Abstract
Parkinson’s Disease (PD) is a neurodegenerative movement disorder characterized by symptoms like resting tremor, rigidity, bradykinesia, and postural instability, mainly due to dopamine depletion and degeneration of dopaminergic neurons. Mitochondrial dysfunction plays a critical role in the disease’s progression, while amyotrophic Lateral Sclerosis (ALS), or Lou Gehrig’s disease, is a fatal progressive neurodegenerative disease characterized by significant motor neuron loss in the primary motor cortex, brainstem, and spinal cord. This loss results in impaired movements such as breathing, leading to death within 2–5 years of diagnosis. Patients experience muscle weakness in the hands, arms, legs, and swallowing muscles and may require breathing aids. This Patent Highlight describes blends, such as microbiome compositions, that can be used to treat various diseases or conditions, particularly those affecting the nervous system, like neurodegenerative diseases (PD and ALS).
Important Compound Classes
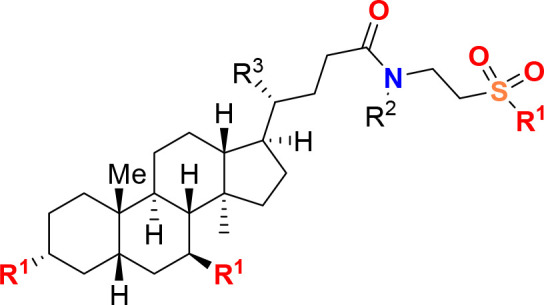
Title
Methods and uses of microbiome compositions, components, or metabolites for treating neurodegenerative diseases.
Patent Publication Number
WO 2023/044076 A1 (URL: https://patents.google.com/patent/WO2023044076A1/en?oq=WO+2023%2f044076+A1).
Publication Date
March 2023, 2023
Priority Application
US 63/330,148
Priority Date
April 12, 2022.
Inventors
Govindan, J. A.; Jayamani, E.; Chatter, P. H.; Chatter, M.
Assignee Company
MarvelBiome Inc. [US/US]; 8 Cabot Road, Suite 3500, Woburn, Massachusetts 01801, United States.
Disease Area
Neurogenerative Diseases
Biological Target
Gut microbiome
Summary
Parkinson’s Disease (PD) is a neurodegenerative movement disorder characterized by resting tremor, rigidity, bradykinesia, and postural instability. PD symptoms are primarily attributed to dopamine depletion and degeneration of dopaminergic neurons in the substantia nigra pars compacta. However, additional neuronal circuits are affected, and nonmotor symptoms are often present, suggesting systemic pathology. Compelling evidence indicates that mitochondrial dysfunction is a primary event in the disease process. It has been reported that PD-related mutations and mitochondrial dynamics have a reciprocal relationship. PD-related mutations can disrupt mitochondrial dynamics, and the consequences of these mutations can be modulated by mitochondrial dynamics.
The disclosure in patent WO 2023/044076 describes a potential effective treatment of PD determined by a reduction in the dose of pharmacological treatments, such as L-DOPA, required to maintain adequate control of PD symptoms. The treatment efficacy is monitored using the Unified Parkinson’s Disease Rating Scale (UPDRS).
PD is the most common motor-related disorder in middle or late life, affecting approximately 6.2 million people worldwide. PD is characterized by the accumulation of alpha-synuclein inclusions in neurons and degeneration and/or loss of dopaminergic neurons. The cardinal clinical symptoms of PD include slow movement, resting tremor, rigidity, and postural instability. While most PD cases have unknown origins and are sporadic, mutations in some genes have been associated with rare, familial forms of the disease.
Several lines of evidence implicate defects in mitochondrial respiration in the etiology and pathogenesis of PD. First, the prodrug 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) gets converted into the neurotoxin MPP+ (1-methyl-4-phenylpyridinium) in the body. MPP+ selectively destroys dopaminergic neurons in the substantia nigra, a region of the brain associated with motor control and movement. MPTP is known to induce PD in humans and animal models. It has been used in research to study the mechanisms of Parkinson’s Disease and test potential therapies. MPTP’s ability to induce PD symptoms is due to its inhibition of complex I of the electron transport chain, which is involved in cellular respiration and energy production. This inhibition leads to oxidative stress, mitochondrial dysfunction, and ultimately the death of dopaminergic neurons.
Second, post-mortem studies of PD patients have found elevated levels of oxidative stress markers/products in the dopaminergic neurons. Third, a reduction of mitochondrial complex I activity by 30% has been observed in the brain and peripheral tissues of PD patients. Fourth, neurotoxins such as rotenone, paraquat, and 6-hydroxydopamine (6-OHDA) induce mitochondrial dysfunction resulting in PD-associated phenotypes in animal models. Finally, PD-associated genes such as alpha-synuclein, LRRK2 (leucine-rich repeat kinase 2), parkin, PINK1, and DJ-1 affect mitochondrial dynamics, trafficking, autophagy, and quality control.
All cells require mitochondria for their energy demands, including neurons, which are critically dependent on proper mitochondrial function. Neurons have high metabolic activity and rely heavily on mitochondria for their bioenergetic demand. Mitochondrial dysfunction, whether primary or secondary, holds promise as a potential therapeutic target. Aging is the greatest risk factor; thus, with increasing average life expectancy worldwide, the number of people affected by PD will rise considerably in the near future. There is a significant clinical unmet need for new therapeutic approaches that can slow down PD progression and potentially serve as preventive measures for the aging population.
While most earlier studies on PD focused solely on brain pathologies, the gastrointestinal (GI) system is now recognized as an essential source for PD pathogenesis. GI symptoms, such as constipation, affect approximately 80% of PD patients, and idiopathic constipation is an important risk factor. In PD, constipation is associated with alpha-synuclein accumulation in the enteric nervous system, gut inflammation, and increased gut permeability. Moreover, intestinal mucosal inflammation is thought to lead to synuclein accumulation in the enteric nerves, which can then spread in a prion-like fashion to the central nervous system via autonomic connections. Many of these GI tract changes are observed even before the onset of neuronal symptoms.
As the understanding of PD pathogenesis continues to evolve, researchers are working on developing new therapeutic strategies to target various aspects of the disease, including mitochondrial dysfunction, neuroinflammation, and GI tract involvement. These emerging approaches aim to not only slow down the progression of PD but also potentially act as preventive measures for the aging population at risk.
Amyotrophic Lateral Sclerosis (ALS), also known as Lou Gehrig’s disease, is a highly fatal progressive neurodegenerative disease. It is characterized by the significant loss of motor neurons in the primary motor cortex, brainstem, and spinal cord. This loss of motor neurons severely impairs essential movements such as breathing and typically results in death within 2–5 years of diagnosis. The progressive deterioration of motor function disrupts patients’ ability to breathe, often necessitating breathing aids for survival. Additional symptoms include muscle weakness in the hands, arms, legs, and swallowing muscles. Some patients, such as those with frontotemporal dementia (FTD-ALS), may also develop cognitive impairments. Two forms of ALS have been identified: sporadic ALS (SALS), which is the most common form, accounting for 90–95% of all cases in the United States; and familial ALS (FALS), which occurs within families, primarily with dominant inheritance, and comprises about 5–10% of all cases in the country. SALS and FALS are clinically indistinguishable.
Pathological studies have found that various cellular processes are disrupted following disease onset, including increased endoplasmic reticulum (ER) stress, generation of reactive oxygen species (ROS), mitochondrial dysfunction, protein aggregation, apoptosis, inflammation, and glutamate excitotoxicity, specifically in motor neurons.
The causes of ALS are complex and heterogeneous. Generally, ALS is considered a multifactorial genetic disorder involving multiple genes and environmental exposures that increase susceptibility. Over a dozen genes associated with ALS have been discovered including SOD1 (Cu/Zn superoxide dismutase), TDP-43 (TARDBP, TAR DNA-binding protein 43), FUS (Fused in Sarcoma/Translocated in Sarcoma), ANG (Angiogenin), ATXN2 (Ataxin-2), VCP (Valosin-containing protein), OPTN (Optineurin), and an expansion of the noncoding GGGGCC hexanucleotide repeat in the chromosome 9 open reading frame 72 (C9ORF72). However, the precise mechanisms underlying motor neuron degeneration remain unclear. Currently, there is no cure for ALS. The only FDA-approved drug is Riluzole, which antagonizes the glutamate response to slow the disease’s pathological progression. However, it has only been reported to extend the life span of early stage ALS patients by about three months, and no therapeutic benefits for late-stage patients have been observed. This indicates a significant lack of treatment options for patients and highlights the need for a new treatment strategy that can effectively halt disease progression.
ALS and PD are complex neurodegenerative diseases with multifaceted etiologies and pathogeneses. Current treatment options are limited, emphasizing the need for continued research to develop more effective therapies to halt or reverse the progression of these devastating diseases. As our understanding of the genetic, molecular, and environmental factors that contribute to ALS and PD deepens, the hope is that novel therapeutic strategies will emerge to improve the quality of life and survival outcomes for patients affected by these disorders.
The disclosure in patent WO 2023/044076 described compositions, such as microbiome compositions, which can be used to treat various diseases or conditions, particularly those affecting the nervous system, like neurodegenerative diseases. The disclosure explains methods to examine the effects of administering these compositions to a subject, as well as identifying or characterizing the effects and/or modulation of metabolite levels in the subject upon administration. The modulated metabolites might be associated with specific diseases. These technologies can help in detecting differences in metabolite levels in a particular subject or population, for instance, before and after administering the disclosed compositions. Furthermore, the disclosure provides technologies that can be helpful in identifying the nature and impact of the disclosed compositions on specific subjects or populations, thus offering personalized information on treating a disease of the nervous system. In some cases, these technologies can be used to identify subject-specific compositions based on the metabolome in the subject’s samples, and treat or prevent a disease by administering the disclosed compositions to modulate the subject’s metabolome. Consequently, the described technologies may serve as therapeutics and tools for reducing the risk of certain diseases and for treating and/or preventing them.
Examples of neurodegenerative diseases, include Amyotrophic Lateral Sclerosis (ALS), Alzheimer’s Disease (AD), Parkinson’s Disease (PD), and Huntington’s Disease (HD). In certain cases, the focus is on treating or preventing ALS. The subject may be suffering from or at risk of developing one or more neurodegenerative diseases or conditions. The features may include (i) cell viability levels; (ii) nucleic acid or protein levels, activity, or forms; (iii) microgliosis; (iv) astrocytosis; (v) ATP levels; (vi) proteasomal function; (vii) lysosomal function; (viii) oxidative stress; or (ix) inflammation.
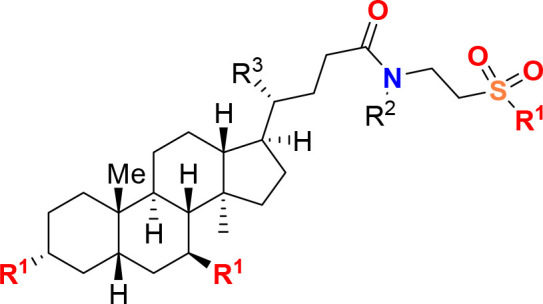
The metabolites can originate from one or more microbial strains, while in other cases, they can be derived from nonmicrobial sources, such as synthetic production, which may include bile acids or specific compounds like tauroursodeoxycholic acid. Examples of such components or metabolites include but are not limited to tauroursodeoxycholic acid, theobromine, propionic acid, picolinic acid, 2-hydroxy-4-methylvaleric acid, urocanic acid, trigonelline, stachydrine, ectine, 5-hydroxylysine, arginine, cholic acid, 2-(4-hydroxyphenyl)propionic acid, N-acetyltryptophan, hydroxyproline, glutamic acid, sarcosine, 5-methoxyindoleacetic acid, indole-3-lactic acid, isovalerylalanine, N-acetylleucine, methionine sulfoxide, N-acetylphenylalanine, proline, and any combination.
The composition can be administered through various routes, including tropically, orally, subcutaneously, intravenously, intramuscularly, intracerebrally, intrathecally, rectally, ophthalmically, intravitreally, or suprachoroidally.
Key Structures
Biological Assay
Mouse model using superoxide dismutase (SOD or SOD1), an enzyme that in humans is encoded by the SOD1 gene, and is implicated in apoptosis and ALS. The ALS-SOD1 transgenic mice exhibits a 4-fold increase in SOD activity and a phenotype resemblance to ALS in humans. In addition, nitric oxide assay, ELISA, and CellTiter-Glo assay.
Biological Data
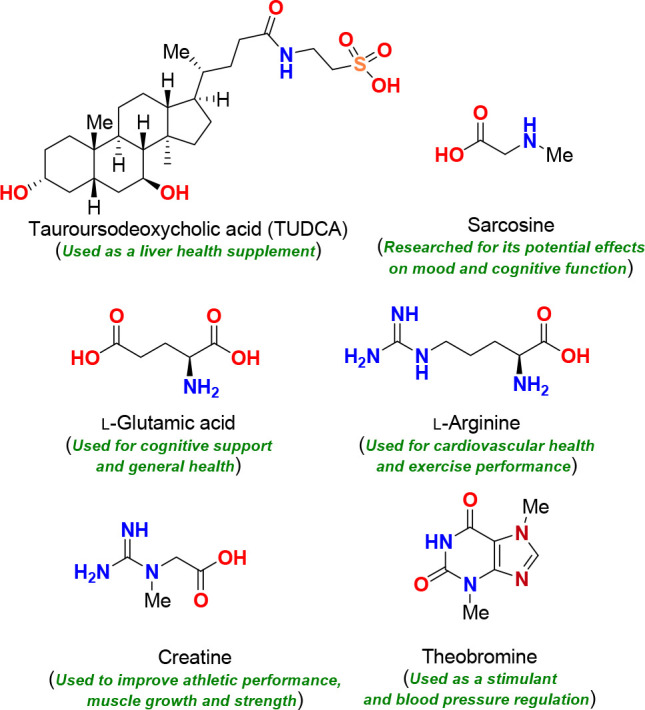
The table below shows exemplary bacterial
strains optimally selected on the basis of their ability to improve
one or more of the target functions: improving mitochondrial function,
resilience to oxidative stress, suppressing neuroinflammation function,
resilience to oxidative stress, suppressing neuroinflammation, M1-M2
microglial polarization, HADC inhibition, and others. Also, *** indicate
high strength, ** indicate medium strength, and * indicate low strength.
Recent Review Articles
References (1−6) present a selection of recent publications.
The author declares no competing financial interest.
References
- Thompson D. S.; Fu C.; Gandhi T.; Fowler J. C.; Frueh B. C.; Weinstein B. L.; Petrosino J.; Hadden J. K.; Carlson M.; Coarfa C.; Madan A. Differential co-expression networks of the gut microbiota are associated with depression and anxiety treatment resistance among psychiatric inpatients. Progress Neuropsychopharmacol. Biol. Psychiatry 2023, 120, 110638. 10.1016/j.pnpbp.2022.110638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A.; Siva Venkatesh I. P.; Basu A. Short-chain fatty acids in the microbiota-gut-brain axis: role in neurodegenerative disorders and viral infections. ACS Chem. Neurosci. 2023, 14, 1045–1062. 10.1021/acschemneuro.2c00803. [DOI] [PubMed] [Google Scholar]
- Yang D.; Huang W.; Wu C. W.; Huang C.; Yang Y. S.; Tung Y. Acute sleep deprivation exacerbates systemic inflammation and psychiatry disorders through gut microbiota dysbiosis and disruption of circadian rhythms. Microbiol. Res. 2023, 268, 127292. 10.1016/j.micres.2022.127292. [DOI] [PubMed] [Google Scholar]
- Modesto Lowe V.; Chaplin M.; Sgambato D. Major depressive disorder and the gut microbiome: what is the link?. Gen. Psychiatry 2023, 36, 100973. 10.1136/gpsych-2022-100973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D.; Spriggs M.; Erritzoe D. Psychedelics therapeutics: What we know, what we think, and what we need to research. Neuropharmacol. 2023, 223, 109257. 10.1016/j.neuropharm.2022.109257. [DOI] [PubMed] [Google Scholar]
- Schellekens H.; Ribeiro G.; Cuesta-Marti C.; Cryan J. F. The microbiome-gut-brain axis in nutritional neuroscience. Nutr. Neurosci. 2022, 1. 10.1080/1028415X.2022.2128007. [DOI] [PubMed] [Google Scholar]


