Abstract
Alcoholic liver disease (ALD) is the most frequent liver disease worldwide, resulting in severe harm to personal health and posing a serious burden to public health. Based on the reported antioxidant and anti-inflammatory capacities of scutellarin (SCU), this study investigated its protective role in male BALB/c mice with acute alcoholic liver injury after oral administration (10, 25, and 50 mg/kg). The results indicated that SCU could lessen serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels and improve the histopathological changes in acute alcoholic liver; it reduced alcohol-induced malondialdehyde (MDA) content and increased glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD) activity. Furthermore, SCU decreased tumor necrosis factor-α ( TNF-α), interleukin-6 ( IL-6), and IL-1β messenger RNA (mRNA) expression levels, weakened inducible nitric oxide synthase (iNOS) activity, and inhibited nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome activation. Mechanistically, SCU suppressed cytochrome P450 family 2 subfamily E member 1 (CYP2E1) upregulation triggered by alcohol, increased the expression of oxidative stress-related nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase-1 (HO-1) pathways, and suppressed the inflammation-related degradation of inhibitor of nuclear factor-κB (NF-κB)-α (IκBα) as well as activation of NF-κB by mediating the protein kinase B (AKT) and p38 mitogen-activated protein kinase (MAPK) pathways. These findings demonstrate that SCU protects against acute alcoholic liver injury via inhibiting oxidative stress by regulating the Nrf2/HO-1 pathway and suppressing inflammation by regulating the AKT, p38 MAPK/NF-κB pathways.
Keywords: Scutellarin, Oxidative stress, Alcoholic liver disease, Inflammation
Abstract
酒精性肝病(ALD)是世界上最常见的肝脏疾病,严重危害个人健康,对公共卫生造成严重负担。基于灯盏花乙素(SCU)抗氧化和抗炎能力的报道,本研究探究了SCU(10、25和50 mg/kg,口服给药)对急性酒精性肝损伤BALB/c小鼠的保护作用。结果表明:SCU可降低血清谷丙转氨酶(ALT)和天冬氨酸转氨酶(AST)水平,改善急性酒精性肝组织病理改变;降低酒精诱导的丙二醛(MDA)含量,提高谷胱甘肽过氧化物酶(GSH-Px)、过氧化氢酶(CAT)和超氧化物歧化酶(SOD)活性。此外,SCU会降低肿瘤坏死因子-α(TNF-α)、白细胞介素6(IL-6)和IL-1β的mRNA表达水平,削弱诱导型一氧化氮合酶(iNOS)活性和抑制NOD样受体蛋白3(NLRP3)炎症小体激活。从机制方面而言,SCU可抑制酒精诱导的CYP450代谢酶家族中的CYP2E1上调,增加氧化应激相关的核因子E2相关因子2(Nrf2)和改为血红素加氧酶-1(HO-1)通路的表达,通过介导蛋白激酶B(AKT)和p38 MAPK通路抑制炎症相关核因子-κB抑制蛋白α因子的降解以及核因子-κB因子的激活。这些结果表明,SCU通过调控Nrf2/HO-1通路抑制氧化应激,通过调控AKT、p38 MAPK/NF-κB通路抑制炎症反应,从而保护急性酒精性肝损伤。
Keywords: 灯盏花乙素, 氧化应激, 酒精性肝病, 炎症
1. Introduction
Alcoholic liver disease (ALD) is a major liver disease, causing approximately 2 million deaths each year worldwide ( Szabo et al., 2019). Long-term excessive intake of alcohol can give rise to a range of diseases on the ALD spectrum. Clinically, the treatment strategy for ALD mainly relies on abstinence and nutritional supplements ( Orman et al., 2013). Some drugs such as glucocorticoids have certain therapeutic effects in the clinical treatment of ALD but result in obvious side effects. Currently, no safe or effective drug therapy exists for ALD. Hence, finding a potent and risk-free approach for ALD treatment is an urgent task.
The liver is the main organ involved in more than 95% of ethanol metabolism, with the rest being excreted through urine, breath, and sweat ( Kong et al., 2019). Alcohol metabolism mainly includes three metabolic pathways: the microsomal ethanol-oxidizing system (MEOS), the alcohol dehydrogenase (ADH) system, and the catalase (CAT) system. Excessive drinking will increase the expression of cytochrome P450 family 2 subfamily E member 1 (CYP2E1), and upregulate reactive oxygen species (ROS) ( Ceni et al., 2014). To eliminate excessive ROS, antioxidant enzymes such as superoxide dismutase (SOD) will be largely depleted ( Itoh et al., 1997; Sporn and Liby, 2012; Iranshahy et al., 2018). The resulting imbalance in the levels of oxidants and antioxidants triggers oxidative stress and leads to liver damage. In addition, alcohol metabolism will induce toxic acetaldehyde release as an endogenous damage-associated molecular pattern (DAMP), which in turn activates the nuclear factor-κB (NF-κB) pathway or nucleotide-binding oligomerization domain (NOD)-like receptor protein 3 (NLRP3) inflammasome pathway, upregulate the transcription of proinflammatory cytokines, such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and finally cause inflammation ( Kubes and Mehal, 2012; Yu et al., 2018). The boost in inflammatory factors leads to immune disorders and impairs hepatic functions. Once the above stimuli are present, nuclear factor erythroid 2-related factor 2 (Nrf2) will be dissociated from oxidized Kelch-like ECH-associated protein 1 (Keap1), enter the nucleus, and then bind to antioxidant response elements ( Otterbein et al., 2003), resulting in the expression of antioxidant and detoxification genes, such as heme oxygenase-1 ( HO-1). Meanwhile, Nrf2 also constrains the NF-κB pathway and alleviates oxidative stress-induced inflammation ( Itoh et al., 1997; Sporn and Liby, 2012; Iranshahy et al., 2018). Therefore, the pursuit for natural drugs with anti-inflammatory and antioxidant properties has broad prospects in preventing and treating ALD.
Scutellarin (SCU) is a flavonoid-active component in the medicinal herb Erigeron breviscapus ( Wang and Ma, 2018). SCU has various pharmacological properties, including antioxidant ( Kim et al., 2016) and anti-inflammation ( Qian et al., 2011; Zhao et al., 2016; Zeng and Cai, 2017). It can alleviate non-alcoholic fatty liver disease (NAFLD) via regulating the Nrf2 and NF-κB pathways ( Zhang XX et al., 2018). In addition, SCU can also attenuate alcohol-induced acute brain injury in mice ( Zhang et al., 2022). Yet, the effect of SCU in acute alcoholic liver injury remains unclear. The purpose of this study was to explore whether SCU can protect against acute alcoholic liver injury as a preventive drug, and to further investigate the relevant mechanism.
2. Materials and methods
2.1. Reagents
SCU was supplied by Shanghai Winherb Medical Technology Co., Ltd. (Shanghai, China). Bifendate was obtained from Beijing Union Pharmaceutical Factory (Beijing, China). Assay kits of glutathione peroxidase (GSH-Px), alanine aminotransferase (ALT), malondialdehyde (MDA), aspartate aminotransferase (AST), SOD, catalase (CAT), and inducible nitric oxide synthase (iNOS) were supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). SB203580 was purchased from MedChemExpress (Shanghai, China). MK-2206 2HCl was obtained from Selleck (Shanghai, China).
2.2. Animals and treatment
Male BALB/c mice (20‒22 g) were obtained from Pizhou Dongfang breeding Co., Ltd. (Xuzhou, China). The animals were kept in a relative humidity of (50±10)%, (23±2) ℃, and a light/dark cycle of 12 h, with adequate food and sterile water. They were given 7 d acclimatization period before the experiment.
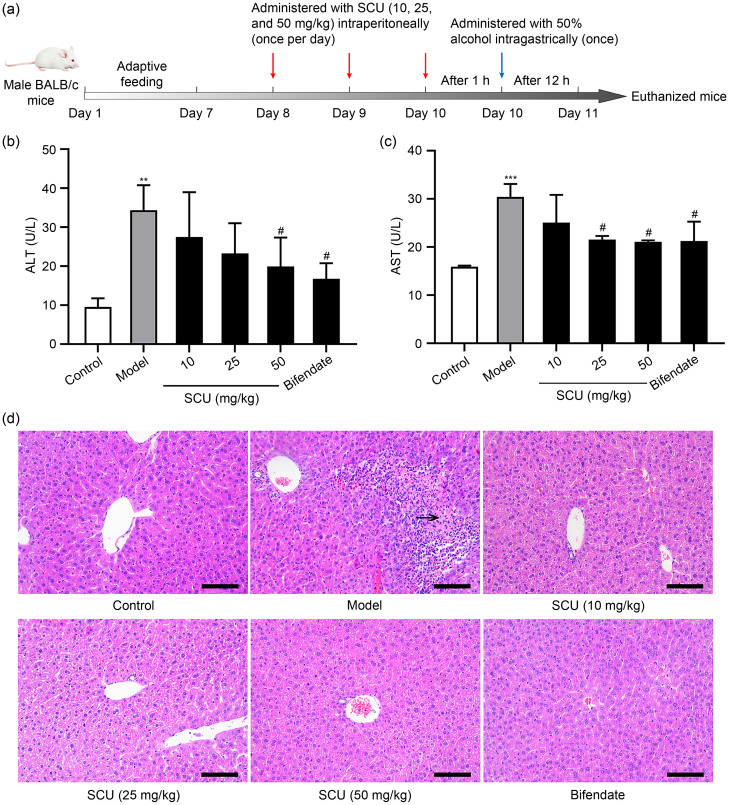
The mice were randomly allocated into six groups ( n=5 each): (1) control group; (2) model group; (3) ethanol+SCU (10 mg/kg); (4) ethanol+SCU (25 mg/kg); (5) ethanol+SCU (50 mg/kg); (6) ethanol+bifendate (150 mg/kg). Animals in the SCU groups were given SCU by intraperitoneal injection once a day for 3 d. The other groups were administered an equal volume of phosphate-buffered saline (PBS) by intraperitoneal injection. Bifendate (150 mg/kg) was administered intragastrically as a positive control ( Wang et al., 2012). Then, all mice received 50% ethanol (12 mL/kg) ( Liu X et al., 2019b) by oral gavage 1 h later on the last day, except for the control group, which was gavaged with an equal amount of sterile water. Blood samples were collected after 12 h ( Fig. 1a). Mice were euthanized via cervical dislocation, and their liver tissue samples were collected for further research.
Fig. 1. Protection of SCU against acute alcoholic liver injury. (a) Experimental protocol for alcoholic liver injury model. (b) ALT level in serum. (c) AST level in serum. (d) Histological analysis of the liver performed using H&E staining (scale bar: 100 µm; black arrow: necrosis). All values ( n=5) are demonstrated as mean±standard deviation (SD). ** P<0.01, *** P<0.001 versus control; # P<0.05 versus model. SCU: scutellarin; ALT: alanine aminotransferase; AST: aspartate aminotransferase.
2.3. Cell culture
HepG2 cells were purchased by the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Servicebio, Wuhan, China) containing 10% (volume fraction) fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 U/mL penicillin at 37 ℃ under 5% CO2 atmosphere.
2.4. Cell viability assay
Monolayer HepG2 cells seeded in 96-well plates (2.5×10 4 cells/well) were treated with SCU (0, 1, 5, 10, 20, 40, 80, and 200 μmol/L), ethanol (0, 50, 100, 200, 400, 600, 800, and 1000 mmol/L), or 600 mmol/L ethanol after SCU (0, 20, 40, and 80 μmol/L) treatment for 1 h. After 24 h of incubation, the cell viability was measured via the cell counting kit-8 (CCK-8) assay according to the manufacturer’s protocol.
2.5. ROS measurement
Monolayer HepG2 cells (2.5×10 4 cells/well) or liver tissue cell suspension (2×10 6 cells/mL) was incubated with 2',7'-dichlorofluorescein diacetate (DCFH-DA; 10 μmol/L, Biosharp, Anhui, China) at 37 ℃ for 30 min away from light. ROS levels were determined by DCFH-DA staining, and fluorescence intensity was measured by a fluorescence microplate reader (BioTek, USA) under excitation/emission wavelength of 488 nm/525 nm.
2.6. Immunofluorescence staining
Monolayer HepG2 cells seeded on 24-well chamber slides were treated with SCU (80 μmol/L) for 1 h, followed by ethanol (600 mmol/L) for 24 h. Immunofluorescence assays were conducted as previously described ( Wang et al., 2018). Specifically, the primary antibody was anti-NF-κB p65 (1:1000 (volume ratio, the same as below); Cell Signaling Technology, MA, USA), and the secondary antibody was fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G (IgG) (1:400; EarthOx, San Francisco, USA). The nucleus was stained with Hoechst 33528 (10 μg/mL; UE, Suzhou, China). Cells were observed under a confocal fluorescence microscope (Eclipse Ti2, Nikon, Japan).
2.7. Histopathological observation
Fresh liver tissues were fixed in 4% (volume fraction) formaldehyde and embedded in paraffin wax. The paraffin sections were stained with hematoxylin and eosin (H&E), and observed through a microscope (DS-Fi2, Nikon).
2.8. Detection of biochemical indicators
Liver SOD, MDA, CAT, and GSH-Px levels, as well as serum AST and ALT levels, were tested by commercially available kits according to the manufacturer’s instructions.
2.9. Western blot
Proteins were extracted from liver and HepG2 cells and western blot assays were conducted as described previously ( Wang et al., 2018). The primary antibodies were Nrf2, CYP2E1, HO-1, glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Proteintech Group, Inc., Wuhan, China), phosphorylated p38 (p-p38), p38, phosphorylated protein kinase B (p-AKT), AKT, p-NF-κB p65, NF-κB p65, inhibitor of NF-κB-α (IκBα), phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2), ERK1/2, phosphorylated c-Jun N-terminal kinase (JNK), JNK (Cell Signaling Technology, MA, USA), caspase-1 and NLRP3 (Adipogen, San Diego, CA, USA), apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) (Wanleibio, Shenyang, China), and β-tubulin (Abmart, Shanghai, China). The horseradish peroxidase (HRP)-conjugated secondary antibodies included goat anti-mouse IgG (H+L) (1:5000; Proteintech Group, Inc.) and goat anti-rabbit IgG (H+L) (1:5000; Proteintech Group, Inc.). The dilution times for each antibody were displayed in Table S1.
2.10. RT-qPCR analysis
Total RNAs from liver tissues were extracted using TRIzol reagent (Vazyme, Nanjing, China) and complementary DNA (cDNA) was synthesized with a reverse transcription kit (Monad, Wuhan, China). Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR) was performed with the SYBR Green qPCR mix (Monad). The reaction system and amplification procedure referred to previously described protocols ( Yu et al., 2021). The messenger RNA (mRNA) expression levels were calculated by the method. The primer sequences were displayed in Table S2.
2.11. Statistical analysis
All values were presented as mean±standard deviation (SD). Comparative analyses among groups were performed by one-way analysis of variance (ANOVA) accompanied by Dunnett’s multiple comparison test, followed by statistics using GraphPad Prism 8.0 software ( https://www.graphpad.com/scientific-software/prism). P<0.05 was considered statistically significant.
3. Results
3.1. Protection of SCU against acute alcoholic liver injury
Blood AST and ALT are the most common biomarkers of liver injury. In comparison to the control group, serum AST and ALT contents after ethanol treatment were markedly increased, demonstrating that the acute ALD animal model was successfully established (Figs. 1b and 1c). In comparison to the model group, SCU at 50 mg/kg pretreatment dose markedly inhibited the elevation of serum ALT and AST levels, and the effect was similar to that of positive control ( P<0.05). To further confirm whether SCU could alleviate acute ALD, liver tissues were observed by H&E staining. Fig. 1d demonstrated that the liver tissue morphology and structure were complete, and the liver cells were arranged neatly, were round-shaped, and appeared normal. However, after ethanol treatment, cell necrosis and many inflammatory cell infiltrations were detected in the model group. SCU administration decreased the number of inflammatory cells and alleviated the hepatocyte injury in contrast to the model group, and the positive group had a similar effect. These results suggested that SCU alleviated alcoholic liver damage in our mouse model.
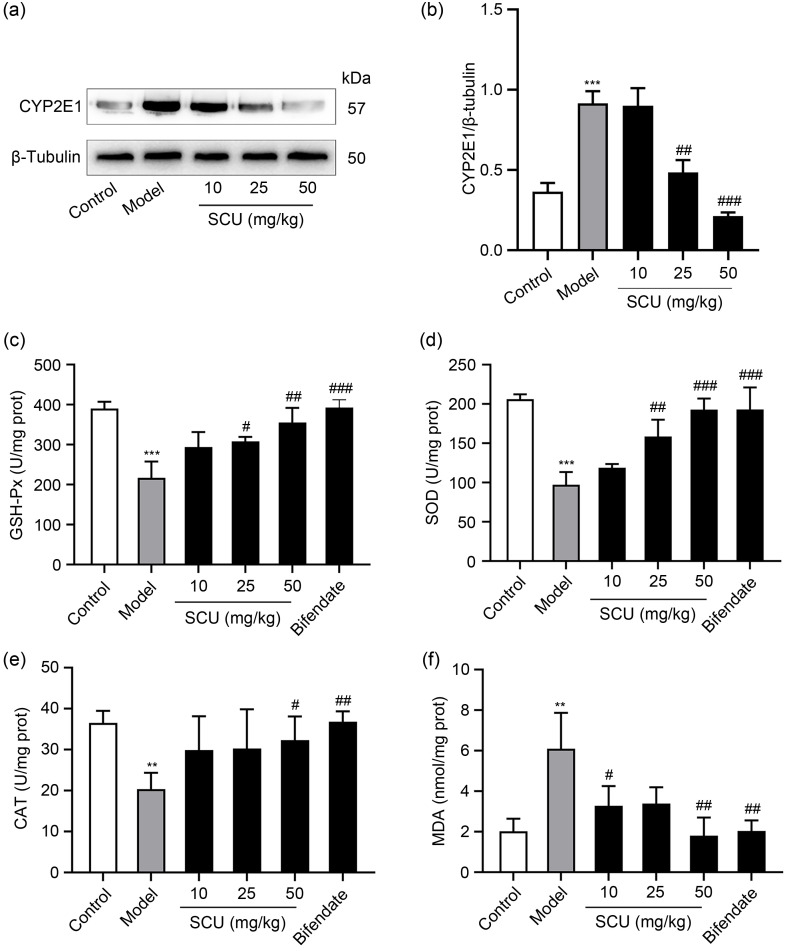
3.2. Effects of SCU on CYP2E1 expression and antioxidant capacity in mice
The process of alcohol consumption can cause the formation of ROS, which initiates the occurrence of oxidative stress ( Zhao et al., 2021). The primary cause of ROS generation was attributed to CYP2E1. As shown in Figs. 2a and 2b, CYP2E1 protein expression in the model group was notably raised. However, the ethanol-induced elevation of CYP2E1 expression was considerably reduced by SCU (25 and 50 mg/kg) pretreatments. SCU (10 mg/kg) did not cause marked change in the model group. To explore the antioxidant capacity of SCU, the related indexes of antioxidant enzymes and peroxides were detected, including GSH-Px, SOD, CAT, and MDA. Ethanol treatment resulted in an elevated MDA level and the reduction in GSH-Px, CAT, and SOD levels in the model group ( Figs. 2c‒2f). However, SCU pretreatment reversed this result. In contrast to the model group, SCU at 25 and 50 mg/kg remarkably restrained the ethanol-induced reduction in GSH-Px and SOD levels (Figs. 2c and 2d). SCU at 50 mg/kg could significantly inhibit the reduction in CAT level induced by ethanol, and SCU at 10 and 50 mg/kg markedly decreased the MDA content compared with the model group (Figs. 2e and 2f). SCU at 25 mg/kg also demonstrated a decreasing tendency of MDA level, yet there was no remarkable change in comparison with the model group (Figs. 2e and 2f). These findings showed that SCU played an antioxidant role by promoting GSH-Px, CAT, and SOD activity while lowering MDA level.
Fig. 2. Effects of SCU on CYP2E1 expression and oxidative stress in the liver of mice. (a) Protein expression of CYP2E1 in the liver analyzed by western blot. (b) Quantification of CYP2E1 protein expression in (a). GSH-Px (c), SOD (d), CAT (e), and MDA (f) levels in the liver with different treatments. All values ( n=5) are demonstrated as mean±standard deviation (SD). ** P<0.01, *** P<0.001 versus control; # P<0.05, ## P<0.01, ### P<0.001 versus model. SCU: scutellarin; CYP2E1: cytochrome P450 family 2 subfamily E member 1; GSH-Px: glutathione peroxidase; SOD: superoxide dismutase; CAT: catalase; MDA: malondialdehyde; prot: protein.
3.3. Effects of SCU on the alcohol-induced Nrf2/HO-1 pathway in vitro and in vivo
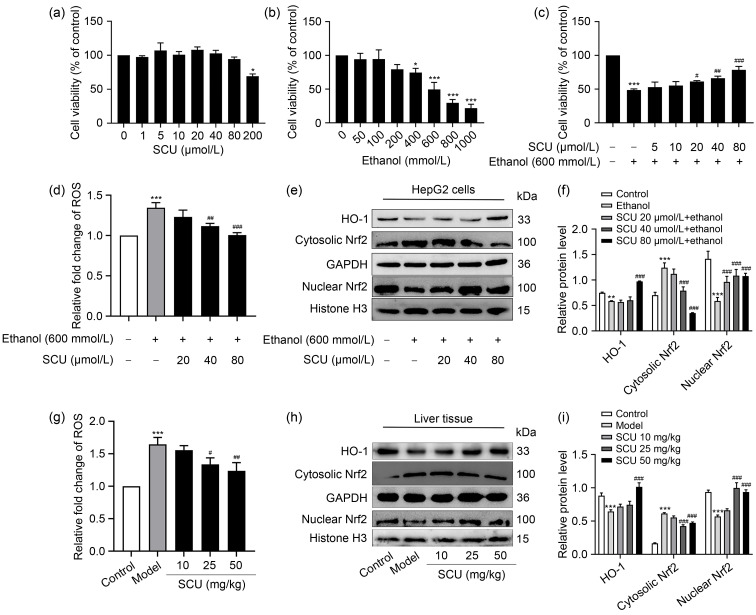
In order to explore the safe concentration of SCU in HepG2 cells in vitro, the CCK-8 kit was used to determine the cell viability. The results showed that SCU below 80 μmol/L had no remarkable effect on the viability of HepG2 cells ( Fig. 3a). To establish a model of ethanol-damaged hepatocytes in vitro, the effects of different ethanol concentrations on the viability of HepG2 cells were determined. At 600 mmol/L ethanol dose, cell viability decreased by about 50% ( Fig. 3b). Therefore, an ethanol concentration of 600 mmol/L was chosen for subsequent experiments. The results highlighted that SCU (20, 40, and 80 μmol/L) pretreatments significantly enhanced the activity of ethanol-induced HepG2 in a dose-dependent manner ( Fig. 3c).
Fig. 3. Effects of SCU on the Nrf2/HO-1 pathway of mice liver both in vivo and in vitro. (a) Effects of SCU on the cell viability of HepG2 cells. (b) Effects of ethanol on the cell viability of HepG2 cells. (c) Protection of SCU against ethanol-induced HepG2 cells injury. (d) ROS level in HepG2 cells after SCU (0, 20, 40, and 80 μmol/L) pretreatments for 1 h and 600 mmol/L ethanol treatment for 24 h. (e) The protein levels of HO-1, cytosolic Nrf2, and nuclear Nrf2 in HepG2 cells analyzed by western blot. (f) Quantification analyses of HO-1, cytosolic Nrf2, and nuclear Nrf2 protein expression in (e). (g) ROS level in liver tissues. (h) Protein levels of HO-1, cytosolic Nrf2, and nuclear Nrf2 in the liver tissues analyzed by western blot. (i) Quantification analyses of HO-1, cytosolic Nrf2, and nuclear Nrf2 protein expression in (h). All values ( n=3) are demonstrated as mean±standard deviation (SD). * P<0.05, ** P<0.01, *** P<0.001 versus control; # P<0.05, ## P<0.01, ### P<0.001 versus model. SCU: scutellarin; Nrf2: nuclear factor erythroid 2-related factor 2; HO-1: heme oxygenase-1; ROS: reactive oxygen species; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
The Nrf2/HO-1 pathway is regarded as one of the most vital anti-oxidative stress mechanisms in cells and has received growing attention ( Li et al., 2020). Hence, to explore the influence of SCU on the Nrf2/HO-1 pathway, the protein levels of Nrf2 and HO-1 were detected in vitro and in vivo. Ethanol resulted in a significant rise in the intracellular level of ROS, whereas SCU pretreatment significantly inhibited the increase in ROS level in a dose-dependent manner both in vitro and in vivo (Figs. 3d and 3g). Ethanol inhibited Nrf2 transport from cytoplasm to nucleus and the HO-1 expression both in vitro and in vivo. Meanwhile, SCU significantly promoted the translocation of cytoplasm Nrf2 to the nucleus and the expression of HO-1 (Figs. 3e, 3f, 3h, and 3i). These results suggested that SCU may play an antioxidant role by mediating the Nrf2/HO-1 pathway.
3.4. Effects of SCU on alcohol-induced pro-inflammatory mediators and NLRP3 inflammasome in mice
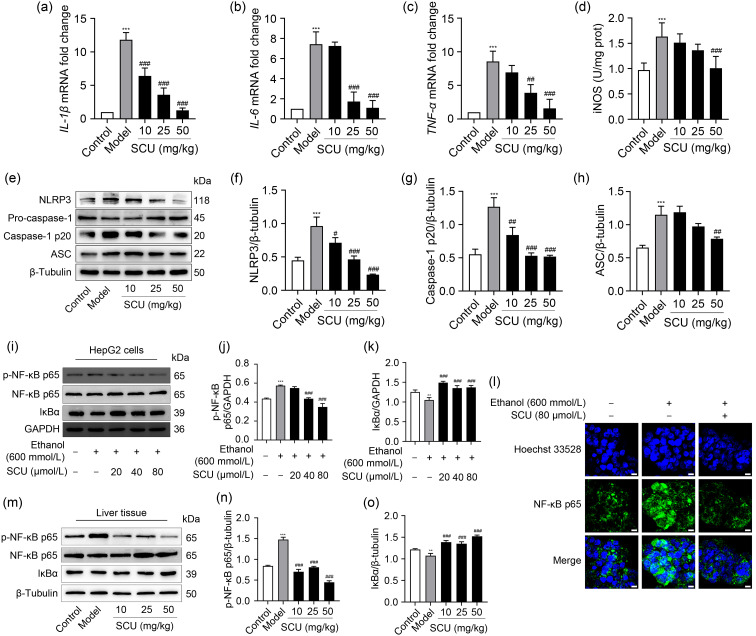
ALD is relevant to the inflammatory response, as it elevates the levels of several pro-inflammatory mediators, such as iNOS and IL-6 ( Kong et al., 2019). The transcription levels of three proinflammatory mediators, IL-1β, IL-6, and TNF-α, were detected in the liver tissues to explore whether SCU had any effect on ethanol-induced liver inflammation. The activity of iNOS was determined by a chemical detection kit. Ethanol raised the levels of IL-1β, IL-6, and TNF-α mRNA levels, and iNOS activity, leading to liver inflammation; however, SCU reversed these changes (Figs. 4a– 4d). A previous study revealed that alcohol exposure caused the activation of NLRP3 inflammasome, which was relevant to the inflammatory response ( Torres et al., 2022). NLRP3, caspase-1 p20, and ASC expression levels were assessed by western blot to further investigate the function of SCU on NLRP3 inflammasomes in acute ALD. Ethanol treatment raised NLRP3, caspase-1 p20, and ASC expression in contrast to the control group, while SCU markedly suppressed these increases (Figs. 4e– 4h). Overall, these results indicated that SCU prevented the inflammatory responses of acute ALD via attenuating pro-inflammatory mediators and activating the NLRP3 inflammasome.
Fig. 4. Effects of SCU on acute ethanol-induced inflammatory mediators, the activation of NLRP3 inflammasome, and NF-κB pathway. (a‒c) mRNA expression of IL-1β, IL-6, and TNF-α in the liver. (d) iNOS activity in the liver. (e) NLRP3, pro-caspase-1, caspase-1 p20, and ASC expression in the liver determined by western blot. (f‒ h) Quantification of NLRP3, caspase-1 p20, and ASC protein expression in (e). (i) Protein levels of p-NF-κB p65 and IκBα analyzed by western blot in HepG2 cells after SCU (0, 20, 40, and 80 μmol/L) pretreatments for 1 h and ethanol (600 mmol/L) treatment for 24 h. (j, k) Quantification analyses of p-NF-κB p65 and IκBα protein expression in HepG2 cells in (i). (l) The nuclear translocation of NF-κB p65 detected by immunofluorescence (scale bar: 10 μm). (m) Protein levels of p-NF-κB p65 and IκBα in the liver analyzed by western blot. (n, o) Quantification analyses of liver p-NF-κB p65 and IκBα protein expression in (m). All values ( n=3) were demonstrated as mean±standard deviation (SD). ** P<0.01, *** P<0.001 versus control; # P<0.05, ## P<0.01, ### P<0.001 versus model. SCU: scutellarin; NLRP3: nucleotide-binding oligomerization domain (NOD)-like receptor protein 3; NF-κB: nuclear factor-κB; IL: interleukin; TNF-α: tumor necrosis factor-α; iNOS: inducible nitric oxide synthase; p-NF- κB: phosphorylated NF- κB; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; ASC: apoptosis-associated speck-like protein containing a caspase recruitment domain; IκBα: inhibitor of NF-κB-α; mRNA: messenger RNA; prot: protein.
3.5 Inhibition of SCU on alcohol-induced activation of the NF- κB pathway in vitro and in vivo
NF-κB, as a key nuclear transcription factor, is thought to control inflammatory responses via regulating the expression of pro-inflammatory mediators ( Nowak and Relja, 2020). Ethanol increased the protein level of p-NF-κB p65 and decreased the protein level of IκBα. SCU significantly inhibited the ethanol-induced elevation of p-NF-κB p65 and the degradation of IκBα in HepG2 cells in vitro (Figs. 4i–4k). Similarly, but more markedly, SCU weakened the ethanol-induced increase in p-NF-κB p65 in RAW264.7 macrophages (Fig. S1). Compared to the model group, SCU pretreatment evidently decreased NF-κB p65 phosphorylation while prominently raising IκBα expression in liver tissues (Figs. 4m– 4o). SCU also inhibited the nuclear translocation of NF-κB p65 induced by ethanol in HepG2 cells ( Fig. 4l). These results indicated that SCU suppressed proinflammatory mediators induced by ethanol via attenuating the NF-κB pathway.
3.6. Inhibition of SCU on ethanol-induced AKT and p38 MAPK pathways in HepG2 cells
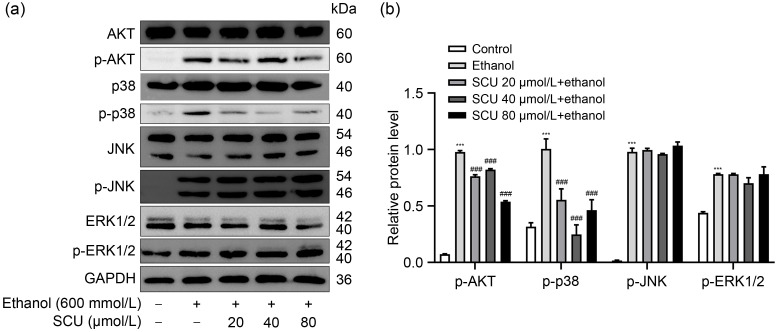
The AKT and mitogen-activated protein kinase (MAPK) pathways are involved in the development of alcoholic liver damage ( Hoek and Pastorino, 2004). To explore the effects of SCU on ethanol-induced activations of AKT and MAPKs, the levels of p-AKT, p-p38, p-JNK, and p-ERK1/2 were measured in liver tissues. The data revealed that ethanol induced remarkable rises in the protein levels of p-AKT, p-p38, p-JNK, and p-ERK1/2, while SCU pretreatment markedly inhibited the ethanol-induced increases in p-AKT and p-p38 protein level but showed no significant difference in the level of p-JNK or p-ERK1/2 ( Fig. 5). At the same time, SCU inhibited the protein levels of p-AKT and p-p38 in ethanol-treated RAW264.7 cells (Figs. S2a and S2b). These results suggested that SCU could inhibit the ethanol-induced activation of AKT and p38 MAPK pathways.
Fig. 5. Effects of SCU on AKT and MAPK pathways in HepG2 cells. (a) Protein levels of p-AKT, p-p38, p-ERK1/2, and p-JNK analyzed by western blot in HepG2 cells after SCU (0, 20, 40, and 80 μmol/L) pretreatments for 1 h and ethanol (600 mmol/L) treatment for 24 h. (b) Quantification analyses of p-AKT, p-p38, p-ERK1/2, and p-JNK protein expression in (a). All values ( n=3) are demonstrated as mean±standard deviation (SD). *** P<0.001 versus control; ### P<0.001 versus model. SCU: scutellarin; AKT: protein kinase B; MAPK: mitogen-activated protein kinase; p-AKT: phosphorylated AKT; ERK1/2: extracellular signal-regulated kinase 1/2; p-ERK1/2: phosphorylated ERK1/2; JNK: c-Jun N-terminal kinase; p-JNK: phosphorylated JNK; GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
3.7. Role of p-AKT and p-p38 inhibition by SCU in the regulation of NF-κB and Nrf2 signaling pathways
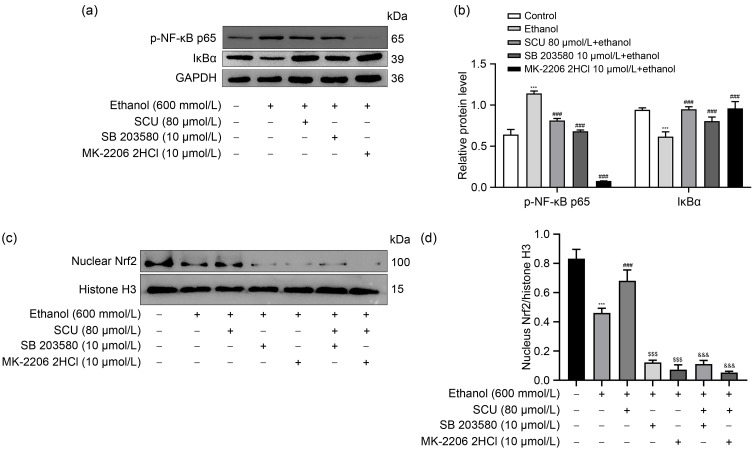
AKT and MAPKs are involved in the regulation of NF-κB activation ( An et al., 2019; Ji et al., 2019). Next, we investigated whether SCU inhibited NF-κB activation by blocking p38 MAPK or AKT activity in ethanol-treated HepG2 cells. Treatment with SB 203580 (a p38 inhibitor) and MK-2206 2HCl (an AKT inhibitor) significantly inhibited ethanol-induced NF-κB activation and IκBα degradation in HepG2 cells (Figs. 6a and 6b). The effects of these agents were similar in RAW264.7 cells (Figs. S2c and S2d). Moreover, both SB 203580 and MK-2206 2HCl remarkably inhibited Nrf2 activation and reversed the SCU-induced Nrf2 activation in ethanol-induced HepG2 cells (Figs. 6c and 6d). These results showed that the inhibition of p-AKT and p-p38 by SCU was involved in the regulation of NF-κB and Nrf2 signaling pathways.
Fig. 6. Inhibition of p-AKT and p-p38 by SCU involved in NF-κB and Nrf2 signaling pathways. (a) Protein levels of p-NF-κB p65 and IκBα analyzed by western blot in HepG2 cells with SCU (80 μmol/L), SB 203580 (10 μmol/L), or MK-2206 2HCl (10 μmol/L) treatment for 1 h and ethanol (600 mmol/L) treatment for 24 h. (b) Quantification analyses of p-NF- κB p65 and IκBα protein expression in HepG2 cells in (a). (c) Protein level of nuclear Nrf2 analyzed by western blot in HepG2 cells with SCU (80 μmol/L), SB 203580 (10 μmol/L), or MK-2206 2HCl (10 μmol/L) treatment for 1 h and ethanol (600 mmol/L) treatment for 24 h. (d) Quantification analysis of nuclear Nrf2 protein expression in HepG2 cells in (c). All values ( n=3) are demonstrated as mean±standard deviation (SD). *** P<0.001 versus control; ### P<0.001, $$$ P<0.001 versus model; &&& P<0.001 versus ethanol+SCU. SCU: scutellarin; NF - κB: nuclear factor- κB; Nrf2: nuclear factor erythroid 2-related factor 2; p-NF - κB: phosphorylated NF-κB; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; IκBα: inhibitor of NF-κB-α; p-AKT: phosphorylated protein kinase B.
4. Discussion
ALD is a serious condition threatening public health, yet no effective strategy or medication has been developed for its treatment ( Liu et al., 2021). Alcohol-induced inflammation and oxidative stress are important pathogenesis mechanisms of ALD. The natural bioactive ingredients in herbal medicine have anti-inflammatory and antioxidant properties and are expected to prevent ALD. This study aimed to determine whether SCU exhibited a hepatoprotective effect in acute alcoholic liver injury and explore its potential mechanism.
Liver injury leads to elevated levels of ALT and AST in the blood ( Xu et al., 2018). Hence, blood AST activity and ALT activity are important biochemical indexes of liver injury. In our study, an acute alcoholic liver injury mouse model was successfully established by alcohol gavage, which was characterized by elevated ALT and AST levels. Similar to other models of acute alcoholic liver injury, H&E-stained liver tissues display considerable structural disruption and inflammation, with an increased infiltration of inflammatory cells ( Liu X et al., 2019a; Fan et al., 2022). SCU was reported to alleviate NAFLD and liver damage induced via diosbulbin B, significantly reducing ALT and AST levels ( Niu et al., 2015; Fan et al., 2017; Zhang XX et al., 2018). In our study, SCU pretreatment markedly decreased the elevation of ethanol-induced serum ALT and AST levels. In addition, the hepatoprotective effect of SCU was further verified by histopathological examination. The above results indicated that SCU could protect from acute alcoholic liver injury.
CYP2E1 is one of the major enzymes in ethanol metabolism ( Leung and Nieto, 2013). Ethanol treatment was shown to enhance transaminase levels and aggravate liver histopathological damage in CYP2E1 overexpressing mice ( Morgan et al., 2002). In contrast, low CYP2E1 levels relieved liver damage induced by ethanol in rats ( Gouillon et al., 2000). Therefore, CYP2E1 could also be used as an index to evaluate liver injury. In our experiment, the CYP2E1 protein was decreased in the liver after SCU treatment, indicating that SCU can protect against acute alcoholic liver injury.
Elevated CYP2E1 levels will give rise to the production of ROS, ultimately leading to oxidative stress ( Chen et al., 2014). Oxidative stress is a major contributing factor to alcoholic liver injury ( Kirpich et al., 2016). The antioxidant enzymes SOD, CAT, and GSH-Px, as well as the lipid peroxidation marker MDA, are generally used to evaluate oxidative stress levels ( Ding et al., 2015; Kirpich et al., 2016). Ethanol treatment lowered antioxidant enzyme levels and increased MDA level in the liver. SCU exerts antioxidant effects in various diseases ( Hu et al., 2019; Bian et al., 2020; Wang et al., 2020). For instance, it decreased oxidative stress in diabetic cardiomyopathy by increasing antioxidant enzyme activity ( Xu et al., 2021). In this research, SCU alleviated oxidative stress by increasing the activity of SOD, CAT, and GSH-Px, and decreasing the level of MDA.
Nrf2 is regarded as a crucial transcription factor mediating redox balance and protecting cells from diseases via regulating downstream antioxidant genes, including HO-1, GSH-Px, glutamate-cysteine ligase catalytic subunit ( GCLC), CAT, and SOD ( Otterbein et al., 2003; Hayes and Dinkova-Kostova, 2014; Galicia-Moreno et al., 2020). Some drugs functioned as protective agents against ALD by activating the Nrf2 pathway ( Sun et al., 2018; Zhao et al., 2018), whereas Nrf2-deficient mice fed with ethanol had an increased mortality rate compared with wild-type mice ( Bataille and Manautou, 2012), indicating that the Nrf2/HO-1 signaling axis may be an effective target to treat ALD ( Li et al., 2020). In addition, SCU exerted a hypoglycemic and renal protective role through the Nrf2/HO-1 signaling pathway ( Liu YG et al., 2019). In this study, SCU activated the Nrf2/HO-1 pathway both in vitro and in vivo, suggesting that SCU might relieve acute alcoholic liver injury via acting on the Nrf2/HO-1 pathway to perform its antioxidant capacity.
Inflammation is another vital cause of acute alcoholic liver injury ( Song et al., 2018). Currently, there are three main inflammatory pathways involved in the development of ALD. First, changes in intestinal permeability trigger an increase in the number of pathogen-associated molecular patterns (PAMPs), which in turn activate Kupffer cells ( Liu, 2014). Second, the DAMP produced by ethanol-induced hepatocyte injury ultimately mediates inflammatory signaling. Third, the migration of inflammatory cells to the liver due to interorgan interactions further promotes inflammation ( Shim and Jeong, 2020). Previous acute alcohol-induced liver injury models exhibited an increase in the mRNA levels of IL-1β, IL-6, and TNF-α, the activations of NLRP3 inflammasome and NF-κB, and the infiltration of inflammatory cells in the liver tissues of BALB/c mice ( Kong et al., 2019; Liu X et al., 2019a; Fan et al., 2022). Consistent with our study, SCU markedly decreased the levels of these pro-inflammatory factors and alleviated inflammation. The NLRP3 inflammasome mediates responses to cellular danger signals that activate and recruit inflammatory cells ( Torres et al., 2022). The activation of NLRP3 inflammasome can cause liver injury ( Chen et al., 2020), whereas the inhibition of NLRP3 inflammasome activation protects against acute liver injury elicited by lipopolysaccharide/D-galactosamine (LPS/D-Gal) ( Xiao et al., 2021). The expression of NLRP3 is regulated by NF-κB ( He et al., 2016), which is involved in regulating inflammatory mediators ( Xu et al., 2018). Herein, SCU decreased the alcohol-induced phosphorylation of NF-κB p65 and inhibited IκBα degradation, effectively inhibiting NF-κB p65 activation. Overall, SCU might reduce inflammation by diminishing the NF-κB pathway to inhibit NLRP3 inflammasome activation, thereby protecting the liver.
A number of studies have suggested that excessive ROS can activate AKT kinase and MAPK family proteins ( Zhang ZH et al., 2018; Chen et al., 2019). AKT and MAPK family proteins, including p38, ERK1/2, and JNK, are involved in various alcohol-induced stress responses ( Hoek and Pastorino, 2004). According to some scholars, ethanol induced the activation of MAPKs in HepG2 cells ( Guo et al., 2016, 2020). However, in some studies related to the alcoholic liver injury model, alcohol treatment inhibited MAPKs activity compared with the control group ( Wang et al., 2010), which might be related to the time, dose, and frequency of alcohol exposure ( Wang et al., 2010; Wu et al., 2012; Li et al., 2015; Zeng et al., 2018). In this paper, ethanol-induced activations of AKT, p38, ERK1/2, and JNK were observed in HepG2 cells. SCU treatment remarkably suppressed the activations of AKT and p38, but had no effect on the activation of ERK1/2 or JNK. This suggested that SCU might play a role in alcohol-induced hepatocyte injury by regulating AKT and p38. Some studies have indicated that AKT and MAPK family proteins are involved in NF-κB activation ( An et al., 2019; Ji et al., 2019). AKT activated the NF-κB pathway by triggering the phosphorylation of IκB kinase (IKK) or RelA/p65 ( Lu and Wahl, 2005). The activation of p38 MAPK can initiate the downstream NF-κB pathway ( Wang et al., 2016). Similarly, SCU may partially inhibit alcohol-induced NF-κB activation via suppressing the activation of AKT and p38 MAPK, thereby inhibiting alcohol-induced inflammatory response and exerting an anti-inflammatory effect.
5. Conclusions
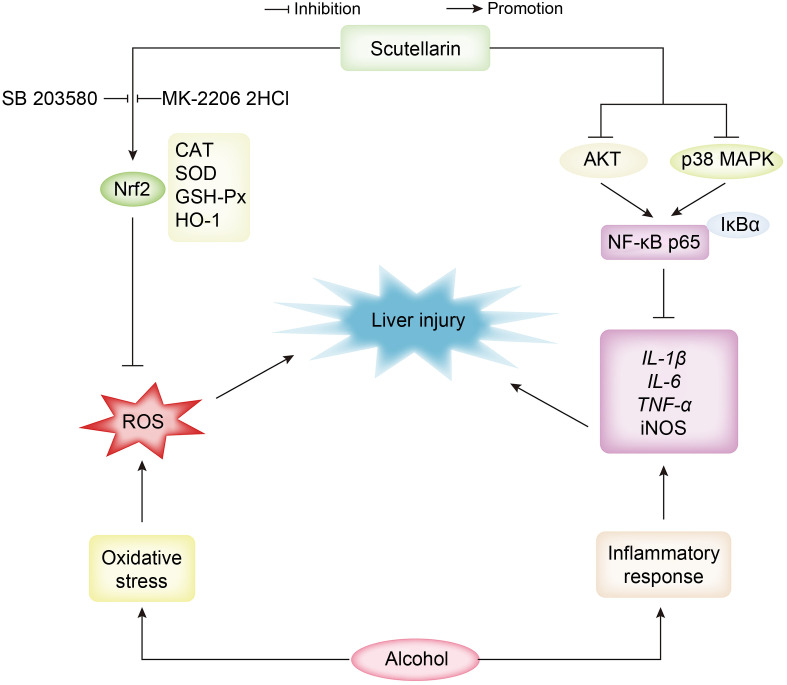
This study established a mouse ALD model and demonstrated that SCU could protect against acute alcoholic liver injury through reducing ALT, AST, and MDA contents, improving CAT, GSH-Px, and SOD activity, decreasing TNF-α, IL-1β, and IL-6 mRNA levels, weakening iNOS activity, and abating NLRP3 inflammasome activation in mice. The potential protective mechanism of SCU may be through inhibiting oxidative stress via regulating the Nrf2/HO-1 signaling pathway, and blocking the inflammatory response by regulating the AKT, p38 MAPK/NF-κB signaling pathways ( Fig. 7). Overall, our results showed that SCU may be an effective candidate for protection against acute alcoholic liver damage.
Fig. 7. Schematic diagram of the protective mechanisms of scutellarin in acute alcoholic liver damage. Nrf2: nuclear factor erythroid 2-related factor 2; CAT: catalase; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; HO-1: heme oxygenase-1; ROS: reactive oxygen species; AKT: protein kinase B; MAPK: mitogen-activated protein kinase; NF- κB: nuclear factor- κB; IκBα: inhibitor of NF-κB- α; IL: interleukin; TNF- α: tumor necrosis factor- α; iNOS: inducible nitric oxide synthase.
Supplementary information
Acknowledgments
This work was supported by the Basic Science (Natural Science) Research Project of Higher Education of Jiangsu Province (Nos. 21KJB230001 and 21KJB350019), the Open Foundation of Jiangsu Key Laboratory of Marine Pharmaceutical Compound Screening (No. HY202101), the Postdoctoral Science Foundation of Lianyungang (No. LYG20220013), and the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Author contributions
Xiao ZHANG, Zhicheng DONG, Hui FAN, and Guili YU performed the experimental research and data analysis. Xiao ZHANG, Nana HE, and Xueqing LI wrote and edited the manuscript. Qiankun YANG and Enzhuang PAN performed the establishment of animal models. Panpan ZHAO, Mian FU, and Jingquan DONG contributed to the study design, data analysis, writing and editing of the manuscript. All authors have read and approved the final manuscript, and therefore, have full access to all the data in the study and take responsibility for the integrity and security of the data.
Compliance with ethics guidelines
Xiao ZHANG, Zhicheng DONG, Hui FAN, Qiankun YANG, Guili YU, Enzhuang PAN, Nana HE, Xueqing LI, Panpan ZHAO, Mian FU, and Jingquan DONG declare that they have no conflicts of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed. The study was approved by the Jiangsu Ocean University Animal Ethics Committee (No. 2020220671), China.
References
- An YN, Zhang HF, Wang C, et al. , 2019. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis. FASEB J, 33(11): 12515- 12527. 10.1096/fj.201802805RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bataille AM, Manautou JE, 2012. Nrf2: a potential target for new therapeutics in liver disease. Clin Pharmacol Ther, 92(3): 340- 348. 10.1038/clpt.2012.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian HT, Wang GH, Huang JJ, et al. , 2020. Scutellarin protects against lipopolysaccharide-induced behavioral deficits by inhibiting neuroinflammation and microglia activation in rats. Int Immunopharmacol, 88: 106943. 10.1016/j.intimp.2020.106943 [DOI] [PubMed] [Google Scholar]
- Ceni E, Mello T, Galli A, 2014. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J Gastroenterol, 20(47): 17756- 17772. 10.3748/wjg.v20.i47.17756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MF, Gong F, Zhang YY, et al. , 2019. Preventive effect of YGDEY from tilapia fish skin gelatin hydrolysates against alcohol-induced damage in HepG2 cells through ROS-mediated signaling pathways. Nutrients, 11(2): 392. 10.3390/nu11020392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YR, Que RY, Lin LB, et al. , 2020. Inhibition of oxidative stress and NLRP3 inflammasome by Saikosaponin-d alleviates acute liver injury in carbon tetrachloride-induced hepatitis in mice. Int J Immunopathol Pharmarol, 34: 2058738420950593. 10.1177/2058738420950593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YY, Zhang CL, Zhao XL, et al. , 2014. Inhibition of cytochrome P4502E1 by chlormethiazole attenuated acute ethanol-induced fatty liver. Chem Biol Interact, 222: 18- 26. 10.1016/j.cbi.2014.08.009 [DOI] [PubMed] [Google Scholar]
- Ding RB, Tian K, Cao YW, et al. , 2015. Protective effect of Panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanol-mediated oxidative stress. J Agric Food Chem, 63(9): 2413- 2422. 10.1021/jf502990n [DOI] [PubMed] [Google Scholar]
- Fan H, Ma XD, Lin P, et al. , 2017. Scutellarin prevents nonalcoholic fatty liver disease (NAFLD) and hyperlipidemia via PI3K/AKT-dependent activation of nuclear factor (erythroid-derived 2)-like 2 (Nrf2) in rats. Med Sci Monit, 23: 5599- 5612. 10.12659/msm.907530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Tu TT, Zhang X, et al. , 2022. Sinomenine attenuates alcohol-induced acute liver injury via inhibiting oxidative stress, inflammation and apoptosis in mice. Food Chem Toxicol, 159: 112759. 10.1016/j.fct.2021.112759 [DOI] [PubMed] [Google Scholar]
- Galicia-Moreno M, Lucano-Landeros S, Monroy-Ramirez HC, et al. , 2020. Roles of Nrf2 in liver diseases: molecular, pharmacological, and epigenetic aspects. Antioxidants, 9(10): 980. 10.3390/antiox9100980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouillon ZQ, Lucas D, Li J, et al. , 2000. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med, 224(4): 302- 308. 10.1111/j.1525-1373.2000.22435.x [DOI] [PubMed] [Google Scholar]
- Guo FF, Xiao M, Wang SY, et al. , 2020. Downregulation of mitogen-activated protein kinases (MAPKs) in chronic ethanol-induced fatty liver. Toxicol Mech Methods, 30(6): 407- 416. 10.1080/15376516.2020.1747126 [DOI] [PubMed] [Google Scholar]
- Guo XL, Cui RB, Zhao JJ, et al. , 2016. Corosolic acid protects hepatocytes against ethanol-induced damage by modulating mitogen-activated protein kinases and activating autophagy. Eur J Pharmacol, 791: 578- 588. 10.1016/j.ejphar.2016.09.031 [DOI] [PubMed] [Google Scholar]
- Hayes JD, Dinkova-Kostova AT, 2014. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci, 39(4): 199- 218. 10.1016/j.tibs.2014.02.002 [DOI] [PubMed] [Google Scholar]
- He Y, Hara H, Núñez G, 2016. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci, 41(12): 1012- 1021. 10.1016/j.tibs.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek JB, Pastorino JG, 2004. Cellular signaling mechanisms in alcohol-induced liver damage. Semin Liver Dis, 24(3): 257- 272. 10.1055/s-2004-832939 [DOI] [PubMed] [Google Scholar]
- Hu X, Wu XF, Zhao B, et al. , 2019. Scutellarin protects human retinal pigment epithelial cells against hydrogen peroxide (H2O2)-induced oxidative damage. Cell Biosci, 9: 12. 10.1186/s13578-019-0276-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranshahy M, Iranshahi M, Abtahi SR, et al. , 2018. The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: a review. Food Chem Toxicol, 120: 261- 276. 10.1016/j.fct.2018.07.024 [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, et al. , 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun, 236(2): 313- 322. 10.1006/bbrc.1997.6943 [DOI] [PubMed] [Google Scholar]
- Ji WL, Liang K, An R, et al. , 2019. Baicalin protects against ethanol-induced chronic gastritis in rats by inhibiting Akt/NF-κB pathway. Life Sci, 239: 117064. 10.1016/j.lfs.2019.117064 [DOI] [PubMed] [Google Scholar]
- Kim MS, Ong M, Qu XQ, 2016. Optimal management for alcoholic liver disease: conventional medications, natural therapy or combination? World J Gastroenterol, 22(1): 8- 23. 10.3748/wjg.v22.i1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpich IA, Miller ME, Cave MC, et al. , 2016. Alcoholic liver disease: update on the role of dietary fat. Biomolecules, 6(1): 1. 10.3390/biom6010001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LZ, Chandimali N, Han YH, et al. , 2019. Pathogenesis, early diagnosis, and therapeutic management of alcoholic liver disease. Int J Mol Sci, 20(11): 2712. 10.3390/ijms20112712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubes P, Mehal WZ, 2012. Sterile inflammation in the liver. Gastroenterology, 143(5): 1158- 1172. 10.1053/j.gastro.2012.09.008 [DOI] [PubMed] [Google Scholar]
- Leung TM, Nieto N, 2013. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol, 58(2): 395- 398. 10.1016/j.jhep.2012.08.018 [DOI] [PubMed] [Google Scholar]
- Li B, Nasser MI, Masood M, et al. , 2020. Efficiency of traditional Chinese medicine targeting the Nrf2/HO-1 signaling pathway. Biomed Pharmacother, 126: 110074. 10.1016/j.biopha.2020.110074 [DOI] [PubMed] [Google Scholar]
- Li XJ, Mu YM, Li TT, et al. , 2015. Gynura procumbens reverses acute and chronic ethanol-induced liver steatosis through MAPK/SREBP-1c-dependent and -independent pathways. J Agric Food Chem, 63(38): 8460- 8471. 10.1021/acs.jafc.5b03504 [DOI] [PubMed] [Google Scholar]
- Liu JY, 2014. Ethanol and liver: recent insights into the mechanisms of ethanol-induced fatty liver. World J Gastroenterol, 20(40): 14672- 14685. 10.3748/wjg.v20.i40.14672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SY, Tsai IT, Hsu YC, 2021. Alcohol-related liver disease: basic mechanisms and clinical perspectives. Int J Mol Sci, 22(10): 5170. 10.3390/ijms22105170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang YN, Wu D, et al. , 2019a. Magnolol prevents acute alcoholic liver damage by activating PI3K/Nrf2/PPARγ and inhibiting NLRP3 signaling pathway. Front Pharmacol, 10: 1459. 10.3389/fphar.2019.01459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hou RL, Yan JJ, et al. , 2019b. Purification and characterization of Inonotus hispidus exopolysaccharide and its protective effect on acute alcoholic liver injury in mice. Int J Biol Macromol, 129: 41- 49. 10.1016/j.ijbiomac.2019.02.011 [DOI] [PubMed] [Google Scholar]
- Liu YG, Wang J, Zhang XR, et al. , 2019. Scutellarin exerts hypoglycemic and renal protective effects in db/db mice via the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev, 2019: 1354345. 10.1155/2019/1354345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YB, Wahl LM, 2005. Production of matrix metalloproteinase-9 by activated human monocytes involves a phosphatidylinositol-3 kinase/Akt/IKKα/NF-κB pathway. J Leukoc Biol, 78(1): 259- 265. 10.1189/jlb.0904498 [DOI] [PubMed] [Google Scholar]
- Morgan K, French SW, Morgan TR, 2002. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology, 36(1): 122- 134. 10.1053/jhep.2002.33720 [DOI] [PubMed] [Google Scholar]
- Niu CW, Sheng YC, Yang R, et al. , 2015. Scutellarin protects against the liver injury induced by diosbulbin B in mice and its mechanism. J Ethnopharmacol, 164: 301- 308. 10.1016/j.jep.2015.02.031 [DOI] [PubMed] [Google Scholar]
- Nowak AJ, Relja B, 2020. The impact of acute or chronic alcohol intake on the NF-κB signaling pathway in alcohol-related liver disease. Int J Mol Sci, 21(24): 9407. 10.3390/ijms21249407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman ES, Odena G, Bataller R, 2013. Alcoholic liver disease: pathogenesis, management, and novel targets for therapy. J Gastroenterol Hepatol, 28(S1): 77- 84. 10.1111/jgh.12030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, et al. , 2003. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol, 24(8): 449- 455. 10.1016/s1471-4906(03)00181-9 [DOI] [PubMed] [Google Scholar]
- Qian LH, Li NG, Tang YP, et al. , 2011. Synthesis and bio-activity evaluation of scutellarein as a potent agent for the therapy of ischemic cerebrovascular disease. Int J Mol Sci, 12(11): 8208- 8216. 10.3390/ijms12118208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim YR, Jeong WI, 2020. Recent advances of sterile inflammation and inter-organ cross-talk in alcoholic liver disease. Exp Mol Med, 52(5): 772- 780. 10.1038/s12276-020-0438-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XL, Liu ZH, Zhang JJ, et al. , 2018. Anti-inflammatory and hepatoprotective effects of exopolysaccharides isolated from Pleurotus geesteranus on alcohol-induced liver injury. Sci Rep, 8: 10493. 10.1038/s41598-018-28785-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn MB, Liby KT, 2012. NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer, 12(8): 564- 571. 10.1038/nrc3278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Fu JQ, Li L, et al. , 2018. Nrf2 in alcoholic liver disease. Toxicol Appl Pharmacol, 357: 62- 69. 10.1016/j.taap.2018.08.019 [DOI] [PubMed] [Google Scholar]
- Szabo G, Kamath PS, Shah VH, et al. , 2019. Alcohol-related liver disease: areas of consensus, unmet needs and opportunities for further study. Hepatology, 69(5): 2271- 2283. 10.1002/hep.30369 [DOI] [PubMed] [Google Scholar]
- Torres S, Segalés P, García-Ruiz C, et al. , 2022. Mitochondria and the NLRP3 inflammasome in alcoholic and nonalcoholic steatohepatitis. Cells, 11(9): 1475. 10.3390/cells11091475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Cui L, Feng L, et al. , 2016. Isoalantolactone inhibits the migration and invasion of human breast cancer MDA-MB-231 cells via suppression of the p38 MAPK/NF-κB signaling pathway. Oncol Rep, 36(3): 1269- 1276. 10.3892/or.2016.4954 [DOI] [PubMed] [Google Scholar]
- Wang JM, Zhang YY, Zhang YS, et al. , 2012. Protective effect of lysimachia christinae against acute alcohol-induced liver injury in mice. Biosci Trends, 6(2): 89- 97. [PubMed] [Google Scholar]
- Wang LP, Ma Q, 2018. Clinical benefits and pharmacology of scutellarin: a comprehensive review. Pharmacol Ther, 190: 105- 127. 10.1016/j.pharmthera.2018.05.006 [DOI] [PubMed] [Google Scholar]
- Wang P, Gao C, Guo N, et al. , 2018. 2'- O-Galloylhyperin isolated from Pyrola incarnata Fisch. attenuates LPS-induced inflammatory response by activation of SIRT1/Nrf2 and inhibition of the NF-κB pathways in vitro and vivo. Front Pharmacol, 9: 679. 10.3389/fphar.2018.00679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YY, Fan XM, Fan B, et al. , 2020. Scutellarin reduce the homocysteine level and alleviate liver injury in type 2 diabetes model. Front Pharmacol, 11: 538407. 10.3389/fphar.2020.538407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZG, Yao T, Song ZY, 2010. Involvement and mechanism of DGAT2 upregulation in the pathogenesis of alcoholic fatty liver disease. J Lipid Res, 51(11): 3158- 3165. 10.1194/jlr.M007948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DF, Wang XD, Zhou R, et al. , 2012. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic Biol Med, 53(6): 1346- 1357. 10.1016/j.freeradbiomed.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao T, Cui YL, Ji HY, et al. , 2021. Baicalein attenuates acute liver injury by blocking NLRP3 inflammasome. Biochem Biophys Res Commun, 534: 212- 218. 10.1016/j.bbrc.2020.11.109 [DOI] [PubMed] [Google Scholar]
- Xu L, Yu YF, Sang R, et al. , 2018. Protective effects of taraxasterol against ethanol-induced liver injury by regulating CYP2E1/Nrf2/HO-1 and NF- κB signaling pathways in mice. Oxid Med Cell Longev, 2018: 8284107. 10.1155/2018/8284107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu LJ, Chen RC, Zhang X, et al. , 2021. Scutellarin protects against diabetic cardiomyopathy via inhibiting oxidative stress and inflammatory response in mice. Ann Palliat Med, 10(3): 2481- 2493. 10.21037/apm-19-516 [DOI] [PubMed] [Google Scholar]
- Yu GL, Wang JX, Zhang W, et al. , 2021. NLRP3 inflammasome signal pathway involves in Vibrio harveyi-induced inflammatory response in murine peritoneal macrophages in vitro . Acta Biochim Biophys Sin, 53(12): 1590- 1601. 10.1093/abbs/gmab137 [DOI] [PubMed] [Google Scholar]
- Yu WZ, Tao MR, Zhao YL, et al. , 2018. 4'-Methoxyresveratrol alleviated AGE-induced inflammation via RAGE-mediated NF-κB and NLRP3 inflammasome pathway. Molecules, 23(6): 1447. 10.3390/molecules23061447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J, Cai SN, 2017. Breviscapine suppresses the growth of non-small cell lung cancer by enhancing microRNA-7 expression. J Biosci, 42(1): 121- 129. 10.1007/s12038-017-9670-0 [DOI] [PubMed] [Google Scholar]
- Zeng T, Zhang CL, Zhao N, et al. , 2018. Impairment of Akt activity by CYP2E1 mediated oxidative stress is involved in chronic ethanol-induced fatty liver. Redox Biol, 14: 295- 304. 10.1016/j.redox.2017.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TM, Wang K, Fan H, et al. , 2022. Ameliorative effect of scutellarin on acute alcohol brain injury in mice. J Zhejiang Univ-Sci B (Biomed & Biotechnol), 23(3): 258- 264. 10.1631/jzus.B2100763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XX, Ji RP, Sun HJ, et al. , 2018. Scutellarin ameliorates nonalcoholic fatty liver disease through the PPARγ/PGC-1α-Nrf2 pathway. Free Radic Res, 52(2): 198- 211. 10.1080/10715762.2017.1422602 [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Ren Z, Chen S, et al. , 2018. ROS generation and JNK activation contribute to 4-methoxy-TEMPO-induced cytotoxicity, autophagy, and DNA damage in HepG2 cells. Arch Toxicol, 92(2): 717- 728. 10.1007/s00204-017-2084-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Mehmood A, Yuan DD, et al. , 2021. Protective mechanism of edible food plants against alcoholic liver disease with special mention to polyphenolic compounds. Nutrients, 13(5): 1612. 10.3390/nu13051612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Guo FF, Xie KQ, et al. , 2018. Targeting Nrf-2 is a promising intervention approach for the prevention of ethanol-induced liver disease. Cell Mol Life Sci, 75(17): 3143- 3157. 10.1007/s00018-018-2852-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YL, Ma X, Wang JB, et al. , 2016. A system review of anti-fibrogenesis effects of compounds derived from Chinese herbal medicine. Mini-Rev Med Chem, 16(2): 163- 175. 10.2174/1389557515666150709121908 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.