Abstract
Venetoclax is a small molecule inhibitor of the pro-survival protein BCL-2 that has gained market approval in BCL-2 dependent hematological cancers such as chronic lymphocytic leukemia (CLL) and acute myeloid leukemia (AML). Neuroblastoma (NB) is a heterogenous pediatric cancer with a five-year survival rate of less than 50% for high-risk patients, which include nearly all cases with amplified MYCN. We have previously demonstrated that venetoclax is active in MYCN-amplified NB but has limited single-agent activity in most models, presumably the result of other pro-survival BCL-2 family protein expression or insufficient pro-death protein mobilization. As the relative tolerability of venetoclax makes it amenable to co-dosing with other therapies, we evaluated the sensitivity of MYCN-amplified NB models to rational combinations of venetoclax with agents that have both mechanistic complementarity as well as active clinical programs. First, MDM2 inhibitor NVP-CGM097 induces an increase in the pro-death BH3-only protein NOXA to sensitize p53-wild-type, MYCN-amplified NBs to venetoclax. Second, the MCL1 inhibitor S63845 sensitizes MYCN-amplified NB through direct neutralization of MCL-1, which induces marked synergistic cell killing when combined with BCL-2 inhibition. Lastly, the standard of care drug cocktail cyclophosphamide and topotecan reduces the apoptotic threshold of NB, thus setting the stage for robust combination efficacy with venetoclax. In all cases, these rational combinations translated to in vivo tumor regressions in MYCN-amplified PDX models. Venetoclax is currently being evaluated in pediatric patients in the clinic, including those with neuroblastoma (NCT03236857). While establishment of safety is still ongoing in this population, the data disclosed herein indicate rational and clinically actionable combination strategies that could potentiate the activity of venetoclax in MYCN-amplified NB patients.
Keywords: neuroblastoma, MYCN, BCL-2, MCL-1, apoptosis
Introduction
MYCN-amplified neuroblastoma (NB) is often fatal and comprises roughly one quarter of all NBs (1). Outcomes for patients with MYCN-amplified tumors continue to be poor despite recent immunotherapy advances with the anti-GD2 antibody dinutuximab (2). Chemotherapy, including topotecan and cyclophosphamide, is the standard of care first-line treatment (3). However, relapse for MYCN-amplified NBs are common, and outside of a subset of tumors with ALK mutations (4), kinase inhibitor-sensitizing alterations are rare, underlining the need for new therapeutic approaches (5, 6). We along with others have demonstrated that while MYCN is a potent oncogene in NB (7), its propensity to prime cells for apoptosis creates treatment opportunities unique to this subset of the disease (8-11).
The BCL-2 inhibitor venetoclax is FDA-approved in chronic lymphocytic leukemia (CLL) (12) and AML, and has demonstrated impressive clinical activity in other blood cancers such as multiple myeloma (13). The relative tolerability of venetoclax makes it an attractive target for combination therapies, where it is currently being explored in a number of clinical trials (14, 15). Indeed, combination of venetoclax with either the BTK inhibitor ibrutinib (17) or the anti-CD20 antibody obinutuzumab (18) have demonstrated potent efficacy and tolerability in CLL (16), including elderly patients with co-existing health conditions (17). In the pediatric population, venetoclax is currently being evaluated in phase I trials, which include both blood cancers and NB (NCT03236857). While unexpected toxicities in the pediatric population cannot be ruled out, venetoclax will likely be tolerable to use in diverse combination therapy strategies in the NB setting.
The BCL-2 family of proteins governs intrinsic apoptosis largely at the mitochondria. Here, a balance of pro-survival (e.g., BCL-2, BCL-xL and MCL-1), and pro-death proteins interact to influence cellular outcome (18). The latter subset of proteins are subdivided into two groups; the BH3-only pro-death members (e.g., BIM, NOXA, PUMA), and the terminal effector molecules (BAK and BAX) which disrupt mitochondrial pores when activated (18). Hogarty and colleagues first demonstrated that models of NB have a dependence on the pro-survival BCL-2 family proteins, thus presenting a potential vulnerability to small molecule inhibitors targeting BCL-2, BCL-xL, or MCL-1 (8, 19-23). For instance, in an unbiased drug screen of the dual BCL-2/BCL-xL inhibitor navitoclax against ~800 solid tumor cell lines, we found MYCN-amplified NB collectively made up the most sensitive subset (8). Importantly, in a follow-up screen with venetoclax, we found a similar response profile, with over 30% of MYCN-amplified NB demonstrating single-agent sensitivity; (24). This sensitivity to venetoclax is realized, at least in part, because amplified MYCN binds to and upregulates PMAIP1 (NOXA), a BH3-only protein and venetoclax sensitizer (8). However, the majority of MYCN-amplified NBs still require additional therapy to unleash the potential of venetoclax to kill these tumors.
We have previously demonstrated that certain agents enhance venetoclax activity in models of MYCN-amplified NBs (8, 23), however, these agents are either not in active clinical trials or do not show sufficient anti-tumor efficacy in NB (25). We were therefore interested in identifying more clinically viable combinations that could rationally modulate the BCL-2 family of proteins in MYCN-amplified NB, thus decreasing the apoptotic threshold to venetoclax and providing a potential and novel treatment option in this patient population.
Materials and Methods
Cell lines
The neuroblastoma cell lines IMR5, SIMA, SMS-SAN, LAN-5, NB-1643, SK-N-AS, SK-N-BE(2), SK-N-DZ, SK-N-SH, 293T, and KELLY were from the Molecular Center Therapeutics Laboratory at Massachusetts General Hospital, which performs routine testing of cell lines by single-nucleotide polymorphism and short tandem repeat analysis. The NB-EBc1 and SK-N-FI cell lines were provided by the Children’s Hospital of Pennsylvania (Y. Mossé). COG-N-415, COG-N-496, COG-N-561, CHLA-20, CHLA-90, CHLA-119, CHLA-297 and CHLA-172 were obtained from the Children’s Oncology Group (COG) Cell Culture and Xenograft Repository. MRC-5 was obtained from the Japanese Cell Research Bank (Osaka, Japan). HCE-T was obtained from RIKEN BioResource Center (Tsukuba, Japan). The NB-EBc1 and 293T cell lines were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). The SK-N-AS, SK-N-BE(2), SK-N-SH, SK-N-DZ, IMR-5, HCE-T, and SK-N-FI cell lines were cultured in DMEM/F12 (50:50) supplemented with 10% FBS. The SIMA, KELLY, NB-1643, LAN-5, and SMS-SAN cell lines were cultured in RPMI 1640 supplemented with 10% FBS. The CHLA-20, CHLA-90, CHLA-119, CHLA-297, and CHLA-172 cell lines were cultured in DMEM supplemented with 20% FBS and 1× insulin-transferrin-selenium (ITS; Thermo Fisher Scientific, catalog #41400045). The COG-N-415, COG-N-561, and COG-N-496 cell lines were cultured in Iscove’s modified Dulbecco’s medium supplemented with 20% FBS and 1× ITS.
Drugs
Venetoclax was provided by AbbVie. NVP-CGM097 and S63845 were provided by ChemieTek. Cyclophosphamide and topotecan were from Sigma-Aldrich.
Western blotting
Cells were lysed with lysis buffer (20mM Tris, 150mM NaCl, 1% NP-40, 1 mM EDTA, 1mM EGTA, 10% glycerol, and protease and phosphatase inhibitors), incubated on ice for 10 min and centrifuged at 10,500 rpm for 10 min at 4 °C. Equal amounts of the detergent-soluble lysates were resolved using the NuPAGE® Novex® Midi Gel system on 4% to 12% Bis-Tris Gels (Invitrogen), transferred to PVDF membranes (PerkinElmer) in between 6 pieces of Whatman paper (Fisher Scientific) set in transfer buffer from Bio-Rad Laboratories with 20% methanol, and following transfer and blocking in 5% non-fat milk in PBS, probed overnight with the antibodies listed below. Chemiluminescence was detected with the Syngene G: Box camera (Synoptics). Antibodies were used at a dilution of 1:1000 in 5% BSA-TBST are as follows: from Cell Signaling Technologies, PUMA (catalog #4976S), NOXA (catalog #14766S), cleaved caspase-3 (catalog #9661), p53 (catalog #2527S), cleaved PARP (catalog #5625); from Santa Cruz Biotechnology, GAPDH (catalog #sc-32233).
Cell viability
Neuroblastoma cells were seeded in quadruplicate in 96-well microtiter plates at a concentration of 4 × 103 cells per well in 180 μl of growth medium and after 24h, cells were treated with drug for 16h or cells were seeded at a concentration of 2 × 103 cells per well and after 24h, cells were treated with drug for 72h. Cell viability was measured by the CellTiter-Glo protocol per the manufacturer (Promega).
Bliss Independence Model
We utilized a Bliss independence model to determine whether there was synergy between the drugs investigated. The Bliss expectation was calculated as: (A+B) A*B, where A is the fractional growth inhibition induced by drug 1, and B is the fractional growth inhibition induced by drug 2, in the respected drug combinations. The Bliss excess is demonstrated for each datum as the difference between the Bliss expectation and the observed growth inhibition induced by each combination (26, 27). The total Bliss sum was calculated by the summation of the bliss scores at the drug concentrations shown.
Crystal violet
Cells were seeded at 5 × 104 cells per well in a six-well dish and treated the following day with drug. Five or six days later, when untreated cells reached confluency, cells were stained with 0.1% crystal violet (Sigma-Aldrich).
FACS apoptosis
Cells were seeded at 1 × 105 cells per well in a six-well plate. After 24h post seeding, cells were treated with drug for 16h. Cells were stained with propidium iodide and annexin V–Cy5 (BD Biosciences) and assayed on a Guava easyCyte flow cytometer (Millipore Sigma). Analysis was performed using FlowJo by separating the measured cells into four quadrants, and cells within annexin V–positive and annexin V/propidium iodide–double positive quadrants were counted as apoptotic.
Statistical analyses
Biological triplicates were used unless explicitly stated otherwise. Statistical analyses were performed using one-way analysis of variance (ANOVA). Results were considered significant when P < 0.05.
Xenograft and patient-derived xenograft models
COG-N-561x and COG-N-415x PDX models from Children’s Oncology Group (COG) Cell Culture and Xenograft Repository were injected into female NOD-SCID-gamma (NSG) mice at 5 × 105 cells per mouse using a 1:1 ratio of cells/Matrigel (Corning, catalog #354248). These mice were randomized when they reached an average tumor size of 170 mm3, with cohorts being five controls, five NVP-CGM097 treated, five venetoclax treated and five combination treated. NVP-CGM097 (50mg/kg) and venetoclax (100 mg/kg) were delivered by oral gavage (100 μl; 60% PHOSAL 50PG, 30% PEG-400, 10% ethanol) 5 days/week. The COG-N-415x PDX model was also injected into female NSG mice at 4.5 × 105 cells per flank, with both flanks being injected, using a 1:1 ratio of cells/Matrigel. Mice were randomized when they reached an average tumor size of 180 mm3, with cohorts being four controls, four S63845 treated, four venetoclax treated, and four combination treated. S63845 (25mg/kg) was delivered via tail vein injection (100ul; 25mM HCl, 20% 2-hydroxy propyl-β-cyclo dextrin) 2 days/week, and venetoclax (100mg/kg) was delivered by oral gavage (200 μl; 60% PHOSAL 50PG, 30% PEG-400, 10% ethanol) 5 days/week. All animal experiments were approved by the Virginia Commonwealth University (VCU) Institutional Animal Care and Use Committee (protocol #AD10001048).
COG-N-453x and COG-N-424x PDX models and NB-1643CRX xenograft model were implanted subcutaneously into the right flank of female CB17 SCID mice. When the tumors reached an average size of 200mm3, the mice were randomized with cohorts being ten vehicles treated, ten chemotherapy (cyclophosphamide plus topotecan, cyclo/topo) treated, ten venetoclax treated, and ten combination treated. Vehicle mice received the solvent for all drugs according to their respective drug routes and schedules. Venetoclax (100 mg/kg) was delivered by oral gavage (60% PHOSAL 50PG, 30% PEG-400, 10% ethanol) 7 days/week. Topotecan (0.5 mg/kg) and cyclophosphamide (20 mg/kg) were delivered by intraperitoneal injection according to the following schedule: 5 days on treatment, 16 days off treatment, cycle resumed on day 21. Mice were weighed and tumors were measured, using a digital caliper, twice a week during the treatment period. All animal studies were approved by The Children’s Hospital of Philadelphia IACUC (protocol # 2012-5-643).
Lentivirus production
The lentiviral short-hairpin RNA (shRNA)-expressing constructs NOXA and PUMA were purchased from Sigma. The constructs were transfected into 293T packaging cells along with the packaging plasmids pMD2.G and psPAX2 (Addgene) and the lentivirus-containing supernatants were used to transduce the NB cells IMR5, SIMA, and SMS-SAN. The pMXs-hu-N-Myc (28) was from Shinya Yamanaka (Addgene plasmid #50772), which was cloned into the pLENTI backbone to form pLENTI-MYCN.
siRNA
NOXA siRNA (cat. #L-005275-00-0005) and scramble (siSCR) control siRNA (cat. #D-001810-10-20) were purchased from Dharmacon. Lipofectamine RNAiMAX Transfection Reagent (ThermoFisher Scientific) was used to produce the knockdown cells with 50 nmol/L of siRNAs, following the manufacturer's protocol.
Results
MDM2 inhibitors increase the BH3-only pro-death protein NOXA and potentiate venetoclax cell killing in MYCN-amplified NB
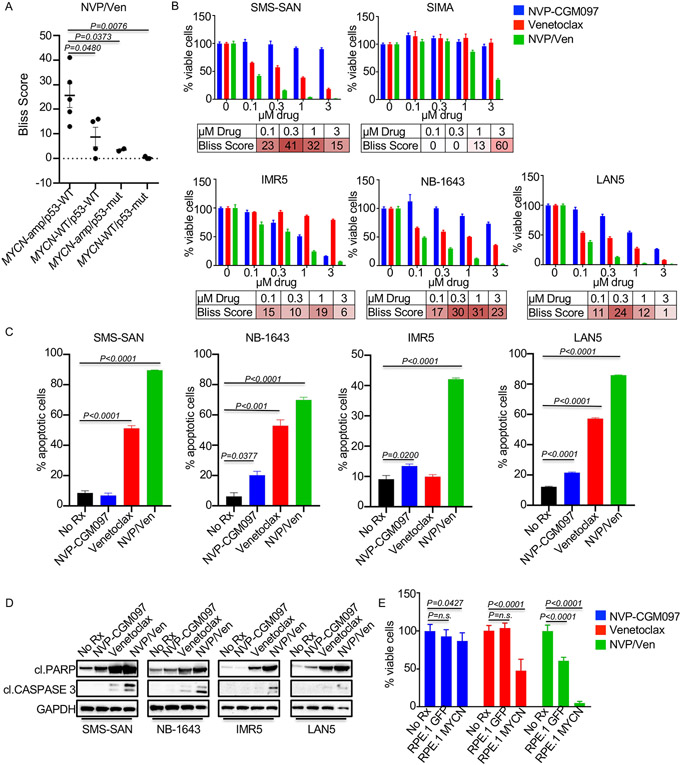
NB tumors, even in the relapsed population, are wild-type for p53 in >90% of cases (5, 6). MDM2 is a direct transcriptional target of MYCN (29), and MDM2 inhibition has been demonstrated to increase the expression of p53 targets in MDM2 expressing cancers, including the BH3-only proteins NOXA and PUMA (30). We hypothesized that MDM2 inhibition (with NVP-CGM097) would synergize with venetoclax in MYCN-amplified, p53-wild-type NB, through increased NOXA and/or PUMA expression. To test this hypothesis, we first evaluated the ability of NVP-CGM097 to sensitize NBs to venetoclax. To do this, we used the Bliss synergy (excess over the bliss) scores. The Bliss score is the difference between the calculated inhibition value if the two agents act independently and the observed combined inhibition values. Positive Bliss scores indicate synergy. We first performed viability assays over 16h; consistent with the sensitivity of MYCN-amplified NBs to venetoclax (8, 31), the combination showed synergistic killing of MYCN-amplified/p53-wild-type NB cell lines compared to the other genotypes (MYCN-WT/p53-WT, MYCN-WT/p53-mutant, MYCN-amp/p53 mutant) (Figs. 1A-B and Sup. Fig. 1). As expected, the efficacy of MDM2 inhibition was ablated in p53 mutant NB, regardless of MYCN status (Fig. 1A and Sup. Fig. 1). The kinetics of cell death were rapid, where just 16h of drug exposure was sufficient for the combination to induce >50% apoptosis in three of the four MYCN-amplified p53-wild-type cell lines investigated (Fig. 1C). We next confirmed apoptosis as a key mechanism of enhanced sensitivity to venetoclax in the MYCN-amplified NBs (Fig. 1D). Demonstrating causality of amplified MYCN, we found expression of exogenous MYCN in the RPE.1 neural crest cell line resulted in increased apoptosis and decreased cell viability to the NVP-CGM097/venetoclax combination compared to GFP expressing RPE.1 control cells (32) (Fig. 1E). Overall, these data demonstrate that NVP-CGM097 enhances the apoptotic potential of MYCN-amplified NB, and that amplified MYCN plays a causal role in the efficacy of venetoclax plus NVP-CGM097 (8, 31).
Figure 1. NVP-CGM097 synergizes with venetoclax in MYCN-amplified neuroblastomas.
(A) Plot of maximum bliss synergy scores up to 1μM from 16h cell viability assays using the combination of NVP-CGM097 and venetoclax. (B) Cell viability assay with the indicated MYCN-amplified, p53-wild-type cell lines treated for 16h with NVP-CGM097, venetoclax, or the combination at indicated concentrations with bliss synergy scores shown. (C) Cells treated for 16h with 1μM NVP-CGM097, venetoclax, or the combination of both drugs were stained for Annexin V/Propidium iodide, and the Annexin V positive, and Annexin V/Propidium iodide–double positive quadrants were counted as apoptotic. (D) Western blot of the cell lines from (C) treated with 1μM of the indicated drug for 8h and probed with the indicated antibodies. (E) Cell viability assay of the RPE.1 cells expressing GFP or MYCN treated for 16h with 10 μM of NVP-CGM097, venetoclax, or the combination of both.
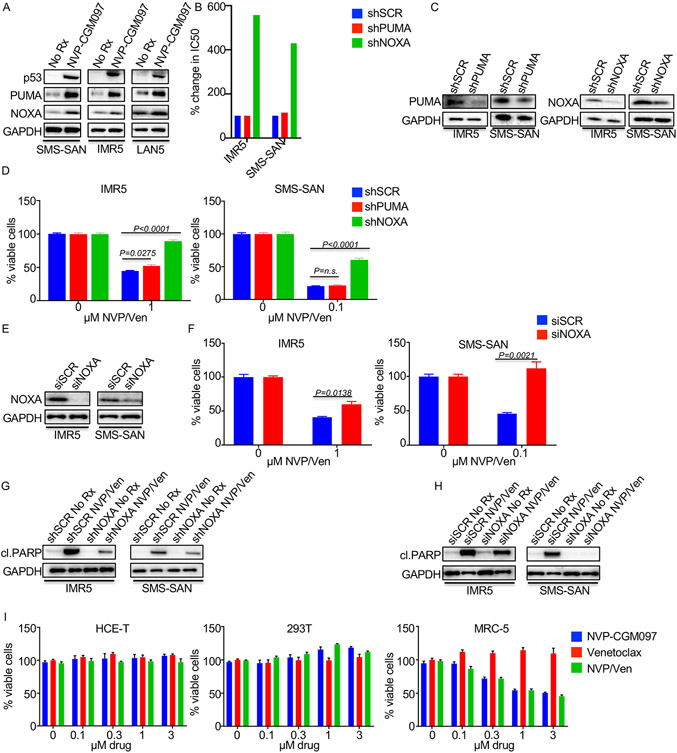
We next sought to determine the mechanistic basis for sensitization. We found that the p53 targets PUMA and NOXA were increased following NVP-CGM097 treatment, indicating activation of the p53 apoptotic pathway (8, 19, 23, 33, 34) (Fig. 2A). We next asked whether the induction of PUMA and NOXA was important for NVP-CGM097 potentiation of venetoclax activity. Interestingly, while knockdown of PUMA had little to no effect on combination efficacy, knockdown of NOXA led to a four-fold decrease in the combination activity across two MYCN-amplified NB models (Figs. 2B-F), which led to a decrease in the cleavage of PARP (Fig. 2G, Fig. 2H). These data demonstrate that NOXA mediates the efficacy of MDM2 inhibitor/venetoclax combination (Figs. 2B-H). While our data demonstrate an increased sensitivity of MYCN-amplified/p53-wild-type NB to the venetoclax-based combination, we wanted to further evaluate the potential therapeutic window. Thus, we treated three normal-tissue derived cells (all with wild-type p53) with the combination for 72h. Despite activity of NVP-CGM097 in the MRC-5 fibroblast cells derived from lung (34), no synergistic activity of the two drugs together was observed in any of the three normal-tissue derived cells tested (Fig. 2I). Altogether, these data imply MDM2i/venetoclax may have a therapeutic window in MYCN-amplified NB.
Figure 2. Activation of the p53 targets, the BH3-only PUMA and NOXA, following NVP-CGM097 therapy.
(A) Western blot of MYCN-amplified, p53-wild-type cell lines treated with 1μM of the indicated drug for 8h and probed with the indicated antibodies. (B) IC50 of MYCN-amplified, p53-wild-type cell lines transduced with shPUMA, shNOXA, or shSCR, and treated for 8h with the combination of NVP-CGM097 and venetoclax. (C) Western blot of the cell lines from (B) probed with the indicated antibodies. (D and E) MYCN-amplified, p53-wild-type cell lines were transduced with shSCR, shPUMA, or shNOXA virus and (D) assayed following 16h of the indicated treatment or (E) Western blotted following the indicated drug treatments for 8h and probed with the indicated antibodies. (F, G and H) MYCN-amplified, p53-wild-type cell lines were transfected with siRNA targeting NOXA or siSCR (F) and (G) assayed following 16h of the indicated treatment or (H) Western blotted following treatment with the indicated drug for 8h and probed with the indicated antibodies. (I) Cell viability assay of normal-tissue derived cell lines treated for 72h with NVP-CGM097, venetoclax, or the combination of the drugs at the indicated concentrations.
MDM2 inhibitors combine with venetoclax to inhibit tumor growth in MYCN-amplified NB PDX models
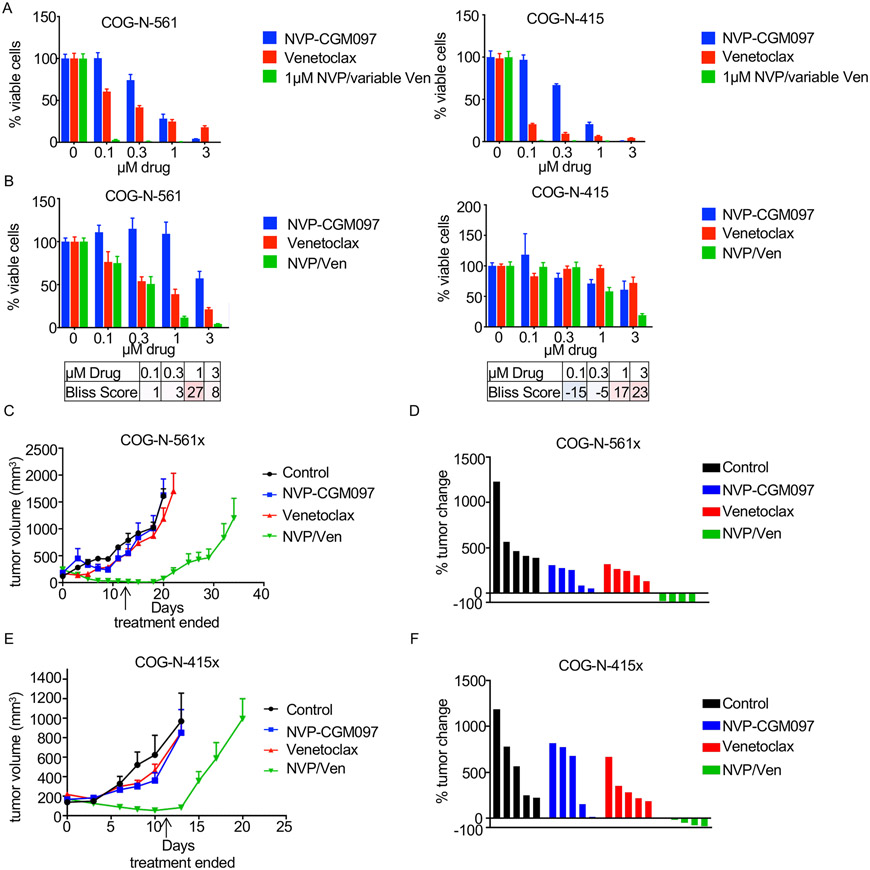
We next evaluated whether NVP-CGM097 could sensitize MYCN-amplified patient-derived xenograft (PDX) models to venetoclax. We chose two MYCN-amplified NB PDX models which had corresponding ex vivo cell cultures: the COG-N-561x and the COG-N-415x, the latter of which was established from a patient following chemotherapy relapse (32). Evaluation of the cell cultures of these two models demonstrated a strong ability of 1μM of NVP-CGM-097 to sensitize to venetoclax (Fig. 3A) and synergistic activity across several doses of each drug (Fig. 3B).
Figure 3. NVP-CGM097 and venetoclax effective in vivo.
(A) Cell viability assays were performed with MYCN-amplified NB ex vivo cell lines corresponding to PDX models treated for 72h with increasing NVP-CGM097, increasing venetoclax, or increasing venetoclax in combination with 1μM NVP-CGM097. (B) Cell viability assays were performed with MYCN-amplified NB ex vivo cell lines corresponding to PDX models treated for 16h with NVP-CGM097, venetoclax, or the combination at the indicated concentrations with bliss synergy scores shown. Red indicates synergy, blue indicates antagonism. (C) The COG-N-561x PDX model was treated with NVP-CGM097, venetoclax, or the combination of both drugs 5 days/week for two weeks and tumor size was monitored for 35 days. (D) Percent change of tumor volume on the day treatment ended for the COG-N-561x PDX model. (E) The COG-N-415x PDX model was treated with NVP-CGM097, venetoclax, or the combination of both drugs 5 days/week for two weeks and tumor size was monitored for 20 days. (F) Percent change of tumor volume on the day treatment ended for the COG-N-415x PDX model.
As the combination of MDM2 inhibition and venetoclax has reached clinical testing (e.g. NCT04029688), we sought to investigate activity of the combination in vivo. Mice were treated with 50mg/kg of NVP-CGM097, 100 mg/kg of venetoclax, or both on a schedule of 5 days/week for two weeks. Consistent with our previous studies (8, 23), venetoclax had limited single agent activity in vivo in both the COG-N-415x and COG-N-561x models (Figs. 3C-F). Similarly, single-agent NVP-CGM097 had only a modest effect in slowing the growth of the tumors; in contrast, the combination was sufficient to shrink tumors in both models (Figs. 3C-F). In particular, the COG-N-561x tumors remained dormant for nearly two weeks without any regrowth following cessation of therapy (Figs. 3C-D). Analyses of the signaling changes in the COG-N-561 ex vivo cells demonstrated cleavage of PARP with corresponding NOXA upregulation (Sup. Fig. 2A). Together, these data indicate that NVP-CGM097 sensitizes venetoclax in PDX models of MYCN-amplified NB and overall, presents a compelling combination therapy strategy in this subset of NB.
Standard induction chemotherapy cyclophosphamide and topotecan sensitizes MYCN-amplified neuroblastoma to venetoclax
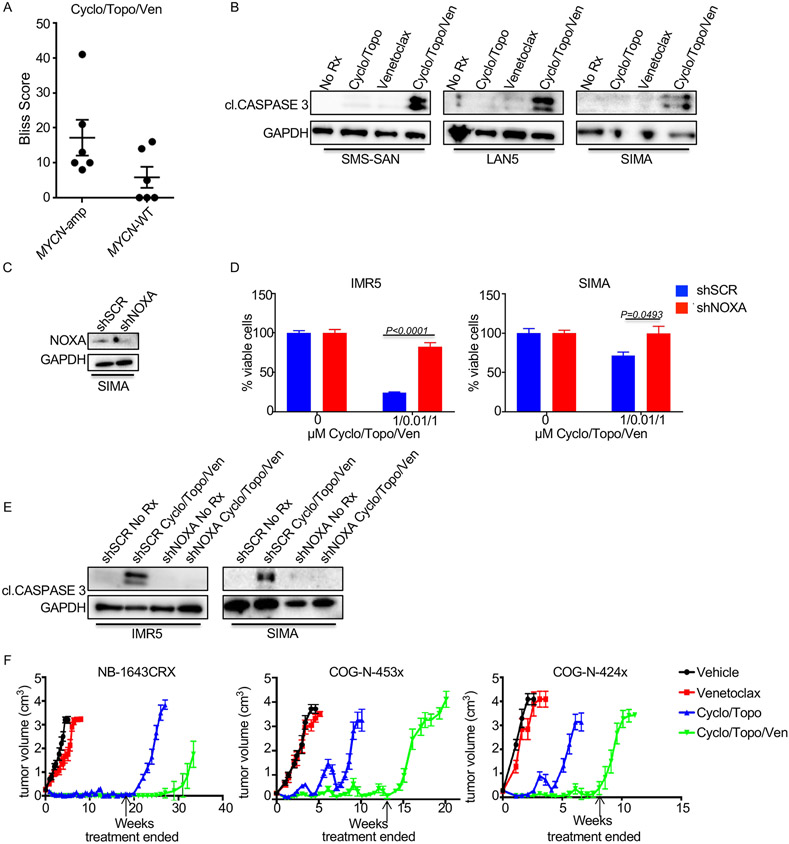
Various chemotherapy regimens have been shown to potentiate the activity of venetoclax by lowering of the apoptotic threshold, with cancers closer to this threshold being most sensitive to the corresponding combinations (35). To extend this observation to models of NB, we tested standard of care chemotherapies in the presence of venetoclax. Cyclophosphamide (DNA crosslinker) and topotecan (DNA intercalator) are components of induction chemotherapy and combine to target the ability and fidelity of DNA replication. Viability assays conducted attested to the combination having the greatest synergistic activity in MYCN-amplified NB cell lines (Fig. 4A and Sup. Fig. 3). Additionally, the combination of cyclophosphamide, topotecan (cyclo/topo) and venetoclax induced marked cell death at early time points post treatment where cell death was not evident by either the chemotherapy cocktail or venetoclax administered alone (Fig. 4B). The knockdown of NOXA in the IMR5 and SIMA cell lines was shown to protect the cells from the loss of cell viability (Fig. 4C) and apoptosis (Fig. 4D).
Figure 4. Chemotherapy and venetoclax combine to control neuroblastoma tumor growth.
(A) Plot of maximum bliss synergy scores up to 1μM from 72h cell viability assays using the combination of chemotherapy (cyclo/topo), and venetoclax. (B) Western blot of MYCN-amplified cell lines treated for 48h with 1μM of venetoclax, 1μM of cyclophosphamide and 10nM of topotecan, or a combination of both conditions. Antibodies used to detect proteins are indicated. (C and D) MYCN-amplified cell lines were transduced with scramble control or shNOXA virus and (C) viability assays were performed with the indicated drugs for 72h, or (D), Western blotted following 48h of treatment with 1μM of venetoclax, 1μM of cyclo and 10nM of topotecan and probed with the indicated antibodies. (E) The indicated PDX models or the NB-1643CRX (crizotinib resistant) xenograft model were treated with chemotherapy, venetoclax, or a combination of both treatments for the indicated timeframes (dose stoppage is indicated by an arrow). Tumor size is presented as cm3.
We next evaluated the chemotherapy combination with venetoclax in vivo. We observed increased efficacy of cyclo/topo plus venetoclax in three models: two MYCN-amplified PDX models, COG-N-453x and COG-N-424x, and the MYCN-amplified, ALK mutant NB-1643CRX xenograft model that developed resistance to crizotinib upon continuous in vivo treatment (36). As anticipated from previous studies (8, 23), venetoclax had limited activity as a single therapy (Fig. 4E). Interestingly, this included the NB-1643CRX model; the parent line from which it is derived (NB-1643) has an ALK sensitizing mutation (R1275Q) and is one of the limited MYCN-amplified NB models that has marked sensitivity to venetoclax in vivo as a monotherapy (8). Therefore, the resistance of NB1643CRX suggests cross-resistance to venetoclax was developed in this model.
The cyclo/topo cocktail showed robust initial activity across all three models, however regrowth occurred during treatment in both the COG-N-453x and COG-N-424x models. In each case the addition of venetoclax was sufficient to suppress this regrowth, which continued throughout the treatment period. The chemotherapy cocktail showed marked regression in the NB-1643CRX model that continues throughout treatment, with regrowth occurring rapidly upon cessation of therapy. The combination of venetoclax extended the tumor regression for several weeks even after the treatment was stopped (Fig. 4E).
Moreover, despite an initial weight loss, mice in the combination group regained weight over the course of the study and did not show other overt signs of toxicity, suggestive of tolerability in patients (Sup. Fig. 4A). These data demonstrate in vivo activity, and suggest that the combination of cyclo/topo and venetoclax is markedly effective in crizotinib resistant NB.
MCL1 inhibition sensitizes MYCN-amplified neuroblastoma to venetoclax
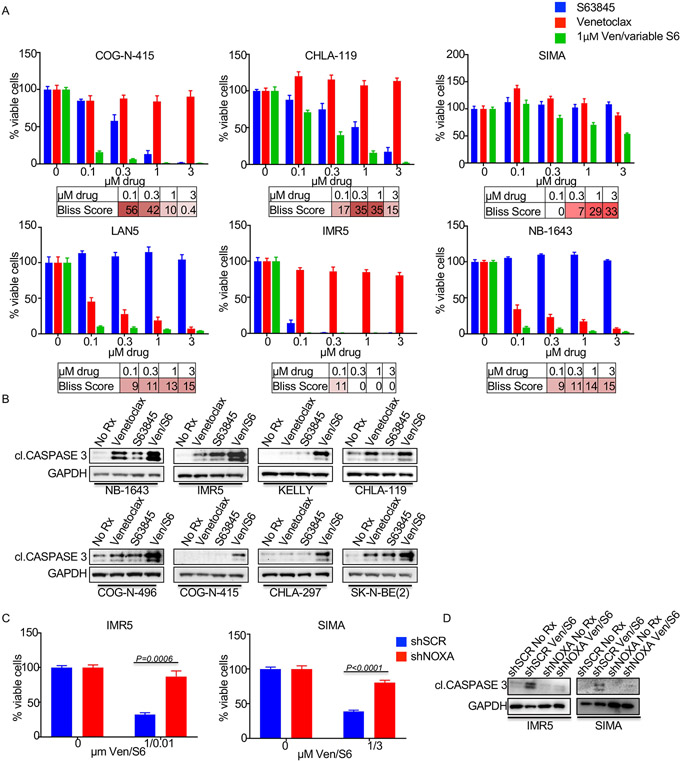
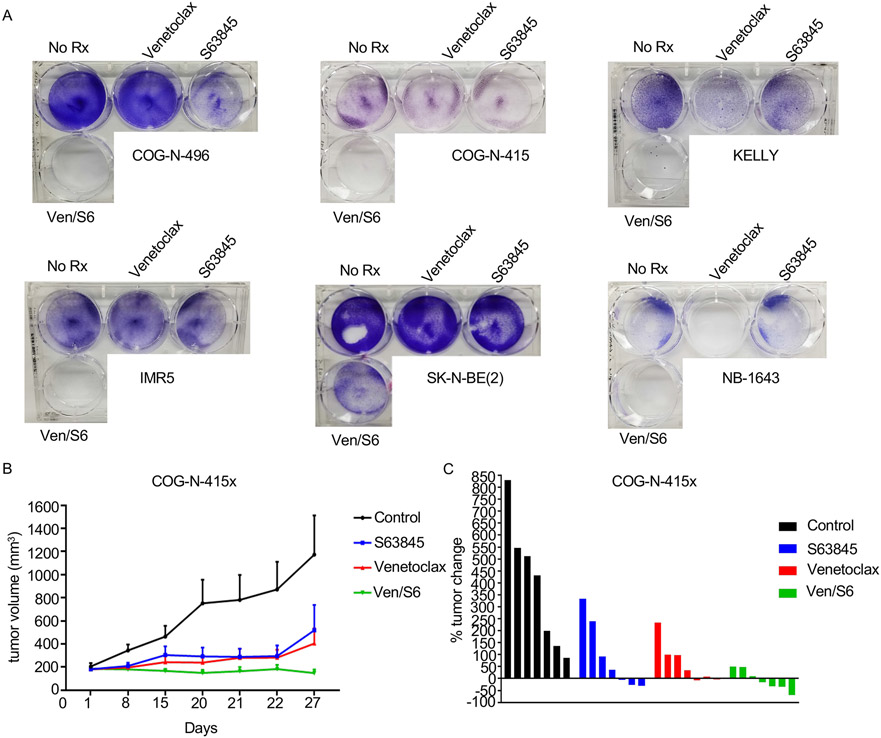
Additional anti-apoptotic BCL-2 family members BCL-xL and MCL-1 are primarily responsible for resistance to venetoclax, through sequestering of pro-apoptotic BCL-2 family members (37-40). We previously reported that BCL-xL levels are low in NB (8), thus suggesting that MCL-1 can protect NB models from BCL-2 inhibition. While upregulation of the endogenous MCL-1 inhibitor NOXA via MDM2 inhibition sensitizes NB cells to venetoclax (Figs. 1 and 2), direct targeting of MCL-1 with recently developed BH3 mimetics provides an alternative strategy for neutralizing this key pro-survival protein. To this end, we treated a series of MYCN-amplified NB cell lines and PDX cell cultures with increasing concentrations of the MCL-1 BH3 mimetic S63845 (41) in the presence or absence of 1μM venetoclax (Fig. 5A). These data demonstrated a considerable synergy across multiple doses of S63845 in these models (Fig. 5A). Western blot analyses indicated synergistic cell death in the combination consistent with their on-target activity (Fig. 5B). Again, knockdown of NOXA led to protection from the combination treatment in terms of both decreased cell viability (Fig. 5C) and cell death (Fig. 5D). In longer (~1 week) assays, with the exception of the NB-1643 cells, which were exquisitely sensitive to venetoclax alone (8), the combination was markedly more effective than monotherapy treatments and led to near total loss of cells together (Fig. 6A).
Figure 5. S63845 and venetoclax combine to decrease cell viability.
(A) Cell viability assays with the MYCN-amplified NB cell lines treated for 72h with increasing S63845, venetoclax, or 1μM venetoclax and increasing S63845 at the indicated concentrations. (B) Western blot of MYCN-amplified NB cell lines treated with 1μM venetoclax, 0.1μM S63845, or the combination of both for 24h with antibodies indicated. (C and D) The MYCN-amplified cell lines IMR5 and SIMA were transduced with scramble control or shNOXA virus and (C) assayed for viability following treatment with the indicated drugs for 72h and (D) Western blotted following the indicated drug treatments for 24h and probed with the indicated antibodies.
Figure 6. S63845 synergizes with venetoclax in MYCN-amplified neuroblastomas.
(A) Crystal violet assay for 5-7 day treatment with 1μM venetoclax, 0.1μM S63845, or the combination in MYCN-amplified NB cell lines. (B) The COG-N-415x PDX model was treated with S63845, venetoclax, or the combination of both drugs for 27 days. (C) Percent of tumor change in COG-N-415x PDX model on day 22 of treatment.
Lastly, we evaluated the efficacy of this combination in a MYCN-amplified PDX (COG-N-415x) model established from a patient following progression of disease/chemotherapy failure (Fig. 6B). Administration of venetoclax (100mg/kg/qd) (24) again had some tumor growth inhibition activity (as in Fig. 3D), and we also found S63845 monotherapy (25mg/kg, 2/week) (41) resulted in moderate tumor growth control as well, in some but not all tumors within each cohort. In contrast, the combination of venetoclax and S63845 controlled the tumor growth of each of the seven tumors, with four of the seven tumors showing clear regression (Fig. 6C).
Discussion
We (8, 23) and others (19) have previously demonstrated that the FDA-approved venetoclax has increased activity in MYCN-amplified NB. More recently, our HTS of ~800 solid tumor cell lines sheds light on the unique sensitivity of MYCN-amplified NB to venetoclax among solid tumors: venetoclax single-agent activity was confined to a subset of high BCL-2 SCLCs and a subset of MYCN-amplified NB (24). However, insofar as preclinical studies provide a good indication, it appears likely the vast majority of MYCN-amplified NB will need rational venetoclax-based combination therapies for clinical responses. We have parlayed the dependency on BCL-2 through MYCN-driven NOXA expression in MYCN-amplified NB to find combination therapies that complimentarily modulate BCL-2 family proteins for a greater therapeutic effect. We have uncovered multiple therapies that are applicable to MYCN-amplified NB patients immediately, as one combining partner (cyclo/topo) is part of current standard therapy, and a second (MDM2i) and a third combining partner (MCL1i) are in clinical trials in combination with venetoclax in several hematological indications (e.g. NCT03940352, NCT02670044 and NCT03797261).
In NB, the MDM2 inhibitor DS-3032b has single-agent activity in p53-wild-type NB cell lines in vitro, including those amplified for MYCN (42); additionally, NVP-CGM097, a structurally similar compound to HDM201 that is currently being evaluated in clinical trials, has been demonstrated to have single-agent activity in p53-wild-type NB in vitro yet minimal single-agent activity in vivo (43). MDM2 inhibitors have been previously demonstrated to possess single-agent preclinical activity in p53-wild-type neuroblastoma (44-48), which represent over 90% of cases even in the relapsed setting (5). In addition, the MDM2 inhibitor Idasanutlin (Genetech) is being combined with venetoclax in early phase trials for relapsed or refractory AML, and has demonstrated both tolerability and encouraging preliminary activity with an ORR approaching 40% (Clinical trial NCT02670044). Others have demonstrated the ability of venetoclax and MDM2 to demonstrate activity in vitro in the MYCN-amplified, p53-wild-type NB cell lines LAN5 and SMS-KCNR; the authors also observed upregulation of BBC3 (PUMA) by Western blot (45).
In our study, we found the combination of NVP-CGM097 and venetoclax was highly effective in MYCN-amplified, p53-wild-type NB. We found that the transcriptional increase of BBC3 (PUMA) and PMAIP1 (NOXA) occurred early after the activation of p53 (Fig. 2A), indicating a rapid response to MDM2 inhibition with NVP-CGM097.
The sensitivity of the MYCN-amplified NB group is likely rooted in a number of reasons. First, from the perspective of BCL-2 inhibition, we had previously demonstrated MYCN is an important modulator of venetoclax-sensitivity in NB through upregulation of PMAIP1 (NOXA) transcription (8). In addition, our recent publication of an unbiased cancer cell line screen with venetoclax, consisting of ~800 solid tumor cell lines (24), further verified that venetoclax demonstrated single-agent activity only in a subset of MYCN-amplified NB and a subset of high BCL-2 SCLC cancer cell lines. It should be noted that venetoclax demonstrated activity in one MYCN-wild-type cell line in the screen, however this cell line is MYC-amplified, which also leads to binding to PMAIP1 and upregulation (49). Indeed, we found knockdown of NOXA, but not PUMA, was sufficient to significantly mitigate the response of MYCN-amplified NB to each combination therapy (Figs. 2B-2H, 4C and 4D, 5C and 5D)
Second, from the perspective of MDM2 inhibition, there is a well formed, albeit incomplete, characterization of an intimate relationship between MYCN and MDM2-p53 in NB. Shokat and colleagues elegantly demonstrated that haploinsufficient MDM2 substantially increases tumor latency period and prolongs survival directly in genetically engineered mouse models (GEMMs) of MYCN-amplified NB (50). In fact, MYCN binds to the p53 promoter to increase p53 transcripts, and consequentially, upregulates targets such as PUMA (51). Additionally, MYCN binds to the MDM2 promoter to increase mdm2 transcripts (29), which contributes to p53 inhibition. Moreover, when amplified MYCN co-localizes with p53, altering the response of certain p53 targets and expanding the target genes p53 binds to, demonstrating an additional level of complexity of p53 function in MYCN-amplified NB (52), which may lead to enhanced anti-NB activity in certain contexts (e.g. BCL-2 inhibition). Our data is in line with a report studying the prototype MDM2 inhibitor nutlin in NB, where the authors demonstrated sensitivity of MYCN-amplified NBs to MDM2 inhibitors (44). In all, MDM2 inhibition induces a strong p53 response that increases both PUMA and NOXA, and the NOXA increase is important for sensitivity (Fig. 2B-2H). While there may be some activity in any cells that have wild-type p53, we believe the data in this paper demonstrates MYCN-amplified NB should be high on the list of cancers that may indeed possess a therapeutic window for the combination of MDM2i and venetoclax.
Expanding on a previous report that targeting MCL-1 in combination with venetoclax is effective in some neuroblastoma models in vitro (19) we demonstrate the MCL-1 BH3 mimetic S63845 sensitizes MYCN-amplified NB to venetoclax in a PDX model of MYCN-amplified neuroblastoma. Preferential activity in MYCN-amplified is still preserved for this chemotherapy-based combination due to the increased sensitivity of these cancers to venetoclax. As with MDM2 inhibitors and venetoclax, clinical trials are exploring the combination of BCL-2 and MCL-1 BH3 mimetics in hematological cancers (e.g. NCT03797261). It should be noted, to the best of our knowledge, it is unknown what the mouse equivalent of the achievable doses of NVP-CGM097 or S63845 are in patients, and thus, the degree of activity we note may not be achievable.
In addition, we also demonstrated that cyclo/topo leads to sensitization of NBs to venetoclax. These data are in line with findings from Goldsmith and colleagues, who demonstrated venetoclax could sensitize twice weekly cyclophosphamide to NB-1643 xenografts (34), resulting in full tumor regressions. We used a clinically parallel schedule to study cyclo/topo in the NB-1643CRX model, which developed resistance to the ALK inhibitor crizotinib upon continuous in vivo treatment; impressively, this model remained sensitive to the combination of venetoclax and cyclo/topo (Fig. 4E). What is likely underlying the sensitivity of the cyclo/topo/venetoclax triple combination is that venetoclax reduces the apoptotic threshold in these cancers to chemotherapy. This is demonstrably important, as Letai and colleagues have demonstrated that the apoptotic threshold is directly correlated with efficacy of chemotherapy; that is, the lower the apoptotic threshold, the better the patient response (35, 53).
This study highlights a true Achilles heel in MYCN-amplified NB, the intricate need to suppress apoptosis for MYCN to exert oncogenic function. As MYCN amplification perseveres through chemotherapy (5), we demonstrate that clinically actionable drug combinations can effectively kill NB with amplified levels of MYCN, and shrink tumors in multiple PDX models, thus offering treatment strategies that may be effective in this challenging pediatric cancer subtype.
Supplementary Material
Acknowledgements
This study was supported by a grant from The Rally Foundation for Childhood Cancer Research and The Truth 365, the National Cancer Institute (R01CA215610), and an American Cancer Society Research Scholar Grant (A.C.F.).
References
- 1.Cooper MJ, Hutchins GM, Cohen PS, Helman LJ, Mennie RJ, Israel MA. Human neuroblastoma tumor cell lines correspond to the arrested differentiation of chromaffin adrenal medullary neuroblasts. Cell Growth Differ. 1990;1(4):149–59. [PubMed] [Google Scholar]
- 2.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM, Children's Oncology G. Anti-GD2 antibody with GM-CSF, interleukin-2 and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donfrancesco A, Jenkner A, Castellano A, Ilari I, Milano GM, De Sio L, Cozza R, Fidani P, Deb G, De Laurentis C, Inserra A, Dominici C. Ifosfamide/carboplatin/etoposide (ICE) as front-line, topotecan/cyclophosphamide as second-line and oral temozolomide as third-line treatment for advanced neuroblastoma over one year of age. Acta Paediatr Suppl. 2004;93(445):6–11. Epub 2004/06/05. [DOI] [PubMed] [Google Scholar]
- 4.Mosse Y, Greshock J, King A, Khazi D, Weber BL, Maris JM. Identification and high-resolution mapping of a constitutional 11q deletion in an infant with multifocal neuroblastoma. Lancet Oncol. 2003;4(12):769–71. Epub 2003/12/10. [DOI] [PubMed] [Google Scholar]
- 5.Padovan-Merhar OM, Raman P, Ostrovnaya I, Kalletla K, Rubnitz KR, Sanford EM, Ali SM, Miller VA, Mosse YP, Granger MP, Weiss B, Maris JM, Modak S. Enrichment of Targetable Mutations in the Relapsed Neuroblastoma Genome. PLoS Genet. 2016;12(12):e1006501. doi: 10.1371/journal.pgen.1006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eleveld TF, Oldridge DA, Bernard V, Koster J, Colmet Daage L, Diskin SJ, Schild L, Bentahar NB, Bellini A, Chicard M, Lapouble E, Combaret V, Legoix-Ne P, Michon J, Pugh TJ, Hart LS, Rader J, Attiyeh EF, Wei JS, Zhang S, Naranjo A, Gastier-Foster JM, Hogarty MD, Asgharzadeh S, Smith MA, Guidry Auvil JM, Watkins TB, Zwijnenburg DA, Ebus ME, van Sluis P, Hakkert A, van Wezel E, van der Schoot CE, Westerhout EM, Schulte JH, Tytgat GA, Dolman ME, Janoueix-Lerosey I, Gerhard DS, Caron HN, Delattre O, Khan J, Versteeg R, Schleiermacher G, Molenaar JJ, Maris JM. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations. Nat Genet. 2015;47(8):864–71. doi: 10.1038/ng.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 1997;16(11):2985–95. Epub 1997/06/02. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ham J, Costa C, Sano R, Lochmann TL, Sennott EM, Patel NU, Dastur A, Gomez-Caraballo M, Krytska K, Hata AN, Floros KV, Hughes MT, Jakubik CT, Heisey DA, Ferrell JT, Bristol ML, March RJ, Yates C, Hicks MA, Nakajima W, Gowda M, Windle BE, Dozmorov MG, Garnett MJ, McDermott U, Harada H, Taylor SM, Morgan IM, Benes CH, Engelman JA, Mosse YP, Faber AC. Exploitation of the Apoptosis-Primed State of MYCN-Amplified Neuroblastoma to Develop a Potent and Specific Targeted Therapy Combination. Cancer cell. 2016;29(2):159–72. doi: 10.1016/j.ccell.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fulda S, Lutz W, Schwab M, Debatin KM. MycN sensitizes neuroblastoma cells for drug-triggered apoptosis. Medical and pediatric oncology. 2000;35(6):582–4. [DOI] [PubMed] [Google Scholar]
- 10.Petroni M, Veschi V, Prodosmo A, Rinaldo C, Massimi I, Carbonari M, Dominici C, McDowell HP, Rinaldi C, Screpanti I, Frati L, Bartolazzi A, Gulino A, Soddu S, Giannini G. MYCN sensitizes human neuroblastoma to apoptosis by HIPK2 activation through a DNA damage response. Mol Cancer Res. 2011;9(1):67–77. doi: 10.1158/1541-7786.MCR-10-0227. [DOI] [PubMed] [Google Scholar]
- 11.Veschi V, Petroni M, Cardinali B, Dominici C, Screpanti I, Frati L, Bartolazzi A, Gulino A, Giannini G. Galectin-3 impairment of MYCN-dependent apoptosis-sensitive phenotype is antagonized by nutlin-3 in neuroblastoma cells. PLoS One. 2012;7(11):e49139. doi: 10.1371/journal.pone.0049139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stilgenbauer S, Eichhorst B, Schetelig J, Hillmen P, Seymour JF, Coutre S, Jurczak W, Mulligan SP, Schuh A, Assouline S, Wendtner CM, Roberts AW, Davids MS, Bloehdorn J, Munir T, Bottcher S, Zhou L, Salem AH, Desai M, Chyla B, Arzt J, Kim SY, Verdugo M, Gordon G, Hallek M, Wierda WG. Venetoclax for Patients With Chronic Lymphocytic Leukemia With 17p Deletion: Results From the Full Population of a Phase II Pivotal Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018:JCO2017766840. doi: 10.1200/JCO.2017.76.6840. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie B, Benboubker L, Facon T, Amiot M, Moreau P, Punnoose EA, Alzate S, Dunbar M, Xu T, Agarwal SK, Enschede SH, Leverson JD, Ross JA, Maciag PC, Verdugo M, Touzeau C. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130(22):2401–9. doi: 10.1182/blood-2017-06-788786. [DOI] [PubMed] [Google Scholar]
- 14.Moreau P, Chanan-Khan A, Roberts AW, Agarwal AB, Facon T, Kumar S, Touzeau C, Punnoose EA, Cordero J, Munasinghe W, Jia J, Salem AH, Freise KJ, Leverson JD, Enschede SH, Ross JA, Maciag PC, Verdugo M, Harrison SJ. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130(22):2392–400. doi: 10.1182/blood-2017-06-788323. [DOI] [PubMed] [Google Scholar]
- 15.Seymour JF, Ma S, Brander DM, Choi MY, Barrientos J, Davids MS, Anderson MA, Beaven AW, Rosen ST, Tam CS, Prine B, Agarwal SK, Munasinghe W, Zhu M, Lash LL, Desai M, Cerri E, Verdugo M, Kim SY, Humerickhouse RA, Gordon GB, Kipps TJ, Roberts AW. Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncol. 2017;18(2):230–40. doi: 10.1016/S1470-2045(17)30012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain N, Keating M, Thompson P, Ferrajoli A, Burger J, Borthakur G, Takahashi K, Estrov Z, Fowler N, Kadia T, Konopleva M, Alvarado Y, Yilmaz M, DiNardo C, Bose P, Ohanian M, Pemmaraju N, Jabbour E, Sasaki K, Kanagal-Shamanna R, Patel K, Jorgensen J, Garg N, Wang X, Sondermann K, Cruz N, Wei C, Ayala A, Plunkett W, Kantarjian H, Gandhi V, Wierda W. Ibrutinib and Venetoclax for First-Line Treatment of CLL. The New England journal of medicine. 2019;380(22):2095–103. doi: 10.1056/NEJMoa1900574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer K, Al-Sawaf O, Bahlo J, Fink AM, Tandon M, Dixon M, Robrecht S, Warburton S, Humphrey K, Samoylova O, Liberati AM, Pinilla-Ibarz J, Opat S, Sivcheva L, Le Du K, Fogliatto LM, Niemann CU, Weinkove R, Robinson S, Kipps TJ, Boettcher S, Tausch E, Humerickhouse R, Eichhorst B, Wendtner CM, Langerak AW, Kreuzer KA, Ritgen M, Goede V, Stilgenbauer S, Mobasher M, Hallek M. Venetoclax and Obinutuzumab in Patients with CLL and Coexisting Conditions. The New England journal of medicine. 2019;380(23):2225–36. doi: 10.1056/NEJMoa1815281. [DOI] [PubMed] [Google Scholar]
- 18.Hata AN, Engelman JA, Faber AC. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer discovery. 2015;5(5):475–87. doi: 10.1158/2159-8290.CD-15-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bate-Eya LT, den Hartog IJ, van der Ploeg I, Schild L, Koster J, Santo EE, Westerhout EM, Versteeg R, Caron HN, Molenaar JJ, Dolman ME. High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition. Oncotarget. 2016;7(19):27946–58. doi: 10.18632/oncotarget.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldsmith KC, Gross M, Peirce S, Luyindula D, Liu X, Vu A, Sliozberg M, Guo R, Zhao H, Reynolds CP, Hogarty MD. Mitochondrial Bcl-2 family dynamics define therapy response and resistance in neuroblastoma. Cancer research. 2012;72(10):2565–77. Epub 2012/05/17. doi: 10.1158/0008-5472.CAN-11-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldsmith KC, Liu X, Dam V, Morgan BT, Shabbout M, Cnaan A, Letai A, Korsmeyer SJ, Hogarty MD. BH3 peptidomimetics potently activate apoptosis and demonstrate single agent efficacy in neuroblastoma. Oncogene. 2006;25(33):4525–33. Epub 2006/03/29. doi: 10.1038/sj.onc.1209489. [DOI] [PubMed] [Google Scholar]
- 22.Nalluri S, Peirce SK, Tanos R, Abdella HA, Karmali D, Hogarty MD, Goldsmith KC. EGFR signaling defines Mcl(−)1 survival dependency in neuroblastoma. Cancer Biol Ther. 2015;16(2):276–86. doi: 10.1080/15384047.2014.1002333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lochmann TL, Powell KM, Ham J, Floros KV, Heisey DAR, Kurupi RIJ, Calbert ML, Ghotra MS, Greninger P, Dozmorov M, Gowda M, Souers AJ, Reynolds CP, Benes CH, Faber AC. Targeted inhibition of histone H3K27 demethylation is effective in high-risk neuroblastoma. Sci Transl Med. 2018;10(441). Epub 2018/05/18. doi: 10.1126/scitranslmed.aao4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lochmann TL, Floros KV, Naseri M, Powell KM, Cook W, March RJ, Stein GT, Greninger P, Maves YK, Saunders LR, Dylla SJ, Costa C, Boikos SA, Leverson JD, Souers AJ, Krystal GW, Harada H, Benes CH, Faber AC. Venetoclax Is Effective in Small-Cell Lung Cancers with High BCL-2 Expression. Clinical cancer research : an official journal of the American Association for Cancer Research. 2018;24(2):360–9. doi: 10.1158/1078-0432.CCR-17-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosse YP, Fox E, Teachey DT, Reid JM, Safgren SL, Carol H, Lock RB, Houghton PJ, Smith MA, Hall D, Barkauskas DA, Krailo M, Voss SD, Berg SL, Blaney SM, Weigel BJ. A Phase II Study of Alisertib in Children with Recurrent/Refractory Solid Tumors or Leukemia: Children's Oncology Group Phase I and Pilot Consortium (ADVL0921). Clinical cancer research : an official journal of the American Association for Cancer Research. 2019;25(11):3229–38. doi: 10.1158/1078-0432.CCR-18-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao W, Sachsenmeier K, Zhang L, Sult E, Hollingsworth RE, Yang H. A New Bliss Independence Model to Analyze Drug Combination Data. J Biomol Screen. 2014;19(5):817–21. Epub 2014/02/05. doi: 10.1177/1087057114521867. [DOI] [PubMed] [Google Scholar]
- 27.Wong M, Tan N, Zha J, Peale FV, Yue P, Fairbrother WJ, Belmont LD. Navitoclax (ABT-263) reduces Bcl-x(L)-mediated chemoresistance in ovarian cancer models. Mol Cancer Ther. 2012;11(4):1026–35. Epub 2012/02/04. doi: 10.1158/1535-7163.MCT-11-0693. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa M, Takizawa N, Narita M, Ichisaka T, Yamanaka S. Promotion of direct reprogramming by transformation-deficient Myc. Proc Natl Acad Sci U S A. 2010;107(32):14152–7. Epub 2010/07/28. doi: 10.1073/pnas.1009374107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slack A, Chen Z, Tonelli R, Pule M, Hunt L, Pession A, Shohet JM. The p53 regulatory gene MDM2 is a direct transcriptional target of MYCN in neuroblastoma. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(3):731–6. doi: 10.1073/pnas.0405495102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(6):1888–93. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bate-Eya LT, den Hartog IJ, van der Ploeg I, Schild L, Koster J, Santo EE, Westerhout EM, Versteeg R, Caron HN, Molenaar JJ, Dolman ME. High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition. Oncotarget. 2016. doi: 10.18632/oncotarget.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harenza JL, Diamond MA, Adams RN, Song MM, Davidson HL, Hart LS, Dent MH, Fortina P, Reynolds CP, Maris JM. Transcriptomic profiling of 39 commonly-used neuroblastoma cell lines. Sci Data. 2017;4:170033. doi: 10.1038/sdata.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh PK, Roukounakis A, Frank DO, Kirschnek S, Das KK, Neumann S, Madl J, Romer W, Zorzin C, Borner C, Haimovici A, Garcia-Saez A, Weber A, Hacker G. Dynein light chain 1 induces assembly of large Bim complexes on mitochondria that stabilize Mcl-1 and regulate apoptosis. Genes Dev. 2017;31(17):1754–69. doi: 10.1101/gad.302497.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanos R, Karmali D, Nalluri S, Goldsmith KC. Select Bcl-2 antagonism restores chemotherapy sensitivity in high-risk neuroblastoma. BMC Cancer. 2016;16:97. doi: 10.1186/s12885-016-2129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ni Chonghaile T, Sarosiek KA, Vo TT, Ryan JA, Tammareddi A, Moore Vdel G, Deng J, Anderson KC, Richardson P, Tai YT, Mitsiades CS, Matulonis UA, Drapkin R, Stone R, Deangelo DJ, McConkey DJ, Sallan SE, Silverman L, Hirsch MS, Carrasco DR, Letai A. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334(6059):1129–33. Epub 2011/10/29. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Infarinato NR, Park JH, Krytska K, Ryles HT, Sano R, Szigety KM, Li Y, Zou HY, Lee NV, Smeal T, Lemmon MA, Mosse YP. The ALK/ROS1 Inhibitor PF-06463922 Overcomes Primary Resistance to Crizotinib in ALK-Driven Neuroblastoma. Cancer discovery. 2016;6(1):96–107. doi: 10.1158/2159-8290.CD-15-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips DC, Xiao Y, Lam LT, Litvinovich E, Roberts-Rapp L, Souers AJ, Leverson JD. Loss in MCL-1 function sensitizes non-Hodgkin's lymphoma cell lines to the BCL-2-selective inhibitor venetoclax (ABT-199). Blood Cancer J. 2016;6:e403. doi: 10.1038/bcj.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nature medicine. 2013;19(2):202–8. Epub 2013/01/08. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 39.Punnoose EA, Leverson JD, Peale F, Boghaert ER, Belmont LD, Tan N, Young A, Mitten M, Ingalla E, Darbonne WC, Oleksijew A, Tapang P, Yue P, Oeh J, Lee L, Maiga S, Fairbrother WJ, Amiot M, Souers AJ, Sampath D. Expression Profile of BCL-2, BCL-XL, and MCL-1 Predicts Pharmacological Response to the BCL-2 Selective Antagonist Venetoclax in Multiple Myeloma Models. Molecular cancer therapeutics. 2016;15(5):1132–44. doi: 10.1158/1535-7163.MCT-15-0730. [DOI] [PubMed] [Google Scholar]
- 40.Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, Boyd KL, Strickland SA, Sensintaffar J, Hogdal LJ, Ayers GD, Olejniczak ET, Fesik SW, Savona MR. A Novel MCL1 Inhibitor Combined with Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer discovery. 2018;8(12):1566–81. doi: 10.1158/2159-8290.CD-18-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotschy A, Szlavik Z, Murray J, Davidson J, Maragno AL, Le Toumelin-Braizat G, Chanrion M, Kelly GL, Gong JN, Moujalled DM, Bruno A, Csekei M, Paczal A, Szabo ZB, Sipos S, Radics G, Proszenyak A, Balint B, Ondi L, Blasko G, Robertson A, Surgenor A, Dokurno P, Chen I, Matassova N, Smith J, Pedder C, Graham C, Studeny A, Lysiak-Auvity G, Girard AM, Grave F, Segal D, Riffkin CD, Pomilio G, Galbraith LC, Aubrey BJ, Brennan MS, Herold MJ, Chang C, Guasconi G, Cauquil N, Melchiore F, Guigal-Stephan N, Lockhart B, Colland F, Hickman JA, Roberts AW, Huang DC, Wei AH, Strasser A, Lessene G, Geneste O. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538(7626):477–82. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 42.Arnhold V, Schmelz K, Proba J, Winkler A, Wunschel J, Toedling J, Deubzer HE, Kunkele A, Eggert A, Schulte JH, Hundsdoerfer P. Reactivating TP53 signaling by the novel MDM2 inhibitor DS-3032b as a therapeutic option for high-risk neuroblastoma. Oncotarget. 2018;9(2):2304–19. doi: 10.18632/oncotarget.23409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang HQ, Halilovic E, Li X, Liang J, Cao Y, Rakiec DP, Ruddy DA, Jeay S, Wuerthner JU, Timple N, Kasibhatla S, Li N, Williams JA, Sellers WR, Huang A, Li F. Combined ALK and MDM2 inhibition increases antitumor activity and overcomes resistance in human ALK mutant neuroblastoma cell lines and xenograft models. Elife. 2017;6. doi: 10.7554/eLife.17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gamble LD, Kees UR, Tweddle DA, Lunec J. MYCN sensitizes neuroblastoma to the MDM2-p53 antagonists Nutlin-3 and MI-63. Oncogene. 2012;31(6):752–63. doi: 10.1038/onc.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Goethem A, Yigit N, Moreno-Smith M, Vasudevan SA, Barbieri E, Speleman F, Shohet J, Vandesompele J, Van Maerken T. Dual targeting of MDM2 and BCL2 as a therapeutic strategy in neuroblastoma. Oncotarget. 2017;8(34):57047–57. doi: 10.18632/oncotarget.18982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lakoma A, Barbieri E, Agarwal S, Jackson J, Chen Z, Kim Y, McVay M, Shohet JM, Kim ES. The MDM2 small-molecule inhibitor RG7388 leads to potent tumor inhibition in p53 wild-type neuroblastoma. Cell Death Discov. 2015;1. doi: 10.1038/cddiscovery.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Maerken T, Rihani A, Dreidax D, De Clercq S, Yigit N, Marine JC, Westermann F, De Paepe A, Vandesompele J, Speleman F. Functional analysis of the p53 pathway in neuroblastoma cells using the small-molecule MDM2 antagonist nutlin-3. Mol Cancer Ther. 2011;10(6):983–93. doi: 10.1158/1535-7163.MCT-10-1090. [DOI] [PubMed] [Google Scholar]
- 48.Komuro H, Hayashi Y, Kawamura M, Hayashi K, Kaneko Y, Kamoshita S, Hanada R, Yamamoto K, Hongo T, Yamada M, et al. Mutations of the p53 gene are involved in Ewing's sarcomas but not in neuroblastomas. Cancer Res. 1993;53(21):5284–8. [PubMed] [Google Scholar]
- 49.Nikiforov MA, Riblett M, Tang WH, Gratchouck V, Zhuang D, Fernandez Y, Verhaegen M, Varambally S, Chinnaiyan AM, Jakubowiak AJ, Soengas MS. Tumor cell-selective regulation of NOXA by c-MYC in response to proteasome inhibition. Proc Natl Acad Sci U S A. 2007;104(49):19488–93. doi: 10.1073/pnas.0708380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z, Lin Y, Barbieri E, Burlingame S, Hicks J, Ludwig A, Shohet JM. Mdm2 deficiency suppresses MYCN-Driven neuroblastoma tumorigenesis in vivo. Neoplasia. 2009;11(8):753–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L, Iraci N, Gherardi S, Gamble LD, Wood KM, Perini G, Lunec J, Tweddle DA. p53 is a direct transcriptional target of MYCN in neuroblastoma. Cancer research. 2010;70(4):1377–88. doi: 10.1158/0008-5472.CAN-09-2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Agarwal S, Milazzo G, Rajapakshe K, Bernardi R, Chen Z, Barberi E, Koster J, Perini G, Coarfa C, Shohet JM. MYCN acts as a direct co-regulator of p53 in MYCN amplified neuroblastoma. Oncotarget. 2018;9(29):20323–38. doi: 10.18632/oncotarget.24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Del Gaizo Moore V, Letai A. BH3 profiling - Measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer letters. 2012. Epub 2012/01/11. doi: 10.1016/j.canlet.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.