Summary
Background
Roflumilast is a targeted inhibitor of phosphodiesterase (PDE)-4 and has been approved for treatment of severe chronic obstructive pulmonary disease for more than a decade. Generic versions are available in the United States. PDE-4 is involved in the psoriasis pathogenesis, but the efficacy and safety of oral roflumilast in patients with psoriasis have not previously been studied.
Methods
A company-independent, multicenter, randomized, double-blind, placebo-controlled trial (ClinicalTrials.govNCT04549870). Patients were randomized 1:1 to receive monotherapy with oral roflumilast 500 μg once daily or placebo. At week 12, placebo patients were switched to open-label roflumilast through week 24. The primary endpoint was a 75% or greater reduction from baseline in the psoriasis area and severity index (PASI75) at week 12.
Findings
In all, 46 patients were randomized (roflumilast, n = 23; placebo, n = 23). At week 12, significantly more patients in the active arm achieved PASI75 (8 of 23 patients [35%]) vs. placebo (0 of 23 patients [0%], with a difference vs. placebo of 8 [35%] patients, 95% CI: 3 [13%]—13 [57%] patients) (p = 0.014). At week 24, 15 (65%), 10 (44%), 5 (22%), and 2 (9%) of patients treated with roflumilast from week 0 had PASI50, PASI75, PASI90, and PASI100 responses (key secondary endpoints), respectively. The most prevalent, drug-related adverse events in both treatment groups were transient gastrointestinal symptoms, weight-loss, headache, and insomnia. A total of three patients (roflumilast n = 2; placebo, n = 1) discontinued therapy due to adverse events.
Interpretation
Oral roflumilast was efficacious and safe in treating moderate-to-severe plaque psoriasis over 24 weeks. With generic versions available, this drug may represent an inexpensive and convenient alternative to established systemic psoriasis treatments.
Funding
Financial support was received from Herlev and Gentofte Hospital, University of Copenhagen, and independent grants from private foundations in Denmark. No pharmaceutical company, including the market authorization holder of roflumilast, was involved in the study at any point.
Keywords: Psoriasis, Roflumilast, PDE4-inhibitor, Phosphodiesterase-4, Therapy, Chronic obstructive pulmonary disease, COPD
Research in context.
Evidence before this study
Prior to study initiation in late 2019, PubMed was searched using the terms ‘roflumilast’ and ‘psoriasis’. Apart from few opinions and experimental publications on topical roflumilast, the search yielded one case report on oral roflumilast. In addition, a PhD dissertation comparing the modes of actions of apremilast and roflumilast was identified with Google Scholar. Psoriasis has a negative impact on quality of life and confers a considerable psychosocial disability to patients. Despite that pharmaceutical treatment options have greatly advanced in modern times, therapy is especially challenged by high costs. Furthermore, patients with psoriasis often report anxiety towards injectable therapies and find these burdensome. Oral roflumilast is approved for severe chronic obstructive pulmonary disease and generic versions are available in the US. The drug inhibits intracellular phosphodiesterase (PDE)-4, which plays a key role in inflammation, including in skin diseases. Oral roflumilast holds a favorable safety and tolerability profile and does not require regular blood monitoring during therapy.
Added value of this study
This company-independent, randomized, multi-center, placebo-controlled trial is the first to investigate the efficacy and safety of oral roflumilast in the treatment of moderate-to-severe psoriasis. It demonstrates that once daily, oral roflumilast was efficacious and well-tolerated, with an effect-size in the same range as both established and new oral therapies for psoriasis.
Implications of all the available evidence
Topical roflumilast was approved for plaque-type psoriasis in 2022. The present results support findings from recently published case reports (2022), showing high clinical efficacy and tolerability of targeted PDE-4 inhibition with roflumilast in patients with psoriasis. Oral roflumilast could likely represent a novel, convenient, safe, and inexpensive treatment alternative, and thereby fill a major unmet need in patients with psoriasis.
Introduction
Psoriasis is a chronic inflammatory skin disease affecting an estimated 60 million people world-wide.1 According to the International Psoriasis Council, patients with an affected body surface area >10%, psoriasis involving special areas, or failure of topical therapy are considered candidates for systemic treatment.2 Psoriasis is associated with an increased mortality risk3 and several comorbidities, including chronic obstructive pulmonary disease (COPD), obesity, type-2 diabetes, and cardiovascular disease.4 Thus, prescriptions of long-term treatments for psoriasis must consider not only the efficacy in improving skin lesions, but also factors such as compatibility with co-occurring diseases, safety, route of administration, and financial burden. Importantly, despite the advent of biosimilars in recent years, affordability of psoriasis therapies remains an issue for many patients and health care systems.5
Roflumilast is a targeted inhibitor of phosphodiesterase (PDE)-4, which is the major hydrolase of cyclic adenosine monophosphate (cAMP) in immune cells. By blocking PDE-4 activity, intracellular cAMP concentrations rise and ultimately result in reduced inflammation.6 Oral roflumilast was approved first-in-class for treatment of severe COPD more than a decade ago, and generic versions are available in the United States (US). A topical formulation of roflumilast has recently been approved by the US Food and Drug Administration (FDA) for treatment of plaque psoriasis,7 but the effect of systemic treatment with roflumilast in patients with psoriasis has not been explored.
The aim of this company-independent, clinical trial was to investigate the efficacy and safety of oral roflumilast in the treatment of patients with moderate-to-severe plaque psoriasis.
Methods
Study protocol
Regulatory approvals were obtained from The Scientific Ethics Committee of the Capital Region of Denmark (H-20013697), the Danish Medicine Agency, and the Danish Data Protection Agency. The trial was registered at EudraCT (2020-000711-76) and conducted in accordance with national data protection acts and the Edinburgh, Scotland, amendment (2000) to the Declaration of Helsinki 1964. Monitoring was performed by the Good Clinical Practice (GCP) units at University of Copenhagen and Aarhus University, Denmark. The trial was registered with ClinicalTrials.gov (NCT04549870). Additional information (e.g. biomarker data) and open-label long-term efficacy and safety outcomes will be disclosed in later publications.
Study design and participants
The Psoriasis Treatment with Oral Roflumilast (PSORRO) study was a company-independent, multicenter, randomized, double-blind, placebo-controlled trial, conducted at three major Danish university hospital dermatology departments between January 1, 2021 and December 12, 2022.
Participants were recruited from study sites and dermatology private practices, and through advertising. Inclusion criteria were age ≥18 years, chronic stable plaque psoriasis (min. six months duration), psoriasis area and severity index (PASI) ≥8, body mass index (BMI) ≥20 kg/m2, indication for systemic treatment of psoriasis, and safe anticonception during the study period and at least one week after end of therapy. Exclusion criteria were severe immunological disease, tuberculosis, hepatitis, heart failure (NYHA III-IV), liver failure (Child-Pugh B–C), breast feeding, confirmed or planned pregnancy within six months, current or previous malignancy, and current or previous depression with suicidal ideation. In addition, patients currently treated with theophylline, phenobarbital, carbamazepine, or phenytoin, or ever treated with apremilast were excluded. Use of topical or systemic prescription drugs for psoriasis was not allowed two and four weeks, respectively, before randomization and during the study period. In case of a significant psoriasis flare-up, topical rescue medication with corticosteroids or calcineurin inhibitors was accepted. Type, dosage, and amount (grams) was recorded.
Prior to enrollment, participants provided written informed consent, which they at any time could withdraw without providing reason(s). In this case, they were encouraged to go through the same final evaluations as participants completing according to study protocol.
Randomization and masking
At baseline, eligble patients were assigned by site investigators to monotherapy with either placebo or oral roflumilast 500 μg once daily (in-label marketed maintenace dose for COPD). No initial dose-titration was applied. The random allocation sequence was implemented with sequentially numbered containers, and the treatment was double-blind until week 12, where participants were unblinded. From week 12 to 24, all patients received open-label treatment with oral roflumilast 500 μg once daily. In-person study visits were conducted at week 0, 4, 8, 12, and 24. Participants switching from placebo to active treatment at week 12 had an additional study visit at week 16.
The Hospital Pharmacy, The Capital Region of Denmark, was responsible for unstratified block randomization with an online generator (alternating block sizes of four and two), labelling, and blinding of study drugs, which was administered free of charge from the participating centers.
Efficacy assessments
The primary study endpoint was defined as the proportion of patients achieving at least 75% reduction from baseline PASI (PASI75) at week 12. PASI is a clinical measure of psoriatic disease severity considering qualitative lesion characteristics (erythema, thickness, and scaling) and degree of skin involvement in defined anatomical regions. It ranges from 0 to 72, with higher values reflecting greater disease severity. All psoriasis lesions, irrespective of size, were included in the PASI in the present trial.
Secondary efficacy endpoints included the proportion of patients achieving at least 50%, 90%, and 100% reduction in baseline PASI (PASI50, PASI90, and PASI100, respectively) at week 12 and PASI50, PASI75, PASI90, and PASI100 at week 24, percent change from baseline PASI at week 12 and 24, absolute change (1 or more points) from baseline in static physician global assessment (sPGA) at week 12 and 24, percentage change from baseline in affected body surface area (BSA) at week 12 and 24, percentage change from baseline in the product of BSA and sPGA (total psoriasis severity index) at week 12 and 24, and absolute change from baseline in dermatology life quality index (DLQI) at week 12 and 24.
The sPGA is a 5-point scale ranging from 0 (clear, except for residual discoloration) to 4 (majority of plaques with severe thickness, erythema, and scaling). Scores for thickness, erythema, and scaling are summed and the mean equals overall sPGA score. Fractional values for the sPGA are rounded to the nearest integer. BSA refers to percentage body area affected by psoriasis. DLQI is a validated, short, self-administered questionnaire that measures the impact of skin disease on quality of life. Questions cover symptoms, feelings, daily activities, personal relationships, and treatment. Each is answered on a scale of 0 (not at all) to 3 (very much); total DLQI ranges from 0 to 30.
Safety assessments
Screening and monitoring were based on recommendations outlined in the summary of product characteristics for roflumilast.8 During the study period, information about all adverse events (AEs) and severe adverse events (SAEs) were recorded, including any possible relation to the study drug, as assessed by the investigator.
Emergency randomization codes were kept at all study sites. In case of AEs necessitating information on the treatment, the code was broken for that patient only. Any assessment to unblind was made in consultation with the research team, yet final decisions to unblind rested with the involved clinicians.
Statistical analysis
Based on the results from an apremilast phase II trial (where PASI75 was achieved by 41% in the apremilast arm, and 6% in the placebo group),9 and assuming that oral roflumilast 500 μg once daily would have similar efficacy as apremilast 30 mg twice daily, a total of 40 patients (20 patients in each treatment arm) would yield at least 80% power to detect a significant difference between roflumilast and placebo in the proportion of patients achieving PASI75 at week 12.
Data analyses were performed non-blinded by authors MLN and AE using Python version 3.7.6 including relevant packages (Python Software Foundation) and the statistical software R version 4.2.0 (The R Foundation). Continuous variables were presented as means and standard deviations (SD) (normally distributed data) or medians and interquartile ranges (IQR) (non-normally distributed data). Categorical variables were reported as frequencies and percentages. Efficacy data were assessed by intention to treat (ITT). Missing data were handled with non-responder imputation (NRI) for binary outcomes and last observation carried forward (LOCF) for continuous outcomes. Binary study outcomes were analyzed with Fisher's exact test and continuous outcomes were analyzed with analysis of covariance (ANCOVA) with baseline value as covariate. AEs were reported as number and percentages of patients as well as exposure-adjusted incidence rates (EAIR) per 100 patient years. The Benjamini-Hochberg procedure was used to adjust p-values for multiple testing, and a false discovery rate of 5% was assumed.
Role of the funding source
The trial was funded through financial support from Herlev and Gentofte Hospital, University of Copenhagen, and independent grants from private foundations in Denmark. None of these had financial interests in roflumilast, access to study data, or influence on publication. No pharmaceutical company, including the market authorization holder of roflumilast, was involved in the study at any point. All authors had full access to all data and accept responsibility to submit for publication. The first and senior author had the final responsibility for manuscript submission.
Results
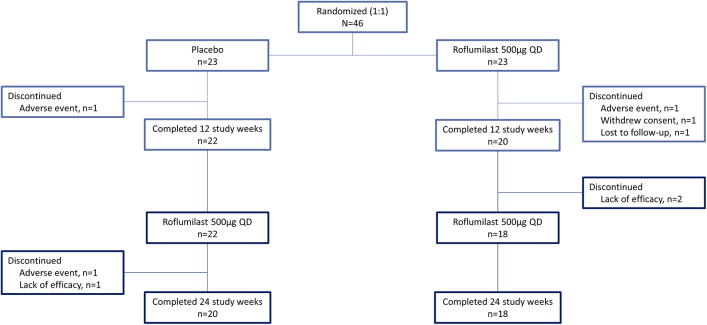
A total of 54 patients were screened. Of these, eight were excluded before randomization as they did not meet inclusion criteria. The remaining 46 patients were enrolled in the trial, of which 42 patients completed week 12 (primary endpoint) and 38 patients the week 24 visit. The patient disposition is displayed in Fig. 1.
Fig. 1.
Patient disposition flow-chart.
Baseline characteristics and demographics were generally well-balanced between the two treatment arms (Table 1). At randomization, median (IQR) PASI was 10.9 (8.5–15.6) and 10.6 (9.7–15.1) and median (IQR) psoriasis duration was 16.0 (12.0–25.5) and 16.0 (10.5–26.0) years in the roflumilast and placebo group, respectively. A total of 15 (65%) patients in the roflumilast group and 16 (70%) patients in the placebo group had previously received systemic psoriasis therapy.
Table 1.
Baseline patient characteristics.
| Roflumilast N = 23 | Placebo N = 23 | |
|---|---|---|
| Demographics | ||
| Age [years], median (IQR) | 38.0 (30.0, 43.0) | 39.0 (32.5, 50.0) |
| Men, n (%) | 15 (65) | 19 (83) |
| Caucasian, n (%) | 23 (100) | 23 (100) |
| Psoriasis duration [years], median (IQR) | 16.0 (12.0, 25.5) | 16.0 (10.5, 26.0) |
| Weight [kg], mean (SD) | 102.0 (23.5) | 105.1 (21.1) |
| BMI [kg/m2], mean (SD) | 33.3 (6.6) | 32.2 (5.9) |
| Previous systemic therapy | ||
| Any, n (%) | 15 (65) | 16 (70) |
| Non-biologic, n (%) | 15 (65) | 16 (70) |
| Biologic, n (%) | 2 (9) | 3 (13) |
| Psoriasis characteristics | ||
| PASI, median (IQR) | 10.9 (8.5, 15.6) | 10.6 (9.7, 15.1) |
| BSA [%], median (IQR) | 14.5 (11.0, 29.0) | 10.5 (9.2, 39.0) |
| sPGA, median (IQR) | 2.0 (2.0, 3.0) | 2.0 (2.0, 3.0) |
| DLQI, median (IQR) | 11.0 (7.5, 14.5) | 9.0 (6.5, 12.5) |
| PsA, n (%)a | 1 (4) | 3 (13) |
Data are shown for all randomized patients. Categorical variables are presented in frequencies and percentages. Numerical data are shown in medians and IQR or mean and SD. PASI ranges from 0 to 72, sPGA from 0 to 4, and DLQI from 0 to 30.
BMI, body mass index; BSA, body surface area; DLQI, dermatology life quality index; IQR, interquartile range; PASI, psoriasis area and severity index; PsA, psoriatic arthritis; SD, standard deviation; sPGA, static physician global assessment.
Diagnosed by a rheumatologist.
Efficacy
Week 0–12 (placebo-controlled period)
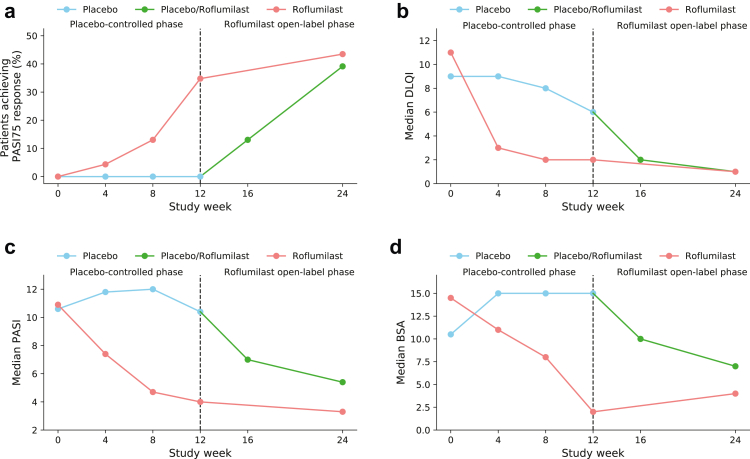
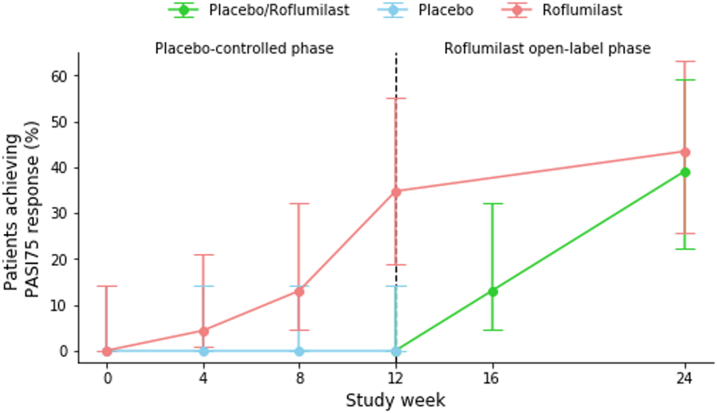
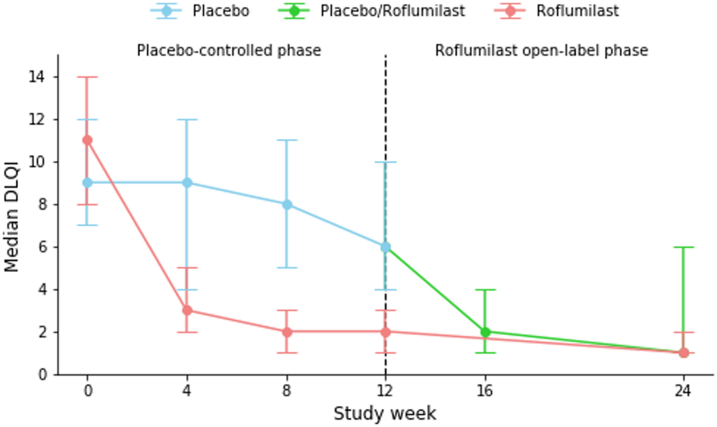
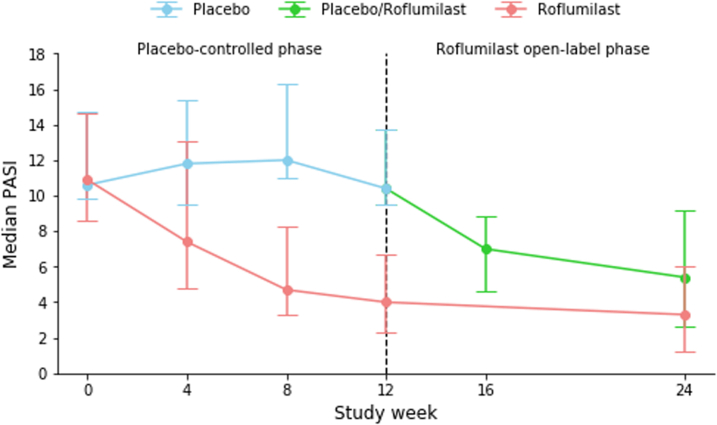
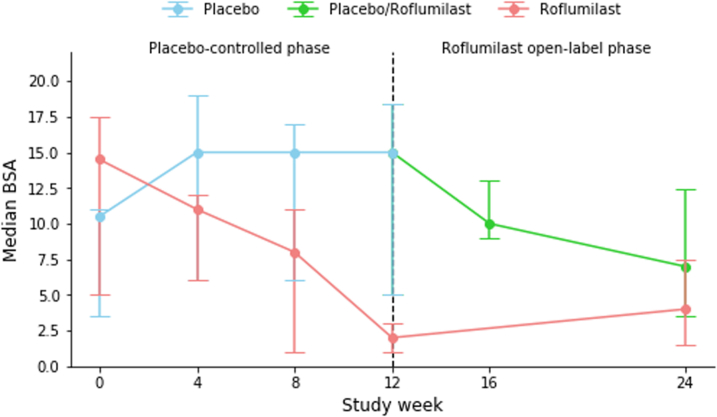
PASI75 at week 12 was achieved by 8 (35%) patients in the roflumilast group compared to 0 (0%) patients in the placebo group (p = 0.014). Significantly more patients treated with roflumilast achieved PASI50 (16 (70%) vs. 1 (4%) with placebo; p < 0.0001). Three patients in the roflumilast group had PASI90 or PASI100 responses, but the difference did not reach statistical significance versus placebo (n = 0 patients; p = 0.25 and p = 0.49, respectively). At week 12, the median change from baseline PASI was −61.7% for roflumilast and −3.7% for placebo (p = 0.014). Seventeen patients (74%) in the roflumilast group had an absolute reduction of ≥1 points from baseline in sPGA, as compared to 4 patients (17%) in the placebo group (p = 0.00011). Median change from baseline for patients receiving roflumilast vs. placebo was −81.8% and 6.2%, respectively, in affected BSA (p = 0.024) and −9.0 to 1.0, respectively, in the product of BSA and sPGA (p = 0.018).
Week 12–24 (open-label period)
Comparable efficacy responses were observed irrespective of baseline randomization. Thus, at week 24, PASI75 and PASI50 were achieved in 9 (39%) and 13 (57%), respectively, of patients assigned to placebo in week 0–12, while corresponding responses in the patients receiving roflumilast from week 0 were 10 (44%) and 15 (65%), respectively. PASI90 was achieved in 5 (22%) of patients treated with roflumilast since week 0, compared to 3 (13%) of patients previously treated with placebo.
Rescue medication was prescribed for three patients in the placebo group (estimated 6.25 g lower-mid potency corticosteroid per patient per week). In the open-label period, no use of topical treatments was registered.
Efficacy outcomes are presented in Table 2 and Fig. 2. All p-values in Table 2 have been adjusted for multiple testing using the Benjamini-Hochberg procedure, which has not changed any significant p-values to non-significant.
Table 2.
Efficacy outcomes.
| Week 0–12: placebo-controlled period |
Week 12–24: open-label period |
||||
|---|---|---|---|---|---|
| Roflumilast n = 23 | Placebo n = 23 | P-value | Roflumilast until week 12 n = 23 | Placebo until week 12 n = 23 | |
| PASI50, n (%) | 16 (70) | 1 (4) | <0.0001 | 15 (65) | 13 (57) |
| PASI75, n (%) | 8 (35) | 0 (0.0) | 0.014 | 10 (44) | 9 (39) |
| PASI90, n (%) | 3 (13) | 0 (0.0) | 0.25 | 5 (22) | 3 (13) |
| PASI100, n (%) | 2 (9) | 0 (0.0) | 0.49 | 2 (9) | 0 (0) |
| PASI, mean (SD) | −7.6 (3.6) | −0.9 (7.2) | 0.014 | −8.0 (4.3) | −5.7 (5.4) |
| PASI %, median (IQR) | −61.7 (−77.8, −48.4) | −3.7 (−18.5, 23.8) | 0.014 | −69.2 (−86.0, −38.1) | −59.5 (−76.6, −16.0) |
| sPGA, median (IQR) | −1.0 (−1.5, −1.0) | 0.0 (0.0, 0.0) | 0.014 | −1.0 (−2.0, 0.0) | −1.0 (−1.0, 0.0) |
| sPGA ≤ -1, n (%) | 17 (74) | 4 (17) | 0.00011 | 14 (61) | 12 (52) |
| BSA, mean (SD) | −11.8 (13.5) | −2.8 (14.7) | 0.083 | −12.5 (8.7) | −13.2 (14.8) |
| BSA %, median (IQR) | −81.8 (−93.8, −30.8) | 6.2 (−15.6, 37.7) | 0.024 | −66.4 (−96.7, −32.4) | −77.4 (−93.4, −26.7) |
| BSA ∗ sPGA, median (IQR) | −9.0 (−19.8, −2.0) | 1.0 (−8.2, 13.3) | 0.018 | −11.5 (−20.0, −3.2) | −11.8 (−18.2, −1.5) |
| DLQI, mean (SD) | −6.8 (5.0) | −3.2 (6.1) | 0.031 | −7.4 (4.6) | −3.7 (6.0) |
Data are shown for all randomized patients. Categorical variables are presented in frequencies and percentages. Numerical data are shown in medians and IQR (non-normally distributed data) or mean and SD (normally distributed data). Δ refers to change from start of current therapy. Missing data are handled with NRI (non-responder imputation) for binary outcomes or LOCF (last observation carried forward) for non-binary outcomes. Bold values denote statistically significant results.
BSA, body surface area; DLQI, dermatology life quality index; IQR, interquartile range; PASI, psoriasis area and severity index; PASI50, at least 50% reduction in baseline PASI; PASI75, at least 75% reduction in baseline PASI; PASI90, at least 90% reduction in baseline PASI; PASI100, 100% reduction in baseline PASI; SD, standard deviation; sPGA, static physician global assessment.
Fig. 2.
Efficacy outcomes. a) Proportion of patients achieving PASI75 [NRI]. b) Median DLQI [LOCF]. c) Median PASI [LOCF]. d) Median BSA [LOCF].
Safety
Week 0–12 (placebo-controlled period)
A total of 20 (87%) patients in the roflumilast group vs. 14 (61%) in the placebo group experienced at least one drug-related AE, as assessed by the investigator. In both treatment groups, transient gastrointestinal symptoms (nausea, abdominal pain, and diarrhea), weight loss, reduced appetite, headache, and insomnia were most frequently reported. Additionally, 14 (61%) and 13 (57%) patients receiving roflumilast and placebo, respectively, had at least one non-drug related AE. A total of two patients discontinued the trial due to AEs: one in the roflumilast group (gastrointestinal symptoms, headache, and insomnia) and one in the placebo group (anxiety/nervousness and gastrointestinal symptoms). No SAEs were reported, and no significant biochemical changes were observed.
Week 12–24 (open-label period)
One or more drug-related AEs were recorded for 30 patients (65%). Most common were gastrointestinal symptoms (nausea, abdominal pain, and diarrhea), weight loss, reduced appetite, headache, and insomnia, as seen in the placebo-controlled period. One patient discontinued therapy (due to gastrointestinal symptoms, vertigo, headache, and insomnia). A total of 23 patients (50%) experienced at least one non-drug related AE. One SAE occurred (non-ischemic chest pain), which by the investigator was determined to be unrelated to roflumilast therapy. No significant biochemical changes were observed during the open-label study period.
Safety data are presented in Table 3.
Table 3.
Adverse events.
| Patients | Week 0–12: placebo-controlled period |
Week 12–24: open-label period |
||||
|---|---|---|---|---|---|---|
| Roflumilast n = 23 |
Placebo n = 23 |
Roflumilast n = 46 |
||||
| n | EAIR/100 patient years | n | EAIR/100 patient years | n | EAIR/100 patient years | |
| Adverse events (AEs) | ||||||
| ≥1 drug-related | 20 (87) | 904 | 14 (61) | 437 | 30 (65) | 787 |
| ≥1 non-drug related | 14 (61) | 414 | 13 (57) | 364 | 23 (50) | 452 |
| Serious AEs (SAEs) | ||||||
| ≥1 drug-related | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| ≥1 non-drug related | 0 (0) | 0 | 0 (0) | 0 | 1 (3)a | 11 |
| ≥1 suspected unexpected serious adverse reaction (SUSAR) | 0 (0) | 0 | 0 (0) | 0 | 0 (0.0) | 0 |
| AEs leading to drug discontinuation | 1 (4)b | 19 | 1 (4)c | 19 | 1 (3)d | 11 |
| Drug-related AEs reported by ≥5 patients in any treatment group in any study phase, n (%) | ||||||
| Weight loss | 19 (83) | 800 | 9 (39) | 229 | 32 (70) | 1128 |
| Reduced appetite | 14 (61) | 434 | 10 (44) | 256 | 21 (46) | 386 |
| Nausea | 12 (52) | 354 | 6 (26) | 131 | 11 (24) | 150 |
| Headache | 11 (48) | 301 | 6 (26) | 134 | 8 (17) | 100 |
| Abdominal pain | 9 (39) | 226 | 6 (26) | 137 | 7 (15) | 86 |
| Insomnia | 9 (39) | 227 | 8 (35) | 188 | 12 (26) | 166 |
| Diarrhea | 7 (30) | 160 | 6 (26) | 136 | 8 (17) | 103 |
| Vertigo | 5 (22) | 110 | 2 (9) | 40 | 4 (9) | 46 |
| Back pain | 3 (13) | 62 | 3 (13) | 64 | 2 (4) | 23 |
| Upper respiratory tract infection | 3 (13) | 62 | 0 (0) | 0 | 5 (11) | 61 |
| Malaise | 2 (9) | 40 | 2 (9) | 41 | 4 (9) | 46 |
| Constipation | 2 (9) | 40 | 0 (0) | 0 | 0 (0) | 0 |
| Nervousness | 2 (9) | 40 | 2 (9) | 40 | 2 (4) | 23 |
| Fatigue | 2 (9) | 40 | 3 (13) | 63 | 4 (9) | 46 |
| Myalgia | 1 (4) | 20 | 3 (13) | 62 | 1 (2) | 11 |
| Palpitations | 1 (4) | 20 | 2 (9) | 40 | 2 (4) | 22 |
| Tremor | 1 (4) | 20 | 2 (9) | 39 | 1 (2) | 11 |
| Tiredness | 0 (0) | 0 | 2 (9) | 40 | 1 (2) | 11 |
| Vomiting | 0 (0) | 0 | 0 (0) | 0 | 3 (7) | 35 |
| Non-drug related AEs reported by ≥5 patients in any treatment group in any study phase, n (%) | ||||||
| Upper airway infection | 3 (13) | 64 | 1 (4) | 20 | 7 (15) | 88 |
| Insomnia | 2 (9) | 41 | 1 (4) | 20 | 0 (0) | 0 |
| Stress | 2 (9) | 40 | 0 (0) | 0 | 2 (4) | 23 |
| Covid-19 infection | 2 (9) | 40 | 1 (4) | 20 | 2 (4) | 22 |
| Selected laboratory abnormalities | ||||||
| ALT >3 × ULN, U/L | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| AST >3 × ULN, U/L | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| Bilirubin >1.8 × ULN, μmol/L | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| HbA1c >75 mmol/mol | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| Total cholesterol >7.8 mmol/L | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| Triglycerides >3.4 mmol/L | 1 (4) | 19 | 1 (4) | 20 | 2 (4) | 11 |
| Lymphocytes <0.8 × 109/L | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
| Neutrophils <1 × 109/L | 0 (0) | 0 | 0 (0) | 0 | 0 (0) | 0 |
Multiple occurrences of an identical event in the same patient figure at the first registration time point within each treatment period. Weight loss is defined by >1 kg.
AE, adverse event; ALT, alanine transaminase; AST, aspartate transaminase; EAIR, exposure-adjusted incidence rate; HbA1c, glycated hemoglobin; SAE, serious adverse event; SUSAR, serious unexpected suspected adverse reaction; ULN, upper limit of normal.
SAE; non-ischemic chest pain.
Gastrointestinal symptoms, headache, and insomnia.
Psychological imbalance/worsening of anxiety, nervousness, and gastrointestinal symptoms.
Gastrointestinal symptoms, vertigo, headache, and insomnia.
Discussion
In this company-independent, randomized, placebo-controlled trial, we found that oral roflumilast 500 μg once daily was efficacious in treating moderate-to-severe psoriasis. A total of 8 (35%) of the patients in the roflumilast group met the primary endpoint of PASI75 at 12 weeks, compared with 0 (0%) in the placebo arm. After 24 weeks of continuous treatment with oral roflumilast, 10 (44%), 5 (22%), and 2 (9%) patients, achieved PASI75, PASI90, and PASI100, respectively. Overall, roflumilast was safe and well-tolerated, and the most common AEs were gastrointestinal and appeared to be transient.
Although the armamentarium of systemic psoriasis therapies has greatly expanded since the 1990's, conventional drugs, in particular methotrexate, still hold a central position in most treatment algorithms.1,10 Methotrexate was the first systemic drug to receive regulatory approval for psoriasis,11 and despite a lower efficacy compared to novel biologic agents,12 methotrexate is a mandatory step before prescription of biologics in many countries. In e.g. Denmark, 95% of patients with psoriasis have received methotrexate prior to starting biologic therapy.13 Advantages of methotrexate include the generally low costs and once-weekly administration, but associated side effects including end-organ toxicities are leading causes of discontinuation.14 Furthermore, methotrexate is contraindicated with several medical conditions and requires regular laboratory monitoring and adherence to alcohol restrictions. Other oral treatments for psoriasis, e.g. cyclosporine, also come with limitations.15 Therefore, alternatives are needed for patients that are not candidates for existing therapies.
PDE-4 activity is increased in lesional skin and peripheral blood from patients with psoriasis, and by blocking the intracellular enzyme, essential inflammatory cytokines in psoriasis pathogenesis, e.g. tumor necrosis factor alpha (TNF-α), interleukin (IL)-17, and IL-23, are downregulated.16 The clinical importance of PDE-4 in psoriasis became evident with apremilast, which has been approved in the US for plaque psoriasis since 2014.9 In vitro studies have previously demonstrated that roflumilast elevate cAMP levels twice as much as other PDE-4 inhibitors, possibly due to higher potency.17 Apart from a small number of case reports,18, 19, 20 the literature on roflumilast and plaque psoriasis is restricted to studies assessing topical drug formulations. In September 2022, data were published from two large phase III trials including patients with mild-to-moderate psoriasis. After eight weeks, PASI75 had been achieved in approximately 40% of patients in the active group compared to <10% in placebo.21 In July 2022, roflumilast cream 0.3% received FDA approval for psoriasis in adults and children 12 years or older.
In the present study, 8 (34%) and 10 (44%) of patients treated with oral roflumilast obtained PASI75 at weeks 12 and 24, respectively. Previously, results from phase III trials found that less than 30% of patients achieved PASI75 after 12 weeks treatment with apremilast 30 mg twice daily. Here, most common side effects were diarrhea and nausea, which occurred in up to 19% of patients compared with 7% in the placebo groups.22,23 Efficacy results similar to apremilast have been observed with subcutaneous methotrexate. In the METOP phase III trial, PASI75 was reached in 41% of patients after 16 weeks compared to 10% in the placebo group.24 Very recently, treatment with the oral tyrosine kinase inhibitor, deucravacitinib, in a phase-3 randomized controlled trial resulted in 53.0% of patients achieving PASI75 at week 16 compared to 9.4% in the placebo arm, i.e. a net difference of 43.6% vs. placebo.25
The defined daily dose list price of roflumilast in Denmark is <$2.5 USD26 (search April 18, 2023), and generics are now available in the US. With low costs, oral administration, convenient safety and tolerability profile, no alcohol restrictions, few contraindications, and no blood test requirements during treatment, oral roflumilast holds important qualities compared to available systemic therapies for psoriasis. Importantly, in addition to COPD, roflumilast may also address other co-existing diseases, e.g. obesity,8 type-2 diabetes,27 and cardiometabolic disease,28 although this needs more confirmation.
An important strength of the present study is the company-independent, randomized, double-blinded design. Limitations include the relatively small number of patients and male domination, which reduces the generalizability of the results, but is in accordance with current data showing that severe psoriasis is more prevalent in the male population. Another limitation is that long-term data are not available from the current material, and that the present findings may not apply for non-plaque types of psoriasis. In addition, baseline PASI was somewhat lower than most company-sponsored trials of newer biologics and small-molecule therapies. It is well-established that a high percent change in PASI is easier to obtain in patients with a greater baseline value, and our results should thus be interpreted accordingly. Finally, the observed efficacy of roflumilast must be viewed in the light of the participating patients’ baseline weight. As oral roflumilast is indicated for patients with severe COPD, which tend to be underweight, it is tempting to speculate whether higher (off-label) dosages could provide even greater PASI improvements. Importantly, considering the weight distribution of the COPD patient populations where the investigated dose (500 μg once daily) is currently approved, oral roflumilast may also be appropriate to use in psoriasis patients that are not overweight or obese.
In conclusion, oral roflumilast at 500 μg once daily was efficacious and safe in treating moderate-to-severe psoriasis through 24 weeks. The drug may represent a novel and convenient alternative to currently available systemic treatments for patients with plaque psoriasis.
Contributors
Mette Gyldenløve: conceptualization, methodology, funding acquisition, investigation, project administration, writing—original draft, writing—review & editing.
Howraman Meteran: investigation, writing—review & editing.
Jennifer A. Sørensen: investigation, writing—review & editing.
Simon Fage: investigation, writing—review & editing.
Yiqiu Yao: investigation, writing—review & editing.
Jesper Lindhardsen: investigation, writing—review & editing.
Christoffer V. Nissen: investigation, writing—review & editing.
Tanja Todberg: data handling, writing—review & editing.
Simon F. Thomsen: resources, writing—review & editing.
Lone Skov: resources, writing—review & editing.
Claus Zachariae: resources, writing—review & editing.
Lars Iversen: investigation, writing—review & editing.
Mia-Louise Nielsen: formal analysis (accessed and verified data), writing—original draft, writing—review & editing.
Alexander Egeberg: conceptualization, methodology, formal analysis (accessed and verified data), writing—original draft, writing—review & editing.
Data sharing statement
Upon reasonable request to the corresponding author, the data that support the findings of the study can be made available with investigator support to public/academic employed researchers.
Declaration of interests
The Danish market authorization holder of roflumilast was informed prior to study initiation but did not provide any financial or in-kind support for the trial. None of the investigators hold any financial interest in the study drug.
Mette Gyldenløve: Research funding from the Kgl. Hofbuntmager Aage Bang Foundation, the Danish Psoriasis Foundation, Fonden af familien Kjærsgaard, Sunds, the Simon Spies Foundation, and the CC. Klestrup og hustru Henriette Klestrups Mindelegat. Four-year postdoctoral stipend (2022–26) from Herlev and Gentofte Hospital, University of Copenhagen.
Howraman Meteran: Research funding from Per Henriksens Fond, Danish Lung Association, and ALK-Abelló. Honoraria as consultant and/or speaker for ALK-Abelló, GSK, Novartis, AstraZeneca, Sanofi-Aventis Denmark, Airsonett AB, and Teva.
Jennifer A. Sørensen: None.
Simon Fage: Investigator for AbbVie, Galderma, UCB, Eli Lilly, Novartis, and LEO Pharma.
Yiqiu Yao: None.
Jesper Lindhardsen: None.
Christoffer Nissen: None.
Tanja Todberg: Investigator for Novartis, AbbVie, Dr. Wolff, Galderma, and Almirall.
Simon F. Thomsen: Research funding from Janssen Pharmaceuticals, LEO Pharma, Novartis, Sanofi and UCB. Honoraria as consultant and/or speaker for Novartis, CSL, Sanofi, Union Therapeutics, LEO Pharma, Pfizer, Sanofi, and AstraZeneca.
Lone Skov: Research funding from Almirall, Bristol-Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish Psoriasis Foundation, the LEO Foundation, and the Kgl. Hofbundtmager Aage Bang Foundation. Honoraria as consultant and/or speaker for AbbVie, Eli Lilly, Novartis, Pfizer, LEO Pharma, Janssen Cilag, UCB, Almirall, Bristol-Myers Squibb, Boehringer Ingelheim, Novo, and Sanofi. Investigator for AbbVie, Pfizer, Sanofi, Janssen Cilag, Boehringer Ingelheim, Eli Lilly, Novartis, Galderma, and LEO Pharma.
Claus Zachariae: Paid speaker for Eli Lilly, Novartis, CSL, and LEO Pharma. Consultant and/or advisory board member for AbbVie, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, Takeda, Amgen, and CSL.
Lars Iversen: Consultant and/or speaker honoraria from AbbVie, BMS, LEO Pharma, Novartis, UCB, Janssen Pharmaceuticals, Boehringer Ingelheim, and Regranion. Part-time employment at MC2 Therapeutics.
Mia-Louise Nielsen: None.
Alexander Egeberg: Research funding from the Danish Psoriasis Foundation, the Kgl. Hofbuntmager Aage Bang Foundation, the Simon Spies Foundation, Pfizer, Eli Lilly, Novartis, AbbVie, and Janssen Pharmaceuticals. Consultant and/or speaker honoraria from AbbVie, Almirall, Bristol-Myers Squibb, Dermavant, Eli Lilly and Company, Galderma, Galápagos NV, Horizon Therapeutics, Janssen Pharmaceuticals, LEO Pharma, Mylan, Novartis, Pfizer, Samsung Bioepis Co., Ltd., UCB, and Union Therapeutics.
Acknowledgements
We wish to thank the participants, departments, and staff at Herlev and Gentofte Hospital, Bispebjerg and Frederiksberg Hospital, and Aarhus University Hospital. Research funding was generously provided from the Kgl. Hofbuntmager Aage Bang Foundation, The Danish Psoriasis Foundation, Fonden af familien Kjærsgaard, Sunds, the Simon Spies Foundation, and the CC. Klestrup og hustru Henriette Klestrups Mindelegat. In addition, Dr. Gyldenløve received a postdoctoral stipend from Herlev and Gentofte Hospital, University of Copenhagen, supporting her work.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100639.
Appendix A. Supplementary data
Supplementary Fig. S1a.
Supplementary Fig. S1b.
Supplementary Fig. S1c.
Supplementary Fig. S1d.
References
- 1.Griffiths C.E.M., Armstrong A.W., Gudjonsson J.E., Barker J. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- 2.Strober B., Ryan C., van de Kerkhof P., et al. Recategorization of psoriasis severity: delphi consensus from the international psoriasis council. J Am Acad Dermatol. 2020;82(1):117–122. doi: 10.1016/j.jaad.2019.08.026. [DOI] [PubMed] [Google Scholar]
- 3.Semenov Y.R., Herbosa C.M., Rogers A.T., et al. Psoriasis and mortality in the United States: data from the national health and nutrition examination survey. J Am Acad Dermatol. 2021;85(2):396–403. doi: 10.1016/j.jaad.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Elmets C.A., Leonardi C.L., Davis D.M.R., et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80(4):1073–1113. doi: 10.1016/j.jaad.2018.11.058. [DOI] [PubMed] [Google Scholar]
- 5.Chen B.K., Yang Y.T., Bennett C.L. Why biologics and biosimilars remain so expensive: despite two wins for biosimilars, the supreme court's recent rulings do not solve fundamental barriers to competition. Drugs. 2018;78(17):1777–1781. doi: 10.1007/s40265-018-1009-0. [DOI] [PubMed] [Google Scholar]
- 6.Hatzelmann A., Morcillo E.J., Lungarella G., et al. The preclinical pharmacology of roflumilast--a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010;23(4):235–256. doi: 10.1016/j.pupt.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Cordoro K.M. Roflumilast for chronic plaque psoriasis. JAMA. 2022;328(11):1049–1050. doi: 10.1001/jama.2022.14663. [DOI] [PubMed] [Google Scholar]
- 8.EMA . Summary of product characteristics (SmPC) for roflumilast. 2010. Last updated: 25/08/2022. [Google Scholar]
- 9.Papp K., Cather J.C., Rosoph L., et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet. 2012;380(9843):738–746. doi: 10.1016/S0140-6736(12)60642-4. [DOI] [PubMed] [Google Scholar]
- 10.Smith C.H., Yiu Z.Z.N., Bale T., et al. British Association of dermatologists guidelines for biologic therapy for psoriasis 2020: a rapid update. Br J Dermatol. 2020;183(4):628–637. doi: 10.1111/bjd.19039. [DOI] [PubMed] [Google Scholar]
- 11.van Huizen A.M., Menting S.P., Gyulai R., et al. International eDelphi study to reach consensus on the methotrexate dosing regimen in patients with psoriasis. JAMA Dermatol. 2022;158(5):561–572. doi: 10.1001/jamadermatol.2022.0434. [DOI] [PubMed] [Google Scholar]
- 12.Sbidian E., Chaimani A., Garcia-Doval I., et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2022;5(5) doi: 10.1002/14651858.CD011535.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loft N., Skov L., Bryld L.E., Gislason G., Egeberg A. Treatment history of patients receiving biologic therapy for psoriasis - a Danish nationwide study. J Eur Acad Dermatol Venereol. 2017;31(8):e362–e363. doi: 10.1111/jdv.14156. [DOI] [PubMed] [Google Scholar]
- 14.Lebwohl M.G., Kavanaugh A., Armstrong A.W., Van Voorhees A.S. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population-based multinational assessment of psoriasis and psoriatic arthritis (MAPP) survey. Am J Clin Dermatol. 2016;17(1):87–97. doi: 10.1007/s40257-015-0169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menter A., Gelfand J.M., Connor C., et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management of psoriasis with systemic nonbiologic therapies. J Am Acad Dermatol. 2020;82(6):1445–1486. doi: 10.1016/j.jaad.2020.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Pincelli C., Schafer P.H., French L.E., Augustin M., Krueger J.G. Mechanisms underlying the clinical effects of apremilast for psoriasis. J Drugs Dermatol. 2018;17(8):835–840. [PubMed] [Google Scholar]
- 17.Walsh N.M. University of Glasgow; 2015. Comparison of the modes of action of apremilast and roflumilast.http://theses.gla.ac.uk/7427/ Available at: [Google Scholar]
- 18.Egeberg A., Meteran H., Gyldenlove M., Zachariae C. Complete clearance of severe plaque psoriasis with 24 weeks of oral roflumilast therapy. Br J Dermatol. 2021;185(6):1251–1252. doi: 10.1111/bjd.20602. [DOI] [PubMed] [Google Scholar]
- 19.Gyldenlove M., Meteran H., Zachariae C., Egeberg A. Long-term clearance of severe plaque psoriasis with oral roflumilast. J Eur Acad Dermatol Venereol. 2022;37(3):e429–e430. doi: 10.1111/jdv.18647. [DOI] [PubMed] [Google Scholar]
- 20.Michels K., Hagner M., El Zein M., Dasa O., Assaly R. Treating 2 diseases with 1 drug: PDE-4 inhibitor for COPD and psoriasis. Am J Ther. 2017;24(1):e103–e104. doi: 10.1097/MJT.0000000000000465. [DOI] [PubMed] [Google Scholar]
- 21.Lebwohl M.G., Kircik L.H., Moore A.Y., et al. Effect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. JAMA. 2022;328(11):1073–1084. doi: 10.1001/jama.2022.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul C., Cather J., Gooderham M., et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2) Br J Dermatol. 2015;173(6):1387–1399. doi: 10.1111/bjd.14164. [DOI] [PubMed] [Google Scholar]
- 23.Papp K., Reich K., Leonardi C.L., et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J Am Acad Dermatol. 2015;73(1):37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 24.Warren R.B., Mrowietz U., von Kiedrowski R., et al. An intensified dosing schedule of subcutaneous methotrexate in patients with moderate to severe plaque-type psoriasis (METOP): a 52 week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10068):528–537. doi: 10.1016/S0140-6736(16)32127-4. [DOI] [PubMed] [Google Scholar]
- 25.Strober B., Thaci D., Sofen H., et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88(1):40–51. doi: 10.1016/j.jaad.2022.08.061. [DOI] [PubMed] [Google Scholar]
- 26.https://medicinpriser.dk/
- 27.Wouters E.F., Bredenbroker D., Teichmann P., et al. Effect of the phosphodiesterase 4 inhibitor roflumilast on glucose metabolism in patients with treatment-naive, newly diagnosed type 2 diabetes mellitus. J Clin Endocrinol Metab. 2012;97(9):E1720–E1725. doi: 10.1210/jc.2011-2886. [DOI] [PubMed] [Google Scholar]
- 28.White W.B., Cooke G.E., Kowey P.R., et al. Cardiovascular safety in patients receiving roflumilast for the treatment of COPD. Chest. 2013;144(3):758–765. doi: 10.1378/chest.12-2332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.