Abstract
Background:
Personalized treatment for clinical T1 renal cortical masses (RCMs) should account for competing risks related to tumor and patient characteristics.
Objective:
To develop treatment-specific prediction models for cancer-specific mortality (CSM), other-cause mortality (OCM), and 90-day Clavien ≥3 complications across radical nephrectomy (RN), partial nephrectomy (PN), thermal ablation (TA), and active surveillance (AS).
Design, Setting, and Participants:
Pretreatment clinical and radiological features were collected for consecutive adult RCM patients treated with initial RN, PN, TA, or AS at four high-volume referral centers (2000–2019).
Outcome Measurement and Statistical Analysis:
Prediction models used competing risks regression for CSM and OCM and logistic regression for 90-day Clavien grade ≥3 complications. Performance was assessed using bootstrap validation.
Results and Limitations:
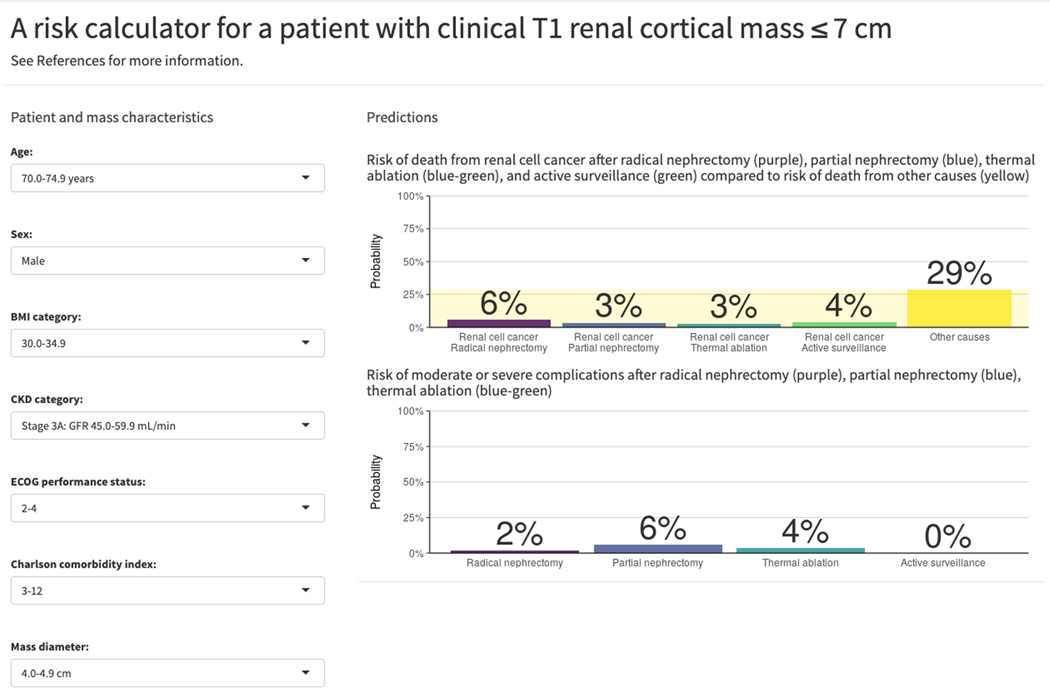
The cohort comprised 5300 patients treated with RN (1277), PN (2967), TA (476), or AS (580). With median follow-up of 5.2 years (IQR 2.5–8.7), there were 117 CSM, 607 OCM, and 198 complication events. C-indices for the predictive models were 0.80, 0.77, and 0.64 for CSM, OCM, and complications, respectively. Predictions from the fitted models are provided in an online calculator (https://small-renal-mass-risk-calculator.fredhutch.org). To illustrate, a hypothetical 74-year-old male with a 4.5cm RCM, BMI of 32 kg/m2, eGFR of 50 mL/min, ECOG PS of 3, and CCI of 3, has a predicted 5-year CSM of 2.9–5.6% across treatments, but a 5-year OCM of 29%, and 90-day risk of Clavien 3–5 complications of 1.9%, 5.8%, and 3.6% for RN, PN, and TA, respectively. Limitations include selection bias, heterogeneity in practice across treatment sites and the study time period, and lack of control for surgeon/hospital volume.
Conclusions:
We present a risk calculator incorporating pretreatment features to estimate treatment-specific competing risks of mortality and complications for use during shared decision-making and personalized treatment election
Patient Summary:
We present a risk calculator that generates personalized estimates of the risks of death from cancer or other causes and complications for surgical, ablation, and surveillance treatment options for patients with stage 1 kidney tumors.
Keywords: Renal Cell Carcinoma, Decision-Aid, Comorbidity, Performance Status, Treatment, Nephrectomy, Ablation, Surveillance, Shared-Decision Making, Competing Risks
Introduction
Patients with clinical T1 (cT1) renal cortical masses (RCMs) may be offered up to four treatment options: radical nephrectomy (RN), partial nephrectomy (PN), thermal ablation (TA), or active surveillance (AS).[1–4] While RN was traditionally considered the gold standard for the management of all renal masses, recent guidelines recommend PN as the preferred treatment modality when feasible to maximally preserve renal function, acknowledging the slight increase in the complication profile with a nephron-sparing approach.[3] Treatment election must balance the competing risks of the tumor with those related to the patient’s health, including comorbidities and performance status. This calculus is complex and involves substantial uncertainty with few validated tools available to assist in quantifying the trade-offs of different therapeutic approaches. Currently, the majority of patients with RCMs are treated operatively, and concern exists over the limited adoption of AS and the potential for overtreatment, especially among older and medically complex patients.[5] Furthermore, to our knowledge, no tools exist that compare treatment-specific cancer-specific (CSM) and other-cause mortality (OCM) as well as treatment-specific morbidity. In this era of personalized medicine, understanding the role of comorbid conditions, age, and treatment-associated quality of life outcomes is imperative when designating appropriate treatment options for patients with localized RCMs.
Conventionally, more aggressive interventions are preferentially offered to young, healthy patients based on generally long life expectancy and the low likelihood of cure with adjuvant or salvage therapies for advanced renal cancers.[6] Conversely, in older patients with generally limited longevity and multimorbidity, the risks of perioperative morbidity and mortality are higher, and less invasive approaches may be preferable.[7–9] Alternatively, observation in the form of AS may be employed for small RCMs or in patients deemed high-risk surgical candidates. However, many patients fall into a gray zone, such as a young patient with multiple comorbidities or a robust older patient. Furthermore, recent retrospective studies support guideline-based recommendations for AS in carefully selected patients with small RCMs, with a low risk of development of metastasis and delayed intervention rates of less than 10%.[10, 11] Likewise, retrospective evaluation of experience at a high-volume center concerning ablative therapies in select patients demonstrated similar rates of local recurrence and CSM to extirpation,[8, 12] while population-based studies demonstrate improved oncologic efficacy over observation.[13]
Currently, counseling patients with cT1 RCMs relies predominantly on a subjective assessment of the risks of the disease and the benefits of procedures. However, the accuracy and precision with which urologists judge a patient’s physiologic reserve and longevity is notoriously inaccurate and highly variable.[14] Thus, the objective of this study was to develop and validate models to estimate individualized treatment-specific risks of CSM, OCM, and moderate-to-severe complications for patients with cT1 RCMs from a large, multi-institutional cohort with heterogeneous clinicopathologic features.
Methods
Study Design, Setting and Participants
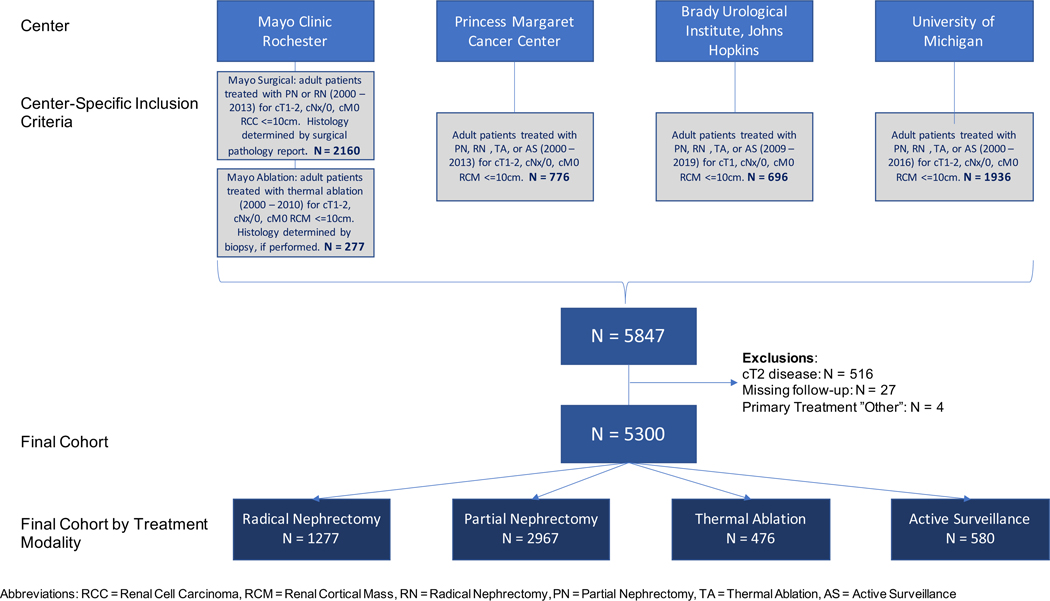
Following institutional review board approval, a registry of 5847 adult (age ≥ 18 years) consecutive patients with sporadic, unilateral, localized (cT1, cNx-0, cM0) RCMs ≤10.0 cm maximal diameter was developed from Mayo Clinic Rochester, Princess Margaret Cancer Center, Brady Urological Institute at Johns Hopkins, and University of Michigan. For the purposes of the current study, we limited our analysis to patients with cT1 masses only. Figure 1 depicts specific inclusion criteria across centers. Excluding patients with cT2+/x RCMs (N=516), no follow-up (N=27), or unspecified treatment (N=4) yielded a final cohort of 5300 evaluable patients.
Figure 1:
CONSORT diagram demonstrating patient inclusion criteria across centers, exclusion criteria, and cohort stratification by treatment.
Variables, Data Sources and Measurement
Following diagnosis and enrollment on AS or treatment with RN, PN, or TA, patients were surveyed for disease recurrence according to institutional practices, including radiographic testing approximately every 3–6 months for the first 2 years and yearly thereafter. The primary outcomes of interest for this study were CSM, OCM, and moderate-to-severe complications within 90 days of surgery or TA (Clavien grade 3–5 complications). For patients who died, timing and cause of death was ascertained by chart review by the treating physicians at the respective site of care.
Quantitative Variables and Statistical Methods
Patient characteristics were stratified by primary treatment (RN, PN, TA, AS) and compared using Kruskal-Wallis or chi-squared tests. Follow-up from the date of treatment (PN, RN, or TA), or after date of the clinic visit at which AS was initiated, was calculated using reverse Kaplan-Meier estimation.[15] Empirical summaries used Aalen-Johansen estimates of cumulative incidence for CSM and OCM and boxplots of continuous clinicopathologic features stratified by 90-day Clavien grade 0–2 versus 3–5. Outcomes of interest for this study were selected based on prior empirical and comparative effectiveness work [16, 17] to provide both intermediate and long-term outcomes that could further inform decision-making, specifically for older patients and those with a high degree of comorbidity.
Variables pre-specified for inclusion in the decision aid were: age (years), sex, Body Mass Index (BMI, categorized according to the World Health Organization thresholds), tumor diameter (cm), Eastern Cooperative Oncology Group Performance Status (ECOG PS), estimated glomerular filtration rate (eGFR, categorized according to Chronic Kidney Disease Stage [18]), and Charlson Comorbidity Index (CCI, excluding RCM). Year of diagnosis (2000–2009 or 2010–2019) was included to account for possible period effects. Predictions for CSM and Clavien grade 3–5 complications also included primary treatment.
Missing data for BMI (7.3%), tumor diameter (1.0%), ECOG PS (23%), eGFR (5.3%), CCI (25%), and year of diagnosis (2.7%) were imputed using fully conditional specification with predictive mean matching (tumor diameter) or polytomous regression (BMI, year of diagnosis, eGFR, ECOG PS, and CCI) accounting for race, year of diagnosis, year of treatment, BMI, eGFR, ECOG PS, CCI, ASA score, constitutional symptoms, calcium level, hematocrit level, diabetes, smoking status, hypertension, neutrophil-to-lymphocyte ratio, pulmonary or liver disease, tumor diameter, and other malignancies. Fitted imputation models were used to generate 10 datasets, and risk prediction models adjusting for the selected decision aid variables were fitted to each dataset. Estimates from the risk prediction models were combined across datasets according to Rubin’s rules after complementary log-log (for CSM and OCM) or logarithmic (for Clavien grade 3–5 complications) transformations.[19]
To evaluate risk prediction model performance, 10 bootstrap samples were drawn from the original dataset. The imputation model and prediction model fitting and procedure for combining across estimates were repeated for each bootstrap sample. Discrimination and calibration of the final risk predictions for 90-day complications and 5-year CSM and OCM from each bootstrap sample were then evaluated using the original dataset.[20] Discrimination between patients with and without events was assessed using the median and interquartile range (IQR) of the concordance index across 10 bootstrap samples and visualized using receiver operator characteristic curves. Calibration of absolute risks was assessed using median and 95% quantile intervals of the empirical proportions of events corresponding to a 10-group partition of the range of predicted probabilities for each outcome across 10 bootstrap samples with 10 imputed datasets for each sample.
Following this bootstrap validation[20, 21], final models based on the full dataset (N=5300) were used to predict outcomes for each treatment over an exhaustive grid of possible clinical and tumor features. An online calculator was developed to provide direct access to individualized predictions. Decision curve analyses for 5-year CSM, OCM, and 90-day severe complications were performed.
Statistical analyses were performed using R version 3.6.2. All tests were two-sided and p-values <0.05 were considered statistically significant. Results of the study were reported according to the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies.[22]
Results
Participants and Descriptive Data
Of the 5300 patients included in the study, 1277 (24%) were treated with RN, 2967 (56%) underwent PN, 476 (9.0%) were treated with TA, and 580 (11%) were managed with AS. Clinical and demographic features of the cohort are presented in Table 1. Among surgical patients, 802/1277 (63%) of patients undergoing RN and 1358/2967 (46%) undergoing PN were treated with a minimally invasive approach. Lymphadenectomy was performed in 147/1277 (12%) RN patients and 30/2967 (1.0%) of patients undergoing PN. Among 476 patients treated with TA, 262 (55%) underwent percutaneous radiofrequency ablation (RFA), 153 (32%) underwent percutaneous cryoablation, 57 (12%) received laparoscopic cryoablation, 3 (0.63%) underwent laparoscopic RFA, and 1 (0.21%) received open cryoablation.
Table 1:
Clinical and demographic features of the study cohort
| Radical nephrectomy | Partial nephrectomy | Thermal ablation | Active surveillance | P* | |
|---|---|---|---|---|---|
| n | 1277 | 2967 | 476 | 580 | |
| Age (n=5300) (median [IQR]) | 63 [54, 72] | 59 [50, 67] | 71 [63, 76] | 71 [64, 79] | <0.001 |
| Sex (n=5300) = Male (%) | 796 (62) | 1916 (64) | 302 (63) | 349 (60) | 0.17 |
| Race Group (n=4865) (%) | <0.001 | ||||
| White | 1003 (89) | 2516 (91) | 406 (90) | 413 (81) | |
| Black/African American | 53 (4.7) | 119 (4.3) | 16 (3.5) | 77 (15.0) | |
| Asian | 29 (2.6) | 60 (2.2) | 5 (1.1) | 14 (2.7) | |
| Other | 38 (3.4) | 84 (3.0) | 24 (5.3) | 8 (1.6) | |
| Site (n=5300) (%) | <0.001 | ||||
| Mayo | 528 (41.3) | 1389 (46.8) | 271 (56.9) | 0 (0.0) | |
| Toronto | 228 (18) | 253 (8.5) | 60 (13) | 153 (26) | |
| Hopkins | 43 (3.4) | 275 (9.3) | 28 (5.9) | 344 (59) | |
| Michigan | 478 (37) | 1050 (35) | 117 (25) | 83 (14) | |
| Year of Diagnosis (n=5155) = 2010–2019 (%) | 376 (30) | 1291 (45) | 101 (23) | 419 (72) | <0.001 |
| Calcium (n=2859) (median [IQR]) | 9.5 [9.2, 9.8] | 9.6 [9.3, 9.9] | 9.6 [9.2, 9.9] | 9.6 [9.1, 9.9] | 0.008 |
| Hemoglobin (n=4134) (median [IQR]) | 14 [13, 15] | 14 [13.1, 15.1] | 14 [12, 15] | 13 [12, 15] | <0.001 |
| Albumin (n=1856) (median [IQR]) | 4.2 [3.9, 4.4] | 4.3 [4.1, 4.5] | 4.2 [3.8, 4.4] | 4.2 [3.9, 4.5] | <0.001 |
| eGFR (n=5020) (median [IQR]) | 70 [53, 85] | 77 [62, 92] | 63 [48, 81] | 68 [50, 83] | <0.001 |
| BMI (n=4909) (median [IQR]) | 29 [26, 34] | 29 [26, 34] | 30 [26, 34] | 28.3 [25, 32] | <0.001 |
| BMI Group (n=4909) (%) | 0.001 | ||||
| 11.25–24.9 | 260 (22) | 510 (18) | 75 (19) | 111 (24) | |
| 25.0–29.9 | 406 (34) | 1002 (35) | 131 (32) | 184 (40) | |
| 30.0–34.9 | 325 (27) | 737 (26) | 112 (28) | 102 (22) | |
| 35.0–39.9 | 119 (9.9) | 341 (12) | 42 (10) | 39 (8.4) | |
| 40.0–74.9 | 93 (7.7) | 245 (8.6) | 45 (11) | 30 (6.4) | |
| Tumor Size (n=5248) (median [IQR]) | 4.6 [3.3, 6.0] | 3.0 [2.1, 4.0] | 2.5 [2.0, 3.2] | 1.9 [1.4, 2.7] | <0.001 |
| Constitutional Symptoms (n=3436) = Yes (%) | 97 (12) | 134 (7.1) | 77 (22) | 21 (5.3) | <0.001 |
| Weight Loss (n=3425) = Yes (%) | 34 (4.3) | 50 (2.6) | 15 (4.2) | 12 (3.0) | 0.097 |
| Night Sweats (n=2878) = Yes (%) | 4 (0.5) | 9 (0.5) | 2 (0.6) | 3 (2.0) | 0.2 |
| Hematuria (n=3562) = Yes (%) | 137 (17) | 154 (8.1) | 10 (2.8) | 47 (9.5) | <0.001 |
| Flank Pain (n=3567) = Yes (%) | 151 (19) | 267 (14) | 38 (11) | 27 (5.4) | <0.001 |
| Flank Mass (n=3567) = Yes (%) | 6 (0.8) | 6 (0.3) | 1 (0.3) | 0 (0.0) | 0.15 |
| Jaundice (n=2879) = Yes (%) | 5 (0.7) | 3 (0.2) | 0 (0.0) | 0 (0.0) | 0.12 |
| Lower Extremity Edema (n=2879) = Yes (%) | 49 (6.5) | 118 (7.2) | 18 (5.4) | 1 (0.7) | 0.01 |
| Thromboembolic Events (n=3552) = Yes (%) | 44 (5.5) | 83 (4.3) | 9 (2.5) | 20 (4.1) | 0.13 |
| ECOG (n=4106) = 2–4 (%) | 47 (5.4) | 46 (2.0) | 52 (13) | 25 (5.2) | <0.001 |
| ASA (n=4208) = 3–4 (%) | 635 (55) | 1181 (43) | 209 (70) | 18 (70) | <0.001 |
| Charlson (n=3949) (%) | <0.001 | ||||
| 1–2 | 369 (41) | 773 (36) | 141 (39) | 195 (38) | |
| 3–12 | 215 (24) | 368 (17) | 112 (31) | 123 (24) | |
| Smoking (n=4486) (%) | <0.001 | ||||
| Never | 452 (45) | 1154 (46) | 186 (44) | 310 (56) | |
| Current | 152 (15) | 407 (16) | 45 (11) | 51 (9.2) | |
| Former | 396 (40) | 948 (38) | 192 (45) | 190 (34) | |
| Unknown | 0 (0.0) | 1 (0.0) | 0 (0.0) | 2 (0.4) |
Continuous characteristics were compared using Kruskal-Wallis tests and categorical characteristics were compared using chi-squared tests.
Outcome Data
Over a median follow-up of 5.2 years (IQR 2.5–8.7), 117 patients died from RCC and 607 died from other causes. Five- and 10-year CSM was 2.0% and 3.7%, respectively; 5- and 10-year OCM was 9.3% and 21%, respectively. A total of 198/4720 (4.2%) patients experienced 90-day Clavien 3–5 complications, including 34/1277 (2.7%), 150/2967 (5.1%), and 14/476 (2.9%) among patients treated with RN, PN, and TA, respectively. Death within 90 days was observed in 2/1277 (0.16%) patients treated with RN and 2/2967 (0.067%) patients treated with PN.
Main Results
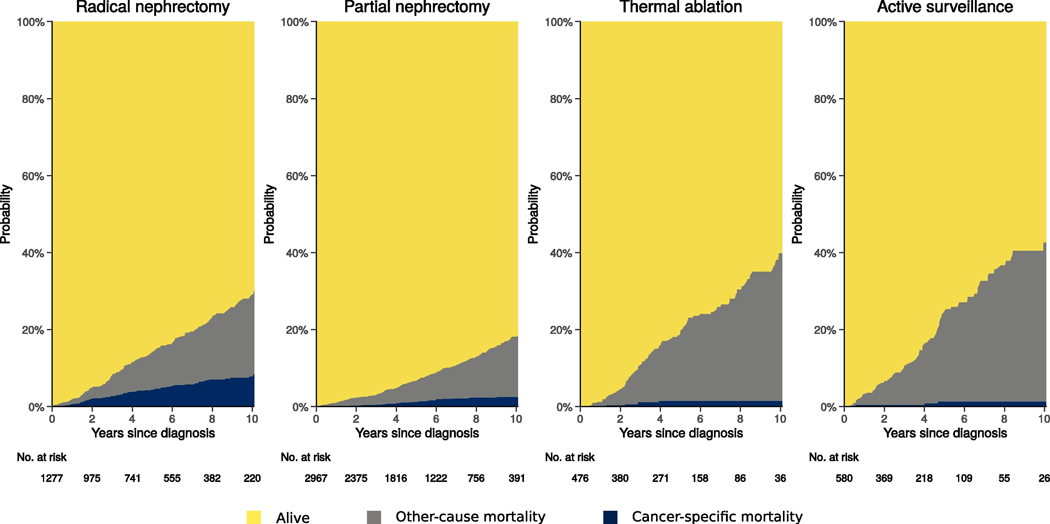
Patients treated with nephrectomy had higher probability of CSM and lower probability of OCM compared to those treated with TA or AS (Figure 2). Unsurprisingly, OCM was higher among individuals with ECOG PS 2–4 or CCI 1–12 (Supplemental Figures 1 and 2). Supplemental Figure 3 demonstrates variation in Clavien 3–5 complications across definitive treatments (RN, PN, and TA). Of note, larger tumors were associated with complications after PN (p<0.001) while lower pretreatment eGFR was associated with complications after RN (p<0.001) after Bonferroni adjustment for the 12 comparisons.
Figure 2:
Cumulative incidence of cancer-specific mortality and other-cause mortality by treatment.
The fitted risk models (Supplemental Tables 1–3) predicted that larger tumor diameter and higher CCI were associated with increased risks of CSM and odds of Clavien 3–5 complications. The risk of CSM was not significantly different across treatments or calendar periods. The odds of Clavien 3–5 complications were higher for patients treated with PN (p<0.001) compared to RN. Male sex, higher ECOG PS, and higher CCI were associated with increased risk of OCM.
Receiver operating curves demonstrated acceptable discrimination for 5-year CSM (median concordance index/area under the curve (AUC) 0.80, IQR 0.79–0.81) and OCM (AUC: 0.77, IQR 0.77–0.77), and a slightly lower discrimination for 90-day complications (AUC 0.64, IQR 0.64–0.65). Calibration plots for 10 bootstrap samples indicate moderate upward bias in predicted risks of 5-year CSM and OCM among patients in the highest risk groups (Supplemental Figure 4A). Decision curve analysis indicated that the risk of calculator outperforms all-or-nothing predictions for these outcomes, although differences are modest when these outcomes are unlikely, as they are for 5-year CSM and 90-day complications (Supplemental Figure 4B). If patients would only consider definitive treatment (RN, PN, or TA) if their risk of 5-year CSM were high (>10%) then the risk calculator is unlikely to be more useful than a simple prediction this outcome is very rare. A similar conclusion applies if their threshold for considering different definitive treatments requires the risk of 90-day complications to be high (>10%). However, if the thresholds for action based on these outcomes are lower, if the risk of 5-year OCM is determinative and their threshold for action is non-trivial (>2%), or if more than one outcome is relevant to decision-making around treatment,[52] then the risk calculator promises greater clinical utility than simple all-or-nothing predictions.
Results from the final risk prediction models are available via an online calculator (https://small-renal-mass-risk-calculator.fredhutch.org), where a user can input individual patient characteristics to obtain personalized treatment-specific risk predictions in a clinical setting. Figures 3A and 3B show exemplar outputs for a patient with a 4.5 cm RCM and varying clinical parameters.
Figure 3:
Example of competing risks predictions including 5-year CSM, OCM, and 90-day complications for (A) a 74-year-old male with a 4.5 cm RCM, BMI of 24 kg/m2, eGFR of 60 mL/min, ECOG PS of 0, and CCI of 1 compared to (B) a 74-year-old male with a 4.5 cm RCM, BMI of 32 kg/m2, eGFR of 50 mL/min, ECOG PS of 3, and CCI of 3.
Discussion
Patients with cT1 RCMs often present a treatment dilemma given that guideline-based care options may include AS, TA, or surgical extirpation via either PN or RN.[1, 2] While decision-making may be straightforward in patients in otherwise good health, for patients with competing comorbidities or significant functional deficits, this calculus is complex.
In this manuscript we present risk prediction models derived from 5300 patients with cT1 RCMs treated with AS, TA, PN, or RN that estimate personalized, treatment-specific 5- and 10-year risks of CSM and OCM as well as 90-day risks of moderate-to-severe complications. The models permit patients and clinicians to evaluate estimates of these short- and long-term outcomes across treatments. Model covariates were selected based on medical and empirical relevance, and traditional statistical models were used to facilitate interpretation and draw inference. The predictions incorporate granular patient-specific data, easily attainable at initial consultation, including performance status, comorbidity burden, BMI, and baseline kidney function.
Notably, especially with smaller masses, patients with localized, node-negative RCMs have low 5-year CSM overall. However, OCM increases significantly with increasing burden of comorbidities and decreasing performance status. Complication rates also increase with ECOG PS and tumor size, specifically for nephron-sparing treatments. Having both short- and long-term estimates offers significant potential benefits for patients with multiple medical risks in terms of providing quantitative predictions underlying the critical trade-offs across treatments. For example, in a patient with a high risk of 5-year OCM, the relevance of risk of major complications within 90 days may be weighed more heavily given the potential impact on short-term quality of life. While this calculus is commonly introduced in shared decision-making, quantification of these trade-offs generally relies on gestalt qualitative estimates made by the treating surgeon based on clinical experience. However, physician estimates of a patient’s life expectancy following an initial cancer diagnosis are frequently inaccurate, underscoring the need for validated estimates to quantify these trade-offs.[14, 23, 24]
Previously, multiple authors have developed nomograms to improve estimates of competing risks. Hollingsworth and colleagues proposed a model including age at diagnosis, race, marital status, and type of surgery, concluding that patients with small renal masses benefit the least from surgery with respect to risk of CSM.[25] However, the model did not incorporate comorbidity. Kutikov and colleagues developed a competing risks nomogram in a SEER-based cohort of over 30,000 patients with surgically resected localized renal cell carcinomas.[17] However, this model similarly did not account for comorbidity, and would not apply to patients who did not undergo surgery. In a subsequent iteration, the authors presented a comorbidity-based competing risks of death model that generated estimates of 5-year CSM, death from other malignancies, and noncancer death based on age, sex, race, tumor size, and CCI; however, the calculator was limited to patients over the age of 66 years treated with surgery.[16] Furthermore, granular data, such as performance status and BMI, was not accounted for.
Importantly, prior studies were limited to patients who underwent surgery. They did not include patients managed expectantly or on AS protocols, nor did they include patients treated with TA. Furthermore, these studies were also limited to patients with confirmed renal cell carcinoma on final pathology, and as such, may have limited generalizability to patients with indolent histology or benign masses. In the current study, patients with a cT1 RCM were included irrespective of pathology. While percutaneous biopsy is an option to discern histology in this scenario,[1] it remains underutilized in contemporary practice [26]. To illustrate, in the current cohort, biopsy was only performed in 24% of patients and was not included in the presented predictive models. In general, treatment decisions are commonly made according to imaging alone; however, as current guidelines would advocate, biopsies should be performed in all patients considering TA and should be considered in patients in whom the histologic diagnosis would influence decision-making[1]. Additionally, few prior studies have included outcomes among patients who did not undergo active intervention, and we are not aware of any studies to date that included performance status. Finally, we are not aware of any nomograms that incorporated individualized quantification of the risks of morbidity/mortality related to the treatment strategies themselves.
Prior studies that quantify competing risks in patients with small and localized renal cell carcinoma relied largely on the SEER database and other administrative datasets, demonstrating the complex interplay of age and comorbidity.[27–31] Additionally, the current study incorporates several patient-specific variables not included in previously published models, including BMI and baseline kidney function.
Regarding BMI, a recent metanalyses of 10,512 patients with renal cell carcinoma demonstrated that increasing BMI was paradoxically associated with decreased CSM but increased OCM.[32] BMI is also variably associated with complications after RN and PN.[33] Previously, Schmit and colleagues reported similar complication rates following percutaneous cryoablation of small renal masses among 367 patients, of whom 161 were obese and 39 were morbidly obese.[34] Consistent with these findings, we did not observe associations between increasing BMI and odds of 90-day Clavien grade 3–5 complications across treatments.
Baseline renal function represents a key clinical parameter assessed during treatment selection for cT1 RCMs given the potential implications for subsequent renal function decline if a patient elects RN vs. a nephron-sparing treatment (PN, TA, or AS). Nephron-sparing approaches are preferred when possible to avoid the risks of severe decline in renal function, eventual end-stage renal dysfunction, and subsequent hypertension, which have significant implications for long-term overall survival[35–37] as well as health-related quality of life.[38] However, a randomized trial and other observational studies have failed to demonstrate an association between overall survival and the risk of chronic kidney disease after RN.[36, 39] For patients with complex or larger masses or with greater surgical risks, the increased risks of prolonged anesthesia and perioperative complications with nephron-sparing approaches must also be weighed.[40] The current risk calculator incorporates baseline renal function in its estimation of competing risks, which may complement the output of previously published tools that predict post-treatment renal function based on preoperative patient-based factors,[41] imaging assessments of tumor volume and renal scintigraphy,[42] and tumor complexity.[43]
This study has several potential limitations. First, selection bias and variation in practice patterns across the treatment sites and over time may influence the results of this retrospective study: as expected, there was substantial heterogeneity in baseline characteristics across the treatment cohorts, and the estimates generated by the final models are subject to unmeasured confounding. We did evaluate heterogeneity across centers and found that associations with age, sex, and tumor diameter were generally robust. There was mixed evidence that associations with ECOG PS varied across centers, which we attribute to differing baseline risks. Additionally, the study cohort may be influenced by variations in practice patterns by surgeon volume and by center, however variation by surgeon volume was not assessed as surgeon identifiers were not available in the dataset. A sensitivity analysis excluding all patients from the center with the majority of missing ECOG PS and CCI data (Michigan) materially altered predicted risks, although differences across ECOG PS and CCI strata were limited relative to the overall differences (data not shown). Consequently, we retained this center in the main analysis for data efficiency, to reflect greater variation across centers, and to improve the generalizability of our results.
Assessments of practice patterns suggest that RN was more commonly employed for cT1a and cT1b renal masses than PN early on, with increasing recent preference for nephron-sparing approaches. Additionally, TA and AS were rarely utilized at the beginning of the study timeframe but have gained increasing acceptance in contemporary practice. We found CSM after TA was significantly lower in later years, possibly owing to a learning curve. To reflect contemporary patients, our online calculator uses predictions of baseline risk of CSM and of complications relevant to the most recent decade of experience. Owing to data limitations, treatment-specific period effects could not be reliably estimated. Furthermore, some centers contributed data for specific treatment groups only, e.g., the Mayo Clinic did not provide data on patients enrolled on AS. As such, there are fewer representative patients in the current cohort managed with AS, which may further limit the generalizability of these risk predictions. Importantly, the multicenter dataset for this cohort included only data on initial treatment strategy and not on subsequent treatments, such as the number of patients who transitioned from AS to definitive treatment or who underwent initial PN or TA and subsequently developed recurrence and received either RN or required systemic therapy. Additionally, there were few patients (n=30/5300, 0.57%) in this dataset with BMI meeting criteria for “underweight” (<18.5 kg/m2). Given the small number of patients in this category and the lack of stability of estimates for this group, these patients were combined with patients with normal weight, potentially limiting the generalizability of our estimates for underweight patients. We also acknowledge that the prediction model for complications in this dataset demonstrated lower discrimination (c-index 0.64) compared to the models for CSM and OCM, which may reflect the relatively low event rate for complications in the dataset. As such, counseling patients regarding individualized risks of complications after PN or RN may benefit from also including other robust, validated risk calculators, such as the American College of Surgeons NSQIP risk calculator, in the risk assessment[44], although this calculator does not predict the risk of adverse outcomes following TA. Similarly, the decision curve analysis indicates limited advantages over all-or-nothing predictions for 5-year CSM and 90-day Clavien complications. However, this evaluation does not account for how a patient might prioritize or weight personalized estimates of both short- and long-term outcomes during treatment decision-making.
Finally, while this study includes carefully collected preoperative personalized covariates, it does not include relevant factors that could influence outcomes, including patient frailty,[45–47] nutritional status,[48] or specific features related to the tumor anatomy, such as RENAL nephrometry score,[49–51] nor does it include all potential outcomes that may be relevant to a specific patient, including discharge disposition following treatment, return vs. maintenance of physical function, preservation of renal function, future burden of surveillance visits and imaging assessments, impact on mental health outcomes (e.g., anxiety, decisional conflict)[52], or intermediate oncologic outcomes, such as recurrence-free survival. To underscore this point, a recent collaborative review by Chandrasekar et al. highlights the complexity and variability in the potential salient outcomes for individuals with localized renal masses who are considering different treatment options[52]. Additionally, while we included the Charlson Comorbidity Index as a surrogate for comorbidity burden, there is increasing awareness of the fact that more granular assessments of comorbidity are available and may be more relevant when quantifying multimorbidity and its relevance in a surgical population.[53, 54] As detailed by the authors, no single tool or statistical model can replace a carefully considered counseling visit based around shared-decision-making with an experienced physician. However, the estimates generated by the models presented herein may further inform these discussions, permitting patients to better understand their personalized risk predictions of periprocedural complications and long-term survival outcomes associated with each treatment. Because the risk predictions are only for an incomplete set of outcomes relevant to shared decision-making, we did not evaluate their clinical utility using established methods like decision curve analysis.[55] It is also of note that outcomes were assessed by treating physicians at each center through retrospective review of electronic health records rather than centralized review of certified death records. By assessing performance using bootstrap samples, the potential for overfitting or unsupported optimism is controlled, and data from all patients can be used in the final fitted models. However, external validation using data from patients treated at other institutions is necessary to establish broader generalizability of the predicted risks.
In summary, we present novel clinical risk prediction models of mortality and 90-day periprocedural moderate-to-severe complications for patients with localized RCM ⍤ 7 cm accounting for tumor size, patient age, sex, BMI, ECOG PS, and CCI across standard treatments. This tool generates personalized, treatment-specific risk estimates of short- and long-term outcomes, providing individualized projections regarding the potential trade-offs of each treatment option for a patient and his or her providers to inform shared decision-making regarding the management of a cT1 RCM.
Supplementary Material
Supplemental Figure 1: Cumulative incidence of cancer-specific mortality and other-cause mortality stratified by treatment and ECOG PS (N=4,106).
Supplemental Figure 2: Cumulative incidence of cancer-specific mortality and other-cause mortality stratified by treatment and Charlson comorbidity index (N=3,949).
Supplemental Figure 3: Prevalence of Clavien grade 0–2 vs. 3–5 complications across definitive treatments by continuous covariates.
Supplemental Figure 4: (A) Bootstrap validation of fitted risk prediction models. Calibration plots for 10 bootstrap samples showing satisfactory calibration for 5-year CSM and OCM and 90-day complications. (B) Decision curve analysis comparing the risk calculator predictions to all-or-nothing predictions.
Funding:
Mr. Gulati is funded under NIH grant R50 CA221836
Footnotes
Financial Disclosures/Conflicts of interest: None
References
- [1].Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, et al. Renal Mass and Localized Renal Cancer: AUA Guideline. The Journal of urology. 2017. [DOI] [PubMed] [Google Scholar]
- [2].Network NCC. NCCN Clinical Practice Guidelines in Oncology: Kidney Cancer Version 2.2021 2021. [Google Scholar]
- [3].L’jungberg BA L; Bedke J; Bex A; Capitanio U; Giles RH; Hora M; Klatte T; Lam T; Marconi L; Powles T; Volpe A . European Association of Urology Guidelines: Renal Cell Carcinoma. Arnhem, The Netherlands: EAU Guidelines Office; 2021. [Google Scholar]
- [4].Chinese guidelines for diagnosis and treatment of renal cell carcinoma 2018 (English version). Chin J Cancer Res. 2019;31:29–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shah PH, Alom MA, Leibovich BC, Thompson RH, Uzzo RG, Kavoussi LR, et al. The Temporal Association of Robotic Surgical Diffusion with Overtreatment of the Small Renal Mass. J Urol. 2018;200:981–8. [DOI] [PubMed] [Google Scholar]
- [6].Kunkle DA, Haas NB, Uzzo RG. Adjuvant therapy for high-risk renal cell carcinoma patients. Current urology reports. 2007;8:19–30. [DOI] [PubMed] [Google Scholar]
- [7].An JY, Ball MW, Gorin MA, Hong JJ, Johnson MH, Pavlovich CP, et al. Partial vs Radical Nephrectomy for T1-T2 Renal Masses in the Elderly: Comparison of Complications, Renal Function, and Oncologic Outcomes. Urology. 2017;100:151–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pierorazio PM, Johnson MH, Patel HD, Sozio SM, Sharma R, Iyoha E, et al. Management of Renal Masses and Localized Renal Cancer: Systematic Review and Meta-analysis. The Journal of urology. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. European urology. 2015;67:252–9. [DOI] [PubMed] [Google Scholar]
- [10].Pierorazio PM, Johnson MH, Ball MW, Gorin MA, Trock BJ, Chang P, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: the DISSRM registry. European urology. 2015;68:408–15. [DOI] [PubMed] [Google Scholar]
- [11].Mir MC, Capitanio U, Bertolo R, Ouzaid I, Salagierski M, Kriegmair M, et al. Role of Active Surveillance for Localized Small Renal Masses. Eur Urol Oncol. 2018;1:177–87. [DOI] [PubMed] [Google Scholar]
- [12].Andrews JR, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A, et al. Oncologic Outcomes Following Partial Nephrectomy and Percutaneous Ablation for cT1 Renal Masses. Eur Urol. 2019;76:244–51. [DOI] [PubMed] [Google Scholar]
- [13].Larcher A, Trudeau V, Sun M, Boehm K, Meskawi M, Tian Z, et al. Population-based assessment of cancer-specific mortality after local tumour ablation or observation for kidney cancer: a competing risks analysis. BJU Int. 2016;118:541–6. [DOI] [PubMed] [Google Scholar]
- [14].Wilson JR, Clarke MG, Ewings P, Graham JD, MacDonagh R. The assessment of patient life-expectancy: how accurate are urologists and oncologists? BJU international. 2005;95:794–8. [DOI] [PubMed] [Google Scholar]
- [15].Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6. [DOI] [PubMed] [Google Scholar]
- [16].Kutikov A, Egleston BL, Canter D, Smaldone MC, Wong YN, Uzzo RG. Competing risks of death in patients with localized renal cell carcinoma: a comorbidity based model. The Journal of urology. 2012;188:2077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kutikov A, Egleston BL, Wong YN, Uzzo RG. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].KDIGO. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney international. 2013;3. [DOI] [PubMed] [Google Scholar]
- [19].Marshall A, Altman DG, Holder RL, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Steyerberg EW, Harrell FE Jr., Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. [DOI] [PubMed] [Google Scholar]
- [21].Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–7. [DOI] [PubMed] [Google Scholar]
- [23].Christakis NA, Lamont EB. Extent and determinants of error in physicians’ prognoses in terminally ill patients: prospective cohort study. West J Med. 2000;172:310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lamont EB, Christakis NA. Complexities in prognostication in advanced cancer: “to help them live their lives the way they want to”. Jama. 2003;290:98–104. [DOI] [PubMed] [Google Scholar]
- [25].Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109:1763–8. [DOI] [PubMed] [Google Scholar]
- [26].Shahait M, Jackman S, Landman J, Lechevallier E, Billiet I, Fossion L, et al. Utilization and Operative Influence of Renal Mass Biopsy in the Small Renal Mass: Analysis from the Clinical Research Office of the Endourological Society Small Renal Mass registry. J Endourol. 2019. [DOI] [PubMed] [Google Scholar]
- [27].Bianchi M, Gandaglia G, Trinh QD, Hansen J, Becker A, Abdollah F, et al. A population-based competing-risks analysis of survival after nephrectomy for renal cell carcinoma. Urologic oncology. 2014;32:46.e1-.e7. [DOI] [PubMed] [Google Scholar]
- [28].Sun M, Abdollah F, Bianchi M, Trinh QD, Jeldres C, Tian Z, et al. A stage-for-stage and grade-for-grade analysis of cancer-specific mortality rates in renal cell carcinoma according to age: a competing-risks regression analysis. European urology. 2011;60:1152–9. [DOI] [PubMed] [Google Scholar]
- [29].Sun M, Bianchi M, Trinh QD, Hansen J, Abdollah F, Hanna N, et al. Comparison of partial vs radical nephrectomy with regard to other-cause mortality in T1 renal cell carcinoma among patients aged >/=75 years with multiple comorbidities. BJU international. 2013;111:67–73. [DOI] [PubMed] [Google Scholar]
- [30].Patel HD, Kates M, Pierorazio PM, Gorin MA, Jayram G, Ball MW, et al. Comorbidities and causes of death in the management of localized T1a kidney cancer. International journal of urology : official journal of the Japanese Urological Association. 2014;21:1086–92. [DOI] [PubMed] [Google Scholar]
- [31].Patel HD, Kates M, Pierorazio PM, Hyams ES, Gorin MA, Ball MW, et al. Survival after diagnosis of localized T1a kidney cancer: current population-based practice of surgery and nonsurgical management. Urology. 2014;83:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bagheri M, Speakman JR, Shemirani F, Djafarian K. Renal cell carcinoma survival and body mass index: a dose-response meta-analysis reveals another potential paradox within a paradox. Int J Obes (Lond). 2016;40:1817–22. [DOI] [PubMed] [Google Scholar]
- [33].Arora K, Hanson K, Habermann EB, Tollefson MK, Psutka SP. Nutritional Predictors of Perioperative Complications and Mortality Following Nephrectomy for Renal Malignancies: A Population-Based Analysis. Kidney Cancer. 2018;2:147–74. [Google Scholar]
- [34].Schmit GD, Thompson RH, Boorjian SA, McDonald RJ, Kurup AN, Weisbrod AJ, et al. Percutaneous renal cryoablation in obese and morbidly obese patients. Urology. 2013;82:636–41. [DOI] [PubMed] [Google Scholar]
- [35].Shah PH, Leibovich BC, Van Houten H, Lyon TD, Yao X, Knoedler M, et al. Association of Partial versus Radical Nephrectomy with Subsequent Hypertension Risk Following Renal Tumor Resection. J Urol. 2019;202:69–75. [DOI] [PubMed] [Google Scholar]
- [36].Gershman B, Thompson RH, Boorjian SA, Lohse CM, Costello BA, Cheville JC, et al. Radical Versus Partial Nephrectomy for cT1 Renal Cell Carcinoma. Eur Urol. 2018;74:825–32. [DOI] [PubMed] [Google Scholar]
- [37].Capitanio U, Larcher A, Cianflone F, Trevisani F, Nini A, Mottrie A, et al. Hypertension and Cardiovascular Morbidity Following Surgery for Kidney Cancer. Eur Urol Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- [38].Lesage K, Joniau S, Fransis K, Van Poppel H. Comparison between open partial and radical nephrectomy for renal tumours: perioperative outcome and health-related quality of life. Eur Urol. 2007;51:614–20. [DOI] [PubMed] [Google Scholar]
- [39].Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. European urology. 2011;59:543–52. [DOI] [PubMed] [Google Scholar]
- [40].Kim SP, Campbell SC, Gill I, Lane BR, Van Poppel H, Smaldone MC, et al. Collaborative Review of Risk Benefit Trade-offs Between Partial and Radical Nephrectomy in the Management of Anatomically Complex Renal Masses. Eur Urol. 2017;72:64–75. [DOI] [PubMed] [Google Scholar]
- [41].Bhindi B, Lohse CM, Schulte PJ, Mason RJ, Cheville JC, Boorjian SA, et al. Predicting Renal Function Outcomes After Partial and Radical Nephrectomy. Eur Urol. 2019;75:766–72. [DOI] [PubMed] [Google Scholar]
- [42].Mitsui Y, Sadahira T, Araki M, Maruyama Y, Nishimura S, Wada K, et al. The 3-D Volumetric Measurement Including Resected Specimen for Predicting Renal Function AfterRobot-assisted Partial Nephrectomy. Urology. 2019;125:104–10. [DOI] [PubMed] [Google Scholar]
- [43].Bertolo R, Garisto J, Li J, Dagenais J, Kaouk J. Development and Internal Validation of a Nomogram for Predicting Renal Function after Partial Nephrectomy. Eur Urol Oncol. 2019;2:106–9. [DOI] [PubMed] [Google Scholar]
- [44].Bilimoria KY, Liu Y, Paruch JL, Zhou L, Kmiecik TE, Ko CY, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217:833–42.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Revenig LM, Canter DJ, Taylor MD, Tai C, Sweeney JF, Sarmiento JM, et al. Too frail for surgery? Initial results of a large multidisciplinary prospective study examining preoperative variables predictive of poor surgical outcomes. Journal of the American College of Surgeons. 2013;217:665–70.e1. [DOI] [PubMed] [Google Scholar]
- [46].Joseph B, Pandit V, Sadoun M, Zangbar B, Fain MJ, Friese RS, et al. Frailty in surgery. The journal of trauma and acute care surgery. 2014;76:1151–6. [DOI] [PubMed] [Google Scholar]
- [47].Ethun CG, Bilen MA, Jani AB, Maithel SK, Ogan K, Master VA. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA: a cancer journal for clinicians. 2017;67:362–77. [DOI] [PubMed] [Google Scholar]
- [48].Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, et al. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. European urology. 2011;59:923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Camacho JC, Kokabi N, Xing M, Master VA, Pattaras JG, Mittal PK, et al. R.E.N.A.L. (Radius, exophytic/endophytic, nearness to collecting system or sinus, anterior/posterior, and location relative to polar lines) nephrometry score predicts early tumor recurrence and complications after percutaneous ablative therapies for renal cell carcinoma: a 5-year experience. J Vasc Interv Radiol. 2015;26:686–93. [DOI] [PubMed] [Google Scholar]
- [50].Dahlkamp L, Haeuser L, Winnekendonk G, von Bodman C, Frey UH, Epplen R, et al. Interdisciplinary Comparison of PADUA and R.E.N.A.L. Scoring Systems for Prediction of Conversion to Nephrectomy in Patients with Renal Mass Scheduled for Nephron Sparing Surgery. J Urol. 2019;202:890–8. [DOI] [PubMed] [Google Scholar]
- [51].Schiavina R, Novara G, Borghesi M, Ficarra V, Ahlawat R, Moon DA, et al. PADUA and R.E.N.A.L. nephrometry scores correlate with perioperative outcomes of robot-assisted partial nephrectomy: analysis of the Vattikuti Global Quality Initiative in Robotic Urologic Surgery (GQI-RUS) database. BJU Int. 2017;119:456–63. [DOI] [PubMed] [Google Scholar]
- [52].Chandrasekar T, Boorjian SA, Capitanio U, Gershman B, Mir MC, Kutikov A. Collaborative Review: Factors Influencing Treatment Decisions for Patients with a Localized Solid Renal Mass. Eur Urol. 2021. [DOI] [PubMed] [Google Scholar]
- [53].Psutka SP, Barocas DA, Catto JWF, Gore JL, Lee CT, Morgan TM, et al. Staging the Host: Personalizing Risk Assessment for Radical Cystectomy Patients. European Urology Oncology. 2018;1:292–304. [DOI] [PubMed] [Google Scholar]
- [54].Garg T, Young AJ, Kost KA, Danella JF, Larson S, Nielsen ME, et al. Burden of Multiple Chronic Conditions among Patients with Urological Cancer. The Journal of urology. 2018;199:543–50. [DOI] [PubMed] [Google Scholar]
- [55].Elkin EB, Vickers AJ, Kattan MW. Primer: using decision analysis to improve clinical decision making in urology. Nat Clin Pract Urol. 2006;3:439–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Cumulative incidence of cancer-specific mortality and other-cause mortality stratified by treatment and ECOG PS (N=4,106).
Supplemental Figure 2: Cumulative incidence of cancer-specific mortality and other-cause mortality stratified by treatment and Charlson comorbidity index (N=3,949).
Supplemental Figure 3: Prevalence of Clavien grade 0–2 vs. 3–5 complications across definitive treatments by continuous covariates.
Supplemental Figure 4: (A) Bootstrap validation of fitted risk prediction models. Calibration plots for 10 bootstrap samples showing satisfactory calibration for 5-year CSM and OCM and 90-day complications. (B) Decision curve analysis comparing the risk calculator predictions to all-or-nothing predictions.