Abstract
Introduction
Prevention of cardiovascular disease (CVD) is of key importance in reducing morbidity, disability and mortality worldwide. Observational studies suggest that digital health interventions can be an effective strategy to reduce cardiovascular (CV) risk. However, evidence from large randomised clinical trials is lacking.
Methods and analysis
The CV-PREVITAL study is a multicentre, prospective, randomised, controlled, open-label interventional trial designed to compare the effectiveness of an educational and motivational mobile health (mHealth) intervention versus usual care in reducing CV risk. The intervention aims at improving diet, physical activity, sleep quality, psycho-behavioural aspects, as well as promoting smoking cessation and adherence to pharmacological treatment for CV risk factors. The trial aims to enrol approximately 80 000 subjects without overt CVDs referring to general practitioners’ offices, community pharmacies or clinics of Scientific Institute for Research, Hospitalization and Health Care (Italian acronym IRCCS) affiliated with the Italian Cardiology Network. All participants are evaluated at baseline and after 12 months to assess the effectiveness of the intervention on short-term endpoints, namely improvement in CV risk score and reduction of major CV risk factors. Beyond the funded life of the study, a long-term (7 years) follow-up is also planned to assess the effectiveness of the intervention on the incidence of major adverse CV events. A series of ancillary studies designed to evaluate the effect of the mHealth intervention on additional risk biomarkers are also performed.
Ethics and dissemination
This study received ethics approval from the ethics committee of the coordinating centre (Monzino Cardiology Center; R1256/20-CCM 1319) and from all other relevant IRBs and ethics committees. Findings are disseminated through scientific meetings and peer-reviewed journals and via social media. Partners are informed about the study’s course and findings through regular meetings.
Trial registration number
Keywords: PREVENTIVE MEDICINE, Health informatics, Cardiac Epidemiology, Clinical trials
Strengths and limitations of this study.
The randomised controlled design of the study and the enrolment of a large population of participants (n~80 000) recruited in different real-world settings, including general medicine, community pharmacies and Scientific Institute for Research, Hospitalization and Health Care (IRCCS).
The adoption of a coordinated network strategy that also envisages the creation of an Information Technology (IT) infrastructure for communication among health operators.
The collection of biological samples for the multisite biobank of the Italian Cardiology Network, according to specific standard operating procedures for sample collection, storage and transfer.
The lack of standardisation of the equipment used for haematological testing and blood pressure measurement, due to the real-world nature of the study, is a possible limitation of the trial.
Due to the nature of the intervention, the trial personnel and participants are not blinded to the treatment allocation.
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death in developed countries.1 In Italy, there are 136 353 deaths annually attributed to atherosclerotic CVD, with acute coronary syndromes and ischaemic strokes accounting for about 22% of total deaths.2 CVDs are also among the major causes of chronic disability, affecting millions of people worldwide.
CVDs are, to a large extent, preventable. Prevention of CVD is of key importance, not only to reduce morbidity, disability and mortality, but also to increase the years of healthy living among the growing elderly population, thus contributing to alleviate the socioeconomic burden associated with cardiovascular (CV) events. However, according to European data,3 only a small percentage of the healthcare budget is allocated to preventive measures. In this context, there is an urgent need for exploring innovative approaches to better address the challenge of CVD prevention. Internet-based tools and smartphone applications have the potential to play a significant role in CVD prevention by enabling remote lifestyle monitoring, diagnosis, self-management of CV risk factors, medication adherence, education and psychological support. Preliminary evidence suggests that digital health interventions can be an easy-to-implement and cost-effective strategy to reduce CV risk in primary prevention.4 However, more robust evidence is still required, which can only be provided by large controlled trials.
In response to this need, and driven by a specific mandate from the Italian Parliament (Law No. 136, 17 December 2018, and Law No. 145, 30 December 2018), the Italian Cardiology Network (ICN), a network of Scientific Institute for Research, Hospitalization and Health Care (Italian acronym IRCCS) engaged in the CV field promoted by the Ministry of Health, launched the ‘Digital Strategies in Primary Cardiovascular Prevention in the Italian Population (CV-PREVITAL)’ study in 2020.
CV-PREVITAL is a multicentre, prospective, randomised, controlled, open-label interventional trial that aims to compare the effectiveness of an educational and motivational mobile health (mHealth) intervention with that of usual care in primary CV prevention. The main hypothesis of the study is that digital technologies can be used efficiently for improving the control of CV risk factors and detrimental lifestyles, thereby reducing CVD incidence and mortality, compared with usual care. The trial also includes several ancillary studies. The purpose of this report is to provide a comprehensive description of the project background and of the study protocol, which is also publicly available at www.clinicaltrials.gov.
Methods and analysis
The study protocol adheres to ‘The Standard Protocol Items: Recommendations for Intervention Trials 2013 statement’5 (online supplemental file 1).
bmjopen-2023-072040supp001.pdf (118.6KB, pdf)
Trial organisation
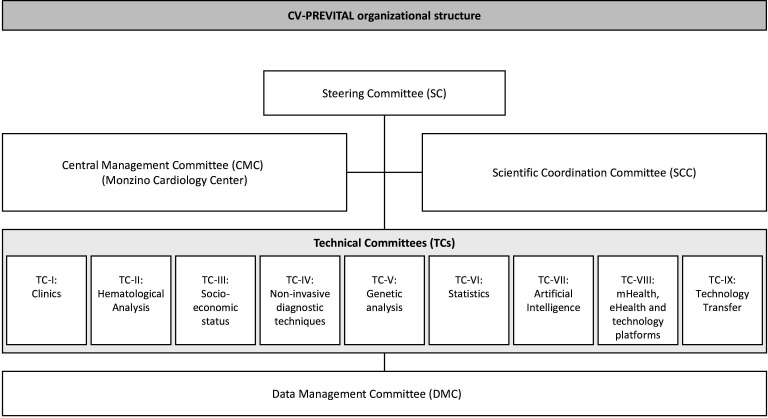
CV-PREVITAL consists of a large clinical trial (the parent study) and of a series of ancillary studies. The organisational structure of CV-PREVITAL is presented in figure 1. The organisational structure includes several integrated committees: the Steering Committee (see also online supplemental table 1); the Central Management Committee (based at the Monzino Cardiology Center) responsible for organising and coordinating the entire study; the Scientific Coordination Committee and nine technical committees (TCs). The TCs are tasked with coordinating specific activities in various areas, including clinics, haematological analysis, socioeconomic status assessment, non-invasive diagnostic techniques, genetic analysis, statistics, artificial intelligence, mHealth, eHealth, technology platforms and technology transfer.
Figure 1.
CV-PREVITAL organisational structure.
bmjopen-2023-072040supp006.pdf (2MB, pdf)
The list of operative units and their role in the study are shown in online supplemental table 2. A part from the Institute of Pharmacological Research Mario Negri IRCCS, which acts as the monitoring centre for the cohort of subjects recruited by general practitioners (GPs), all other IRCCSs participate as recruiting centres. The working group also includes Consorzio Sanità (Co.S.), a consortium of cooperatives of GPs working in the National Health Service.
Study data are collected and managed using REDCap electronic data capture tools hosted at the Consortium of Bioengineering and Medical Informatics.6 7
Trial design
CV-PREVITAL is a multicentre, prospective, parallel-arm, randomised, open-label interventional study. It aims to recruit approximately 80 000 participants (aged ≥45 years) nationwide. Of these, 50 000 subjects are selected among those who daily access the participanting GPs offices. Additionally, to evaluate the effectiveness of the mHealth intervention in settings other than primary care, several specific cohorts are enrolled by IRCCSs (online supplemental table 2). These cohorts consist of approximately 34 000 subjects from outpatient clinics, diagnostic centres, blood donor centres, company cohorts, the general population and pharmacies. The actual study start date (first participant enrolled) is 10 June 2022; the estimated completion date is June 2029.
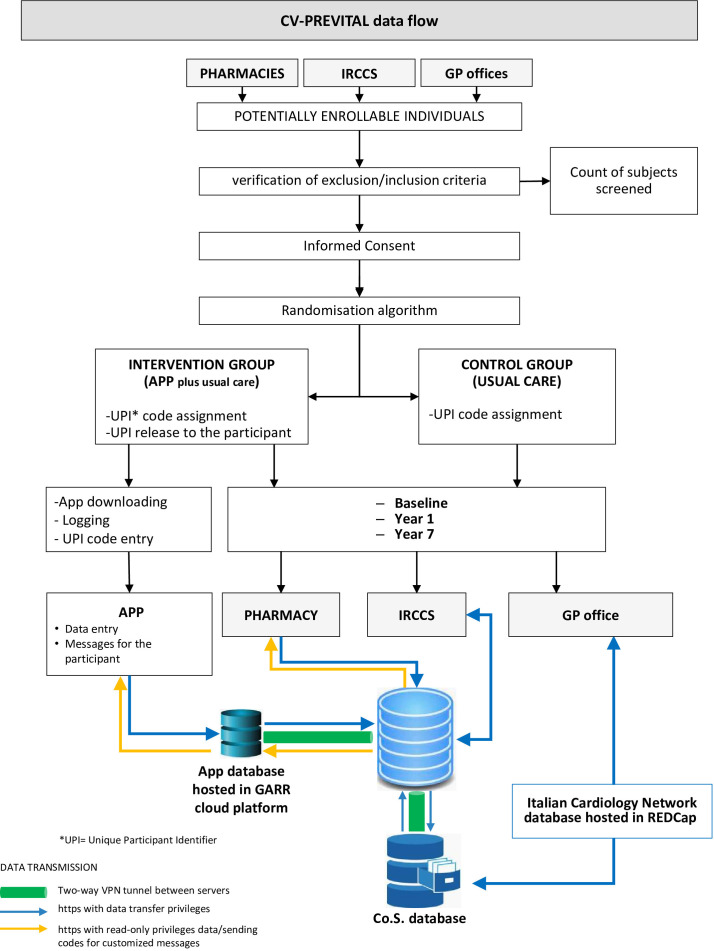
The full list of study sites is available on ClinicalTrials.gov. A structured summary of the trial based on the WHO Trial Registration Data Set is provided in online supplemental file 2. The data flow of CV-PREVITAL is illustrated in figure 2.
Figure 2.
CV-PREVITAL data flow. Co.S., Consorzio Sanità; GARR, Gruppo per l'Armonizzazione della Rete della Ricerca; GP, general practitioner; IRCCS, Scientific Institute for Research, Hospitalization and Health Care (Istituto di Ricovero e Cura a Carattere Scientifico in Italian language); VPN, Virtual Private Network.
bmjopen-2023-072040supp002.pdf (130.2KB, pdf)
Eligibility
Participants of both sexes are eligible to participate in the study if they meet the following criteria: (a) they are in primary CV prevention, (b) they are ≥45 years old, (c) they possess a smartphone and (d) they have provided their informed consent by signing the relevant documents. Individuals who meet any of the following conditions are not eligible for the study: (a) informed consent not signed, (b) age lower than 45 years, (c) history of overt CVD (myocardial infarction (MI), angina pectoris, stroke, transient ischaemic attack (TIA), aortic aneurysm or arteriopathy obliterating lower limb pathologies, or congestive heart failure (NYHA Class III–IV)). Prior to randomisation, eligible participants are asked by the study investigators to sign the informed consent forms (online supplemental file 3). To promote participation across all recruitment settings, leaflets and posters promoting the study and explanatory videos emphasising the importance of proper management of CV risk factors have been realised. The number of screened individuals who are deemed ineligible is centrally recorded in the ICN database.
bmjopen-2023-072040supp003.pdf (474.5KB, pdf)
Randomisation
To assess the effectiveness of the intervention, the participants are allocated randomly in a 1:1 fashion into two groups: (1) the control group, receiving conventional care (usual care); and (2) the intervention group, receiving mHealth intervention in addition to usual care (mHealth group). Randomisation is carried out in three different ways depending on whether the participants are enrolled by GPs, by community pharmacies or IRCCSs. For participants enrolled by GPs, the randomisation procedure ensures that the number of physicians assigned to the control group is balanced with the number assigned to the intervention group within each group practice (referred to as Centro Sanitario Polifunzionale). For participants enrolled by community pharmacies, the randomisation procedure ensures that the number of pharmacies assigned to the control group is balanced with the number assigned to the intervention group in each geographic area. For participants enrolled by IRCCSs, the procedure directly randomises the participants themselves. The randomisation process is carried out by means of a central randomisation service developed in-house. Allocation concealment is ensured, as the randomisation code is not revealed until the participant is recruited and baseline measurements are completed. Additional details are provided in the online supplemental material.
Intervention
At baseline, participants allocated to the mHealth group receive, in addition to usual care, a smartphone application (CV-PREVITAL app) designed for managing a personalised primary CV prevention programme. The app serves the following purposes: (a) education on CV risk, remote monitoring and self-management of CV risk factors, (b) education on and remote monitoring and self-management of psycho-behavioural variables and (c) detection and/or modification of harmful lifestyles. The CV risk factors monitored by the app include high blood pressure, dyslipidaemia, diabetes mellitus, obesity, abdominal obesity and sleep disorders. Psycho-behavioural variables include stress, depression, anxiety and factors related to aspects of the human sphere relevant for patients’ empowerment, such as risk propensity, self-efficacy and locus of control. Harmful lifestyles include unhealthy diet, excessive alcohol intake, smoking habits and a sedentary lifestyle. The app is organised in several educational sections and tools for monitoring the variables under consideration, allowing participants to track their progress over time. It delivers personalised educational contents and provides guided access to different sections based on the participant’s profile (eg, subjects with hypertension or hypercholesterolemia or diabetes and so on). Profiling is performed through specific algorithms according to data collected at baseline (see below), which are recorded in a pseudonymised form on the ICN platform database. Lifestyle monitoring is carried out through active participation, where participants periodically provide information relevant to their health, such as dietary habits, assumption of medications, specific anthropometric parameters, sleep quality and level of physical activity practiced. The app may integrate with native wellness apps to automatically track step counts and sleep duration with the user’s permission. The app also provides reminders, personalised motivational feedback and evaluation of tasks and goal achievements on a periodic basis. These elements are implemented using a gamification logic,8 that is, an approach that seeks to create experiences reminiscent of gaming and that implies not only a combination of concepts such as rewards (eg, points, achievement badges and challenges), but also the use of narrative storylines, avatar-based self-representation and onboarding tutorials. Gamification logic has been proposed for a twofold purpose: to make study participation and data compilation tasks more enjoyable, and to encourage long-term commitment to tasks that may be perceived as boring or demotivating over time. The ultimate goal is to help users complete the required tasks, improve health literacy and adhesion to healthy behaviours and/or maintaining healthy habits. Data collected through the app during the follow-up period are transmitted to the ICN database. This allows the treating doctor to access the information and personalise further prevention activities based on the collected data.
Control group
Participants allocated to the control group are followed by the conventional approach (usual care) based on regular visits respecting the usual schedule dictated by the rules of general practice. As a part of the baseline assessment, they receive counselling and are encouraged to maintain or improve their current physical activity level, dietary habits, medical adherence and so on depending on their individual goals and needs.
Hypotheses and outcomes
The primary hypothesis of the trial is that a personalised intervention of CV primary prevention based on mHealth technology can be more effective than usual care in controlling conventional risk factors and harmful lifestyles in the short term and in reducing vascular events in the long term. The primary outcome used to measure the efficacy of the mHealth intervention at short term (12 months) is the change in a risk score developed specifically for the Italian population. The score, referred to as the ‘modified Moli-Sani score’ (details in online supplemental material), was created by analysing the combined impact of different modifiable risk factors on the risk of developing CVD during the follow-up of the Moli-Sani study, which collects data from the general population of Molise, a region in south-central Italy.9 10 An improvement of one unit (approximately 33% reduction) in the modified Moli-Sani score between the baseline and final assessment in the intervention group (App), compared with the score change observed in the control group (Usual care), is indicative of a clinically meaningful intervention effectiveness in the short term. This is because, according to the construction of the Moli-Sani risk score, a one-point improvement in the Moli-Sani risk score is equivalent (in terms of CV risk) to an increase of 1 year of age. The primary outcome used to measure the efficacy of the mHealth intervention in the long term (7 years) includes major adverse cardiovascular events (MACE), that is, CV death, MI, stroke, TIA, peripheral artery disease, new diagnoses of angina, hospitalisations for CVD and need for revascularisation.
Several short-term secondary outcomes are also pre-specified. These include: (a) a combined endpoint including the simultaneous change in hypertension, diabetes and hypercholesterolemia; (b) the change in at least one of the risk factors considered in the score; (c) the percentage of subjects who agree to complete questionnaires; (d) the percentage of subjects who interrupts the use of the app during the follow-up and (e) adherence to recommended therapies.
Besides these clinical outcomes, other outputs of the project include the development and validation of a new algorithm for estimating CV risk, the estimation of the costs and effectiveness of the intervention and the identification of new socioeconomic and behavioural risk factors.
Measurements performed at baseline and follow-up
At baseline, subjects identified as potentially eligible for recruitment receive information material and consent forms for study participation. Those who agree to participate are invited to complete a series of questionnaires. Questionnaires can be completed in two ways: (a) on-site by a direct access to an electronic ‘Case Report Form’ (eCRF), with the assistance of a healthcare professional; or (b) remotely via web access to the eCRF, with the help of computer tutorials or phone assistance, after having provided a digital informed consent and using a secure access. The remote option was provided to cope with the limitations due to the COVID-19 pandemic, which required social distancing to limit the spread of SARS-CoV-2.
Self-report questionnaires administered at baseline cover the following areas:
Family and personal history of diseases (cardiovascular and cerebrovascular disease; metabolic disease).
Ethnicity, socioeconomic status and marital status.
Smoking habits.
Alcohol consumption (PREDIMED questionnaire).11
Adherence to Mediterranean diet (PREDIMED questionnaire)11 and Moli-Sani questionnaire—an adaptation of the MEDAS questionnaire.12
Salt consumption (MiniSal questionnaire).13
Physical activity (IPAQ—International Physical Activity Questionnaire).14
Personal history of sleep disorder and sleep quality (PSQI—Pittsburgh Sleep Quality Index).15
-
Psycho-behavioural factors:
9.1 Perceived stress (PSS—Perceived Stress Scale).16
9.2 Anxiety and depression (PHQ 4 questionnaire).17
9.3 Self-efficacy (GSE—General Self-Efficacy Scale).18
9.4 Locus of control (Multidimensional Health Locus of Control Scale).19
9.5 Risk propensity (RPS—Risk Propensity Scale).20
Personal history of COVID-19.
Baseline evaluation is completed by healthcare professionals (nurses, physicians or pharmacists) with the collection of the following data: (a) ongoing pharmacological treatments (chronic therapies); (b) personal history of organ damage from diabetes and hypertension; (c) measurements of anthropometric parameters (weight, height, body mass index, waist circumference,21 blood pressure and heart rate) and (d) biochemical variables (total, low-density lipoprotein and high-density lipoprotein cholesterol, triglycerides and glycated haemoglobin) assessed by point-of-care testing or by standard laboratory methods. Based on these data, by using validated algorithms a series of risk scores are estimated, including scores assessing the risk of developing metabolic diseases such as diabetes (Findrisc),22 and hypertension,23 and a score assessing the risk of developing vascular events (the modified Moli-Sani score). Other risk algorithms, such as those developed within the ‘Progetto Cuore’ framework,24 the European and the American risk algorithms (ie, SCORE-Risk25 and Framingham Risk Score,26 respectively) and the ASCVD (ie, the score proposed within the American College of Cardiology/American Heart Association Task Force on Practice guidelines),27 are also calculated for comparison.
At the 12-month follow-up, participants are invited to return to the recruitment centre to complete the baseline questionnaires again and to repeat the anthropometric and biochemical measurements made during the first assessment. At the 7-year follow-up, participants are contacted to monitor the occurrence of new MACE. In the case of fatal events, information is obtained by contacting the participant’s family.
All follow-up visits adhere to routine clinical practice for CV prevention. Reasons for discontinuation are documented using a dedicated eCRF form.
A schematic diagram illustrating the data collected at the three time points of the study protocol (baseline, 12 months and 7 years) is shown in online supplemental file 4.
bmjopen-2023-072040supp004.pdf (82.4KB, pdf)
Sample size
The sample size has been calculated based on the long-term endpoint (ie, incidence of CV events). Using data from the IMPROVE study, which included approximately 1000 Italians (around 50% men and 50% women) aged 55–79 years with a 7-year follow-up (Italian groups of the IMPROVE study),28 the annual incidence of MACE was estimated. With an assumed incidence rate of 0.0116 /year, it was projected that with a sample size of 50 000 participants, with an approximately equal distribution of men and women, a total of 3921 events would occur over 7 years. This sample size was determined to be sufficient to detect as statistically significant (alpha=0.05) and with a power of 80% an 8.5% reduction in MACE incidence in the intervention group compared with the usual care group, with an HR of 0.915. Additionally, on the basis of the data of the Moli-Sani study, which included 21 806 subjects with a median follow-up of 8.2 years and 862 events, the incidence rate is 0.0048 and the expected number of events in the CV-PREVITAL population is 1687. This sample size provides a power of 80% to detect as significant (alpha=0.05) a 12.8% reduction in MACE incidence in the intervention group compared with the usual care group, with an HR of 0.872. Based on past experience of prevention studies with very long follow-ups (≥5 years), for example, IMPROVE, the number of lost at follow-up is particularly high (even >50%). In order to take account of such a potentially high rate of loss to follow-up, the calculated sample size (n=50 000) was increased by approximately 60% to a final number of ~80 000. It is worth noting that being calculated on the vascular events at 7 years, this sample size yields a very high power (>95%) to detect even very small differences in short-term endpoints, in both risk scores and single risk factors (eg, <1 mg/dL for total cholesterol and blood glucose, and 1 mm Hg for systolic blood pressure). So, all the results obtained derived from a sample sufficiently powered (1−β=80%) to perform also sex stratified analyses. Results obtained from different cohorts, such as those enrolled by GPs and various IRCCSs, are combined using a meta-analytic approach to ensure a comprehensive analysis.
Statistical analysis
Continuous data will be presented as means and SDs and as medians and IQRs, categorical data as frequencies and %.
Three classes of pre-specified statistical analyses have been planned. The first class involves analysing variables collected at enrolment to estimate the baseline prevalence of different risk factors and conditions among the recruited cohorts. The second class focuses on variables collected at the end of the short-term follow-up (12 months) to assess the effectiveness of the intervention (App vs Usual care) on CV risk. The third class of analyses pertains to variables collected at the end of the long-term follow-up (7 years) to assess the effectiveness of the intervention on the incidence of fatal and non-fatal CV events.
Cross-sectional analyses on data collected at baseline will be carried out using logistic regression and general linear models (GLMs). The short-term primary endpoint, that is, the change in CV risk score in the two treatment arms, will be analysed with GLMs adjusting for potential confounders possibly unbalanced between the two groups. Secondary endpoints, that is, changes in the level of individual risk factors, will be analysed with GLMs and Bonferroni correction will be applied to account for the number of tests performed. The long-term primary endpoint, that is, the incidence of fatal and nonfatal CV events, will be analysed by Cox regression models adjusting for potential confounders. As long-term secondary endpoints, new risk algorithms will be developed using Cox models and validated using Receiver-operating characteristic (ROC) curve analysis and reclassification techniques. Results generalisability will be tested with cross-validation approaches. Subgroup analyses stratified by gender are also planned.
Missing outcomes for the primary endpoint will be imputed using multiple imputation, and a sensitivity analysis on the imputed data will be performed. Drop-outs will not be replaced.
The efficacy analyses will be performed according to the intention to treat principle on the full analysis set (ITT analysis). A sensitivity analysis will also be performed in the population with adherence to protocol (Per-protocol (PP) analysis).
Cost-effectiveness assessment
In the assessment of cost-effectiveness for different screening scenarios, the economic aspects of digital-health interventions are recognised as complex due to their nature as ‘complex interventions in a complex system’. Instead, in the intervention involving health professionals the costs are relatively limited and include: (a) cost of implementing and mainteining the Information Technology (IT) platform required for the intervention; (b) cost for developing the smartphone application that, once implemented, has virtually negligible installation costs and (c) cost of the time spent by health professionals for training, using the IT platform, and engaging and educating the participants. For an analytical evaluation of the economic aspects of the intervention, two approaches are applied: cost/efficacy analysis (CEA) and cost/utility analysis (CUA). CEA is the simplest and most frequently used form of evaluation in health economics. It aims to estimate the relationship between the costs of the resources used and the effectiveness achieved through their use. Effectiveness is estimated using a single indicator in two ways: first, as the number of participants who achieve the target in the main risk factors (hypertension, diabetes and hypercholesterolemia); and second, as the number of CV events (both fatal and non-fatal) avoided during the 7-year follow-up, relative to the incurred costs. CUA, on the other hand, considers not only the duration, but also the quality of life that participants experience as a result of the intervention. Quality-adjusted life years (QALYs) are used as summary measures to comprehensively assess the overall health and well-being of the individuals. To estimate QALYs, validated instruments such as WHO Quality Of Life or similar tools will be used and administered during the follow-up period.
Web-based trial management
To establish an effective communication network between GPs and physicians from the IRCCSs, all the data collected are stored in the IT platform of the ICN, as shown in figure 2. This platform is integrated with the Co.S. IT platform, which is the interface used by GPs participating in the study (figure 2). Additionally, the ICN database communicates with the mHealth interface (App for smartphones) dedicated to the population, which serves for both educational purposes and additional data collection (figure 2).
To ensure data protection and comply with security recommendations specified by the National Institute of Standards and Technology29 and European data protection regulations,30 various measures have been implemented. These measures are designed to safeguard the confidentiality, integrity and availability of the collected data. A detailed description of the web-based system for data management and the specific data protection measures implemented is provided in the online supplemental material.
Staff training, standard operating procedures (SOPs) and quality control
A detailed description of models for staff training, standard operating procedures (SOPs; available on request) and quality control activities is reported in the online supplemental material.
Ancillary studies (CV-PREVITAL sub-studies)
CV-PREVITAL also includes a series of ancillary studies that are conducted by the various IRCCSs already participating in the parent study. Ancillary studies are designed to evaluate a series of additional risk biomarkers in groups or selected sub-groups included in the parent study. A detailed description of the specific variables evaluated in each ancillary study is reported in the online supplemental material.
The steering committee reviewed all the ancillary study protocols to ensure that the specific objectives did not duplicate or interfere with those of the parent study and that all the adopted procedures were consistent with those established in the main protocol. Beyond the specific aims of single sub-studies, a particularly relevant goal, common to all the sub-studies, is the collection of biological samples (eg, serum, plasma, DNA or RNA) for the multisite biobank of the ICN. For this purpose, the research consortium developed specific SOPs for collection, storage and samples transfer, for example, towards centres acting as core lab. A brief description of the plan for collection, processing and storage of biological specimens for genetic or molecular analysis in the current trial and for future use in ancillary studies is provided in online supplemental file 5. A biobank informed consent is obtained to specifically address the collection of these samples.
bmjopen-2023-072040supp005.pdf (105.5KB, pdf)
Patient and public involvement
Patients and/or the public are not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Ethics and dissemination
The CV-PREVITAL study has been approved by all relevant IRBs and ethics committees. Their list and the study approval number-IDs are provided in online supplemental table 3. Any protocol amendment is promptly reported to all relevant parties (namely, investigators, ethical committees/IRBs, ClinicalTrials.gov). Personal data are processed in compliance with the provisions set out in Regulation (UE) 2016/679 (the ‘GDPR’) and in Legislative Decree. 196/2003 (Personal Data Protection Code, added by the Legislative Decree 101/2018). Personal information is available to researchers using password-protected files. In addition, all data for presentations are anonymised and aggregated, so the participants’ identity is not revealed in any way. CV-PREVITAL results will be disseminated at conferences, through publication in peer-reviewed journals and through other channels (eg, web sites of the ICN and of all the hospital involved, social media) in order to reach a diverse community of researchers, GPs, pharmacists and other stakeholders, including citizen and policymakers.
Discussion
To the best of our knowledge, this is the first randomised, controlled trial designed to evaluate the effects of an individualised digital intervention delivered through an app containing tools for education on CV risk, remote monitoring and self-management of CV risk factors, detection and/or modification of harmful lifestyles, and patient empowerment.
Preliminary data show that smartphone applications might actually have a great potential in the remote monitoring and self-management of CV risk factors and in improving therapy adherence in hypertensive, diabetic and dyslipidemic patients.31 32 However, more evidence is needed to confirm their effectiveness in primary CV prevention programmes. CV-PREVITAL, by collecting information in a prospective, randomised and controlled way on a large-scale, has the potential to provide strong evidence to support policy makers in making informed decisions about strategic planning and resources allocation in primary CV prevention.
If the proposed intervention proves to be workable and successful, the study will provide robust evidence that digital medicine can be a useful strategy to engage, motivate and empower people towards primary prevention of CVD. In addition, due to the large sample size and the different types of cohorts involved, the study has also the potential to generate reliable evidence for implementing digital technology-based CV primary prevention programmes not only in general or specialist medicine, but also in other settings such as occupational medicine, blood donors centres and community pharmacies.
A significant strength of the CV-PREVITAL study design is the adoption of a coordinated network strategy involving IRCCSs with proven experience in primary prevention programmes, epidemiology and biomedical statistics, along with a large number of GPs spread throughout the national territory. We expect that such strategy, which also envisages the creation of an IT infrastructure for communication among GPs and IRCCSs, may provide the basis for their permanent collaboration, increase the opportunity for future real-world research and enhances knowledge transfer among healthcare professionals.
The participation of a large number of pharmacies is another significant strength, considering their wide distribution and frequent access by citizens, which makes them capable of taking an active role as an outpost of the national health systems for the delivery of health services and the implementation of primary prevention programmes. In this regard, it is worth mentioning that it is estimated that approximately 4 000 000 people enter the ~20 000 pharmacies existing in Italy every day.33
Another important strength of the study is that it allows to make inference on (a) the level of adherence to the digital prevention programmes, (b) the rate of drop-outs associated with this type of programmes in different cohorts and in different age and sex classes and (c) the barriers to participation based on participants’ feedbacks. These insights are crucial for decision makers to understand and address barriers that can hinder the successful implementation of digital health in primary prevention of CVD, including digital literacy, internet access, concerns about privacy and data security, and perceptions of digital approaches’ usefulness.
A last important aspect of the CV-PREVITAL study is that it was designed in the pre-COVID-19 era, but is in fact being carried out during the pandemic. While this presented challenges, it also provided new opportunities to highlight the usefulness of digital tools and accelerate their adoption in remote monitoring and CVD management worldwide.34
A possible limitation of the study is the lack of standardised equipment for haematological testing and blood pressure measurement. However, blood pressure measurement devices validated according to internationally acknowledged validation protocols are used.35 Although aware of the inevitable increase in variability of measurements associated to this type of choice, the decision was made to obtain data better reflecting what happens in real-world prevention programmes.
Overall, the CV-PREVITAL study holds promise to contribute significant evidence and insights into the effectiveness and implementation of digital interventions in primary CV prevention.
Supplementary Material
Acknowledgments
This manuscript is dedicated to the memory of Professor Alberto Zanchetti, who passed away on 24 March 2018, and Professor Luigi Rossi Bernardi, who passed away on 16 December 2019, whose thoughts and experiences have deeply inspired the study. The authors wish to thank Ms Manuela Pettignano (Centro Cardiologico Monzino IRCCS) for her valuable support in preparing the manuscript.
Footnotes
Collaborators: CV-PREVITAL Study Group: Giulio Pompilio, Damiano Baldassarre, Roberta Baetta, Mauro Amato, José Pablo Werba, Stefano Genovese, Gualtiero Colombo, Chiara Vavassori, Maria Luisa Biondi, Beatrice Frigerio, Alessio Ravani, Daniela Sansaro, Daniela Coggi, Alessandra Romandini, Monica Giroli, Mattia Giuliani, Alice Bonomi, Maurizio Rondinelli, Catia Trudu, Carmen Cinieri, Massimo Monturano, Francesca Colazzo (Centro Cardiologico Monzino IRCCS). Gianfranco Parati, Cecilia Invitti, Grzegorz Bilo, Martino Pengo, Francesca Gorini, Luciana Auteri, Andrea Faini, Carolina Lombardi, Anna Di Blasio, Debora Rosa, Camilla Torlasco, Luisa Gilardini, Davide Soranna, Antonella Zambon, Elisa Perger, Lucia Zanotti, Luigi Badano, Lidia Cova, Davide Gentilini, Luca Grappiolo (Istituto Auxologico Italiano IRCCS). Gianluigi Condorelli, Giuseppe Ferrante, Laura Papa, Victor Savevski, Francesca Ieva, Ignazio Romano (IRCCS Humanitas Research Hospital). Giuseppe Remuzzi, Maria Carla Roncaglioni, Marta Baviera, Luisa Ojeda, Andreana Foresta, Fiorenza Clerici, Angela Palumbo (Istituto di Ricerche Farmacologiche Mario Negri IRCCS). Gian Franco Gensini, Giuseppe Ambrosio, Alberico Catapano, Roberto Mattioli, Ermanno Longhi, Livio Luzi, Lorenzo Giovanni Mantovani, Fabiana Madotto (IRCCS MultiMedica). Luigi Frati, Licia Iacoviello, Augusto Di Castelnuovo, Alessandro Gialluisi, Amalia De Curtis, Marialaura Bonaccio, Francesco Gianfagna, Anwal Ghulam, Sara Magnacca, Fabrizia Noro, Simona Costanzo, Simona Esposito, Sabatino Orlandi, Mariarosaria Persichillo, Francesca Bracone, Teresa Panzera, Emilia Ruggiero, Roberta Parisi, Sabrina Franciosa, Martina Morelli, Fiorella De Rita, Chiara Cerletti, Giovanni de Gaetano, Maria Benedetta Donati (IRCCS Istituto Neurologico Mediterraneo NEUROMED). Lorenzo Menicanti, Serenella Castelvecchio, Alexis Elias Malavazos, Massimiliano Marco Corsi Romanelli, Rosanna Cardani, Ambra Cerri, Carola Dubini, Manuel Bruno Trevisan, Laura Valentina Renna, Valentina Milani, Sara Boveri, Paola Giubbilini, Lucia Ramputi, Irene Baroni, Giada De Angeli (IRCCS Policlinico San Donato). Walter Ricciardi, Maria Teresa La Rovere, Simonetta Scalvini, Antonia Pierobon, Alessandra Gorini, Francesca Olmetti, Maurizio Bussotti, Carlo Gaetano, Paola Baiardi, Tiziana Bachetti, Martina Balbi, Laura Comini, Monica Lorenzoni, Adriana Olivares, Egidio Traversi, Camilla Garrè, Riccardo Sideri (Istituti Clinici Scientifici Maugeri IRCCS). Pier Giulio Conaldi, Valentina Agnese, Francesco Clemenza, Giovanni Gentile, Giuseppe Caruana, Nicola Cuscino, Gabriele Di Gesaro, Alessio Greco, Italia Loddo, Fabio Tuzzolino (IRCCS ISMETT (Mediterranean Institute for Transplantation and Advanced Specialized Therapies)). Antonio Uccelli, Bianca Pane, Domenico Palombo, Giovanni Spinella, Gaddiel Mozzetta, Pietro Ameri, Gabriele Zoppoli, Alice Finotello, Italo Porto, Giovanni Pratesi (IRCCS Ospedale Policlinico San Martino). Fabio Blandini, Daniele Prati, Luca Valenti, Laura Spinardi, Margherita Clerici, Serena Pelusi, Cristiana Bianco, Rossana Carpani, Giulia Periti, Sara Margarita (Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico). Giovanni Scambia, Giovanna Liuzzo, Gaetano Antonio Lanza, Anna Severino, Daniela Pedicino, Domenico D’Amario, Alessia D’Aiello, Ramona Vinci, Alice Bonanni, Mattia Brecciaroli, Simone Filomia, Roberta Pastorino, Stefania Boccia, Andrea Urbani, Maurizio Sanguinetti, Angelo Santoliquido, Luca Proto, Dalila Tarquini, Maria Chiara Grimaldi (Fondazione Policlinico Universitario Agostino Gemelli IRCCS). Eloisa Arbustini, Catherine Klersy, Sergio Leonardi, Arianna Elia, Alessia Currao, Mario Urtis, Alessandro Di Toro, Lorenzo Giuliani (Fondazione IRCCS Policlinico San Matteo). Massimo Fini, Maurizio Volterrani, Giuseppe Caminiti, Federica Marcolongo, Barbara Sposato, Fiorella Guadagni, Valentina Morsella, Angelica Marziale (IRCCS San Raffaele). Antonio Di Malta, Marco Visconti (Consorzio Sanità (Co.S.)). Annarosa Racca, Manuela Bandi, Giulia Protti (Federfarma Lombardia & Fondazione Guido Muralti).
Contributors: GPo, DB, RB, MA, PW, SG, GCol, CV, MLB, BF, ARa, DSa, DC, ARo, MGir, MGiu, ABono, MR, CTr, CCi, MMon, FCo, GPa, CI, GB, MPen, FGo, LA, AFa, CL, ADiB, DR, CTo, LGi, DSo, AZ, EP, LZ, LB, LCov, DG, LGr, GCon, GF, LPa, VS, FI, IR, GR, MCR, MBav, LOF, AFo, FCler, APa, GFG, GA, ACa, RM, EL, LL, LGM, FMad, LF, LI, ADiC, AGi, ADeC, MBo, FGi, AGh, SMag, FN, SCo, SE, SO, MPer, FBr, TP, ER, RPar, SFr, MMor, FD, CCe, GDeG, MBD, LM, SCa, AEM, MMC, RCard, ACe, CD, MBT, LVR, VMi, SBov, PG, LR, IB, GDeA, WR, MTLR, SS, APi, AGo, FO, MBu, CGae, PB, TB, MBal, LCom, ML, AO, ET, CGar, RS, PGC, VA, FClem, GG, GCar, NC, GDiG, AGr, IL, FT, AUc, BP, DPa, GSp, GM, PA, GZ, AFi, IP, GPra, FBl, DPr, LV, LS, MC, SP, CB, RCarp, GPe, SMar, GSc, GL, GAL, ASe, DPe, DD, AD’Ai, RV, ABona, MBr, SFi, RPas, SBoc, AUr, MS, ASa, LPr, DT, MCG, EA, CK, SL, AE, ACu, MU, ADiT, LG, MF, MVo, GCam, FMar, BS, FGu, VMo, ADM, ADiM, MVi, ARac, MBan, GPro, ETre, FV, SBos substantially contributed to the conception of the work and final approval of the version to be published. DB, GPa, CI, GB, MPen, GCon, GF, MCR, GFG, GA, ACa, LF, LI, ADiC, LM, MTL, PGC, GL, EA, MVo, ADiM, MVi, ETre, FV contributed to the design of the work. DB, RB, MA, SG, GCol, CV, MLB, BF, ARa, DSa, DC, ARo, CTr, CCi, GB, MPen, FGo, ADiB, DR, GF, LPa, IR, ACa, ADeC, SMag, SCo, MPer, FBr, TP, ER, RPar, SCa, RCard, CD, LVR, LR, GDeA, MTL, SS, TB, MBal, LCom, ML, AO, ET, CGar, RS, VA, GDiG, GM, PA, DPr, LV, SP, CB, RCarp, GPe, SMar, GL, ASe, DPe, AD’Ai, LPr, DT, MCG, EA, SL, AE, ACu, MU, ADiT, MVo, FGu, VMo, GPro contributed to the acquisition of data. DB, MA, ABono, DSo, GF, FI, LGM, ADiC, VMi, SBov, PB, FT, RPas, SBoc, CK, FV contributed to the statistical advice. DB, RB, ABono, MPen, GF, MCR, ACa, SCa, MTL, BP, DPr, LV, GL, EA, MVo, FV contributed to the drafting of the work. GPo, DB, RB, MA, PW, DC, ABono, GPa, MPen, FGo, GCon, GF, MCR, MBav, LOF, AFo, ACa, LI, ADiC, SCa, MTL, AGo, VA, AUc, BP, GM, DPr, LV, GL, EA, CK, MVo, FV contributed to revising the work critically for important intellectual content. DB, RB, MA contributed to the agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GPa and GPo are joint last authors.
Funding: The CV-PREVITAL study is carried out through funding by the Italian Ministry of Health (Ministero della Salute), Project Code RCR-2019-23669116_001. The finantial support includes the cost of developing the app used in the study. The app was built with the technical collaboration of a mobile solutions provider (YouCo s.r.l.) selected through a European tender carried out under Article 60 of Legislative Decree 50/2016 as amended. Ownership of the app is held by the Italian Cardiology Network. Funding source (Italian Ministry of Health) and technical partner (YouCo) are not involved in study execution, data analyses and interpretation, or decision to submit results.
Competing interests: Authors and collaborators disclosed the relationships/activities/interests reported below. Grants or contracts from any entity: GSc, ACa, DPr, PW, ADiC, LV, LB, FCler, APa, LGM; Consulting fees: GSc, GL, SG, LV, LL, LGM, SL, IP; Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: GSc, GA, ACa, DPr, GL, PW, SG, LV, LL, GPa, LB, LGi, EP, PA; Support for attending meetings and/or travel: DPr, LV, LL, EP, PA; Patents planned, issued or pending: LL, CGae; Participation on a Data Safety Monitoring Board or Advisory Board: GA, DPr, SG, LV, LL, LB, PA; Leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: GA, LV; Receipt of equipment, materials, drugs, medical writing, gifts or other services: LB, PA. Details are available in the individual ICMJE online disclosure forms (available upon request).
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
CV-PREVITAL Study Group:
Giulio Pompilio, Damiano Baldassarre, Roberta Baetta, Mauro Amato, José Pablo Werba, Stefano Genovese, Gualtiero Colombo, Chiara Vavassori, Maria Luisa Biondi, Beatrice Frigerio, Alessio Ravani, Daniela Sansaro, Daniela Coggi, Alessandra Romandini, Monica Giroli, Mattia Giuliani, Alice Bonomi, Maurizio Rondinelli, Catia Trudu, Carmen Cinieri, Massimo Monturano, Francesca Colazzo, Gianfranco Parati, Cecilia Invitti, Grzegorz Bilo, Martino Pengo, Francesca Gorini, Luciana Auteri, Andrea Faini, Carolina Lombardi, Anna Di Blasio, Debora Rosa, Camilla Torlasco, Luisa Gilardini, Davide Soranna, Antonella Zambon, Elisa Perger, Lucia Zanotti, Luigi Badano, Lidia Cova, Davide Gentilini, Luca Grappiolo, Gianluigi Condorelli, Giuseppe Ferrante, Laura Papa, Victor Savevski, Francesca Ieva, Ignazio Romano, Giuseppe Remuzzi, Maria Carla Roncaglioni, Marta Baviera, Luisa Ojeda, Andreana Foresta, Fiorenza Clerici, Angela Palumbo, Gian Franco Gensini, Giuseppe Ambrosio, Alberico Catapano, Roberto Mattioli, Ermanno Longhi, Livio Luzi, Lorenzo Giovanni Mantovani, Fabiana Madotto, Luigi Frati, Licia Iacoviello, Augusto Di Castelnuovo, Alessandro Gialluisi, Amalia De Curtis, Marialaura Bonaccio, Francesco Gianfagna, Anwal Ghulam, Sara Magnacca, Fabrizia Noro, Simona Costanzo, Simona Esposito, Sabatino Orlandi, Mariarosaria Persichillo, Francesca Bracone, Teresa Panzera, Emilia Ruggiero, Roberta Parisi, Sabrina Franciosa, Martina Morelli, Fiorella De Rita, Chiara Cerletti, Giovanni de Gaetano, Maria Benedetta Donati, Lorenzo Menicanti, Serenella Castelvecchio, Alexis Elias Malavazos, Massimiliano MarcoCorsi Romanelli, Rosanna Cardani, Ambra Cerri, Carola Dubini, Manuel Bruno Trevisan, Laura Valentina Renna, Valentina Milani, Sara Boveri, Paola Giubbilini, Lucia Ramputi, Irene Baroni, Giada DeAngeli, Walter Ricciardi, Maria Teresa La Rovere, Simonetta Scalvini, Antonia Pierobon, Alessandra Gorini, Francesca Olmetti, Maurizio Bussotti, Carlo Gaetano, Paola Baiardi, Tiziana Bachetti, Martina Balbi, Laura Comini, Monica Lorenzoni, Adriana Olivares, Egidio Traversi, Camilla Garrè, Riccardo Sideri, Pier Giulio Conaldi, Valentina Agnese, Francesco Clemenza, Giovanni Gentile, Giuseppe Caruana, Nicola Cuscino, Gabriele Di Gesaro, Alessio Greco, Italia Loddo, Fabio Tuzzolino, Antonio Uccelli, Bianca Pane, Domenico Palombo, Giovanni Spinella, Gaddiel Mozzetta, Pietro Ameri, Gabriele Zoppoli, Alice Finotello, Italo Porto, Giovanni Pratesi, Fabio Blandini, Daniele Prati, Luca Valenti, Laura Spinardi, Margherita Clerici, Serena Pelusi, Cristiana Bianco, Rossana Carpani, Giulia Periti, Sara Margarita, Giovanni Scambia, Giovanna Liuzzo, Gaetano Antonio Lanza, Anna Severino, Daniela Pedicino, Domenico D’Amario, Alessia D’Aiello, Ramona Vinci, Alice Bonanni, Mattia Brecciaroli, Simone Filomia, Roberta Pastorino, Stefania Boccia, Andrea Urbani, Maurizio Sanguinetti, Angelo Santoliquido, Luca Proto, Dalila Tarquini, Maria Chiara Grimaldi, Eloisa Arbustini, Catherine Klersy, Sergio Leonardi, Arianna Elia, Alessia Currao, Mario Urtis, Alessandro Di Toro, Lorenzo Giuliani, Massimo Fini, Maurizio Volterrani, Giuseppe Caminiti, Federica Marcolongo, Barbara Sposato, Fiorella Guadagni, Valentina Morsella, Angelica Marziale, Antonio Di Malta, Marco Visconti, Annarosa Racca, Manuela Bandi, and Giulia Protti
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Comitato Etico degli IRCCS Istituto Europeo di Oncologia e Centro Cardiologico Monzino (Parent study; R1256/20-CCM 1319); Comitato Etico degli IRCCS Istituto Europeo di Oncologia e Centro Cardiologico Monzino (Ancillary studies of Monzino; R1579/21-CCM 1677; R1617/22-CCM 1723); Comitato Etico dell’IRCCS Istituto Auxologico Italiano (Ancillary study of Istituto Auxologico Italiano; 2022_03_08_06); Comitato Etico Indipendente dell’Istituto Clinico Humanitas (Ancillary study of Humanitas; 2860); Comitato Etico IRCCS Multimedica - Sezione del Comitato Etico Centrale IRCCS Lombardia (Ancillary study of Multimedica; MM: 472.2021); Comitato Etico dell’Istituto Neurologico Mediterraneo Neuromed (Ancillary study of Neuromed; Session of 28/09/2020); Comitato Etico IRCCS Ospedale San Raffaele (Ancillary study of San Donato; 197/INT/2021); Comitato Etico degli Istituti Clinici Scientifici Maugeri IRCCS (Ancillary study of Maugeri; 2575 CE); Comitato Etico IRCCS Sicilia Sezione ISMETT IRCCS srl (Ancillary study of ISMETT; IRRB/16/22); Comitato Etico Regionale della Liguria (Ancillary study of San Martino; 173/2021); Comitato Etico Milano Area 2 (Ancillary study of Ca’ Granda; 887_2020); Comitato Etico della Fondazione Policlinico Universitario A. Gemelli IRCCS - Università Cattolica del Sacro Cuore (Ancillary study of Gemelli; 3614); Comitato Etico Pavia (Ancillary studies of San Matteo; 2022-3.11/91; 2022-3.11/493); Comitato Etico IRCCS San Raffale Roma (Ancillary study of San Raffaele; 21/21). Participants gave informed consent to participate in the study before taking part.
References
- 1. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation 2020;141:e139–596. 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2. European Commission . State of health in the EU – Italy, country health profile. 2021. Available: https://www.euro.who.int/__data/assets/pdf_file/0008/355985/Health-Profile-Italy-Eng.pdf
- 3. Healthcare expenditure Statistics: Eurostat (Hlth_Sha11_Hc). 2019. Available: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Healthcare_expenditure_statistics#Healthcare_expenditure_by_function
- 4. Widmer RJ, Collins NM, Collins CS, et al. Digital health interventions for the prevention of cardiovascular disease: a systematic review and meta-analysis. Mayo Clin Proc 2015;90:469–80. 10.1016/j.mayocp.2014.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan A-W, Tetzlaff JM, Altman DG, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med 2013;158:200–7. 10.7326/0003-4819-158-3-201302050-00583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harris PA, Taylor R, Minor BL, et al. The redcap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (Redcap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sardi L, Idri A, Fernández-Alemán JL. A systematic review of gamification in E-health. J Biomed Inform 2017;71:31–48. 10.1016/j.jbi.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 9. Di Castelnuovo A, Costanzo S, Persichillo M, et al. Distribution of short and lifetime risks for cardiovascular disease in Italians. Eur J Prev Cardiol 2012;19:723–30. 10.1177/1741826711410820 [DOI] [PubMed] [Google Scholar]
- 10. Salvetti M, Paini A, Di Castelnuovo A, et al. Correction of QRS voltage for body mass index does not improve the prediction of fatal and nonfatal cardiovascular events. The Moli-Sani study. Nutr Metab Cardiovasc Dis 2020;30:426–33. 10.1016/j.numecd.2019.10.013 [DOI] [PubMed] [Google Scholar]
- 11. Martínez-González MA, García-Arellano A, Toledo E, et al. A 14-item mediterranean diet assessment tool and obesity indexes among high-risk subjects: the PREDIMED trial. PLoS One 2012;7:e43134. 10.1371/journal.pone.0043134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schröder H, Fitó M, Estruch R, et al. A short screener is valid for assessing mediterranean diet adherence among older Spanish men and women. J Nutr 2011;141:1140–5. 10.3945/jn.110.135566 [DOI] [PubMed] [Google Scholar]
- 13. D’Elia L, Manfredi M, Strazzullo P, et al. Validation of an easy questionnaire on the assessment of salt habit: the MINISAL-SIIA study program. Eur J Clin Nutr 2019;73:793–800. 10.1038/s41430-018-0204-0 [DOI] [PubMed] [Google Scholar]
- 14. Mannocci A, Di Thiene D, Del Cimmuto A, et al. International physical activity questionnaire: validation and assessment in an Italian sample. Int J Public Health 2010;7:369–76. 10.2427/5694 [DOI] [Google Scholar]
- 15. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Research 1989;28:193–213. 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 16. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 17. Kroenke K, Spitzer RL, Williams JBW, et al. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics 2009;50:613–21. 10.1176/appi.psy.50.6.613 [DOI] [PubMed] [Google Scholar]
- 18. Zotti AM, Balestroni G, Cerutti P, et al. Application of the general perceived self-efficacy scale in cardiovascular rehabilitation. Monaldi Arch Chest Dis 2007;68:178–83. 10.4081/monaldi.2007.451 [DOI] [PubMed] [Google Scholar]
- 19. Gala C, Musicco F, Durbano F, et al. Italian validation of the multidimensional scale of "health locus of control. New Trends Exp Clin Psychiat 1995;11:79–86. Available: https://csrc.nist.gov/CSRC/media/Publications/sp/800-155/draft/documents/draft-SP800-155_Dec2011.pdf [Google Scholar]
- 20. Meertens RM, Lion R. Measuring an individual’s tendency to take risks: the risk propensity scale1. J Appl Social Pyschol 2008;38:1506–20. 10.1111/j.1559-1816.2008.00357.x [DOI] [Google Scholar]
- 21. Neeland IJ, Ross R, Després J-P, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7:715–25. 10.1016/S2213-8587(19)30084-1 [DOI] [PubMed] [Google Scholar]
- 22. Wilson PWF, Meigs JB, Sullivan L, et al. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham offspring study. Arch Intern Med 2007;167:1068–74. 10.1001/archinte.167.10.1068 [DOI] [PubMed] [Google Scholar]
- 23. Parikh NI, Pencina MJ, Wang TJ, et al. A risk score for predicting near-term incidence of hypertension: the Framingham heart study. Ann Intern Med 2008;148:102–10. 10.7326/0003-4819-148-2-200801150-00005 [DOI] [PubMed] [Google Scholar]
- 24. Palmieri L, Panico S, Vanuzzo D, et al. Evaluation of the global cardiovascular absolute risk: the progetto CUORE individual score. Ann Ist Super Sanita 2004;40:393–9. [PubMed] [Google Scholar]
- 25. Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. 10.1016/s0195-668x(03)00114-3 [DOI] [PubMed] [Google Scholar]
- 26. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008;117:743–53. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 27. Goff DC, Lloyd-Jones DM, Bennett G, et al. ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 2014;129:S49–73. 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 28. Baldassarre D, Nyyssönen K, Rauramaa R, et al. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. Eur Heart J 2010;31:614–22. 10.1093/eurheartj/ehp496 [DOI] [PubMed] [Google Scholar]
- 29. Regenscheid A, Scarfone K. BIOS integrity measurement guidelines (draft) recommendations of the National institute of standards and technology. NIST National institute of standards and technology (US Department of commerce) (Special Publication 800-155). [Google Scholar]
- 30. Regulation (EU) 2016/679 of the European parliament and of the council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing directive 95/46/EC (General data protection regulation) (text with EEA relevance) OJ L 119 27.04.2016 [P. 1, CELEX]; 2016. Available: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32016R0679OJ
- 31. Ramachandran A, Snehalatha C, Ram J, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2013;1:191–8. 10.1016/S2213-8587(13)70067-6 [DOI] [PubMed] [Google Scholar]
- 32. Albini F, Xiaoqiu L, Torlasco C, et al. An ICT and mobile health integrated approach to optimize patients' education on hypertension and its management by physicians: the patients optimal strategy of Treatment(POST) pilot study. Annu Int Conf IEEE Eng Med Biol Soc 2016;2016:517–20. 10.1109/EMBC.2016.7590753 [DOI] [PubMed] [Google Scholar]
- 33. Cossolo M. La Farmacia Italiana 2020/2021. n.d. Available: https://www.federfarma.it/Documenti/farmacia_italiana2020-2021.aspx
- 34. Cowie MR, Lam CSP. Remote monitoring and digital health tools in CVD management. Nat Rev Cardiol 2021;18:457–8. 10.1038/s41569-021-00548-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stergiou GS, Alpert B, Mieke S, et al. A universal standard for the validation of blood pressure measuring devices: association for the advancement of medical instrumentation/European society of hypertension/international organization for standardization (AAMI/ESH/ISO). J Hypertens 2018;36:472–8. 10.1097/HJH.0000000000001634 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-072040supp001.pdf (118.6KB, pdf)
bmjopen-2023-072040supp006.pdf (2MB, pdf)
bmjopen-2023-072040supp002.pdf (130.2KB, pdf)
bmjopen-2023-072040supp003.pdf (474.5KB, pdf)
bmjopen-2023-072040supp004.pdf (82.4KB, pdf)
bmjopen-2023-072040supp005.pdf (105.5KB, pdf)