Abstract
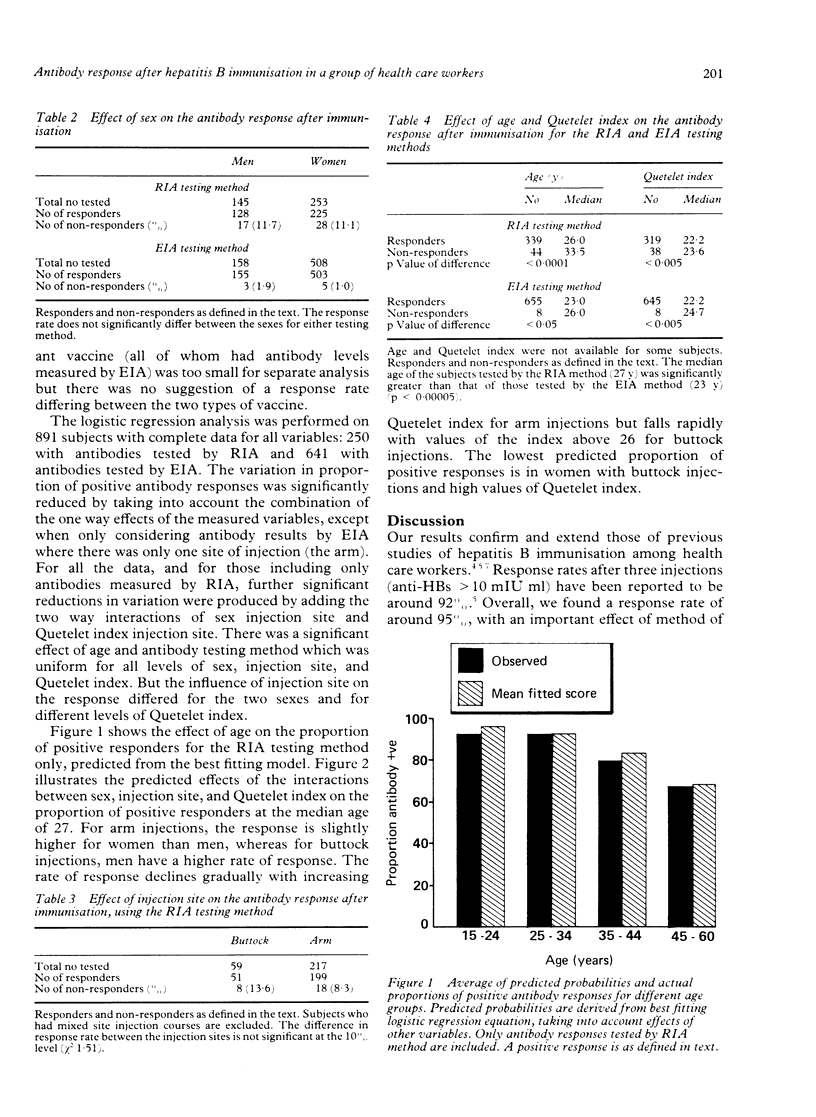
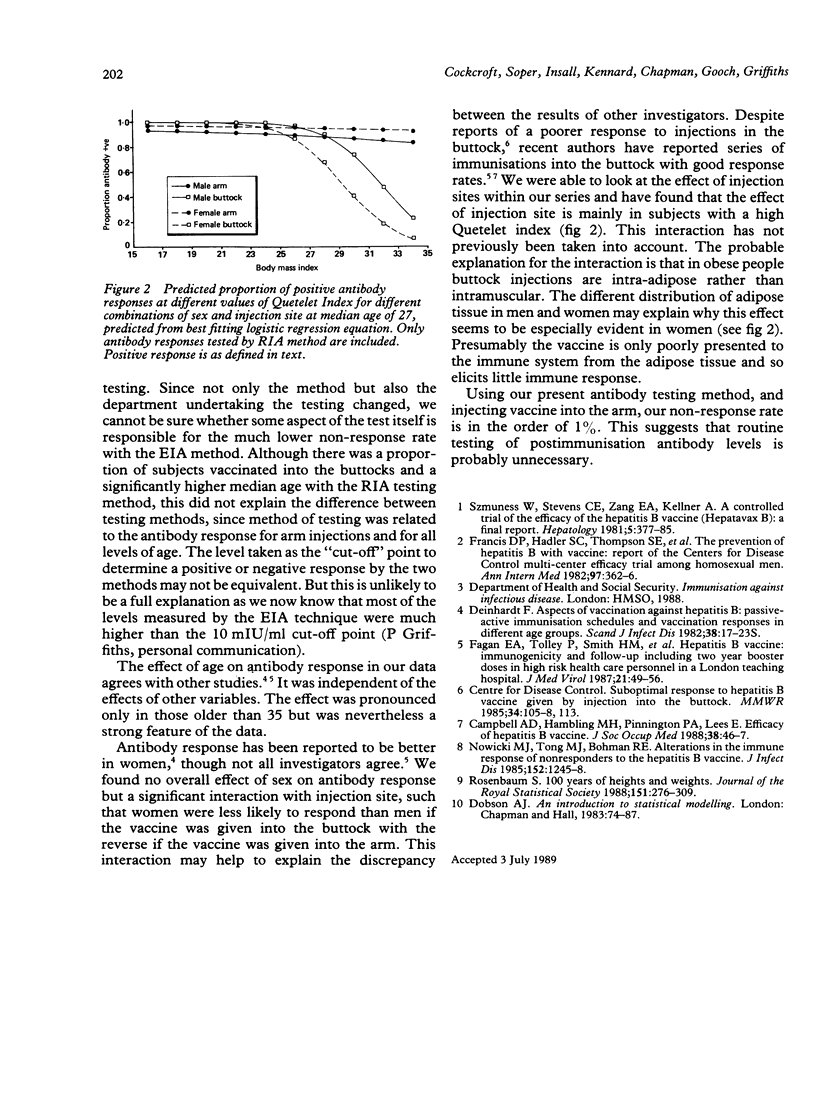
Hepatitis B immunisation has been offered to staff of Hampstead Health Authority since 1982 and is now offered to all staff with clinical contact. Three doses of 20 micrograms of vaccine are given at zero, one, and six months and the antibody response is measured three months later. Results were analysed to seek for associations with the antibody response. At the time of analysis, 2739 people had started vaccination and 1067 had completed the course and had a measurement of antibody response. Vaccine injections were initially into the buttock and later into the arm; measurement of antibody levels was initially by radioimmunoassay (RIA) and later by enzyme immunoassay (EIA). A positive antibody response was defined as a positive/negative ratio of greater than 10 for RIA or a level of greater than 10 mIU/ml for EIA. Associations between antibody response and other variables were tested by chi 2 and a multiple logistic regression analysis was undertaken to examine the effects of variables in combination. The overall antibody response rate was 95%. Men and women did not respond differently but there were significantly more positive responses with the EIA testing method and a tendency for more positive responses with arm injections. The responders were significantly younger than the non-responders and had significantly lower values of body mass index (wt/ht2).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell A. D., Hambling M. H., Pinnington P. A., Lees E. Efficacy of hepatitis B vaccine. J Soc Occup Med. 1988 Spring-Summer;38(1-2):46–47. doi: 10.1093/occmed/38.1-2.46. [DOI] [PubMed] [Google Scholar]
- Deinhardt F. Aspects of vaccination against hepatitis B; passive-active immunization schedules and vaccination responses in different age groups. Scand J Infect Dis Suppl. 1983;38:17–23. [PubMed] [Google Scholar]
- Fagan E. A., Tolley P., Smith H. M., Peters M. P., Coleman J., Elliott P., Williams R., Eddleston A. L. Hepatitis B vaccine: immunogenicity and follow-up including two year booster doses in high-risk health care personnel in a London teaching hospital. J Med Virol. 1987 Jan;21(1):49–56. doi: 10.1002/jmv.1890210107. [DOI] [PubMed] [Google Scholar]
- Francis D. P., Hadler S. C., Thompson S. E., Maynard J. E., Ostrow D. G., Altman N., Braff E. H., O'Malley P., Hawkins D., Judson F. N. The prevention of hepatitis B with vaccine. Report of the centers for disease control multi-center efficacy trial among homosexual men. Ann Intern Med. 1982 Sep;97(3):362–366. doi: 10.7326/0003-4819-97-3-362. [DOI] [PubMed] [Google Scholar]
- Nowicki M. J., Tong M. J., Bohman R. E. Alterations in the immune response of nonresponders to the hepatitis B vaccine. J Infect Dis. 1985 Dec;152(6):1245–1248. doi: 10.1093/infdis/152.6.1245. [DOI] [PubMed] [Google Scholar]
- Szmuness W., Stevens C. E., Zang E. A., Harley E. J., Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981 Sep-Oct;1(5):377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]


