Abstract
Background and Objectives
One of the most prevalent chronic diseases, osteoarthritis (OA), may work in conjunction with APOE-ε4 to accelerate Alzheimer disease (AD) alterations, particularly in the primary motor (precentral) and somatosensory (postcentral) cortices. To understand the reasoning behind this, we investigated how OA and APOE-ε4 influence the accumulation of β-amyloid (Aβ) and tau accumulation in primary motor and somatosensory regions in Aβ-positive (Aβ+) older individuals.
Methods
We selected Aβ+ Alzheimer Disease Neuroimaging Initiative participants, defined by baseline 18F-florbetapir (FBP) Aβ PET standardized uptake value ratio (SUVR) of AD summary cortical regions, who had longitudinal Aβ PET, the records of OA medical history, and APOE-ε4 genotyping. We examined how OA and APOE-ε4 relate to baseline and longitudinal Aβ accumulation and tau deposition measured at follow-up in precentral and postcentral cortical areas and how they modulate Aβ-associated future higher tau levels, adjusting for age, sex, and diagnosis and using multiple comparison corrections.
Results
A total of 374 individuals (mean age 75 years, 49.2% female, 62.8% APOE-ε4 carriers) who underwent longitudinal FBP PET with a median follow-up of 3.3 years (interquartile range [IQR] 3.4, range 1.6–9.4) were analyzed, and 96 people had 18F-flortaucipir (FTP) tau PET measured at a median of 5.4 (IQR 1.9, range 4.0–9.3) years postbaseline FBP PET. Neither OA nor APOE-ε4 was related to baseline FBP SUVR in precentral and postcentral regions. At follow-up, OA rather than APOE-ε4 was associated with faster Aβ accumulation in postcentral region (β = 0.005, 95% CI 0.001–0.008) over time. In addition, OA but not the APOE-ε4 allele was strongly linked to higher follow-up FTP tau levels in precentral (β = 0.098, 95% CI 0.034–0.162) and postcentral (β = 0.105, 95% CI 0.040–0.169) cortices. OA and APOE-ε4 were also interactively associated with higher follow-up FTP tau deposition in precentral (β = 0.128, 95% CI 0.030–0.226) and postcentral (β = 0.124, 95% CI 0.027–0.223) regions.
Discussion
This study suggests that OA was associated with faster Aβ accumulation and higher Aβ-dependent future tau deposition in primary motor and somatosensory regions, providing novel insights into how OA increases the risk of AD.
Osteoarthritis (OA) is one of the most common chronic health conditions, leading to clinical manifestations including pain, stiffness, swelling, and limitations in joint function.1 All over the world, approximately 240 million individuals experience symptomatic OA, including 10% of men and 18% of women aged 60 years and older.2 Besides, more than 11% of those aged 65 years and older experience dementia, mainly caused by Alzheimer disease (AD), accounting for 60%–80% of patients with dementia.3 PET imaging has been commonly used to measure the aggregation of extracellular β-amyloid (Aβ) plaques and intracellular neurofibrillary tau tangles,4,5 the key hallmarks of AD.6 According to the National Institute on Aging and Alzheimer's Association research framework,7 Aβ positive (Aβ+) individuals defined by PET imaging are in the AD continuum, who are more likely to undergo cognitive decline than Aβ biomarker normal individuals.8 Of importance, OA may increase the AD risk in older individuals, according to previous reports.9,10 Both animal11 and human tissue12 studies have suggested that OA may accelerate Aβ pathology. Furthermore, 2 human cohort studies found that OA was related to a higher incidence of dementia13 or faster hippocampal atrophy14 in older individuals.
The precentral gyrus, also known as the primary motor cortex, is responsible for executing motor movements, while the postcentral gyrus, referred to as the primary somatosensory cortex, processes the sensations from the body.15 One previous autopsy study16 found similar significant senile plaques in primary motor and somatosensory cortices to that found in other cortical areas in patients with AD. By contrast, the number of neurofibrillary tangles in these 2 regions was marginally lower than those in the entorhinal, frontal, and parietal associative areas. Our group recently observed significant tau deposition measured by PET imaging in precentral and postcentral regions but not in medial temporal areas of individuals who were Aβ PET positive but CSF Aβ42/Aβ40 negative,17 suggesting that tau tangles aggregation in primary motor and somatosensory cortices may also occur in the early stage of AD. Another study18 reported that the somatosensory responses measured by magnetoencephalography were affected early in AD progression and may affect their behavioral and functional performances. Moreover, several studies19-21 found significant gray matter atrophy in precentral and postcentral regions in patients with OA compared with that in healthy controls. In addition, it has been reported that patients with knee OA had a significant disrupted representation of the knee in both primary motor22 and primary sensory23 cortices. Together, these studies16-23 suggest that primary motor and somatosensory cortices are significantly involved in OA and AD disorders.
Furthermore, 2 animal studies24,25 revealed that inflammatory OA mice model with an expression of the human APOE-ε4 gene, the most important genetic risk factor of sporadic AD,26 developed a more severe OA compared with mice with the human APOE-ε3 gene. It has also been demonstrated that the APOE-ε4 allele could promote inflammatory changes in microglia and astrocytes.27 Together, it raises the possibility that OA and APOE-ε4 alleles may interact to affect the abnormal changes of AD pathophysiology in primary motor and somatosensory regions. However, the rationale behind this is still not fully understood.
In this study, we analyzed Aβ+ older individuals from the Alzheimer Disease Neuroimaging Initiative (ADNI) who had longitudinal 18F-florbetapir (FBP) Aβ PET scans, APOE-ε4 genotype, and OA medical history records. Parted of them had follow-up 18F-flortaucipir (FTP) tau PET scan measured at a median of 5.4 years postbaseline Aβ PET scan. We investigated how OA and APOE-ε4 independently and interactively affect the aggregation of cortical Aβ plaques and tau tangles in precentral and postcentral cortical regions in the AD continuum.7 The ultimate goal is to provide imaging evidence to underlie the mechanism behind OA-related high risk of AD and help better health management of older individuals, especially individuals with both OA and AD disorders.
Methods
Participants
Data used in this study were obtained from the ADNI database (ida.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of mild cognitive impairment (MCI) and early AD as we previously described.28 For up-to-date information, see adni-info.org. We identified cognitively unimpaired (CU) individuals and cognitively impaired (CI) individuals (MCI and dementia due to AD) with longitudinal Aβ PET, the records of OA medical history, and APOE-ε4 genotyping and who were Aβ+ at baseline. The OA (key words “Osteoarth” or “arthritis”) history was determined as with or without OA disorder according to the participants' self-reported medical history (RECMHIST.csv and INITHEALTH.csv files downloaded from the ADNI website on December 30, 2021). Individuals with and without OA disease histories were defined as OA+ and OA−, respectively, and carrying and noncarrying 1 APOE-ε4 allele were defined as APOE-ε4+ and APOE-ε4−, respectively.
Standard Protocol Approvals, Registrations, and Patient Consents
The ADNI study was approved by institutional review boards of all participating centers, and written informed consent was obtained from all participants or their authorized representatives.
Aβ PET Imaging
The details of FBP Aβ PET image acquisition are provided on the ADNI website (adni-info.org). In brief, from 50 to 70 minutes after injection, FBP PET data were collected in 4 × 5-minute frames. The T1-weighted structural MRI and completely preprocessed FBP PET images were downloaded from the LONI website (ida.loni.usc.edu). FBP scans were coregistered to their corresponding structural MRI scans.4 The Desikan-Killiany atlas29 was referred to define 34 regions of interest (ROIs) in FreeSurfer (version 7.1.0), and these ROIs were used to extract cortical tracer retention of FBP. The mean FBP uptake in the whole cerebellum was calculated as the reference, and the regional FBP uptake was normalized to generate FBP standardized uptake value ratios (SUVRs). Aβ+ was defined as FBP SUVR in AD summary cortical regions (composed of frontal, cingulate, parietal, and lateral temporal cortical regions) ≥1.11 as previously described.5 Because 1 big composite (made up of brainstem, whole cerebellum, and eroded white matter) reference has shown less variance in longitudinal changes of FBP SUVRs,30s we used the big composite reference to calculate the SUVRs in precentral and postcentral regions for the following analyses.
Tau PET Imaging
The information on FTP tau PET image acquisition was also provided on the ADNI website (adni-info.org). From 75 to 105 minutes after injection, 6 × 5-minute frames of FTP PET data were collected. The fully preprocessed FTP PET scans were coregistered to their corresponding T1-weighted MRI scans. FTP uptakes in 34 cortical ROIs were also extracted in individual structural MRI space as previously described.29 A mean inferior cerebellar gray matter FTP uptake was referred to calculate regional FTP SUVR.31 The intensity-normalized and spatially normalized FTP PET images at the voxel-wise level in the Montreal Neurological Institute space and finally smoothed using a Gaussian kernel of 8 mm in statistical parametric mapping (SPM) 12 (Welcome Department of Imaging Neuroscience, London, United Kingdom) were used for the voxel-wise analyses as previously described.17
Statistical Analysis
Unless otherwise stated, statistical analyses were conducted using R (version 4.0.4; The R Foundation for Statistical Computing, Vienna, Austria). The Shapiro-Wilk test and a visual examination of the data histograms were used to determine whether the distributions were normal. The differences in continuous and categorical characteristics at baseline between OA+ and OA− were compared using 2-tailed Mann-Whitney U and Fisher exact tests, respectively. Data were presented as median (interquartile range, IQR) or n (%) unless otherwise noted. A false discovery rate of <0.05 using the Benjamini-Hochberg approach was used for multiple comparison corrections.
To determine the associations of OA disease and APOE-ε4 allele with cortical Aβ plaques in AD, we first investigated how OA and APOE-ε4 relate to baseline FBP SUVRs in precentral and postcentral cortical regions using generalized linear models (GLMs) as follows:
Model 1: Baseline FBP SUVR − OA × APOE-ε4 + age + sex + diagnosis
Model 1 investigated the main effect of OA and APOE4 status and their interaction on baseline FBP SUVR, controlling for age, sex, and diagnosis.
We subsequently investigated how OA and APOE-ε4 relate to longitudinal changes of FBP SUVR in precentral and postcentral cortical regions using linear mixed-effects models (lme4 package) as follows:
Model 2: FBP SUVR − Time × OA × APOE-ε4 + age + sex + diagnosis + (1 + Time|Subject)
Model 2 investigated how OA status and APOE4 status and their interaction are associated with longitudinal changes of FBP SUVR over time, including the same covariates above.
Furthermore, we studied how OA and APOE-ε4 relate to follow-up FTP SUVRs in precentral and postcentral cortices measured at a median of 5.4 years postbaseline Aβ PET scan and how they modulate the association between baseline FBP SUVR and follow-up FTP SUVRs using GLMs as follows:
Model 3: Follow-up FTP SUVR − OA × APOE-ε4 + baseline FBP SUVR+ age + sex + diagnosis
Model 3 investigated how OA status and APOE4 status and their interaction are related to the follow-up FTP SUVR levels, including baseline FBP SUVR, age, sex, and diagnosis.
Model 4: Follow-up FTP SUVR − baseline FBP SUVR × OA × APOE-ε4 + age + sex + diagnosis
Model 4 investigated how baseline FBP SUVRs, OA status, and APOE4 and their interaction are related to the follow-up FTP SUVR levels, including the same covariates above.
Notably, to rectify the data's non-normal distribution, we used a “log” link function from the Gaussian family in GLMs for the FTP SUVR.
We also compared the voxel-wise FTP SUVR PET images between the OA+ group and the OA− (ref) group using a 2-sample t test in SPM12, controlling for age, sex, and diagnosis. The voxel-wise comparisons between the OA+ and OA− groups were presented using an uncorrected voxel threshold of p < 0.001. We performed these analyses separately in APOE-ε4 carriers, APOE-ε4 noncarriers, and the whole cohort.
Data Availability
The ADNI database (adni.loni.usc.edu) provided all the data used in this study. Any qualified investigator may request the derived data from the corresponding author upon the terms of a data use agreement.
Results
Demographic Characteristics
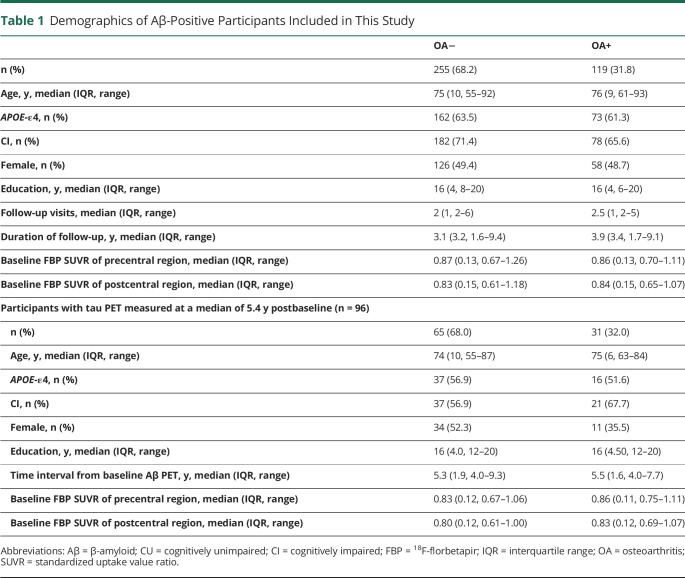
The demographic characteristics of participants at baseline are summarized in Table 1. We analyzed 374 Aβ+ individuals (114 CU, 212 MCI, and 48 dementia due to AD) with longitudinal Aβ PET (median duration 3.3 [IQR 3.4, range 1.6–9.4] years), records of OA medical history, and APOE-ε4 genotyping. Of them, 260 (69.5%) were CI, 235 (62.8%) were APOE-ε4 carriers, 184 (49.2%) were female individuals, and 119 (31.8%) were OA+. Of them, 97 individuals (38 CU, 58 MCI, and 1 with dementia due to AD) had follow-up FTP PET scans measured at a median of 5.4 years postbaseline FBP PET. Notably, 1 MCI individual whose follow-up FTP SUVRs in precentral and postcentral regions were 5.8 and 6.7 standard deviations above the sample's mean value were excluded from the following analysis. No significant differences were found in age, education, baseline FBP SUVR in precentral and postcentral regions, and percentages of female individuals, APOE-ε4 carriers, and CI between OA+ and OA− groups.
Table 1.
Demographics of Aβ-Positive Participants Included in This Study
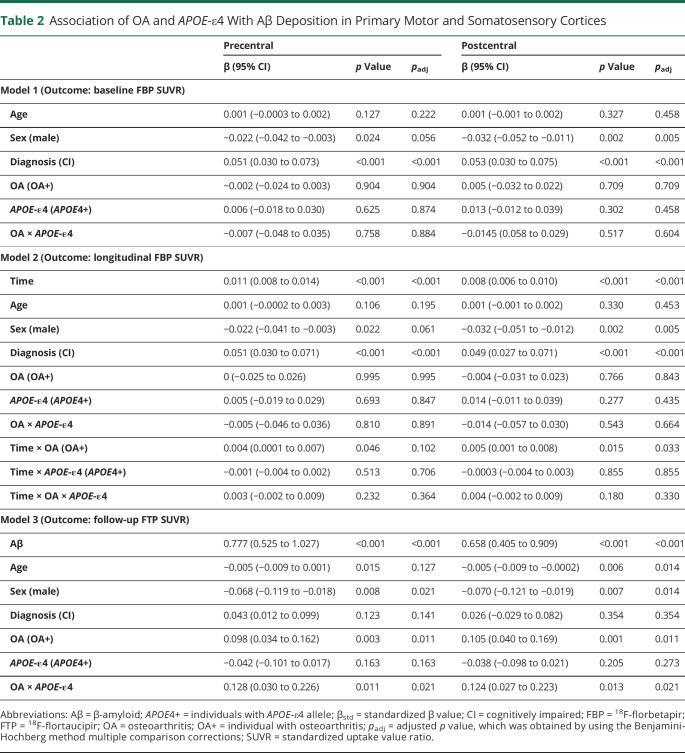
Association of OA and APOE-ε4 Allele With Aβ Accumulation in Primary Motor and Somatosensory Cortices
At baseline, female individuals and impaired cognition were related to higher baseline FBP SUVRs of precentral and postcentral cortices. By contrast, neither OA nor APOE-ε4 allele was associated with baseline FBP SUVRs of precentral and postcentral cortices (Table 2). Longitudinally, OA+ individuals showed significantly faster rates of Aβ accumulation in precentral and postcentral regions than OA− individuals. Notably, the rapid rates of Aβ accumulation in the precentral region disappeared after multiple comparison corrections. By contrast, APOE-ε4 did not have an independent influence or interactive effect with OA in longitudinal Aβ accumulation in precentral and postcentral cortices (Table 2). The other factors did not significantly correlate with longitudinal Aβ accumulation either.
Table 2.
Association of OA and APOE-ε4 With Aβ Deposition in Primary Motor and Somatosensory Cortices
Association of OA and APOE-ε4 Allele With Follow-up Tau Deposition in Primary Motor and Somatosensory Cortices
In 96 participants with follow-up FTP tau PET measured at a median of 5.4 years postbaseline Aβ PET, OA disorder was associated with a higher follow-up FTP SUVR in precentral and postcentral cortices (Table 2). In addition, the association between OA disorder and follow-up FTP SUVR was much more robust in APOE-ε4 carriers than in APOE-ε4 noncarriers. Younger ages and female individuals were related to higher tau deposition in precentral and postcentral regions. However, the APOE-ε4 allele did not show an independent effect in follow-up tau aggregation of these regions.
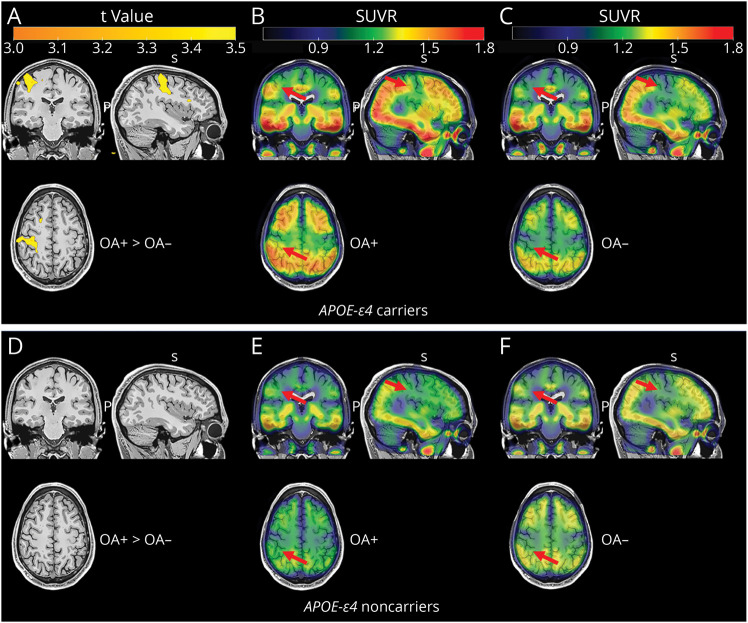
The voxel-wise analysis supported the predefined ROI analysis. Specifically, OA status was associated with higher FTP tau deposition in the precentral and postcentral cortices in 53 APOE-ε4 carriers (Figure 1, A–C). However, the significance did not survive after correction with a family-wise error corrected p < 0.05 at the cluster level. In addition, no significant difference was found in FTP tau between OA+ and OA− groups in 43 APOE-ε4 noncarriers (Figure 1, D–F) and the whole cohort (data not shown).
Figure 1. Voxel-wise Comparisons of Follow-up Tau PET Between OA+ and OA− Groups in Aβ+ Older Individuals.
(A) Comparison of FTP tau PET between OA+ and OA−, and the mean tau PET images of (B) OA+ group and (C) OA− group in APOE-ε4 carriers. (D) Comparison of FTP tau PET between OA+ group and OA− group, and the mean tau PET images of (E) OA+ group and (F) OA− group in APOE-ε4 noncarriers. Brain maps were created at p < 0.001 at the voxel level without cluster correction and overlayed in an MRI template in MNI space. Red arrows indicate cortical regions of interest. Aβ = β-amyloid; MNI = Montreal Neurological Institute and Hospital; OA+ = participants with osteoarthritis; OA− = participants without osteoarthritis; SUVR = standardized uptake value ratio.
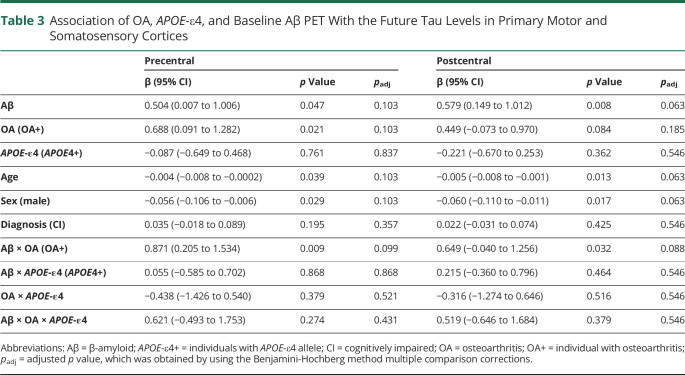
Association of OA and APOE-ε4 Allele With Aβ-Related Tau Accumulation in Primary Motor and Somatosensory Cortices
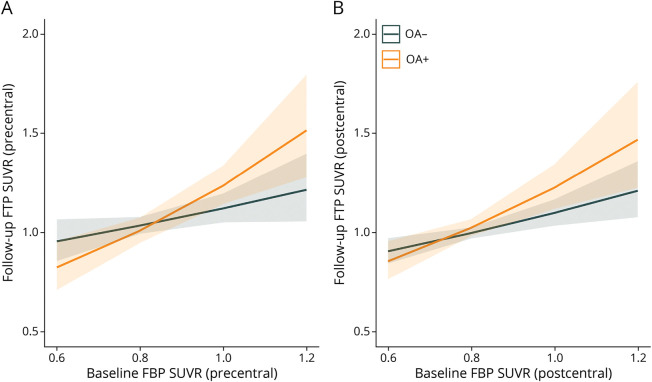
Table 3 summarizes and Figure 2 shows that higher baseline Aβ PET and OA disorder were independently and interactively related to significant or marginal higher follow-up tau deposition in precentral and postcentral cortices. Younger ages and female individuals were also associated with greater tau deposition. Notably, these associations became marginal or disappeared after multiple comparison corrections. By contrast, APOE-ε4 had no independent influence or interactive effect with OA or baseline Aβ PET in follow-up tau accumulation in precentral and postcentral cortices (Table 3).
Table 3.
Association of OA, APOE-ε4, and Baseline Aβ PET With the Future Tau Levels in Primary Motor and Somatosensory Cortices
Figure 2. Associations Between Baseline FBP SUVR and Follow-up FTP SUVR in Precentral and Postcentral Cortical Regions.
The comparisons of Aβ-related higher tau levels between OA+ and OA− groups in (A) precentral and (B) postcentral regions. Aβ = β-amyloid; FBP = 18F-florbetapir; FTP = 18F-flortaucipir; OA+ = participants with osteoarthritis; OA− = participants without osteoarthritis; SUVR = standard uptake value ratio.
Discussion
In this study, we investigated how OA disorder and APOE-ε4 allele relate to longitudinal changes of Aβ plaques in precentral and postcentral cortices over a median of 3.3 years and corresponding follow-up tau tangles measured at a median of 5.4 years postbaseline Aβ PET. This showed that OA disorder but not the APOE-ε4 allele was associated with faster longitudinal Aβ accumulation and follow-up tau deposition in precentral and postcentral cortices. The OA disorder showed a significant positive association with the follow-up tau deposition in precentral and postcentral cortices independently and interactively with the APOE-ε4 allele. In addition, we found that OA disease but not the APOE-ε4 allele was related to higher Aβ-associated tau levels in the precentral cortex. These results offer novel insights into why OA disorders, a common chronic disease in older individuals, increase the risk of AD.
The previous animal study11 observed increased numbers of Aβ plaques and higher expression of neuroinflammatory factors in the brain in OA-induced amyloid precursor protein/presenilin 1 (APP/PS1) mice compared with those in the age-matched and sex-matched APP/PS1 mice, but less was known about how OA affects cortical Aβ accumulation in the human brain. Our analyses with a large longitudinal Aβ PET dataset revealed that OA disease could accelerate the rates of Aβ accumulation in primary motor and somatosensory cortices related to general bodily movement and sensation. One animal study found that early peripheral joint injury may trigger central spinal microglia activation, which increases the release of proinflammatory cytokine.32 The proinflammatory cytokine might facilitate the production of Aβ and promote the seeding of Aβ plaques.33 Together, it is likely that the peripheral inflammation induced by OA might trigger a proinflammatory response in the central neuron system and subsequently accelerate Aβ plaques accumulation in AD.
Consistent with previous study,34,35 we also found APOE-ε4 allele is not related to faster Aβ accumulation in Aβ+ individuals. However, we observed OA-associated faster Aβ accumulation in primary motor and somatosensory cortices in Aβ+ individuals. Although Aβ accumulation in precentral and postcentral regions are usually involved in the late stage of AD,36 OA disease may accelerate Aβ aggregation in precentral and postcentral cortices in early or intermediate stages of AD (73% of individuals in this study were non-demented). Consequently, OA-related faster Aβ accumulation in precentral and postcentral regions should be paid more attention to in the future.
No PET study has investigated the association between OA and tau aggregation in AD. This study found that OA disorder was positively related to elevated follow-up tau tangles in precentral and postcentral cortical regions measured approximately 5 years later. The OA disorder also interacted with the APOE-ε4 allele in predicting higher follow-up tau deposition. The voxel-wise findings were in line with the region-wise analysis. Notably, our laboratory4,8,37 and other groups38-41 have demonstrated that Aβ deposition can drive cortical tau aggregation in AD. Intriguingly, we further found that OA disorder was significantly associated with higher Aβ-related follow-up tau aggregation in the precentral and postcentral regions. However, the APOE-ε4 allele did not show an independent or interactive effect in Aβ-related follow-up tau aggregation in either precentral or postcentral areas. Altogether, these findings suggest that OA disease may be associated with faster Aβ accumulation over time and higher future tau aggregation in primary motor and somatosensory cortices in the AD continuum,7 and the more prominent tau deposition in the precentral region may be explained by the more substantial Aβ-dependent tau accumulation in OA+ individuals.
One functional MRI study22 found that patients with knee OA showed a significant anterior shift in the knee representation and swap of the relative position of the knee and ankle representations in the motor cortex, providing further evidence for the link between OA and motor cortex. In addition, Stanton et al.23 reported that tactile acuity at the knee was decreased in painful knee OA, implying a disrupted representation of the knee in the primary sensory cortex. Furthermore, a few brain stimulation studies42-44 demonstrated the critical function of the primary motor cortex in reducing pain for older individuals with OA diseases. These studies suggest that primary motor and somatosensory cortices are significantly involved in OA disease. Our findings provide novel PET imaging evidence that OA may be associated with more aggregation of Aβ plaques and tau tangles in primary motor and somatosensory cortices in Aβ+ older individuals. Together with previous findings that an elevated tau level was closely linked to neurodegeneration,4,45,46 the higher tau aggregation in primary motor and somatosensory cortices may lead to more neurodegeneration in these regions and result in more motor and sensory dysfunction, including olfaction, hearing, and even walking speed.47,48 Considering the high prevalence of OA disease in older individuals, more attention and better clinical management are extremely important for individuals in the AD continuum with an OA disease history.
The major strength of this study is that we analyzed a series of Aβ PET and tau PET image data, which revealed the relationship between OA, APOE-ε4, and AD key pathologies in Aβ+ older individuals. This is important for understanding how OA increases the risk of AD. However, this study has a few limitations. First, the OA medical history was defined by referring to self-reported medical history in the ADNI cohort. Thus, future studies with more precise assessments of OA disease in other datasets would be beneficial. Second, the OA disorder most commonly affects joints in the patient's hands, knees, hips, and spine, although it can damage any joint, while this study cannot explain the association between any subtypes of OA and AD. Third, the sample size of participants with follow-up tau PET was relatively small, which may need to be validated in other cohorts with large sample sizes and more longitudinal tau PET data. Moreover, we did not adjust for body mass index and physical activity as the covariates because we investigated the association between OA and AD, but they may be associated with faster Aβ accumulation according to the previous literature.49,50
Overall, this longitudinal Aβ PET and follow-up tau PET imaging study suggest that OA diseases may accelerate faster Aβ accumulation and induce higher Aβ-related tau deposition in primary motor and somatosensory cortices in Aβ+ older individuals. The association between OA and AD pathologies, such as faster Aβ plaque accumulation and more significant Aβ-dependent tau deposition in precentral and postcentral cortices, highlights the importance of considering the influence of other common chronic health conditions (e.g., OA) for better health management of AD patients in future.
Glossary
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer Disease Neuroimaging Initiative
- CI
cognitively impaired
- CU
cognitively unimpaired
- GLM
generalized linear model
- IQR
interquartile range
- MCI
mild cognitive impairment
- OA
osteoarthritis
- FBP
18F-florbetapir
- FTP
18F-flortaucipir
- ROI
region of interest
- SPM
statistical parametric mapping
- SUVR
standardized uptake value ratio
Appendix 1. Authors

Appendix 2. Coinvestigators

Study Funding
This work was supported by the Guangdong Basic and Applied Basic Science Foundation for Distinguished Young Scholars (2023B1515020113). Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). The ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer Association; Alzheimer Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC; Johnson & Johnson Pharmaceutical Research & Development LLC; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115-2126. [DOI] [PubMed] [Google Scholar]
- 2.Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthr Cartil. 2022;30(2):184-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alzheimer's Association. 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. [DOI] [PubMed] [Google Scholar]
- 4.Guo T, Korman D, Baker SL, Landau SM, Jagust WJ. Longitudinal cognitive and biomarker measurements support a unidirectional pathway in Alzheimer's disease pathophysiology. Biol Psychiatry. 2021;89(8):786-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T, Brendel M, Grimmer T, Rominger A, Yakushev I. Predicting regional pattern of longitudinal β-amyloid accumulation by baseline PET. J Nucl Med. 2017;58(4):639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci. 2018;19(11):687-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T, Landau SM, Jagust WJ. Detecting earlier stages of amyloid deposition using PET in cognitively normal elderly adults. Neurology. 2020;94(14):e1512-e1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikram M, Innes K, Sambamoorthi U. Association of osteoarthritis and pain with Alzheimer's diseases and related dementias among older adults in the United States. Osteoarthr Cartil. 2019;27(10):1470-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Innes KE, Sambamoorthi U. The association of osteoarthritis and related pain burden to incident Alzheimer's disease and related dementias: a retrospective cohort study of U.S. Medicare beneficiaries. J Alzheimers Dis. 2020;75(3):789-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyrkanides S, Tallents RH, Miller JH, et al. Osteoarthritis accelerates and exacerbates Alzheimer's disease pathology in mice. J Neuroinflammation. 2011;8(1):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li S, Liu B, Zhang L, Rong L. Amyloid beta peptide is elevated in osteoporotic bone tissues and enhances osteoclast function. Bone. 2014;61:164-175. [DOI] [PubMed] [Google Scholar]
- 13.Huang S-W, Wang W-T, Chou L-C, Liao C-D, Liou T-H, Lin H-W. Osteoarthritis increases the risk of dementia: a nationwide cohort study in Taiwan. Sci Rep. 2015;5(1):10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Tong Q, Gao J, Liu C, Liu Y. Osteoarthritis was associated with a faster decline in hippocampal volumes in cognitively normal older people. Front Aging Neurosci. 2020;12:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garey LJ. Brodmann's Localisation in the Cerebral Cortex. World Scientific; 1999. [Google Scholar]
- 16.Suvà D, Favre I, Kraftsik R, Esteban M, Lobrinus A, Miklossy J. Primary motor cortex involvement in Alzheimer disease. J Neuropathol Exp Neurol. 1999;58(11):1125-1134. [DOI] [PubMed] [Google Scholar]
- 17.Jiang C, Wang Q, Xie S, et al. β-Amyloid discordance of cerebrospinal fluid and positron emission tomography imaging shows distinct spatial tau patterns. Brain Commun. 2022;4(2):fcac084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephen JM, Montaño R, Donahue CH, et al. Somatosensory responses in normal aging, mild cognitive impairment, and Alzheimer's disease. J Neural Transm. 2010;117(2):217-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barroso J, Vigotsky AD, Branco P, et al. Brain gray matter abnormalities in osteoarthritis pain: a cross-sectional evaluation. Pain. 2020;161(9):2167-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundermann B, Dehghan Nayyeri M, Pfleiderer B, et al. Subtle changes of gray matter volume in fibromyalgia reflect chronic musculoskeletal pain rather than disease‐specific effects. Eur J Neurosci. 2019;50(12):3958-3967. [DOI] [PubMed] [Google Scholar]
- 21.Liao X, Mao C, Wang Y, et al. Brain gray matter alterations in Chinese patients with chronic knee osteoarthritis pain based on voxel-based morphometry. Medicine (Baltimore). 2018;97(12):e0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanahan CJ, Hodges PW, Wrigley TV, Bennell KL, Farrell MJ. Organisation of the motor cortex differs between people with and without knee osteoarthritis. Arthritis Res Ther. 2015;17(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanton TR, Lin CWC, Bray H, et al. Tactile acuity is disrupted in osteoarthritis but is unrelated to disruptions in motor imagery performance. Rheumatology. 2013;52(8):1509-1519. [DOI] [PubMed] [Google Scholar]
- 24.van den Bosch M, Kruisbergen N, de Munter W, et al. More severe OA joint pathology in human APOE-ε4 as compared to APOE-ε3 transgenic mice: APOE-isoforms as possible risk factor for inflammatory osteoarthritis development? Osteoarthr Cartil. 2018;26:S123. [Google Scholar]
- 25.de Munter W, Ascone G, Blom A, et al. Human APOε4 results in more severe experimental osteoarthritis in comparison to APOε3: Apoe-isoforms as possible risk factor for inflammatory osteoarthritis development? Osteoarthr Cartil. 2017;25:S270. [Google Scholar]
- 26.Serrano-Pozo A, Das S, Hyman BT. APOE and Alzheimer's disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021;20(1):68-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzioras M, Davies C, Newman A, Jackson R, Spires-Jones T. Invited review: APOE at the interface of inflammation, neurodegeneration and pathological protein spread in Alzheimer's disease. Neuropathol Appl Neurobiol. 2019;45(4):327-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lan G, Cai Y, Li A, Liu Z, Ma S, Guo T. Association of presynaptic loss with Alzheimer's disease and cognitive decline. Ann Neurol. 2022;92(6):1001-1015. [DOI] [PubMed] [Google Scholar]
- 29.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968-980. [DOI] [PubMed] [Google Scholar]
- 30.Landau SM, Fero A, Baker SL, et al. Measurement of longitudinal β-amyloid change with 18 F-florbetapir PET and standardized uptake value ratios. J Nucl Med. 2015;56(4):567-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maass A, Landau S, Baker SL, et al. Comparison of multiple tau-PET measures as biomarkers in aging and Alzheimer's disease. Neuroimage. 2017;157:448-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mousseau M, Burma NE, Lee KY, et al. Microglial pannexin-1 channel activation is a spinal determinant of joint pain. Sci Adv. 2018;4(8):eaas9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat Rev Neurol. 2021;17(3):157-172. [DOI] [PubMed] [Google Scholar]
- 34.Lim YY, Mormino EC. APOE genotype and early β-amyloid accumulation in older adults without dementia. Neurology. 2017;89(10):1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burnham SC, Laws SM, Budgeon CA, et al. Impact of APOE-ε4 carriage on the onset and rates of neocortical Aβ-amyloid deposition. Neurobiol Aging. 2020;95:46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo T, Dukart J, Brendel M, Rominger A, Grimmer T, Yakushev I. Rate of β-amyloid accumulation varies with baseline amyloid burden: implications for anti-amyloid drug trials. Alzheimers Dement. 2018;14(11):1387-1396. [DOI] [PubMed] [Google Scholar]
- 37.Leal SL, Lockhart SN, Maass A, Bell RK, Jagust WJ. Subthreshold amyloid predicts tau deposition in aging. J Neurosci. 2018;38(19):4482-4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanseeuw BJ, Betensky RA, Jacobs HIL, et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease. JAMA Neurol. 2019;76(8):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack CR, Wiste HJ, Weigand SD, et al. Predicting future rates of tau accumulation on PET. Brain. 2020;143(10):3136-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knopman DS, Lundt ES, Therneau TM, et al. Association of initial β-amyloid levels with subsequent flortaucipir positron emission tomography changes in persons without cognitive impairment. JAMA Neurol. 2021;78(2):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doré V, Krishnadas N, Bourgeat P, et al. Relationship between amyloid and tau levels and its impact on tau spreading. Eur J Nucl Med Mol Imaging. 2021;48(7):2225-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahn H, Woods AJ, Kunik ME, et al. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: an experimenter- and participant-blinded, randomized, sham-controlled pilot clinical study. Brain Stimul. 2017;10(5):902-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavares DRB, Okazaki JEF, Santana MVdA, et al. Motor cortex transcranial direct current stimulation effects on knee osteoarthritis pain in elderly subjects with dysfunctional descending pain inhibitory system: a randomized controlled trial. Brain Stimul. 2021;14(3):477-487. [DOI] [PubMed] [Google Scholar]
- 44.Apkarian AV, Bushnell MC, Treede R-D, Zubieta J-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463. [DOI] [PubMed] [Google Scholar]
- 45.La Joie R, Visani AV, Baker SL, et al. Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. 2020;12(524):eaau5732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison TM, La Joie R, Maass A, et al. Longitudinal tau accumulation and atrophy in aging and alzheimer disease. Ann Neurol. 2019;85(2):229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchman AS, Bennett DA. Loss of motor function in preclinical Alzheimer's disease. Expert Rev Neurother. 2011;11(5):665-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albers MW, Gilmore GC, Kaye J, et al. At the interface of sensory and motor dysfunctions and Alzheimer's disease. Alzheimers Dement. 2015;11(1):70-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gottesman RF, Schneider ALC, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabin JS, Klein H, Kirn DR, et al. Associations of physical activity and β-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol. 2019;76(10):1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The ADNI database (adni.loni.usc.edu) provided all the data used in this study. Any qualified investigator may request the derived data from the corresponding author upon the terms of a data use agreement.