Abstract
With advances in pediatric and obstetric surgery, pediatric patients are subject to complex procedures under general anesthesia. The effects of anesthetic exposure on the developing brain may be confounded by several factors including pre-existing disorders and surgery-induced stress. Ketamine, a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, is routinely used as a pediatric general anesthetic. However, controversy remains about whether ketamine exposure may be neuroprotective or induce neuronal degeneration in the developing brain. Here, we report the effects of ketamine exposure on the neonatal nonhuman primate brain under surgical stress. Eight neonatal rhesus monkeys (postnatal days 5–7) were randomly assigned to each of two groups: Group A (n = 4) received 2 mg/kg ketamine via intravenous bolus prior to surgery and a 0.5 mg/kg/h ketamine infusion during surgery in the presence of a standardized pediatric anesthetic regimen; Group B (n = 4) received volumes of normal saline equivalent to those of ketamine given to Group A animals prior to and during surgery, also in the presence of a standardized pediatric anesthetic regimen. Under anesthesia, the surgery consisted of a thoracotomy followed by closing the pleural space and tissue in layers using standard surgical techniques. Vital signs were monitored to be within normal ranges throughout anesthesia. Elevated levels of cytokines interleukin (IL)-8, IL-15, monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein (MIP)-1β at 6 and 24 h after surgery were detected in ketamine-exposed animals. Fluoro-Jade C staining revealed significantly higher neuronal degeneration in the frontal cortex of ketamine-exposed animals, compared with control animals. Intravenous ketamine administration prior to and throughout surgery in a clinically relevant neonatal primate model appears to elevate cytokine levels and increase neuronal degeneration. Consistent with previous data on the effects of ketamine on the developing brain, the results from the current randomized controlled study in neonatal monkeys undergoing simulated surgery show that ketamine does not provide neuroprotective or anti-inflammatory effects.
Keywords: Brain, developmental, anesthesia, neurotoxicology, neuroscience, surgery
Impact Statement
Despite the widespread clinical use of ketamine as a pediatric anesthetic, limited data are available regarding its pharmacokinetics and pharmacodynamics in infants and neonates. There is a need to do further studies in appropriate animal models using clinically relevant doses of drug in the presence of surgical stress. The similarity of the physiology, pharmacology, and reproductive systems of the nonhuman primate (NHP) to that of the human, especially during pregnancy and the neonatal period, make the monkey the most appropriate animal model for use in the preclinical studies. This study is designed to explore differences in various outcomes and neurologic effects of ketamine in an NHP infant animal model and to better understand the consequences of ketamine exposure during surgical procedures in children. Our findings indicated that ketamine administration prior to and throughout surgery in a clinically relevant model appears to increase neuronal degeneration and does not provide anti-inflammatory effects.
Introduction
Surgery in the human neonatal period and early infancy is associated with significant neurologic morbidity including long-term cognitive deficits.1–6 Putative mechanisms for this neurologic injury include ischemia, hypoxia, micro-emboli and activation of an exaggerated inflammatory state, otherwise referred to as systemic inflammatory response syndrome (SIRS). At the cellular level, glial activation and neuronal cell death caused by ischemia and inflammation depend upon glutamate excitotoxicity, mediated via N-Methyl D-Aspartate (NMDA) receptors. Thus, the use of NMDA receptor antagonists such as ketamine can theoretically prevent this type of cellular injury. Ketamine, an anesthetic agent used commonly in children, is a potent, non-competitive NMDA receptor antagonist, which also has anti-inflammatory effects. Despite its widespread use, limited data are available regarding the pharmacokinetics (PK) and pharmacodynamics of ketamine in infants. 7
Glutamate-mediated signaling controls every aspect of neuronal cell development in an activity-dependent manner. 7 The mammalian brains undergo a growth spurt when profuse neural cells proliferate and exuberant synaptogenesis occur. Among the proliferated cells, some cells are destined to mature successfully from undifferentiated and unconnected cells into neuronal cells with complex three-dimensional morphologies. Other cells will die naturally by programmed apoptotic cell death. Simultaneously, the excessive synaptic connections are systemically eliminated by pruning. 8 Some animal studies have suggested that the use of ketamine in neonatal animals may lead to neuronal degeneration.9–11 Other in vitro and in vivo rodent and primate models showed that both brain immaturity and prolonged exposures to ketamine were necessary to produce neuroapoptotic cell death.12–14 On the other hand, studies have also shown that lower and more clinically relevant doses of ketamine (5–10 mg/kg given via the subcutaneous route) administered as a single dose or multiple doses to neonatal animals do not induce apoptosis and may be neuroprotective if given prior to an inflammatory stimulus. 15 It is difficult to thoroughly explore the adverse effects of anesthetic exposure on human infants and children. Ultimately, the nonhuman primate more accurately reflects the human condition because of their physiological similarity to humans with regard to the reproduction, development, neuroanatomy, and cognition13,14,16,17
The published literature suggests that the dose of ketamine administered, the presence or absence of surgical stress, and the maturity of the developing brain are important determinants of the central nervous system effects of ketamine. 17 Thus, there is a need to conduct further pre-clinical studies that use clinically relevant doses of ketamine to mimic the clinical situation in the presence of surgical stress in neonatal animals.
Materials and methods
Animals and procedures
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences and the National Center for Toxicological Research (NCTR) and conducted in accordance with the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals. All nonhuman primates were born and housed at the NCTR nonhuman primate research facility. Animal procedures were designed to minimize the number of animals required and any pain or distress associated with the experimental procedures. Neonatal rhesus monkeys (Macaca mulatta) were obtained from the NCTR breeding colony as previously described. 14 Briefly, breeder monkeys were housed in separate cages under a 12:12 h light/dark cycle, provided with water ad libitum and fed high protein jumbo monkey diet supplemented routinely with fresh fruit. All births occurred via natural delivery and the day of birth was designated as postnatal day (PND) 0. Neonatal monkeys remained with their mothers except during anesthetic exposure or control sequestrations.
Eight neonatal rhesus monkeys (all males) at PND 5–7 were anesthetized using a standardized mixture of inhaled and intravenous (IV) anesthetics consisting of sevoflurane, fentanyl, midazolam, and pancuronium with doses commonly used during pediatric surgical procedures. A central venous line was placed for IV drug administration. Monkeys were intubated using an endotracheal tube and positive pressure ventilation was maintained. Animals randomized to the ketamine group (Group A; n = 4) received a 2 mg/kg IV bolus of ketamine followed by an IV infusion of 0.5 mg/kg/h for surgery. Animals in the Placebo Group (Group B; n = 4) received equivalent amounts of normal saline. Animals were monitored using vital signs and pulse oximetry. Surgery consisted of thoracotomy with a standard incision size followed by entry into the pleural space and manipulations with retractors to simulate surgical procedures. This was followed by closing the pleural space and tissue in layers using standard surgical techniques. Anesthesia was stopped and animals were extubated. Surgical and anesthetic regimens were designed in consultation with pediatric cardiovascular experts and surgeons at Arkansas Children’s Hospital (ACH).
Physiological parameters or vital signs of all subjects were monitored following procedures previously described.14,16 Briefly, non-invasive pulse oximetry (N-395 Pulse Oximeter, Nellcor, Pleasanton, CA; MouseOX Plus Vital Sign Monitor, StarrTM Life Sciences, Oakmont, PA), capnography (Tidal Wave Hand-held capnography, Respironics, Murrysville, PA), sphygmomanometry (Critikon Dynamap Vital Signs Monitor, GE Healthcare, Waukesha, WI), and a rectal temperature probe were used to verify the physiological status of subjects during the exposures and sequestrations. Heart and respiration rates, arterial blood O2 saturation levels, expired CO2 concentrations, rectal temperatures, and systolic and diastolic blood pressures were recorded in anesthetized and control animals. Venous blood (approximately 300 µL) was collected for measurement of plasma glucose concentrations, venous blood gases, pH values, and hematocrits (GEM® PremierTM 4000, Instrumentation Laboratory, Lexington, MA).
Blood samples were obtained at set intervals and total blood drawn was not more than 7 mL total for each animal. Blood samples were collected from the subjects (from all animals) immediately after the induction of anesthesia and prior to the start of the surgical procedure through a central venous line placed for clinical purposes into a heparinized vacuum tube, and additional blood samples were also collected at 5, 10, 30, 60 min, 2, 6, 12, and 24 h after ketamine administration. All blood samples were centrifuged to remove plasma, and plasma aliquots were stored in a −70 °C freezer for later use. Using one plasma aliquot from each sampling time, ketamine and nor-ketamine levels were determined by liquid chromatography with tandem mass spectrometry (LC/MS/MS) and isotope dilution quantification (deuterated internal standards). 14 The elimination half-life, clearance, apparent volume of distribution and other pharmacokinetic parameters were calculated using classical model-fitting techniques.13,14
Mass spectrometry. Analyses were conducted using a Quattro Micro or Quattro Premier triple quadrupole mass spectrometer (Waters, Milford, MA) equipped with an electrospray source. Positive ions were monitored in selected ion recording mode. Source and desolvation temperatures were set to 100oC and 300oC, respectively, with dwell times of 0.2 s. Capillary potential was 0.5 kV. Resolution was set to give peak widths at half-height of 0.9 Th. Optimized m/z ratios were acquired (m/z 242-labeled ketamine, m/z 238-unlabeled ketamine and m/z 228-labeled nor-ketamine, m/z 224-unlabeled nor-ketamine) using cone voltages of 25 and 20 V, respectively, for quantification of the protonated molecules from ketamine and nor-ketamine. In addition, two pairs of confirmatory ions were acquired for ketamine (m/z 211.1-labeled, m/z 207.1 unlabeled) and nor-ketamine (m/z 170.1-labeled, m/z 166.1-unlabeled). 14
Method performance. The method described was optimized with respect to the solid-phase extraction cartridges, solvents, and laboratory hardware available to give the highest recoveries based on fortified samples (>80%) and MS response. Limits of detection and quantification for ketamine and nor-ketamine were approximately 6 and 17 pg on column, respectively. When using 10–11 plasma samples, this corresponds to concentrations of approximately 0.0012 and 0.0036l g/mL, respectively (0.005 and 0.014l M).13,14
Luminex xMAP™-based technology (Luminex, Corp., Austin, TX) was utilized to assay multiple cytokines. A multiplex antibody kit (Billerica, MA) was used to enable measurement of multiple cytokines simultaneously. The entire assay was performed according to the instructions provided with the kit. The samples were centrifuged at 13,000g for 10 min at 4°C. Manufacturer-supplied polystyrene beads conjugated to protein-specific capture antibodies were added to the wells of the micro plate. Eight working standards were prepared in duplicates by serially diluting the reconstituted standard. The samples were diluted 1:2 with supplied assay buffer. The beads were washed with the wash buffer followed by addition of incubation buffer. After reconstitution, 100 µL of standard and samples were added into the wells of the microplate containing the beads. This reaction mixture was allowed to incubate with agitation for a period of 2 h during which the proteins bind to the capture antibodies. After incubation, the beads were washed with the wash buffer followed by addition of protein-specific biotinylated detector antibodies and incubated with the beads for 1 h. After the incubation, the bead mixture was washed thoroughly again to remove the excess antibodies. This was followed by the addition of streptavidin conjugated to the fluorescent protein R phycoerythrin (Streptavidin RPE) and a 30-min incubation during which a solid phase sandwich was formed. After washing to remove unbound streptavidin, the beads were analyzed using the Luminex detection system.
To evaluate if intravenous ketamine administration prior to and throughout surgery may cause elevated neural degeneration, and/or to determine the severity and the nature of ketamine-induced neuronal damage, after 24-h recovery, the brains from monkeys in Group A (ketamine; n = 3) and Group B (placebo; n = 3) were processed for Fluoro-Jade C staining. The frontal lobe/cortex was selected for pathological analysis. Fluoro-Jade C detects all degenerating neurons, regardless of mechanism of cell death. Coronal sections (40 µm), obtained via a cryostat, were processed as previously described. 18 Prior to staining, sections were mounted onto polylysinized slides. The mounted sections were bathed in a solution of 1% sodium hydroxide in 80% ethanol for 5 min, and washed in 70% ethanol and distilled water for 2 min, respectively, followed by an incubation in 0.06% potassium permanganate solution for 10 min, and finally in a 0.0001% solution of Fluoro-Jade C (Histo-Chem, Inc., Jefferson, AR) dissolved in 0.1% acetic acid vehicle for 10 min. The slides were rinsed through three changes of distilled water for 1 min per change. The air-dried slides were cleaned in xylene and a coverslip was applied with DPX nonfluorescent mounting media (Sigma, USA).
Statistical analyses
Summary statistics and plots were used to check normality assumptions. Statistical analyses were performed, and graphs were produced using SigmaPlot. The vital signs or physiological parameters and cytokine data were analyzed with a General Linear Mixed Model. Student’s t-test was used for the comparison of Fluoro-Jade C staining between control (placebo) and ketamine-exposed monkeys. Generalized least square models for repeated measures were used in the comparison of plasma cytokine levels between control and ketamine-treated animals collected at hours 0, 6, and 24. Statistical tests in the analyses are two-sided. Data are expressed as mean ± SD, unless denoted otherwise. Statistical significance was assessed at the 5% nominal level (alpha = 0.05). All analyses were statistically different with a P value <0.05.
Results
To prevent hypoventilation, tracheal intubation and mechanical ventilation were applied to neonatal monkeys after induction. The vital signs and/or physiological parameters including the heart rate, blood pressure, rectal temperature, respiratory rate, hemoglobin, peripheral blood oxygen saturation, CO2 concentration, and blood glucose levels at intervals of 1–2 h during anesthetic exposure were monitored continuously. All parameters were maintained within normal limits. The differences between the ketamine-exposed and control groups in the measurements of these physiological parameters were analyzed using a general linear mixed model and were recorded at hours 1, 4, 8, 12, 16, 20, and 24 following ketamine administration. The differences in heart rate, respiratory rate, systolic blood pressure, and peripheral blood oxygen saturation between the ketamine-exposed neonatal monkeys and the control animals were statistically insignificant. All monkeys tolerated the procedures well and fully recovered from anesthesia. No statistically significant differences were observed between the ketamine-exposed and control groups in these parameters (Figure 1). The blue dots in Figure 1 represent the means of the difference of indicated indexes between the ketamine group and control group over time. The blue error bars indicate the 95% confidence intervals of the differences in the indexes over time.
Figure 1.
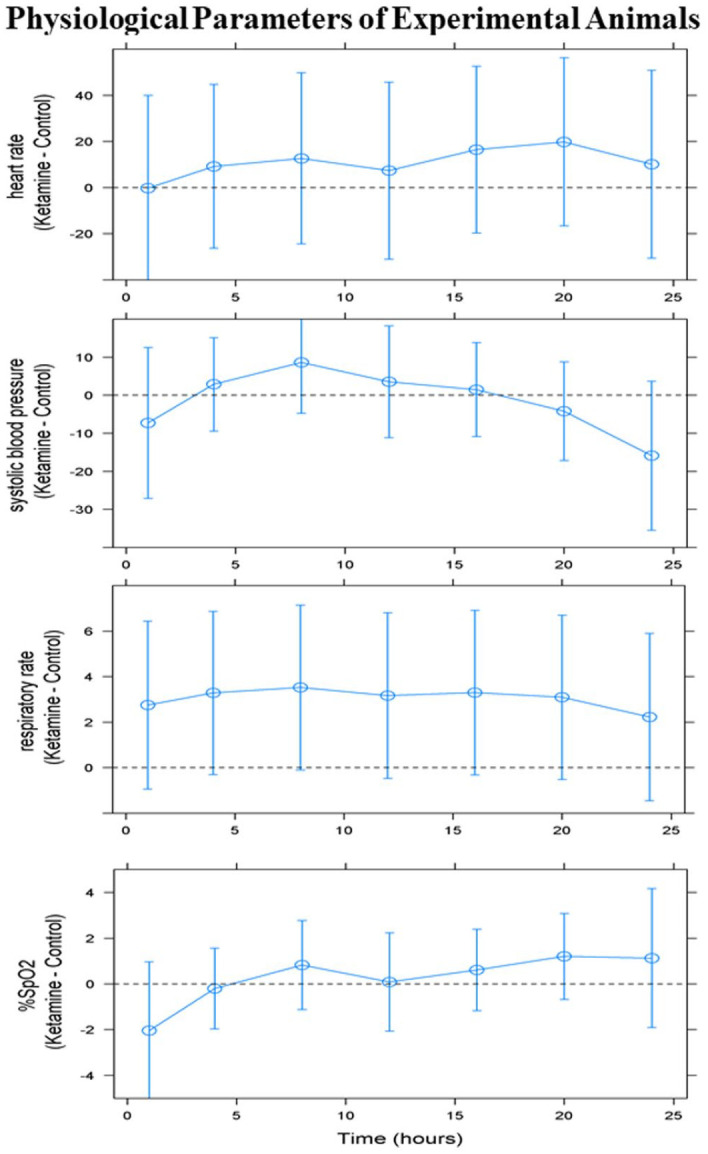
All physiological parameters were maintained within normal limits. The differences between the ketamine-exposed and control groups in the measurements of these physiological parameters were analyzed using a general linear mixed model and were projected at hours 1, 4, 8, 12, 16, 20, and 24 following ketamine administration. The differences in heart rate, respiratory rate, systolic blood pressure, and peripheral blood oxygen saturation between ketamine-exposed neonatal monkeys and controls were statistically insignificant. The blue dots represent the means of the difference of indicated indexes between the ketamine group and control group over time. The blue error bars indicate the 95% confidence intervals of the differences in the indexes over time.
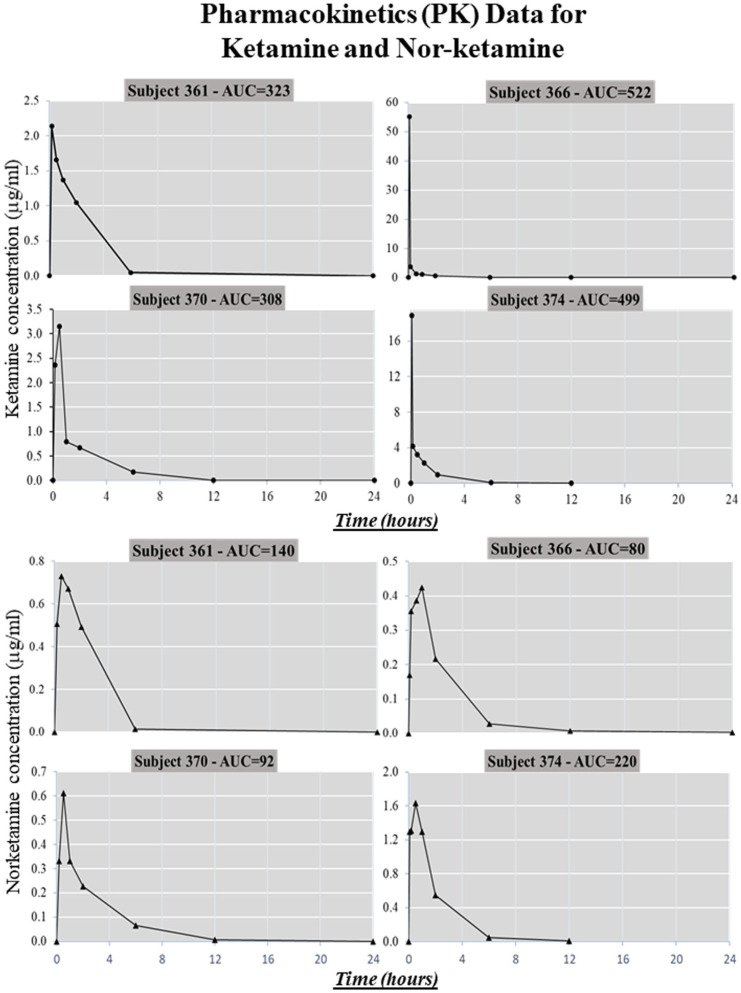
In this study, plasma concentrations of ketamine and nor-ketamine, the main active metabolite of ketamine, were monitored continually from the beginning of ketamine administration until 24 h after surgery (blood samples), to determine PK and drug disposition. Plasma ketamine/nor-ketamine concentrations ranged around µg/mL levels 14 for each individual monkey at the time of simulated surgery (Figure 2). Present data show the trend of the clearance of ketamine, the metabolism of ketamine, and the relationship between ketamine and nor-ketamine in animals undergoing surgery.
Figure 2.
Plasma ketamine/nor-ketamine concentrations ranged in the µg/mL levels 14 for each individual monkey at the time of simulated surgery. The data show the trend for the clearance of ketamine, the metabolism of ketamine and the relationship between ketamine and nor-ketamine in animals undergoing surgery.
To evaluate plasma markers of neurotoxicity and inflammation factors in the blood samples, plasma cytokine and/or chemokine levels were examined using a Luminex protein assay. In the present study, among the analyzed cytokines and chemokines, the abnormal alternation/elevation of cytokines including interleukin (IL)-8, IL-15, monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein (MIP)-1β were remarkably increased at hours 6 and 24 in the ketamine-exposed (Group A) compared with control (Group B) animals (Table 1). Specifically, IL-8 (control vs ketamine: 225 pg/mL vs 833 pg/mL), MCP-1 (control vs sevoflurane: 1641 pg/mL vs 5293 pg/mL), and MIP-1β (control vs ketamine: 2.4 pg/mL vs 8.4 pg/mL) were all significantly elevated in the ketamine-exposed monkeys compared with control animals (Table 1). At hour 24, the MCP-1 remained significantly higher in ketamine exposed animals. In addition, the original data of the statistical report with the full list of cytokines are attached as supplementary material.
Table 1.
Cytokine levels in blood samples.
| Cytokine (pg/mL) | 6 h (n = 4 per group) | 24 h (n = 4 per group) | ||
|---|---|---|---|---|
| Control | Ketamine | Control | Ketamine | |
| IL-15 | 4.7 (1.1–8.4) |
9.7 (5.7–13.8) |
6.0 (2.4–9.7) |
7.7 (3.6–11.8) |
| IL-8 | 225.2 (42.2–408.3) |
833 (628–1037)* |
177 (0–360) |
253.4 (48.7–458.0) |
| MCP-1 | 1641 (0–3698) |
5293 (2993–7593)** |
1501 (0–3558) |
5029 (2729–7329)** |
| MIP-1b | 2.4 (0.5–4.4) |
8.4 (6.0–10.8)* |
2.4 (0–4.8) |
2.4 (0–5.8) |
IL: interleukin; MCP: monocyte chemoattractant protein; MIP: macrophage inflammatory protein; CI: confidence interval.
Cytokines levels: mean (lower bound 95% CI–upper bound 95% CI).
p < 0.001; **p < 0.05.
Fluoro-Jade C staining of the frontal cortex of neonatal rhesus monkeys was conducted 24 h after the administration of standard pediatric general anesthesia and simulated thoracotomy. The IV administration of a clinically relevant concentration of ketamine produced extensive neural damage as indicated by increased numbers of Fluoro-Jade C-positive neuronal cells in the frontal cortex of ketamine-exposed monkeys when compared with controls animals (Figure 3). Fluro-Jade C is an anionic fluorochrome capable of selectively staining degenerating neurons in brain tissue. Quantitative analysis of Fluoro-Jade C data with simulated surgery shows that the number of Fluoro-Jade C-positive neurons was significantly increased in ketamine-exposed monkeys compared with the control monkeys (Figure 3).
Figure 3.
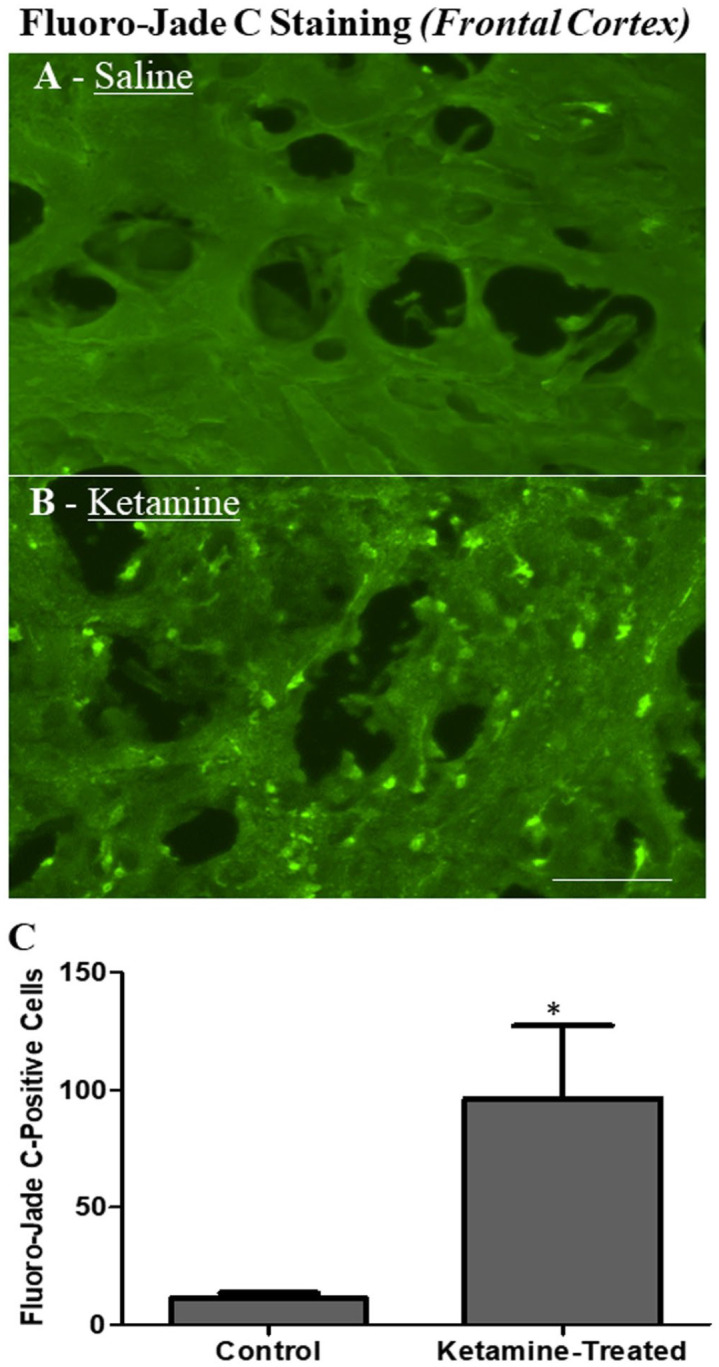
Fluoro-Jade C staining in the frontal cortex of PND 5 rhesus monkeys 24 h after the simulated thoracotomy under standard pediatric general anesthesia. The IV administration of a clinically relevant concentration of ketamine produced extensive neural damage as indicated by increased numbers of Fluoro-Jade C-positive neuronal cells in the frontal cortex in ketamine-exposed monkeys (B) compared with controls (A). Fluro-Jade C is an anionic fluorochrome capable of selectively staining degenerating neurons in brain tissue. Scale bar = 90 µm. IV, intravenous; PND, postnatal day.
Discussion
With advances in pediatric and obstetric surgery, pediatric patients are subject to complex procedures under general anesthesia. 19 Although general anesthetics are diverse chemically, they exert their functions either as agonists to GABAA receptors or as antagonists to NMDA receptors.20–22 The general anesthetic agent ketamine exerts its actions as an NMDA receptor antagonist20,21,23 and is frequently used in pediatric clinics and general anesthesia. Both preclinical and clinical research have been carried out to expand the knowledge regarding potential mechanisms of neurotoxicity induced by prescribed drugs, including general anesthetics.24–26 Various animals, including rodents or non-human primates14,27–30 have been used in pediatric anesthetic neurotoxicity studies where animals were exposed to general anesthetic agents without confounding preexisting diseases or surgical procedures, and neural tissue sampled for examination following exposures. However, many hurdles, such as various sensitivity to anesthetics among species, 12 prevent any direct extrapolation of results obtained in preclinical studies to clinical practice in humans. Being a noncompetitive NMDA receptor antagonist, exposure to ketamine at anesthetic doses resulted in increased apoptotic neurodegeneration in preclinical studies where surgical procedures were not involved14,31–33 Alternatively, ketamine has also been shown to protect the immature brain from cortical and subcortical cell death elicited by inflammatory pain. 15
Evidence suggests that developmental neurotoxicity testing on rodent models may only provide gross evaluations and the information on mechanisms is quite limited. In contrast, nonhuman primates, for example, rhesus monkeys, being 93% genetically similar to humans 34 can in many instances more accurately predict how pathological conditions arise in humans, and the vulnerability of the primate brain to toxicants/chemicals is closely related to the maturity of brain development. 17
Ketamine is routinely used in pediatric clinics. The doses used are 1–2 mg/kg, often given as bolus. Ketamine infusions are also commonly cited in the literature with doses ranging from 0.2 to 2 mg/kg/h.1–4 This study is an attempt to create an animal model to duplicate clinical situations and, thus, the doses being used are in line with the doses being used clinically. It should also be pointed out that other anesthetic agents were used to ensure the animals remained well anesthetized under the surgical plane of anesthesia during the entire experiment.
Due to confounding factors, and because it is difficult to disentangle the effect of anesthesia per se from the effects of surgery or pre-existing pathologies that necessitate surgery, it remains uncertain if the neurodevelopmental deficits may be caused by general anesthesia in itself.35,36 In this study, to delineate the effects of ketamine on the developing brain in the milieu of surgery, a randomized controlled study was carried out in neonatal rhesus monkeys PND 5–7 in the presence of a standard pediatric anesthesia regimen. 37 Simulated surgery, a thoracotomy, was conducted to mimic the clinical surgical situation. The size and physiological parameters of nonhuman primates approximate human infants and children providing the opportunity to test this hypothesis. Therefore, it is suitable/essential to utilize the neonatal nonhuman primate to obtain valuable information on potential neuroprotective effects of ketamine and/or ketamine-induced neural degeneration.
Data in support of a correlation between surgery and subsequent physiological changes have accumulated.1–6 In this study, during general anesthesia the procedures followed for the maintenance and monitoring of the experimental subjects were similar to those previously detailed 14 and to those used in the clinical settings. During anesthetic exposures, vital signs of neonatal monkeys were continuously monitored including heart rate, respiratory rate, blood pressure, and rectal temperature. The homeostatic status in the exposed neonatal monkeys was also evaluated by continuously monitoring physiological parameters, including hemoglobin, oxygen saturation, CO2 concentration, and blood glucose levels at intervals of 1–2 h during anesthetic exposure. The vital signs or physiological parameters were analyzed with a general linear mixed model. The general linear mixed model provides a useful approach for analyzing longitudinal data with a wide variety of data structures. Importantly, vital sign monitoring showed no major physiological disturbance. No statistically significant differences were observed between the ketamine-exposed and control groups in these parameters, including heart rate, respiratory rate, systolic blood pressure, and oxygen saturation at hours 1, 4, 8, 12, 16, 20, and 24 following ketamine administration.
Ketamine is one of the commonly used agents for conscious sedation in pediatrics. Despite the widespread clinical use of ketamine as a pediatric anesthetic, there are limited data available regarding the PK of ketamine in infants. Ketamine is highly permeable to the blood-brain barrier and the concentration of ketamine on the action sites is an important correlate to its effects on the developing brain. Thus, there is a need to do preclinical studies that use clinically relevant doses of ketamine, in the presence of surgical stress in neonatal animals. One of the main purposes of the study was to investigate the PK of ketamine in a nonhuman primate infant animal model so that we can better understand the consequences of ketamine exposure during surgical procedures in children. It is well known that ketamine undergoes N-demethylation to its primary active metabolite, nor-ketamine. In this study, a clinically relevant model in which neonatal animals were randomized to receive pre-emptive ketamine followed by an infusion of ketamine or placebo during the procedure. PK and drug disposition were examined by measuring plasma levels of ketamine and its major active metabolite, nor-ketamine in animals undergoing surgery. The data (Figure 2) clearly delineate the clearance of ketamine, the metabolism of ketamine and the relationship between ketamine and nor-ketamine in animals undergoing surgery. The data also indicate that, although nor-ketamine is one-third to one-fifth as potent as ketamine, it still effectively provided prolonged (has a longer half-life than ketamine) analgesic/anesthesia action.
It is well known that surgery in pediatric patients is associated with significant neurologic disturbances, such as long-term behavioral deficits.1–6 Immature neurons in the infant brain are more susceptible to excitotoxic cell death than the adult brain because of the specific characteristics of their NMDA receptors. 17 It is hypothesized that by blocking the action of glutamate with ketamine, the potential neurotoxicity associated with surgical procedures in neonates and infants may also be blocked or at least lessened. On the other hand, early-life exposure to commonly used general anesthetics caused massive apoptotic neurodegeneration in the developing animal brain. 38
To measure plasma markers of neurotoxicity and inflammation in neonatal primates that received ketamine anesthesia versus placebo during simulated cardiac surgery, Luminex protein analysis of the blood samples was applied and the potential involvement of cytokines in ketamine-induced neurotoxicity was examined. Cytokines are signaling molecules that bind to cell surface receptors, initiate the activation of signal transduction pathways, and mediate cell to cell communication.39–41 In this study, Luminex protein analysis showed that among the analyzed cytokines, the levels (blood samples) of IL-8, IL-15, MCP-1, and MIP-1β were remarkably elevated in ketamine exposed monkeys compared with the controls at hours 6 and 24 following ketamine administration. Particularly, IL-8, MCP-1, and MIP-1β were significantly elevated at 6 h in ketamine-exposed monkeys. IL-8 and MIP-1β have been found to contribute to neuronal damage in various situations. 42 Also, MIP-1β and MCP-1 play vital roles in the process of inflammation where they attract or enhance the expression of other inflammatory factors/cells 43 and may contribute to inflammatory pathologies in the CNS. Perturbations of the nervous system should be reflected in changes in cytokine levels, and elevated levels of MIP-1β and MCP-1 suggest that ketamine exposure may have stimulated an inflammatory reaction in the CNS. Thus, elevated cytokine secretions could be critical for the development of neuronal damage induced by ketamine. Also, based on our findings, it looks like cytokine dysregulation could be transitory or time dependent.
In this study, following a 24-h recovery, the brains were processed for Fluoro-Jade C staining. Fluoro-Jade C is an anionic fluorochrome capable of selectively staining degenerating neurons in brain tissue. Consistent with the cytokine data, it was shown that a clinically relevant concentration of ketamine produced extensive neuronal damage as evidenced by elevated numbers of Fluoro-Jade C-positive neuronal cells in the frontal cortex compared with controls. Quantitative analysis of Fluoro-Jade C data with simulated surgery shows that the number of Fluoro-Jade C-positive neurons is significantly increased in ketamine-exposed monkeys compared with controls.
Neurotransmission mediated by NMDA receptors is of primary importance to synaptogenesis, myelination, and neuronal cell apoptosis in the late gestation period until infancy.8,44 Persistent disruption of neurotransmission mediated by NMDA receptors with general anesthetics can be detrimental to the nervous system. 45 In this study, the plasma markers of neurotoxicity (cytokine data) and histological evidence (Fluoro-Jade C data) are closely correlated in neonatal primates who receive ketamine anesthesia versus placebo during simulated cardiac surgery. These findings clearly indicate that intravenous ketamine administration prior to and throughout surgery appeared to increase neuronal degeneration and did not provide neuroprotective effects. It should be mentioned that to prevent hypoventilation, mechanical ventilation was applied to neonatal monkeys after induction. Meanwhile, the ability of maintaining physiological status within normal limits/range provides additional confidence that observed changes are the result of ketamine-anesthesia related neurotoxicity and not the consequence of non-specific effects. These findings/data are consistent with the hypothesis 17 that: immature neurons in the infant brain are more susceptible to excitotoxic cell death because of the specific characteristics of their NMDA receptors. Continuous blockade of NMDA receptors on immature neurons by ketamine can cause a compensatory upregulation of NMDA receptors. Activation of these upregulated NMDA receptors by endogenous glutamate after ketamine washout can elevate calcium influx and interfere with electron transport in a manner that results in elevated production of reactive oxygen species (ROS) and/or increased neuronal damage.
Summary
By using a randomized controlled model, the current study was designed to: (1) determine the pharmacokinetic parameters, (2) disentangle the effect of anesthesia per se from the effects of surgery, and (3) evaluate plasma markers of neurotoxicity and histological evidence in neonatal primates who receive ketamine anesthesia versus placebo during simulated cardiac surgery under general pediatric anesthesia. Our data show that the PK, clearance, and metabolism of ketamine, as well as vital sign monitoring during the surgery and experimental duration are consistent and show no major physiological disturbances. Ketamine exposure prior to and throughout surgery in a neonatal primate model appears to elevate cytokine levels. Ketamine exposure increases neuronal degeneration as evidenced by a significant elevation of Fluoro-Jade C staining positive neurons. The results from the current study show that ketamine does not provide neuroprotective or anti-inflammatory effects in this model of neonatal surgery. In general, the dose of anesthetics, duration of anesthesia, route of administration, presence or absence of surgical stress, and the maturity of the developing brain are all critical elements to consider in the design of future pediatric anesthesia studies.
Supplemental Material
Supplemental material, sj-docx-1-ebm-10.1177_15353702231168144 for Development of a primate model to evaluate the effects of ketamine and surgical stress on the neonatal brain by Cheng Wang, Adnan Bhutta, Xuan Zhang, Fang Liu, Shuliang Liu, Leah E Latham, John C Talpos, Tucker A Patterson and William Slikker in Experimental Biology and Medicine
Acknowledgments
Clinical collaborators and support team: Surgical and anesthetic regimens were designed by Dr Adnan Bhutta (Anesthesiologist) in consultation with Dr Robert D. Jaquiss (Chief of Pediatric Cardiovascular Surgery at Arkansas Children’s Hospital) and Dr Michael Schmitz (Chief of the Pediatric Cardiovascular Intensive Care Unit). Surgery was performed by Dr Adnan T. Butta, who has completed training in General Pediatrics and pediatric critical care medicine and is Board Certified in Pediatrics and Pediatric Critical Care Medicine. The authors would also thank UAMS personnel – Jiang Bian, Jeffrey M. Gossett, and Xinyu Tang – for their critical participation in the above-mentioned experiments.
Footnotes
Authors’ Contributions: CW helped with experimental design, conducted part of the experiments, drafted/wrote, and revised the manuscript; AB helped with experimental design, conducted part of the experiments, and revised the manuscript; XZ helped with experimental design and revised the manuscript; FL conducted part of the experiments, summarized information and revised the manuscript; SL helped with analysis of the data and revised the manuscript; LL helped with manuscript revision; JT helped with interpretation of the studies, revised, and edited the manuscript; TP revised and edited the manuscript; WS initiated the NHP projects and was involved in experimental design, revised, and edited the manuscript.
Authors’ Note: This manuscript reflects the views of the authors and does not necessarily reflect those of the U.S. Food and Drug Administration.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by NCTR/US Food and Drug Administration.
ORCID iDs: Cheng Wang  https://orcid.org/0000-0002-8315-6232
https://orcid.org/0000-0002-8315-6232
Adnan Bhutta  https://orcid.org/0000-0003-3362-5835
https://orcid.org/0000-0003-3362-5835
Fang Liu  https://orcid.org/0000-0002-4568-3859
https://orcid.org/0000-0002-4568-3859
William Slikker  https://orcid.org/0000-0002-9616-9462
https://orcid.org/0000-0002-9616-9462
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Ashraf S, Bhattacharya K, Tian Y, Watterson K. Cytokine and S100B levels in paediatric patients undergoing corrective cardiac surgery with or without total circulatory arrest. Eur J Cardiothorac Surg 1999;16:32–7 [DOI] [PubMed] [Google Scholar]
- 2.Bhutta AT, Anand KJ. Vulnerability of the developing brain. Neuronal mechanisms. Clin Perinatol 2002;29:357–72 [DOI] [PubMed] [Google Scholar]
- 3.Galli KK, Zimmerman RA, Jarvik GP, Wernovsky G, Kuypers MK, Clancy RR, Montenegro LM, Mahle WT, Newman MF, Saunders AM, Nicolson SC, Spray TL, Gaynor JW. Periventricular leukomalacia is common after neonatal cardiac surgery. J Thorac Cardiovasc Surg 2004; 127:692–704 [DOI] [PubMed] [Google Scholar]
- 4.Hintz SR, Kendrick DE, Stoll BJ, Vohr BR, Fanaroff AA, Donovan EF, Poole WK, Blakely ML, Wright L, Higgins R, Network NNR. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005;115:696–703 [DOI] [PubMed] [Google Scholar]
- 5.Rice D, Barone S., Jr.Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000;108:511–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith PL. The systemic inflammatory response to cardiopulmonary bypass and the brain. Perfusion 1996;11:196–9 [DOI] [PubMed] [Google Scholar]
- 7.Hua JY, Smith SJ. Neural activity and the dynamics of central nervous system development. Nat Neurosci 2004;7:327–32 [DOI] [PubMed] [Google Scholar]
- 8.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev 2010;20:327–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999;283:70–4 [DOI] [PubMed] [Google Scholar]
- 10.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science 1989;244:1360–2 [DOI] [PubMed] [Google Scholar]
- 11.Olney JW, Labruyere J, Wang G, Wozniak DF, Price MT, Sesma MA. NMDA antagonist neurotoxicity: mechanism and prevention. Science 1991;254:1515–8 [DOI] [PubMed] [Google Scholar]
- 12.Scallet AC, Schmued LC, Slikker W, Jr., Grunberg N, Faustino PJ, Davis H, Lester D, Pine PS, Sistare F, Hanig JP. Developmental neurotoxicity of ketamine: morphometric confirmation, exposure parameters, and multiple fluorescent labeling of apoptotic neurons. Toxicol Sci 2004;81:364–70 [DOI] [PubMed] [Google Scholar]
- 13.Slikker W, Jr., Paule MG, Wright LK, Patterson TA, Wang C. Systems biology approaches for toxicology. J Appl Toxicol 2007;27:201–17 [DOI] [PubMed] [Google Scholar]
- 14.Slikker W, Jr., Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci 2007;98:145–58 [DOI] [PubMed] [Google Scholar]
- 15.Anand KJ, Garg S, Rovnaghi CR, Narsinghani U, Bhutta AT, Hall RW. Ketamine reduces the cell death following inflammatory pain in newborn rat brain. Pediatr Res 2007;62:283–90 [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss CE, Wang C, Slikker W., Jr.Effect of prolonged ketamine exposure on cardiovascular physiology in pregnant and infant rhesus monkeys (Macaca mulatta). J Am Assoc Lab Anim Sci 2007;46:21–8 [PubMed] [Google Scholar]
- 17.Wang C, Liu S, Liu F, Bhutta A, Patterson TA, Slikker W., Jr.Application of nonhuman primate models in the studies of pediatric anesthesia neurotoxicity. Anesth Analg 2022;134:1203–14 [DOI] [PubMed] [Google Scholar]
- 18.Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Research 2005;1035:24–31 [DOI] [PubMed] [Google Scholar]
- 19.Grabowski J, Goldin A, Arthur LG, Beres AL, Guner YS, Hu YY, Kawaguchi AL, Kelley-Quon LI, McAteer JP, Miniati D, Renaud EJ, Ricca R, Slidell MB, Smith CA, Sola JE, Sømme S, Downard CD, Gosain A, Valusek P, St Peter SD, Jagannathan N, Dasgupta R. The effects of early anesthesia on neurodevelopment: a systematic review. J Pediatr Surg 2021;56:851–61 [DOI] [PubMed] [Google Scholar]
- 20.Campagna JA, Miller KW, Forman SA. Mechanisms of actions of inhaled anesthetics. N Engl J Med 2003;348:2110–24 [DOI] [PubMed] [Google Scholar]
- 21.Chau PL. New insights into the molecular mechanisms of general anaesthetics. Br J Pharmacol 2010;161:288–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Preckel B, Weber NC, Sanders RD, Maze M, Schlack W. Molecular mechanisms transducing the anesthetic, analgesic, and organ-protective actions of xenon. Anesthesiology 2006;105:187–97 [DOI] [PubMed] [Google Scholar]
- 23.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature 1994;367:607–14 [DOI] [PubMed] [Google Scholar]
- 24.Bellinger DC, Calderon J. Neurotoxicity of general anesthetics in children: evidence and uncertainties. Curr Opin Pediatr 2019;31:267–73 [DOI] [PubMed] [Google Scholar]
- 25.Brambrink AM, Orfanakis A, Kirsch JR. Anesthetic neurotoxicity. Anesthesiol Clin 2012;30:207–28 [DOI] [PubMed] [Google Scholar]
- 26.Jevtovic-Todorovic V. General anesthetics and neurotoxicity: how much do we know. Anesthesiol Clin 2016;34:439–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 2003;23: 876–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanungo J, Cuevas E, Ali SF, Paule MG. Ketamine induces motor neuron toxicity and alters neurogenic and proneural gene expression in zebrafish. J Appl Toxicol 2013;33:410–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzi S, Carter LB, Ori C, Jevtovic-Todorovic V. Clinical anesthesia causes permanent damage to the fetal guinea pig brain. Brain Pathol 2008;18:198–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loepke AW, Istaphanous GK, McAuliffe JJ, III, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg 2009;108:90–104 [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Sadovova N, Fu X, Schmued L, Scallet A, Hanig J, Slikker W. The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience 2005;132:967–77 [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG, Slikker W., Jr.Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci 2006;91:192–201 [DOI] [PubMed] [Google Scholar]
- 33.Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W, Wang C. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci 2009;108:149–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhesus Macaque Genome S, Analysis C, Gibbs RA, Rogers J, Katze MG, Bumgarner R, Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC, Wilson RK, Batzer MA, Bustamante CD, Eichler EE, Hahn MW, Hardison RC, Makova KD, Miller W, Milosavljevic A, Palermo RE, Siepel A, Sikela JM, Attaway T, Bell S, Bernard KE, Buhay CJ, Chandrabose MN, Dao M, Davis C, Delehaunty KD, Ding Y, Dinh HH, Dugan-Rocha S, Fulton LA, Gabisi RA, Garner TT, Godfrey J, Hawes AC, Hernandez J, Hines S, Holder M, Hume J, Jhangiani SN, Joshi V, Khan ZM, Kirkness EF, Cree A, Fowler RG, Lee S, Lewis LR, Li Z, Liu YS, Moore SM, Muzny D, Nazareth LV, Ngo DN, Okwuonu GO, Pai G, Parker D, Paul HA, Pfannkoch C, Pohl CS, Rogers YH, Ruiz SJ, Sabo A, Santibanez J, Schneider BW, Smith SM, Sodergren E, Svatek AF, Utterback TR, Vattathil S, Warren W, White CS, Chinwalla AT, Feng Y, Halpern AL, Hillier LW, Huang X, Minx P, Nelson JO, Pepin KH, Qin X, Sutton GG, Venter E, Walenz BP, Wallis JW, Worley KC, Yang SP, Jones SM, Marra MA, Rocchi M, Schein JE, Baertsch R, Clarke L, Csuros M, Glasscock J, Harris RA, Havlak P, Jackson AR, Jiang H, Liu Y, Messina DN, Shen Y, Song HX, Wylie T, Zhang L, Birney E, Han K, Konkel MK, Lee J, Smit AF, Ullmer B, Wang H, Xing J, Burhans R, Cheng Z, Karro JE, Ma J, Raney B, She X, Cox MJ, Demuth JP, Dumas LJ, Han SG, Hopkins J, Karimpour-Fard A, Kim YH, Pollack JR, Vinar T, Addo-Quaye C, Degenhardt J, Denby A, Hubisz MJ, Indap A, Kosiol C, Lahn BT, Lawson HA, Marklein A, Nielsen R, Vallender EJ, Clark AG, Ferguson B, Hernandez RD, Hirani K, Kehrer-Sawatzki H, Kolb J, Patil S, Pu LL, Ren Y, Smith DG, Wheeler DA, Schenck I, Ball EV, Chen R, Cooper DN, Giardine B, Hsu F, Kent WJ, Lesk A, Nelson DL, O’Brien W E, Prufer K, Stenson PD, Wallace JC, Ke H, Liu XM, Wang P, Xiang AP, Yang F, Barber GP, Haussler D, Karolchik D, Kern AD, Kuhn RM, Smith KE, Zwieg AS. Evolutionary and biomedical insights from the rhesus macaque genome. Science 2007;316:222–34 [DOI] [PubMed] [Google Scholar]
- 35.O’Leary JD, Janus M, Duku E, Wijeysundera DN, To T, Li P, Maynes JT, Faraoni D, Crawford MW. Influence of surgical procedures and general anesthesia on child development before primary school entry among matched sibling pairs. JAMA Pediatr 2019;173:29–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walkden GJ, Pickering AE, Gill H. Assessing long-term neurodevelopmental outcome following general anesthesia in early childhood: challenges and opportunities. Anesth Analg 2019;128:681–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhutta AT, Schmitz ML, Swearingen C, James LP, Wardbegnoche WL, Lindquist DM, Glasier CM, Tuzcu V, Prodhan P, Dyamenahalli U, Imamura M, Jaquiss RD, Anand KJ. Ketamine as a neuroprotective and anti-inflammatory agent in children undergoing surgery on cardiopulmonary bypass: a pilot randomized, double-blind, placebo-controlled trial. Pediatr Crit Care Med 2012;13:328–37 [DOI] [PubMed] [Google Scholar]
- 38.Walters JL, Paule MG. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol 2017;60:2–23 [DOI] [PubMed] [Google Scholar]
- 39.Guo CJ, Douglas SD, Lai JP, Pleasure DE, Li Y, Williams M, Bannerman P, Song L, Ho WZ. Interleukin-1beta stimulates macrophage inflammatory protein-1alpha and -1beta expression in human neuronal cells (NT2-N). J Neurochem 2003;84:997–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coughlan CM, McManus CM, Sharron M, Gao Z, Murphy D, Jaffer S, Choe W, Chen W, Hesselgesser J, Gaylord H, Kalyuzhny A, Lee VM, Wolf B, Doms RW, Kolson DL. Expression of multiple functional chemokine receptors and monocyte chemoattractant protein-1 in human neurons. Neuroscience 2000;97:591–600 [DOI] [PubMed] [Google Scholar]
- 41.Boutet A, Salim H, Leclerc P, Tardieu M. Cellular expression of functional chemokine receptor CCR5 and CXCR4 in human embryonic neurons. Neuroscience Letters 2001;311:105–8 [DOI] [PubMed] [Google Scholar]
- 42.Wang HK, Park UJ, Kim SY, Lee JH, Kim SU, Gwag BJ, Lee YB. Free radical production in CA1 neurons induces MIP-1alpha expression, microglia recruitment, and delayed neuronal death after transient forebrain ischemia. J Neurosci 2008;28:1721–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh S, Anshita D, Ravichandiran V. MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol 2021;101:107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sur M, Leamey CA. Development and plasticity of cortical areas and networks. Nat Rev Neurosci 2001;2:251–62 [DOI] [PubMed] [Google Scholar]
- 45.Ikonomidou C. Triggers of apoptosis in the immature brain. Brain Dev 2009;31:488–92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-ebm-10.1177_15353702231168144 for Development of a primate model to evaluate the effects of ketamine and surgical stress on the neonatal brain by Cheng Wang, Adnan Bhutta, Xuan Zhang, Fang Liu, Shuliang Liu, Leah E Latham, John C Talpos, Tucker A Patterson and William Slikker in Experimental Biology and Medicine