Abstract
A broad range of AOAC Official Methods of AnalysisSM (OMA) have been developed and approved for the measurement of dietary fiber (DF) and DF components since the adoption of the Prosky method (OMA 985.29). OMA 985.29 and other OMA were developed to support the Trowell definition of DF. However, these methods do not measure DF as defined by the “new,” physiologically relevant, Codex Alimentarius definition. Methodology to support the Codex definition has been developed and updated in recent years. In this article, the relevance of each OMA in supporting the Codex definition of DF is described and suggestions are presented on the most appropriate method, together with proposals for changes in title and application statements for the “historic” OMA methods.
Interest in dietary fiber (DF), and its measurement in human foods, is a consequence of the belief that DF contributes positively to the health and quality of life of the consumer. DF is a multicomponent mixture including:
plant cell-wall polysaccharides such as cellulose, modified xylans, and pectins (with associated lignin, cutin, suberin, and waxes);
storage polysaccharides such as galactomannans, glucomannan, arabinoxylan, xyloglucans, and fructans;
microbial polysaccharides (xanthan);
resistant starch (RS; natural and manufactured);
exudate gums (gum arabic and larch arabinogalactan);
algal polysaccharides (agar, alginates and carrageenans);
nondigestible oligosaccharides such as fructooligosaccharides, galactosyl-sucrose oligosaccharides, galactooligosaccharides; and
resistant oligosaccharides derived from starch and glucose (e.g., Fibersol 2 and polydextrose).
Thus, a clear, all-inclusive definition of DF is required for both regulatory purposes and to facilitate the development of analytical methodology to support the definition. As our understanding of the physiological role and multi-component complexity of DF have advanced, physiologically relevant methodology has been developed.
In 1929, McCance and Laurence (1) classified dietary carbohydrates as either “available” or “unavailable.” Unavailable carbohydrates were defined as those that are not hydrolyzed by any (human-derived) enzymes secreted into the human digestive tract. This definition parallels the food component “dietary fiber,” as coined by Hipsley in 1953 (2). In this definition, Hipsley included lignin, cellulose, and hemicellulose. Southgate (the natural heir to McCance and Widdowson at the Dunn Nutrition Unit, Cambridge), still employing the terminology of McCance and Laurence (1), defined “unavailable carbohydrates” on a more physiological basis as “not hydrolysed by any enzyme secreted into the human digestive tract” (3, 4).
In 1972, after consultation with David Southgate (5), Trowell published a definition of DF as “the skeletal remains of plant cells that are resistant to enzymes of man” (6). This definition was subsequently refined to include polysaccharides and lignin that are not digested in the human small intestine (7). Methodology to support this definition was separately developed by Furda (8, 9), Schweizer and Wursch (10, 11), and Asp and Johansson (12), leading to the enzymatic gravimetric Official Method of AnalysisSM (OMA) for total DF (TDF) [Prosky et al. (13); OMA 985.29] (Figure 1) as well as for insoluble DF (IDF) and soluble DF that precipitates in 78% aqueous ethanol (SDFP) [Lee et al. (14); OMA 991.43] (Figure 2) and various other related DF methodologies (15), such as the Matsutani modification of OMA 985.29 to allow measurement of the nondigestible oligosaccharide component of Fibersol 2, which does not precipitate in 78% aqueous ethanol (16) (OMA 2001.03; Figure 3).
Figure 1.
Schematic representation of the steps involved in OMA 985.29.
Figure 2.
Schematic representation of the steps involved in OMA 991.43.
Figure 3.
Schematic representation of the steps involved in OMA 2001.03.
Since the early 1990s, the recognition of the need for a more definite physiological definition of DF was realized. In response to this, the American Association of Cereal Chemists International (AACCI) (now Cereals & Grains Association) began a critical review of the current state of DF science including consideration of the state of the DF definition. Consequently, an updated definition of DF was published (17), namely:
Dietary fiber is the edible parts of plants or analogous carbohydrates that are resistant to digestion and absorption in the human small intestine with complete or partial fermentation in the large intestine. Dietary fiber includes polysaccharides, oligosaccharides, lignin, and associated plant substances. Dietary fibers promote beneficial physiological effects including laxation, and/or blood cholesterol attenuation, and/or blood glucose attenuation.
Several other definitions of DF were subsequently published. The Food Nutrition Board (FNB) of the Institute of Medicine of the National Academies (USA) (2001) defined DF (18) as follows:
Dietary fiber consists of nondigestible carbohydrates and lignin that are intrinsic and intact in plants. Added fiber consists of isolated, nondigestible carbohydrates that have beneficial physiological effects in humans. Total fiber is the sum of dietary fiber and added fiber.
The definition for DF adopted in 2009 by the Codex Alimentarius Commission (CAC) (19) lists three categories of carbohydrates that are not hydrolyzed by the endogenous enzymes in the small intestine of humans; however, the decision concerning the inclusion, or not, of oligosaccharides with degrees of polymerization (DP) in the range of 3 to 9 was left to the discretion of national authorities:
Dietary fiber consists of carbohydrate polymers with ten or more monomeric units, which are not hydrolysed by the endogenous enzymes in the small intestine of humans and belong to the following categories: edible carbohydrate polymers naturally occurring in the food as consumed; carbohydrate polymers which have been obtained from food raw material by physical, enzymatic or chemical means and which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities, and; synthetic carbohydrate polymers which have been shown to have a physiological effect of benefit to health as demonstrated by generally accepted scientific evidence to competent authorities.
When derived from a plant origin, DF may include fractions of lignin and/or other compounds when associated with polysaccharides in the plant cell walls and if these compounds are quantified by the AOAC gravimetric analytical method for DF analysis: fractions of lignin and the other compounds (proteic fractions, phenolic compounds, waxes, saponins, phytates, cutin, phytosterols, etc.) intimately “associated” with plant polysaccharides in the AOAC 991.43 method.
Decision on whether to include carbohydrates of 3 to 9 monomeric units should be left up to national authorities.
The FDA released its definition of DF in 2016 (20). Two general classes of fiber were identified, naturally occurring fibers that are “intrinsic and intact” in plants and isolated or synthetic non-digestible carbohydrates (with three or more monomeric units) determined by FDA to have physiological effects that are beneficial to human health. The first category includes plant fiber that is intrinsic and intact and occurs naturally in foods such as whole grains, cereal bran, flaked cereal and flours, vegetables, and fruits. It is considered “intact” because this kind of fiber has not been removed from the food. According to the FDA, “foods containing these fibers have been shown to be beneficial, and manufacturers do not need to demonstrate that they provide beneficial physiological effects to human health.
The second category of fiber—isolated or synthetic—includes nondigestible carbohydrates that are separated from their original plant form or that do not arise naturally in plants. These are commonly used in processed foods to boost fiber content or otherwise improve the product. The FDA allows these ingredients to be called fiber only if they have a “beneficial effect on human health,” such as lowering cholesterol or glucose levels, increasing frequency of bowel movements, or improving mineral absorption. The FDA released a list of the “isolated or synthetic” category that the agency determined had scientifically proven health benefits and, subsequently, a list of fibers that the FDA proposes to add to the original list has been published.
Most of the confusion associated with the measurement of DF can be attributed to the changing definition of this group of food components. The initial DF methods, the Prosky-type methods (OMA 985.29, 991.42, 991.43, 993.19, 994.13, and 2001.03) based on AOAC OMA 985.29 (15) (Table 1), were developed to support the Trowell definition of DF (6, 7). In these methods, starch removal was achieved using a combination of heat-stable α-amylase at a high temperature followed by amyloglucosidase (AMG) (Figure 1), and it was assumed either that all starch was removed or that which remained was insignificant. Subsequently, with the release of commercial starches high in RS and the realization that this RS behaves physiologically as DF, a need for analytical methodology that more accurately measured physiologically relevant RS, i.e., methods that simulate the in vivo conditions in the human small intestine, was required.
Table 1.
AOAC (OMA), AACCI, ICC, and Codex official methods for the analysis of dietary fiber and what they claim to measure

|
Not included in the original Codex list of accepted DF methods.
In 2012, the Codex Committee on Methods of Analysis and Sampling (CCMAS) recommended 14 OMA methods (Table 1) for the measurement of DF (21), and further updates have subsequently been made. This is an extraordinarily large number of methods, and it is not surprising that considerable confusion exists in selection of the most appropriate method for a specific analysis. Many of these 14 methods were initially included in the Codex list because it was not clear what each of these measured in view of the new Codex definition of DF (19). Many of the methods that effectively supported the Trowell definition (7) (OMA 985.29, 991.42, 993.19, 991.43, 994.13, and 2001.03) (15) have limited applicability under the new definition. In Table 2, these methods are listed showing what the method claims to measure along with what it actually measures, according to the Codex definition. Of the Prosky-type methods, only OMA 2001.03 includes measurement of SDFS, and none of these methods give an accurate measurement of physiologically relevant RS. In the current article, attention will be focused on the evolution from the Prosky-type methods to the more physiologically relevant methods (OMA 2017.16 and 2022.01) for the measurement of DF, which employ pancreatic α-amylase plus AMG and incubation conditions (for starch hydrolysis) that match those in the human small intestine. Since methods for the measurement of non-starch polysaccharides (NSP) are not OMA methods, these will not be discussed in this article.
Table 2.
AOAC OMA: What is measured and what is not measured with particular methods

|
Nomenclature and Acronyms
With the change in definition of DF, new nomenclature needed to be introduced to facilitate the identification of the specific fiber fractions, and thus to allow a comparison of DF values obtained with different methodologies. DF as defined under the Trowell definition (7) is different from that under the Codex 2009 definition (19). In the Prosky-type methods for TDF (except OMA 2001.03), only the polysaccharide fraction is measured, comprising both the soluble and insoluble polymers (termed high molecular weight DF; HMWDF) (22) (Table 3). Nondigestible oligosaccharides that remained soluble in 78% aqueous ethanol (SDFS) are not measured in those methods because at that point in time these oligosaccharides were not considered to be DF. In the Codex discussions on the definition of DF, one of the sticking points was the definition of oligosaccharides. In the classical definition, this would include sugars with a DP of 3–9, but from a practical, analytical viewpoint, the oligosaccharides contained in the 78% (v/v) aqueous ethanol fraction (SDFS) clearly includes all oligosaccharides of DP ≥3 that are soluble in this solvent.
Table 3.
Terminology and acronyms used for the various fractions of DF

|
Aqueous ethanol at 78% (v/v) precipitates higher molecular weight polysaccharides and oligosaccharides and lower molecular weight oligosaccharides remain in solution.
The solubility of oligosaccharides in aqueous ethanol or water is dictated by the nature of the oligosaccharide: α-linked oligosaccharides such as maltodextrins are very soluble in water and 78% aqueous ethanol. Maltooligosaccharides up to ∼DP 15 remain soluble in 78% aqueous ethanol. β-linked oligosaccharides such as cellooligosaccharides, β-mannooligosaccharides, and β-xylooligosaccharides are much less soluble, e.g., cellooligosaccharides above DP 7 are insoluble in water.
To clarify these discussions, definitions of the various DF fractions that matched the experimental procedures employed were introduced (Table 3). Thus, IDF is the DF that is insoluble in water, and SDF is the DF that is soluble in water. The SDF fraction is composed of soluble DF that precipitates in the presence of 78% (v/v) aqueous ethanol (SDFP) and the water-soluble DF that remains soluble in 78% aqueous ethanol (SDFS) (equivalent to the nondigestible oligosaccharide fraction described by McCleary et al. (22).
Prosky-Type Methods for DF Measurement
The Prosky-type DF methods (OMA 985.29, 991.42, 993.19, 991.43, 994.13, and 2001.03) all employ thermostable α-amylase (at ∼98–100°C) and AMG (at 60°C) to hydrolyze starch and any other α-1,4:1,6-glucans to glucose and lower degree of polymerization (DP) maltodextrins, which are subsequently removed by washing the sample with aqueous ethanol (Figures 1–3). At the time of development and accreditation of OMA 985.29 (Prosky) for measurement of total DF, the concept of RS was just emerging. Whether at that time the researchers did not realize that some of the starch resisted hydrolysis or, alternatively, they considered that the amount remaining was so low as to be negligible is not clear. However, with the general recognition by nutritionists, food manufacturers, and regulators that RS is a valuable DF component, as well as its recognition by Codex as DF, a need for accurate measurement became clear. The concurrent acceptance of nondigestible oligosaccharides as DF further highlighted the need to relook at the original DF methods and determine what they actually measure. While OMA 985.29 gave a good measure of DF according to the Trowell definition (7), it does not measure TDF according to the Codex definition (19). It clearly does not measure nondigestible oligosaccharides, and values obtained for the various RS fractions are either underestimated or overestimated (Table 2).
Methods for the Measurement of Specific DF Components
Prosky-type OMA procedures for measurement of DF do not quantitatively measure RS and do not measure soluble DF that remains soluble in 78% aqueous ethanol (SDFS). Consequently, methods for measurement of specific nondigestible oligosaccharides such as fructooligosaccharides (FOS) [(OMA 997.08) (15, 23) and 999.03 (15, 24)] and galactooligosaccharides (GOS) [(OMA 2001.02) (15, 25)] were developed and validated. An ion-chromatography method for measurement of polydextrose (OMA 2000.11) (15, 26) was developed and validated, and a modified Prosky-type method for the measurement of Fibersol 2 (OMA 2001.03) (15, 16), which includes fiber as measured by the Prosky method as well as the SDFS component of this fiber, was also developed. For the measurement of RS, a method based on considerable research by Englyst et al. (27), Berry (28), Muir and O’Dea (29), Champ (30), Faisant et al. (31), Goni et al. (32), McCleary and Monaghan (33), and others was developed and validated as OMA 2002.02 (15, 24) following an extensive interlaboratory study. The specificity of each of these methods is summarized in Table 4. The methods have found considerable application in the measurement of the specific DF components. However, total DF cannot simply be calculated by the addition of the weight of the specific DF (e.g., RS, FOS, or polydextrose) to the weight of DF determined with OMA 985.29. A problem of “double counting” is experienced, as shown in Figure 4. Thus, if RS values obtained using OMA 2002.02 are added to DF measured with OMA 985.29, then there is “double counting” of the overlapping area shown in Figure 4. This problem highlighted the need for an integrated, all-inclusive TDF procedure that gives accurate measurement of all DF components and avoids double counting.
Table 4.
AOAC OMA: Methods for particular DF components and their specificity

|
Figure 4.
Dietary fiber components measured and not measured with OMA 985.29; identifying the problem of “double counting” of some components when a combination of methods are employed. First presented at Symposium on Dietary Fiber, Osaka, Japan, September 17–19, 2004. McCleary, B.V., Mills, C., & Draga, A. (2009). Quality Assurance and Safety of Crops & Foods, 1, 213–224.
An Integrated Procedure for the Measurement of Total Dietary Fiber (INTDF)
To allow measurement of DF as defined by Codex Alimentarius (19), an integrated method for the measurement of total DF was developed and published in 2007 (34). This method measures the traditional fibers such as cellulose, pectins, and hemicelluloses, but also RS and SDFS. The sample being analyzed is incubated with pancreatic α-amylase and AMG under conditions very similar to those employed in OMA 2002.02 for the measurement of RS, but with a 10-fold increase in sample size, buffer volumes, and quantity of enzymes employed. An incubation time of 16 h was used to remain consistent with the incubation time in OMA 2002.02 for RS (15, 24). The reaction is terminated by pH adjustment and heating to ∼95°C, and after incubation with protease and pH adjustment (Figure 5, LHS), high molecular weight resistant polysaccharides are precipitated from solution in 78% (v/v) aqueous ethanol and the insoluble and alcohol precipitated soluble polysaccharides (SDFP) recovered by filtration, washed, dried, and weighed. The aqueous ethanol extract is concentrated, desalted, and analyzed for SDFS by HPLC using a Waters SugarPak® column. With this procedure, the recovery of RS for most RS-containing samples is in line with published values obtained with OMA 2002.02 (Table 5) and with ileostomy studies. Values obtained for native potato starch, Actistar® (insoluble linear maltodextrins), and green banana starch with this integrated procedure are lower than those determined with OMA 2002.02, and this is attributed to the fact that in the integrated procedure the RS is completely dissolved and then reprecipitated with alcohol, whereas in OMA 2002.02, the RS remains insoluble. In comparison, the RS determined for these three samples with OMA 985.29 is very low (Table 3 in reference 34), inconsistent with ileostomy data.
Figure 5.
Schematic representation of the steps involved in OMA 2009.01 and 2017.16.
Table 5.
A comparison of the resistant starch values determined for a range of samplesa using AOAC OMA 2002.02 and 2009.01 (with modifications)

|
Samples were freeze-dried and had a final moisture content of 2–3%.
HylonVII and Novelose 240 are native, high-amylose maize starches.
Novelose 330 and CrystaLean are retrograded high-amylose maize starches.Reprinted from McCleary, B.V. (2007) Anal. Bioanal. Chem. 389, 291–308.
This integrated method for measurement of TDF was subjected to an extensive interlaboratory study under the auspices of AOAC INTERNATIONAL and accepted as OMA 2009.01 (15, 35). The method was then modified to allow separate measurement of soluble and IDF (Figure 6), and after interlaboratory validation became OMA 2011.25 (36). These methods were widely evaluated internationally for over 5 years, and various deficiencies or areas of possible improvement were identified, namely:
Figure 6.
Schematic representation of the steps involved in OMA 2011.25.
The time of incubation with pancreatic α-amylase/AMG was excessive. This time does not match with the likely time of residence of food in the human small intestine. It was suggested that this could impact on the accurate measurement of certain RS materials such as phosphate crosslinked starch (RS4).
In the analysis of samples with high starch contents, a resistant maltodextrin was produced under the conditions employed, and this is erroneously measured as DF.
FOS are underestimated when analyzed using a Waters SugarPak column. One of the oligosaccharide fractions in hydrolyzed inulin, namely fructotriose (F3), elutes from the HPLC column at the same point as sucrose (a disaccharide), and thus is not measured as DF.
The inclusion of sodium azide, a known carcinogen, as a preservative in the assay buffer was deemed to be unacceptable. This antimicrobial agent was important in the buffers under the incubation conditions employed (16 h incubation at neutral pH, 37°C and in the presence of sugars).
The preparation of the NDO fraction for HPLC is tedious.
Development of an Improved Procedure for the Measurement of Total Dietary Fiber, the Rapid Integrated TDF Procedure (RINTDF)
Each of the suggestions and criticisms of the integrated TDF procedures (OMA 2009.01 and 2011.25) were critically evaluated, and solutions were devised.
The incubation time with pancreatic α-amylase/AMG was reduced from 16 h to 4 h; the likely time of residence of food in the human small intestine (37–39). Concurrently, the levels of pancreatic α-amylase and AMG were increased (Figure 5) to ensure that the determined RS values for several control samples remained consistent with ileostomy data and that DF values for a range of other samples were generally consistent with data using OMA 2009.01 (Table 6).
Under the incubation conditions employed, the much higher levels of AMG ensured complete hydrolysis of the resistant oligosaccharides (63,65-di-α-D-glucosyl maltopentaose and 63-α-D-glucosyl maltotriose) produced from non-RS in OMA 2009.01 (22, 40).
Using the TSK columns as employed in OMA 2001.03 (the Matsutani method), F3 chromatographed with other trisaccharides (41) and was thus correctly measured.
With the shorter incubation time of 4 h, the possibility of microbial contamination was minimal; thus, sodium azide could be removed from the incubation buffers.
In OMA 2001.03, recovery, concentration, and deionization of the fraction containing the oligosaccharides is tedious. This has been greatly simplified by handling just 25% of the aqueous ethanol fraction and removal of the salt in the sample by deionization in a tube containing resins followed by in-line deionization using Bio-Rad deashing cartridges (42, 43) (Figure 5, RHS).
Table 6.
A comparison of DF values obtained for a range of starch containing samples using AOAC OMA 2009.01 and 2017.16

|
Reprinted from McCleary, B.V., Sloane, N., & Draga, A. (2015) Starch, 67, 860–883.
Commercial product in cans was strained, washed with water, and freeze-dried before analysis.
The principle of the method that resulted from this research, the rapid integrated TDF method (OMA 2017.16) (40, 42), is shown in Figure 5 (RHS) and compared to OMA 2009.01 (LHS). The novel element of the RINTDF method (OMA 2017.16) is that starch hydrolysis is performed under conditions that are as close to physiological conditions as is possible in a routine analytical laboratory situation (Table 7), which should future-proof the method as new starch-based DF components are introduced. The protein hydrolysis, precipitation, and recovery steps are consistent with those employed in the traditional Prosky-type methods. In application, samples are suspended in buffer at pH 6.0 and incubated with pancreatic α-amylase and AMG with shaking or stirring at 37°C for 4 h. The reaction is terminated by pH adjustment to ∼8.2, followed by heating to ∼95°C. At pH 8.2, AMG has essentially no activity, which is essential to ensure no hydrolysis of RS as it is partially solubilized when the reaction suspension is heated to ∼95°C to denature proteins and inactivate pancreatic α-amylase and AMG. Pancreatic α-amylase is inactivated at temperatures above 50°C, well below the temperature of starch gelatinization (∼65°C).
Table 7.
A comparison of incubation conditions employed for the hydrolysis of starch in AOAC OMA 2009.01 and AOAC OMA 2017.16 to in vivo conditions in the human small intestine

|
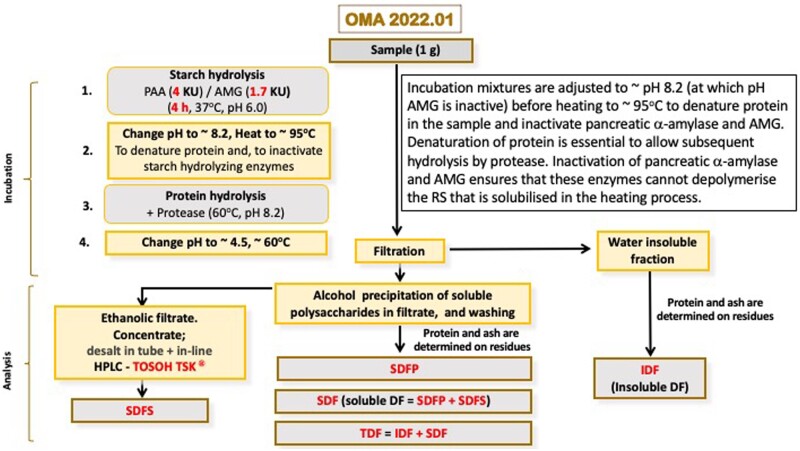
After cooling to 60°C, samples are incubated with protease, consistent with other DF procedures. Solution pH is adjusted to pH 4.5, and, for TDF determination, ethanol is added to a concentration of 78% (v/v) with stirring and the suspension allowed to stand for at least 1 h. The insoluble residue is recovered by filtration, washed, dried, and weighed to obtain residue weights. Ash and residual protein weights are determined and subtracted from residue weights to determine DF values. For separate measurement of IDF and SDF, suspensions are filtered before the addition of ethanol. The fraction containing IDF is captured on the sintered glass funnel and the filtrate is treated with ethanol to 78% (v/v), mixed and filtered as described above, to separate the SDFP and SDFS fractions. SDF is the sum of SDFP and SDFS (OMA 2022.01; Figure 7).
Figure 7.
Schematic representation of the steps involved in OMA 2022.01.
This RINTDF method has been applied to the analysis of a wide range of food matrixes, pure polysaccharides, and oligosaccharides and a broad range of starches, chemically modified starch, and starch-derived fibers. The DF values for a range of control oligosaccharides and polysaccharides is shown in Table 8. The higher value obtained for Raftilose P95® with OMA 2017.16 is due to the use of TSKgel G2500PWXL® gel permeation columns in this procedure, compared to a Waters SugarPak column in OMA 2009.01. With the TSKgel G2500PWXL gel permeation columns, FOS are correctly separated and the F3 fraction is accurately measured. The low DF values obtained for AdvantaFiber® isomaltooligosaccharides is because these oligosaccharides are hydrolyzed in the incubations, consistent with results obtained using OMA 2001.03 (unpublished results) and those from Tanabe et al. (44). With Fibersym® (RS4) and Hylon VII® (RS2), the higher DF values obtained with OMA 2017.16 than with OMA 2009.01 relates to the shorter time of incubation with the former method. This value is considered to be a more accurate reflection of the RS (and DF) content because the assay procedure more closely parallels in vivo conditions in the human small intestine, i.e., an incubation time of 4 h (Table 7). The higher DF values obtained for Fibersym with OMA 985.29 compared to OMA 2017.16 are due to the nonphysiological incubation conditions employed in OMA 985.29. In fact, slight alteration of the incubation temperature with heat stable α-amylase (otherwise performed under the incubation conditions of OMA 985.29) results in dramatically different DF values (Figure 8); e.g., compare the DF values obtained at 90°C to those at ∼100°C and the digested Fibersym (measured as free glucose) at 95 and 100°C.
Table 8.
TDF values obtained for a range of reference polysaccharides and oligosaccharides with AOAC OMA 2009.01 and AOAC OMA 2017.16

|
The FOS analyzed had a high content of inulinotriose. Analyzed using Sugar Pak column.
Figure 8.

Hydrolysis of wheat starch and Fibersym under conditions exactly as described for AOAC Method 985.29, but with incubations with heat-stable α-amylase performed at 70, 80, 90, 95, and 100°C. Digestible starch was calculated from the determined glucose value and the DF value was determined by gravimetric analysis. Reprinted from McCleary, B.V., McLoughlin, C., Charmier, L.M.J. & McGeough, P. (2019) Cereal Chem., 97, 114–137.
Following extensive in-house evaluation of the rapid integrated TDF method, it was then subjected to interlaboratory evaluation under the auspices of AOAC INTERNATIONAL, AACC International, and the International Association for Cereal Science and Technology (ICC). For the measurement of total DF, the method is now accepted as OMA 2017.16 (Final Approval) (15, 42), AACC Method 32–60.01, and ICC Standard No 185. The method was also successfully evaluated for the separate measurements of insoluble, soluble, and TDF: OMA 2022.01 (First Approval) (15, 43) and ICC Standard No. 191. OMA 2017.16 was recently accepted by Codex (21) as a replacement for OMA 2009.01 (15, 22). When OMA 2022.01 (SDF and IDF) (43) has gained Final Approval status with AOAC, the method will be presented to Codex as a replacement for OMA 2011.25.
Other Considerations and Requirement for Further Research
OMA 2017.16 and 2022.01 are designed for the measurement of TDF or SDF and IDF in foods “as eaten.” After all, it is the fate of the food component in the human digestion system that is of relevance to consumers, nutritionists, and regulators. In applying these methods to the analysis of raw ingredients, food and food ingredient manufacturers will face some challenges. For example, a bread flour may contain a high level of RS1, but this will “disappear” during the baking step of bread making. Thus, a high DF or RS value for the flour may not be reflected in the final product. It may be essential to develop and incorporate pre-incubation steps to predict what might happen to a starch material in a particular process. A defined pre-cooking step could be useful for ingredients that form part of a final product that is to be cooked or baked before being consumed. The effect of pre-cooking for 15 min at ∼100°C on the determined RS values of a range of starch and food samples is shown in Table 9. RS in green bananas and native potato starch is solubilized by cooking and subsequently digested. There is considerable digestion of RS2 in native high amylose starches (Hylon VII, Novelose 240® and high amylose maize starch), but RS3 in Novelose 330® (retrograded starch) is not affected by heating.
Table 9.
Effect of pre-cooking of starch containing samples on the determined resistant starch valuesa

|
Reprinted from McCleary, B.V, Sloane, N. & Draga, A. (2015) Starch, 67, 860–863.
Accurate analysis of DF requires the complete removal of fatty materials from samples. This may not be a significant challenge with simple plant-based foods; however, problems could be experienced in analyzing complex food products. Removal of coconut fat from foods, for example, poses challenges and requires exhaustive defatting of samples before analysis.
By definition, DF includes oligosaccharides of DP 3 and higher. Effective separation on TSKgel G2500PWXL gel permeation columns of the disaccharide and trisaccharide fractions is complicated by certain disaccharides (e.g., lactose and isomaltose) that overlap with the trisaccharide fraction on elution. If overlapping of the di- and trisaccharide fractions is observed, the offending disaccharides can be “removed” by hydrolysis to monosaccharides with the appropriate enzyme before performing the HPLC (43).
Which Method to Use for the Measurement of Dietary Fiber
The decision on which method to use for measurement of DF content of a sample is dictated by the fiber component that is being measured (TDF, IDF or SDF) and by the nature of the sample that is being analyzed (Table 10).
Table 10.
Choosing the AOAC OMA to use to measure DF in samples containing, or devoid of resistant starch

|
If a sample is known to contain no RS, then:
TDF can be measured with either OMA 2001.03 or 2017.16.
TDF as IDF plus SDFP can be measured with OMA 985.29, 991.43, 2001.03, or 2017.16. With OMA 985.29 and 991.43, SDFS is measured as described in OMA 2022.01.
TDF as IDF and SDFP measured separately can be determined with OMA 2022.01 or, alternatively, with OMA 991.43 with measurement of SDFS using the HPLC procedure described in OMA 2022.01 (Table 10). Alternatively, IDF can be measured with OMA 991.42 and SDFP with OMA 993.19.
If no information is available on the sample being analyzed, or if the sample is known to contain RS, then:
TDF is measured with OMA 2017.16.
SDF (as SDFP and SDFS separately) and IDF are measured with OMA 2022.01 (Table 10).
Concluding Comment and Recommendation
Numerous methods have been approved by AOAC for the measurement of DF. Many of these methods claim to measure total DF, and while this was the case under the Trowell (7) definition of DF, it no longer is the case under the accepted Codex definition (19). Each of the OMA methods needs critical re-evaluation in terms of scope and application, and this can best be done by an appropriate Analytical Solutions Forum (ASF) within AOAC. As a first step in this process, the titles of the OMA need to be updated in line with the Codex DF definition. Some suggestions on possible OMA title changes are given in Tables 11 and 12, and possible updated scope and application statements for some of the OMA are given in Table 13. In the interim, to prevent continued confusion and potential future conflict, the author recommends that all DF values supplied to customers by analytical laboratories, or published in literature, should include details of the AOAC OMA employed to generate these values.
Table 11.
Suggested title update of OMA DF methods in line with the Codex definition of DF (part 1)

|
Table 12.
Suggested title update of OMA DF methods in line with the Codex definition of DF (part 2)

|
Table 13.
Suggested updating of the scope statements of traditional AOAC OMA for DF, in line with the Codex definition of DF

|
Acknowledgments
The views expressed in this manuscript are those of the author. The need for an integrated procedure for the measurement of TDF to service the evolving Codex Alimentarius definition of DF, became evident to the author early in the 2000’s. Subsequent research within Megazyme led to OMA 2009.01, 2011.25, 2017.16 and 2022.01. The development and validation of these methods involved several Megazyme and external scientists, the names of whom appear on the references cited in this paper. These methods form the basis of commercial Megazyme test kits, now supplied by Neogen Plc.
Conflict of Interest
None declared.
Funding
This work was completely funded by FiberCarb.
References
- 1. McCance R.A., Lawrence R.D. (1929) Medical Research Council Special Report Series No. 135. London: H.M. Stationery Office [Google Scholar]
- 2. Hipsley E. (1953) Br. Med. J. 2, 420–422. doi: 10.1136/bmj.2.4833.420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Southgate D.A.T. (1969) J. Sci. Food Agric. 20, 326–330. doi: 10.1002/jsfa.2740200602 [DOI] [PubMed] [Google Scholar]
- 4. Southgate D.A.T. (1969) J. Sci. Food Agric. 20, 331–335. doi: 10.1002/jsfa.2740200603 [DOI] [PubMed] [Google Scholar]
- 5. Cummings J.H., Engineer A. (2018) Nutr. Res. Rev. 31, 1–15. doi: 10.1017/S0954422417000117 [DOI] [PubMed] [Google Scholar]
- 6. Trowell H.C. (1972) Atherosclerosis 16, 138–140. doi: 10.1016/0021-9150(72)90017-2 [DOI] [PubMed] [Google Scholar]
- 7. Trowell H.C., Southgate D.A.T., Wolever T.M.S., Leeds A.R., Gassull M.A., Jenkins D.J.A. (1976) Lancet 307, 967. doi: 10.1016/s0140-6736(76)92750-1 [DOI] [PubMed] [Google Scholar]
- 8. Furda I. (1977) Cereal Foods World 22, 252–254 [Google Scholar]
- 9. Furda I. (1981) in The Analysis of Dietary Fiber in Foods, James W.P.T., Theander O. (Eds), Marcel Dekker, New York, pp 163–172 [Google Scholar]
- 10. Schweizer T.F., Wursch P. (1979) J. Sci. Food Agric. 30, 613–619. doi: 10.1002/jsfa.2740300610 [DOI] [PubMed] [Google Scholar]
- 11. Schweizer T.F., Wursch P. (1981) in The Analysis of Dietary Fiber in Foods, James W.P.T., Theander O. (Eds), Marcel Dekker, New York, pp 203–216 [Google Scholar]
- 12. Asp N.-G. (1981) in The Analysis of Dietary Fiber in Foods, James W.P.T., Theander O. (Eds), Marcel Dekker, New York, pp 173–189 [Google Scholar]
- 13. Prosky L., Asp N.G., Furda I., DeVries J.W., Schweizer T.F., Harland B.F. (1985) J. AOAC Int. 68, 677–679 [PubMed] [Google Scholar]
- 14. Lee S.C., Prosky L., Vries J.W.D. (1992) J. AOAC Int. 75, 395–416 doi: 10.1093/jaoac/75.3.395 [DOI] [Google Scholar]
- 15. Official Methods of Analysis (2020) 21st Ed., Appendix D, AOAC INTERNATIONAL, Rockville, MD, http://eoma.aoac.org/app_d.pdf [Google Scholar]
- 16. Gordon D.T., Okuma K. (2002) J. AOAC Int. 85, 435–444 [PubMed] [Google Scholar]
- 17. Anon (2001) Cereal Foods World 46, 112–126 [Google Scholar]
- 18. Institute of Medicine (2002) in Dietary Reference Intakes: Energy, Carbohydrates, Fiber, Fat, Fatty Acids, Cholesterol, Protein and Amino Acids, National Academies Press, Washington, DC [Google Scholar]
- 19. Codex Alimentarius, Food and Agriculture Organization (2010) Guidelines on Nutrition Labelling CAC/GL 2-1985 as Last Amended
- 20. Miller-Jones J. (2014) Nutr J. 13, Article Number 34, 1–1024405527 [Google Scholar]
- 21.Codex Alimentarius Recommended Methods of Analysis and Sampling. CXS 234-1999. Amendments adopted by the 44th Session of the Codex Alimentarius Commission in 2021
- 22. McCleary B.V., Sloane N., Draga A., Lazewska I. (2013) Cereal Chem. 90, 396–414. doi: 10.1094/CCHEM-10-12-0135-FI [DOI] [Google Scholar]
- 23. Hoebregs H., Balis P., De Vries J., Eekelen J. v., Farnell P., Gray K., Goedhuys B., Hermans M., Heroff J., van Leeuwen M., Li B.W., Martin D., Pieters M., Quemener B., Roomans H., Slaghek T., Thibault J.-F., van der Waal W., de Wit D; Collaborators (1997) J. AOAC Int. 80, 1029–1039. doi: 10.1093/jaoac/80.5.1029 [DOI] [Google Scholar]
- 24. McCleary B.V., McNally M., Rossiter P. (2002) J. AOAC Int. 85, 1103–1111. doi: 10.1093/jaoac/85.5.1103 [DOI] [PubMed] [Google Scholar]
- 25. De Slegte J. (2002) J. AOAC Int. 85, 417–423. doi: 10.1093/jaoac/85.2.417 [DOI] [PubMed] [Google Scholar]
- 26. Craig S.A.S., Holden J.F., Khaled M.J. (2000) J. AOAC Int. 83, 1006–1012 [PubMed] [Google Scholar]
- 27. Englyst H.N., Kingman S.M., Cummings J.H. (1992) Eur. J. Clin. Nutr. 46 (Suppl 2), S33–S50 [PubMed] [Google Scholar]
- 28. Berry C.S. (1986) J. Cereal Sci. 4, 301–314. doi: 10.1016/S0733-5210(86)80034-0 [DOI] [Google Scholar]
- 29. Muir J.G., O'Dea K. (1992) Am. J. Clin. Nutr. 56, 123–127. doi: 10.1093/ajcn/56.1.123 [DOI] [PubMed] [Google Scholar]
- 30. Champ M. (1992) Eur. J. Clin. Nutr. 46 (Suppl 2), S51–S62 [PubMed] [Google Scholar]
- 31. Faisant N., Planchot V., Kozlowski F., Pacouret M.-P., Colonna P., Champ M. (1995) Sci. Aliment. 15, 83–89 [Google Scholar]
- 32. Goñi I., García-Diz L., Mañas E., Saura-Calixto F. (1996) Fd. Chem. 56, 445–449. doi: 10.1016/0308-8146(95)00222-7 [DOI] [Google Scholar]
- 33. McCleary B.V., Monaghan D.A. (2002) J. AOAC Int. 85, 665–675 doi: 10.1093/jaoac/85.3.665 [DOI] [PubMed] [Google Scholar]
- 34. McCleary B.V. (2007) Anal. Bioanal. Chem. 389, 291–308. doi: 10.1007/s00216-007-1389-6 [DOI] [PubMed] [Google Scholar]
- 35. McCleary B.V. (2010) Cereal Foods World 55, 24–28 [Google Scholar]
- 36. McCleary B.V., DeVries J.W., Rader J.I., Cohen G., Prosky L., Mugford D.C., Champ M., Okuma K. (2012) J. AOAC Int. 95, 824–844 doi: 10.5740/jaoacint.CS2011_25 [DOI] [PubMed] [Google Scholar]
- 37. Read N.W., Cammack J., Edwards C., Holgate A.M., Cann P.A., Brown C. (1982) Gut 23, 824–828. doi: 10.1136/gut.23.10.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sadik R., Abrahamsson H., Bjornsson E., Gunnarsdottir A., Stotzer P.-O. (2003) Scand. J. Gastroenterol. 38, 1039–1044 doi: 10.1080/00365520310004939 [DOI] [PubMed] [Google Scholar]
- 39. Camilleri M., Thorne N.K., Ringel Y., Hasler W.L., Kuo B., Esfandyari T., Gupta A., Scott S.M., McCallum R.W., Parkman H.P., Soffer E., Wilding G.E., Semler J.R., Rao S.S.C. (2010) Neurogastroenterol. Motil. 22, 874–882.e233. doi: 10.1111/j.1365-2982.2010.01517.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCleary B.V., Sloane N., Draga A. (2015) Starch ‐ Stärke 67, 860–883. doi: 10.1002/star.201500017 [DOI] [Google Scholar]
- 41. McCleary B.V., Cox J. (2017) Luminacoids Res. 21, 9–21 [Google Scholar]
- 42. McCleary B.V., Ames N., Cox J., Iilians S., Jin Y., Johnson M., McCarthy S., McKie V., Nishibata T., Pastell H., Plank D., Salman H., Sanders P., Santi A., Steegmans M., Yoshida M; Collaborators (2019) J. AOAC Int. 102, 196–207. doi: 10.5740/jaoacint.18-0180 [DOI] [Google Scholar]
- 43. McCleary B.V., McLoughlin C. (2022) J. AOAC Int. 127, 127–145 doi: 10.1093/jaoacint/qsac098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tanabe K., Nakamura S., Oku T. (2014) Food Chem. 151, 539–546. doi: 10.1016/j.foodchem.2013.11.121 [DOI] [PubMed] [Google Scholar]