 and on 1 June 2015)
Searching other resources
We searched the International Society for the Study of Trophoblastic Diseases (ISSTD) website. In addition, we searched the following online registries for ongoing trials (15 June 2008 and 17 September 2012):
Australian New Zealand Clinical Trial Registry (www.anzctr.org.au/)
Chinese Clinical Trial Register (www.chictr.org)
ClinicalTrials.gov (www.clinicaltrials.gov)
Current Controlled Trials (www.controlled‐trials.com)
WHO ICTRP Search Portal (www.who.int/ictrp/network/en/index.html)
the National Research Register (www.update‐software.com)
the ISRCTN Register (isrctn.org)
Data collection and analysis
Selection of studies
Two review authors (DLY, XY) sifted the results of the original search and TL sifted the updated search for potentially relevant trials. DLY and XY independently assessed trials for inclusion in the review. Differences were resolved by discussion between review authors (DLY, XY, WT). Failure to resolve differences would have been brought to the attention of the Contact Editor (WT) responsible for the review and a majority decision taken.
Data extraction and management
DLY and XY independently extracted data using a piloted data extraction form. Extracted data on study characteristics included methods, participants, interventions and outcomes. Differences were resolved by discussion between the review authors. Failure to resolve differences would have been brought to the attention of the Contact Editor responsible for the review and a majority decision taken. If data from the trial reports were insufficient or missing the authors would have been contacted for additional information.
Where possible we extracted data to allow for an intention‐to‐treat analysis (the analysis included all the participants in the groups to which they were originally randomly assigned). If the number randomised and the number analysed were inconsistent, the percentage lost to follow‐up was calculated. For dichotomous outcomes, we recorded the number of participants experiencing the event in each group of the trial out of the number in whom the event was assessed in each group. For continuous outcomes, we extracted the arithmetic mean and standard deviation (SD) for each group.
Assessment of risk of bias in included studies
Using The Cochrane Collaboration's tool (Higgins 2011), we assessed the following:
selection bias: random sequence generation and allocation concealment;
performance bias: blinding of participants and personnel (patients and treatment providers);
detection bias: blinding of outcome assessment;
attrition bias: incomplete outcome data;
reporting bias: selective reporting of outcomes;
other possible sources of bias.
We classified the risk of these biases as 'low', 'high' or 'unclear' for each included study. For more details see Appendix 5.
Measures of treatment effect
Only dichotomous data were available for this review, therefore we used the risk ratio (RR) with 95% confidence intervals (CIs) for all outcomes. In future versions of this review, if other types of outcome data are available, we will also use the following measures of the effect of treatment:
for time to event data, the hazard ratio (HR) with 95% CIs; and
for continuous outcomes that use the same scales, the mean difference (MD) between treatment arms with 95% CIs; for trials that measured the same outcome but use different scales, we plan to use the standardised mean difference (SMD).
Assessment of heterogeneity
Heterogeneity among trials would have been assessed by visual inspection of the forest plots and by formal statistical tests for heterogeneity, including the T2, I2 and Chi2 statistics (Deeks 2001; Higgins 2003); however there were insufficient trials for meta‐analyses. In future versions of this review, we will regard heterogeneity as substantial if T2 is greater than zero and either I2 is greater than 30% or there is a low P value (< 0.10) in the Chi2 test for heterogeneity.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011) and used the random‐effects model to produce an overall summary of the mean treatment effect across pooled studies (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses in order to explore effect size differences according to:
prognostic scores, for example high‐risk (scores of 7 to 13) and very high‐risk (scores >13); and
duration of follow‐up.
Sensitivity analysis
Had there been sufficient included studies, sensitivity analyses would have been performed to examine the effects of lower methodological quality studies on the results and to explore the reasons for heterogeneity.
Results
Description of studies
There were no new studies to include (Figure 1; Figure 2) in the updated versions of the review. We included one prospective randomised study (Curry 1989) in the original review. This North American study was a multi‐institutional study conducted between November 1981 and May 1986. Prognostic risk factors were evaluated using the criteria of Hammond (Hammond 1973) and the prognostic score from Wong (Wong 1986). Forty‐two women entered the study, with 22 receiving the MAC regimen and 20 receiving a modified CHAMOCA (or CHAMOMA) regimen. The regimens consisted of methotrexate, actinomycin D, chlorambucil (MAC) or methotrexate, actinomycin D, cyclophosphamide, doxorubicin, melphalan, hydroxyurea and vincristine (CHAMOCA). Each regimen was repeated every 21 days with a window of 12 to 14 days or 10 to 14 days between courses, respectively. If there was cerebral or hepatic metastasis, radiotherapy was administered with the commencement of chemotherapy. Where treatment failed, surgical procedures, chemotherapy or both, were initiated as directed by the principal investigator. Outcomes evaluated included remission (primary or secondary), death from the disease, treatment failures, the number of courses to achieve normal hCG titre, haematological toxicity and total toxicity were evaluated in this study. Treatment failure was measured by a plateau or rising hCG titre or a new metastasis.
1.
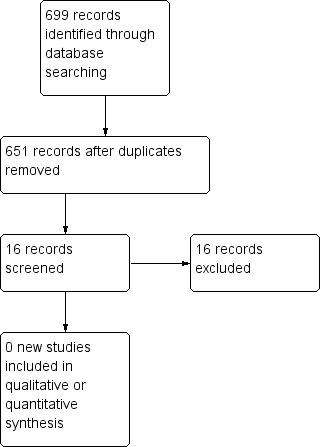
Study flow diagram of updated search (12 September 2012).
2.
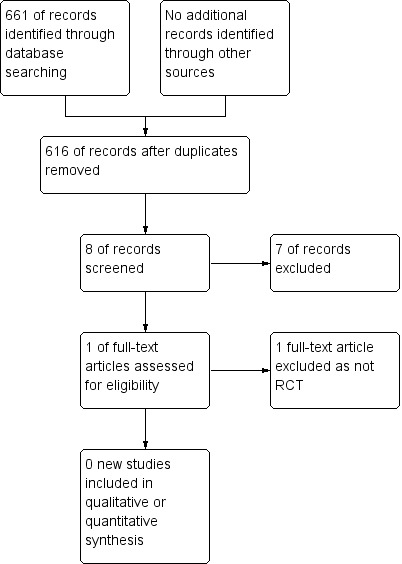
Study flow diagram of update search (May 2015)
Risk of bias in included studies
The Curry 1989 study randomly allocated 42 patients into two groups; however, the randomisation procedure was not described and we could not determine whether allocation concealment or blinding was used. Selection bias, performance bias and detection bias may therefore be present in this study. No patients withdrew and there was no loss to follow‐up. Baseline 'risk' characteristics were similar in each allocation group; however the mean age of participants was not described. As several aspects of the methodology of this study were unclear, we considered it to be at an unclear risk of bias (Figure 3).
3.
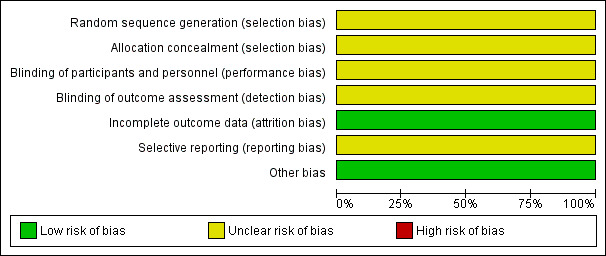
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Only one study (Curry 1989), comparing MAC with the modified CHAMOCA regimen, was included in this review. The results of this study were as follows:
Primary outcomes
Death rates were 4% (1 out of 22) and 30% (6 out of 20) for the MAC and CHAMOCA treatment groups, respectively (Analysis 1.1). Primary remission rates were 73% (16 out of 22) and 65% (13 out of 20), respectively and secondary remission rates were 23% (5 out of 22) and 5% (1 out of 20), respectively. These death and remission rate differences were not statistically significant (Analysis 1.2).
1.1. Analysis.
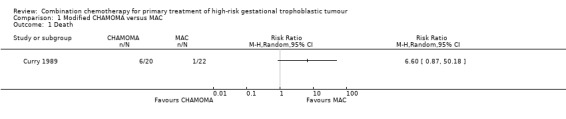
Comparison 1 Modified CHAMOMA versus MAC, Outcome 1 Death.
1.2. Analysis.
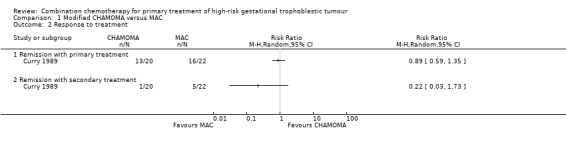
Comparison 1 Modified CHAMOMA versus MAC, Outcome 2 Response to treatment.
Primary treatment with MAC failed in six (27.3%) women. Five of these women received a total abdominal hysterectomy or the CHAMOMA regimen and survived; one woman died of seizure and aspiration, having achieved normal hCG titres and normal laboratory data. Primary treatment with CHAMOCA failed in seven women (35%); one of these women received a thoracotomy with additional chemotherapy and survived. The remaining six women received chemotherapy, adrenal resection or total abdominal hysterectomy but died of the disease.
Secondary outcomes
Severe toxicity (grade 3 to 4) occurred significantly less frequently with the MAC regimen compared with the CHAMOCA regimen (P = 0.014; 11 out of 22 women (50%) versus 18 out of 20 women (90%), respectively; Analysis 1.3). These events were life‐threatening in two out of 22 (9%) and eight out of 20 (45%) women, respectively. Similarly, severe haematological toxicity (grade 3 to 4) occurred significantly less frequently with the MAC regimen than with the CHAMOCA regimen (P = 0.004; eight out of 22 women (36%) versus 17 out of 20 women (85%), respectively; Analysis 1.4).
1.3. Analysis.
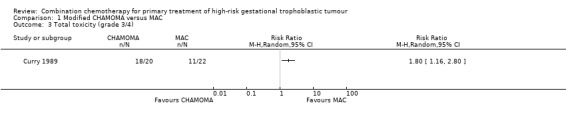
Comparison 1 Modified CHAMOMA versus MAC, Outcome 3 Total toxicity (grade 3/4).
1.4. Analysis.
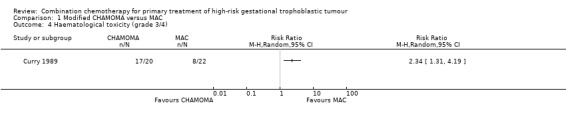
Comparison 1 Modified CHAMOMA versus MAC, Outcome 4 Haematological toxicity (grade 3/4).
Discussion
Summary of main results
Only one RCT (Curry 1989) comparing two combinations of chemotherapy for high‐risk GTN was identified and could be included. This study was at an unclear risk of bias. Overall, 42 women were enrolled and randomised to receive the MAC regimen or the modified CHAMOCA regimen. Not all review outcomes could be assessed for this comparison. However, the MAC regimen was at least as effective as the modified CHAMOCA regimen, and statistically significantly less toxic overall (P = 0.014), and to the bone marrow (P = 0.004).
Overall completeness and applicability of evidence
The Curry 1989 trial was closed when just over half the study sample had been recruited due to a statistically significant increase in severe haematological toxicity in the CHAMOCA group. The quality of evidence relating to CHAMOCA toxicity was good, despite the fact that Curry 1989 was an older study and more modern treatment consists of bone marrow support with granulocyte‐colony stimulating factor (G‐CSF) frequently being used to prevent and treat haematological complications of chemotherapy for GTN (Seckl 2010). However, the fact that the CHAMOCA regimen was not shown to be more effective than MAC means that no further studies of this significantly more toxic regimen are necessary.
EMA/CO is the preferred regimen for treating high‐risk GTN; however high‐quality evidence in support of this is still lacking. There have been no RCTs comparing EMA/CO and MAC, for example. Retrospective studies have reported primary remission rates for EMA/CO ranging from 54% to 91% (see Table 4). Newlands 1998 described the results of 272 consecutive patients treated with EMA/CO, including 121 for secondary/salvage treatment: the median follow‐up duration was 4.5 years (range 1 to 16 years) and the cumulative five‐year survival rate was 86.2% (95% CI 81.9% to 90.5%). Drug resistance occurred in 47 out of 272 patients (17%) and 34 patients died (12.5%), 31 from GTN and three from other causes. The investigators noted a significant increase in secondary tumours including AML, colon cancer, melanoma and breast cancer compared with expected rates (P = 0.011).
4. Cohort studies of EMA‐CO for primary treatment of high‐risk GTN.
| Study ID | Study period | Country | Participants with high‐risk GTN undergoing primary treatment |
CT regimen | Primary remission rate (CR) |
Median follow‐up (months) |
OS |
| Bolis 1988 | 1980 to 1985 | Italy | 22 | EMA/CO | 94% | 32 | 88% |
| Soper 1994 | 1966 to 1992 | North America | 6 | EMA/CO | 67% | ‐ | ‐ |
| Schink 1992 | 1986 to 1991 | North America | 12 | EMA/CO | 83% | 26 | 100% |
| Newlands 1998; Bower 1997 | 1979 to 1995 | UK | 272 ¹ | EMA/CO | 78% | 54 | 87% |
| Kim 1998 | 1971 to 1995 | Korea | 227 ² | EMA/CO (96); MA (49); MAC (40); CHAMOCA (42) |
EMA/CO: 91%; MA: 63%; MAC: 68%; CHAMOCA: 76% | All follow‐up exceeded 24 months | ‐ |
| Escobar 2003 | 1986 to 2001 | North America | 25 | EMA/CO | 76% | 36 | 92% |
| Turan 2006 | 1994 to 2004 | Turkey | 23 | EMA/CO | ‐ | 53 | 91% |
| Lu 2008 | 1996 to 2005 | China | 45 | EMA/CO | 78% | 56 ⁴ | 93% |
| Lurain 2010 | 1989 to 2009 | North America | 26 | EMA/CO | 62%⁷ | ‐ | 92% |
| Chauhan 2010 | 1995 to 2008 | India | 67³ | EMA/CO | 75% (43/57) | range, 12 to 72 | 93% |
| Cyriac 2011a | 1997 to 2006 | India | 29 | EMA/CO | 76% | 47 | 71% |
| Bianconi 2012 | 1990 to 2011 | Argentina | 23 | EMA/CO (for scores 7 to 13); EMA/EP for scores > 13 |
83% | ‐ | 93% |
| Cagayan 2012 | 2006 to 2010 | Phillipines | 67 | EMA/CO ⁵ | 71% | ‐ | 86% |
| Fülöp 2012 | 1977‐ to 2010 | Hungary | 174 | EMA/CO (26); MAC (118); BEP (17) ⁶ |
EMA/CO: 81%; MAC: 93% BEP: 94% |
‐ | 97% |
| CT: chemotherapy; CR: complete response; OS: overall survival; EMA/CO: etoposide, methotrexate, actinomycin D/cyclophosphamide, vincristine; EMA/EP: etoposide, methotrexate, actinomycin D/etoposide, cisplatin; MAC: methotrexate, actinomycin D, cyclophosphamide or chlorambucil; BEP: bleomycin, etoposide, cisplatin. | |||||||
¹ This study included 121 women undergoing secondary/salvage treatment.
² MA and MAC were used mainly between 1971 and 1982; CHAMOCA was used between 1982 and 1985; EMA/CO was used from 1985 to 1995. Mean age and prognostic scores were similar in these groups.
³ Only 57 were treated with EMA/CO, the other 10 were treated with EMA.
⁴ Mean not median.
⁵ 56 women completed treatment.
⁶ Prognostic scores differed between these treatment groups as follows: MAC = 11.5, EMA/CO = 13.2 and BEP = 15.5.
⁷ Two patients relapsed from remission at 4 and 5 months therefore the overall durable CR was 54%.
Kim 1998 reported on a cohort of 227 patients with high‐risk GTN who were treated with four different chemotherapy regimens during the period 1971 to 1995, including MA (methotrexate, folinic acid and actinomycin‐D) and MAC from 1971 to 1982, CHAMOCA from 1982 to 1985 and EMA/CO from 1985 to 1995. Mean age and risk scores (mean, 10.6) of these treatment groups were comparable; however, it is not clear whether these women were all undergoing primary treatment. The remission rate of the EMA/CO regimen was 91% (87 out of 96) compared with the remission rates of 63% (31 out of 49), 68% (27 out of 40) and 76% (32 out of 45) for MA, MAC and CHAMOCA, respectively, and remission was achieved in fewer courses in the EMA/CO group. Nine deaths (9.4%) occurred in the EMA/CO group compared with 18 (37%), 13 (33%) and 10 (22%) in the MA, MAC and CHAMOCA groups, respectively. Toxicity was considered to be lower in the EMA/CO group; however, these are not detailed in the report.
Lurain 2010 conducted a retrospective study of 40 women with metastatic high‐risk GTN who were treated with EMA/CO between 1986 and 2009; 26 women underwent primary treatment and 14 underwent secondary treatment following failed single‐agent therapy. Primary remission rates for primary and secondary treatment groups were 62% (16 out of 26) and 50% (7 out of 14), respectively; however, two women in the primary treatment group relapsed soon after remission, reducing the durable remission rate to 54% for primary treatment. The primary remission rates in Lurain 2010 are thus the lowest reported with EMA/CO treatment, although salvage chemotherapy for relapsed and resistant cases resulted in an overall survival of 90% in this study.
More recent data on other regimens are limited; however, in women diagnosed with high‐risk GTN in Hungary between 1977 and 2010, Fülöp 2012 reports primary remission rates of 93% and 94% for the MAC (110 out of 118 women) and bleomycin, etoposide and cisplatin (BEP) (16 out of 17 women) regimens, respectively. The EMA/CO regimen, introduced in 1993, achieved a primary remission rate of 81% (21 out of 26 women). Mean risk scores for these three groups were 11.5 (MAC), 15.5 (BEP) and 13.2 (EMA/CO). Overall survival was 96.6%.
Toxicity from EMA/CO is often described as well tolerated; however all 56 patients in Cagayan 2012 experienced adverse drug reactions, with haematological toxicity occurring in 36%. In Chauhan 2010, two women died from acute haematological toxicity (Chauhan 2010). Although stem cell support with G‐CSF may be used to prevent or reduce myelosuppression, it is very expensive and not always an option, particularly in developing countries where the bulk of GTN disease occurs.
In addition, the long‐term risk of secondary cancers is still to be determined. Etoposide‐related secondary leukaemias have a significantly worse prognosis that other types of leukaemia (Ezoe 2012), therefore it is important to understand the risk to patients with high‐risk GTN who undergo treatment with this agent. The risk of etoposide‐induced AML is related to the cumulative drug dose and possibly the dose method (higher dose frequency or prolonged duration of treatment) (Ezoe 2012). Furthermore, it may be increased when combined with high‐dose platinum agents or concomitant radiotherapy (Ezoe 2012). As etoposide/cisplatin regimens (e.g. EMA/EP) are being used more frequently as primary (Cyriac 2011b; Ghaemmaghami 2004) or salvage therapy (Newlands 2000) (or both), researchers and clinicians should ensure that adequate long‐term follow‐up and surveillance for secondary cancers is conducted.
The importance of randomising participants to treatment groups in studies of chemotherapy interventions cannot be over‐emphasised. Baseline differences between study groups in prognostic risk scores, brain and liver metastases, surgery and adjuvant therapies (e.g. G‐CSF or radiotherapy), and in those undergoing primary or secondary treatment may have a substantial impact on remission, survival and toxicity rates, therefore trials comparing chemotherapy regimens need to control for these variables.
We consider the available evidence to be incomplete and believe that there is a need for carefully planned and well‐conducted, multicentred RCTs to determine the most clinically effective, cost‐effective and least toxic therapy for high‐risk GTN. The possible regimens with which EMA/CO could be compared to in these studies include MAC, FAV and EMA/EP.
Potential biases in the review process
To our knowledge there were no potential biases in the review process.
Authors' conclusions
Implications for practice.
CHAMOCA is not recommended for GTN treatment. EMA/CO is currently the most widely used first‐line combination chemotherapy for high‐risk GTN, although this regimen has not been rigorously compared in RCTs to other combinations, such as MAC or FAV. Other regimens may be reasonable alternatives to EMA/CO; however, there is no good evidence to support this and proper evaluation in high‐quality RCTs that include long‐term surveillance for secondary cancers is required. We acknowledge that, given the low incidence of GTN, RCTs in this field are difficult to conduct, hence multicentre collaboration is necessary.
Implications for research.
A rigorously designed, international multicentre RCT is required to evaluate EMA/CO chemotherapy versus other combination chemotherapies for GTN. Baseline data should include risk scores, metastases, adjuvant therapies, and whether the participants have primary high‐risk GTN or whether they are originally low‐risk cases that have failed single‐agent therapy. Outcomes should include overall survival, progression‐free survival, hCG titres, toxicity, QoL and cost. Toxicity should be critically assessed using CTCAE 2010 guidelines, and long‐term surveillance should be conducted for secondary cancers.
What's new
| Date | Event | Description |
|---|---|---|
| 23 March 2016 | Review declared as stable | No new studies identified in this or the previous update and currently there no on‐going studies. Therefore, the review is considered stable and not for future update. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 4 October 2012 | New citation required but conclusions have not changed | Review updated. No new studies included or excluded. Conclusions unchanged. |
| 3 September 2012 | New search has been performed | Search updated. |
| 9 February 2009 | New citation required but conclusions have not changed | New author byline. |
| 7 July 2008 | New search has been performed | Minor update 13 June 08 |
Acknowledgements
An Ruifang (AR) and Xue Yan (XY) were co‐authors of the original review. AR assisted with development of the original review. XY assisted with protocol development, searching for trials, quality assessment of trials, data extraction, data analysis and review development. We thank them for their contributions.
In addition, we thank Gail Quinn and Clare Jess (Managing Editors), and Jo Morrison and Chris Williams (Co‐ordinating Editors), of the Cochrane Gynaecological Cancer Group (CGCG). We are most grateful to them all for their advice and support.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Chinese Biomedical (CBM) search strategy
The search strategy in the Chinese Biomedical Base (CBM Base) #1 gestational trophoblastic tumour #2 gestational trophoblastic disease #3 invasive mole #4 choriocarcinoma #5 hydatidiform #6 persistent trophoblastic disease #7 treatment #8 chemotherapy #9 etoposide #10 methotrexate #11 actinomycin #12 cyclophosphamide #13 vincristine #14 chlorambucilum #15 doxorubicin #16 melphalan #17 hydroxyurea #18 Hammond #19 Goldstein #20 CHAMOCA #21 EMA/CO #22 (#1 or #2 or #3 or #4 or #5 or #6) and (#7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21)
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Gestational Trophoblastic Disease explode all trees #2 gestational and trophoblastic and (neoplasm* or tumor* or tumour* or disease*) #3 GTT or GTD or GTN #4 choriocarcinoma* #5 ((hydatidiform or invasive) and mole*) #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Antineoplastic Agents explode all trees #8 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols, this term only #9 chemotherap* #10 (etoposide or methotrexate or dactinomycin or actinomycin or cylophosphamide or vincristine or chlorambucil* or doxorubicin or melphalan or hydroxyurea or Hammond or Goldstein or EMA/CO or EMA‐CO or CHAMOCA) #11 (#7 OR #8 OR #9 OR #10) #12 (#6 AND #11)
Appendix 3. MEDLINE (Ovid) search strategies
1 exp Gestational Trophoblastic Disease/ 2 (gestational trophoblastic adj (neoplasm* or tumor* or tumour* or disease*)).mp. 3 (GTT or GTD or GTN).mp. 4 choriocarcinoma*.mp. 5 ((hydatidiform or invasive) and mole*).mp. 6 1 or 2 or 3 or 4 or 5 7 exp Antineoplastic Agents/ 8 Antineoplastic Combined Chemotherapy Protocols/ 9 chemotherap*.mp. 10 (etoposide or methotrexate or dactinomycin or actinomycin or cyclophosphamide or vincristine or chlorambucil* or doxorubicin or melphalan or hydroxyurea or Hammond or Goldstein or EMA?CO or EMA‐CO or CHAMOCA).mp. 11 7 or 8 or 9 or 10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 drug therapy.fs. 17 randomly.ab. 18 trial.ab. 19 groups.ab. 20 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21 6 and 11 and 20 22 exp animals/ not humans.sh. 23 21 not 22
key: mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier ab=abstract fs=floating subheading pt=publication type
Appendix 4. EMBASE (Ovid) search strategy
1 exp trophoblastic tumor/ 2 (gestational trophoblastic adj (neoplasm* or tumor* or tumour* or disease*)).mp. 3 (GTT or GTD or GTN).mp. 4 choriocarcinoma*.mp. 5 ((hydatidiform or invasive) and mole*).mp. 6 1 or 2 or 3 or 4 or 5 7 exp antineoplastic agent/ 8 exp chemotherapy/ 9 chemotherap*.mp. 10 (etoposide or methotrexate or dactinomycin or actinomycin or cyclophosphamide or vincristine or chlorambucil* or doxorubicin or melphalan or hydroxyurea or Hammond or Goldstein or EMA?CO or EMA‐CO or CHAMOCA).mp. 11 7 or 8 or 9 or 10 12 crossover procedure/ 13 double‐blind procedure/ 14 randomized controlled trial/ 15 single‐blind procedure/ 16 random*.mp. 17 factorial*.mp. 18 (crossover* or cross over* or cross‐over*).mp. 19 placebo*.mp. 20 (double* adj blind*).mp. 21 (singl* adj blind*).mp. 22 assign*.mp. 23 allocat*.mp. 24 volunteer*.mp. 25 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 6 and 11 and 25
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 5. Risk of bias assessment
We assessed the risk of bias of included RCTs in accordance with guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) as follows.
Randomisation
The method of randomisation was noted on the data extraction form. We assessed the randomisation as:
low risk of bias: e.g. a computer‐generated random sequence or a table of random numbers;
high risk of bias: e.g. date of birth, clinic id‐number or surname;
unclear risk of bias: e.g. details not reported.
Allocation concealment
We assessed the concealment of allocation sequence from treatment providers and participants as:
low risk of bias: e.g. where the allocation sequence could not be foretold;
high risk of bias: e.g. the computer‐generated random sequence was displayed so treatment providers could see which arm of the trial the next participant was assigned to, or kept in a sealed opaque envelope;
unclear risk of bias: allocation concealment not reported.
Blinding
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received, as well as whether the outcome assessors were blind to the group allocation. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed the methods of blinding as:
low, high or unclear risk of bias for participants, personnel or both;
low, high or unclear risk of outcome assessor bias.
Incomplete outcome data
We recorded the proportion of participants whose outcomes were not reported at the end of the study and we noted if loss to follow‐up was not reported.
We assessed methods as:
low risk of bias, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
high risk of bias, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between the treatment arms;
unclear risk of bias if loss to follow‐up was not reported.
Selective reporting
We assessed the methods of outcome reporting as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest have been reported);
high risk of bias (where not all the study's pre‐specified outcomes were reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so could not be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
Other bias
We described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias and assessed the risk as follows:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
Data and analyses
Comparison 1. Modified CHAMOMA versus MAC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Response to treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Remission with primary treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Remission with secondary treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total toxicity (grade 3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Haematological toxicity (grade 3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Curry 1989.
| Methods | Multicentre RCT conducted in the US from 1981 to 1986 by the Gynecologic Oncology Group | |
| Participants | 42 women with metastatic GTN with at least 1 high‐risk factor including: duration of disease > 4 months; brain or liver metastases; hCG > 42,000 mIU/mL; failed previous chemotherapy; and post‐term pregnancy | |
| Interventions | Group 1 (22 participants): methotrexate, actinomycin D, chlorambucil (MAC) regimen, repeated every 21 days (12‐ to 14‐day window) Group 2 (20 participants): cyclophosphamide, hydroxyurea, actinomycin D, methotrexate, doxorubicin, melphalan, vincristine (CHAMOMA) regimen, repeated every 21 days (10‐ to 14‐day window). Patients with cerebral or liver metastases also received radiation | |
| Outcomes | Death: 4% (1/22) with MAC vs. 30% (6/20)with CHAMOMA Response to therapy with primary treatment: 73% (16/22) with MAC vs. 65% (13/20) with CHAMOMA Response to therapy with secondary treatment: 23% (5/220) with MAC vs. 5% (91/20) with CHAMOMA Haematological toxicity: 36% (8/22) with MAC vs. 85% (17/20) with CHAMOMA Total toxicity: 50% (11/22) with MAC vs. 90% (18/20) with CHAMOMA Life‐threatening haematological toxicity: 9% (2/22) with MAC vs. 40% (8/20) with CHAMOMA Life‐threatening total toxicity: 9% (2/22) with MAC vs. 45% (9/20) with CHAMOMA Treatment was considered to have failed if there was a hCG plateau over 3 weeks, a rise of > 20% over 2 weeks, or if new metastases occurred. The treatment was then discontinued and patients received other chemotherapy or operative procedures |
|
| Notes | Baseline risk characteristics were similar between the 2 groups but mean age was not reported Investigators had intended to enrol 40 patients to each arm but the trial was stopped early because the CHAMOMA regimen was considered "significantly more toxic and possibly less effective" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes (toxicity and effectiveness) were reported. Mean age was not reported |
| Other bias | Low risk | No other risks noted |
GTN: gestational trophoblastic disease; hCG: human chorionic gonadotrophin; RCT: randomised controlled trial.
Differences between protocol and review
The 'Methods' section has been updated in accordance with the CGCG guidelines to include a revised 'Assessment of risk of bias of included studies', 'Measures of treatment effect' and 'Assessment of heterogeneity' section.
'Secondary cancers' has been added to the list of 'Secondary outcomes'.
The WHO ICTRP and its Primary Trial Registries have been added to the 'Search methods for identification of studies' section for ongoing studies.
The updated version of the review differs from the original review in that study data have been added to the analyses figures. However, meta‐analyses could not be performed as only one study (Curry 1989) contributed data.
Contributions of authors
Deng Linyu (DLY): responsible for updating the review (2008).
Zhang Jing (ZJ): searching for trials, quality assessment of trials, data extraction, data analysis and review development.
Wu Taixiang (WT): protocol development, searching for trials, quality assessment of trials, data extraction, data analysis and review development and updating this version.
Tess Lawrie (TL) assisted with the writing of the updated 2012 review.
Sources of support
Internal sources
Chinese Cochrane Centre, China.
West China Hospital of Sichuan University, China.
External sources
-
National Institute for Health Research (NIHR), UK.
This review received methodological, statistical and editorial support as part of the 10/4001/12 NIHR Cochrane Programme Grant Scheme: Optimising care, diagnosis and treatment pathways to ensure cost effectiveness and best practice in gynaecological cancer: improving evidence for the NHS.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Curry 1989 {published data only}
- Curry SL, Blessing JA, Disaia PJ, Soper JT, Twiggs LB. A prospective randomised comparison of methotrexate, dactinomycin and chlorambucil versus methotrexate, dactinomycin, cyclophosphamide, doxorubicin, melphalan, hydroxyurea, and vincristine in "poor prognosis" metastatic gestational trophoblastic disease: a Gynecologic Oncology Group Study. Obstetrics and Gynecology 1989;73(3 (part 1)):357‐62. [PubMed] [Google Scholar]
Additional references
Alazzam 2012a
- Alazzam M, Tidy J, Hancock BW, Osborne R, Lawrie T. First‐line chemotherapy in low‐risk gestational trophoblastic neoplasia. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD007102.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alazzam 2012b
- Alazzam M, Tidy J, Osborne R, Coleman R, Hancock B, Lawrie TA. Chemotherapy for resistant or recurrent gestational trophoblastic neoplasia. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008891.pub2] [DOI] [PubMed] [Google Scholar]
Bagshawe 1976
- Bagshawe KD. Risk and prognostic factors in trophoblastic neoplasia. Cancer 1976;38:1373‐85. [DOI] [PubMed] [Google Scholar]
Bagshawe 1977
- Bagshawe KD. Treatment of trophoblastic tumours. Recent Results Cancer Research 1977;62:192‐9. [DOI] [PubMed] [Google Scholar]
Bagshawe 1986
- Bagshawe KD, Dent J, Webb J. Hydatidiform mole in England and Wales, 1973‐1983. Lancet 1986;ii:673. [DOI] [PubMed] [Google Scholar]
Bianconi 2012
- Bianconi MI, Otero S, Moscheni O, Alvarez L, Storino C, Jankilevich G. Gestational trophoblastic disease: a 21‐year review of the clinical experience at an Argentinean public hospital. Journal of Reproductive Medicine 2012;57(7‐8):341‐9. [PubMed] [Google Scholar]
Bolis 1988
- Bolis G, Bonazzi C, Landoni F, Mangili G, Vergadoro F, Zanaboni F, et al. EMA/CO regimen in high‐risk gestational trophoblastic tumour. Gynecologic Oncology 1988;31:439‐44. [DOI] [PubMed] [Google Scholar]
Bower 1997
- Bower M, Newlands ES, Holden L, Short D, Brock C, Rustin GJ, et al. EMA‐CO for high‐risk gestational trophoblastic tumours: results from a cohort of 272 patients. Journal of Clinical Oncology 1997;15:2639. [DOI] [PubMed] [Google Scholar]
Cagayan 2012
- Cagayan MS. High‐risk metastatic gestational trophoblastic neoplasia. Primary management with EMA‐CO (etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine) chemotherapy. Journal of Reproductive Medicine 2012;57(5‐6):231‐6. [PubMed] [Google Scholar]
Chauhan 2010
- Chauhan A, Dave K, Desai A, Mankad M, Patel S, Dave P. High‐risk gestational trophoblastic neoplasia at Gujarat Cancer and Research Institute: thirteen years of experience. Journal of Reproductive Medicine 2010;55(7‐8):333‐40. [PubMed] [Google Scholar]
CTCAE 2010
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), v 4.0, June 2010. evs.nci.nih.gov/ftp1/CTCAE/About.html. (accessed 12 November 2012).
Cyriac 2011a
- Cyriac S, Rajendranath R, Sridevi V, Sagar TG. Management of high‐risk gestational trophoblastic neoplasia with etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine chemotherapy. Journal of Reproductive Medicine 2011;56(6):219‐23. [PubMed] [Google Scholar]
Cyriac 2011b
- Cyriac S, Rajendranath R, Sridevi V, Sagar TG. Etoposide, cisplatin‐etoposide, methotrexate, actinomycin‐D as primary treatment for management of very‐high‐risk gestational trophoblastic neoplasia. International Journal of Gynaecology and Obstetrics 2011;115(1):37‐9. [DOI] [PubMed] [Google Scholar]
Decherney 2003
- Decherney AH, Nathan L. The care and treatment of women. China: Ren‐ming Wei‐sheng Chu‐banshe, 2003. [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001:285‐312. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dobson 2000
- Dobson LS, Lorigan PC, Coleman RE, Hancock BW. Persistent gestational trophoblastic disease: results of MEA (methotrexate, etoposide and dactinomycin) as first‐line chemotherapy in high risk disease and EA (etoposide and dactinomycin) as second‐line therapy for low risk disease. British Journal of Cancer 2000;82:1547‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Driscoll 1981
- Driscoll SG. Trophoblastic growths: morphologic aspects and taxonomy. Journal of Reproductive Medicine 1981;26:2181. [PubMed] [Google Scholar]
Escobar 2003
- Escobar PF, Lurain JR, Singh DK, Bozorgi K, Fishman DA. Treatment of high‐risk gestational trophoblastic neoplasia with etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine chemotherapy. Gynecological Oncology 2003;91:552‐7. [DOI] [PubMed] [Google Scholar]
Ezoe 2012
- Ezoe S. Secondary leukemia associated with the anti‐cancer agent, etoposide, a topoisomerase II inhibitor. International Journal of Environmental Research and Public Health 2012;9:2444‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feng 2011
- Feng F, Xiang Y, Wan X, Geng S, Wang T. Salvage combination chemotherapy with floxuridine, dactinomycin, etoposide and vincristine (FAEV) for patients with relapsed/chemoresistant gestational trophoblastic neoplasia. Annals of Oncology 2011;22:1588‐94. [DOI] [PubMed] [Google Scholar]
FIGO 2009
- FIGO Committee on Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary and gestational trophoblastic neoplasia. International Journal of Gynaecology and Obstetrics 2009;105:3‐4. [DOI] [PubMed] [Google Scholar]
Fisher 2009
- Fisher R. Genetics. In: Hancock BW, Seckl M, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. www.isstd.org/isstd/book.html. 3rd Edition. International Society for the Study of Trophoblastic Diseases, 2009:6‐48. [Google Scholar]
Fülöp 2012
- Fülöp V, Szigetvári I, Szepesi J, Végh G, Singh M, Berkowitz RS. Clinical epidemiology and management of gestational trophoblastic neoplasia in Hungary in the past 34 years. Journal of Reproductive Medicine 2012;57(7‐8):310‐8. [PubMed] [Google Scholar]
Ghaemmaghami 2004
- Ghaemmaghami F, Modares M, Arab M, Behtash N, Moosavi AZ, Khanafshar N, et al. EMA‐EP regimen, as first line multiple agent chemotherapy in high‐risk GTT patients (stage II‐IV). International Journal of Gynecological Cancer 2004;14(2):360‐5. [DOI] [PubMed] [Google Scholar]
Goldstein 2012
- Goldstein DP, Berkowitz RS. Current management of gestational trophoblastic neoplasia. Hematology Oncology Clinics of North America 2012;26:111‐31. [DOI] [PubMed] [Google Scholar]
Hammond 1973
- Hammond CB, Borchert LG, Tyrey L, Creasman WT, Parker RT. Treatment of metastatic trophoblastic disease: good and poor prognosis. American Journal of Obstetrics and Gynecology 1973;115:451‐7. [DOI] [PubMed] [Google Scholar]
Harma 2005
- Harma M, Harma M, Yurtseven S, Gungen N. Gestational trophoblastic disease in Sanliurfa, southeast Anatolia, Turkey. European Journal of Gynaecologic Oncology 2005;26(3):306‐8. [PubMed] [Google Scholar]
Hayashi 1982
- Hayashi K, Bracken MB, Freeman DH, Hellenbrand K. Hydatidiform mole in the United States (1970‐1977): a statistical and theoretical analysis. American Journal of Epidemiology 1982;115:67‐77. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011. Available from www.cochrane‐handbook.org.
ICH 1997
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Good clinical practice consolidated guideline. Federal Register. 1997;62, issue 90:25691‐709.
Kim 1998
- Kim SJ, Bae SN, Kim JH, Kim CT, Han KT, Lee JM, et al. Effects of multi agent chemotherapy and independent risk factors in the treatment of high‐risk GTT ‐ 25 years experience of KRI‐TRD. International Journal of Gynaecology and Obstetrics 1998;60 Suppl 1:85‐96. [PubMed] [Google Scholar]
Kohorn 2001
- Kohorn EI. The new FIGO 2000 staging and risk factor scoring system for gestational trophoblastic disease: description and critical assessment. International Journal of Gynecologic Cancer 2001;11:73‐7. [DOI] [PubMed] [Google Scholar]
Kurman 1984
- Kurman RJ, Main CS, Chen HC. Intermediate trophoblast: a distinctive form of trophoblast with specific morphological, biochemical, and functional features. Placenta 1984;5:349‐70. [DOI] [PubMed] [Google Scholar]
Lee 2009
- Lee C, Smith HO, Kim SJ. Epidemiology. In: Hancock BW, Newlands ES, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Diseases. www.isstd.org/isstd/book.html. 3rd Edition. International Society for the Study of Trophoblastic Diseases, 2009:49‐96. [Google Scholar]
Lu 2008
- Lu WG, Ye F, Shen YM, Fu YF, Chen HZ, Wan XY, et al. EMA‐CO chemotherapy for high‐risk gestational trophoblastic neoplasia: a clinical analysis of 54 patients. International Journal of Gynecological Cancer 2008;18(2):357‐62. [DOI] [PubMed] [Google Scholar]
Lurain 1982
- Lurain JR, Brewer JI. Invasive mole. Seminars Oncology 1982;9:174‐80. [PubMed] [Google Scholar]
Lurain 2010
- Lurain JR, Singh DK, Schink JC. Management of metastatic high‐risk gestational trophoblastic neoplasia: FIGO stages II‐IV: risk factor score > or = 7. Journal of Reproductive Medicine 2010;55(5‐6):199‐207. [PubMed] [Google Scholar]
Lurain 2011
- Lurain JR. Gestational trophoblastic disease II: classification and management of gestational trophoblastic neoplasia. American Journal of Obstetrics & Gynecology 2011;January:11‐18. [DOI] [PubMed] [Google Scholar]
Matsui 2000
- Matsui H, Suzuka K, Iitsuka Y, Seki K, Sekiya S. Combination chemotherapy with methotrexate, etoposide, and actinomycin D for high‐risk gestational trophoblastic tumors. Gynecologic Oncology 2000;78(1):28‐31. [DOI] [PubMed] [Google Scholar]
Mazur 1987
- Mazur MT, Szulman AE, Buchsbaum HJ (editors). Choriocarcinoma and placental site trophoblastic tumour (in gestational trophoblastic disease). New York Springer‐Verlag. 1987.
Newlands 1986
- Newlands ES, Bagshawe KD, Begent RH, Rustin GJ, Holden L, Dent J. Developments in chemotherapy for medium and high risk patients with gestational trophoblastic tumours (1979‐1984). British Journal of Obstetrics and Gynaecology 1986;93:63‐9. [DOI] [PubMed] [Google Scholar]
Newlands 1998
- Newlands ES, Bower M, Holden L, Short D, Seckl MJ, Rustin GJ, et al. Management of resistant gestational trophoblastic tumors. Journal of Reproductive Medicine 1998;43(2):111‐8. [PubMed] [Google Scholar]
Newlands 2000
- Newlands ES, Mulholland PJ, Holden L, Seckl MJ, Rustin GJ. Etoposide and cisplatin/etoposide, methotrexate, and actinomycin D (EMA) chemotherapy for patients with high‐risk gestational trophoblastic tumors refractory to EMA/cyclophosphamide and vincristine chemotherapy and patients presenting with metastatic placental site trophoblastic tumors. Journal of Clinical Oncology 2000;18:854‐9. [DOI] [PubMed] [Google Scholar]
Pastorfide 1974
- Pastorfide GB, Goldstein DP, Kosasa TS. The use of a radioimmunoassay specific for human chorionic gonadotropin in patients with molar pregnancy and gestational trophoblastic disease. American Journal of Obstetrics and Gynecology 1974;120:1025‐8. [DOI] [PubMed] [Google Scholar]
Popiolek 2006
- Popiolek DA, Yee H, Mittal K, Chiriboga L, Prinz MK, Caragine TA, et al. Multiplex short tandem repeat DNA analysis confirms the accuracy of p57(KIP2) immunostaining in the diagnosis of complete hydatidiform mole. Human Pathology 2006;37(11):1426‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Schink 1992
- Schink JC, Singh DK, Rademaker AW, Miller DS, Lurain JR. Etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine for the treatment of metastatic, high‐risk gestational trophoblastic disease. Obstetrics & Gynecology 1992;80:817‐20. [PubMed] [Google Scholar]
Schorge 2003
- Schorge JO. Contemporary therapy in obstetrics and gynecology. China: Ren‐ming Wei‐sheng Chu‐banshe, 2003. [Google Scholar]
Seckl 2009a
- Seckl MJ. Presentation and management of persistent gestational trophoblastic disease (GTD) and gestational trophoblastic tumours (GTT) in the United Kingdom. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational trophoblastic disease. www.isstd.org/isstd/book.html. 3rd Edition. International Society for the Study of Trophoblastic Diseases, 2009:277‐298. [Google Scholar]
Seckl 2009b
- Seckl MJ. Investigation and treatment of patients with persistent gestational trophoblastic disease and gestational trophoblastic tumours/neoplasia in the United Kingdom. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. www.isstd.org/isstd/book.html. 3rd Edition. International Society for the Study of Trophoblastic Diseases, 2009:335‐66. [Google Scholar]
Seckl 2010
- Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet 2010;376:717‐29. [DOI] [PubMed] [Google Scholar]
Song 2004
- Song HZ. Diagnosis and treatment of trophoblastic tumours. China: Ren‐ming Wei‐sheng Chu‐banshe, 2004. [Google Scholar]
Soper 1994
- Soper JT, Evans AC, Clark‐Pearson DL, Berchuck A, Rodriguez G, Hammond CB. Alternating weekly chemotherapy with etoposide‐methotrexate‐dactinomycin/cyclophosphamide‐vincristine for high‐risk gestational trophoblastic disease. Obstetrics & Gynecology 1994;83(1):113‐7. [PubMed] [Google Scholar]
Szulman 1978
- Szulman AE, Surti U. The syndromes of hydatidiform mole: I. cytogenic and morphologic correlations and II morphologic evolution of the complete and partial mole. American Journal Obstetrics and Gynecology 1978;131 and 132:665‐71;20‐7. [DOI] [PubMed] [Google Scholar]
Tang 2003
- Tang Zhao‐you. Current Tumourology. China: Ren‐ming Wei‐sheng Chu‐banshe, 2003. [Google Scholar]
Turan 2006
- Turan T, Karacay O, Tulunay G Boran N, Koc S, Bozok S, et al. Results with EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) chemotherapy in gestational trophoblastic neoplasia. International Journal of Gynecological Cancer 2006;16:1432‐8. [DOI] [PubMed] [Google Scholar]
Wang 2006
- Wang S, An R, Han X, Zhu K, Xue Y. Combination chemotherapy with 5‐fluorouracil, methotrexate and etoposide for patients with high‐risk gestational trophoblastic tumors: a report based on our 11‐year clinical experiences. Gynecologic Oncology 2006;103(3):1105‐8. [DOI] [PubMed] [Google Scholar]
WHO 1983
- WHO Scientific Group. Gestational trophoblastic disease. Technical Report Series No 692. 1990. [PubMed]
Wong 1986
- Wong LC, Choo YC, Ma HK. Modified Bagshawe's regimen in high‐risk gestational trophoblastic disease. Gynecologic Oncology 1986;23:87‐93. [DOI] [PubMed] [Google Scholar]
Zhao 2000
- Zhao Xia‐yu. Gestational trophoblastic tumours with EMA/CP regime: an analysis of 22 cases. Journal of Bengbu Medical School 2000;25(5):334‐5. [Google Scholar]
Zhao 2009
- Zhao Y, Zhang W, Duan W. Management of gestational trophoblastic neoplasia with 5‐fluorouracil and actinomycin D in northern China. Journal of Reproductive Medicine 2009;54(2):88‐94. [PubMed] [Google Scholar]
References to other published versions of this review
Deng 2009
- Deng L, Yan X, Zhang J, Wu T. Combination chemotherapy for high‐risk gestational trophoblastic tumour. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD005196.pub3] [DOI] [PubMed] [Google Scholar]


