Abstract
Background and Objectives
Patients with Lewy body disease (LBD) often show a co-occurring Alzheimer disease (AD) pathology. CSF biomarkers allow the detection in vivo of AD-related pathologic hallmarks included in the amyloid-tau-neurodegeneration (AT(N)) classification system. Here, we aimed to investigate whether CSF biomarkers of synaptic and neuroaxonal damage are correlated with the presence of AD copathology in LBD and can be useful to differentiate patients with LBD with different AT(N) profiles.
Methods
We retrospectively measured CSF levels of AD core biomarkers (Aβ42/40 ratio, phosphorylated tau protein, and total tau protein) and of synaptic (β-synuclein, α-synuclein, synaptosomal-associated protein 25 [SNAP-25], and neurogranin) and neuroaxonal proteins (neurofilament light chain [NfL]) in 28 cognitively unimpaired participants with nondegenerative neurologic conditions and 161 participants with a diagnosis of either LBD or AD (at both mild cognitive impairment, AD-MCI, and dementia stages, AD-dem). We compared CSF biomarker levels in clinical and AT(N)-based subgroups.
Results
CSF β-synuclein, α-synuclein, SNAP-25, neurogranin, and NfL levels did not differ between LBD (n = 101, age 67.2 ± 7.8 years, 27.7% females) and controls (age 64.8 ± 8.6 years, 39.3% females) and were increased in AD (AD-MCI: n = 30, AD-dem: n = 30, age 72.3 ± 6.0 years, 63.3% females) compared with both groups (p < 0.001 for all comparisons). In LBD, we found increased levels of synaptic and neuroaxonal degeneration biomarkers in patients with A+T+ (LBD/A+T+) than with A−T− profiles (LBD/A−T−) (p < 0.01 for all), and β-synuclein showed the highest discriminative accuracy between the 2 groups (area under the curve 0.938, 95% CI 0.884–0.991). CSF β-synuclein (p = 0.0021), α-synuclein (p = 0.0099), and SNAP-25 concentrations (p = 0.013) were also higher in LBD/A+T+ than in LBD/A+T− cases, which had synaptic biomarker levels within the normal range. CSF α-synuclein was significantly decreased only in patients with LBD with T− profiles compared with controls (p = 0.0448). Moreover, LBD/A+T+ and AD cases did not differ in any biomarker level.
Discussion
LBD/A+T+ and AD cases showed significantly increased CSF levels of synaptic and neuroaxonal biomarkers compared with LBD/A−T− and control subjects. Patients with LBD and AT(N)-based AD copathology showed, thus, a distinct signature of synaptic dysfunction from other LBD cases.
Classification of Evidence
This study provides Class II evidence that CSF levels of β-synuclein, α-synuclein, SNAP-25, neurogranin, and NfL are higher in patients with AD than in patients with LBD.
Parkinson disease (PD) and dementia with Lewy bodies (DLBs) (together Lewy body diseases [LBDs]) are progressive neurodegenerative disorders caused by misfolding and intraneuronal aggregation of the presynaptic protein α-synuclein.1 The clinical heterogeneity of motor and nonmotor symptoms observed in patients with LBD is only partially explained by the underlying Lewy-type pathology and its topographical distribution across the brain. In fact, α-synuclein pathology often coexists with several other proteinopathies, such as Alzheimer disease (AD) pathology, which is reported in more than 50% of all LBD cases and significantly influences the disease course and cognitive outcome.2,3
AD pathology can be accurately assessed in vivo by means of CSF biomarkers reflecting the neuropathologic hallmarks within the amyloid-tau-neurodegeneration (AT(N)) classification system, namely, β-amyloidosis (A: decreased amyloid-β1–42/amyloid-β1–40 ratio, Aβ42/40), tauopathy (T: increased phosphorylated tau protein, p-tau), and neurodegeneration (N: increased total tau protein, t-tau).4 Conversely, Lewy-related pathology is only modestly associated with decreased CSF levels of α-synuclein as a possible biomarker of α-synucleinopathy.5,6 Instead, increased α-synuclein concentrations may reflect the ongoing synaptic derangement, as reported in AD and Creutzfeldt-Jakob disease and in advanced stages of PD.7,8
The quantification in CSF levels of other presynaptic and postsynaptic proteins, such as synaptosomal-associated protein 25 (SNAP-25) and neurogranin, may help detecting early synaptic leakage from degenerating terminals in AD.9,10 Similarly, β-synuclein is a presynaptic protein closely related to α-synuclein and has been recently exploited in CSF and blood as a novel candidate biomarker of synaptic damage in AD and prion disease.11-15 Available data on CSF synaptic biomarkers in the LBD spectrum are, to date, not conclusive because they have been reported either slightly increased or not altered in comparison with control subjects.10,16,17 In addition, to the best of our knowledge, no study has tested to date whether they could be associated with AD copathology in LBD.
Hence, in this study, we first sought to investigate different CSF biomarkers reflecting synaptic damage, namely, β-synuclein, α-synuclein, SNAP-25, and neurogranin, in patients at different cognitive stages within the LBD spectrum (PD, PD with dementia [PDD], and DLB). Second, we aimed to verify the hypothesis that synaptic dysfunction markers were differently expressed in AT(N)-based subgroups of LBD. In respect to this, we included 2 convenience groups for comparisons: (1) patients with AD at the mild cognitive impairment (AD-MCI) and dementia (AD-dem) stages as a reference AT(N) biomarker–positive group; (2) cognitively unimpaired patients with nondegenerative neurologic conditions as a reference AT(N) biomarker–negative group. Finally, we measured in all patients CSF levels of neurofilament light chain protein (NfL), a biomarker of neuroaxonal injury in primary and nonprimary neurologic disorders,18,19 to evaluate whether biomarkers reflecting neurodegeneration and synaptic derangement may show different behaviors in the LBD spectrum.
These facts considered that the primary research question being addressed in this study was as follows: are CSF levels of β-synuclein, α-synuclein, SNAP-25, neurogranin, and NfL different in LBD and AD.
Methods
Study Population
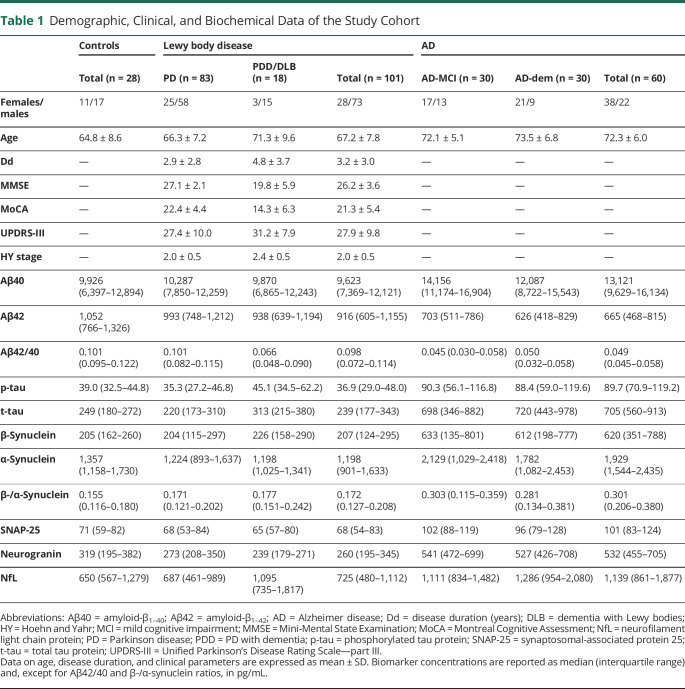
We retrospectively analyzed CSF samples of 189 patients admitted to the Section of Neurology of the University of Perugia (Perugia, Italy) from 2015 to 2020. The cohort included 28 cognitively unimpaired controls, 101 patients with LBD, and 60 patients with AD. All patients underwent, at baseline, neuropsychological evaluation, neuroimaging (MRI or, when not feasible, CT), and lumbar puncture as parts of the routine diagnostic workup.
The LBD group included 83 patients with clinically established PD, 12 with probable PDD, and 6 with probable DLB, diagnosed according to the currently available clinical criteria.20-22 We considered patients affected by PDD and DLB within the same group (PDD/DLB) for analyses on clinical groups. The Movement Disorders Society Unified Parkinson's Disease Rating Scale—part III (UPDRS-III) score and the Hoehn and Yahr (HY) staging system were used to assess the motor impairment and the disease-related disability, respectively.23,24 The global cognitive functioning was assessed in all LBD cases with the Mini-Mental State Examination (MMSE)25 and the Montreal Cognitive Assessment (MoCA) score.26
The diagnosis of AD was supported by the analysis of CSF core biomarkers, according to the most updated National Institute on Ageing and Alzheimer's Association recommendations.4 All AD cases showed a CSF A+T+N+ biomarker profile, except 1 AD-MCI case with a CSF A+T+N− profile (see below for biomarker cutoffs). Patients with AD were stratified into 2 clinical subgroups according to the Clinical Dementia Rating (CDR) score, namely, AD-MCI (CDR = 0.5, n = 30) and AD-dem (CDR ≥1, n = 30).27,28
The control (Ctrl) group included 10 patients with subjective memory complaints, who did not fulfill criteria for MCI,29 and 18 cognitively unimpaired controls with nondegenerative neurologic diseases (epilepsy n = 2, cerebrovascular disorders n = 2, psychiatric disorders n = 3, polyneuropathy n = 6, and optic neuritis n = 5). All control subjects had Aβ42/40 and p-tau levels within the normal range (Table 1).
Table 1.
Demographic, Clinical, and Biochemical Data of the Study Cohort
CSF Sampling and Analysis of AD Core Biomarkers
CSF sampling was performed according to international guidelines.30 Following standardized procedures, 10–12 mL of CSF was collected in sterile polypropylene tubes and centrifuged at 2,000g for 10 minutes. Aliquots (0.5 mL) were stored at −80°C until analysis. CSF levels of Aβ42, Aβ40, p-tau, and t-tau were measured by means of Lumipulse G600-II (Lumipulse) Aβ40, Lumipulse Aβ42, Lumipulse p-tau 181, and Lumipulse t-tau assays, respectively (Fujirebio Europe, Gent, Belgium).
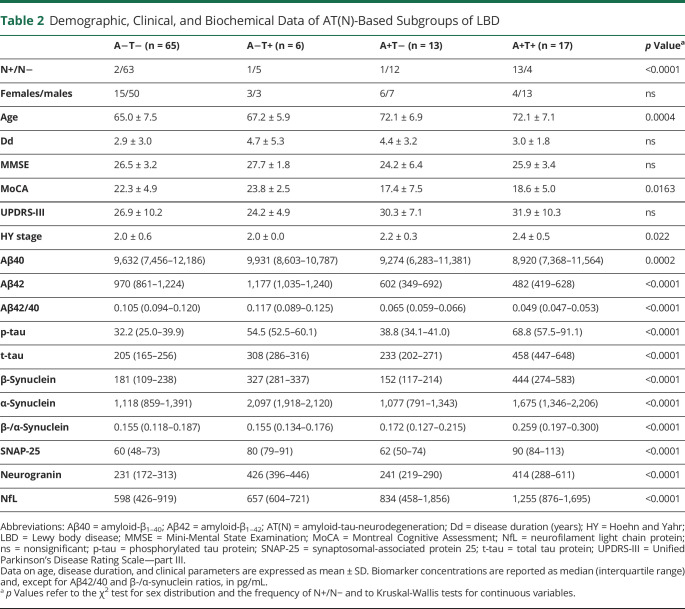
By applying the AT(N) classification scheme,4 AD core biomarker values were classified as pathologic (+) or nonpathologic (−) according to previously described cluster-based cutoffs.31 We considered Aβ42/40 ratio <0.072 as A+, p-tau >50.0 pg/mL as T+, and total tau >392 pg/mL as N+. The frequency of AT(N) profiles across clinical groups is reported in eTable 1 (links.lww.com/WNL/C804). Given the scarce number of N+ cases in patients with LBD with profiles other than A+T+ (4 of 84), we did not perform comparisons between N+ and N− profiles.
Quantification of CSF Synaptic and Neuroaxonal Proteins
CSF synucleins were quantified by using a commercial α-synuclein immunoassay (Euroimmun, Lübeck, Germany) and an in-house established immunoassay for β-synuclein (14). We measured CSF levels of NfL with a commercially available kit for the ELLA microfluidic system (Bio-Techne, Minneapolis, MN),32 SNAP-25 with a commercial Simoa kit (Quanterix Inc., Lexington, MA), and neurogranin with a commercial immunoassay (Euroimmun). For all measurements, the coefficients of intra-assay and interassay variability were <10% and <15%, respectively.
Statistical Analysis
All statistical analyses were performed by using SPSS Statistics 22.0 (IBM Inc., Armonk, NY) and GraphPad 8 (GraphPad Software, La Jolla, CA). We adopted the χ2 test to compare categorical variables and the Mann-Whitney U or Kruskal-Wallis test (followed by the Dunn-Bonferroni post hoc test) to compare continuous variables, as appropriate. Multivariable regression models were adopted for age- and/or sex-adjusted comparisons after transformation of the biomarker levels into logarithmic scales. Correlations between non-normally distributed variables were computed by means of the Spearman's ρ coefficient. We performed receiver operating characteristic (ROC) analyses to assess the diagnostic accuracy of biomarkers. A p value of <0.05 was considered as the first level of statistical significance.
Standard Protocol Approvals, Registrations, and Patient Consents
All participants gave written informed consent to research, and the Ethics Committee of the University of Perugia approved the study (approval number: Perugia 19369/08/AV, registry number: 1287/08, date: October 9, 2008). This study was conducted in accordance with the Helsinki Declaration and its recent modifications.
Data Availability
Anonymized data will be shared with qualified investigators on reasonable request to the corresponding author.
Results
Demographic and Clinical Data of the Study Population
Complete data on the study cohort are available in Table 1. Patients with LBD (mean age 67.2 ± 7.8 years) had the same age as Ctrls (mean age 64.8 ± 8.6 years) but were younger than patients with AD (mean age 72.3 ± 6.0 years, p < 0.0001). The male gender was more represented in the LBD (73/101, 72.3%) than in the AD group (22/60, 36.7%, p < 0.0001). Among LBD clinical subgroups, we found a longer disease duration (p = 0.013), lower MMSE scores (p < 0.0001), lower MoCA scores (p < 0.0001), and higher HY stages (p = 0.036) in PDD/DLB vs PD cases.
After classification of patients with LBD according to their AT(N) profile (Table 2), we found no significant difference in sex distribution, disease duration, MMSE, UPDRS-III, and HY stage. Patients with LBD with A+T+ profiles (LBD/A+T+) had lower MoCA scores than LBD/A−T− cases, but statistical significance did not survive to the post hoc test (p = 0.072, unadj p = 0.011). In relation to age, LBD/A−T− cases were younger than LBD/A+T− (p = 0.016) and LBD/A+T+ (p = 0.0022).
Table 2.
Demographic, Clinical, and Biochemical Data of AT(N)-Based Subgroups of LBD
Associations of CSF Biomarkers With Clinical Variables and With Each Other
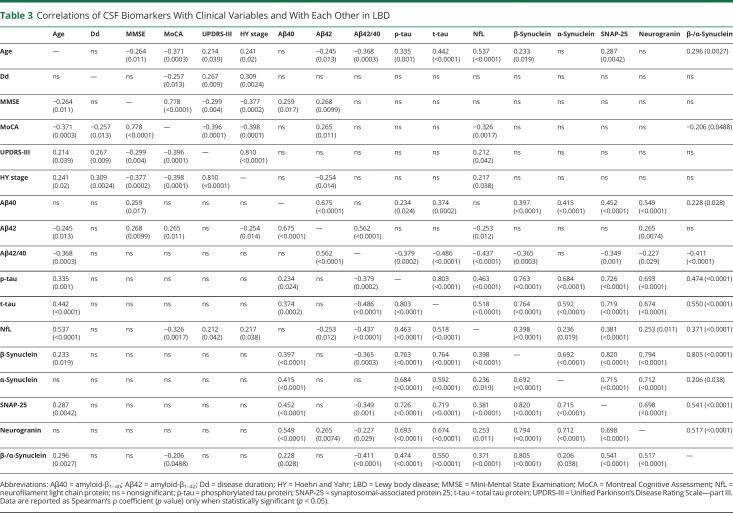
In LBD, male and female patients did not differ in any biomarker concentrations, whereas age correlated significantly with CSF levels of NfL (r = 0.537, p < 0.0001), β-synuclein (r = 0.233, p = 0.019), β-/α-synuclein ratio (r = 0.296, p = 0.0027), and SNAP-25 (r = 0.287, p = 0.0042) (Table 3). CSF synaptic biomarkers did not correlate with disease duration or clinical scores, whereas NfL showed weak but significant correlations with the HY stage (r = 0.217, p = 0.038), the MoCA score (r = −0.326, p = 0.0017), and the UPDRS-III score (r = 0.212, p = 0.042). The correlation between β-/α-synuclein ratio values and the MoCA score was of marginal significance (r = −0.206, p = 0.0488) (eFigures 1–5, links.lww.com/WNL/C804).
Table 3.
Correlations of CSF Biomarkers With Clinical Variables and With Each Other in LBD
In LBD, CSF levels of synaptic proteins, NfL, and AD core biomarkers were well associated one with each other (Table 3). However, α-synuclein was the only biomarker that did not significantly correlate with the Aβ42/40 ratio (p = 0.209). The strongest correlations were observed between CSF synucleins and either SNAP-25 (β-synuclein r = 0.820, α-synuclein r = 0.715, p < 0.0001 for both) or neurogranin levels (β-synuclein r = 0.794, α-synuclein r = 0.712, p < 0.0001 for both). Equally, we found significant correlations between β-synuclein and α-synuclein concentrations (r = 0.692, p < 0.0001), as well as between SNAP-25 and neurogranin concentrations (r = 0.698, p < 0.0001). Of interest, all 4 synaptic biomarkers were significantly correlated with both t-tau and NfL, but correlations with t-tau were stronger in all cases. Similar findings were also observed in the AD and Ctrl groups (eTables 2 and 3, links.lww.com/WNL/C804).
Distribution of CSF Synaptic and Neuroaxonal Biomarkers Among Clinical and AT(N)-Based Groups
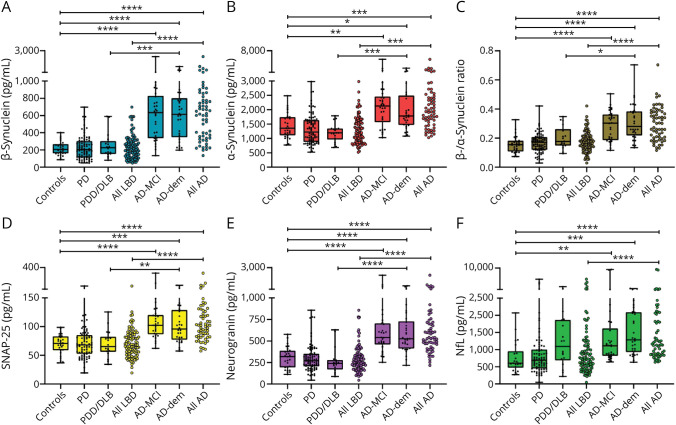
CSF levels of β-synuclein, α-synuclein, β-/α-synuclein ratio, SNAP-25, neurogranin, and NfL, as well as those of AD core biomarkers, did not differ between LBD and Ctrls. Instead, all biomarkers were significantly increased, and the Aβ42/40 ratio significantly decreased, in AD compared with both LBD and Ctrl groups (p < 0.001 for all comparisons) (Figure 1, eFigure 6, links.lww.com/WNL/C804). The above-mentioned findings were confirmed in multivariable regression models after adjustment for age (AD vs Ctrls) or for age + sex (AD vs LBD) (eTable 4). Analyses on AD core biomarkers and among clinical subgroups are reported in eResults. Of notice, in a subanalysis on DLB cases (n = 6), we found no significant differences in any biomarker level compared with patients with PD except for a higher β-/α-synuclein ratio in DLB (p = 0.026) (eFigure 7).
Figure 1. CSF Biomarkers Across Clinical Groups.
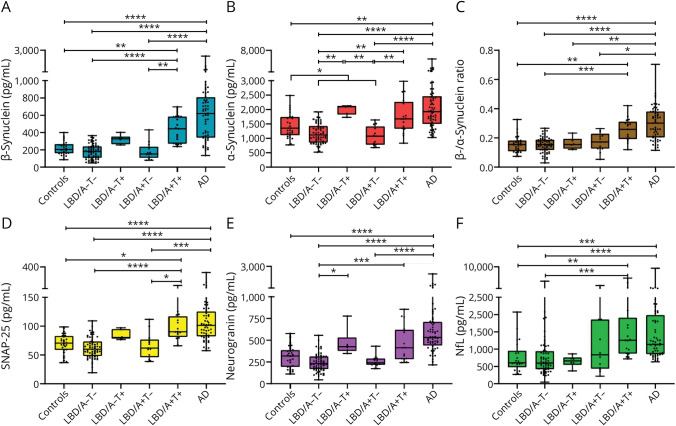
Panels show CSF levels of (A) β-synuclein, (B) α-synuclein, (C) β-/α-synuclein ratio, (D) SNAP-25, (E) neurogranin, and (F) NfL in Ctrls (n = 28), PD (n = 83), PDD/DLB (n = 18), all LBD (n = 101), AD-MCI (n = 30), AD-dem (n = 30), and all AD (n = 60). *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001. AD = Alzheimer disease; AD-dem = AD with dementia; AD-MCI = AD with mild cognitive impairment; Ctrls = controls; DLB = dementia with Lewy bodies; LBD = Lewy body disease; NfL = neurofilament light chain protein; PD = Parkinson disease; PDD = PD with dementia; SNAP-25 = synaptosomal-associated protein 25.
In comparison with AD biomarker–negative subgroups (i.e., Ctrls and LBD/A−T−), LBD/A+T+ cases showed significantly higher concentrations of β-synuclein (p = 0.0076 vs Ctrls; p < 0.0001 vs LBD/A−T−), α-synuclein (p = 0.0018 vs LBD/A−T−), β-/α-synuclein ratio (p = 0.0032 vs Ctrls; p = 0.0004 vs LBD/A−T−), SNAP-25 (p = 0.011 vs Ctrls; p < 0.0001 vs LBD/A−T−), neurogranin (p = 0.0008 vs LBD/A−T−), and NfL (p = 0.0047 vs Ctrls; p = 0.0001 vs LBD) (Figure 2). However, at the age-adjusted analysis, only β-synuclein (p = 0.001), α-synuclein (p = 0.049), and the β-/α-synuclein ratio (p = 0.007) maintained statistical significance in the comparison between LBD/A+T+ and LBD/A−T− (eTable 4, links.lww.com/WNL/C804).
Figure 2. CSF Biomarkers Across AT(N)-Based Subgroups.
Panels show CSF levels of (A) β-synuclein, (B) α-synuclein, (C) β-/α-synuclein ratio, (D) SNAP-25, (E) neurogranin, and (F) NfL in Ctrls (n = 28), LBD/A−T− (n = 65), LBD/A−T+ (n = 6), LBD/A+T− (n = 13), LBD/A+T+ (n = 17), and AD (n = 60). *p < 0.05; **p < 0.01; ***p < 0.001; and ****p < 0.0001. AD = Alzheimer disease; AT(N) = amyloid-tau-neurodegeneration; Ctrls = controls; LBD = Lewy body disease; NfL = neurofilament light chain protein; SNAP-25 = synaptosomal-associated protein 25.
Of notice, we found significantly decreased levels of CSF α-synuclein in patients with LBD with p-tau levels within the normal range (i.e., LBD/A−T− and LBD/A+T−) in comparison with Ctrls (p = 0.0448). Moreover, β-synuclein (p = 0.0021), α-synuclein (p = 0.0099), and SNAP-25 (p = 0.013) levels were significantly increased also in LBD/A+T+ compared with LBD/A+T− cases. Finally, LBD/A+T+ patients did not differ from patients with AD in any biomarker level (Figure 2). In a subanalysis on the PD subgroup, we could confirm the finding of increased CSF biomarker levels in patients with A+T+ profiles (i.e., PD/A+T+) compared with controls and patients with PD with A−T− profiles (i.e., PD/A−T−) (eResults, eFigure 8, links.lww.com/WNL/C804).
Discriminative Accuracy of CSF Biomarkers in LBD and AD Spectra
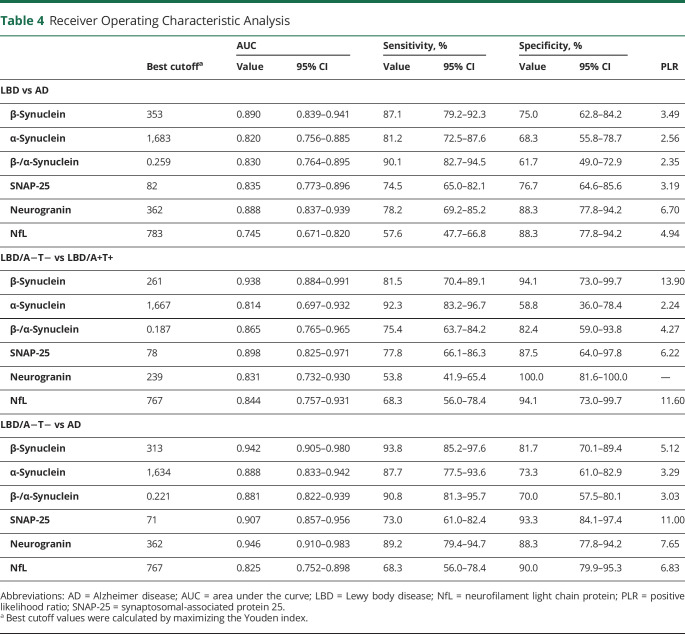
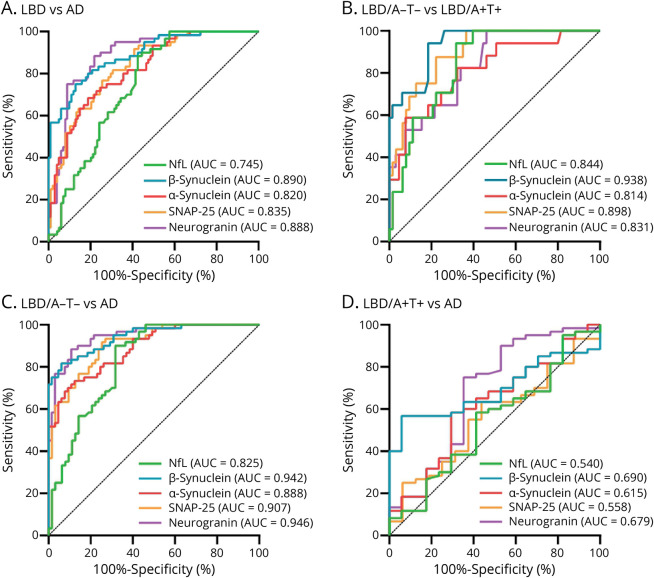
Complete results of ROC analyses are summarized in Table 4 and eTable 5 (links.lww.com/WNL/C804). CSF synaptic biomarkers could well discriminate patients with AD from patients with LBD with an area under the curve (AUC) of 0.890 (95% CI 0.839–0.941) for β-synuclein, 0.820 (95% CI 0.756–0.885) for α-synuclein, 0.835 (95% CI 0.773–0.896) for SNAP-25, and 0.888 (95% CI 0.837–0.939) for neurogranin. In comparison, the performance of NfL was suboptimal (AUC 0.745, 95% CI 0.671–0.820) and that of the β-/α-synuclein ratio was not superior to β-synuclein alone (Figure 3, Table 4).
Table 4.
Receiver Operating Characteristic Analysis
Figure 3. ROC Analysis of CSF Biomarkers.
Panels show the results of ROC analyses in the comparisons between (A) LBD and AD, (B) LBD/A−T− and LBD/A+T+, (C) LBD/A−T− and AD, and (D) LBD/A+T+ and AD. AD = Alzheimer disease; LBD = Lewy body disease; NfL = neurofilament light chain protein; ROC = receiver operating characteristic; SNAP-25 = synaptosomal-associated protein 25.
In AT(N)-based subgroups, we found a high accuracy of CSF synaptic biomarkers to discriminate LBD/A−T− and LBD/A+T+ cases, with best results for β-synuclein (AUC 0.938, 95% CI 0.884–0.991), followed by SNAP-25 (AUC 0.898, 95% CI 0.824–0.971). Similarly, LBD/A−T− could be well distinguished from AD cases by β-synuclein (AUC 0.942, 95% CI 0.905–0.980) and neurogranin levels (AUC 0.946, 95% CI 0.910–983). Conversely, no biomarker could discriminate LBD/A+T+ from AD with AUC >0.7 (Figure 3, eTable 5, links.lww.com/WNL/C804). We replicated these results in the PD subgroup for the comparison between PD/A+T+ and PD/A−T− cases, with the highest accuracy for β-synuclein (AUC 0.956, 95% CI 0.898–1.00) and SNAP-25 (AUC 0.913, 95% CI 0.822–1.00) (in detail in eResults and eTable 5).
Classification of Evidence
This study provides Class II evidence that CSF levels of β-synuclein, α-synuclein, SNAP-25, neurogranin, and NfL are higher in patients with AD than in patients with LBD.
Discussion
In this study, we described distinct CSF patterns of synaptic and neuroaxonal biomarkers in AT(N)-based subgroups of LBD. In summary, we provided evidence that (1) CSF synaptic biomarkers were overall higher in AD compared with LBD; (2) patients with LBD with a CSF AD-like profile (i.e., LBD/A+T+) disclosed a remarkable increase of CSF biomarkers of synaptic and neuroaxonal damage, similarly to what observed in AD; (3) in LBD, α-synuclein levels were correlated with CSF p-tau and t-tau concentrations but not with Aβ42/40 ratio values; and (4) synaptic and neuroaxonal biomarkers may show different trajectories across the LBD-AD spectrum.
Our data on CSF synaptic proteins might be interpreted as a tentative to systemically dissect, from a neurochemical point of view, the effect of AD pathology on the synaptic compartment in LBD. In this regard, we selected synaptic proteins that contribute in various ways to the overall synapse wellness and may, thus, reflect different aspects of synaptic derangement. Physiologically, α-synuclein and β-synuclein participate to neurotransmitter metabolism in the presynaptic terminal.6 Similarly, SNAP-25 actively contributes to neurotransmitter release by modulating presynaptic vesicle fusion in a calcium-dependent fashion.33 On the other hand, neurogranin exerts activities in modulating the postsynaptic response.9 The finding of increased levels of all synaptic proteins in LBD/A+T+ and AD, thus, suggests the massive disruption of different synaptic pathways, which might underlie the clinical heterogeneity of the 2 disease spectra.
Although belonging to the same protein family and sharing structural and functional properties, β-synuclein and α-synuclein have shown distinct profiles as CSF biomarkers in LBD. As evidenced by several meta-analyses, CSF α-synuclein levels have been frequently found to be lower in patients with LBD compared with healthy and neurologic controls, as a possible marker of α-synucleinopathy, but current data do not support the use of this biomarker for routine diagnostic purposes (reviewed in references 5, 6). Nevertheless, reports from cross-sectional and longitudinal studies widely vary, in part because of preanalytical confounders affecting α-synuclein quantification and the clinical heterogeneity of the study populations, which often did not account for the presence of AD copathology.34-36 Moreover, the biomarker was also reported to increase in AD since early stages, but its role in the pathophysiology of the disease needs further clarification.8,11,15 In our cohort, we observed decreased CSF α-synuclein levels in LBD only when excluding those patients with elevated CSF p-tau levels. Hence, the presence of an undiagnosed AD copathology in LBD cohorts might represent an additional source of variability among studies.
Conversely, CSF β-synuclein was not influenced by the presence of a synucleinopathy and, similarly to other synaptic markers, could well discriminate LBD/A+T+ and AD patients from other groups. Our previous studies revealed a stage-independent association of CSF β-synuclein with AD because patients at the preclinical, MCI, and dementia stages showed similar elevated values.11,15,17 CSF β-synuclein might be, thus, associated with the presence of AD pathology but not with clinical progression. Furthermore, the first results obtained in serum encourage investigations on β-synuclein as a peripheral biomarker of synaptic damage in AD and other neurodegenerative disorders, given the selective neuronal expression of the protein.12-14 As a comparison, the diagnostic performance of neurogranin, SNAP-25, and α-synuclein in CSF failed to date to be replicated in blood because of their extracerebral synthesis or the lack of sensitive assays.37,38,e1 Thus, considering its less invasive nature, the quantification of blood β-synuclein might be more suitable to assess and monitor in vivo AD-related synaptic derangement.
As an intriguing complementary measure, the β-/α-synuclein ratio has shown some potential in discriminating LBD/A+T+ patients from controls and disease groups. In a previous study, the β-/α-synuclein ratio was reported to be increased in only PDD vs controls and PD, but the presence of AD copathology was not assessed.11 Our data show that although all patients with LBD with T+ profiles had higher levels of both β-synuclein and α-synuclein, the β-/α-synuclein ratio was specifically elevated in only LBD/A+T+ cases, thus suggesting it as a more suitable candidate biomarker of AD-like profiles in LBD. Of note, the β-/α-synuclein ratio was also the single marker that increased selectively in patients with DLB vs PD, whereas other synaptic proteins did not differ between the 2 groups. However, given the high neuropathologic heterogeneity in the LBD spectrum, we cannot exclude that the small simple size might have led to underestimate the potential influence of variable regional patterns of neuronal and synaptic loss on CSF biomarker levels (see also below).
Within this frame, CSF synucleins may provide new insights into AD pathophysiology in addition to more established synaptic biomarkers such as SNAP-25 and neurogranin, which disclosed a progressive increase along the AD continuum and tighter associations with the cognitive stage.9,10,16,39
Previous studies reported the increase of CSF SNAP-25 and neurogranin concentrations since the earliest stages of Aβ deposition in AD, but tau pathology seemed to have a stronger influence on the elevation of both CSF protein levels.9,10,39,40 Interestingly, we observed increased CSF synaptic biomarkers only LBD cases with elevated p-tau levels but not in those with a positive Aβ status and normal p-tau concentrations (i.e., LBD/A+T−), confirming the detrimental role of the tauopathy in determining synaptic derangement. Of notice, although only in a small group, we also found elevated CSF synaptic protein levels in patients with LBD with isolated increase of p-tau (i.e., LBD/A−T+). According to a very recent study, patients without dementia with A−T+ profiles do not display an increased risk of cognitive impairment or hippocampal atrophy compared with biomarker-negative controls.41 Because synaptic dysfunction is supposed to precede neuronal degeneration by yearse2 and can be tracked in the AD continuum before the rise of biomarkers of neuroaxonal degeneration,15 synapse loss may be an early consequence of tau pathology but not be sufficient alone to lead to cognitive decline in the absence of significant neurodegeneration.
Concerning CSF neuroaxonal biomarkers, previous studies showed that NfL may better reflect disease progression in later LBD stages as a nonspecific marker of worse cognitive and clinical outcome.18,42 In our study, we found increased CSF NfL levels in only in patients with LBD with a CSF AD-like profile (i.e., LBD/A+T+) but also significant correlations with Aβ peptides (Aβ42 and Aβ42/40 ratio), MoCA, UPDRS-III, and HY stage, hence with a different pattern than synaptic biomarkers.
Overall, our results support the hypothesis of common pathologic pathways between patients with AD and distinct LBD subgroups. On the one hand, α-synuclein pathology might be alone a pivotal cause of synaptic dysfunction in brains affected by LBD, especially in dopaminergic neurons.e3 On the other hand, our biomarker panel surely does not picture the global and heterogeneous synaptic dysfunction that characterizes AD and LBD, rather the extent of the specific synaptic damage expressed by these 4 biomarkers. Therefore, we speculate that the synaptic loss signature, which is mirrored by β-synuclein, α-synuclein, SNAP-25, and neurogranin, might be similar in AD and LBD-AD cases, and it seems to be related to AD pathology. Moreover, patients with mixed LBD-AD might also experience higher degrees of synaptic dysfunction compared with pure LBD cases, given that α-synuclein, tau, and amyloid-β pathologies may trigger one each other.43 Therefore, it should be better clarified whether the associations between CSF levels of synaptic biomarkers and AD pathology depend more on their regional expression (e.g., greater in temporal vs occipital regions)14 or on the direct interactions between synaptic proteins and misfolded aggregates.
These relevant issues must be evaluated in the stratification of patients for future clinical trials. Indeed, experimental disease-modifying therapies for AD may be also effective in some LBD subgroups. In this direction, CSF biomarkers for synaptic damage could represent surrogate endpoints for synaptic integrity44 and be implemented when assessing synapse-targeting therapies. Furthermore, when testing novel therapeutic strategies for LBD, an underlying AD copathology might affect the individual response to treatment and, if undetected, lead to misleading interpretation of results. Hence, complementary biomarkers reflecting different pathologic mechanisms of LBD, such as α-synuclein aggregation, synaptic derangement, and neuroaxonal degeneration, should be combined to enroll study cohorts with more homogeneous biochemical profiles.45,46
As the main limitation of this study, we acknowledge the lack of a validated biomarker of α-synucleinopathy (e.g., α-synuclein real-time quaking-induced conversion [RT-QuIC] and protein misfolding cyclic amplification [PMCA])46 or autopsy-based confirmation of LBD and AD pathology. α-Synuclein RT-QuIC has demonstrated so far exceptionally high accuracy in correctly identifying autoptic cases of LBD,46 also in the case of AD copathology.47 Similarly, the presence of α-synuclein copathology should be also assessed in the AD group, given its potential influence on CSF biomarker levels (i.e., lower CSF neurogranin in AD-LBD mixed cases compared with pure AD cases).48 Nevertheless, α-synuclein RT-QuIC is to date available only in specialized centers, with most of them using in-house established assays, and no standardized materials and/or protocols are available.46 Moreover, studies assessing the cross-validity of RT-QuIC/PMCA assays among centers with reference samples are scanty.e4 Second, although previous investigations supported the high reliability of AD core biomarkers in detecting AD copathology in definite LBD-AD cases,49 our results need further validation in independent autopsied cohorts. Third, although clinical diagnoses were formulated by expert movement disorder specialists, the presence of misdiagnosed alternative diagnoses in the LBD group (e.g., atypical parkinsonian syndromes) could have led to biased interpretation of our results because patients with typical and atypical parkinsonian disorders may differ in their CSF biomarker profiles.e5 However, previous studies reported only slight, if any, differences between the 2 groups in CSF levels of synaptic markers, such as β-synuclein,11 α-synuclein,e5 and neurogranin.50
In conclusion, patients with LBD with AT(N)-based AD copathology showed a distinct signature of synaptic and neuroaxonal biomarkers and were well distinguishable from other LBD cases. These biomarkers may, hence, aid the identification of AD-related events in LBD.
Acknowledgment
The authors are thankful to Stephen Meier (Department of Neurology, Ulm University, Germany) and Katrin Schulz (Department of Neurology, Martin-Luther-University of Halle-Wittenberg, Germany) for their expert technical assistance and to all patients for participating in this study.
Glossary
- Aβ40
amyloid-β1–40
- Aβ42
amyloid-β1–42
- AD
Alzheimer disease
- AD-dem
AD with dementia
- AD-MCI
AD with mild cognitive impairment
- AT(N)
amyloid-tau-neurodegeneration
- AUC
area under the curve
- Ctrl
control
- CDR
Clinical Dementia Rating
- DLB
dementia with Lewy bodies
- HY
Hoehn and Yahr
- LBD
Lewy body disease
- MMSE
Mini-Mental State Examination
- MoCA
Montreal Cognitive Assessment
- NfL
neurofilament light chain protein
- PD
Parkinson disease
- PDD
Parkinson disease with dementia
- PMCA
protein misfolding cyclic amplification
- p-tau
phosphorylated tau protein
- ROC
receiver operating characteristic
- RT-QuIC
real-time quaking-induced conversion
- SNAP-25
synaptosomal-associated protein 25
- t-tau
total tau protein
- UPDRS-III
Unified Parkinson's Disease Rating Scale—part III
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This study was supported by the EU Joint Programme-Neurodegenerative Diseases networks Genfi-Prox (01ED2008A), the German Federal Ministry of Education and Research (FTLDc 01GI1007A), the EU Moodmarker program (01EW2008), the German Research Foundation/DFG (SFB1279), the foundation of the state Baden-Württemberg (D.3830), Boehringer Ingelheim Ulm University BioCenter (D.5009), the Thierry Latran Foundation (D.2468), and the Roux program of the Martin-Luther-University Halle-Wittenberg. S. Abu-Rumeileh received support from the Medical Faculty of Martin-Luther-University Halle-Wittenberg (Clinician Scientist Programm No. CS22/06). G. Bellomo is supported by the Postdoctoral Fellowship for Basic Scientists grant of the Parkinson's Foundation (award ID: PF-PRF-934916).
Disclosure
L. Barba, S. Abu-Rumeileh, S. Halbgebauer, G. Bellomo, F. Paolini Paoletti, L. Gaetani, P. Oeckl, and P. Steinacker report no disclosures relevant to the manuscript. F. Massa received a speaker honorarium from Roche Diagnostics. L. Parnetti gave scientific advice to and received funds by Fujirebio. M. Otto gave scientific advice for Fujirebio, Roche, Biogen, and Axon; the foundation of the state Baden-Wuerttemberg handed in a patent for the measurement of beta-synuclein and relevant authors are M. Otto, S. Halbgebauer, and P. Oeckl. Go to Neurology.org/N for full disclosures.
References
- 1.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers. 2017;3:17013. doi: 10.1038/NRDP.2017.13 [DOI] [PubMed] [Google Scholar]
- 2.Irwin DJ, Grossman M, Weintraub D, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol. 2017;16(1):55-65. doi: 10.1016/S1474-4422(16)30291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson JL, Lee EB, Xie SX, et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain. 2018;141(7):2181-2193. doi: 10.1093/BRAIN/AWY146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. doi: 10.1016/J.JALZ.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parnetti L, Gaetani L, Eusebi P, et al. CSF and blood biomarkers for Parkinson's disease. Lancet Neurol. 2019;18(6):573-586. doi: 10.1016/S1474-4422(19)30024-9 [DOI] [PubMed] [Google Scholar]
- 6.Barba L, Paolini Paoletti F, Bellomo G, et al. Alpha and beta synucleins: from pathophysiology to clinical application as biomarkers. Mov Disord. 2022;37(4):669-683. doi: 10.1002/MDS.28941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mastrangelo A, Baiardi S, Zenesini C, et al. Diagnostic and prognostic performance of CSF α-synuclein in prion disease in the context of rapidly progressive dementia. Alzheimers Dement (Amst). 2021;13(1):e12214. doi: 10.1002/DAD2.12214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Twohig D, Nielsen HM. α-synuclein in the pathophysiology of Alzheimer's disease. Mol Neurodegener. 2019;14(1):23. doi: 10.1186/S13024-019-0320-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarawneh R, D'Angelo G, Crimmins D, et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol. 2016;73(5):561-571. doi: 10.1001/JAMANEUROL.2016.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halbgebauer S, Steinacker P, Hengge S, et al. CSF levels of SNAP-25 are increased early in Creutzfeldt-Jakob and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2022;93(10):1059-1065. doi: 10.1136/JNNP-2021-328646 [DOI] [PubMed] [Google Scholar]
- 11.Oeckl P, Metzger F, Nagl M, et al. Alpha-, beta-, and gamma-synuclein quantification in cerebrospinal fluid by multiple reaction monitoring reveals increased concentrations in Alzheimer's and Creutzfeldt-Jakob disease but no alteration in synucleinopathies. Mol Cell Proteomics. 2016;15(10):3126-3138. doi: 10.1074/MCP.M116.059915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oeckl P, Halbgebauer S, Anderl-Straub S, et al. Targeted mass spectrometry suggests beta-synuclein as synaptic blood marker in Alzheimer's disease. J Proteome Res. 2020;19(3):1310-1318. doi: 10.1021/ACS.JPROTEOME.9B00824 [DOI] [PubMed] [Google Scholar]
- 13.Halbgebauer S, Abu-Rumeileh S, Oeckl P, et al. Blood β-synuclein and neurofilament light chain during the course of prion disease. Neurology. 2022;98(14):E1434-E1445. doi: 10.1212/WNL.0000000000200002 [DOI] [PubMed] [Google Scholar]
- 14.Oeckl P, Anderl-Straub S, Danek A, et al. Relationship of serum beta-synuclein with blood biomarkers and brain atrophy. Alzheimers Dement. 2022;19(4):1358-1371. doi: 10.1002/ALZ.12790 [DOI] [PubMed] [Google Scholar]
- 15.Barba L, Abu Rumeileh S, Bellomo G, et al. Cerebrospinal fluid β-synuclein as a synaptic biomarker for preclinical Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2022;94:83-86. doi: 10.1136/jnnp-2022-329124 [DOI] [PubMed] [Google Scholar]
- 16.Bereczki E, Bogstedt A, Höglund K, et al. Synaptic proteins in CSF relate to Parkinson's disease stage markers. NPJ Parkinsons Dis. 2017;3(1):7. doi: 10.1038/S41531-017-0008-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Halbgebauer S, Oeckl P, Steinacker P, et al. Beta-synuclein in cerebrospinal fluid as an early diagnostic marker of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2021;92(4):349-356. doi: 10.1136/jnnp-2020-324306 [DOI] [PubMed] [Google Scholar]
- 18.Gaetani L, Blennow K, Calabresi P, di Filippo M, Parnetti L, Zetterberg H. Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry. 2019;90(8):870-881. doi: 10.1136/jnnp-2018-320106 [DOI] [PubMed] [Google Scholar]
- 19.Abu-Rumeileh S, Abdelhak A, Foschi M, et al. The multifaceted role of neurofilament light chain protein in non-primary neurological diseases. Brain. 2023;146(2):421-437. doi: 10.1093/BRAIN/AWAC328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. 2015;30(12):1591-1601. doi: 10.1002/MDS.26424 [DOI] [PubMed] [Google Scholar]
- 21.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689-1707. doi: 10.1002/MDS.21507 [DOI] [PubMed] [Google Scholar]
- 22.McKeith IG, Boeve BF, DIckson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. doi: 10.1212/WNL.0000000000004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19(9):1020-1028. doi: 10.1002/MDS.20213 [DOI] [PubMed] [Google Scholar]
- 24.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. doi: 10.1002/MDS.22340 [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. doi: 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 26.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord. 2008;23(7):1043-1046. doi: 10.1002/MDS.22017 [DOI] [PubMed] [Google Scholar]
- 27.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270-279. doi: 10.1016/J.JALZ.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263-269. doi: 10.1016/J.JALZ.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183-194. doi: 10.1111/J.1365-2796.2004.01388.X [DOI] [PubMed] [Google Scholar]
- 30.Teunissen CE, Petzold A, Bennett JL, et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology. 2009;73(22):1914-1922. doi: 10.1212/WNL.0B013E3181C47CC2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellomo G, Indaco A, Chiasserini D, et al. Machine learning driven profiling of cerebrospinal fluid core biomarkers in Alzheimer's disease and other neurological disorders. Front Neurosci. 2021;15:647783. doi: 10.3389/FNINS.2021.647783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halbgebauer S, Steinacker P, Verde F, et al. Comparison of CSF and serum neurofilament light and heavy chain as differential diagnostic biomarkers for ALS. J Neurol Neurosurg Psychiatry. 2022;93(1):68-74. doi: 10.1136/JNNP-2021-327129 [DOI] [PubMed] [Google Scholar]
- 33.Kádková A, Radecke J, Sørensen JB. The SNAP-25 protein family. Neuroscience. 2019;420:50-71. doi: 10.1016/j.neuroscience.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 34.Hall S, Surova Y, Öhrfelt A, Zetterberg H, Lindqvist D, Hansson O. CSF biomarkers and clinical progression of Parkinson disease. Neurology. 2015;84(1):57-63. doi: 10.1212/WNL.0000000000001098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mollenhauer B, Parnetti L, Rektorova I, et al. Biological confounders for the values of cerebrospinal fluid proteins in Parkinson's disease and related disorders. J Neurochem. 2016;139(suppl 1):290-317. doi: 10.1111/JNC.13390 [DOI] [PubMed] [Google Scholar]
- 36.Mollenhauer B, Caspell-Garcia CJ, Coffey CS, et al. Longitudinal analyses of cerebrospinal fluid α-Synuclein in prodromal and early Parkinson's disease. Mov Disord. 2019;34(9):1354-1364. doi: 10.1002/MDS.27806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vos A, Jacobs D, Struyfs H, et al. C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer's disease. Alzheimers Dement. 2015;11(12):1461-1469. doi: 10.1016/J.JALZ.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 38.Nilsson J, Ashton NJ, Benedet AL, et al. Quantification of SNAP-25 with mass spectrometry and Simoa: a method comparison in Alzheimer's disease. Alzheimers Res Ther. 2022;14(1):78. doi: 10.1186/S13195-022-01021-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milà-Alomà M, Brinkmalm A, Ashton NJ, et al. CSF synaptic biomarkers in the preclinical stage of Alzheimer disease and their association with MRI and PET: a cross-sectional study. Neurology. 2021;97(21):E2065-E2078. doi: 10.1212/WNL.0000000000012853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lan G, Cai Y, Li A, Liu Z, Ma S, Guo T. Association of presynaptic loss with Alzheimer's disease and cognitive decline. Ann Neurol. 2022;92(6):1001-1015. doi: 10.1002/ANA.26492 [DOI] [PubMed] [Google Scholar]
- 41.Oberstein TJ, Schmidt MA, Florvaag A, et al. Amyloid-β levels and cognitive trajectories in non-demented pTau181-positive subjects without amyloidopathy. Brain. 2022;145(11):4032-4041. doi: 10.1093/BRAIN/AWAC297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bäckström DC, Domellöf ME, Linder J, et al. Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol. 2015;72(10):1175-1182. doi: 10.1001/JAMANEUROL.2015.1449 [DOI] [PubMed] [Google Scholar]
- 43.Coughlin D, Xie SX, Liang M, et al. Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann Neurol. 2019;85(2):259-271. doi: 10.1002/ANA.25392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colom-Cadena M, Spires-Jones T, Zetterberg H, et al. The clinical promise of biomarkers of synapse damage or loss in Alzheimer's disease. Alzheimers Res Ther. 2020;12(1):21. doi: 10.1186/S13195-020-00588-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howard E, Irwin DJ, Rascovsky K, et al. Cognitive profile and markers of Alzheimer disease-type pathology in patients with Lewy body dementias. Neurology. 2021;96(14):e1855-e1864. doi: 10.1212/WNL.0000000000011699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellomo G, de Luca CMG, Paoletti FP, Gaetani L, Moda F, Parnetti L. α-Synuclein seed amplification assays for diagnosing synucleinopathies: the way forward. Neurology. 2022;99(5):195-205. doi: 10.1212/WNL.0000000000200878 [DOI] [PubMed] [Google Scholar]
- 47.Hall S, Orrù CD, Serrano GE, et al. Performance of αSynuclein RT-QuIC in relation to neuropathological staging of Lewy body disease. Acta Neuropathol Commun. 2022;10(1):90. doi: 10.1186/S40478-022-01388-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Quilico Cousins KA, Arezoumandan S, Shellikeri S, et al. CSF biomarkers of Alzheimer disease in patients with concomitant α-synuclein pathology. Neurology. 2022;99(20):e2303-e2312. 10.1212/WNL.0000000000201202. doi: 10.1212/WNL.0000000000201202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Irwin DJ, Xie SX, Coughlin D, et al. CSF tau and β-amyloid predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology. 2018;90(12):e1038-e1046. doi: 10.1212/WNL.0000000000005166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall S, Janelidze S, Zetterberg H, et al. Cerebrospinal fluid levels of neurogranin in Parkinsonian disorders. Mov Disord. 2020;35(3):513-518. doi: 10.1002/mds.27950 [DOI] [PubMed] [Google Scholar]
- eReferences are listed at; links.lww.com/WNL/C804.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared with qualified investigators on reasonable request to the corresponding author.